Abstract
The intraorgan renin-angiotensin system (RAS) plays an important role in the pathophysiology of a variety of diseases and has been implicated in fibrogenesis. The role of RAS in the development of chronic pancreatitis is not well established. The blockade of RAS in rat models with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor 1 (AT1) blockers (ARBs) mostly have reduced pancreatic inflammation and fibrosis with a few exceptions. At the same time, the use of ACEi and ARBs in humans is associated with a modest risk of acute pancreatitis. The aim of this study was to elucidate the effect of the AT1 signaling pathway in the development of pancreatitis using AT1a- and AT1b-deficient mice as well as the ARB losartan. Chronic pancreatitis was induced by repetitive cerulein administration in C57BL/6J wild-type (WT) and AT1a- and AT1b-deficient mice (AT1a−/− and AT1b−/−), and pancreatic injury was assessed at day 10. Pancreatic weight of cerulein treated groups was significantly reduced. There was severe parenchymal atrophy and fibrosis assessed by histological examination. Fibrosis was accompanied by activation of pancreatic stellate cells (PSC) evaluated by Western blot analysis for α-smooth muscle actin. No differences were seen between cerulein-treated WT, AT1a−/− , AT1b−/− mice, or losartan treated-WT mice with regards to morphological or molecular alterations induced by cerulein. Our results demonstrate that AT1a and AT1b receptor pathways do not seem to be essential for the development of pancreatitis in the mouse model of pancreatitis induced by repetitive cerulein injury.
Keywords: renin-angiotensin system, losartan, angiotensin receptor 1
chronic pancreatitis is a condition characterized by irreversible damage of the exocrine and to a lesser extent the endocrine pancreas, leading to pain, malnutrition, and diabetes. The causes of pancreatitis include alcohol abuse, gallstones, and hereditary predisposition although often the cause of chronic pancreatitis cannot be determined (idiopathic). The main histological features of chronic pancreatitis are chronic inflammation, progressive parenchymal atrophy, and extensive fibrosis of the exocrine pancreas (for review see Ref. 47). The pathophysiology of chronic pancreatitis is not completely understood although a number of advances have been made in recent years. The most accepted theory is that repeated acute attacks of pancreatic necroinflammation together with a dysregulated ability to repair organ damage leads to activation of a fibrotic cascade and loss of parenchymal mass (the necrosis-fibrosis concept). Pancreatic stellate cells (PSC) are now established as the key cells initiating fibrosis and as a primary source of the fibrotic collagen extracellular matrix (see review in Ref. 29). Despite the better understanding of the mechanism of pancreatitis, no specific treatment is available for this disease.
Traditionally, renin-angiotensin system (RAS) has been considered to be an endocrine system regulating blood pressure and body-fluid homeostasis (31). It is now recognized that RAS also acts as an intraorgan local mediator of different pathophysiological processes, including cell proliferation, apoptosis, inflammation, and fibrosis (30). The main bioactive peptide of RAS is angiotensin II (AII). AII is produced from its hepatic precursor angiotensinogen (AGT) by sequential proteolytic action of two enzymes: renal synthesized renin (producing angiotensin I peptide) and pulmonary-bound angiotensin-converting enzyme (ACE) (producing AII peptide). The main AII receptors are G protein-coupled receptors designated as angiotensin receptor type 1 (AT1) and angiotensin receptor type 2 (AT2). There are two subtypes of the AT1 receptor (AT1a and AT1b) in rodents. The majority of the pathophysiological functions of AII are mediated through the AT1 receptor. Treatment with blockers of AII actions, such as ACE inhibitors or AT1 receptor blockers (ARBs), are well-established therapies in ameliorating heart failure, hypertension, and kidney damage in human. Recent studies have shown that these inhibitors can also decrease inflammation and fibrosis of the heart, kidney, and liver in animal models of fibrotic diseases (34, 37, 51).
All components of RAS are intrinsically present in the pancreas, and the level of RAS is enhanced in animal models of pancreatic diseases (for the review see Ref. 18), suggesting a role for RAS in the development of pancreatitis. The inhibition of RAS with ACE inhibitors and ARBs was reported mostly to ameliorate the development of acute and chronic pancreatitis in rat models (17, 48–49). However, there are some controversies concerning the effects of RAS inhibitors on the course of pancreatitis. For example, Tsang et al. (41) reported that losartan, an ARB, ameliorated cerulein-induced acute pancreatitis in rats, but the ACE inhibitor ramiprilat enhanced acute pancreatitis in the same model (42). The mechanisms involved in the anti-inflammatory and antifibrotic effects of ACE inhibitors and ARBs are not completely understood. Their effects may not be attributed solely to their inhibition of AII pathways. There is accumulating evidence that some of these inhibitors are partial peroxisome proliferator-activated receptor (PPAR)-γ agonists and therefore may exert their effects through the PPAR-γ nuclear receptor pathway (7, 38).
The use of ACE inhibitors and ARBs in humans has been associated with an increased risk of acute pancreatitis (2, 8–9, 36). The mechanism of this adverse reaction is not clear. One of the effective ways to investigate the specific role of AII signaling is to use genetically engineered animals deficient in angiotensin receptors. Recently, using AT2-deficient mice, we have shown that AT2 receptor signaling has protective effects in a mouse model of cerulein-induced pancreatic fibrogenesis (44). Nagashio et al. (25) evaluated AT1a-deficient mice in a cerulein-induced model of pancreatitis and found that the AT1a receptor pathway was not essential in the development of acute pancreatitis but plays a role in the development of pancreatic fibrosis. The AT1a isoform is the predominant receptor in a number of mouse tissues (21). In contrast, we and others have demonstrated that both isoforms, AT1a and AT1b, are expressed at comparable levels in the pancreas (19, 25, 44). Interestingly, repetitive cerulein treatment caused differential regulation of AT1 receptor isoform mRNA, increasing AT1b and decreasing AT1a transcript levels (44). It is unclear which isoform functions most like the human AT1 gene product. The aim of the present study was to clarify the role of AT1 receptor signaling in pancreatitis by examining both isoforms in a model of chronic pancreatitis.
To clarify the role of the AT1 receptor signaling in the pathophysiology of chronic pancreatic injury, we compared the response to repetitive cerulein-induced pancreatic injury in wild-type (WT) and AT1a- and AT1b-deficient mice (AT1a−/− and AT1b−/−). Double knockout mice deficient in both AT1a and AT1b are not sufficiently viable to rigorously evaluate, so we also studied the effect of the ARB losartan, an inhibitor of both AT1a and AT1b isoforms on the course of repetitive cerulein-induced pancreatic injury in mice.
MATERIALS AND METHODS
Animals.
AT1a+/− and AT1b−/− mice on a C57BL/6J background were kindly provided by Dr. Thomas Coffman (Duke University) (14, 28). AT1a+/− mice were intercrossed to generate AT1a−/− mice. WT control C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in standard facilities under controlled conditions of temperature, humidity and a 12-h:12-h light/dark cycle and were maintained on standard rodent chow with free access to water. Animal care and all procedures were approved by the institutional animal care committee of Saint Louis University.
Experimental pancreatitis and tissue processing.
