Abstract
Dietary salt restriction is a well-established approach to lower blood pressure and reduce cardiovascular disease risk in hypertensive individuals. However, little is currently known regarding the effects of salt restriction on central and peripheral hemodynamic responses to exercise in those with hypertension. Therefore, this study sought to determine the impact of salt restriction on the central and peripheral hemodynamic responses to static-intermittent handgrip (HG) and dynamic single-leg knee extension (KE) exercise in individuals with hypertension. Twenty-two subjects (14 men and 8 women, 51 ± 10 yr, 173 ± 11 cm, 99 ± 23 kg) forewent their antihypertensive medication use for at least 2 wk before embarking on a 5-day liberal salt (LS: 200 mmol/day) diet followed by a 5-day restricted salt (RS: 10 mmol/day) diet. Subjects were studied at rest and during static intermittent HG exercise at 15, 30, and 45% of maximal voluntary contraction and KE exercise at 40, 60, and 80% of maximum KE work rate. Salt restriction lowered resting systolic blood pressure (supine: −12 ± 12 mmHg, seated: −17 ± 12 mmHg) and diastolic blood pressure (supine: −3 ± 9 mmHg, seated: −5 ± 7 mmHg, P < 0.05). Despite an ~8 mmHg lower mean arterial blood pressure during both HG and KE exercise following salt restriction, neither central nor peripheral hemodynamics were altered. Therefore, salt restriction can lower blood pressure during exercise in subjects with hypertension, reducing the risk of cardiovascular events, without impacting central and peripheral hemodynamics during either arm or leg exercise.
NEW & NOTEWORTHY This is the first study to examine the potential blood pressure-lowering benefit of a salt-restrictive diet in individuals with hypertension without any deleterious effects of exercising blood flow. While mean arterial pressure decreased by ~8 mmHg following salt restriction, these findings provide evidence for salt restriction to provide protective effects of reducing blood pressure without inhibiting central or peripheral hemodynamics required to sustain arm or leg exercise in subjects with hypertension.
Keywords: blood flow, essential hypertension, handgrip exercise, knee extension exercise
INTRODUCTION
Hypertension affects more than half of adults globally and represents a major risk factor for cardiovascular disease (28). Disconcertingly, the cardiovascular response to exercise in individuals with hypertension is characterized by altered central and peripheral hemodynamic responses, specifically, an exaggerated increase in blood pressure (i.e., the exercise pressor response) and diminished blood flow to the active skeletal muscle (3, 9, 15, 19, 32, 36, 39). This exaggerated response has been linked to increased risk of cardiovascular events, such as stroke and coronary ischemia both during and following exercise (17, 23, 29–31, 47). This presents an interesting paradox, calling into question the utility of exercise to control blood pressure in hypertensive individuals (41). Furthermore, the impaired skeletal muscle perfusion may limit exercise tolerance, leading to the early onset of fatigue and limiting physical activity, as well as exercise capacity.
Lifestyle modifications, including exercise and dietary interventions, represent a multifactorial approach to ameliorate high blood pressure in hypertensive individuals (45). In addition to exercise, lowering one’s salt intake is often recommended, as high-salt intake is associated with elevated blood pressure (12) and contributes to the increased cardiovascular event risk (1). Indeed, lowering dietary salt intake is a well-studied lifestyle modification that can quickly lower resting blood pressure and, subsequently, the risk of cardiovascular disease (16). However, less is known regarding the effects of salt restriction on central and peripheral hemodynamic responses to exercise in hypertensive individuals, which are altered by hypertension and contribute to exercise tolerance (3, 9, 15, 19, 32, 36, 39).
Few studies have examined the effects of salt restriction on central or peripheral hemodynamics during exercise in hypertension. Previous work in rodents revealed that a high-salt diet augmented the exercise pressor response, measured as the change in blood pressure from rest to exercise, suggesting an association between salt intake and the exercise pressor response (48). Furthermore, mild salt restriction (120 mmol/day) modestly lowered resting blood pressure but increased peripheral resistance in adults with essential hypertension, effects that were carried over to whole body cycling exercise, but did not lower the magnitude of the blood pressure response to exercise (34). If blood flow is to be maintained in the presence of lower blood pressure during exercise, elevated vascular conductance likely contributes to the maintained hyperemic response. However, this has yet to be investigated in individuals with hypertension. Furthermore, mild salt restriction improves vascular function in essential hypertension, as assessed by the vasodilatory response to the brachial artery flow-mediated dilation test (21), which may facilitate enhanced vasodilation during exercise. In combination, these previous findings suggest that lowering dietary salt may attenuate the blood pressure response to exercise and augment peripheral vasodilation in hypertensive individuals. A better understanding of the hemodynamic responses to exercise with salt restriction may, therefore, provide evidence for a nonpharmacological approach to alleviate symptoms of hypertension and allow for improved central and peripheral hemodynamic responses to exercise, ultimately, improving the safety, efficacy, and utility of exercise prescription for the treatment of hypertension.
Therefore, this study sought to comprehensively investigate the impact of salt restriction on central and peripheral hemodynamics during arm and leg exercise in subjects with essential hypertension. We hypothesized that salt restriction would lower resting blood pressure, attenuate the blood pressor response to exercise, and improve blood flow and vascular conductance during both handgrip (HG) and knee extension (KE) exercise.
METHODS
Subjects.
A total of 22 subjects with diagnosed essential hypertension (14 men and 8 women) were recruited to participate in this study. Antihypertensive medications were discontinued for at least 2 wk before experimentation, according to a well-defined and previously used protocol (43, 44). The protocol was approved by and written informed consent was obtained according to the Institutional Review Board of the University of Utah and the Salt Lake City Veterans Affairs Medical Center.
Protocols.
