Abstract
Despite its success as a potent antineoplastic agent, ∼25% of patients receiving cisplatin experience acute kidney injury (AKI) and must discontinue therapy. Impaired magnesium homeostasis has been linked to cisplatin-mediated AKI, and because magnesium deficiency is widespread, we examined the effect of magnesium deficiency and replacement on cisplatin-induced AKI in physiologically relevant older female mice. Magnesium deficiency significantly increased cisplatin-associated weight loss and markers of renal damage (plasma blood urea nitrogen and creatinine), histological changes, inflammation, and renal cell apoptosis and modulated signaling pathways (e.g., ERK1/2, p53, and STAT3). Conversely, these damaging effects were reversed by magnesium. Magnesium deficiency alone significantly induced basal and cisplatin-mediated oxidative stress, whereas magnesium replacement attenuated these effects. Similar results were observed using cisplatin-treated LLC-PK1 renal epithelial cells exposed to various magnesium concentrations. Magnesium deficiency significantly amplified renal platinum accumulation, whereas magnesium replacement blocked the augmented platinum accumulation after magnesium deficiency. Increased renal platinum accumulation during magnesium deficiency was accompanied by reduced renal efflux transporter expression, which was reversed by magnesium replacement. These findings demonstrate the role of magnesium in regulating cisplatin-induced AKI by enhancing oxidative stress and thus promoting cisplatin-mediated damage. Additional in vitro experiments using ovarian, breast, and lung cancer cell lines showed that magnesium supplementation did not compromise cisplatin's chemotherapeutic efficacy. Finally, because no consistently successful therapy to prevent or treat cisplatin-mediated AKI is available for humans, these results support developing more conservative magnesium replacement guidelines for reducing cisplatin-induced AKI in cancer patients at risk for magnesium deficiency.
Keywords: apoptosis, hypomagnesemia, inflammation, nephrotoxicity, oxidative stress
cisplatin (CIS) is a major chemotherapeutic drug used for cancer treatment, including ovarian, breast, testicular, non-small cell lung, gastric, and other cancers (12, 50, 57, 69). In fact, testicular cancer has shown complete remission in ∼70–80% of men after CIS treatment (23). Despite this remarkable success, CIS is associated with acute kidney injury (AKI) in ∼25% of patients after repeated dosing (50, 57, 69). Many patients exhibit irreversible kidney injury due to cumulative damage to the proximal and distal tubules, requiring dose reduction or CIS discontinuation (50, 69). While the pathogenesis of CIS-induced AKI includes localized inflammation, oxidative stress, DNA damage, and tubular epithelial cell apoptosis along with impaired renal handling of magnesium (Mg) (12, 41, 50, 57, 69, 71), no therapies have been shown to consistently reduce or prevent CIS-induced AKI in humans.
Mg is required (300–400 mg/day) for optimal metabolic function (e.g., synthesis/stability of DNA, RNA, and protein, mitochondrial function, and as a cofactor for ATP activity and >300 enzymes) (4, 26, 33, 53, 67). Surprisingly, <50% of the United States population consumes the reference daily intake of Mg (68). Mg deficiency, characterized by increased inflammation and oxidative stress (47, 49, 56), can result from an imbalance between Mg intake, absorption, and renal losses as well as increased metabolic demands (22, 47, 49, 56, 68). Mg deficiency is more common among the elderly (22, 82), who are also more susceptible to AKI due to renal parenchymal loss, ATP depletion, and mitochondrial dysfunction (2, 73).
The synergistic effects of CIS and Mg deficiency are believed to contribute to renal dysfunction (42). Precisely how Mg deficiency promotes CIS-induced AKI is not known, and little has been done to prevent it. Because advanced age and the female sex are risk factors for CIS-induced AKI (50, 69), we investigated the effects of Mg deficiency and Mg replacement/supplementation after Mg deficiency on CIS-induced AKI in older female mice.
METHODS
Animals and Cell Lines
The Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research approved the animal experiments (no. 2012-009). C57BL/6 mice [female (retired breeders), 11 mo old, Taconic Farms, Germantown, NY] were acclimatized under normal environmental conditions and allowed free access to standard chow and tap water for 1 wk before experimentation. LLC-PK1 renal epithelial and MCF-7 human breast cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). The H460 human large cell lung cancer cell line was provided by Dr. H Simpkins (The Feinstein Institute for Medical Research, Manhasset, NY). The A2780 human ovarian tumor cell line was obtained from Dr. T. C. Hamilton (Fox Chase Cancer Center, Philadelphia, PA).
Experimental Model of CIS-Induced AKI
Mice were randomized to receive either 1) control diet (normal chow containing 100% of the recommended amount of Mg) or 2) Mg-deficient (MgD) diet (containing 10% of the recommended amount of Mg, prepared by Teklad/Harlan, Madison, WI) for 2 wk before the administration of either saline or CIS (12 mg/kg ip, n = 12–15 mice/group). All mice were weighed before CIS administration and just before euthanasia 48 h post-CIS (thus, MgD mice were on the 10% Mg diet for a total of 16 days). In addition, one group of mice [n = 10, Mg-supplemented (MgS) or replacement group] received the MgD diet for 16 days followed by the control (100% Mg) diet along with 0.3% MgCl2 (wt/vol) in their drinking water for 11 days before CIS (12 mg/kg ip). After CIS treatment, this group continued 100% Mg-containing chow with 0.3% MgCl2 (wt/vol) drinking water and received MgSO4 (100 mg/kg/day sc) twice daily until euthanasia 48 h later by CO2 asphyxiation. Blood was collected by exsanguination via cardiac puncture into heparinized needles/syringes; after centrifugation, isolated plasma was collected and frozen at −80°C until analysis. Kidneys were collected and either flash frozen in liquid N2 (outer medulla and cortex only) or fixed in 10% formalin [1/2 kidney (sagittal sections)].
Antibodies and Reagents
ERK1/2 (rabbit anti-mouse), phosphorylated (p-)ERK1/2 (rabbit anti-mouse), STAT3 (rabbit anti-mouse), p-STAT3 (Tyr705, rabbit anti-mouse), p53 (mouse anti-mouse), p-p53 (Ser15, rabbit anti-mouse), multidrug resistance protein (MRP)4 [ATP-binding cassette subfamily C (ABCC)4, rabbit anti-mouse], and GAPDH (rabbit anti-mouse) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Organic cation transporter (OCT)1 (rabbit anti-mouse), copper transporter 1 (CTR1; rabbit anti-mouse), MRP2 (rabbit anti-mouse), and MRP6 (rabbit anti-mouse) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). OCT2 antibody (OCT21-A, rabbit anti-mouse) was purchased from Alpha Diagnostic (San Antonio, TX). CIS [cis-dicholorodiammineplatinum(II)] was purchased from Acros Organics (Pittsburgh, PA). MgCl2·6H2O and MgSO4 (anhydrous) were purchased from Fisher Scientific. 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Neutral red and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO).
Determination of Plasma Mg, Blood Urea Nitrogen, and Creatinine Levels
Plasma ionized Mg2+ (mg/dl) and blood urea nitrogen (BUN; in mg/dl) levels were determined using Quantichrom Magnesium and Quantichrom Urea Assay kits, respectively (BioAssay Systems, Hayward, CA). Plasma creatinine (Cr; in mg/dl) was measured using an enzymatic assay (Diazyme Laboratories, Poway, CA).
Real-Time Quantitative PCR
High-quality RNA was isolated from frozen kidneys using a RNeasy Universal Plus Mini kit (Qiagen, Valencia, CA). The purity/concentration of total RNA was assayed using a Nanodrop spectrophotometer (Wilmington, DE). Quantitative PCRs using specific primers (Table 1; Roche Universal Probe Library) were performed in duplicate/triplicate using Eurogentec One Step RT qPCR mastermix, 100 ng RNA, and a Roche 480 Light Cycler using the following conditions: 48°C for 30 min and 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Relative changes in gene expression were calculated as fold changes using the comparative ΔΔCt method (where Ct is threshold cycle). Mouse Gapdh was used as the housekeeping gene for normalizing transcript levels (16).
Table 1.
Mouse quantitative PCR primers
| Gene Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| Ncf1 (p47phox) | 5′-GGACACCTTCATTCGCCATA-3′ | 5′-CTGCCACTTAACCAGGAACAT-3′ |
| Cxcl2 | 5′-AAAATCATCCAAAAGATACTGAACAA-3′ | 5′-CTTTGGTTCTTCCGTTGAGG-3′ |
| Ccl2 | 5′-CATCCACGTGTTGGCTCA-3′ | 5′-GATCATCTTGCTGGTGAATGAGT-3′ |
| Cxcl10 | 5′-GCTGCCGTCATTTTCTGC-3′ | 5′-TCTCACTGGCCCGTCATC-3′ |
| Tnfa | 5′-CTGTAGCCCACGTCGTAGC-3′ | 5′-TTGAGATCCATGCCGTTG-3′ |
| Bak1 | 5′-GGAATGCCTACGAACTCTTCA-3′ | 5′-CCAGCTGATGCCACTCTTAAA-3′ |
| Oct1 | 5′-GGCTCTGCCTGAGACTATTGA-3′ | 5′-CGTGTTTTCTTTGGCCTTTG-3′ |
| Oct2 | 5′-CAATTTGCCGTGACTCTGC-3′ | 5′-GAGACTCCGGTATGCACCA-3′ |
| Ctr1 | 5′-GGGATCCAGTTCTGAGAGGA-3′ | 5′-GAAAAAGATGAGATTCAGTGGAAAA-3′ |
| Abcc2 | 5′-CAAATCCAATTCTCTACCTATGCAC-3′ | 5′-GCCTGCAGTGTTGGATCA-3′ |
| Abcc4v1 | 5′-CCACATGATTTACCGGAAGG-3′ | 5′-AGGTTAACTATCTGGCCTGTGG-3′ |
| Abcc4v3 | 5′-GAGCACACGGACGAGGAG-3′ | 5′-TCTTCAATGGCCTCTTTAAGTTG-3′ |
| Abcc6 | 5′-CATCTTGCCAGGAATCAACAC-3′ | 5′-GGGCTCCTGACGGAAGTT-3′ |
| Gapdh | 5′-GAGCCAAACGGGTCATCA-3′ | 5′-CATATTTCTCGTGGTTCACACC-3′ |
Ncf, neutrophil cytosolic factor; Cxcl, chemokine (C-X-C motif) ligand; Ccl, chemokine (C-C motif) ligand; Tnfa, TNF-α; Oct, organic cation transporter; Ctr, copper transporter; Abcc, ATP-binding cassette subfamily C.