Chronic pancreatitis was induced by repeated intraperitoneal (IP) injections of 50 μg/kg per hour cerulein (Sigma, St. Louis, MO) as described previously (26, 44). Control mice from all genotypes received comparable injections of sterile 0.9% sodium chloride (saline). Six hourly injections given in 1 day constituted one treatment. Treatments were given every other day for a total of three treatments. To allow resolution of acute changes, mice were euthanized by CO2 asphyxiation 3 days after their final cerulein treatment. Pancreatic tissues were harvested, weighed, and divided into sections. Sections were immediately frozen in liquid nitrogen and stored at −80°C for subsequent protein extraction and Western blot analysis, fixed in 10% neutral buffered formalin solution (Sigma) for histological analysis, or placed in an RNA stabilization solution (RNAlater; Ambion, Austin, TX) and stored overnight at 4°C for RNA isolation and subsequent RT-PCR analysis.
To assess the effect of AT1a and AT1b receptor blockade, the AT1 receptor antagonist losartan (gift from DuPont Pharmaceuticals, Wilmington, DE) was given to mice by IP injection twice daily starting 2 days before the induction of pancreatitis with cerulein and then every day during the experiment (11 days total). Different doses of losartan (20 mg/kg per day, 5 mg/kg per day, 2 mg/kg per day, and 0.5 mg/kg per day) were evaluated.
Histological analysis.
Formalin-fixed pancreatic sections were embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin according to standard methods. Severity of pancreatitis was blindly graded by an experienced pathologist using a semiquantitative scoring system similar to systems previously described (5, 45). Within pancreatic sections, areas of abnormal pancreatic tissue architecture were graded as follows: 0 = absent, 1 = rare, 2 = <10%, 3 = 10–50%, 4 = >50%. Within these areas, glandular atrophy, presence of pseudotubular complexes, and necrosis were graded as follows: 0 = absent, 1 = minimal <10%, 2 = moderate 10–50%, and 3 = severe >50%. In addition, the presence of acute inflammatory cells (mainly neutrophils) and chronic inflammatory cells (mononuclear cells) was graded as: 0 = absent, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe. To assess pancreatic collagen content, sections were stained with Sirius red. Sections were pretreated to remove paraffin and stained in 0.1% Sirius red (F3B) solution in saturated picric acid for 1 h. Slides were then washed in two changes of 0.09 N acetic acid, dehydrated in three changes of 100% ethanol, cleared in xylene, and finally mounted in Permount (Fisher Scientific, Fair Lawn, NJ). The extent of collagen accumulation was evaluated by morphometric analysis (10). Images were captured with all exposures manually set at equal times for all sections using a Leica DM4000 B microscope equipped with a QICAM FAST 1394 (Surrey, BC, Canada) digital camera by an investigator blinded to treatment group. Nonoverlapping images from each pancreas were acquired using the ×20 objective. Image analysis was performed using ImageJ software (ImageJ 1.37v; National Institute of Health, Bethesda, MD) as previously described (44). The amount of collagen was expressed relative to the amount of collagen in the WT saline control group.
Western blotting.
Pancreatic tissue sections were homogenized in ice-cold RIPA buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, and a freshly added mixture of a protease inhibitor cocktail (Sigma). Protein extracts (20 μg total protein) were resolved by SDS-PAGE and blotted to polyvinylidene fluoride membranes. Blots were blocked in 5% nonfat dried milk in Tris-buffered saline-Tween buffer (10 mM Tris·HCl, pH 7.4, 0.9% NaCl, 0.05% Tween-20) and probed with monoclonal antibody to α-smooth muscle actin (α-SMA) (Sigma). For the loading control, blots were probed with an antibody to histone deacetylase (HDAC2) (Santa Cruz Biotechnology, Santa Cruz, CA). Signals were developed using horseradish peroxidase-conjugated anti-mouse IgG (Sigma) for α-SMA and anti-rabbit IgG (Santa Cruz Biotechnology) for HDAC2 and ECL Plus Western Blotting Detection Reagent (Amersham, Buckinghamshire, UK) followed by detection with X-ray film. Protein band intensities were quantified using Personal Densitometer SI and ImageJ software (ImageJ 1.37v; National Institute of Health).
Real-time RT-PCR.
To prepare total RNA, pancreatic tissue in RNA stabilization solution (RNAlater, Ambion) was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The quantity and purity of RNA was verified by measuring absorbance at 260 and 280 nm. For real-time RT-PCR assays, 2 μg of total RNA was treated with Turbo-DNase (Ambion) and reverse transcribed to complementary DNA (cDNA) using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen).
RT-PCR was performed with MiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) using iQ SYBR Green Supermix (Bio-Rad Laboratories) according to the manufacturer's instructions. PCR primers were either designed using Primer Express software (Applied Biosystems, Foster City, CA) or were based on the sequences from Primer Bank (46). AT1a primers were designed according Kudoh et al. (16). The specificity of the PCR primers used to identify AT1b receptor was confirmed previously (44). Primer sequences of transcripts evaluated by real-time quantitative PCR are shown in Table 1. Threshold cycle numbers were determined using iCycler software version 1.0 (Bio-Rad). Amplification products were verified by melting curves. Control reactions in the absence of template were used as negative controls. Results were calculated with normalization to acidic ribosomal phosphoprotein P0 mRNA, a reliable housekeeping gene for detecting fibrotic changes in the pancreas (12, 24). Relative changes in mRNA abundance were calculated using the comparative threshold cycle (Ct) method (20).
Table 1.
GenBank accession numbers and primer sequences of genes evaluated by real-time quantitative PCR
| Gene | Accession Number | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| ARP | NM_007475 | 5′-AGATTCGGGATATGCTGTTGGC-3′ | 5′-TCGGGTCCTAGACCAGTGTTC-3′ |
| Agt | NM_007428 | 5′-GTTCGCCATC TACGAGCA-3′ | 5′-TGCTCGTAGATGGCGAACAGG-3′ |
| AT1a | NM_177322 | 5′-TCACCTGCATCATCATCTGG-3′ | 5′-AGCTGGTAAGAATGATTAGG-3′ |
| AT1b | NM_175086 | 5′-TGGCTTGGCTAGTTTGCCG-3′ | 5′-ACCCAGTCCAATGGGGAGT-3′ |
| AT2 | NM_007429 | 5′-AACTGGCACCAATGAGTCCG-3′ | 5′-CAAAAGGAGTAAGTCAGCCAAG-3′ |
ARP, acidic ribosomal phosphoprotein P0; Agt, angiotensinogen; AT1a, angiotensin receptor type 1a; AT1b, angiotensin receptor 1b; AT2, angiotensin receptor 2.
Statistical analysis.
The results were expressed as means ± SE. Statistical analysis was performed using one-way ANOVA followed by two-tailed t-tests with P values <0.05 being considered statistically significant (SigmaPlot 9.0; Systat Software, San Jose, CA).
RESULTS
Expression of AT1a and AT1b mRNA in the pancreas.
