Abstract
Mammary gland involution, a tightly regulated process of tissue remodeling by which a lactating mammary gland reverts to the prepregnant state, is characterized by the most profound example of regulated epithelial cell death in normal tissue. Defects in the execution of involution are associated with lactation failure and breast cancer. Initiation of mammary gland involution requires upregulation of lysosome biogenesis and acidification to activate lysosome-mediated cell death; however, specific mediators of this initial phase of involution are not well described. Zinc transporter 2 [ZnT2 (SLC30A2)] has been implicated in lysosome biogenesis and lysosome-mediated cell death during involution; however, the direct role of ZnT2 in this process has not been elucidated. Here we showed that ZnT2-null mice had impaired alveolar regression and reduced activation of the involution marker phosphorylated Stat3, indicating insufficient initiation of mammary gland remodeling during involution. Moreover, we found that the loss of ZnT2 inhibited assembly of the proton transporter vacuolar ATPase on lysosomes, thereby decreasing lysosome abundance and size. Studies in cultured mammary epithelial cells revealed that while the involution signal TNFα promoted lysosome biogenesis and acidification, attenuation of ZnT2 impaired the lysosome response to this involution signal, which was not a consequence of cytoplasmic Zn accumulation. Our findings establish ZnT2 as a novel regulator of vacuolar ATPase assembly, driving lysosome biogenesis, acidification, and tissue remodeling during the initiation of mammary gland involution.
Keywords: acidification, involution, lysosome, mammary gland, SLC30A2, v-ATPase, zinc, ZnT2
INTRODUCTION
Mammary gland involution, a highly controlled process by which the lactating mammary gland reverts to the prepregnant state, is the most profound example of normal tissue remodeling (6, 63). This stage of mammary gland development is characterized by extensive mammary epithelial cell (MEC) death and proceeds through a series of tightly regulated events (63). The initial, reversible phase of involution involves lysosome-mediated cell death (LCD) of secreting MECs, which occurs <24 h postweaning (28). This event is supervened by an irreversible phase (>48 h postweaning) that includes MEC apoptosis (39, 67), stromal remodeling, and immune cell infiltration (39, 68). Premature execution of mammary gland remodeling can lead to lactation failure, while impaired remodeling is associated with breast cancer (13, 41, 49). Although the overall process of mammary gland remodeling has been well characterized, the molecular mechanisms regulating key events upstream of LCD are poorly understood, yet they are critical to understanding the progression of normal cell death.
As the initial process, LCD is the driving force behind involution and requires extensive upregulation of lysosome biogenesis, acidification, and activity (1, 28, 44, 57). Lysosome biogenesis requires both biosynthesis of lysosome proteins and proper trafficking and endocytic delivery of these proteins (54). One of the mechanisms by which lysosome biogenesis is regulated is through expression of the coordinated lysosome expression and regulation (CLEAR) network (56). This gene network, encoding a cluster of lysosome-related proteins, is transcriptionally regulated by transcription factor EB (TFEB) (56). In addition, the generation of a lysosome is dependent on intravesicular acidification, which is critical for activation of lysosomal proteases (30) and maintenance of lysosomal calcium homeostasis (32), and therefore, is essential for proper lysosome function. During involution, the acidic lysosome environment is critical for the activation of LCD, apoptosis, and mammary gland remodeling (28). The proton pump vacuolar ATPase (v-ATPase) is responsible for establishing the proton gradient across the lysosomal membrane and creating the acidic environment of the lysosome lumen (16, 40). v-ATPase activity is primarily regulated by modulation of the association of cytoplasmic (V1) and membrane-embedded (V0) domains (16). v-ATPase is expressed in MECs (47), and both pharmacological inhibition and genetic deletion of v-ATPase activity in cultured MECs impair vesicle acidification (34). Moreover, deletion of the v-ATPase subunit V0a2 in V0a2-null mice impairs mammary gland development and function, underscoring the need to better understand factors that regulate vesicle acidification and tissue remodeling (47).
Several studies have reported that Zn drives proton-coupled transport (43) and that vesicular Zn is required to reduce the pH of intracellular compartments by inhibiting the extraluminal proton leak (18, 36). We recently reported that the vesicular Zn transporter ZnT2 physically interacts with v-ATPase (34) and is critical for generating acidified secretory vesicles during lactation (20, 21). In addition, our previous studies support a role for ZnT2 during involution. The overexpression of ZnT2 in the mammary gland during lactation leads to Zn accumulation in lysosomes and precocious mammary gland remodeling in mice (21). Additionally, the proinvolution signal TNFα dephosphorylates ZnT2, permitting adaptor protein 3 binding and ZnT2 relocalization to lysosomes, driving the activation of LCD in MECs (20). However, a direct role for ZnT2 in lysosome biogenesis and acidification during mammary gland involution has not been reported. In this study we hypothesized that ZnT2 modulates proton transport, mediating lysosome biogenesis and acidification and driving proper execution of mammary gland involution. We found that the initiation of involution was insufficient in ZnT2-null (ZnT2ko) mice, resulting from impaired cell death and stromal remodeling, compared with their wild-type (WT) littermates. Loss of ZnT2 impaired v-ATPase assembly and lysosome acidification, leading to impaired lysosome formation and reduced lysosome abundance, and these defects were not a consequence of aberrant cytoplasmic Zn accumulation. Together, our results indicate that ZnT2 is an important regulator of v-ATPase function and lysosome acidification in MECs and, thus, is a principal and essential factor in driving the initial phase of mammary gland remodeling.
MATERIALS AND METHODS
Mice and tissue collection.
All animal protocols were approved by the Institutional Animal Care and Use Committee at the Penn State University Milton S. Hershey College of Medicine, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. ZnT2 transgenic mice were generated as previously described (33). Male and female heterozygous mice were mated to generate ZnT2ko mice and their WT littermates. Offspring were genotyped as previously described (33) using primers 1 (5′-CATTGCCCGCTTACCCTGAG-3′), 2 (5′-GACTGATGGAGGGCCAACCCCATTC-3′), and 3 (5′-CAGCAGCCTCTGTTCCACATACACTTCAT-3′). Mice were housed in a controlled environment under consistent humidity and temperature. They were maintained on a 12:12-h light-dark cycle and fed ad libitum a standard commercial rodent diet (Envigo, Huntingdon, UK).
Involuting mice.
To study the involuting mammary gland, nulliparous WT and ZnT2ko female mice (10–13 wk of age) were mated with WT male mice and allowed to deliver normally. Litter size was normalized to six pups per dam on lactation day 2. On lactation day 5, the offspring were removed, and the dams were euthanized by CO2 asphyxiation 24 or 48 h later. Mammary glands were immediately excised and fixed in 4% phosphate-buffered paraformaldehyde, snap-frozen in isopentane, or stored in RNAlater (Sigma-Aldrich, St. Louis, MO) at −80°C until analysis.
Whole-mount analysis.
Mammary glands were fixed overnight in Carnoy’s solution and stained with carmine alum, as previously described (21). Sections were examined on a wide-field microscope (model BX60, Olympus, Tokyo, Japan).
Histological analysis.
To assess post-LCD consequences from the loss of ZnT2 on mammary gland involution, mammary glands collected at 48 h of involution were fixed in 4% phosphate-buffered paraformaldehyde overnight, washed in PBS and ethanol, and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin, as previously described (12). Digital images of three, ×10 fields of view per section (n = 3 mice/genotype) were collected, and the area occupied by adipocytes was measured using Adobe Photoshop (Adobe, San Jose, CA) and expressed as percentage of total area, as previously described (5, 42, 64). Phosphorylated (Tyr705) Stat3 (pStat3, 1:400 dilution; Cell Signaling Technology, Beverly, MA) was detected using the ImPRESS peroxidase polymer detection kit (Vector Laboratories, Burlingame, CA), counterstained with toluidine blue, and examined by light microscopy (model DM IL LED, Leica Microsystems, Wetzlar, Germany). The number of pStat3-positive MECs in three, ×10 fields of view per section (n = 3 mice/genotype) was quantified and expressed as a percentage of the total MECs. Immunofluorescence imaging of key molecules was conducted on mammary glands collected at 48 h of involution, as previously described (21). Antibodies for immunofluorescence imaging included ZnT2 (4 μg/ml; Santa Cruz Biotechnology, Dallas, TX), v-ATPase B1/2 (V1 domain, 1:25 dilution; Santa Cruz Biotechnology), and lysosome-associated membrane protein 1 (Lamp1, a lysosome marker, 1 μg/ml; Abcam, Cambridge, MA) and were detected with secondary antibodies [rabbit or mouse IgG labeled with Alexa Fluor 488 or Alexa Fluor 568 (Life Technologies, Carlsbad, CA)]. Nuclei were stained with 4′,6-diamidino-2-phenylindole, dilactate (DAPI, 1:20 dilution; Invitrogen). To determine association of the cytoplasmic v-ATPase subunit V1B with ZnT2 and the lysosome marker Lamp1, digital images of four, ×63 fields of view per section (n = 3 mice/genotype) were collected with an inverted confocal microscope (model SP8, Leica Microsystems). Mander's correlation coefficients were determined by correlation analysis using Imaris software. To determine the number of lysosomes, Lamp1-positive vesicles in five, ×63 fields of view per section (n = 3 mice/genotype) were counted, and data are expressed as total number of lysosomes. To determine the size of lysosomes, images of Lamp1-positive vesicles in five, ×63 fields of view per section (n = 3 mice/genotype) were collected, and vesicle area was determined using Adobe Photoshop.