Cytokine and Chemokine Assays
Frozen cortical renal tissue specimens (100 mg) were homogenized in 250 μl lysis buffer [Tris-buffered saline (pH 7.3) containing 0.25% Triton X-100 and protease and phosphatase inhibitor cocktail] on ice using a Dounce homogenizer; supernatants were collected after centrifugation. Kidney homogenates and plasma were assayed for cytokines and chemokines using the Meso Scale Discovery (MSD) multiplex platform and MSD Sector Imager 2400 plate reader (Meso Scale Diagnostics, Rockville, MD). Raw data were measured as electrochemiluminescence signals and analyzed using Discovery Workbench 3.0 software (MSD). In addition, renal chemokine (C-X-C motif) ligand (CXCL)2 and chemokine (C-C motif) ligand (CCL)2 levels were determined by ELISA (R&D Systems, Minneapolis, MN). Renal inflammatory mediator values were adjusted for protein concentrations (using the Bio-Rad protein assay, Bio-Rad, Hercules, CA).
Measurement of Tissue ATP Levels and Myeloperoxidase
Renal cortical tissues were homogenized, and ATP levels were measured using a colorimetric ATP assay kit (Biovision, San Francisco, CA) according to the manufacturer's instructions. For the assessment of myeloperoxidase (MPO) levels, renal cortex tissues were homogenized in lysis buffer (described above); protein concentrations were measured using the Bio-Rad assay. MPO levels were measured by ELISA (Hycult Biotechnologies) and adjusted for protein concentrations according to the manufacturer's instructions.
Western Blot Analysis
Renal cortex tissues were homogenized in lysis buffer (described above); protein concentrations were measured using the Bio-Rad assay. Proteins (50 μg/lane) were separated by SDS-PAGE (Invitrogen) and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). After being blocked for 1 h, membranes were then incubated with each primary antibody (1:1,000, Cell Signaling antibodies/1:300, Santa Cruz Biotechnology antibodies) overnight at 4°C. After being washed, blots were then incubated with the appropriate near-infrared fluorescently labeled secondary antibody (1:15,000, LI-COR Biosciences) for 1 h and washed before bands were revealed using the Odyssey infrared imaging system (LI-COR Biosciences). Band densities were determined using the appropriate controls or housekeeping proteins; quantitation was determined using ImageJ Software (National Institutes of Health).
Histological Assessment of Renal Cell Apoptosis, Neutrophil Infiltration, and Renal Injury
Formalin-fixed kidneys were embedded in paraffin and sectioned (5μm). Renal apoptosis (in the cortex and outer medulla regions) was measured by TUNEL using an ApoTag kit (Millipore). Slides were scored by counting the number of densely stained apoptotic cells per high-power field (using >5 random fields/section, 4–5 mice/group). Neutrophilic infiltration was assessed by Napthol AS-D chloroacetate esterase (Leder staining, Sigma-Aldrich). Slides were scored by counting the number of neutrophils per high-power field, as described for TUNEL. Similarly, sections were stained with hematoxylin and esosin and scored using a semiquantitative scale designed to assess AKI-associated tubular injury (tubular epithelial cell loss, necrosis, tubular epithelial simplification, intratubular debris, and casts) by a pathologist unaware of the experimental groups (using >5 random fields/section, 4–5 mice/group). Tubule injury scores (ranging between 0 and 4) were based on the percentage of tubules affected as follows: 0 = <10%, 1 = 10–25%, 2 = 26–50%, 3 = 51–75%, and 4 = >75%.
Quantification of Platinum Accumulation in the Kidney
Renal platinum (Pt) analysis was performed at the Biomarker Mass Spectrometry Facility of the University of North Carolina at Chapel Hill using a modification of a previously described method (51). Briefly, frozen kidneys were digested with 100 μl of concentrated (70%) nitric acid along with 150 ng rhenium (Re) in a 7-ml polypropylene screw cap vial and left at room temperature for 5 h. After being vented, vials were placed at 85°C overnight. Once cooled, 100 μl of 30% H2O2 was added. Vials were vented 20 min after being returned to 85°C. Digestion continued for 4–6 h, after which samples were centrifuged at 3,500 rpm for 5 min. Dilution to 3 ml with 18-MΩ water resulted in a final matrix of 2% nitric acid and 50 ng/g Re (which served as a yield monitor). Matrix-matched calibration standards (Pt/Re, ranging from 0 to 1,000 ng/g) were prepared with 100 μl; mouse plasma (Bioreclamation, Liverpool, NY). Samples and standards were processed in a single batch. Pt and Re were measured using an Agilent 7500cx (Tokyo, Japan) inductively coupled plasma mass spectrometer and quantified against an external calibration curve (r2 = 0.9999). Validity of the data was assessed through use of the Re yield monitor, replicate sample analysis, and quality control standards. Tissue concentrations of Pt were corrected individually for yield from the Re recovery. Re recovery for all samples was 98 ± 1.5%. The difference in replicate sample analyses was ≤5%. Quality control standards varied <10% from known values.
In Vitro LLC-PK1 Oxidative Stress and CIS-Induced Cytotoxicity Assays
DCFH-DA (oxidative stress) assay.
Briefly, LLC-PK1 cells were grown in medium 199 containing 10% FBS and penicillin-streptomycin and glutamine (PSQ). Four days before experimentation, media were replaced with MEM containing 5% FBS, Ca2+ (2.7 g/dl), nonessential amino acids, PSQ, and either 100% (9.63 mg/dl) or 10% of the recommended amount of Mg (0.963 mg/dl) as MgSO4. Approximately 16 h before experimentation, one set of cells maintained in 10% Mg was supplemented with MgSO4 to 100% Mg (i.e., to a final concentration of 100% Mg to mimic Mg replacement after Mg deficiency). On the day of the experiment, LLC-PK1 cells were harvested, resuspended (1 × 106cells/ml), treated with either MEM (vehicle) or CIS (25 μg/ml or 83.3 μM), and then incubated at 37°C in 5% CO2 for 3.5 h. The dose of CIS used for these experiments was based on the results of pilot studies testing the amount of CIS required to induce consistently measurable yet modest increases in oxidative stress in these cells within 3.5 h, which allowed us to identify conditions that enhanced and reduced oxidative stress. After the incubation period, cells were washed with HBSS and labeled with DCFH-DA (20 μM) for 20 min. Labeled cells were washed and analyzed for oxidative stress by the conversion of nonfluorescent DCFH-DA into a fluorescent compound, dichlorofluorescein, by ROS intermediates (24), which was measured 0.5 h later in a quantitative manner using a Victor3 fluorescence plate reader (Perkin-Elmer) at an excitation wavelenght of 485 nm and emission wavelength of 535 nm.
LLC-PK1 cytotoxicity assay.
LLC-PK1 cells were grown as described above; media were replaced with 100% Mg-MEM or 10% Mg-MEM, and cells were incubated for 3 days. A portion of cells maintained on 10% Mg media were supplemented with 190% Mg (final concentration: 200% Mg) 1 day before CIS treatment (prepared from a stock solution of 1 mg/ml in prewarmed saline). MEM or CIS (diluted in MEM, 25 μM final concentration) was added, and cells were assayed for cytotoxicity using the neutral red assay (65) 29 h later and read using a spectrometer (optical density at 570 nm). The dose of CIS used for these experiments was determined through preliminary experiments [the dose that killed ∼50% of the cells within ∼30 h (which allowed us to identify conditions that improved cell viability or worsened cell viability)].
In Vitro CIS-Mediated Tumor Cell Killing (A2780 Human Ovarian Cancer, MCF-7 Human Breast Cancer, and H460 Human Lung Cancer Cells)
A2780 cells were grown in RPMI 1640 media containing 10% FBS and PSQ in 96-well plates and allowed to reach 60% confluence. The media were replaced with MEM containing either 100% Mg (4.9 mg/dl) or 10% Mg (0.49 mg/dl) supplied as MgSO4, Ca2+ (2.7 g/dl), nonessential amino acids, and PSQ, and cells were incubated for 3 days. A portion of cells maintained in 10% Mg media were supplemented with 90% Mg (final concentration: 100% Mg as MgSO4) ∼36 h before CIS treatment. MEM or CIS (diluted in MEM, 0–40 μM final concentration) was added, and cells were assayed for cytotoxicity using a MTT assay (optical density at 570/690 nm) 24 h later. IC50 values for tumor cell killing by CIS were calculated using nonlinear regression to fit the data to the log (inhibitor-CIS dose) versus response (variable slope-percent viable) curve using GraphPad Prism (GraphPad Prism Software, San Diego, CA). The same procedures were followed for MCF-7 and H460 cell lines except for MCF-7 breast cancer cells, which were originally grown in DMEM (where 100% Mg = 9.63 mg/dl and 10% Mg = 0.963 mg/dl).
Statistical Analyses
Experiments were performed at least twice, and data are expressed as means ± SE (or means ± SD) as indicated. One-way ANOVAs were used for multiple comparisons followed by Bonferroni post hoc testing using GraphPad Prism (GraphPad Software). P values of <0.05 were considered significant.