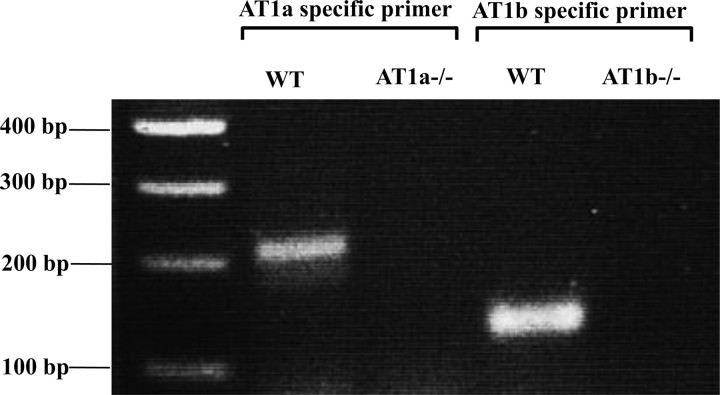
To confirm the expression of AT1a and AT1b receptors in the mouse pancreas and the absence of AT1a receptor in AT1a knockout mice and AT1b receptor in AT1b knockout mice, we performed real-time RT-PCR analysis (Fig. 1). WT mice expressed both AT1a and AT1b receptor mRNA. As expected, AT1a receptor expression was absent in AT1a knockout mice, and AT1b receptor expression was absent in AT1b knockout mice. Antibodies that distinguish AT1a from AT1b are not available to assess differential expression at the protein level.
Fig. 1.
Angiotensin receptor type 1a (AT1a) and AT1b mRNA expression in the pancreas of wild-type (WT), AT1a−/−, and AT1b−/− mice. An aliquot of each real-time RT-PCR product was loaded on 2% agarose gel and stained with ethidium bromide after electrophoresis. Pancreas of WT mice expressed AT1a and AT1b receptor mRNA. As expected, AT1a receptor expression was undetectable in AT1a−/− mice, and AT1b receptor expression was undetectable in AT1b−/− mice.
Effect of AT1a receptor deletion on the severity of chronic pancreatic injury following repeated episodes of acute pancreatic injury.
To evaluate the role of the AT1a receptor in pancreatic remodeling and fibrosis associated with repeated injury, 7-wk-old AT1a−/− and WT mice (8 mice per group) were subjected to three episodes of acute cerulein injury per week and euthanized 3 days after the last cerulein injection. The ratios of pancreatic weight to body weight as well as histological changes in the pancreas were evaluated in the cerulein-treated mice and saline-treated control groups. Pancreatic weights were significantly lower (about twofold) in cerulein-treated groups compared with control mice (Fig. 2A), indicating significant organ atrophy with repetitive injury. No differences were found between saline-treated WT and AT1a−/− mice, and no differences were found between cerulein-treated WT and AT1a−/− mice. Morphological changes in the pancreas were assessed by hematoxylin and eosin staining. All mice treated with repeated cerulein injections displayed histopathological signs of chronic pancreatitis as reflected by abnormal architecture, glandular atrophy, pseudotubular complexes, necrosis, and inflammatory cell infiltrate (Fig. 2B and Table 2). However, no statistically significant differences were found in any histological parameters between AT1a−/− and WT groups.
Fig. 2.
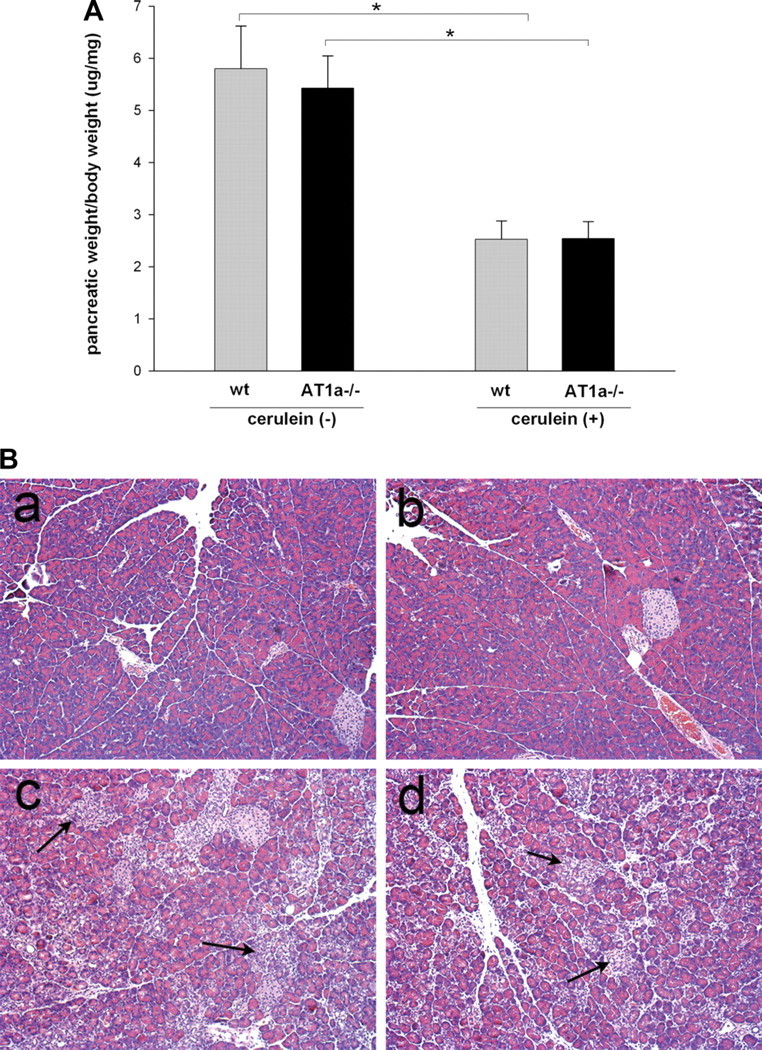
Severity of cerulein-induced chronic pancreatitis in AT1a −/− and WT male C57BL6/J mice. Mice were subjected to 3 episodes of acute pancreatitis (6 cerulein treatments per day every other day for 3 treatments) and killed 3 days after the last treatment. A: loss of pancreatic weight relative to total body weight in cerulein-treated mice suggests significant atrophy (8 mice per group), *P < 0.001. B: histological changes in the pancreas of AT1a −/− and WT mice after repetitive episodes of acute pancreatitis (representative picture, hematoxylin and eosin stain, original magnification, ×200). a and b: pancreas from WT (a) and AT1a −/− mice (b) after control saline treatment show no abnormalities in untreated AT1a −/− mice. c and d: pancreas from cerulein-treated WT (c) and cerulein-treated AT1a−/− (d) mice show severe parenchymal atrophy, dedifferentiation to tubular complexes, and interstitial inflammation. There were no differences in these parameters of chronic pancreatitis between WT and AT1a −/− mice.
Table 2.