Real-time PCR.
To assess the effects on gene expression of key molecules involved in LCD, real-time (RT) PCR was performed on RNA isolated from mammary glands of WT and ZnT2ko mice at 24 h of involution. RNA was isolated using TRIzol (Invitrogen) according to the manufacturer’s instructions. RNA concentration was quantified by spectrophotometry, and integrity was assessed by the quality of 28S and 18S bands following gel electrophoresis through 1% agarose. cDNA was synthesized from 1 μg of RNA using the ImProm-II reverse transcription system (Promega, Madison, WI). RT-PCR was performed with the QuantStudio 12K Flex machine (Thermo Fisher, Waltham, MA) using FastStart Universal SYBR green master (ROX) mix (Roche, Manheim, Germany). PCR was performed using the following protocol: 50.0°C for 2 min, 95.0°C for 10 min, 40 cycles of 95.0°C for 30 s, 60.0°C for 30 s, and 72.0°C for 1 min. Each sample was analyzed in triplicate (n = 5 mice/genotype). The linearity of the dissociation curve was assessed using ExpressionSuite software (Thermo Fisher). Relative gene expression was calculated using the 2ΔΔCt method, where Ct represents the cycle threshold. ΔCt values were calculated as the difference between the target genes and β-actin, and ΔΔCt values were calculated as the difference in relative expression between ZnT2ko and WT mice. The data are represented as fold change in expression relative to WT mice. The PCR primers are listed in Table 1.
Table 1.
Primer sequences for CLEAR network genes assessed using RT-PCR
| Primer Sequence | |
|---|---|
| Ctsd | |
| Forward | 5′-TACTCAAGGTATCGCAGGGTG-3′ |
| Reverse | 5′-CCAATGAAGACATCGCCCAG-3′ |
| Lamp1 | |
| Forward | 5′-GTAACAACGGAACCTGCCTG-3′ |
| Reverse | 5′-TCTGGTCACCGTCTTGTTGT-3′ |
| Mcoln1 | |
| Forward | 5′-GGCATTGAGGCAAAGAACCT-3′ |
| Reverse | 5′-GAATGACACCGACCCAGACT-3′ |
| Ppt1 | |
| Forward | 5′-CTCTGCTGCCTTGGTGCT-3′ |
| Reverse | 5′-AGCTGTCCCCCATCCCAT-3′ |
| Tnfa | |
| Forward | 5′-AGGGTCTGGGCCATAGAACT-3′ |
| Reverse | 5′-CCACCACGCTCTTCTGTCTAC-3′ |
| Actb | |
| Forward | 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Reverse | 5′-AGCCATGTACGTAGCCATCC-3′ |
CLEAR, coordinated lysosome expression and regulation; Ctsd, cathepsin D; Lamp1, lysosome-associated membrane protein; Mcoln1, mucolipin 1 (TRPML1); Ppt1, palmitoyl-protein thioesterase 1; Tnfa, TNF-α; Actb, β-actin.
Cell fractionation.
Mammary glands from WT and ZnT2ko mice at 24 h of involution were homogenized on ice in homogenization buffer (20 mM HEPES, pH 7.4, 1 mM EDTA, 250 mM sucrose, and protease inhibitors) and centrifuged at 5,000 g for 15 min. The pellet was resuspended in homogenization buffer and pottered 50 times in a tight-fitting Dounce homogenizer. Cell debris was pelleted by centrifugation at 380 g for 5 min. Centrifugation of the postnuclear supernatant at 50,000 g for 2 h resulted in membrane (pellet) and cytosolic (supernatant) fractions.
Immunoblotting.
Mammary glands from WT and ZnT2ko mice at 24 h of involution were homogenized on ice in homogenization buffer and centrifuged at 2,000 g for 5 min. The postnuclear supernatant was collected as the total lysate or centrifuged at 100,000 g for 30 min to isolate the crude membrane fraction. Protein concentration was determined using the Bradford assay, and samples (20 μg) were prepared, electrophoresed, and immunoblotted as previously described (38). The following antibodies were used: cathepsin B (1:1,000 dilution; Santa Cruz Biotechnology), Lamp1 (1:10,000 dilution; Abcam), v-ATPase B1/2 (V1 domain, 1:500 dilution; Santa Cruz Biotechnology), ATP6V0a2 (V0 domain, 1 µg/µl; Sigma-Aldrich), calnexin (1 µg/µl; Abcam), GAPDH (1:1,000 dilution; Santa Cruz Biotechnology), ZnT2 (1;1,000 dilution; Biorbyt, San Francisco, CA), and β-actin (1:5,000 dilution; Sigma-Aldrich). Antibodies were detected with IRDyes (1:20,000 dilution; LI-COR Biosciences, Lincoln, NE). Blots were scanned on the Odyssey CLX imaging system (LI-COR Biosciences). Relative band signal intensity was quantified using AlphaView software (ProteinSimple, San Jose, CA).
Zinpyr-1 imaging.
Frozen mammary glands were sectioned (5 μm) and stained with Zinpyr-1 (ZP-1; 20 μM) and DAPI (0.5 μM) diluted in saline, as previously described (33). Sections were rinsed with saline and immediately imaged on an inverted confocal microscope (model SP8, Leica Microsystems).
siRNA transfection of mammary epithelial cells and immunofluorescence.
Mouse MECs were a gift from Dr. Jeffery Rosen (Baylor College of Medicine, Houston, TX) and used with the permission of Dr. Bernd Groner (Institute for Biomedical Research, Frankfort, Germany). Cells were cultured in growth medium (RPMI 1640 supplemented with 10% fetal bovine serum, 5 μg/ml insulin, 10 ng/ml epidermal growth factor, and gentamicin). Cells were transfected with ZnT2 siRNA (5′-CCAUCUGCCUGGUGU UCAU-3′; Sigma-Aldrich) using Lipofectamine 2000 (Life Technologies), as previously described, to attenuate ZnT2 (ZnT2KD) (58). ZnT2 attenuation was confirmed by immunoblotting, as described above. Transfected cells were stimulated with TNFα (15 ng/ml) for 24 h in serum-free medium at 37°C to initiate lysosome biogenesis, as previously reported (21). To selectively chelate Zn, cells were treated with a small amount of N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN, 10 µM) for 2 h. Immunofluorescence imaging was carried out as previously described (21). Cells were incubated with the lysosome marker anti-Lamp1 (1 μg/ml; Abcam), and antibody was detected with Alexa Fluor 568-conjugated anti-mouse IgG (1 μg/ml; Life Technologies). Nuclei were stained with DAPI (1:20 dilution). Cells were visualized using an inverted confocal microscope (model SP8, Leica Microsystems).
FluoZin-3 staining.
HC11 MECs transfected with siRNA, as described above, were loaded with FluoZin-3 (2 μM; Invitrogen), as previously described (21). Cells were visualized by live-cell imaging using an inverted confocal microscope (model SP8, Leica Microsystems).
LysoSensor staining and lysosome pH measurement.