RESULTS
Mg Status Regulates CIS-Induced Markers of AKI
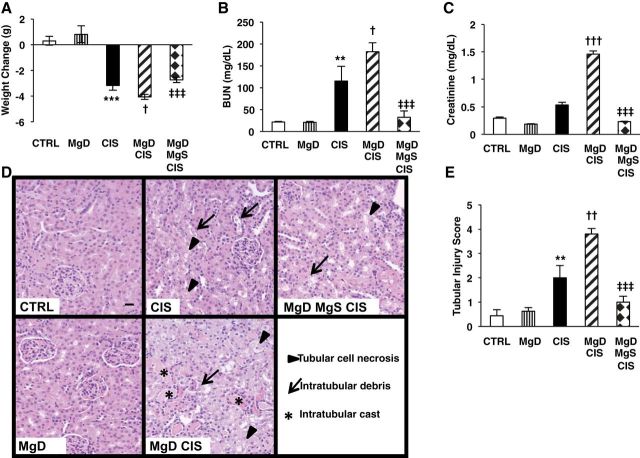
Consumption of the MgD diet (10% Mg) by older female mice reduced plasma Mg2+ levels by 37% (1.39 ± 0.15 mg/dl for MgD vs. 2.21 ± 0.06 mg/dl for control, means ± SE, P < 0.001); Mg supplementation after Mg deficiency increased plasma Mg2+ levels by 23% (1.7 ± 0.1 mg/dl, P = 0.058) versus MgD mice. CIS induced significant weight loss (P < 0.001; Fig. 1A), whereas Mg deficiency alone did not significantly affect weight; MgD + CIS treatment significantly enhanced weight loss compared with CIS alone (P < 0.05; Fig. 1A), which was attenuated by Mg supplementation (P < 0.001; Fig. 1A). Mg deficiency alone had no effect on kidney function as assessed by plasma BUN (Fig. 1B) and Cr levels (Fig. 1C). CIS alone significantly elevated BUN levels (P < 0.01; Fig. 1B) and only slightly (not significantly) elevated Cr levels (Fig. 1C) compared with controls. When combined with Mg deficiency, CIS significantly augmented BUN (P < 0.05; Fig. 1B) and Cr levels (P < 0.001; Fig. 1C) versus CIS-treated mice, whereas Mg supplementation significantly attenuated CIS-induced BUN and Cr levels versus MgD + CIS mice (Fig. 1, B and C).
Fig. 1.
Magnesium (Mg) deficiency before cisplatin (CIS) treatment enhances and Mg supplementation protects against CIS-induced weight loss and kidney damage. Mice were maintained on either 100% Mg or 10% Mg-deficient (MgD) diets, as described in methods, and then treated with either saline [control (CTRL)] or CIS (12 mg/kg). Another group of mice maintained on 10% Mg was switched to the 100% Mg diet and given a Mg-supplemented (MgS) diet, as described in methods. A: changes in weight from just before CIS to 48 hrs post-CIS. B and C: all mice were euthanized 48 h post-CIS (or saline), and blood urea nitrogen (BUN; B) and plasma creatinine levels (C) were determined. Data are shown as means ± SE (in mg/dl). Fixed kidney tissues were stained with hematoxylin and eosin and evaluated for histology. D: representative images for each group (×200 magnification). E: histological damage scores (ranging between 0 and 4) were based on the percentage of tubules affected (0 = <10%, 1 = 10–25%, 2 = 26–50%, 3 = 51–75%, and 4 = >75%). Data are shown as means ± SE. Scale bar = 20 μm. **P < 0.01 vs. CTRL; ***P < 0.001 vs. CTRL; †P < 0.05 vs. CIS; ††P < 0.01 vs. CIS; †††P < 0.001 vs. CIS; ‡‡‡P < 0.001 vs. MgD + CIS.
Renal Tubular Damage/Necrosis After CIS Is Regulated by Mg Status
Mg deficiency alone (Fig. 1, D and E) did not affect renal histology. However, CIS treatment induced significant renal injury versus controls (Fig. 1, D and E). When combined with Mg deficiency, CIS significantly increased tubular injury compared with CIS alone (Fig. 1, D and E). In contrast, MgDMgS + CIS kidneys showed significantly less tubular injury compared with MgD + CIS kidneys (P < 0.001; Fig. 1, D and E).
CIS-Mediated Renal Chemokine Expression Is Upregulated by Mg Deficiency and Downregulated by Mg Supplementation
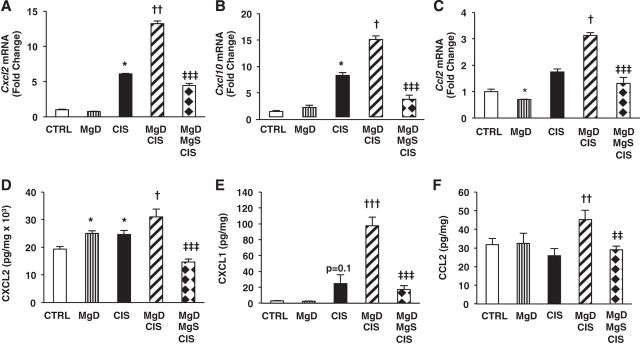
Renal expression of several chemokines is increased after CIS treatment, and their expression is associated with increased renal injury (12, 50, 57, 69). CIS increased both renal Cxcl2 (P < 0.05; Fig. 2A) and Cxcl10 (P < 0.05; Fig. 2B) mRNA expression but only slightly upregulated renal Ccl2 mRNA expression above controls (Fig. 2C). In combination with Mg deficiency, CIS significantly enhanced renal Cxcl2, Cxcl10, and Ccl2 mRNA expression above the levels observed after CIS alone; this was reversed by Mg supplementation (P < 0.001; Fig. 2, A–C).
Fig. 2.
CIS-induced renal chemokine expression is upregulated by Mg deficiency and downregulated by Mg replacement. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). A–C: all mice were euthanized 48 h post-CIS (or saline), and chemokine (C-X-C motif) ligand (Cxcl)2 (A), Cxcl10 (B), and chemokine (C-C motif) ligand (Ccl2)2 (C) mRNA expression in renal cortical tissues was measured by quantitative PCR. Data are shown as means ± SE (in fold changes vs. Gapdh). D–F: CXCL2 (D), CXCL1 (E), and CCL2 (F) renal protein levels were measured by Meso Scale Discovery (MSD)/ELISA. Data are shown as means ± SE (per mg protein). *P < 0.05 vs. CTRL; †P < 0.05 vs. CIS; ††P < 0.01 vs. CIS; †††P < 0.001 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS; ‡‡‡P < 0.001 vs. MgD + CIS; P = 0.1 vs. CTRL.
Mg deficiency alone significantly increased renal CXCL2 protein levels versus controls (Fig. 2D). CIS significantly enhanced renal CXCL2 levels compared with controls (Fig. 2D) and slightly increased CXCL1 (P = 0.1; Fig. 2E) but did not increase CCL2 levels above controls (Fig. 2F). MgD mice treated with CIS showed enhanced renal CXCL2 (P < 0.05; Fig. 2D), CXCL1 (P < 0.001; Fig. 2E), and CCL2 (P < 0.01; Fig. 2F) compared with CIS-treated mice; chemokine expression induced by Mg deficiency was attenuated by Mg supplementation (P < 0.01; Fig. 2, D–F).
CIS-Induced Renal Neutrophil Infiltration Is Exacerbated by Mg Deficiency and Improved by Mg Supplementation
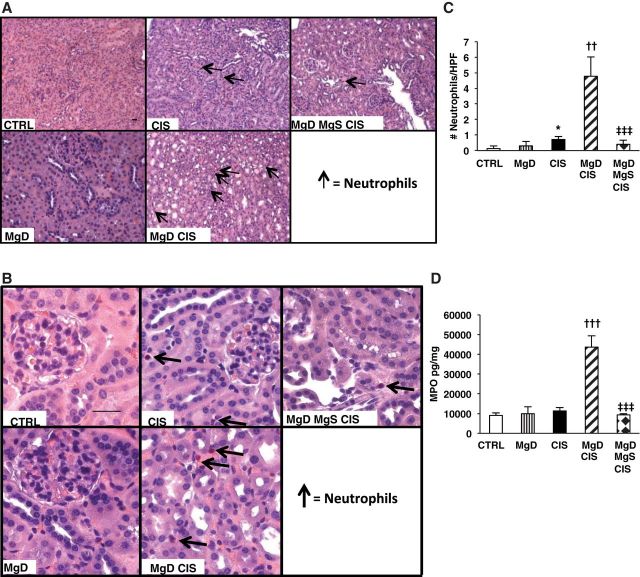
Because we observed a significant increase in CXCL1 and CCL2, neutrophil chemotactic factors, in the kidneys (Fig. 2, E and F) and because renal neutrophil infiltration is associated with CIS-induced AKI (25, 44), we examined the effect of Mg status on CIS-induced renal neutrophil infiltration. CIS slightly enhanced renal neutrophil infiltration compared with controls (P < 0.05) without increasing renal MPO levels (Fig. 3, A–D). Both renal neutrophil accumulation and MPO levels were exacerbated when CIS was given to MgD mice (P < 0.01; Fig. 3, A–D), and these effects were improved by Mg supplementation (P < 0.001; Fig. 3, A–D).
Fig. 3.
CIS treatment after Mg deficiency is associated with enhanced neutrophil infiltration and renal myeloperoxidase (MPO). Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline); fixed kidney tissues were evaluated for neutrophils by Leder staining. A and B: representative images for each group at ×200 (A) and ×400 (B) magnification. C: mean numbers of neutrophils per high-power field (HPF) ± SE. D: frozen renal tissues were analyzed for MPO. Data are shown as mean MPO concentrations ± SE (in pg/mg protein). Scale bars = 20 μm. *P < 0.05 vs. CTRL; ††P < 0.01 vs. CIS; †††P < 0.001 vs. CIS; ‡‡‡P < 0.001 vs. MgD + CIS.