Severity of cerulein-induced pancreatitis in AT1a−/− mice and WT controls
| Mice | WT | AT1a−/− | WT | AT1a−/− | P‡ |
|---|---|---|---|---|---|
| Cerulein | – | – | + | + | |
| Abnormal architecture | 0 ± 0 | 0 ± 0 | 2.0 ± 0.7 | 2.8 ± 0.5 | 0.29 |
| Glandula atrophy | 0 ± 0 | 0 ± 0 | 2.2 ± 0.8 | 2.8 ± 0.5 | 0.54 |
| Psedotubular complexes | 0 ± 0 | 0 ± 0 | 2.4 ± 0.6 | 3.0 ± 0 | 0.20 |
| Necrosis | 0 ± 0 | 0 ± 0 | 1.0 ± 0 | 1.25 ± 0.5 | 0.29 |
| Total score of above | 0 ± 0 | 0 ± 0 | 1.95 ± 0.8 | 2.44 ± 0.8 | |
| Acute inflammation* | 0 ± 0 | 0 ± 0 | 1.4 ± 0.6 | 1.75 ± 0.5 | 0.20 |
| Chronic inflammation† | 0.5 ± 1.0 | 0 ± 0 | 2.4 ± 0.6 | 2.3 ± 0.5 | 0.85 |
Data are means ±SE (n = 4 mice per group).
For the acute inflammation, the content of acute inflammatory cells (neutrophils) was graded, and
for the chronic inflammation, the content of mononuclear cells was graded. For the description of the histopathology score see materials and methods.
P values were calculated between cerulein-treated wild-type (WT) and AT1a−/− groups.
Effect of AT1a receptor deletion on collagen deposition following repeated episodes of acute pancreatitis.
Sirius red staining was used as a measure of collagen deposition in the pancreas (22). In control saline-treated mice, Sirius red staining was detected only in interlobular areas and around vessels and pancreatic ducts (Fig. 3 Aa). AT1a deletion did not change the pattern or intensity of Sirius red staining under these conditions (Fig. 3Ab). Repetitive cerulein treatment strongly increased interlobular and periacinar staining in both WT and AT1a−/− mice (Fig. 3, Ac and Ad). To quantify these fibrogenic changes, the extent of Sirius red staining was evaluated with morphometric image analysis (Fig. 3B). The analysis confirmed that, although cerulein treatment caused a marked increase in the pancreatic collagen deposition, there was no difference in collagen staining between WT and AT1a−/− mice.
Fig. 3.
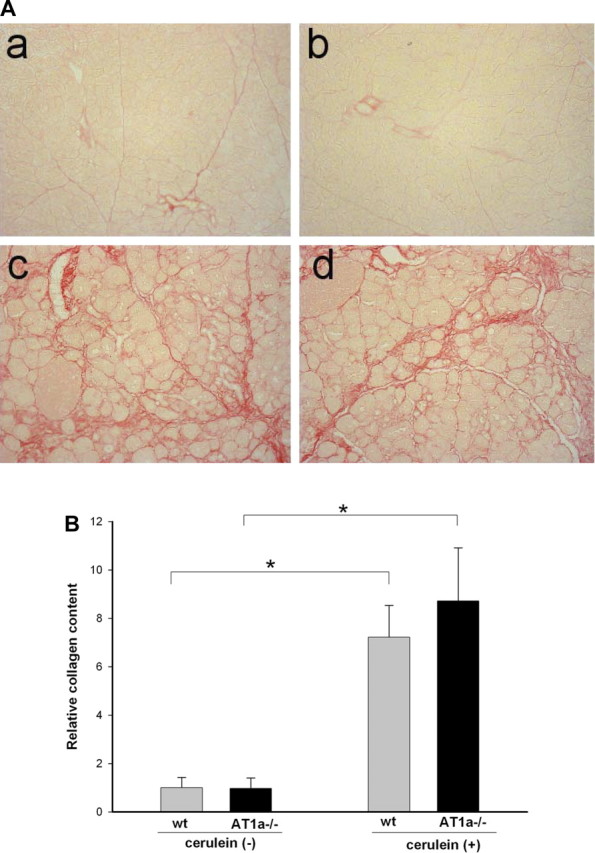
Collagen content in the pancreas assessed by Sirius red staining after repetitive episodes of acute pancreatitis as described in Fig. 1. A: representative pancreatic sections of pancreas from saline treated control WT (a) and AT1a−/− (b) mice and from cerulein-treated WT (c) and AT1a−/− (d) mice. Collagen staining appears red; control mice demonstrate some perilobular collagen, and there was no difference between control WT and AT1a −/− mice. Cerulein-treated mice demonstrated extensive interlobular and periacinar collagen staining, indicating robust fibrogenesis in this model of chronic pancreatitis. Blinded analysis identified no difference between WT and AT1a −/− mice (original magnification ×200). B: relative amount of pancreatic collagen quantified by morphometric analysis demonstrates no differences between WT and AT1a −/− mice. Results are expressed as means ± SE, n = 6 mice in each group. *P < 0.01.
Effect of AT1a receptor deletion on PSC activation following repeated episodes of acute pancreatitis.
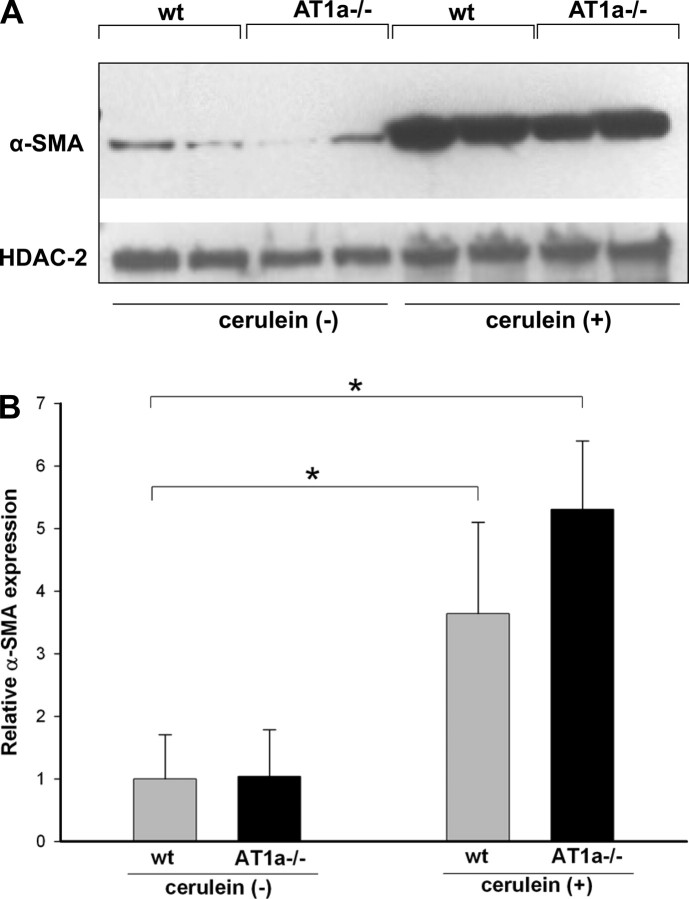
Activated PSC are known to mediate fibrogenesis in the pancreas. When activated, PSC express α-SMA. To analyze the level of PSC activation, we measured α-SMA expression in pancreas of mice treated with repeated episodes of cerulein injections or saline. Western blot analysis demonstrated that α-SMA expression was strongly increased in cerulein-treated mice (Fig. 4A). No significant differences in the increased α-SMA expression were noted between WT and AT1a−/− mice with cerulein treatment (Fig. 4B).