Lysosome pH was measured using LysoSensor blue/yellow DND-160 (Thermo Fisher), as previously described (34). Cells were imaged using an inverted confocal microscope (model SP8, Leica Microsystems). The cells were excited at 405 nm, and fluorescence was emitted at 455 nm for LysoSensor blue (pH 9) and 540 nm for LysoSensor yellow (pH 3). Imaris 8.2 software (Bitplane, Belfast, UK) was used to measure the fluorescence intensity of LysoSensor blue and LysoSensor yellow in all vesicles in five randomly selected cells in a ×63 image (n = 5 images/group). Results are presented as the ratio of fluorescence intensity of LysoSensor blue to fluorescence of LysoSensor yellow. Vesicle pH was determined using a calibration curve, as previously described (34).
Statistical analysis.
All experiments were performed in triplicate and repeated three times unless indicated otherwise. Values are means (SD) unless otherwise noted. Statistical analysis was performed using Student’s t-test or one-way analysis of variance, where appropriate (Prism GraphPad, Berkeley, CA), and a significant difference was demonstrated at P < 0.05.
RESULTS
Involution is impaired in ZnT2ko mice.
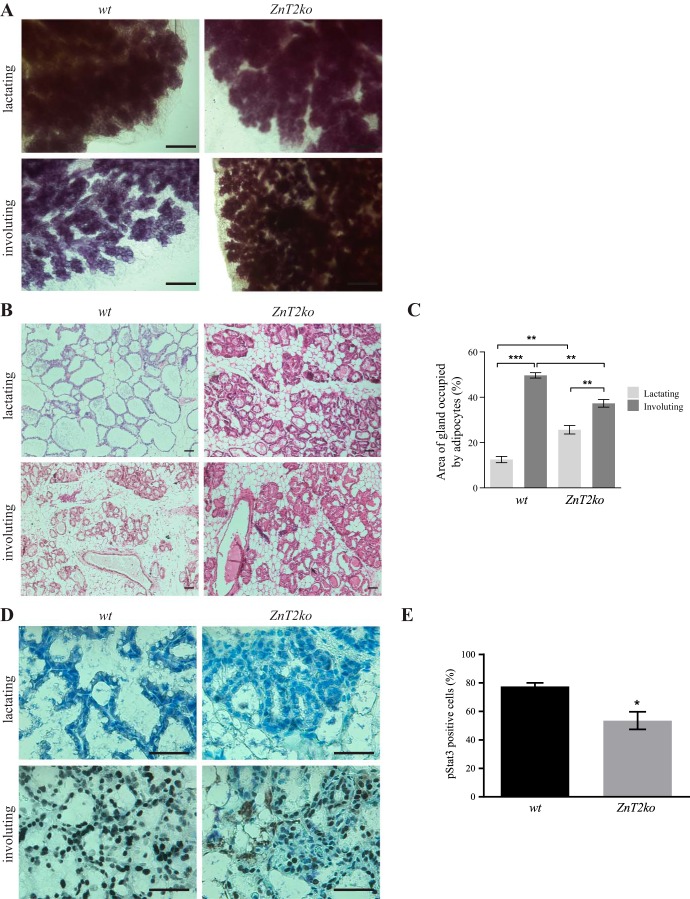
To understand the effects of loss of ZnT2 on involution, female WT and ZnT2ko mice were mated and allowed to deliver naturally. Successful deletion of ZnT2 was previously confirmed by the absence of protein expression determined by both immunoblot and immunofluorescence imaging (33). On lactation day 5, offspring were removed, the mice were euthanized, and mammary glands were collected 48 h later. Whole-mount analysis revealed impaired alveolar regression in ZnT2ko compared with WT mice (Fig. 1A). As expected, the involuting mammary glands of WT mice displayed sparse ductal branching and alveolar budding; however, the involuting mammary glands of ZnT2ko mice appeared to more closely resemble the lactating mammary gland. To confirm that involution was impaired in ZnT2ko mice, we measured the area of the mammary gland occupied by adipocytes, as adipocyte reappearance in the stromal compartment is a hallmark of involution (8). During involution, the area occupied by adipocytes increased by ~37% in mammary glands from WT mice but only by 12% in mammary glands from ZnT2ko mice (Fig. 1, B and C). Because a reduction in adipocyte refilling could be influenced by the initial difference in adipocyte area or reflect fewer MECs in ZnT2ko mice during lactation, we assessed an additional index of involution: we measured the number of MECs expressing pStat3, a transcription factor required for execution of LCD and initiation of involution (28). As expected, no pStat3-positive cells were observed in either genotype during lactation. However, mammary gland sections from involuting ZnT2ko mice had ~35% fewer (P < 0.05) pStat3-positive cells than their WT littermates (Fig. 1, D and E). Collectively, these data indicate that mammary gland involution was insufficient in the absence of ZnT2.
Fig. 1.
Involution is impaired in Zn transporter 2 (ZnT2)-null (ZnT2ko) mice. A: representative whole-mount images of mammary glands from wild-type (WT) and ZnT2ko mice. Scale bars = 1 mm. B: representative hematoxylin-eosin-stained images of mammary gland sections from lactating and involuting WT and ZnT2ko mice. Magnification ×10; scale bars = 100 µm. C: percentage of total area of gland occupied by adipocytes in hematoxylin-eosin-stained sections. Values are means (SD); data were collected from three, ×10 images/section; n = 3 mice/genotype. **P < 0.001; ***P < 0.0001. D: representative images of phosphorylated Stat3 (pStat3) in mammary gland sections from lactating and involuting WT and ZnT2ko mice. Magnification ×40; scale bars = 100 µm. E: abundance of pStat3-postive cells as percentage of total cells. Values are means (SD); data were collected from three, ×10 images/section; n = 3 mice/genotype. *P < 0.05.
ZnT2ko mice have fewer, smaller lysosomes.
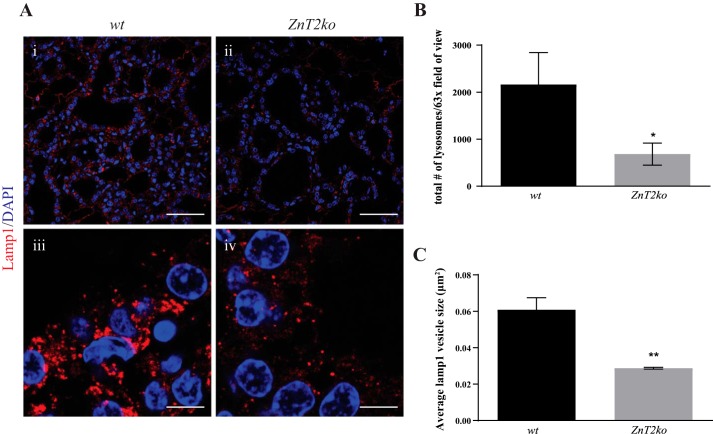
Zn plays a critical role in lysosome biogenesis (23), and lysosomes are vital for the initiation of MEC death during involution (28, 57, 63). Moreover, we previously showed that ZnT2 accumulation in lysosomes paralleled lysosomal Zn accumulation during involution (21). Here, we hypothesized that the loss of lysosomal Zn accumulation in mammary glands of involuting ZnT2ko mice would lead to fewer lysosomes than in their WT counterparts. Immunofluorescence imaging of the lysosomel marker Lamp1 showed that the number of lysosomes was approximately threefold less in the mammary glands of involuting ZnT2ko mice than their WT littermates (Fig. 2, A and B; P < 0.05). In addition, the size of Lamp1-positive vesicles that were detected in the mammary glands of involuting ZnT2ko mice was significantly smaller (~3-fold, P < 0.01) than in the mammary glands of WT mice, indicating an impairment in lysosome formation and morphology (Fig. 2, A and C).
Fig. 2.
Zn transporter 2 (ZnT2)-null (ZnT2ko) mice have fewer and smaller lysosomes. A: representative images of the lysosome marker lysosome-associated membrane protein 1 (Lamp1, red) and DAPI (blue) in involuting mammary glands from wild-type (WT; i and iii) and ZnT2ko (ii and iv) mice. Magnification ×63; scale bars = 50 µm (i and ii) and 5 µm (iii and iv). B: total number of lysosomes shown as number of Lamp1-positive vesicles. Values are means (SD); data were collected from five, ×63 images/section; n = 3 mice/genotype. *P < 0.05. C: average size of lysosomes shown as size of Lamp1-positive vesicles. Values are means (SD); data were collected from five, ×63 images/section; n = 3 mice/genotype. **P < 0.01.
Biosynthesis of lysosome proteins is not affected in ZnT2ko mice.