Mg Deficiency Leads to Dysregulated Renal Cytokine Expression in CIS-Treated Mice: Reversal by Mg Supplementation
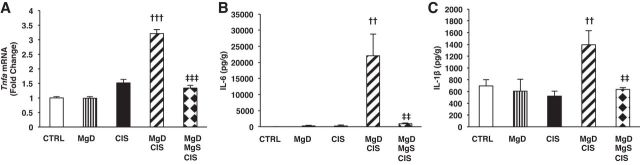
Next, we investigated the effects of Mg deficiency (with or without Mg supplementation) on established cytokine markers of inflammation associated with CIS-induced AKI. CIS upregulated renal Tnfa mRNA expression versus saline-treated controls, but this was not significant (Fig. 4A). When CIS was combined with Mg deficiency, renal Tnfa mRNA was significantly enhanced (P < 0.001) compared with CIS alone (Fig. 4A), and this increase was reduced by Mg supplementation (P < 0.001; Fig. 4A). Renal TNF-α protein was not detectable under any conditions. CIS alone did not alter renal IL-6 (Fig. 4B) or IL-1β (Fig. 4C) levels above controls. However, MgD + CIS mice showed significantly enhanced renal IL-6 (Fig. 4B) and IL-1β (Fig. 4C) levels compared with CIS-treated mice. Mg supplementation significantly attenuated CIS-mediated kidney cytokines after Mg deficiency (Fig. 4, A–C).
Fig. 4.
Mg deficiency before CIS treatment upregulates the expression of renal cytokines: reversal by Mg replacement. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). A: all mice were euthanized 48 h post-CIS (or saline), and renal TNF-α (Tnfa) mRNA expression in renal cortical tissues was measured using quantitative PCR. Data are shown as means ± SE (in fold changes vs. Gapdh). B and C: IL-6 (B) and IL-1β (C) renal protein levels were measured by MSD. Data are shown as mean cytokine concentrations ± SE (in pg/g protein). ††P < 0.01 vs. CIS; †††P < 0.001 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS; ‡‡‡P < 0.001 vs. MgD + CIS.
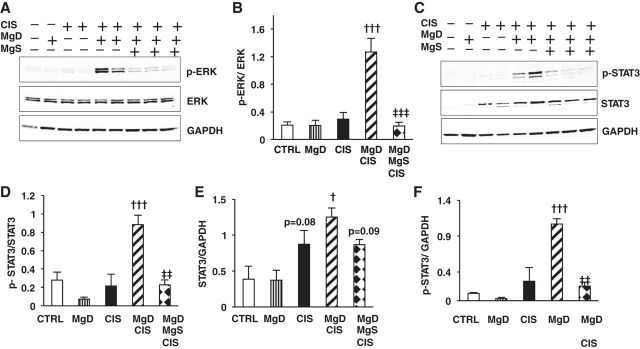
Mg Status Regulates CIS-Induced Renal ERK1/2 and STAT3 Activation
Based on the effect of Mg status on renal inflammation in the CIS model, we determined the effect of Mg on CIS-induced activation of ERK1/2 and STAT3, two proinflammatory signaling pathways implicated in CIS-induced AKI (38, 59). CIS alone slightly increased renal ERK1/2 phosphorylation (p-ERK1/2) over basal levels, but this was significantly enhanced by Mg deficiency (Fig. 5, A and B). In contrast, decreased renal p-ERK1/2 was observed after MgDMgS + CIS versus MgD + CIS treatment (Fig. 5, A and B). Total ERK1/2 was not affected by CIS or Mg deficiency; therefore, p-ERK1/2 expression was adjusted for ERK1/2 expression (Fig. 5, A and B). Phosphorylation of STAT3 (p-STAT3, Tyr705) was not altered by Mg deficiency alone or CIS alone (Fig. 5, C, D, and F) but was significantly enhanced by MgD + CIS treatment (Fig. 5, C, D, and F). Because CIS alone increased total STAT3 expression (Fig. 5, C and E), p-STAT3 levels were also corrected for GAPDH (Fig. 5F). Mg supplementation significantly decreased CIS-mediated renal p-STAT3 induced by MgD + CIS treatment (Fig. 5, C, D, and F).
Fig. 5.
Mg status regulates CIS-induced activation of ERK1/2 and STAT3 inflammatory signaling pathways. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline), and ERK1/2 and STAT3 protein expression and phosphorylation in renal cortical tissues were measured by Western blot analysis. GAPDH, total ERK1/2, and total STAT3 were used as loading controls. A: representative blots for ERK1/2. B: quantitation of phosphorylated (p-)ERK-to-total ERK1/2 band densities (means ± SE). C: representative blots for STAT3 (total STAT3), p-STAT3 (Tyr305), and GAPDH. D–F: quantitation of p-STAT3-to-total STAT3 (D), total STAT3-to-GAPDH (E), and p-STAT3-to-GAPDH (F) band densities. All data are expressed as mean band densities ± SE. †P < 0.05 vs. CIS; †††P < 0.001 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS; ‡‡‡P < 0.001 vs. MgD + CIS; P = 0.08 vs. CTRL; P = 0.09 vs. MgD + CIS.
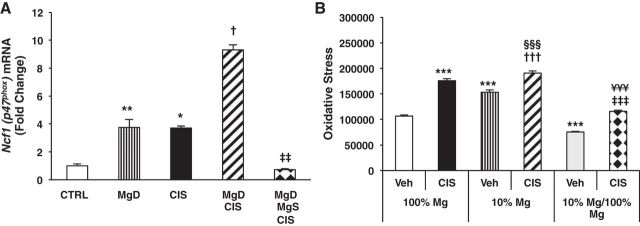
Oxidative Stress Before and During CIS-Mediated AKI Is Regulated by Mg Status
In conjunction with ongoing inflammation, oxidative stress is another characteristic feature of CIS-induced AKI (12). Mg deficiency alone and CIS alone significantly upregulated renal neutrophil cytosolic factor 1 (Ncf1) mRNA expression (P < 0.01 and P < 0.05, respectively) compared with controls (Fig. 6A). MgD + CIS treatment further increased renal Ncf1 mRNA expression by ∼2.5-fold versus CIS alone (Fig. 6A), and this was reversed by Mg supplementation (Fig. 6A).
Fig. 6.
Mg deficiency upregulates and Mg supplementation downregulates basal and CIS-induced oxidative stress in vivo and in renal epithelial cells. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with either saline (CTRL) or CIS (12 mg/kg). A: all mice were euthanized 48 h post-CIS (or saline), and neutrophil cytosolic factor 1 (Ncf1) mRNA expression in renal cortical tissues was measured using quantitative PCR. Data are shown as means ± SE (in fold changes vs. Gapdh). *P < 0.05 vs. CTRL; **P < 0.01 vs. CTRL; †P < 0.05 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS. B: LLC-PK1 renal epithelial cells were grown in either 100% Mg media, 10% Mg (deficient) media, or 10% Mg media followed by 100% Mg (10% Mg/100% Mg) media for 4 days and then treated with MEM [vehicle (Veh)] or CIS (83.3 μM) and assayed for oxidative stress using a 2′,7′-Dichlorodihydrofluorescein diacetate assay. Data are shown as mean oxidative stress (or fluorescence) ± SD. ***P < 0.001 vs. Veh + 100% Mg; †††P < 0.001 vs. Veh + 10% Mg; ‡‡‡P < 0.001 vs. Veh + 10% Mg/100% Mg; §§§P < 0.001 vs. CIS + 100% Mg; ¥¥¥P < 0.001 vs. CIS + 10% Mg.
Using LLC-PK1 renal epithelial cells to further examine the effect of Mg status and CIS on oxidative stress in real time, we found that growth in 10% Mg media increased basal oxidative stress compared with cells maintained in 100% Mg media (P < 0.001; Fig. 6B) and 100% Mg after 10% Mg suppressed the elevated basal oxidative stress (P < 0.001; Fig. 6B). CIS treatment increased oxidative stress (P < 0.001) in 100% Mg-exposed LLC-PK1 cells (Fig. 6B) and even further enhanced oxidative stress in 10% Mg-exposed cells (P < 0.001; Fig. 6B). In contrast, 100% Mg after 10% Mg reduced both basal and CIS-induced oxidative stress compared with untreated 10% Mg-exposed cells and CIS-treated 10% Mg-exposed cells, respectively (P < 0.001; Fig. 6B).
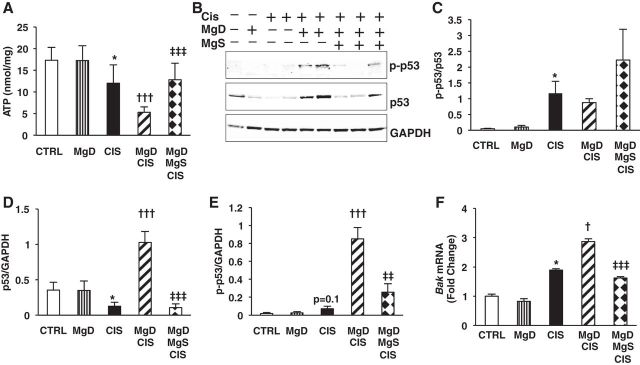
CIS-Mediated Renal ATP Depletion and Apoptosis Is Amplified by Mg Deficiency and Spared by Mg Supplementation
Next, we investigated the effect of Mg status on renal ATP levels, p53 activation, and apoptosis in our model system. While Mg deficiency alone did not affect renal ATP levels compared with controls, CIS reduced renal ATP levels by 30% versus controls (P < 0.05; Fig. 7A), which were further reduced in MgD + CIS mice (P < 0.001; Fig. 7A); Mg supplementation significantly protected MgD + CIS mice against renal ATP loss (P < 0.001; Fig. 7A).
Fig. 7.
Mg deficiency before CIS treatment enhances renal ATP depletion, activation of the p53 proapoptotic signaling pathway, and Bak mRNA expression in vivo: reversed by Mg replacement. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline). A: renal ATP levels [shown as mean ATP concentrations ± SD (in nmol/mg), corrected for protein levels]. B: kidney total p53 and p-p53 (Ser15) were measured by Western blot analysis. GAPDH and total p53 were used as loading controls. Representative blots are shown. C–E: ratios of p-p53 to total p53 (C), total p53 to GAPDH (D), and p-p53 to GAPDH (E) are shown as band densities (means ± SEM). F: renal Bak mRNA expression was measured using quantitative PCR and expressed as means ± SE (in fold changes vs. Gapdh). *P < 0.05 vs. CTRL; †P < 0.05 vs. CIS; †††P < 0.001 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS; ‡‡‡P < 0.001 vs. MgD + CIS; P = 0.1 vs. CTRL.