Fig. 4.
Activation of pancreatic stellate cells (PSC) during the course of cerulein-induced chronic pancreatitis. α-Smooth muscle actin (α-SMA) was used as a marker of PSC activation. A: representative Western blots of α-SMA in pancreatic extracts show marked increases in α-SMA expression with repetitive cerulein treatment. Histone deacetylase (HDAC2) was used as a loading control. B: densitometric analysis of α-SMA protein expression after repetitive cerulein treatment. Although there was a trend toward more α-SMA expression in cerulein-treated AT1a −/− mice, this difference was not significant. Data are means of 8 mice per group ± SE, *P < 0.05.
Effect of AT1a receptor deletion on AGT and AT1b and AT2 receptor mRNA expression in the pancreas.
Transcript levels of AGT and AT1b and AT2 receptors were analyzed in the pancreas of WT as well as AT1a−/− mice following control saline or repetitive cerulein treatment using real-time RT-PCR. The expression of AGT mRNA without cerulein treatment was found to be similar in WT and AT1a−/− mice. Cerulein treatment induced significant increases in AGT expression with no difference in the degree of this increase between WT and AT1a−/− mice (Fig. 5A). The level of AT1b receptor mRNA was also similar between WT and AT1a−/− mice. It increased slightly with the cerulein treatment in WT and AT1−/− mice, but there was not a statistically significant difference in this increase between cerulein-treated WT and AT1a−/− mice (Fig. 5B). Expression of AT2 receptor mRNA could not be detected in WT or AT1a −/− mice without cerulein treatment. After repetitive cerulein treatment, AT2 mRNA expression could be detected in the pancreas of WT and AT1a−/− mice although at significantly lower levels than AT1b mRNA (Fig. 5C). There was no statistically significant difference between AT2 mRNA expression between WT and AT1a−/− mice.
Fig. 5.
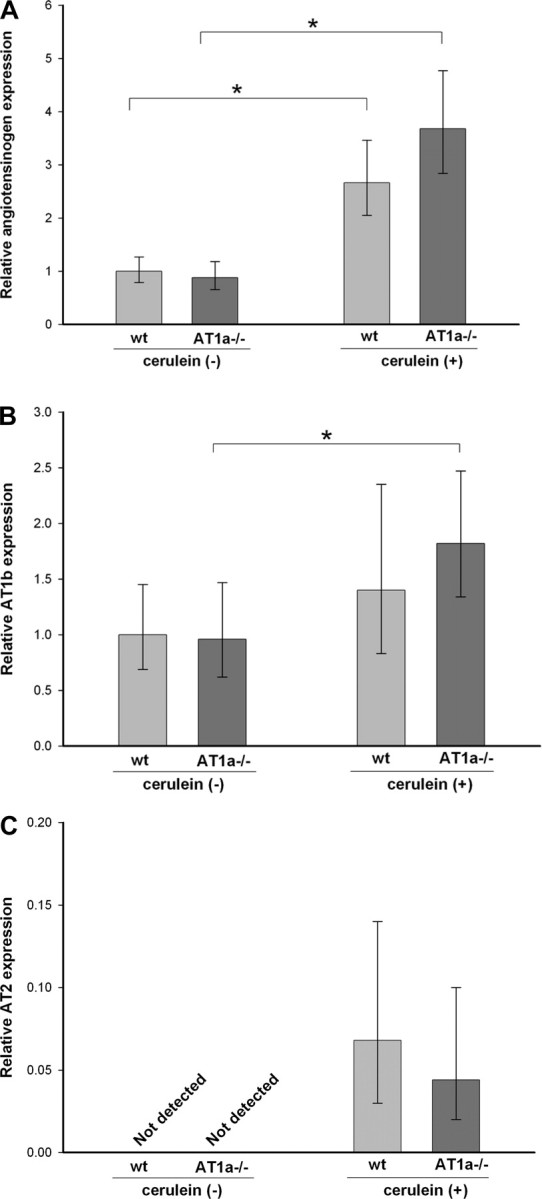
Pancreatic angiotensinogen (A), AT1b (B), and AT2 (C) receptor mRNA expression in WT and AT1a−/− mice under normal conditions and after repetitive cerulein-induced pancreatitis. Expression of angiotensinogen and AT1b mRNA is normalized to acidic ribosomal phosphoprotein P0 and expressed as fold increase over the level in WT control mice. Because pancreatic AT2 mRNA level was undetectable in control mice, its level is expressed as fold increase over the level of AT1b mRNA expression in WT mice. Angiotensinogen was significantly induced by cerulein treatment with no difference between WT and AT1a −/− mice. AT1b mRNA was also induced by cerulein but to a lesser degree. The bars represent means (n = 8), and error bars denote 95% confidence intervals. Confidence intervals were calculated using the ΔCt values before exponential transformation to fold increase in mRNA and are therefore asymmetric about the means; *P < 0.05.
Effect of AT1b receptor deletion on the severity of chronic pancreatic injury following repeated episodes of acute pancreatic injury.
To evaluate the role of the AT1b receptor in pancreatic remodeling and fibrosis, AT1b−/− and WT control mice (8 mice per group) were subjected to three episodes of acute cerulein injury per week and euthanized 3 days after the last cerulein injection according to the same protocol used for AT1a−/− mice. The ratios of pancreatic weight to body weight and histological changes in the pancreas were evaluated in the cerulein-treated mice and saline-treated control groups. Similar to the AT1a−/− experiment, pancreatic weights were significantly (about twofold) lower in cerulein-treated groups compared with control mice (Fig. 6A), indicating significant organ atrophy. No differences were noted between WT and AT1b−/− groups of mice without or with cerulein treatment. Morphological changes in the pancreas were assessed by hematoxylin and eosin staining (Fig. 6B). Repetitive cerulein treatment induced notable changes in the pancreas similar to those described above for AT1a−/− mice (Fig. 6B and Table 3). As with AT1a−/− mice, no differences were noted between AT1b−/− and WT groups.
Fig. 6.