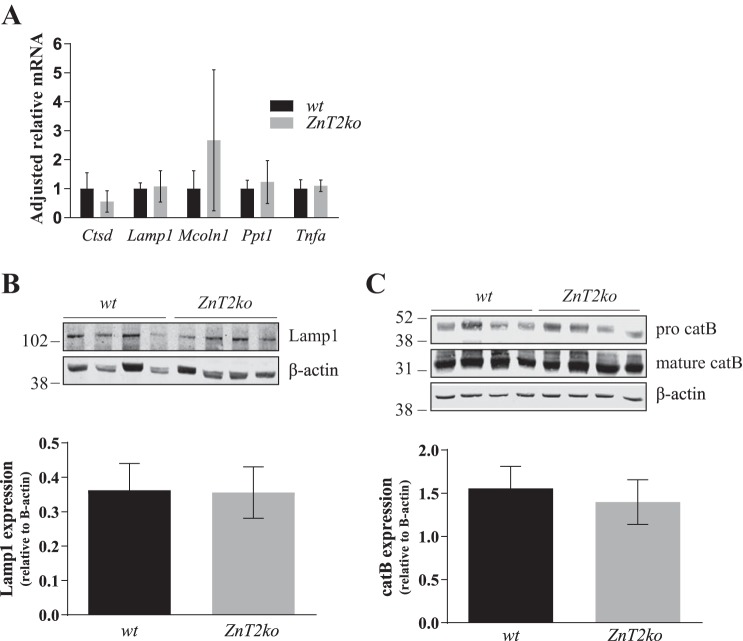
Lysosome biogenesis is dependent on both the synthesis and endocytic delivery of lysosome proteins (54). The synthesis of lysosome proteins is transcriptionally regulated by TFEB, a transcription factor that promotes expression of genes within the CLEAR network (56). To determine if the lysosome phenotype observed in ZnT2ko mice was a result of reduced expression of lysosome genes, we used RT-PCR to measure mRNA levels of five genes in the CLEAR network: Ctsd, which encodes the lysosomal protease cathepsin D; Lamp1, which encodes the lysosomal membrane protein Lamp1; Mcoln1 (mucolipin 1), which encodes the lysosomal calcium and Zn channel TRPML-1; Ppt1, which encodes the lysosomal lipid-catabolizing enzyme palmitoyl-protein thioesterase 1; and Tnfa, which encodes TNFα. No difference in expression between WT and ZnT2ko mice was observed for any of these genes (Fig. 3A). To confirm the lack of effect, immunoblot analysis of two key CLEAR network genes (total cathepsin B and Lamp1) revealed similar protein abundance in the mammary glands of WT and ZnT2ko mice (Fig. 3, B and C), collectively suggesting that the loss of ZnT2 does not impact transcriptional regulation of proteins belonging to the CLEAR network.
Fig. 3.
Expression of lysosome proteins is not impaired in Zn transporter 2 (ZnT2)-null (ZnT2ko) mice. A: relative gene expression of Ctsd (cathepsin D), Lamp1 [lysosome-associated membrane protein 1 (Lamp1)], Mcoln1 [mucolipin 1 (TRPML1)], Ppt1 (palmitoyl-protein thioesterase 1), and Tnfa (TNFα) in involuting mammary glands from wild-type (WT) and ZnT2ko mice. Data were normalized to β-actin. Values are means (SD); n = 5 mice/genotype. B: representative immunoblot of Lamp1 in total membrane fractions of mammary glands prepared from WT and ZnT2ko involuting mammary glands. Data were normalized to β-actin. Values are means (SD); n = 4 mice/genotype. Experiment was repeated 3 times. β-Actin served as a loading control. C: representative immunoblot of pro- and mature cathepsin B (catB) expression in cell lysates prepared from WT and ZnT2ko involuting mammary glands. Data represent sum of pro- and mature catB abundance relative to β-actin. Values are means (SD); n = 4 mice/genotype. Experiment was repeated 3 times.
Loss of ZnT2 impairs v-ATPase assembly and lysosome acidification.
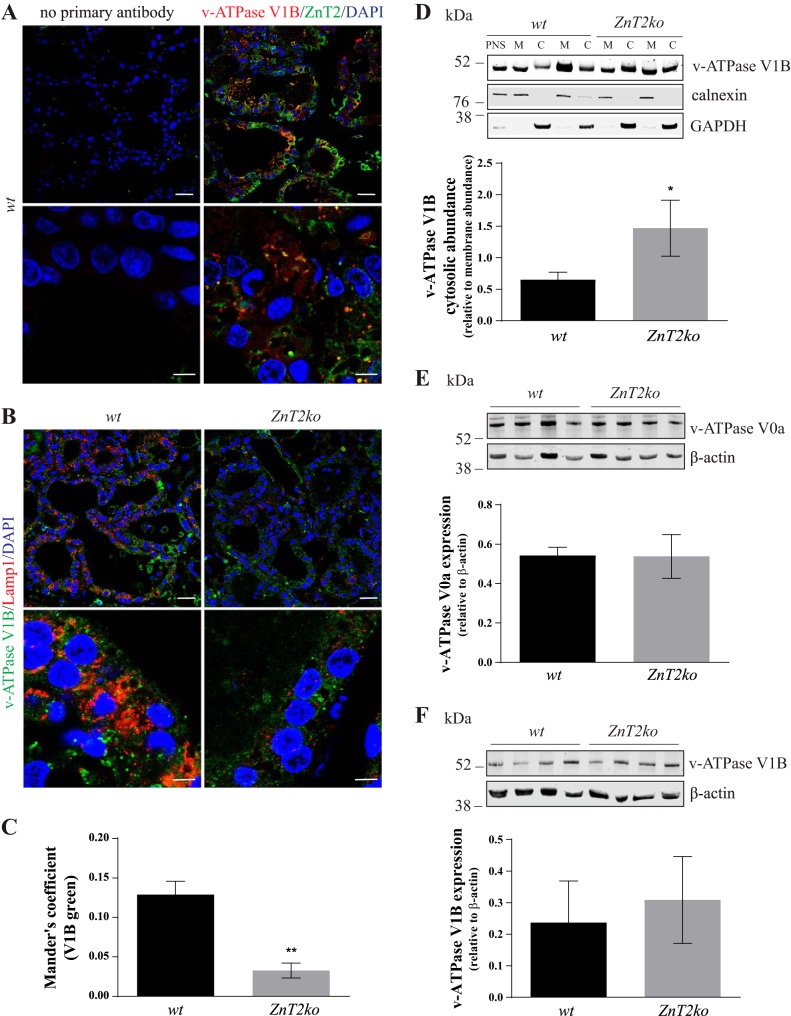
The initial phase of mammary gland involution is characterized by increased lysosome acidification and enzyme activity (28). Lysosome acidification, mediated by v-ATPase, is essential for the activation of lysosomal enzymes and, therefore, is critical for lysosome function (30). The primary mechanism by which v-ATPase activity is regulated is reversible dissociation of the cytoplasmic (V1) domain from the membrane-bound (V0) domain (16). Since we previously showed that ZnT2 interacts with the V1B subunit on vesicle membranes (34), we hypothesized that loss of ZnT2 would impair v-ATPase assembly and lysosome acidification. We first confirmed that ZnT2 interacts with the V1 subunit in the involuting mammary gland by performing coimmunofluorescence studies of ZnT2 and V1B. Indeed, we found that ZnT2 and V1B were colocalized (Mander’s coefficient = 0.145 ± 0.033, n = 3 mice; Fig. 4A). Reasoning that less membrane-associated V1B would indicate impaired v-ATPase assembly on lysosomes and reduced lysosome proton transport, we next performed coimmunofluorescence studies of Lamp1 and V1B to investigate the effects of loss of ZnT2 on v-ATPase assembly (Fig. 4B). Colocalization between Lamp1 and V1B was observed in the mammary glands of involuting WT mice, while colocalization of V1B with Lamp1 was negligible (~75% decrease, P < 0.01) in ZnT2ko mice (Fig. 4C). To confirm that loss of ZnT2 reduced v-ATPase assembly, we assessed the relative abundance of V1B in the membrane and cytosol by immunoblotting following cellular fractionation. We confirmed that the amount of V1B retained in the cytosol was significantly greater in ZnT2ko (~56%, P < 0.05) than WT mice (Fig. 4D). To determine whether reduced lysosomal V1B abundance in the mammary glands of involuting ZnT2ko mice was a result of reduced expression of the V1B or V0a subunits, the relative abundance of V1B and V0a was assessed by immunoblotting. Similar to our rationale for choosing the cytosolic V1B subunit, the membrane-associated V0a subunit was chosen based on the requirement of this subunit for v-ATPase assembly and function (50, 51). No significant difference in the abundance of either subunit was detected between WT and ZnT2ko mice (Fig. 4, E and F), suggesting that differences in assembly were not driven by reduced expression of these key v-ATPase subunits. Together, these findings indicate that ZnT2 plays a key role in the assembly of v-ATPase during mammary gland involution, which is essential for lysosome biogenesis and function.