All subjects were studied in a thermoneutral environment, reporting to the laboratory fasted and forgoing caffeine and exercise for 24 h before each study visit. Subjects reported to the laboratory on a preliminary day to complete a health history questionnaire, physical examination, and perform a graded single-leg, knee-extensor test to determine maximal work rate. During the experimental phase of the protocol, participants completed 5 days of liberal salt (LS) (200 mmol/day) followed by 5 days of restricted salt (RS) (10 mL/day) with each diet also containing 100 mmol/day potassium and 20 mmol/day calcium, as previously described (43, 44). Testing of the two experimental conditions was separated by 7 days, and all medications (non-antihypertensive) and supplements remained consistent between trials. All food and liquids consumed during the RS diet were prepared by the University of Utah’s Center for Clinical and Translation Science bionutritionist using the Harris-Benedict equation to calculate daily energy requirements with adjustments for activity level (38).
Resting blood pressure.
Blood pressure was assessed in both the supine and seated positions by automated sphygmomanometry (Tango M2, SunTech Medical, Morrisville, NC). Following instrumentation, participants were asked to relax and avoid movement and conversation for 5 min. Blood pressure was assessed in triplicate with 1 min of recovery between assessments. Values were averaged to determine resting blood pressure (2), which has previously been shown to closely approximate 24-h blood pressure in most hypertensive individuals (7).
HG exercise.
Before performing HG exercise, subjects rested in the supine position for ~10 min with their right arm abducted at 90°. The elbow joint was extended at heart level to allow subjects to perform HG exercise. First, maximal voluntary contraction (MVC) was established by taking the highest of three maximal contractions using a HG dynamometer (TSD121C, Biopac Systems, Goleta, CA). Static-intermittent HG exercise was performed at three relative workloads (15, 30, and 45% of MVC). Each exercise intensity was performed for 3 min to ensure the attainment of steady-state hemodynamics, and 2 min of recovery was allotted between bouts. Guidance was provided by a metronome set at a rate of 1 Hz, and real-time force output was displayed to provide visual feedback to the subjects. Rating of perceived exertion (RPE) was assessed during the last 30 s of HG exercise, according the Borg 1–10 scale (6). Handgrip exercise was performed in 22 subjects (14 men and 8 women).
KE exercise.
The KE paradigm implemented in this study has been described in detail previously (24). Briefly, subjects were positioned on an adjustable chair with a cycle ergometer (model 828E; Monark Exercise, Vansbro, Sweden) positioned behind them. Resistance was created by applying friction to the flywheel, which was turned by the subject via a bar connecting the crank arm of the ergometer to a metal boot worn by the subject. Subjects rested in this position for ~10 min before starting KE exercise, where they maintained 60 contractions/min using visual feedback from a cadence sensor. Each exercise intensity was performed for 3 min to ensure the attainment of steady-state hemodynamics and 2 min of recovery was allotted between bouts. RPE was assessed during the last 30 s of KE exercise according the Borg 1–10 scale (6). KE exercise was performed in 18 subjects (10 men and 8 women).
Peripheral hemodynamics.
Both at rest and during HG and KE exercise, blood velocity and vessel diameter of the brachial and common femoral artery were determined in the right arm and leg, respectively, using an ultrasound Doppler system (GE Medical Systems, Milwaukee, WI) operating in duplex mode. The brachial artery was insonated approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle. The common femoral artery was insonated 2–3 cm proximal to the bifurcation of the common femoral artery. Blood velocity was measured using a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2–25 kHz). Sample volume was optimized in relation to vessel diameter and centered within the vessel. Vessel artery diameter was obtained during end diastole (corresponding to each R wave documented by the simultaneous ECG signal) using the same transducer at an imaging frequency ranging from 9 to 14 MHz. An angle of insonation of ≤60° was maintained for all measurements (27). Brachial artery diameter, at rest and during HG exercise, was determined offline from end-diastolic, ECG R wave-triggered images using automated edge-detection software (Medical Imaging Applications, Coralville, Iowa), which has been described in detail previously (35). Common femoral artery diameter measured at rest was used as a constant throughout the KE exercise, as the common femoral artery does not dilate during exercise (37). Ultrasound Doppler measurements were performed continuously, with the last 60 s of each exercise intensity used for the determination of limb blood flow. Blood flow, assessed in the arm (ABF) and leg (LBF), was calculated with the formula: Blood flow (mL/min) = (Vmean × π(vessel diameter/2)2 × 60, and vascular conductance (VC), assessed in the arm (AVC) and leg (LVC), was calculated as VC (mL·min−1·mmHg) = blood flow/mean arterial blood pressure (MAP).
Central hemodynamics.
Heart rate (HR) was monitored from a standard three-lead ECG. Stroke volume (SV) was calculated from photoplethysmography (Finometer, Finapres Medical Systems BV, Amsterdam, Netherlands) measurements and then calculated using the Modelflow method, which accounts for age, sex, height, and weight (Beatscope version 1.1; Finapres Medical Systems, Amsterdam, The Netherlands) (5) and has been documented to accurately track SV during a variety of experimental protocols, including exercise (8). Cardiac output (CO) was then calculated from the product of HR and SV. Systolic (SBP), diastolic (DBP), and MAP were measured by automated plethysmography (Tango M2, SunTech Medical, Morrisville, NC). During exercise, blood pressure was measured in duplicate at minute 1.5 and minute 2.5 of each exercise bout. MAP was calculated as MAP (mmHg) = DBP + (pulse pressure × 0.33). Total peripheral resistance (TPR) was calculated as TPR = MAP/CO.
Statistical analyses.