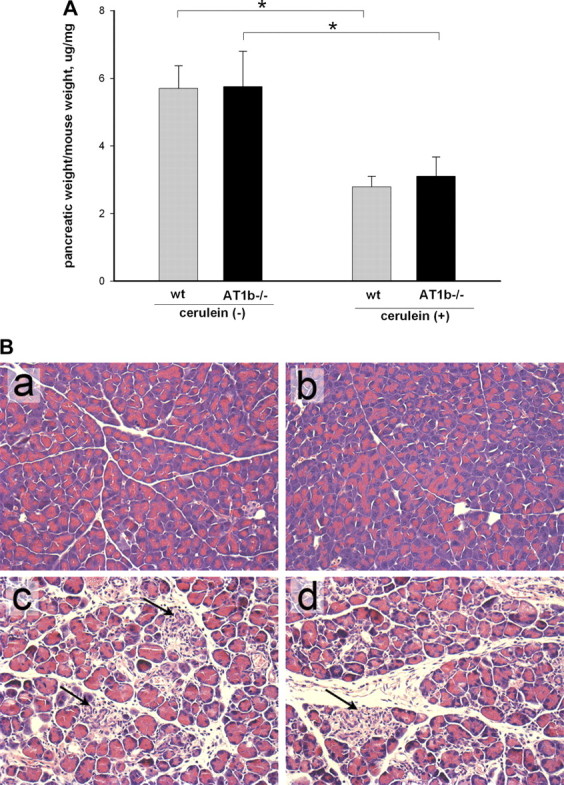
Pancreatic weight and hematoxylin and eosin stains in AT1b −/− mice after repetitive episodes of acute pancreatitis (as described in Fig. 1). A: loss of pancreatic weight relative to total body weight in cerulein-treated mice suggests significant atrophy (8 mice per group), *P < 0.001. B: histological changes in the pancreas of AT1b −/− and WT mice after repetitive episodes of acute pancreatitis (representative picture, hematoxylin and eosin stain, original magnification, ×400). a and b: pancreas from WT (a) and AT1b −/− mice (b) after control saline treatment showed no abnormalities in untreated AT1b −/− mice. c and d: pancreas from cerulein treated WT (c) and cerulein-treated AT1b−/− (d) mice showed severe parenchymal atrophy, dedifferentiation to tubular complexes, and interstitial inflammation. There were no differences in these parameters of chronic pancreatitis between WT and AT1b −/− mice.
Table 3.
Severity of cerulein-induced pancreatitis in AT1b−/− mice and WT controls
| Mice | WT | AT1b−/− | WT | AT1b−/− | ‡P |
|---|---|---|---|---|---|
| Cerulein | – | – | + | + | |
| Abnormal architecture | 0 ± 0 | 0 ± 0 | 2.5 ± 0.6 | 2.0 ± 0.0 | 0.37 |
| Glandula atrophy | 0 ± 0 | 0 ± 0 | 2.3 ± 0.5 | 2.8 ± 0.5 | 0.12 |
| Psedotubular complexes | 0 ± 0 | 0 ± 0 | 1.5 ± 0.6 | 1.0 ± 0 | 0.37 |
| Necrosis | 0 ± 0 | 0 ± 0 | 1.5 ± 0.6 | 1.0 ± 0.0 | 0.37 |
| Total score of above | 0 ± 0 | 0 ± 0 | 1.94 ± 0.7 | 1.69 ± 0.8 | |
| Acute inflammation* | 0 ± 0 | 0 ± 0 | 1.5 ± 1.0 | 1.0 ± 0.0 | 0.37 |
| Chronic inflammation† | 0.25 ± 0.5 | 0 ± 0 | 2.3 ± 0.5 | 2.3 ± 0.5 | 0.37 |
Data are mean ±SE (n = 4 mice per group). For the acute inflammation
the content of acute inflammatory cells (neutrophils) was graded, and for the chronic inflammation
the content of mononuclear cells was graded. For the description of the histopathology score see materials and methods.
P values were calculated between cerulein-treated WT and AT1b−/− groups.
Effect of AT1b receptor deletion on collagen deposition following repeated episodes of acute pancreatitis.
Collagen deposition in the pancreas was measured and calculated using the same method as for AT1a−/− mice (see above). In control saline-treated mice, collagen staining was detected only in interlobular areas and around vessels and pancreatic ducts (Fig. 7Aa). AT1b deletion did not change the pattern or intensity of collagen staining in these saline-treated control mice (Fig. 7Ab). Repetitive cerulein treatment strongly increased interlobular and periacinar collagen staining both in WT and in AT1b−/− mice (Fig. 7, Ac and Ad). Morphometric analysis confirmed that, although cerulein treatment caused a significant increase in the pancreatic collagen deposition, there was no difference in collagen content between WT and AT1b−/− mice (Fig. 7B).
Fig. 7.
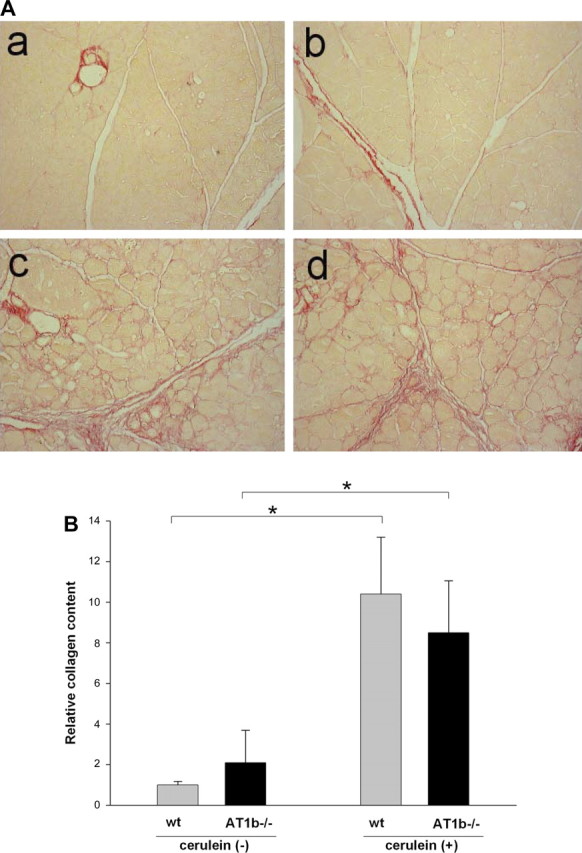
Collagen content in the pancreas of WT and AT1b −/− mice after repetitive episodes of acute pancreatitis. A: representative Sirius red staining of pancreatic sections of control pancreas from WT (a) and AT1b−/− (b) mice and from cerulein-treated WT (c) and AT1b−/− (d) mice. Collagen staining appears red (original magnification ×200). B: relative amount of pancreatic collagen quantified by morphometric analysis. Similar to AT1a −/− mice, the absence of AT1b did not alter the accumulation of collagen. Results are expressed as means ± SE, n = 6. *P < 0.01.
Effect of the AT1a and AT1b receptor antagonist losartan on repetitive cerulein-induced pancreatic injury.
To evaluate whether simultaneous blockade of AT1a and AT1b receptor isoforms affects the course of cerulein-induced pancreatitis, WT cerulein-treated mice (8 mice/group) were treated with AT1 receptor antagonist losartan and compared with control cerulein-treated mice (8 mice/group). Losartan was delivered by IP injection because it was demonstrated in several studies that IP delivery of losartan produces biological effects in mice (27, 35). Several doses of losartan, 0.5 mg/kg per day, 2 mg/kg per day, 5 mg/kg per day, and 20 mg/kg per day were evaluated. As a measure of severity of pancreatitis, we evaluated pancreatic weight relative to body weight, assessed histological changes by hematoxylin and eosin staining and evaluated fibrosis by Sirius red staining, analogous to what was described above for AT1a and AT1b receptor knockout mice. No differences were found in any of these parameters at any of the concentrations of losartan tested. Specifically, the pancreatic weight, pancreatic atrophy, and parenchymal changes evident by hematoxylin and eosin staining and Sirius red staining of collagen were identical to the changes observed with the AT1a−/− and AT1b−/− mice as described above.