Fig. 4.
Lysosome acidification and vacuolar ATPase (v-ATPase) assembly are impaired in Zn transporter 2 (ZnT2)-null (ZnT2ko) mice. A: representative images of ZnT2 (red), vacuolar-ATPase (v-ATPase) subunit V1B (V1B, green), and DAPI (blue) in involuting mammary glands from wild-type (WT) mice. Sections stained with secondary antibody only (no primary antibody) illustrate primary antibody specificity. Magnification ×63; scale bars = 50 µm (top) and 5 µm (bottom). B: representative images of lysosome-associated membrane protein 1 (Lamp1, red), V1B (green), and DAPI (blue) in involuting mammary glands from WT (left) and ZnT2ko (right) mice. Magnification ×63; scale bars = 50 µm (top) and 5 µm (bottom). C: Mander's correlation coefficient for colocalization of Lamp1 and V1B. Values are means (SD); data were collected from 5 images/section, n = 3 mice/genotype. **P < 0.01. D: representative immunoblot of V1B expression in postnuclear supernatant (PNS), membrane (M), and cytosol (C) fractions prepared from WT and ZnT2ko involuting mammary glands. Calnexin and GAPDH abundance verified the quality of membrane and cytosolic separation. Data represent cytosolic V1B relative to membrane V1B abundance. Values are means (SD); n = 4 mice/genotype. Experiment was repeated 3 times. *P < 0.05. E: representative immunoblot of v-ATPase subunit V0a (V0a) abundance in total tissue homogenates from WT and ZnT2ko involuting mammary glands. Data represent V0a expression relative to β-actin. Values are means (SD); n = 4 mice/genotype. Experiment was repeated 3 times. β-Actin served as a loading control. F: representative immunoblot of V1B in total membrane fractions prepared from WT and ZnT2ko involuting mammary glands. Data represent V1B expression relative to β-actin. Values are means (SD); n = 4 mice/genotype. Experiment was repeated 3 times. β-Actin served as a loading control.
Lysosome phenotype and impaired lysosome acidification are not attributable to Zn toxicity.
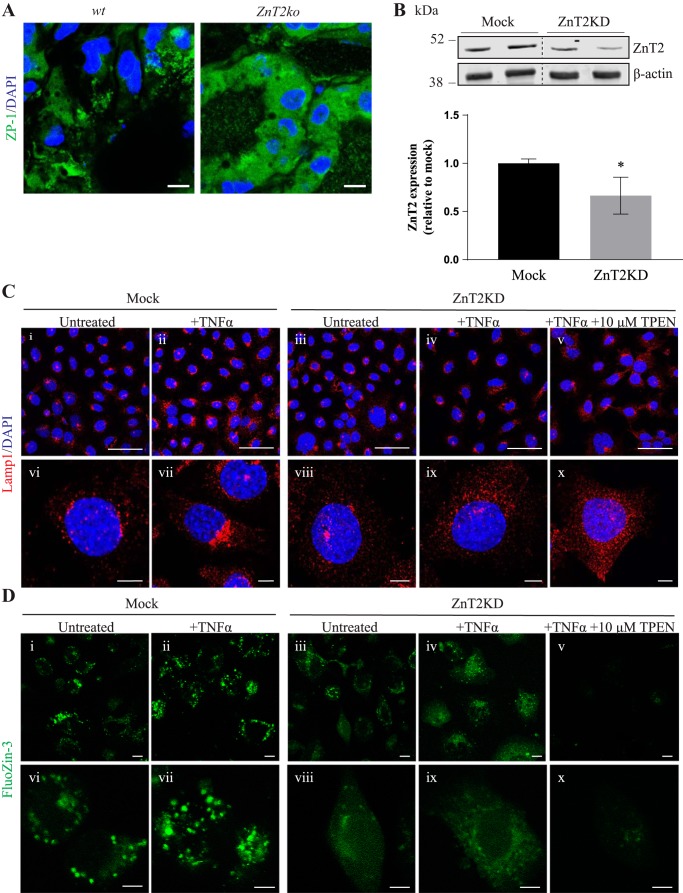
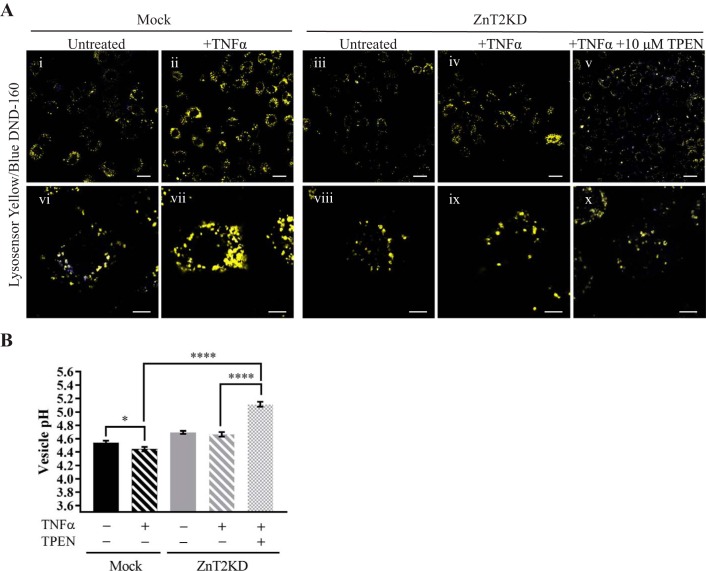
We previously reported that Zn accumulates in lysosomes during involution and that this process is associated with the accumulation of ZnT2 in lysosomes (21). Here, we used the cell-permeable, fluorescence-labile Zn reporter ZP-1 and found that ZP-1 fluorescence accumulated in punctate vesicles in the MECs of involuting WT mice, reflecting Zn accumulation in lysosomes (Fig. 5A). In contrast, ZP-1 fluorescence in the MECs of involuting ZnT2ko mice appeared hazy and not contained within discrete puncta, consistent with the accumulation of Zn in the cytoplasm. These findings are consistent with our previous report of cytoplasmic Zn accumulation in the mammary glands of lactating ZnT2ko mice and are expected, given the role of ZnT2 in mediating vesicular Zn sequestration (33, 37, 46). Given this finding, we hypothesized that the aberrant lysosome phenotype observed in ZnT2ko mice was a result of Zn toxicity due to accumulation of Zn in the cytoplasm. To test this hypothesis, we utilized HC11 cells in which ZnT2 expression was attenuated using ZnT2 siRNA (ZnT2KD) (Fig. 5B). To observe differences in lysosome phenotype, we treated cells with TNFα to stimulate lysosome biogenesis and acidification (21). Using immunofluorescence of Lamp1 to visualize lysosome morphology and abundance, we found that, upon treatment with TNFα, lysosomes in mock-transfected cells were larger and clustered in the perinuclear region, a classic hallmark of highly acidified lysosomes (25), whereas lysosomes in ZnT2KD cells appeared smaller and were dispersed throughout the cell (Fig. 5C). When ZnT2KD cells were briefly treated with a modest amount of the Zn chelator TPEN, the lysosome phenotype was not reversed, suggesting that the defect in lysosome formation observed with the loss of ZnT2 is not a result of cytoplasmic Zn accumulation. To confirm that these results were associated with differences in vesicular Zn between genotypes, we used the Zn-specific fluorophore FluoZin-3 to visualize labile Zn in vitro (Fig. 5D). FluoZin-3 fluorescence in mock-transfected cells appeared punctate, similar to our findings when we used ZP-1 in the involuting mammary glands of WT mice. In contrast, ZnT2 attenuation led to cytoplasmic Zn accumulation, as indicated by the hazy fluorescence throughout the cells, which was eliminated by TPEN treatment. To confirm that chelation of cytoplasmic Zn in ZnT2-attenuated cells did not restore lysosome acidification, the ratiometric fluorescent pH reporter LysoSensor blue/yellow DND-160 was used to directly measure pH (Fig. 6A). As expected, vesicle pH was significantly reduced in mock-transfected cells treated with TNFα compared with untreated cells, confirming our observations of lysosome biogenesis in MECs in response to TNFα (Fig. 6B). However, TNFα did not reduce vesicle pH in ZnT2KD cells, confirming a role for ZnT2 in vesicle acidification. Importantly, when ZnT2KD cells were treated with TPEN, lysosome pH was not reduced to the level observed in mock-transfected cells but, in fact, was profoundly increased. These findings confirm that cytoplasmic Zn accumulation is not the cause of impaired lysosome acidification and biogenesis observed in the absence of ZnT2.