Statistics were performed using commercially available software (SigmaStat 3.10; Systat Software, Point Richmond, CA). Baseline comparisons were made using a paired t-test. A 2 × 3 repeated-measures ANOVA (α < 0.05) (group, two levels: LS vs. RS) (workload, three levels: HG exercise: 15, 30, and 45% MVC; KE exercise: 40, 60, 80% KE Max) was performed to determine the hemodynamic responses. The Holm-Sidak method was used for α-adjustment and post hoc analysis. Because of potential differences in hemodynamic responses based on sex and body mass, LS and RS changes in each variable were compared between men and women using an unpaired t-test, and a Pearson correlation was used to assess relationships between body mass index and salt-induced changes. These analyses revealed that neither sex nor BMI influenced the impact of RS on any of the measured variables; for this reason, men and women, regardless of BMI, were combined into the overall analysis. Subject characteristics presented in the text and tables are expressed as means ± SD. Group hemodynamic data presented in figures are expressed as means ± SE for clarity.
RESULTS
Subject characteristics.
Subject characteristics and blood chemistries are reported in Table 1. Body weight decreased significantly following RS (LS: 99.4 ± 23.7 kg, RS: 96.5 ± 22.4 kg, P < 0.001). In general, blood characteristics fell into the normal range. Red blood cell (LS: 5.03 ± 0.57 M/μL, RS: 5.20 ± 0.57 M/μL), hemoglobin (LS: 14.9 ± 1.3 g/dL, RS: 15.5 ± 1.5 g/dL), and hematocrit (LS: 44.2 ± 4.0%, RS: 45.6 ± 1.5%) increased in response to RS (P < 0.05).
Table 1.
Subject characteristics during liberal salt condition
| Liberal Salt | Reference Range | |
|---|---|---|
| Anthropometric measures | ||
| Age, yr | 50.5 ± 10.1 | |
| Sex (men/women), n | 14/8 | |
| Height, cm | 173.1 ± 10.8 | |
| Weight, kg | 99.4 ± 23.3 | |
| BMI, kg/m2 | 33.0 ± 5.7 | |
| KE Max work rate, W | 35.3 ± 16.7 | |
| HG Max force, kg | 20.1 ± 4.1 | |
| Blood measures | ||
| WBC, K/uL | 6.38 ± 1.96 | 3.20–10.60 |
| Sodium, mmol/L | 141.4 ± 3.1 | 136–144 |
| Potassium, mmol/L | 4.4 ± 0.4 | 3.3–5.0 |
| Chloride, mmol/L | 103.0 ± 4.2 | 102–110 |
| Carbon dioxide, mmol/L | 23.3 ± 2.3 | 20–26 |
| BUN, mg/dL | 15.1 ± 3.1 | 8–24 |
| Creatinine, mg/dL | 0.94 ± 0.15 | 0.72–1.25 |
| Glucose, mg/dL | 98.8 ± 11.9 | 64–128 |
| Calcium, mg/dL | 9.6 ± 0.6 | 8.4–10.5 |
| Protein, total, g/dL | 7.1 ± 0.5 | 6.5–8.4 |
| Albumin g/dL | 4.5 ± 0.2 | 3.5–5.0 |
| HgbA1C, % | 5.7 ± 0.4 | <6.0 |
Values are means ± SD. BMI, body mass index; KE Max work rate, knee extension exercise maximum work rate; HG Max force, handgrip exercise maximum force; WBC, white blood cell; BUN, blood urea nitrogen; HgbA1C, glycated hemoglobin.
Resting hemodynamics.
Resting hemodynamic variables for LS and RS are reported in Table 2. SBP, DBP, and MAP were significantly reduced following RS compared with LS (P < 0.001) (Table 2). HR, SV, CO, and TPR were, generally, not different between LS and RS in both the supine and seated positions at rest. ABF and AVC were not different between LS and RS conditions before HG exercise in the supine position. LBF was significantly lower during RS compared with LS in the seated position before knee extension exercise (P < 0.05), although LVC was unaltered by RS.
Table 2.
Resting hemodynamics
| Liberal Salt | Restricted Salt | |
|---|---|---|
| Supine hemodynamics | ||
| Brachial artery blood flow, mL/min | 87 ± 42 | 86 ± 42 |
| Brachial artery vascular conductance, mL·min−1·mmHg−1 | 0.8 ± 0.4 | 0.9 ± 0.4 |
| Systolic blood pressure, mmHg | 135 ± 15 | 122 ± 13* |
| Diastolic blood pressure, mmHg | 84 ± 10 | 80 ± 7* |
| Mean arterial pressure, mmHg | 102 ± 9 | 94 ± 9* |
| Heart rate, beats/min | 64 ± 11 | 64 ± 13 |
| Stroke volume, mL | 120 ± 34 | 118 ± 53 |
| Cardiac output, L/min | 7.5 ± 2.0 | 7.3 ± 3.0 |
| Total peripheral resistance, mmHg·mL−1·min | 14.3 ± 3.4 | 14.6 ± 4.5 |
| Seated hemodynamics | ||
| Femoral artery blood flow, mL/min | 391 ± 129 | 339 ± 128* |
| Femoral artery vascular conductance, mL·min−1·mmHg−1 | 3.9 ± 1.4 | 3.7 ± 1.6 |
| Systolic blood pressure, mmHg | 141 ± 15 | 124 ± 13* |
| Diastolic blood flow, mmHg | 84 ± 9 | 78 ± 10* |
| Mean arterial pressure, mmHg | 103 ± 9 | 94 ± 10* |
| Heart rate, beats/min | 66 ± 11 | 65 ± 10 |
| Stroke volume, mL | 111 ± 42 | 100 ± 37 |
| Cardiac output, L/min | 7.2 ± 2.5 | 6.5 ± 2.4 |
| Total peripheral resistance, mmHg·mL−1·min | 15.7 ± 5.1 | 15.8 ± 5.0 |
Values are means ± SD; n = 22 (14 men/8 women). Resting comparisons were made using a paired t-test.
