Abstract
Engineered cardiac tissue and cardiomyocyte cell cultures offer wide opportunities for improved therapeutic intervention and laboratory heart models. Electrical field excitation is a common intervention in the production of engineered tissue and the investigation of the electrical properties of in vitro cell cultures. In this work, we use strength-duration relationships to investigate systematically factors influencing electrical excitability of two- (2D) and three-dimensional (3D) neonatal rat ventricular myocyte cultures. We find that the strength of the voltage pulse is negatively correlated with the threshold duration, as predicted by the Lapicque-Hill equation, and show that higher pacing frequencies require higher thresholds to capture paced cultures. We also study the impact of properties intrinsic to the 2D and 3D cultures on strength-duration relationships. We show that a smaller culture dimension, perpendicular anisotropic culture orientation with respect to electrical field, higher proportion of added fibroblasts, and TBX18-induced pacemaker reprogramming independently result in higher stimulation thresholds. These properties reflect the characteristics of the well-insulated endogenous pacemaking tissue in the heart (sinoatrial node) and should guide the engineering of biological pacemakers for improved outcomes.
NEW & NOTEWORTHY Gaps exist in the availability of in vitro functional assessment tools that can emulate the integration of regenerative cells and tissues to the host myocardium. We use strength-duration relationships of electrically stimulated two- and three-dimensional myocardial constructs to study the effects of pacing frequency, culture dimensions, anisotropic cell alignment, fibroblast content, and pacemaker phenotype on electrical excitability. Our study delivers electrical strength-duration as a quantifiable parameter to evaluate design parameters of engineered cardiac tissue constructs.
INTRODUCTION
Approaches to regenerate damaged myocardium by replacing dead and dying cells with de novo cardiac myocytes rely on the premise that the donor myocytes closely replicate the electromechanical properties of the host myocardium. Electrical or contractile inhomogeneity between the donor and host myocardium would lead to ineffective functional regeneration at best or potentially fatal consequences at worst. Probing the structure and function of the engineered cardiac cells and tissues is inherently multifactorial. For example, the myocardium is continuously exposed to physiological electrical fields on the order of ~1 V/cm, oscillatory tensile and shear stresses due to muscular contraction and blood flow, and neurohumoral stimuli that further control the electrical excitation and contraction (13).
Extrinsic electrical pacing has long been considered for improving the function of engineered cardiac tissue (15, 41). For example, electrical stimulation of pluripotent stem cell-derived cardiac myocytes resulted in anisotropic cell alignment, improved sarcomere organization, and increased electrical cell-to-cell coupling (43) as well as protection against dedifferentiation, arrhythmogenicity, and reduced viability (9, 27, 35, 37). These “biomimetic approaches” to tissue engineering (40) seek to mimic the natural cardiac milieu (12, 25) and to optimize the structure and function of the derived tissues (40). Akin to the multifactorial efforts in generating cardiac derivatives, methods and criteria to evaluate the functional properties of the engineered cardiac tissues are also complex and often require terminal processes that are incompatible with longitudinal studies (14, 21, 32, 42). A functional readout that can be standardized and reports the electromechanical properties of cardiomyocytes could provide a much needed comparative tool across many laboratories.
The electrically excitable membranes of cardiac myocytes exhibit the classic strength-duration relationship, where decreased stimulation strength requires a longer stimulus duration and vice versa (34). The Lapicque-Hill equation relates the strength of a transmembrane current source to the threshold duration for excitation (34), approximates the behavior of single cardiomyocytes (39), and qualitatively resembles the strength-duration relationships of primary cardiomyocyte tissue constructs (36). In the present study, we apply routine, label-free live-cell imaging to discern quantitatively the strength-duration properties of cardiac tissue constructs. By constructing two-dimensional (2D) monolayers and three-dimensional (3D) spheroids of primary neonatal rat ventricular myocytes (NRVMs), we illustrate the relationship between threshold stimulus strength and stimulus duration needed to elicit syncytial contraction of the cardiomyocytes by extrinsically imposed electrical field. We investigate the effect on excitability of tissue size, anisotropic cellular alignment, cardiomyocyte density, and proportion of fibroblasts with variable pacing frequency. We then demonstrate the validity of this method by comparing the excitability of two contrasting cardiac tissue constructs: 1) ventricular cardiomyocyte constructs and 2) cardiac tissue constructs consisting of induced pacemaker cells (16–18) as a surrogate for the native pacemaker cells in the sinoatrial node. We test the hypothesis that the induced pacemaker constructs are electrically well-insulated and are less prone to be driven by an external electrical stimulation, owing to their lower myocyte content and isotropic cell alignment compared with ventricular myocyte constructs.