Fig. 5.
Lysosome phenotype observed with the loss of Zn transporter 2 (ZnT2) is not a result of Zn toxicity. A: representative images of Zinpyr-1 (ZP-1) fluorescence (green) in frozen mammary gland sections from involuting wild-type (WT) and ZnT2-null (ZnT2ko) mice. Nuclei were counterstained with DAPI (blue). Note punctate, vesicular pattern staining in WT mammary glands and hazy green fluorescence in mammary epithelium of ZnT2ko mice. Magnification ×63; scale bars = 200 µm. B: representative immunoblot of ZnT2 in total membrane fraction from untransfected (Mock) and ZnT2-attenuated (ZnT2KD) mammary epithelial cells (MECs). β-Actin served as a loading control. Dotted lines indicate spliced sections taken from a single immunoblot. Data represent ZnT2 expression relative to β-actin. Values are means (SD); n = 4 samples/group. Experiment was repeated 3 times. *P < 0.05. C: representative images of lysosome-associated membrane protein 1 (Lamp1, red) and DAPI (blue) in Mock (i, ii, vi, and vii) and ZnT2KD (iii–v and viii–x) MECs treated with TNFα (+TNFα) or TNFα + N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (+TNFα +10 µM TPEN) or unstimulated (untreated) MECs. Magnification ×63; scale bars = 50 µm (i–v) and 5 µm (vi–x). D: representative images of FluoZin-3 fluorescence in Mock (i, ii, vi, and vii) and ZnT2KD (iii–v and viii–x) MECs treated with TNFα (+TNFα) or TNFα + TPEN (+TNFα +10 µM TPEN) or unstimulated (untreated) MECs. Magnification ×63; scale bars = 10 µm (i–v) and 5 µm (vi–x).
Fig. 6.
Impaired lysosome acidification with loss of Zn transporter 2 (ZnT2) is not a result of Zn toxicity. A: representative images of LysoSensor yellow/blue DND-160 fluorescence in untransfected (Mock; i, ii, vi, and vii) and ZnT2-attenuated (ZnT2KD; iii–v and viii–x) mammary epithelial cells (MECs) treated with TNFα (+TNFα) or TNFα + N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (+TNFα +10 µM TPEN) or unstimulated (untreated) MECs. Magnification ×63; scale bars = 25 µm (i–v) and 8 µm (vi–x). B: vesicular pH. Values are means ± SE of all vesicles from five, ×63 images/group; n = 5 replicates per group. *P < 0.05; ****P < 0.0001.
DISCUSSION
Mammary gland involution is a tightly coordinated process initially driven by lysosome biogenesis followed by lysosomal membrane permeabilization and the activation of LCD, for which the underlying molecular mechanisms are poorly understood (28). A role for Zn and the Zn transporter ZnT2 in LCD and mammary gland involution was first proposed when we documented that overexpression of ZnT2 in the lactating mammary gland leads to lysosomal Zn accumulation and precocious involution (21). Further studies suggested that ZnT2 plays a direct role in LCD, as the proinvolution signal TNFα was shown to dephosphorylate ZnT2, redirecting it from secretory vesicles to lysosomes, activating lysosome biogenesis, and executing LCD in vivo and in cultured MECs (20, 21); however, the precise role of ZnT2 was not elucidated. In this study we used ZnT2ko mice to determine the direct role of ZnT2 in mammary tissue remodeling.
We recently reported that ZnT2 interacts with the multisubunit proton pump v-ATPase in MECs, and this interaction is critical for vesicle acidification (34). It is reasonable to assume that v-ATPase plays a role in mammary gland remodeling for several reasons. First, lysosome pH ranges from 4.3 to 4.5, and this acidic environment is required for activity of lysosomal proteases that are released during lysosomal membrane permeabilization to initiate LCD and mammary gland involution (30). Moreover, deletion of v-ATPase subunit V0a2 in mice (V0a2ko) is associated with impaired degradation of Notch and TGFβ pathway mediators, resulting in aberrant activation of these signaling pathways in the mammary gland (47). Both Notch and TGFβ signaling are known to affect the balance between proliferation, differentiation, and apoptosis in different cell types (4, 14) and regulate mammary tissue remodeling during pregnancy, lactation, and involution (7, 19, 61). Therefore, v-ATPase and vesicle acidification are likely required for appropriate involution. Here we found that loss of ZnT2 impaired lysosomal v-ATPase assembly, which profoundly impaired mammary gland remodeling during involution. As a result, ZnT2ko mice had fewer and smaller lysosomes and, thus, suffered from suboptimal LCD. The interaction between v-ATPase and ZnT2 and the ability to generate acidified vesicles may be of vital importance in maintaining optimal mammary gland function. Similar developmental defects are observed in the mammary glands of ZnT2ko and V0a2ko mice (47), and both mouse strains exhibit profound defects during lactation (34, 47). However, the molecular mechanisms responsible for the defects during lactation, and now involution, have only been elucidated in ZnT2ko mice (34). Further studies are required to understand molecular interaction between ZnT2 and v-ATPase and the regulatory factors that govern this interaction in the mammary gland.
Several potential mechanisms could account for reduced lysosome abundance with the loss of ZnT2. First, lysosome biogenesis is regulated by TFEB-mediated transcription and the correct trafficking of lysosome proteins. The CLEAR network is a cluster of lysosome-related genes that are commonly regulated by TFEB, a transcription factor that is activated in response to starvation or lysosome stress (56, 59). Downregulation of lysosome biogenesis has been shown to be protective against LCD, while promotion of lysosome biogenesis promotes the accumulation of large lysosomes and exacerbates LCD in Caenorhabditis elegans (3). Our data suggest that the lysosome defect in ZnT2ko mice is a TFEB-independent process, as expression of these key proteins was not affected by the loss of ZnT2, nor did the loss of ZnT2 affect the total abundance of V0a or V1B. This suggests that the loss of ZnT2 may impair other aspects of lysosome biogenesis, such as lysosomal Zn accumulation or the endocytic delivery of lysosome components. For example, here we showed that the loss of ZnT2 prevents accumulation of vesicular Zn during mammary gland involution. Vesicular Zn binds to lysobisphosphatidic acid (27), a negatively charged phospholipid enriched in late endosomal and lysosomal membranes that is important in membrane sorting in late endosome and lysosome maturation (52). Interestingly, there were fewer Lamp1-positive vesicles involuting mammary glands from ZnT2ko than WT mice, yet levels of Lamp1 protein were similar in involuting mammary glands from ZnT2ko and WT mice. This discrepancy could be explained by impaired membrane sorting/trafficking in ZnT2ko mice. Additionally, it is possible that more Lamp1 accumulates in prelysosome delivery compartments in ZnT2ko mice, such as early and late endosomes, thereby creating endosomes with Lamp1-enriched membranes (10). Thus, while lysosome components are produced in ZnT2ko mice, the inability to import Zn into vesicles to stabilize lysobisphosphatidic acid may lead to a major defect in the delivery of lysosome proteins and the formation of a large enough pool of active lysosomes to efficiently execute mammary gland remodeling.