P < 0.05, significant difference between liberal and restricted salt groups.
Hemodynamic response to HG exercise.
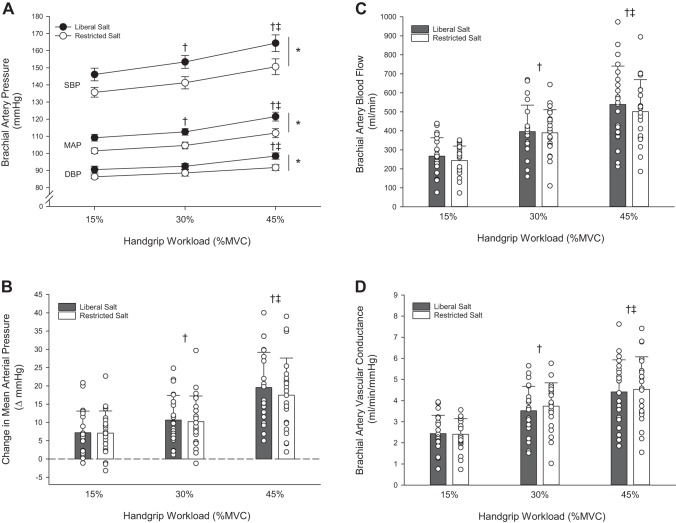
During HG exercise, SBP, DBP, and MAP increased significantly with exercise intensity (P < 0.001) (Fig. 1A). The RS induced significant reductions in SBP (−12 mmHg), DPB (−5 mmHg), and MAP (−8 mmHg) at rest persisted during HG exercise, as blood pressure remained significantly lower across all exercise intensities compared with LS (P < 0.001). As such, the magnitude of the change in MAP during HG exercise was not different between LS and RS conditions (Fig. 1B). There were no differences in HR, SV, CO, and TPR between LS and RS conditions. HR and CO increased significantly with HG exercise intensity (P < 0.001) (Table 3), while SV and TPR were not different across exercise intensities or condition. ABF and AVC increased significantly with HG exercise intensity (Fig. 1, C and D) (P < 0.001), but there were no differences between the LS and RS conditions. RPE increased with each increase in intensity (P < 0.001) but was not different between LS and RS conditions (Table 3).
Fig. 1.
Peripheral hemodynamic response to handgrip exercise in subjects with hypertension during liberal or restricted salt intake. A: systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) response to handgrip (HG) exercise. B: change in mean arterial pressure from baseline during handgrip exercise at 15, 30, and 45% maximum voluntary contraction (MVC). C: brachial artery blood flow response to HG exercise. D: brachial artery vascular conductance response to HG exercise. Values are expressed as means ± SE for A; individual (○) and mean (bars) data with SD are presented in B, C, and D. Data includes n = 22 (14 men and 8 women). A 2 × 3 repeated-measures ANOVA (group, 2 levels: liberal vs. restricted salt) (workload, 3 levels: HG exercise: 15, 30, and 45% MVC) was performed to compare hemodynamic responses. The Holm-Sidak method was used for α-adjustment and post hoc analysis. *P < 0.05, treatment effect. †P < 0.05, vs. 15%, both liberal and restricted salt groups. ‡P < 0.05, vs. 30%, both liberal and restricted salt groups.
Table 3.
Central hemodynamics and ratings of perceived exertion during handgrip exercise
| Liberal Salt | Restricted Salt | |
|---|---|---|
| Heart rate, beats/min | ||
| 15% MVC | 71 ± 14 | 69 ± 15 |
| 30% MVC | 74 ± 12 | 72 ± 15 |
| 45% MVC | 79 ± 13†‡ | 77 ± 15†‡ |
| Stroke volume, mL | ||
| 15% MVC | 115 ± 43 | 116 ± 50 |
| 30% MVC | 120 ± 36 | 119 ± 54 |
| 45% MVC | 117 ± 36 | 114 ± 46 |
| Cardiac output, L/min | ||
| 15% MVC | 8.1 ± 2.5 | 7.7 ± 2.8 |
| 30% MVC | 8.5 ± 2.6 | 8.1 ± 3.4 |
| 45% MVC444 | 9.1 ± 2.8†‡ | 8.3 ± 3.2† |
| Total peripheral resistance, mmHg·mL−1·min | ||
| 15% MVC | 14.5 ± 3.9 | 14.5 ± 3.8 |
| 30% MVC | 14.2 ± 3.6 | 14.3 ± 4.1 |
| 45% MVC | 14.3 ± 3.4 | 14.7 ± 4.1 |
| Rating of perceived exertion, AU | ||
| 15% MVC | 2.7 ± 1.0 | 2.2 ± 0.6 |
| 30% MVC | 5.1 ± 1.4† | 4.5 ± 1.2† |
| 45% MVC | 7.9 ± 2.0†‡ | 7.7 ± 1.6†‡ |
Values are means ± SD; n = 22 (14 men/8 women). A 2 × 3 repeated-measures ANOVA (group, 2 levels: liberal vs. restricted salt) (workload, 3 levels: HG exercise: 15, 30, and 45% MVC) was performed to determine the hemodynamic responses. The Holm-Sidak method was used for α-adjustment and post hoc analysis.
P < 0.05, vs. 15%.
P < 0.05, vs. 30%.
Hemodynamic response to KE exercise.