MATERIALS AND METHODS
Ethics statement.
All procedures were approved by the institutional animal care and use committee at Emory University and performed in accordance with the guidelines for federal research published by the National Institutes of Health.
2D and 3D cultures of neonatal rat ventricular myocytes.
NRVMs and cardiac fibroblasts were isolated from postnatal days 1–3 Sprague-Dawley rat pups as previously described (18). NRVMs were seeded overnight at a density of 2 × 105 cells/cm2 on standard polyvinyl coverslips coated with 1–2 μg/cm2 fibronectin (Corning, Corning, NY). Fibronectin was diluted from a 200× stock solution in phosphate-buffered saline (PBS), then added to the substrate, and incubated for 1 h at 37°C and 5% CO2. NRVMs were cultured and transduced in standard NRVM media composed of Medium 199 supplemented with the following: 10 mM HEPES, 0.1 mM nonessential amino acids, 3.5 mg/ml glucose, 2 mM GlutaMAX, 4 μg/ml vitamin B12, 100 U/ml penicillin, and heat-inactivated fetal bovine serum at 10% (transduction and 1st 2 days of culture) or 2% (after 2 days of culture) final concentration. When applicable, coverslips were abraded with 15-μm Alumina lapping film (PACE Technologies, Tucson, AZ) to create alignment grooves, then incubated in 70% ethanol for 30 min, and washed with PBS thrice. The coverslips were then placed in the cell culture wells and incubated in fibronectin at 1–2 μg/cm2 for 1–2 h. NRVM spheroids were produced using AggreWell 400 (STEMCELL Technologies, Vancouver, Canada). The plate was washed with rinsing solution (STEMCELL Technologies), then 0.5 ml of 10% FBS culture media was added per well, and the plate was centrifuged for 5 min at 2,000 g. Then, 6 × 105 cells were added per well, which results in 500 cells per aggregated sphere, and the plate was incubated at 37°C and 5% CO2 for 3 days, after which the spheroids were fully compacted. The usual media changes described above were performed on days 1–3. After aggregation, the spheres were dislodged by pipetting, filtered using a 40-μm-pore-size filter, and then seeded on fibronectin-coated, 6-well plates.
Electrical stimulation and live-cell imaging.
Electrical stimulation of monolayer and spheroid cell cultures on coverslips was performed using 6-well C-Dish (IonOptix, Westwood, MA) connected to a C-Pace EP Culture Pacer (IonOptix) applying a square, bimodal, biphasic voltage waveform with controllable amplitude, duration, and frequency. The cell culture coverslips were placed at the center of the 6-well plate, and the strength of the applied electrical field was determined by dividing the applied voltage by the distance between the carbon electrodes of the C-Dish (IonOptix). All recordings >5 min were done at 37°C and 5% CO2 using a custom-made microscope stage-top incubator (Tokai Hit, Shizuoka, Japan) that allows placement of the C-Dish (IonOptix) inside the incubator. All transmitted light and fluorescent imaging was performed using a fully motorized DMi8 Leica inverted microscope (Leica Microsystems, Wetzlar, Germany). Live-cell imaging was performed in a stage-top physiological environmental chamber with temperature, CO2, and humidity control (Tokai Hit), which was customized to fit the electrical pacing system. Real-time cardiomyocyte contraction images were recorded with an sCMOS camera (ORCA-Flash4.0; Hamamatsu Photonics, Hamamatsu, Japan).
Anisotropy analysis.
The anisotropy of the NRVM monolayers cultured on abraded coverslips was quantitated by Fourier component analysis of the transduced fluorescent marker (Ad-GFP) using the Directionality plugin in ImageJ (23), which analyzes the spectrum in polar coordinates and outputs a histogram representing the distribution of powers per angle. The direction of the abrasions was chosen randomly, and the alignment of the cells was compared with the power spectrum of a bright-field image of the same location, which features clear abrasions without the transparent cells.
Immunostaining, fluorescence intensity, and morphometric analysis.