DISCUSSION
Therapeutic interventions for chronic pancreatitis are limited and address symptoms and the complications of the disease rather than the underlying pathogenesis. Although the classical function of RAS is regulation of vascular homeostasis, RAS signaling has been implicated in the progression of inflammation and fibrosis in the heart, kidney, and liver (30). Drug therapies targeting the RAS by inhibiting AII formation (ACEi) or by blocking ARBs are now in widespread clinical use and have been shown to reduce tissue injury and fibrosis in cardiac and renal diseases independently of their effects on blood pressure (3). All major components of RAS are present in the pancreas (19, 43) and are activated during pancreatic injury (18). Using rat models of pancreatitis and different RAS inhibitors, it has been suggested that RAS blockade may be beneficial in the treatment and prevention of pancreatitis, but the data are controversial ranging from protection to worsening of pancreatitis depending on the animal species used, the model of pancreatitis, or the properties of individual blocking agent (7, 17, 38, 41–42, 48). Moreover, in humans, RAS inhibitors occasionally cause acute pancreatitis (2, 8–9, 36).
The objective of this study was to clarify the role of AT1 signaling in chronic pancreatitis to determine whether modulating this signaling pathway might be beneficial for preventing chronic pancreatitis. Although there are two major AII receptors, AT1 and AT2, the majority of the pathophysiological functions of AII are mediated through the AT1 receptor (40). To evaluate the effects of AII signaling through the AT1 receptor, we utilized AT1 receptor knockout mice and the cerulein-induced mouse model of chronic pancreatitis, which we previously developed and validated (26, 44). In contrast to humans, where there is only one AT1 receptor, rodents have two highly homologous AT1 receptors, AT1a and AT1b. The AT1a receptor is predominant in most tissues, but in the pancreas both of these receptors are expressed at a comparable level (19, 25, 44). To evaluate the role of AII signaling in pancreatic injury, we compared the parameters of chronic pancreatitis in AT1a and AT1b knockout mice separately to age- and sex-matched WT controls.
A different AT1a−/− mouse has been evaluated in a similar model of chronic pancreatitis by a different group (25) and was found to have an alleviated response to pancreatic injury and decreased fibrosis. In contrast with this report, we did not find any differences in the degree of pancreatic injury and fibrosis in WT and AT1a−/− mice as assessed by pancreatic weight changes, parenchymal injury as assessed by hematoxylin and eosin staining, the degree of collagen deposition, and stellate cell activation. The mRNA expression of AGT was similar between WT and AT1a−/− mice with or without cerulein treatment. AT1b receptor mRNA expression was similar between WT and AT1a−/− mice without cerulein treatment and was slightly higher but not statistically significant in the AT1a−/− cerulein-treated group. Yamada et al. (48) found that, in Wistar Bonn/Kobori rats, which spontaneously develop chronic pancreatitis, blockade of AT1 receptor with candesartan enhanced the mRNA expression of AT2 receptor. They speculated that AII interaction with AT2 receptor may be involved in attenuation of pancreatic inflammation and fibrosis (48). We reported recently that AT2−/− mice have increased severity of pancreatic fibrosis in cerulein model of pancreatitis (44). The expression of AT2 receptor in the pancreas, as in most adult tissues, is very low but increases in response to injury (11, 44). We evaluated the expression of AT2 mRNA in WT and AT1a−/− mice with and without cerulein-induced injury. Similar to our previous results, we were not able to detect the AT2 transcript without cerulein treatment both in WT and AT1a−/− mice; cerulein treatment raised the level of AT2 transcript into the measurable range at the same extent in WT and in AT1a−/− mice although it was about 10-fold less than the expression of AT1b receptor. It seems that the absence of AT1a receptor does not affect the level of AT2 expression in this model.
The role of AT1b in the development of pancreatitis has not been previously examined. We compared AT1b−/− mice and WT mice in a cerulein-induced model of chronic pancreatitis using the same parameters as for AT1a−/− mice. Similar to AT1a−/− mice, we did not find any differences in the degree of pancreatic injury and fibrosis between WT and AT1b−/− mice. The expression of AGT, AT1b, and AT2 receptors mRNA was not different between WT, AT1a−/−, and AT1b−/− mice with or without cerulein treatment (data not shown).
There is a possibility that AT1a and AT1b may have similar roles in pancreatic fibrosis and could compensate for each other. In this case, the evaluation of an AT1a−/−, AT1b−/− double knockout mouse would be useful. It is possible to obtain double knockouts by crossing AT1a−/− and AT1b−/− mice, but the resulting animals have diminished survival and a severe kidney phenotype (28). We were not able to breed enough double knockout mice and have them live long enough for our experiments. Nevertheless, we evaluated three AT1a−/−, AT1b−/− mice using this pancreatitis model and did not find any improvement in the degree of pancreatic injury and fibrosis compared with WT mice on the basis of hematoxylin and eosin staining and collagen measurements (unpublished observations).
Another approach to blocking both AT1a and AT1b receptor signaling is to use ARBs. We choose to use ARB losartan because it was reported that losartan ameliorates biochemical and histopathological changes of cerulein-induced acute pancreatic injuries in rats (41). We are not aware of any reports using an ARB in a mouse model of pancreatitis. IP delivery of losartan was chosen because it was shown that IP injections of losartan in mice caused biological effects such as prevention of sepsis-induced acute lung injury (35) and regulation of blood pressure (27). We evaluated a broad range of the doses, but we did not find any difference in the degree of cerulein-induced pancreatic injury and fibrosis in our model between losartan-treated and control mice. The losartan used in this study was an effective ARB because it was able to block effects of AII in isolated mouse hepatic stellate cells (unpublished observations). The ARB candesartan was reported to suppress pancreatic inflammation and fibrosis in WBN/Kob rats, a strain that spontaneously develops chronic pancreatitis with age (48). These differences could be attributed to a different mechanism of pancreatitis or may be species specific.
Overall, our findings indicate that AT1 signaling likely does not play a major role in the development of cerulein-induced chronic pancreatic injury and fibrosis in mice. The negative findings of this study are in contrast with the results of Nagashio et al. (25), who, studying a different AT1a knockout mouse, reported that AT1a receptor signaling is not important in the pancreatic injury in acute pancreatitis but plays a role in pancreatic fibrosis. The source of this discrepancy is not clear because the background mouse strain, mouse age, and the injury model were similar between the studies. The different observations might be attributed to the length of cerulein treatment, which was longer in experiments reported by Nagashio et al. (25). Of note, this type of discrepancy also happens in other experimental models of organ fibrosis. Two recent studies reported conflicting results on the role of losartan in bleomycin-induced pulmonary fibrosis (15, 50). It seems that the effects of AT1 signaling on the fibrotic response to injury are highly species or model dependent. For example, blockade of AII signaling with an ARB attenuated the development of hepatic fibrosis in several animal models, such as the bile duct ligation model in rats or the model of pig serum-induced fibrosis (1), but did not influence liver injury and fibrogenic events in the choline-deficient diet model of steatohepatitis in rats (13). In the pancreas, blockade of the AT1 pathway with losartan was reported to ameliorate pancreatic edema and amylase secretion in the “mild” model of cerulein-induced acute injury in rats (two injections of cerulein) (41) but failed to do so in a more severe model (six injections of cerulein) (4).