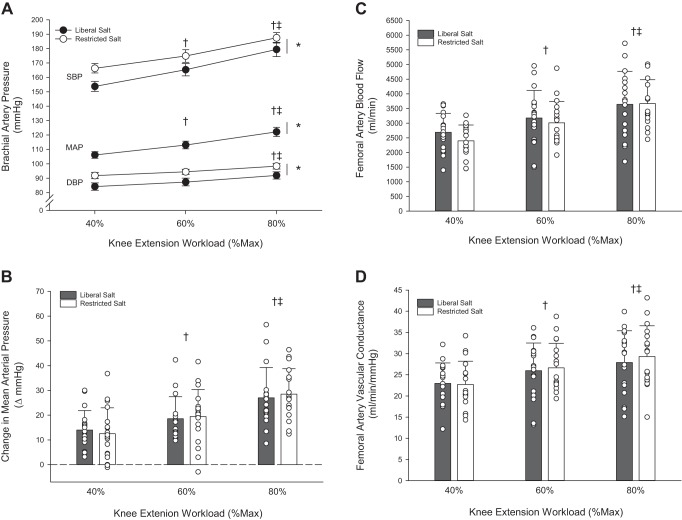
During KE exercise, SBP, DBP, and MAP increased with KE exercise intensity (P < 0.001) (Fig. 2A). The RS induced significant reductions in SBP (−10 mmHg), DPB (−7 mmHg), and MAP (−9 mmHg) at rest, which persisted during KE exercise, as blood pressure remained significantly lower across all exercise intensities compared with LS (P < 0.001). Similar to HG exercise, the magnitude of the change in MAP during knee extension exercise was not different between LS and RS conditions (Fig. 2B). There were no differences in HR, SV, CO, and TPR between LS and RS conditions. HR and CO increased significantly with KE exercise intensity (P < 0.001), while SV and TPR were not different across exercise intensities and conditions (Table 4). LBF and LVC increased significantly with KE exercise intensity (P < 0.001) (Fig. 2, C and D); however, there were no differences between LS and RS conditions. RPE increased with each increase in intensity (P < 0.001) but was not different between LS and RS conditions (Table 4).
Fig. 2.
Peripheral hemodynamic response to knee extension exercise in subjects with hypertension during liberal or restricted salt intake. A: systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) response to knee extension (KE) exercise. B: change in mean arterial pressure from baseline during KE exercise. C: femoral artery blood flow response to knee extension (KE) exercise. D: femoral artery vascular conductance response to KE exercise. Values are expressed as means ± SE for A, individual (circles) and mean (bars) data with SD are presented in B, C, and D. Data includes n = 18 (10 men/8 women). A 2 × 3 repeated-measures ANOVA (group, 2 levels: liberal vs. restricted salt group) [workload, 3 levels: KE exercise: 40, 60, 80% KE maximum (Max)] was performed to compare the hemodynamic responses. The Holm-Sidak method was used for α-adjustment and post hoc analysis. *P < 0.05, group effect. †P < 0.05, vs. 40%, both liberal and restricted salt groups. ‡P < 0.05, vs. 60%, both liberal and restricted salt.
Table 4.
Central hemodynamics and ratings of perceived exertion during knee extension exercise
| Liberal Salt | Restricted Salt | |
|---|---|---|
| Heart rate, beats/min | ||
| 40% Max | 81 ± 13 | 82 ± 14 |
| 60% Max | 89 ± 14† | 90 ± 16† |
| 80% Max | 100 ± 19†‡ | 102 ± 21†‡ |
| Stroke volume, mL | ||
| 40% Max | 113 ± 44 | 112 ± 44 |
| 60% Max | 115 ± 46 | 103 ± 47 |
| 80% Max | 115 ± 53 | 111 ± 45 |
| Cardiac output, L/min | ||
| 40% Max | 9.5 ± 4.0 | 9.1 ± 3.8 |
| 60% Max | 10.2 ± 3.9 | 9.8 ± 3.8 |
| 80% Max | 11.5 ± 4.9†‡ | 11.2 ± 4.6†‡ |
| Total peripheral resistance, mmHg/mL/min | ||
| 40% Max | 13.6 ± 4.0 | 13.3 ± 4.9 |
| 60% Max | 12.9 ± 3.5 | 12.9 ± 4.3 |
| 80% Max | 12.5 ± 3.7 | 12.2 ± 4.2 |
| Rating of perceived exertion, AU | ||
| 40% Max | 3.9 ± 1.3 | 3.4 ± 1.3 |
| 60% Max | 6.3 ± 1.4† | 6.2 ± 1.1† |
| 80% Max | 7.7 ± 1.7†‡ | 8.1 ± 1.1†‡ |
Values are means ± SD; n = 18 (10 men/8 women). A 2 × 3 repeated-measures ANOVA (group, 2 levels: LS vs. RS) [workload, 3 levels: KE exercise: 40, 60, and 80% KE maximum (Max)] was performed to compare hemodynamic responses. The Holm-Sidak method was used for α-adjustment and post hoc analysis.
P < 0.05, vs. 40% Max.
P < 0.05 vs. 60% Max.
DISCUSSION
Lifestyle modifications, including lowering dietary salt intake and exercise, are often prescribed as a first-line therapy for the treatment of hypertension, yet little is known regarding the effects of salt restriction on central and peripheral hemodynamics during exercise. To our knowledge, this is the first study to comprehensively investigate the effects of restricted salt (RS) on the central and regional, peripheral hemodynamic responses to both arm and leg exercise in subjects with hypertension. Novel findings from this study include marked reductions in resting SBP, DBP, and MAP evoked by RS, which were maintained over a range of HG and KE exercise intensities (low, moderate, and high). Contrary to our hypothesis of an exercise pressor-lowering effect, the magnitude of change in MAP during both HG and KE exercise was not different between RS and LS conditions, suggesting a similar exercise pressor response, despite a lower starting and exercise-induced blood pressure. Finally, RS did not alter blood flow or vasodilation during HG or KE exercise. Collectively, these results indicate that restricting dietary salt intake evokes a significant and clinically meaningful reduction in blood pressure at both rest and during exercise but does not alter the peripheral hemodynamic response to exercise in hypertensive individuals.