NRVM cell cultures were fixed using 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and incubated with the primary antibodies α-sarcomeric actinin (Sigma-Aldrich 7811; 1:800), connexin-43 (Sigma-Aldrich C6219; 1:800), and vimentin (Abcam ab24525) and Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific) A-11031, A-11034, and A-21449. Immunofluorescence imaging was taken at the same illumination intensity and exposure in each channel. Morphometric analysis was performed using ImageJ by drawing a polygonal perimeter around randomly selected cells and measuring area, perimeter, and mean gray value in the α-sarcomeric actinin channel. Major and minor cell axes were obtained by fitting an ellipse to the selection. Connexin-43 and N-cadherin were quantified by applying an intensity threshold using the Triangle algorithm in ImageJ (44) and then performing particle analysis with an area upper limit of 100 μm2 per particle.
Virus transduction.
Virus production and somatic gene transfer of green fluorescent protein (GFP) or TBX18 to the neonatal rat ventricular cardiomyocytes was performed as previously described (17, 18). Briefly, NRVMs were transduced in suspension in routine NRVM media on the day of isolation with Ad-GFP or Ad-TBX18 vectors, respectively, with multiplicity of infection of 1 for 2 h at room temperature, and then seeded on polyvinyl coverslips or aggregation wells as described above.
Curve fitting and statistical analysis.
The Lapicque-Hill model is expressed by the exponential function , also termed the Weiss-Lapicque equation (34), where Irh is rheobasic current and τ is a time constant. Nonlinear fitting was performed by minimizing adjusted χ2, using the functional form y = a(1 − e−bx)c while restricting the value of c to −1, and goodness of fit was assessed using the coefficient of determination r2. Linear correlation was measured by calculating a Pearson correlation factor, and significance was assessed assuming a Gaussian distribution by performing an F test against the horizontal. Exponential and linear fitting and statistical tests were done using OriginPro. Gaussian fitting of the Fourier power spectra was done using the Directionality plugin in ImageJ (23). The mean, standard deviation, and standard error of the mean (SE) were calculated for each set of measurements. Mean and SE were plotted unless otherwise indicated. Data sets were compared using unpaired or one-sample t-test unless otherwise indicated, and a confidence level of P < 0.05 was reported as statistically significant.
RESULTS
NRVM 2D and 3D constructs exhibit Lapicque-Hill strength-duration relationship.
We employed NRVMs as a model of ventricular myocardium for electrical stimulation. NRVMs are a well-characterized in vitro model for primary cardiomyocytes (11, 24, 29), and the functional immaturity of these myocytes may render them an adequate reflector of pluripotent stem cell-derived cardiomyocytes. Electrical stimulation was delivered to the cardiomyocytes by applying a biphasic voltage square wave (Fig. 1A). A “capture” voltage/millisecond pair was defined as the pair that led to a syncytial contraction of the myocytes under routine, transmitted light microscopy at a frequency that is equivalent to that of the pacing frequency for 30 s of observation time, equivalent to >95% instantaneous capture rate (Fig. 1B). A closed-environment chamber system was customized to fit on the stage of the microscope to maintain a physiological condition of pH 7.4 and 37°C with >95% relative humidity throughout the electrical stimulation and visual recording (Fig. 1C). A representative relationship between threshold duration and voltage plotted for an NRVM monolayer is shown in Fig. 1D. Figure 1E shows a representative relationship between threshold voltage and duration for a 3D spheroid composed of a 500-cell aggregate and assessed for contraction in a similar manner. Both the monolayer and spheroid relationships are fit with the Lapicque-Hill equation and displaying a classic strength-duration relationship with the strength of the required field rising steeply at low stimulation durations and reaching an almost vertical asymptote and reaching an almost horizontal asymptote at large stimulation durations. The same data are replotted on logarithmic axes in the insets.
Fig. 1.
Example strength-duration relationships for neonatal rat ventricular myocyte 2-dimensional (2D) monolayer (D) and 3-dimensional (3D) spheroid aggregates (E) under electrical stimulation, fitted with Lapicque-Hill model (B). Insets are replotted on a log-log scale. A: waveform diagram indicating interpretation of strength, duration, and frequency of stimulation. C: customized closed-environment chamber system maintaining physiological conditions (pH 7.4, 37°C, >95% relative humidity) for live-cell electrical stimulation and visual recording. Electrical field stimulation was performed at 1 Hz using a square bimodal, biphasic voltage waveform. τ, Time constant; Irh, rheobasic current; t, time.