In past years the classical concept of RAS has experienced significant conceptual changes from the limited-proteolysis linear cascade to a cascade with multiple mediators, multiple receptors, and multifunctional enzymes. Among the recent discoveries contributing to this evolving paradigm was the identification of ACE2 (6, 39), the A(1–7)-forming enzyme, and of the G protein-coupled receptor Mas (33) as an A(1–7) receptor (reviewed in Ref. 32). ACE2-A(1–7)-Mas is coming to be viewed as the principle counterregulatory axis of RAS. This pathway was reported to participate in pathophysiology of different organs, including liver fibrosis (23). This pathway has not been evaluated in the development of pancreatitis but may contribute to the observed species- and model-specific differences in the course of pancreatitis.
In conclusion, the results of the present study suggest that AT1 receptor signaling may not be essential for the development of cerulein-induced chronic pancreatic injury and fibrosis in mice and that ARBs might not be beneficial in the treatment or prevention of chronic pancreatitis. Whether this is also true in humans requires clinical trials because AT1 receptor signaling might be different in humans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal : 1346–1355, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Birck R, Keim V, Fiedler F, van der Woude FJ, Rohmeiss P. Pancreatitis after losartan. Lancet : 1178, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bommer WJ. Use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker therapy to reduce cardiovascular events in high-risk patients: Part 1. Prev Cardiol : 148–154, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Chan YC, Leung PS. AT1 receptor antagonism ameliorates acute pancreatitis-associated pulmonary injury. Regul Pept : 46–53, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, Deviere J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol : G1105–G1112, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res : E1–E9, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Ernsberger P, Koletsky RJ. Metabolic actions of angiotensin receptor antagonists: PPAR-gamma agonist actions or a class effect? Curr Opin Pharmacol : 140–145, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Famularo G, Minisola G, Nicotra GC, De Simone C. Acute pancreatitis associated with irbesartan therapy. Pancreas : 294–295, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Fisher AA, Bassett ML. Acute pancreatitis associated with angiotensin II receptor antagonists. Ann Pharmacother : 1883–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 10.French SW, Miyamoto K, Wong K, Jui L, Briere L. Role of the Ito cell in liver parenchymal fibrosis in rats fed alcohol and a high fat-low protein diet. Am J Pathol : 73–85, 1988. [PMC free article] [PubMed] [Google Scholar]
- 11.Gallinat S, Busche S, Raizada MK, Sumners C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am J Physiol Endocrinol Metab : E357–E374, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-κB activation is associated with hormone-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol : G1402–G1414, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez P, Solis N, Pizarro M, Aguayo G, Duarte I, Miquel JF, Accatino L, Arrese M. Effect of losartan on early liver fibrosis development in a rat model of nonalcoholic steatohepatitis. J Gastroenterol Hepatol : 846–851, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA : 3521–3525, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keogh KA, Standing J, Kane GC, Terzic A, Limper AH. Angiotensin II antagonism fails to ameliorate bleomycin-induced pulmonary fibrosis in mice. Eur Respir J : 708–714, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kudoh S, Komuro I, Hiroi Y, Zou Y, Harada K, Sugaya T, Takekoshi N, Murakami K, Kadowaki T, Yazaki Y. Mechanical stretch induces hypertrophic responses in cardiac myocytes of angiotensin II type 1a receptor knockout mice. J Biol Chem : 24037–24043, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, Nakazawa T, Ohara H, Nomura T, Joh T, Shirai T, Itoh M. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology : 1010–1019, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol : 31–37, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol : 13–19, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods : 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension : 538–548, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-De Leon A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem : 737–743, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol : 1327–1338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugea A, Gukovsky I, Gukovskaya AS, Pandol SJ. Nonoxidative ethanol metabolites alter extracellular matrix protein content in rat pancreas. Gastroenterology : 1845–1859, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Nagashio Y, Asaumi H, Watanabe S, Nomiyama Y, Taguchi M, Tashiro M, Sugaya T, Otsuki M. Angiotensin II type 1 receptor interaction is an important regulator for the development of pancreatic fibrosis in mice. Am J Physiol Gastrointest Liver Physiol : G170–G177, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci : 665–674, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol : R685–R691, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA : 15496–15501, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest : 50–59, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev : 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev : 313–370, 1977. [DOI] [PubMed] [Google Scholar]
- 32.Santos RA, Ferreira AJ, Simoes E Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol : 519–527, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA : 8258–8263, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol : 317–325, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Shen L, Mo H, Cai L, Kong T, Zheng W, Ye J, Qi J, Xiao Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock : 500–506, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Singh S. Angiotensin-converting enzyme (ACE) inhibitor-induced acute pancreatitis: in search of the evidence. South Med J : 1327–1328, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Smits JF, van Krimpen C, Schoemaker RG, Cleutjens JP, Daemen MJ. Angiotensin II receptor blockade after myocardial infarction in rats: effects on hemodynamics, myocardial DNA synthesis, and interstitial collagen content. J Cardiovasc Pharmacol : 772–778, 1992. [PubMed] [Google Scholar]
- 38.Storka A, Vojtassakova E, Mueller M, Kapiotis S, Haider DG, Jungbauer A, Wolzt M. Angiotensin inhibition stimulates PPARgamma and the release of visfatin. Eur J Clin Invest : 820–826, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem : 33238–33243, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev : 639–672, 2000. [PubMed] [Google Scholar]
- 41.Tsang SW, Ip SP, Leung PS. Prophylactic and therapeutic treatments with AT 1 and AT 2 receptor antagonists and their effects on changes in the severity of pancreatitis. Int J Biochem Cell Biol : 330–339, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Tsang SW, Ip SP, Wong TP, Che CT, Leung PS. Differential effects of saralasin and ramiprilat, the inhibitors of renin-angiotensin system, on cerulein-induced acute pancreatitis. Regul Pept : 47–53, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol : 1084–1096, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulmasov B, Xu Z, Tetri LH, Inagami T, Neuschwander-Tetri BA. Protective role of angiotensin II type 2 receptor signaling in a mouse model of pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol : G284–G294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Westerloo DJ, Florquin S, de Boer AM, Daalhuisen J, de Vos AF, Bruno MJ, van der Poll T. Therapeutic effects of troglitazone in experimental chronic pancreatitis in mice. Am J Pathol : 721–728, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res : e154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology : 1557–1573, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Yamada T, Kuno A, Masuda K, Ogawa K, Sogawa M, Nakamura S, Ando T, Sano H, Nakazawa T, Ohara H, Nomura T, Joh T, Itoh M. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther : 17–23, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Yamada T, Kuno A, Ogawa K, Tang M, Masuda K, Nakamura S, Ando T, Okamoto T, Ohara H, Nomura T, Joh T, Shirai T, Itoh M. Combination therapy with an angiotensin-converting enzyme inhibitor and an angiotensin II receptor blocker synergistically suppresses chronic pancreatitis in rats. J Pharmacol Exp Ther : 36–45, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration : 236–242, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology : 745–750, 2001. [DOI] [PubMed] [Google Scholar]