Renal p-p53 (Ser15) was significantly increased by CIS (Fig. 7, B and C). Because CIS reduced total p53 expression (Fig. 7, B and D), p-p53 (Ser15) levels were corrected for GAPDH (Fig. 7E). MgD + CIS treatment significantly enhanced the phosphorylation of p53 at the Ser15 position compared with CIS alone (Fig. 7E). Mg supplementation after Mg deficiency significantly decreased CIS-mediated phosphorylation of p53 compared with MgD + CIS mice (Fig. 7E). Likewise, renal Bak mRNA expression was significantly increased by CIS compared with controls (Fig. 7F). Whereas Mg deficiency alone had no effect on Bak mRNA expression, MgD + CIS treatment significantly enhanced renal Bak mRNA expression compared with CIS alone (Fig. 7F), and this was reversed by Mg supplementation after Mg deficiency (Fig. 7F).
Using TUNEL staining, we confirmed the significant increase in CIS-induced renal cell apoptosis (Fig. 8, A–C), which was further and significantly increased by MgD + CIS treatment (Fig. 8, A–C). Mg supplementation before and during CIS treatment significantly reduced the number of renal apoptotic cells compared with MgD + CIS mice (Fig. 8, A–C).
Fig. 8.
Mg deficiency enhances CIS-induced renal cell apoptosis in vivo and increases CIS-mediated killing of LLC-PK1 cells: reversal by Mg replacement. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48h post-CIS (or saline). Renal apoptosis was measured by TUNEL staining. A and B: representative photomicrographs (at ×200 magnification) in a complete representative section (A) and a selected area in the section (B). C: apoptosis was determined by counting the number of TUNEL-positive cells per HPF using random sections, and mean apoptosis scores ± SE are shown. Scale bar = 20 μm. *P < 0.05 vs. CTRL; †P < 0.05 vs. CIS; ‡‡‡P < 0.001 vs. MgD + CIS. D: LLC-PK1 cells were maintained in either 100% Mg or 10% Mg (Mg-deficient) media or 10% Mg media followed by 200% Mg replacement (10% Mg/200% Mg). Cell viability was measured 24 h post-CIS using a neutral red assay, and data are shown as means ± SD (as %viability). ***P < 0.001 vs. 100% Mg + CIS; ‡‡‡P < 0.001 vs. 10% Mg + CIS.
Using the LLC-PK1 cell line, which is shown to exhibit increased CIS-induced oxidative stress after Mg deficiency (Fig. 6B), we examined the effect of Mg status on CIS-mediated renal epithelial cell cytotoxicity in vitro. LLC-PK1 renal epithelial cells maintained in MgD media had significantly reduced cell viability (P < 0.001) after CIS compared with 100% Mg + CIS-treated cells (Fig. 8D); Mg supplementation before CIS reversed CIS-induced LLC-PK1 cytotoxicity (P < 0.001; Fig. 8D).
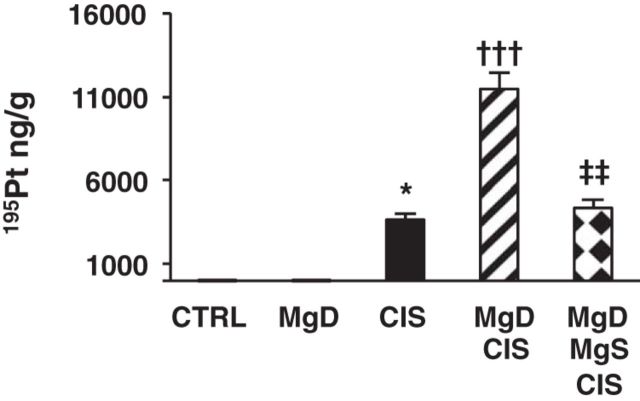
Mg Status Regulates Renal Pt Accumulation
Because Mg status affected all aspects of CIS-induced AKI (e.g., inflammation, oxidative stress, and apoptosis), we investigated the effect of Mg status on Pt accumulation in the kidneys. CIS-treated mice showed renal Pt accumulation compared with controls (P < 0.05; Fig. 9). Pt accumulation was amplified in MgD + CIS mice (P < 0.001; Fig. 9), and this increase was completely blocked by Mg supplementation (P < 0.01; Fig. 9).
Fig. 9.
CIS-induced renal platinum accumulation is enhanced by Mg deficiency and decreased by Mg replacement. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline), and renal platinum (195Pt) accumulation, as measured by inductively coupled plasma mass spectrometry, is shown as means ± SE (in ng/g kidney tissue). *P < 0.05 vs. CTRL; †††P < 0.001 vs. CIS; ‡‡ P < 0.01 vs. MgD + CIS.
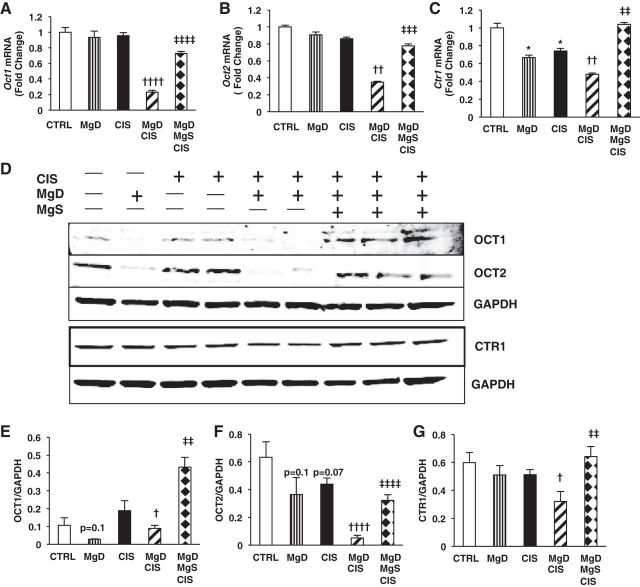
Mg Status Affects Renal Expression of CIS Uptake and Efflux Transporters
Renal Pt accumulation is balanced by both CIS uptake and efflux. Mg deficiency alone and CIS alone did not affect Oct1 (Fig. 10A) or Oct2 (Fig. 10B) mRNA expression but significantly decreased Ctr1 (P < 0.05; Fig. 10C) mRNA expression compared with controls. MgD + CIS mice showed significantly decreased Oct1 (P < 0.0001; Fig. 10A) versus CIS alone and Mg deficiency alone and Oct2 (P < 0.01; Fig. 10B) and Ctr1 (P < 0.01; Fig. 10C) mRNA expression compared with mice treated with CIS alone. OCT1 (P < 0.05; Fig. 10, D and E), OCT2 (P < 0.0001; Fig. 10, D and F), and CTR1 (P < 0.05; Fig. 10, D and G) proteins were reduced by MgD + CIS versus CIS alone, and this was reversed by Mg supplementation (Fig. 10, D–G).
Fig. 10.
Mg status regulates CIS uptake transporter expression in the kidneys. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline), and renal cortical tissues were assessed for uptake transporter mRNA expression by quantitative PCR. A: organic cation transporter (Oct)1. B: Oct2. C: copper transporter 1 (Ctr1). Data are shown as means ± SE (in fold changes vs. Gapdh). D: representative Western blots showing renal OCT1, OCT2, and CTR1 protein expression. E–G: quantitation of band ratios for OCT1 to GAPDH (E), OCT2 to GAPDH (F), and CTR1 to GAPDH (G). Mean band densities ± SE are shown. * P < 0.05 vs. CTRL, † P < 0.05 vs. CIS, †† P < 0.01 vs. CIS, †††† P < 0.0001 vs. CIS, ‡‡ P < 0.01 vs. MgD CIS, ‡‡‡ P < 0.001 vs. MgD CIS, ‡‡‡‡ P < 0.0001 vs. MgD CIS, P = 0.1 vs. CTRL, P = 0.07 vs. CTRL.
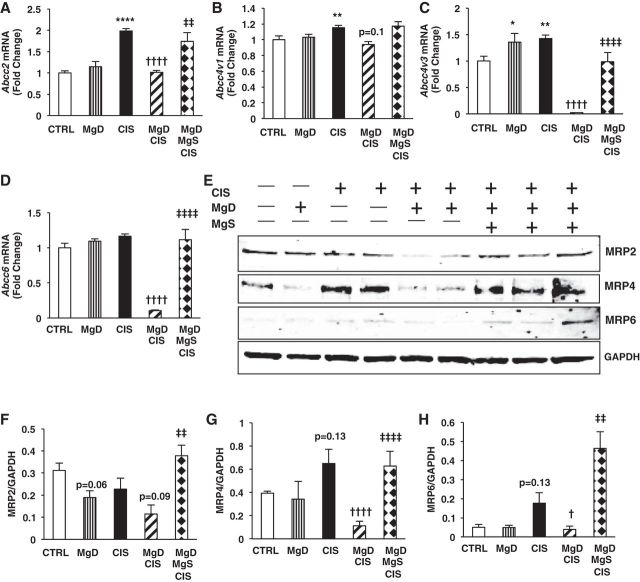
Whereas CIS alone significantly increased renal Abcc2 (MRP2, P < 0.0001; Fig. 11A) and Abcc4 (MRP4, variants 1 and 3, P < 0.01; Fig. 11, B and C) mRNA expression versus saline-treated control mice, CIS alone only increased MRP4 and MRP6 protein levels but not MRP2 (Fig. 11, E–H). Mg deficiency alone did not affect efflux transporter expression at mRNA or protein levels. When combined with CIS, Mg deficiency significantly decreased Abcc2, Abcc4v3, and Abcc6 (P < 0.0001; Fig. 11, A–D) and slightly decreased Abcc4v1 (P = 0.1; Fig. 11B) mRNA expression compared with CIS alone. Both MRP4 (P < 0.0001) and MRP6 (P < 0.05) protein levels were decreased by MgD + CIS versus CIS alone (Fig. 11, E, G, and H). MgDMgS + CIS treatment significantly increased Abcc2 (P < 0.01; Fig. 11A), Abcc4v3 (P < 0.0001; Fig. 11C), and Abcc6 (P < 0.0001; Fig. 11D) and slightly increased Abcc4v1 (Fig. 11B) mRNA expression versus MgD + CIS treatment. Mg supplementation significantly increased the protein expression of all efflux transporters (MRP2, MRP4, and MRP6, P < 0.01; Fig. 11, E–H) compared with MgD + CIS mice.