Increased pacing frequency requires higher strength-duration thresholds.
Stimulation frequency is an important parameter in studies concerning the effect of electrical pacing on cells (9). It is not obvious that capture thresholds of cardiomyocyte cultures are independent of stimulation frequency, and action potential or contraction-dependent effects of electrical field stimulation will depend on the capture fidelity. We hypothesized that the strength and duration thresholds will rise, resulting in a rightward shift of the strength-duration curves at higher stimulation frequencies. Thus we investigated the effect of pacing frequency on the strength-duration relationships of NRVM 2D monolayers and 3D spheroids. As above, for each spheroid, we plotted the threshold field strength at which the spheroid was synchronously captured, as assessed by light microscopy, versus the stimulation duration, with the pacing frequency set to 1, 2, or 3 Hz. Our data indicate that for any given stimulation duration, a spheroid required a higher field strength to be captured when the stimulation frequency was higher (Fig. 2A). For example, the required field strength to capture the example spheroid at a stimulation duration of 6 ms was 5 V/cm at 1 Hz, 8 V/cm at 2 Hz, and 13 V/cm at 3 Hz. The ratio of the threshold field strength at 2 versus 1 Hz is plotted against the stimulation duration in Fig. 2B. The ratios were analyzed using a one-sample unpaired t-test against a ratio of 1 and were significantly (P < 0.05) greater at all durations for which we had a complete data set. Note that for durations shorter than the minimum ones shown, capture was not possible at the maximum voltage output (13 V/cm) of our setup.
Fig. 2.
Stimulation frequency is inversely correlated with electrical excitability. A: example strength-duration relationship for the same neonatal rat ventricular myocyte 3-dimensional (3D) spheroid aggregate paced at 1, 2, and 3 Hz. B: ratio of threshold voltage for a constant stimulation duration while pacing at 2 vs. 1 Hz, n = 3–4 spheroids. C: example strength-duration relationship for the same neonatal rat ventricular myocyte monolayer culture paced at 1 and 3 Hz using a voltage source with a bimodal, biphasic square waveform. Inset is replotted on a log-log scale. D: ratio of threshold duration for a constant stimulation strength while pacing at 3 vs. 1 Hz, n = 3 monolayers. Mean and SE are plotted. *P < 0.05. 2D, 2-dimensional.
We investigated the effect of pacing frequency on NRVM monolayers as well (Fig. 2, C and D). In this case, for each voltage magnitude of stimulation, we increased the stimulation duration until the monolayer was captured, and the threshold duration was recorded. Strength-duration relationships of the NRVM monolayers at 1- and 3-Hz stimulation frequencies indicate positive correlation of capture threshold with stimulation frequency (Fig. 2C). Normalizing the stimulation duration at 3 Hz over 1 Hz against the field strength illustrates that significantly longer stimulation duration is required at strengths ≤2 V/cm (Fig. 2D). Notably, the quasihyperbolic shape of the Lapicque-Hill fit was more pronounced in the monolayers than in the 3D spheroids, with steeper slopes at the inflection point in the monolayers.
Myocardial construct size correlates negatively with electrical excitability.
Engineered cardiac tissue constructs to treat or replace the infarcted heart need to span a significant area to generate mechanical force. Therefore, understanding the excitability properties of cardiomyocyte constructs as a function of their physical dimensions is useful for determining the parameters needed for successful electrical field pacing in vitro and physiological integration in vivo. To test this, we generated 3D NRVM spheroids of varying sizes (Fig. 3A) and subjected each spheroid to electrical field stimulation. For each spheroid, the electrical field strength is increased at a given duration of stimulation until the spheroid is captured. Each curve represents the strength-duration curve of one spheroid of a given diameter (Fig. 3B). At a stimulation duration of 10 ms, the threshold voltage strength for stimulation correlated negatively with the spheroid diameter (r = −0.89; Fig. 3C). This negative correlation is preserved across five stimulation durations of 2–18 ms (Fig. 3D). We also examined the effect of 2D culture dimensions on the strength-duration relationship by comparing the strength-duration curves of square monolayers seeded at the same density (200,000 cells/cm2, full confluence) but of different areas, 16 and 81 mm2. The strength-duration relationships for individual monolayers are plotted in Fig. 3E, and the mean and SE are plotted in the inset. Although there is a trend toward lower stimulation thresholds for the larger monolayers (81 mm2), the difference did not reach significance due to higher variability in the smaller monolayers. We frequently observed that the NRVMs could show patches of directionality in 2D cultures, but this was not by design but by chance. Such random anisotropic cell alignment could lead to larger variability in excitability in smaller monolayers. To study this in a controlled manner, we next implemented anisotropic cell plating.