Impact of salt restriction on resting and exercise-induced blood pressure.
The impact of salt intake on blood pressure and cardiovascular disease outcomes in hypertensive individuals has been well described (46). The strong link between dietary salt and health is exemplified in the manifestation of elevated blood pressure in most, but not all, individuals upon increasing salt intake (46). Interestingly, the negative impact of a high-salt diet has also been associated with vascular dysfunction, independent of blood pressure effects (4), suggesting a salt-induced increase in blood pressure is not a prerequisite for diminished cardiovascular health. Indeed, the underlying mechanism(s) mediating altered cardiovascular function in response to increased salt intake in hypertensive individuals has been the topic of much investigation and remains to be fully elucidated. Currently, the pathophysiology of salt-induced hypertension has been linked to numerous pathways, including the renin-angiotensin-aldosterone system, the endothelin system, nitric oxide (NO), and oxidative stress, the sympathetic nervous system, atrial natriuretic peptides, and CYP450-derived metabolites of arachidonic acids (11). By examining the impact of salt restriction on central and peripheral hemodynamics during exercise, the current study has the potential to provide useful insight into how these aforementioned mechanisms, all of which impact peripheral hemodynamics during exercise, contribute to the pathophysiology of salt-induced hypertension.
Previous work using nearly identical dietary salt interventions as the current study reported congruent reductions in resting MAP (approximately −10 mmHg) in hypertensive individuals (43, 44). A more conservative salt restriction study (100–120 mmol/day) reported a significant reduction in SBP (−7 mmHg), albeit substantially less of a reduction than the current study (−17 mmHg), suggestive of a dose-response relationship between salt intake and the reduction in resting blood pressure afforded by RS (34). However, baseline health status (i.e., normotensive vs. hypertensive) likely accounts for some of these observed differences, as a greater drop in blood pressure would be expected in hypertensive individuals. In the present investigation, RS lowered resting blood pressure in the supine and upright positions (Table 2), quite possibly through a diuretic-like effect by reducing plasma volume, as indicated by the ~3-kg decrease in body weight and concomitant increases in red blood cell count, hemoglobin concentration, and hematocrit. Interestingly, this reduction in blood pressure was not accompanied by concomitant changes in cardiac output or total peripheral resistance (Table 2). Regardless of the precise mechanism(s), a lowering of blood pressure at rest and at any given exercise intensity is advantageous and represents a reduced risk of further cardiovascular complications.
During exercise, individuals with hypertension exhibit an exaggerated blood pressure response to exercise, which has been linked to an increased risk of future cardiovascular disease (3, 29, 36, 39, 47) and has been postulated to contribute to an increased risk of cardiovascular events during and following exercise (17, 23, 30, 31). Interestingly, this exaggerated exercise blood pressure response may be partially attributable to heightened group III and IV afferent feedback (25), which results in augmented sympathetic activation (9), the latter of which is strongly associated with reductions in vascular conductance (13). This reduction in vascular conductance creates a hemodynamic challenge, which may limit exercise capacity and increase the risk of future cardiovascular risks (17, 23, 30, 31). Reducing resting and exercising blood pressure in hypertension is critical to improve the safety, efficacy, and utility of exercise in this prominent form of cardiovascular disease.
Given previous findings that salt augments sympathetic nervous system responsiveness (18, 20), it is reasonable to hypothesize that alterations in salt intake may alter the magnitude of the exercise pressor reflex. Yamauchi et al. (48) reported an amplification of the exercise pressor reflex with high-salt feeding in rodents, suggesting salt-mediated neurovascular control, and potential sensitization of the medullary circuits involved in sympathetic outflow. However, a change in resting blood pressure, as is commonly observed in humans following alterations in dietary salt intake (18, 20), was not observed, potentially indicating a fundamentally different physiology compared with human hypertensive individuals. In the current study, the RS-induced reduction in blood pressure at rest persisted during both HG and KE exercise; however, there was no indication that the magnitude of the change in blood pressure during exercise was altered. Omvik and Lung-Johansen (34), using a relatively mild salt restriction (140 mmol/day) regimen, reported a decrease in resting seated systolic (−7 mmHg) and diastolic (−5 mmHg) blood pressure in response to 9 mo of mild RS (100–120 mmol/day), which also remained consistent across a range of exercise intensities, supporting the current findings.
Impact of salt restriction on resting and exercise-induced blood flow and vascular conductance.
Attenuated blood flow during exercise is characteristic of hypertension arising from increased peripheral resistance and diminished vasodilatory capacity, as assessed by vascular conductance (15, 32). Considering this impaired blood flow in hypertensive individuals, along with the vascular impact of dietary salt (10), the current RS intervention afforded a unique opportunity to evaluate the impact of alterations in dietary salt on peripheral hemodynamics during exercise. In contrast to our hypothesis, blood flow and vascular conductance during HG and KE exercise were unaltered by RS. Because of the novelty of this finding, there is currently no clear explanation for these null changes following RS in the literature. Dietary salt has been linked to changes in endothelial function, as measured by flow-mediated dilation (FMD) and ACh infusion in hypertensive, normotensive, salt-sensitive, and salt-resistant individuals (22, 42). However, it is important to recognize that endothelial and vascular function, while related to blood flow, do not dictate blood flow during exercise, as the contribution of endothelium-derived vasodilating factors, including NO and prostaglandins, to exercise hyperemia is minimal (22). Given previous findings that salt augments sympathetic nervous system responsiveness (18, 20), it is reasonable to hypothesize that alterations in salt intake may alter the magnitude of the exercise pressor reflex. In agreement with the current findings, Yamauchi et al. (48) reported a reduction in the exercise pressor reflex following a low-salt diet in rats, yet this change in pressure was not associated with a concomitant change in blood flow during simulated exercise. Together, these findings suggest that high blood pressure is not obligatory to maintain blood flow in hypertension, and salt restriction may represent a practical approach to lower exercising blood pressure, without compromising skeletal muscle blood flow, and improve safety during exercise in individuals with hypertension.