Fig. 11.
Mg status regulates CIS efflux transporter expression in the kidneys. Mice were maintained on either 100% Mg or 10% Mg-deficient diets or were maintained on a 10% Mg diet followed by Mg supplementation, as described in methods, and then treated with saline (CTRL) or CIS (12 mg/kg). All mice were euthanized 48 h post-CIS (or saline), and renal efflux transporter mRNA expression was measured by quantitative PCR. A: ATP-binding cassette subfamily C (ABCC)2. B: Abcc4v1. C: Abcc4v3. D: Abcc6. Data are shown as means ± SE (in fold changes vs. Gapdh). E: representative Western blots for renal ABCC2 [multidrug resistance protein (MRP)2], ABCC6 (MRP6), and ABCC4 (MRP4) expression. F–H: quantitation of band ratios of ABCC2 (MRP2) to GAPDH (F), ABCC4 (MRP4) to GAPDH (G), and ABCC6 (MRP6) to GAPDH (H). Mean band densities ± SE are shown. *P < 0.05 vs. CTRL; **P < 0.01 vs. CTRL; ****P < 0.0001 vs. CTRL; †P < 0.05 vs. CIS; ††††P < 0.0001 vs. CIS; ‡‡P < 0.01 vs. MgD + CIS; ‡‡‡‡P < 0.0001 vs. MgD + CIS; P = 0.06 vs. CTRL; P = 0.13 vs. CTRL; P = 0.09 vs. CIS; P = 0.1 vs. CIS.
Mg Supplementation Does Not Compromise CIS-Mediated Tumor Cell Killing In Vitro
CIS is commonly used for the treatment of ovarian cancer, breast cancer, and lung cancer, among others. Using human A2780 (ovarian), MCF-7 (breast), and H460 (lung) cancer cell lines, we investigated whether Mg status affects the antitumor efficacy of CIS in vitro. As expected, A2780, MCF-7, and H460 cancer cell lines grown in 100% Mg media were sensitive to killing by CIS (Table 2). Neither Mg deficiency (10% Mg) nor Mg replacement after Mg deficiency (10% Mg/100% Mg) significantly affected the IC50 for CIS-mediated killing of A2780 ovarian and H460 lung cancer cells in vitro (Table 2). Mg deficiency significantly increased the IC50 for CIS-mediated killing of MCF-7 breast cancer cells (P < 0.05; Table 2), whereas Mg replacement slightly reduced the IC50 for CIS-mediated killing of MCF-7 cells compared with MgD cells (although not significantly; Table 2). No significant differences in IC50 were observed between 100% Mg-treated MCF-7 cells and MgS MCF-7 cells (Table 2).
Table 2.
Mg supplementation does not compromise cisplatin-mediated tumor cell killing in vitro
| 100% Mg | 10% Mg | 10%/100% Mg | |
|---|---|---|---|
| A2780 cells | 12.71 ± 2.91 | 11.08 ± 4.39 | 12.4 ± 1.39 |
| MCF-7 cells | 32.78 ± 2.29 | 41.29 ± 4.08* | 39.77 ± 1.33 |
| H460 cells | 20.24 ± 0.63 | 20.23 ± 8.45 | 19.23 ± 3.92 |
Data are means ± SD of cisplatin IC50 values (in μM). *P < 0.05, 10% magnesium (Mg)-treated cells vs. 100% Mg-treated cells.
DISCUSSION
In addition to increased oxidative stress, inflammation, and apoptosis in the kidneys (50, 57, 69), CIS leads to hypomagnesemia in up to 90% of patients (41). Although Mg deficiency has been proposed to amplify CIS-mediated renal damage, little is known regarding the mechanism(s) involved. Using a model of CIS-mediated AKI in older female mice, here we report the deleterious effects of Mg deficiency on CIS-induced renal damage. Based on plasma Mg levels and previous reports, MgD diet (10% Mg) given for 16 days induces mild to moderate Mg deficiency (9, 76), which is not uncommon in the United States, particularly among the elderly (22, 82). A moderate dose of CIS (12 mg/kg) was used to assess the effect of Mg deficiency (with or without Mg supplementation) because a higher dose of CIS (20 mg/kg, commonly used) was lethal to MgD mice (data not shown), limiting our ability to study mechanism(s) and reversal by Mg supplementation. Conversely, Mg supplementation, which increased serum Mg2+ levels by almost 23% above that found in MgD animals, exerted significant renoprotective effects against CIS-induced AKI. Most CIS studies have used male mice, and, to our knowledge, this is the first study to use older mice to study CIS-mediated AKI. Older female C57BL/6 mice (11 mo old) were used because 1) they better represent a CIS-treated cohort; 2) older kidneys exhibit increased susceptibility to AKI (in humans and mice) (2, 62, 73, 86); 3) Mg deficiency is more common among the elderly (22, 82); and 4) women are more susceptible to CIS-induced AKI (50, 69). Additional experiments were performed to examine the effect of Mg on CIS-induced oxidative stress and cytotoxicity using the LLC-PK1 cell line. The LLC-PK1 cell line is a porcine renal tubular epithelial cell line most commonly used to study nephrotoxic drugs and mechanisms to block drug-induced nephrotoxocity (34). The LLC-PK1 cell line was chosen because it expresses OCT2 [the major influx transporter expressed by the human kidney (32, 77)] as well as numerous efflux transporters (75).
Numerous studies have connected Mg deficiency with enhanced inflammation in the intestines (14, 72), lungs (54), and heart (14) with elevated cytokine levels (6, 47, 84). Although CIS-induced AKI is typically accompanied by renal inflammation (50, 57, 69), CIS (12 mg/kg) did not increase renal cytokines at 48 h (Fig. 4). However, when CIS was combined with Mg deficiency, renal Tnfa mRNA and renal IL-6 and IL-1β protein levels were significantly elevated (Fig. 4, A–C). In addition to renal cytokine dysregulation, MgD + CIS mice exhibited elevated renal chemokine expression (Fig. 2, A–F) with increased renal neutrophil infiltration and MPO levels (Fig. 3, A–D); these effects were reversed by Mg supplementation given with MgD + CIS treatment. Consistent with our observations, several studies have reported anti-inflammatory effects of MgSO4 treatment (10, 20, 39, 78).
In this study, elevated cytokine/chemokine expression in the kidneys of MgD + CIS mice was accompanied by increased p-ERK and p-STAT3, whereas Mg supplementation suppressed both ERK1/2 and STAT3 activation (Fig. 5, A–F). ERK1/2 signaling has been linked to CIS-induced cytokine production in vivo (38), and the IL-6/STAT3 pathway has been implicated in renal inflammation (55). In addition, both ERK1/2 and STAT3 activation have been implicated in oxidative stress (21, 70).
Mg deficiency alone was accompanied by enhanced renal oxidative stress, as shown by increased expression of Ncf1 (which encodes for p47phox; Fig. 6A). p47phox, a cytosolic regulatory subunit of NADPH oxidase, is expressed by most cell types, including nonimmune cells (11), such as renal cells (37) and auditory cells (40), after CIS. Although Mg deficiency has been previously reported to increase oxidative stress in liver, heart, and skeletal muscles (63, 66), this is the first study to demonstrate increased renal oxidative stress during Mg deficiency and exaggerated CIS-induced ROS production after Mg deficiency. Similar to previous studies showing that Mg deficiency enhances oxidative stress in several cell types (19, 29, 89), we observed increased basal ROS production by LLC-PK1 renal epithelial cells after Mg deficiency (Fig. 6B). Increased oxidative stress accompanying Mg deficiency in vitro and in vivo was further enhanced by CIS (Fig. 6, A and B), and this was reversed by Mg supplementation in vitro and in vivo. Consistent with our results, treatment of rats with the antioxidant apocynin, which targets the NADPH oxidase system, blocking p47phox translocation to the membrane, prevents CIS-induced AKI (13). Taken together, these observations suggest that an initial priming of the kidneys by Mg deficiency promotes ROS production, which is exacerbated by CIS, and this can be alleviated by Mg supplementation.
Both oxidative stress and inflammation have been shown to mediate CIS-induced renal injury (12, 50, 69). More specifically, ERK1/2 phosphorylates p53 at Ser15 [p-p53 (Ser15)] (18, 60), and the roles of both ERK1/2 and p53 in CIS-mediated renal apoptosis have been previously described (17, 83, 85). MgD + CIS mice showed enhanced renal ERK1/2 activation, total p53, and p-p53 (Ser15) compared with CIS-treated control mice (Figs. 5 and 7, respectively). CIS-induced apoptosis is associated with ATP depletion (8). In this study, Mg deficiency alone did not decrease renal ATP, but, when combined with CIS, Mg deficiency significantly decreased renal ATP (Fig. 7A). This decrease was attenuated by Mg supplementation, suggesting a role for Mg in reducing mitochondrial dysfunction during CIS-mediated AKI. In the mitochondrial pathway of apoptosis, BAK activation after CIS induces mitochondrial pore formation with concomitant cytochrome c release (50, 57, 69). We observed that renal Bak mRNA expression (Fig. 7F) and renal cell apoptosis (Fig. 8, A–C) were significantly increased in MgD + CIS mice compared with CIS-treated mice and attenuated by Mg supplementation. Although previous studies have shown that Mg deficiency induces apoptosis in the heart (79), thymus (48), and retina (30), to our knowledge, this is the first study revealing the apoptotic role of Mg deficiency in CIS-mediated AKI. In rats, Mg supplementation given during CIS-induced AKI had no nephroprotectant effect (5); however, prolonged Mg supplementation was provided before CIS in the absence of Mg deficiency, suggesting that excess Mg may not be beneficial.