Fig. 3.
Culture size is inversely correlated with electrical excitability. B: strength-duration relationships for 3-dimensional neonatal rat ventricular myocyte spheroid aggregates under electrical field stimulation with diameters ranging from 100 to 210 μm as measured in A. C: linear fit to stimulation field threshold strength vs. spheroid diameter at a constant stimulation duration (10 ms), n = 6. D: overlay of stimulation field threshold strength vs. spheroid diameter at a range of constant durations. E: strength-duration relationships for 16 and 81 mm2 neonatal rat ventricular myocyte monolayers. Inset is mean and SE. n = 3 Monolayers per group. All stimulation was performed at 1 Hz using a voltage source with a biphasic, bimodal waveform.
Anisotropic cell alignment parallel to the electrical field lowers capture thresholds.
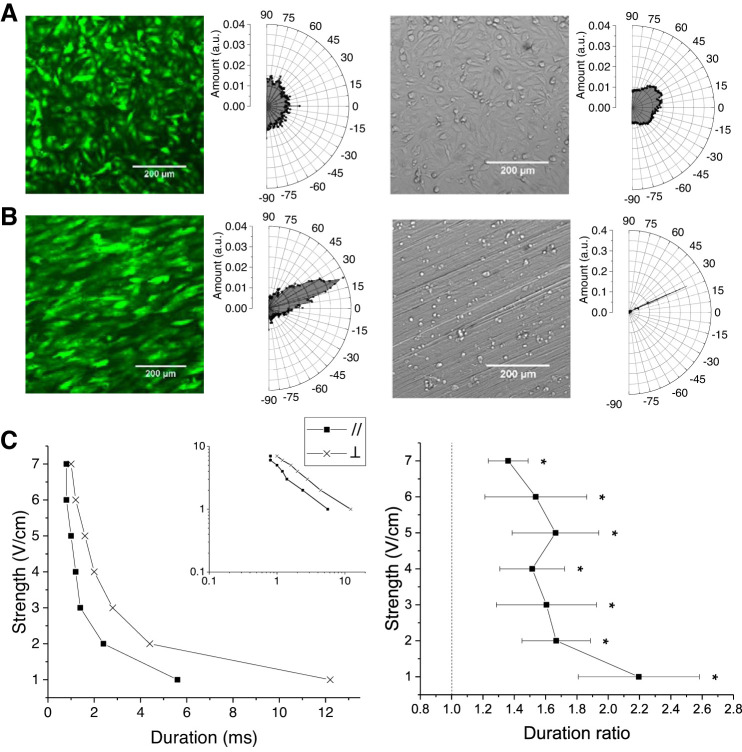
Electrical pacing has been shown to be easier along the direction of the long axis of single cardiomyocytes (39). Conversely, electrically paced cells, including cardiomyocytes, have been shown to align and elongate in the direction of the electrical field (28, 35). We studied the effect of anisotropic cell alignment with respect to the electrical field on the strength-duration relationships of 2D NRVM monolayers. Cells were transduced with a fluorescent reporter gene, GFP, and seeded at full confluence (200,000 cells/cm2) on plastic coverslips. Monodirectional abrasions were created by scraping the coverslips with lapping film of 15-µm grade. Three days after cell seeding, we analyzed cell alignment of NRVM monolayers seeded on abraded coverslips as well as normal, unabraded coverslips. The directional power spectrum of the GFP-labeled NRVM monolayers ranged uniformly on normal coverslips (Fig. 4A) but peaked at 23 ± 14° on abraded coverslips (Fig. 4B). The orientation of the anisotropic NRVM alignment matched perfectly with the angle of the abrasions (24 ± 1°) in the same field of view imaged with a bright-field mode through the myocytes (Fig. 4B, right). To investigate the effect of cell culture anisotropy on strength-duration relationships, the coverslips were subjected to an electrical field that is parallel or perpendicular to the direction of cell alignment. An example strength-duration relationship for the same coverslip with 3-Hz stimulation frequency is shown in Fig. 4C, left, and replotted on a log-log scale in the inset. The duration of stimulation was increased at each electrical field strength until the field of view contracted synchronously with the 3-Hz pacing. For each field strength, the threshold duration of stimulation was recorded. The threshold duration was increased when the cells were aligned perpendicularly relative to the electrical field compared with parallel alignment. The ratio of threshold stimulation durations in the perpendicular case versus the parallel case were plotted against electrical field strength in Fig. 4C, right. The ratio of the means was significantly greater than 1 at all tested strengths (1–7 V/cm). These data indicate that misaligned cardiomyocytes are more difficult to capture.