Surprisingly, the observed reductions in MAP did not translate to an improvement in vasodilation, as assessed by vascular conductance (VC), during HG or KE exercise (Figs. 1 and 2). According to Ohm’s law, a change in blood pressure would be expected to evoke a corresponding change in VC when blood flow remains constant, as was the case during HG and KE exercise. However, as elegantly described by O’Leary (33), under high-flow conditions, such as exercise, the relationship between blood flow and VC is quite steep and nonlinear, indicating that a rather large change in MAP evokes only a modest change in VC. Furthermore, during the small muscle mass exercise used in the current study, blood flow increased ~6-fold during HG exercise and ~10-fold during KE exercise, whereas MAP only increased by 20 to 30 mmHg from rest to exercise. Clearly, the much larger magnitude of change in blood flow dictated the change in VC, even though reductions in blood pressure evoked by RS were physiologically impressive and clinically meaningful.
Perspectives.
Although the magnitude of change in blood pressure from rest to exercise was similar between RS and LS, indicating that alterations in salt intake did not modify the exercise pressor reflex, the absolute reduction in blood pressure during exercise was quite impressive. This finding is potentially of high clinical importance, considering the associations between absolute blood pressure, end organ damage, the development of cardiovascular disease, and mortality (29, 47). As a first-line defense in the treatment of hypertension, the current findings highlight the importance and utility (no hemodynamic consequences) of combining dietary modifications, through salt restriction, with exercise training to lower the risk of cardiovascular events during and after exercise. Furthermore, although the current investigation used only Caucasian males and females, it should be noted that African-Americans suffer disproportionately from hypertension (26) and may benefit even more from salt restriction, as was indicated from the DASH diet study (40). Salt sensitivity typically affects half of all Caucasian Americans and nearly 75% of all African Americans (14). Therefore, follow-up studies examining hemodynamic changes to exercise following salt restriction based on race are warranted. Additionally, the use of only 5 days of salt restriction to significantly lower blood pressure and examine the effects of peripheral hemodynamic changes during exercise is a rather short intervention compared with previous salt restriction studies, which have lasted weeks to months. Future studies using long-term salt restriction may be necessary to see additional alterations to peripheral hemodynamics and vascular function associated with exercising blood flow. Finally, the observed changes in blood pressure represent a highly integrative and complex response at rest and during exercise. Additional studies using microneurography, neck pressure/neck suction, and postexercise cuff occlusion may help elucidate the mechanisms by which dietary salt impacts muscle sympathetic nerve activity, baroreflex, metaboreflex, and mechanoreflex sensitivity.
Experimental considerations.
The restricted salt diet followed the liberal salt diet; therefore, it is possible that an ordering effect may have occurred. To avoid such an ordering effect, all subjects were familiarized with the testing procedures before the salt interventions to avoid any unwanted learning effect. Additionally, careful attention was made to follow guidelines for the assessment of blood pressure. Lastly, when compared with previous investigations using identical dietary interventions in a counterbalanced experimental design, the reported changes in BP between studies are similar. Together, these precautions suggest that an ordering effect likely did not occur. In the present investigation, 5 of the 22 subjects studied were salt-insensitive and did not have a decrease in resting mean arterial blood pressure of greater than 5 mmHg following salt restriction. Regardless of salt sensitivity, dietary salt restriction is thought to have pleiotropic effects on blood vessels, heart, kidneys, and brain, which warranted not excluding these individuals, as they may have experienced additional alterations to exercising hemodynamics independent of blood pressure. The current study did not have a control, nonhypertensive group; instead, the hypertensive subjects acted as their own control group, performing both liberal salt and restricted salt diets. Further investigation examining the impact of salt restriction on exercising hemodynamics in nonhypertensive or salt-insensitive hypertensive individuals is warranted and may provide additional evidence for salt restriction having universal benefits regardless of starting blood pressure.
Conclusions.
This study is the first to comprehensively investigate the effects of RS on both central and peripheral hemodynamics during rest, as well as during arm and leg exercise in individuals with hypertension. Salt restriction evoked marked reductions in resting blood pressure that persisted over a wide range of exercise intensities during HG and KE exercise. The magnitude of the blood pressure change from rest to exercise was similar between RS and LS, implying that dietary salt may not play a major role in modifying the exercise pressor reflex in hypertensive men and women. Despite substantial reductions in blood pressure, neither blood flow nor vascular conductance was altered during exercise. Overall, restricted salt intake provides a safe and effective means to lower blood pressure in salt-sensitive hypertensive individuals, while not altering the central and peripheral hemodynamic response to exercise regardless of salt sensitivity.
GRANTS
This study was supported by Veterans Affairs Rehabilitation Research and Development Career Development (IK2RX001215), Merit (E6910-R and E1697-R), Spire (E1433-P), Senior Research Career Scientist (E9275-L), and Advanced Fellowship in Geriatrics awards; American Heart Association Grant 14S-DG-18850039; National Heart, Lung, and Blood Institute Grant HL-091830; and the Ruth L. Kirschstein National Research Service Award (1T32-HL-139451).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.N.H., R.S.R., and J.D.T. conceived and designed research; S.M.R., R.M.B., D.T.L.S., O.-S.K., S.-Y.P., and J.D.T. performed experiments; S.M.R., R.M.B., D.T.L.S., and J.D.T. analyzed data; S.M.R., R.M.B., D.T.L.S., P.N.H., R.S.R., and J.D.T. interpreted results of experiments; S.M.R. and J.D.T. prepared figures; S.M.R. and J.D.T. drafted manuscript; S.M.R., R.M.B., D.T.L.S., O.-S.K., S.-Y.P., P.N.H., R.S.R., and J.D.T. edited and revised manuscript; S.M.R., R.M.B., D.T.L.S., O.-S.K., S.-Y.P., P.N.H., R.S.R., and J.D.T. approved final version of manuscript.