Mg is a required cofactor for >300 enzymes in the body (26), and, therefore, it is not surprising that Mg status regulates multiple pathways associated with CIS-induced AKI (e.g., oxidative stress, inflammation, and apoptosis). It is equally plausible to hypothesize that Mg simultaneously regulates these pathways by controlling renal CIS/Pt accumulation (Fig. 9). Here, we show that Mg deficiency increased and Mg supplementation decreased Mg deficiency-induced renal Pt accumulation despite decreased influx transporter expression in MgD + CIS mice. Although the precise mechanisms regulating cellular CIS uptake are not completely understood, it has been attributed, in part, to specific influx transporters including OCT1, OCT2, and CTR1 as well as passive diffusion through the plasma membrane (for reviews, see Refs. 57 and 74). Using a rat model, Yokoo et al. (90) showed that Mg deficiency increased renal CIS accumulation and renal OCT2 expression after CIS. It is important to note that OCT2 knockout (OCT2−/−) mice are incompletely protected from CIS-mediated AKI, with a 50% reduction in BUN and serum Cr levels compared with wild-type mice (27), indicating that OCT2 is only partially responsible. Likewise, CTR1 can mediate intracellular CIS accumulation in both human and rodent models (36, 45, 58); however, CTR1 knockout cells can accumulate CIS (36, 43). Caution should be taken when translating results obtained in mice to humans because the expression of CIS influx/efflux transporters is different [e.g., mouse kidneys express Oct1 and Oct2 (15, 27, 28), whereas human kidneys predominantly express Oct2 (3, 31)]. Based on our observations and the fact that diffusion into the cell accounts for up to 50% of CIS uptake (57), we propose that Mg deficiency enhances CIS diffusion into kidney epithelial cells through lipid changes in the cell membrane, as previously shown (46, 64, 81). Preliminary studies using LLC-PK1 renal epithelial cells showed that Mg deficiency significantly increased 16:1 (palmitoleic acid) by >200% and decreased 18:1ω-9 (oleic acid) by 20%. Although little is known about 16:1 with respect to the plasma membrane, 18:1ω-9 has been shown to alter the cell membrane's lipid matrix (80). Thus, it is possible that Mg deficiency-mediated lipid changes influence CIS uptake despite decreased influx transporter expression. In addition, our data revealed a dramatic decrease in two important renal CIS efflux transporters, MRP4 and MRP6 (1), by MgD + CIS treatement (Fig. 11, E, G, and H), and these effects were reversed by Mg supplementation (Fig. 11, E, G, and H). Taken together, these results may explain the increased CIS accumulation in MgD mice and decreased CIS accumulation in MgS mice. However, our results showing the protective role of Mg in CIS-mediated AKI do not rule out the possibility that other mechanisms, such as changes in osmolality, as previously described for mannitol (61), might also be involved.
It can be argued that the protective role of Mg could extend to tumors, whereby Mg supplementation may interfere with CIS-mediated killing of tumors. However, in highly proliferative tumor cells, Mg concentrations are higher and more tightly regulated compared with nontumor cells (88). Therefore, they are more resistant to changes in Mg concentrations than nontumor cells (88). Consistent with these observations, we did not observe any significant alterations in the IC50 of CIS for killing A2780 ovarian or H460 lung cancer cells related to Mg supplementation or Mg deficiency (Table 2). In the case of MCF-7 breast cancer cells, Mg deficiency increased the IC50 for CIS-mediated killing (P < 0.05; Table 2), suggesting that cells were less sensitive to CIS-mediated killing when in MgD media. The IC50 for MCF-7 breast cancer cells was slightly reduced (although not significantly) by Mg replacement (Table 2) compared with cells in MgD media. However, additional tumor cell lines and in vivo tumor models will be required to better understand the effects of Mg status on the chemotherapeutic efficacy of CIS.
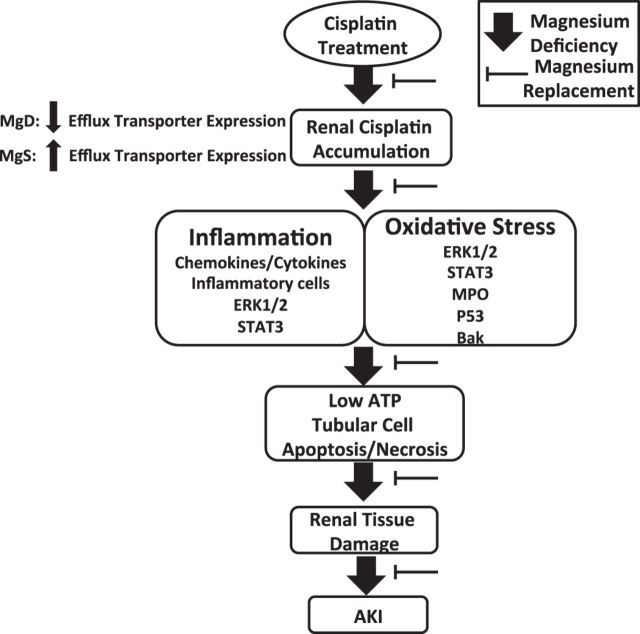
Based on our findings, we propose a mechanistic model describing the role of Mg in CIS-induced AKI (Fig. 12). These observations, together with the results of four small clinical trials reporting the renoprotective effects of Mg supplementation in CIS-treated patients with testicular cancer [n = 16, Willox et al. (87)], ovarian cancer [n = 41, Bodnar et al. (7)], head/neck cancer [n = 23, Hirai et al. (35)], and non-small cell lung cancer [n = 50, Muraki et al. (52)], strongly support maintaining Mg at homeostatic levels to reduce the number of patients who succumb to CIS-induced AKI. Studies are underway in our laboratory to examine the effect of Mg status on tumor cell growth and CIS-mediated cytotoxicity using a wide range of tumor cells as well as the effect of Mg status on CIS-induced AKI in tumor-bearing animals. Finally, much larger randomized control trials with extended followup periods to study cancer progression and patient outcomes will be required to develop better guidelines for Mg supplementation on CIS-mediated AKI and to investigate the role of Mg on the antineoplastic efficacy of CIS in various cancer populations.
Fig. 12.
Proposed mechanisms by which Mg regulates CIS-induced acute kidney injury (AKI). Mg deficiency leads to enhanced renal CIS accumulation via decreased efflux transporter expression and increased CIS-induced inflammation and oxidative stress with reduced ATP levels in the kidneys. Activated signaling pathways, including ERK1/2, STAT3, and p53, associated with inflammation and oxidative stress merge to promote renal cell apoptosis/necrosis, resulting in renal tissue damage and, ultimately, AKI. Mg replacement after Mg deficiency protects against CIS-induced AKI by decreasing CIS accumulation, increasing efflux transporter expression, inflammation, oxidative stress, and the activation of pathways that lead to kidney cell apoptosis/necrosis.
GRANTS
This work was supported by the Feinstein Institute for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.H.S. and C.N.M. conception and design of research; M.H.S., P.K.C., M.G., X.X., M.H.M., R.M., and C.N.M. performed experiments; M.H.S., A.P., R.M., P.C.S., and C.N.M. interpreted results of experiments; M.H.S., A.P., and C.N.M. prepared figures; M.H.S. and C.N.M. drafted manuscript; M.H.S., P.K.C., and C.N.M. edited and revised manuscript; M.H.S., P.K.C., M.G., X.X., A.P., M.H.M., R.M., P.C.S., and C.N.M. approved final version of manuscript; C.N.M. analyzed data.
ACKNOWLEDGMENTS
Present addresss of A. Plagov: Department of Pathology and Laboratory Medicine, Drexel University College of Medicine, Philadelphia, Pennsylvania.