Alternatively, because ZnT2 interacts with both V0 and V1 domains of v-ATPase in MECs (34), another possibility is that the loss of ZnT2 impairs v-ATPase assembly and, thus, impairs proton transport and vesicular acidification. This is consistent with our studies in cultured MECs that reported a reduction in vesicle acidification upon attenuation of ZnT2 expression (34). Regulation of vesicle pH is acutely dependent on the assembly of the V0 and V1 domains on the membrane (11, 26, 66). A defect in assembly in ZnT2ko mice is supported by the reduction in the colocalization of Lamp1 and V1B, despite no reduction in Lamp1 or V1B proteins, indicating that the amount of V1B associated with lysosomal membranes was specifically reduced. Moreover, a defect in v-ATPase assembly is likely due to the loss of ZnT2 specifically, as we previously showed that the proinvolution signal TNFα stimulates the dephosphorylation of ZnT2, which activates adaptor protein 3 binding and relocalization of ZnT2 to lysosomes (21). We speculate that, without ZnT2 relocalization to lysosomes, a key partner critical for v-ATPase assembly and, thus, lysosome acidification is lost. It is also possible that loss of ZnT2 leads to accumulation of cytoplasmic Zn, which impairs v-ATPase activity (17). The cytosolic subunit V1A contains two cysteine residues, Cys254 and Cys532, which are implicated in regulation of v-ATPase activity (15). Cysteine residues have a high affinity for Zn; therefore, Zn binding to V1A may inhibit v-ATPase assembly or its proton-translocating activity (45). However, we showed that gentle chelation of Zn in ZnT2-attenuated cells was not sufficient to restore the normal lysosome phenotype. This finding is consistent with our previous findings that Zn chelation did not rescue vesicle acidification in ZnT2-attenuated MECs (34) and suggests that the interaction between ZnT2 and v-ATPase proteins is critical for v-ATPase assembly and lysosome acidification and function. Further studies are required to understand the molecular mechanisms through which ZnT2 and/or Zn interacts with v-ATPase.
Another possibility that cannot be ruled out is that, in addition to impairing v-ATPase assembly and proton transport, the loss of ZnT2-mediated lysosomal Zn accumulation may exacerbate the extraluminal proton leak, thereby inhibiting the formation of acidic vesicles (18, 36). Given that we found that Zn chelation in ZnT2-attenuated cells further increased lysosome pH, it is likely that the role of ZnT2 in lysosome acidification during involution involves both lysosomal Zn accumulation and interaction with v-ATPase. Sufficient lysosome acidification is critical for several of its functions, including cargo release, protease activation and maturation, autophagy, membrane trafficking, and inactivation of internalized pathogens (16, 40, 62). In addition, the acidic environment of the vesicle lumen is essential for endosome maturation (22). At an elevated lysosome pH, the low H+-sensitive endolysosomal Ca2+ channel TRPML1 is activated, depleting intralysosomal Ca2+ stores and elevating cytosolic Ca2+ (32), which would inhibit trafficking, recycling, and fusion of vesicles (48, 53). Given that our findings suggest that the loss of ZnT2 increases intraluminal pH, lysosomal Ca2+ homeostasis may be altered, thereby indirectly inhibiting lysosome protein trafficking and impacting lysosome and endosome fusion (9, 32).
In addition, the loss of MEC polarity we previously observed in ZnT2ko mice (34) may contribute to dysregulation of involution. Loss of polarity interferes with the trafficking of receptors to the cell surface (60). In the lactating mammary gland of ZnT2ko mice, this translates to failed trafficking of the prolactin receptor to the cell membrane, impairing downstream signaling and mammary gland development during pregnancy and lactation (34). Given that the initial phase of mammary gland involution is regulated by local factors and cytokines with cell surface receptors, including leukemia inhibitory factor (29), oncostatin M (65), and TNFα (21), it is possible that a loss of polarity in ZnT2ko mice impairs the response of MECs to key involution signals. Furthermore, a loss of polarity in MECs has been shown to interfere with spatially regulated apoptotic signaling (69), providing another mechanism by which the loss of polarity in ZnT2ko mammary glands may impair involution.
Perspectives and Significance
The importance of understanding the role of ZnT2 in mammary gland remodeling stems from the fact that numerous mutations and nonsynonymous variants in ZnT2, resulting in altered Zn transport and cellular dysfunction, have been reported in humans (2, 24, 31, 35, 38). Furthermore, elevated milk sodium-to-potassium ratios have been detected in women expressing common ZnT2 variants (i.e., D103E, T288S), suggesting breast dysfunction and loss of the tight junction barrier function (2), which is associated with precocious mammary gland remodeling during lactation. However, no studies have assessed the impact of ZnT2 variants on lactation performance in breastfeeding women. Our findings suggest that breastfeeding women who harbor defective variants in ZnT2 may have impaired or accelerated lysosome biogenesis, which affects milk volume and lactation performance. Here we report impaired initiation of mammary gland involution in the absence of ZnT2; however, whether the process of involution is inhibited or delayed is not known. Assessment of mammary gland morphology throughout the remodeling and postremodeling period would provide insight into additional mechanisms that may underlie or compensate for the effect of a ZnT2ko phenotype on involution. Although it is beyond the scope this study, such exploration has implications in terms of the impact of ZnT2 function on the success of subsequent lactation, as well as mammary neoplasia and tumorigenesis (13, 41, 49, 55). Further studies are needed to determine the consequence of harboring ZnT2 variants on breast health, lactation, and infant health.
GRANTS
This work was funded by intramural funds from the Penn State Hershey Department of Surgery to S. L. Kelleher and the Ruth Pike Graduate Fellowship to S. R. Hennigar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.C.R., S.R.H., and S.L.K. conceived and designed research; O.C.R. and S.R.H. performed experiments; O.C.R., S.R.H., and S.L.K. analyzed data; O.C.R., S.R.H., and S.L.K. interpreted results of experiments; O.C.R. prepared figures; O.C.R. drafted manuscript; O.C.R., S.R.H., and S.L.K. edited and revised manuscript; O.C.R., S.R.H., and S.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Sooyeon Lee and Samina Alam for technical support. The authors also acknowledge Dr. Kirill Kiselyov for providing primers for the selected CLEAR network genes. Confocal imaging was performed at the Microscopy Imaging Facility, Section of Research Resources, Penn State Hershey College of Medicine.
Present address for S. R. Hennigar: Military Nutrition Division, US Army Research Institute of Environmental Medicine (USARIEM), 10 General Green Ave, Natick, MA 01760.
REFERENCES
- 1.Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci 126: 1905–1912, 2013. doi: 10.1242/jcs.091181. [DOI] [PubMed] [Google Scholar]
- 2.Alam S, Hennigar SR, Gallagher C, Soybel DI, Kelleher SL. Exome sequencing of slc30a2 identifies novel loss- and gain-of-function variants associated with breast cell dysfunction. J Mammary Gland Biol Neoplasia 20: 159–172, 2015. [Erratum in J Mammary Gland Biol Neoplasia 2016.] doi: 10.1007/s10911-015-9338-z. [DOI] [PubMed] [Google Scholar]
- 3.Artal-Sanz M, Samara C, Syntichaki P, Tavernarakis N. Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J Cell Biol 173: 231–239, 2006. doi: 10.1083/jcb.200511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 284: 770–776, 1999. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 5.Bagci H, Laurin M, Huber J, Muller WJ, Côté JF. Impaired cell death and mammary gland involution in the absence of Dock1 and Rac1 signaling. Cell Death Dis 5: e1375, 2014. doi: 10.1038/cddis.2014.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter FO, Neoh K, Tevendale MC. The beginning of the end: death signaling in early involution. J Mammary Gland Biol Neoplasia 12: 3–13, 2007. doi: 10.1007/s10911-007-9033-9. [DOI] [PubMed] [Google Scholar]
- 7.Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3: 429–441, 2008. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev 13: 2604–2616, 1999. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115: 599–607, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Row PE, Davidson HW. Lysosome associated membrane protein 1 (Lamp1) traffics directly from the TGN to early endosomes. Traffic 5: 685–699, 2004. doi: 10.1111/j.1600-0854.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 11.Cotter K, Stransky L, McGuire C, Forgac M. Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem Sci 40: 611–622, 2015. doi: 10.1016/j.tibs.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey C, McCormick NH, Croxford TP, Seo YA, Grider A, Kelleher SL. Marginal maternal zinc deficiency in lactating mice reduces secretory capacity and alters milk composition. J Nutr 142: 655–660, 2012. doi: 10.3945/jn.111.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, Michels KB, Palmer JR, Schedin P, Stuebe AM, Watson CJ, Sherman ME. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst 105: 166–174, 2013. doi: 10.1093/jnci/djs505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng XH, Derynck R. Specificity and versatility in TGFβ signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Forgac M. Inhibition of vacuolar H+-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J Biol Chem 269: 13224–13230, 1994. [PubMed] [Google Scholar]