REFERENCES
- 1.Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Curr Hypertens Rep 14: 193–201, 2012. doi: 10.1007/s11906-012-0275-6. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 11: 532–546, 2014. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J 47: 802–809, 1983. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- 4.Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. J Vasc Res 50: 458–467, 2013. doi: 10.1159/000355270. [DOI] [PubMed] [Google Scholar]
- 5.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 6.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 7.de la Sierra A, Banegas JR, Divisón JA, Gorostidi M, Vinyoles E, de la Cruz JJ, Segura J, Ruilope LM. Ambulatory blood pressure in hypertensive patients with inclusion criteria for the SPRINT trial. J Am Soc Hypertens 10: 947–953.e5, 2016. doi: 10.1016/j.jash.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 8.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- 9.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens 24: 8–13, 2015. doi: 10.1097/MNH.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council . Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68: e7–e46, 2016. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 12.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, Marmot M; Intersalt Cooperative Research Group . Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 312: 1249–1253, 1996. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 25, Suppl: 247S–255S, 2006. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 15.Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension 58: 943–949, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176529. [DOI] [PubMed] [Google Scholar]
- 16.He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension 42: 1093–1099, 2003. doi: 10.1161/01.HYP.0000102864.05174.E8. [DOI] [PubMed] [Google Scholar]
- 17.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kübler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65: 583–589, 1990. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 18.Huang BS, Leenen FH. Dietary Na and baroreflex modulation of blood pressure and RSNA in normotensive vs. spontaneously hypertensive rats. Am J Physio Heart Circ Physiol l 266: H496–H502, 1994. doi: 10.1152/ajpheart.1994.266.2.H496. [DOI] [PubMed] [Google Scholar]
- 19.Inrig JK, Van Buren P, Kim C, Vongpatanasin W, Povsic TJ, Toto RD. Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 6: 2016–2024, 2011. doi: 10.2215/CJN.11351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 276: R1600–R1607, 1999. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis 3: 347–356, 2009. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007. doi: 10.1113/jphysiol.2007.135889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determina4nts of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil 22: 178–183, 2002. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 25.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: 948–954, 2010. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 27.Logason K, Bärlin T, Jonsson ML, Boström A, Hårdemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50–69% and 70–99% carotid artery stenosis. Eur J Vasc Endovasc Surg 21: 311–313, 2001. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 28.Mackay J, Mensah GA. The Atlas of Heart Disease and Stroke. Geneva, Switzerland: World Health Organization, 2004. [Google Scholar]
- 29.Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol 51: 29–35, 1998. doi: 10.1016/S0895-4356(97)00223-0. [DOI] [PubMed] [Google Scholar]
- 30.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE; Determinants of Myocardial Infarction Onset Study Investigators . Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. N Engl J Med 329: 1677–1683, 1993. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 31.Mittleman MA, Siscovick DS. Physical ex4ertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin 14: 263–270, 1996. doi: 10.1016/S0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 32.Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 590: 1481–1494, 2012. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- 34.Omvik P, Lund-Johansen P. Hemodynamic effects at rest and during exercise of long-term sodium restriction in mild essential hypertension. Acta Med Scand Suppl 220:S714: 71–74, 1986. doi: 10.1111/j.0954-6820.1986.tb08971.x. [DOI] [PubMed] [Google Scholar]
- 35.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickering TG. Exercise and hypertension. Cardiol Clin 5: 311–318, 1987. doi: 10.1016/S0733-8651(18)30553-8. [DOI] [PubMed] [Google Scholar]
- 37.Rådegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol 274: H314–H322, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 40: 168–182, 1984. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 39.Seguro C, Sau F, Zedda N, Scano G, Cherchi A. [Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension]. Cardiologia 36: 867–877, 1991. [PubMed] [Google Scholar]
- 40.Spencer A, Jablonski R, Loeb SJ. Hypertensive African American women and the DASH diet. Nurse Pract 37: 41–46, 2012. doi: 10.1097/01.NPR.0000410278.75362.a2. [DOI] [PubMed] [Google Scholar]
- 41.Trinity J, Lui C. Is exercise safe in hypertension. Austin J Clin Cardiol 1: 1001, 2014. [Google Scholar]
- 42.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008. doi: 10.1161/HYPERTE4NSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 43.Underwood PC, Chamarthi B, Williams JS, Vaidya A, Garg R, Adler GK, Grotzke MP, Staskus G, Wadwekar D, Hopkins PN, Ferri C, McCall A, McClain D, Williams GH. Nonmodulation as the mechanism for salt sensitivity of blood pressure in individuals with hypertension and type 2 diabetes mellitus. J Clin Endocrinol Metab 97: 3775–3782, 2012. doi: 10.1210/jc.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, Adler GK. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension 61: 886–893, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 32: 3–15, 2014. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 46.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996. doi: 10.1161/01.HYP.27.3.481. [DOI] [PubMed] [Google Scholar]
- 47.Wilson MF, Sung BH, Pincomb GA, Lovallo WR. Exaggerated pressure response to exercise in men at risk for systemic hypertension. Am J Cardiol 66: 731–736, 1990. doi: 10.1016/0002-9149(90)91139-W. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol 306: H450–H454, 2014. doi: 10.1152/ajpheart.00813.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]