REFERENCES
- 1.Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology : 82–88, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O'Hare AM, Schmader KE, High KP. Acute kidney injury in older adults. J Am Soc Nephrol : 28–38, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Aoki M, Terada T, Kajiwara M, Ogasawara K, Ikai I, Ogawa O, Katsura T, Inui K. Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Renal Physiol : F165–F170, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Arnaud MJ. Update on the assessment of magnesium status. Br J Nutr Suppl : S24–S36, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafi F, Haghshenas S, Nematbakhsh M, Nasri H, Talebi A, Eshraghi-Jazi F, Pezeshki Z, Safari T. The role of magnesium supplementation in cisplatin-induced nephrotoxicity in a rat model: no nephroprotectant effect. Int J Prev Med : 637–643, 2012. [PMC free article] [PubMed] [Google Scholar]
- 6.Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, Prost M, Gaume V, Adrian M, Laurant P, Berthelot A. Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med : 277–284, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar L, Wcislo G, Gasowska-Bodnar A, Synowiec A, Szarlej-Wcislo K, Szczylik C. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer : 2608–2614, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest : 1275–1285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RC, Bidlack WR. Dietary magnesium depletion: p-nitroanisole metabolism and glucuronidation in rat hepatocytes and hepatic microsomal membranes. Proc Soc Exp Biol Med : 85–90, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol : e291–e299, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene : 131–140, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol : 223–242, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Chirino YI, Sanchez-Gonzalez DJ, Martinez-Martinez CM, Cruz C, Pedraza-Chaverri J. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicology : 18–23, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Chmielinska JJ, Tejero-Taldo MI, Mak IT, Weglicki WB. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol Cell Biochem : 53–57, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol : 1477–1484, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cikos S, Bukovska A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol : 113, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther : 8–17, 2002. [DOI] [PubMed] [Google Scholar]
- 18.DeHaan RD, Yazlovitskaya EM, Persons DL. Regulation of p53 target gene expression by cisplatin-induced extracellular signal-regulated kinase. Cancer Chemother Pharmacol : 383–388, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Dickens BF, Weglicki WB, Li YS, Mak IT. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS Lett : 187–191, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Dowling O, Chatterjee PK, Gupta M, Tam Tam HB, Xue X, Lewis D, Rochelson B, Metz CN. Magnesium sulfate reduces bacterial LPS-induced inflammation at the maternal-fetal interface. Placenta : 392–398, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Duan W, Yang Y, Yi W, Yan J, Liang Z, Wang N, Li Y, Chen W, Yu S, Jin Z, Yi D. New role of JAK2/STAT3 signaling in endothelial cell oxidative stress injury and protective effect of melatonin. PLOS ONE : e57941, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durlach J, Bac P, Durlach V, Rayssiguier Y, Bara M, Guiet-Bara A. Magnesium status and ageing: an update. Magnes Res : 25–42, 1998. [PubMed] [Google Scholar]
- 23.Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol : 1777–1781, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol : 57–72, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther : 8–15, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth : 302–320, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther : 396–402, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franke RM, Kosloske AM, Lancaster CS, Filipski KK, Hu C, Zolk O, Mathijssen RH, Sparreboom A. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-acetyl-β-d-glucosaminidase. Clin Cancer Res : 4198–4206, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman AM, Mak IT, Stafford RE, Dickens BF, Cassidy MM, Muesing RA, Weglicki WB. Erythrocytes from magnesium-deficient hamsters display an enhanced susceptibility to oxidative stress. Am J Physiol Cell Physiol : C1371–C1375, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Gong H, Amemiya T, Takaya K. Retinal changes in magnesium-deficient rats. Exp Eye Res : 23–32, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, Baumann C, Lang F, Busch AE, Koepsell H. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol : 871–881, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Grundemann D, Babin-Ebell J, Martel F, Ording N, Schmidt A, Schomig E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 cells. J Biol Chem : 10408–10413, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Guerrera MP, Volpe SL, Mao JJ. Therapeutic uses of magnesium. Am Fam Physician : 157–162, 2009. [PubMed] [Google Scholar]
- 34.Gunness P, Aleksa K, Kosuge K, Ito S, Koren G. Comparison of the novel HK-2 human renal proximal tubular cell line with the standard LLC-PK1 cell line in studying drug-induced nephrotoxicity. Can J Physiol Pharmacol : 448–455, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Hirai S, Kaida S, Ito T, Hasebe S, Ueno M, Udagawa H, Hayashi M. [Magnesium premedication prevents cisplatin-induced nephrotoxicity in patients with esophageal and hypopharyngeal cancer]. Gan To Kagaku Ryoho : 743–747, 2013. [PubMed] [Google Scholar]
- 36.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA : 14298–14302, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T. Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int : 77–88, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int : 458–466, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Kao MC, Jan WC, Tsai PS, Wang TY, Huang CJ. Magnesium sulfate mitigates lung injury induced by bilateral lower limb ischemia-reperfusion in rats. J Surg Res : e97–e106, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci : 3933–3946, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev : 47–58, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Lajer H, Kristensen M, Hansen HH, Nielsen S, Frokiaer J, Ostergaard LF, Christensen S, Daugaard G, Jonassen TE. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol : 535–542, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Landon CD, Benjamin SE, Ashcraft KA, Dewhirst MW. A role for the copper transporter Ctr1 in the synergistic interaction between hyperthermia and cisplatin treatment. Int J Hyperthermia : 528–538, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Webb HK, Fukushima H, Micheli J, Markova S, Olson JL, Kroetz DL. Attenuation of cisplatin-induced renal injury by inhibition of soluble epoxide hydrolase involves nuclear factor kappaB signaling. J Pharmacol Exp Ther : 725–734, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H. Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int : 196–202, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Mahfouz MM, Smith TL, Kummerow FA. Changes of linoleic acid metabolism and cellular phospholipid fatty acid composition in LLC-PK cells cultured at low magnesium concentrations. Biochim Biophys Acta : 70–74, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Malpuech-Brugere C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, Lebreton J, Mazur A, Rayssiguier Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta : 91–98, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Malpuech-Brugere C, Nowacki W, Gueux E, Kuryszko J, Rock E, Rayssiguier Y, Mazur A. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br J Nutr : 405–411, 1999. [PubMed] [Google Scholar]
- 49.Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys : 48–56, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) : 2490–2518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J Ovarian Res : 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muraki K, Koyama R, Honma Y, Yagishita S, Shukuya T, Ohashi R, Takahashi F, Kido K, Iwakami S, Sasaki S, Iwase A, Takahashi K. Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-small cell lung cancer. J Thorac Dis : 562–568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadler JL, Rude RK. Disorders of magnesium metabolism. Endocrinol Metab Clin North Am : 623–641, 1995. [PubMed] [Google Scholar]
- 54.Nasulewicz A, Zimowska W, Bayle D, Dzimira S, Madej J, Rayssiguier Y, Opolski A, Mazur A. Changes in gene expression in the lungs of Mg-deficient mice are related to an inflammatory process. Magnes Res : 259–263, 2004. [PubMed] [Google Scholar]
- 55.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol : 1106–1115, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev : 333–340, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int : 994–1007, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol : F505–F511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan H, Shen Z, Mukhopadhyay P, Wang H, Pacher P, Qin X, Gao B. Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol : F496–F504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem : 35778–35785, 200. [DOI] [PubMed] [Google Scholar]
- 61.Polycarpe E, Arnould L, Schmitt E, Duvillard L, Ferrant E, Isambert N, Duvillard C, Beltramo JL, Chevet D, Chauffert B. Low urine osmolarity as a determinant of cisplatin-induced nephrotoxicity. Int J Cancer : 131–137, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci : 830–839, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Rayssiguier Y, Gueux E, Bussiere L, Durlach J, Mazur A. Dietary magnesium affects susceptibility of lipoproteins and tissues to peroxidation in rats. J Am Coll Nutr : 133–137, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Rayssiguier Y, Gueux E, Weiser D. Effect of magnesium deficiency on lipid metabolism in rats fed a high carbohydrate diet. J Nutr : 1876–1883, 1981. [DOI] [PubMed] [Google Scholar]
- 65.Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc : 1125–1131, 2009. [DOI] [PubMed] [Google Scholar]
- 66.Rock E, Astier C, Lab C, Vignon X, Gueux E, Motta C, Rayssiguier Y. Dietary magnesium deficiency in rats enhances free radical production in skeletal muscle. J Nutr : 1205–1210, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Romani AM. Cellular magnesium homeostasis. Arch Biochem Biophys : 1–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev : 153–164, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol : 803–821, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, Sofroniew MV. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLOS ONE : e9532, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sartori S, Nielsen I, Tassinari D, Rigolin F, Arcudi D, Abbasciano V. Changes in intracellular magnesium concentrations during cisplatin chemotherapy. Oncology : 230–234, 1993. [DOI] [PubMed] [Google Scholar]
- 72.Scanlan BJ, Tuft B, Elfrey JE, Smith A, Zhao A, Morimoto M, Chmielinska JJ, Tejero-Taldo MI, Mak Iu T, Weglicki WB, Shea-Donohue T. Intestinal inflammation caused by magnesium deficiency alters basal and oxidative stress-induced intestinal function. Mol Cell Biochem : 59–69, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol : F1265–F1272, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Schneider V, Krieger ML, Bendas G, Jaehde U, Kalayda GV. Contribution of intracellular ATP to cisplatin resistance of tumor cells. J Biol Inorg Chem : 165–174, 2013. [DOI] [PubMed] [Google Scholar]
- 75.Spears KJ, Ross J, Stenhouse A, Ward CJ, Goh LB, Wolf CR, Morgan P, Ayrton A, Friedberg TH. Directional trans-epithelial transport of organic anions in porcine LLC-PK1 cells that co-express human OATP1B1 (OATP-C) and MRP2. Biochem Pharmacol : 415–423, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Standley CA, Cotton DB. Brain ionized magnesium and calcium levels during magnesium supplementation and deficiency in female Long-Evans rats. Obstet Gynecol : 184–188, 1996. [DOI] [PubMed] [Google Scholar]
- 77.Sweet DH, Pritchard JB. The molecular biology of renal organic anion and organic cation transporters. Cell Biochem Biophys : 89–118, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Tam Tam HB, Dowling O, Xue X, Lewis D, Rochelson B, Metz CN. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am J Obstet Gynecol : e361–e368, 2011. [DOI] [PubMed] [Google Scholar]
- 79.Tejero-Taldo MI, Chmielinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes Res : 208–212, 2007. [PubMed] [Google Scholar]
- 80.Teres S, Barcelo-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, Escriba PV. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc Natl Acad Sci USA : 13811–13816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tongyai S, Rayssiguier Y, Motta C, Gueux E, Maurois P, Heaton FW. Mechanism of increased erythrocyte membrane fluidity during magnesium deficiency in weanling rats. Am J Physiol Cell Physiol : C270–C276, 1989. [DOI] [PubMed] [Google Scholar]
- 82.Vaquero MP. Magnesium and trace elements in the elderly: intake, status and recommendations. J Nutr Health Aging : 147–153, 2002. [PubMed] [Google Scholar]
- 83.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem : 39435–39443, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF. Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem : 169–173, 1992. [DOI] [PubMed] [Google Scholar]
- 85.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol : F1282–F1291, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis : 302–307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willox JC, McAllister EJ, Sangster G, Kaye SB. Effects of magnesium supplementation in testicular cancer patients receiving cis-platin: a randomised trial. Br J Cancer : 19–23, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolf FI, Cittadini AR, Maier JA. Magnesium and tumors: ally or foe? Cancer Treat Rev : 378–382, 2009. [DOI] [PubMed] [Google Scholar]
- 89.Wolf FI, Trapani V, Simonacci M, Ferre S, Maier JA. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res : 58–64, 2008. [PubMed] [Google Scholar]
- 90.Yokoo K, Murakami R, Matsuzaki T, Yoshitome K, Hamada A, Saito H. Enhanced renal accumulation of cisplatin via renal organic cation transporter deteriorates acute kidney injury in hypomagnesemic rats. Clin Exp Nephrol : 578–584, 2009. [DOI] [PubMed] [Google Scholar]