- 16.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 17.Fukao Y, Ferjani A. V-ATPase dysfunction under excess zinc inhibits Arabidopsis cell expansion. Plant Signal Behav 6: 1253–1255, 2011. doi: 10.4161/psb.6.9.16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerbino A, Hofer AM, McKay B, Lau BW, Soybel DI. Divalent cations regulate acidity within the lumen and tubulovesicle compartment of gastric parietal cells. Gastroenterology 126: 182–195, 2004. doi: 10.1053/j.gastro.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q, Betts C, Pennock N, Mitchell E, Schedin P. Mammary gland involution provides a unique model to study the TGF-β cancer pParadox. J Clin Med 6: E10, 2017. doi: 10.3390/jcm6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennigar SR, Kelleher SL. TNFα post-translationally targets ZnT2 to accumulate zinc in lysosomes. J Cell Physiol 230: 2345–2350, 2015. doi: 10.1002/jcp.24992. [DOI] [PubMed] [Google Scholar]
- 21.Hennigar SR, Seo YA, Sharma S, Soybel DI, Kelleher SL. ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci Rep 5: 8033, 2015. doi: 10.1038/srep08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huotari J, Helenius A. Endosome maturation. EMBO J 30: 3481–3500, 2011. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci 28: 3114–3122, 2008. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itsumura N, Inamo Y, Okazaki F, Teranishi F, Narita H, Kambe T, Kodama H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS One 8: e64045, 2013. doi: 10.1371/journal.pone.0064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212: 677–692, 2016. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane PM. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70: 177–191, 2006. doi: 10.1128/MMBR.70.1.177-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol 1: 113–118, 1999. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 28.Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, Poli V, Flavell RA, Clarkson RW, Watson CJ. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 13: 303–309, 2011. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 29.Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 130: 3459–3468, 2003. doi: 10.1242/dev.00578. [DOI] [PubMed] [Google Scholar]
- 30.Kubisch R, Fröhlich T, Arnold GJ, Schreiner L, von Schwarzenberg K, Roidl A, Vollmar AM, Wagner E. V-ATPase inhibition by archazolid leads to lysosomal dysfunction resulting in impaired cathepsin B activation in vivo. Int J Cancer 134: 2478–2488, 2014. doi: 10.1002/ijc.28562. [DOI] [PubMed] [Google Scholar]
- 31.Lasry I, Golan Y, Berman B, Amram N, Glaser F, Assaraf YG. In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J Biol Chem 289: 7275–7292, 2014. doi: 10.1074/jbc.M113.533786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Reports 12: 1430–1444, 2015. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Hennigar SR, Alam S, Nishida K, Kelleher SL. Essential role for zinc transporter 2 (ZnT2)-mediated zinc transport in mammary gland development and function during lactation. J Biol Chem 290: 13064–13078, 2015. doi: 10.1074/jbc.M115.637439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Rivera OC, Kelleher SL. Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells. J Biol Chem 292: 21598–21613, 2017. doi: 10.1074/jbc.M117.794461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liew HM, Tan CW, Ho CK, Chee JN, Koh MJ. Transient neonatal zinc deficiency caused by a novel mutation in the SLC30A2 gene. Pediatr Dermatol 34: e104–e105, 2017. doi: 10.1111/pde.13065. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Kohler JE, Blass AL, Moncaster JA, Mocofanescu A, Marcus MA, Blakely EA, Bjornstad KA, Amarasiriwardena C, Casey N, Goldstein LE, Soybel DI. Demand for Zn2+ in acid-secreting gastric mucosa and its requirement for intracellular Ca2+. PLoS One 6: e19638, 2011. doi: 10.1371/journal.pone.0019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez V, Foolad F, Kelleher SL. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in malignant metallothionein-null T47D breast tumor cells. Cancer Lett 304: 41–51, 2011. doi: 10.1016/j.canlet.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J 422: 43–52, 2009. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund LR, Rømer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Danø K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 122: 181–193, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426, 2008. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monks J, Henson PM. Differentiation of the mammary epithelial cell during involution: implications for breast cancer. J Mammary Gland Biol Neoplasia 14: 159–170, 2009. doi: 10.1007/s10911-009-9121-0. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 139: 269–275, 2012. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- 43.Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem 284: 17677–17686, 2009. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ono K, Wang X, Han J. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol Cell Biol 21: 8276–8288, 2001. doi: 10.1128/MCB.21.24.8276-8288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pace NJ, Weerapana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules 4: 419–434, 2014. doi: 10.3390/biom4020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J 15: 1784–1791, 1996. [PMC free article] [PubMed] [Google Scholar]
- 47.Pamarthy S, Mao L, Katara GK, Fleetwood S, Kulshreshta A, Gilman-Sachs A, Beaman KD. The V-ATPase a2 isoform controls mammary gland development through Notch and TGF-β signaling. Cell Death Dis 7: e2443, 2016. doi: 10.1038/cddis.2016.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062, 2000. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: transgenic models and clinical studies. J Mammary Gland Biol Neoplasia 14: 181–191, 2009. doi: 10.1007/s10911-009-9123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raines SM, Rane HS, Bernardo SM, Binder JL, Lee SA, Parra KJ. Deletion of vacuolar proton-translocating ATPase V0a isoforms clarifies the role of vacuolar pH as a determinant of virulence-associated traits in Candida albicans. J Biol Chem 288: 6190–6201, 2013. doi: 10.1074/jbc.M112.426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rane HS, Bernardo SM, Hayek SR, Binder JL, Parra KJ, Lee SA. The contribution of Candida albicans vacuolar ATPase subunit V1B, encoded by VMA2, to stress response, autophagy, and virulence is independent of environmental pH. Eukaryot Cell 13: 1207–1221, 2014. doi: 10.1128/EC.00135-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reaves BJ, Row PE, Bright NA, Luzio JP, Davidson HW. Loss of cation-independent mannose 6-phosphate receptor expression promotes the accumulation of lysobisphosphatidic acid in multilamellar bodies. J Cell Sci 113: 4099–4108, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, Wessel GM, Parrington J, Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr Biol 20: 703–709, 2010. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635, 2009. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 55.Sandgren EP, Schroeder JA, Qui TH, Palmiter RD, Brinster RL, Lee DC. Inhibition of mammary gland involution is associated with transforming growth factor-α but not c-myc-induced tumorigenesis in transgenic mice. Cancer Res 55: 3915–3927, 1995. [PubMed] [Google Scholar]
- 56.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science 325: 473–477, 2009. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 57.Sargeant TJ, Lloyd-Lewis B, Resemann HK, Ramos-Montoya A, Skepper J, Watson CJ. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat Cell Biol 16: 1057–1068, 2014. doi: 10.1038/ncb3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo YA, Lee S, Hennigar SR, Kelleher SL. Prolactin (PRL)-stimulated ubiquitination of ZnT2 mediates a transient increase in zinc secretion followed by ZnT2 degradation in mammary epithelial cells. J Biol Chem 289: 23653–23661, 2014. doi: 10.1074/jbc.M113.531145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429–1433, 2011. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinde SR, Maddika S. PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7. Nat Commun 7: 10689, 2016. doi: 10.1038/ncomms10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sizemore GM, Balakrishnan S, Hammer AM, Thies KA, Trimboli AJ, Wallace JA, Sizemore ST, Kladney RD, Woelke SA, Yu L, Fernandez SA, Chakravarti A, Leone G, Ostrowski MC. Stromal PTEN inhibits the expansion of mammary epithelial stem cells through Jagged-1. Oncogene 36: 2297–2308, 2017. doi: 10.1038/onc.2016.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sobota JA, Bäck N, Eipper BA, Mains RE. Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J Cell Sci 122: 3542–3553, 2009. doi: 10.1242/jcs.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia 12: 25–35, 2007. doi: 10.1007/s10911-007-9035-7. [DOI] [PubMed] [Google Scholar]
- 64.Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, Hennighausen L, Huang AM, Sterneck E. C/EBPδ is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 132: 4675–4685, 2005. doi: 10.1242/dev.02050. [DOI] [PubMed] [Google Scholar]
- 65.Tiffen PG, Omidvar N, Marquez-Almuina N, Croston D, Watson CJ, Clarkson RW. A dual role for oncostatin M signaling in the differentiation and death of mammary epithelial cells in vivo. Mol Endocrinol 22: 2677–2688, 2008. doi: 10.1210/me.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science 299: 1400–1403, 2003. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 67.Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res 8: 203, 2006. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med 8: 1–15, 2006. doi: 10.1017/S1462399406000196. [DOI] [PubMed] [Google Scholar]
- 69.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 135: 865–878, 2008. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]