Fig. 4.
Cell alignment with respect to electrical field impacts electrical excitability. A: fluorescence (left) and bright-field images (right) of neonatal rat ventricular myocytes (NRVMs) cultured on smooth substrate and directionality analysis histograms with peaks at angles −23.97, 20.18° and standard deviations of 176.91, 29.16°, respectively, by normal distribution curve fitting. B: fluorescence (left) and bright-field images (right) of NRVMs cultured on anisotropic substrate and directionality analysis histograms with peaks at angles 22.99, 24.22° and standard deviations of 13.85, 1.04°, respectively, by normal distribution fitting. C, left: example strength-duration relationship for an NRVM monolayer cultured on anisotropic substrate and oriented parallel or perpendicular to the stimulating electrical field. Inset is replotted on a log-log scale. C, right: ratio of threshold duration for a constant stimulation strength with cells aligned perpendicular vs. parallel to the electrical field, n = 5. Electrical stimulation was performed using a voltage source with a biphasic, bimodal waveform at 3-Hz stimulation rate. Mean and SE are plotted. *P < 0.05. a.u., Arbitrary units.
Fibroblasts negatively impact excitability by electrical field stimulation.
Nonmyocytes in the myocardium such as fibroblasts account for up to two-thirds of the total cell number in the heart (1, 2), although estimates vary (33). This fraction is particularly increased in the sinoatrial node, which can have up to 95% collagen and fibroblasts and as little as 5–20% myocytes by volume (30, 31), and the proportion of connective tissue increases with aging (5). To determine the effects of the presence of fibroblasts on electrical field excitability in vitro, we recorded the strength-duration relationships for NRVM monolayers with or without added fibroblasts. We seeded NRVMs and fibroblasts as square monolayers of 100 mm2 in three different configurations: 1) the usual NRVM density (200,000 cells/cm2, fully confluent), 2) NRVMs at half of the usual density (100,000 cells/cm2) mixed with the same number of fibroblasts (100,000 fibroblasts/cm2), and 3) NRVMs at the usual density (200,000 cells/cm2) mixed with the same number of fibroblasts (200,000 fibroblasts/cm2; Fig. 5, A and B). We recorded the strength-duration relationships at 2-Hz stimulation for all three groups by increasing the duration of stimulation at a given electrical field magnitude until the monolayer is captured. We recorded the threshold duration of stimulation against the strength of the electrical field. The threshold duration of stimulation was significantly increased for the group seeded at the usual density with added fibroblasts when stimulated at a strength of 1 or 4 V/cm. There was a trend toward increased threshold durations at higher stimulation strengths, but the effect was not significant. The cultures were not capturable at strengths <1 V/cm (Fig. 5C).
Fig. 5.
Fibroblasts negatively impact electrical excitability. A and B: differential interference contrast (DIC) imaging (A) and immunostaining for α-sarcomeric actinin (α-SA), vimentin, and 4′,6′-diamidino-2-phenylindole (DAPI; B) of neonatal rat ventricular myocyte (NRVM) monolayers with and without added fibroblasts (FBs). k, Thousand. C, top: strength-duration relationships for NRVM monolayer cultures with added fibroblasts (A and B, middle and right) vs. control (ctrl; A and B, left), n = 6 monolayers per group. C, bottom: data replotted on a log-log scale. D: strength-duration relationships for NRVM spheroids with and without added fibroblasts. n = 6 Spheroids per group (as shown in inset). ns, Not significant. E: immunostaining for α-SA, vimentin, and DAPI in spheroid NRVM cultures at day 2 postplating of spheroids on a flat substrate (day 6 from start of aggregation). All electrical stimulation was performed using a voltage source with a biphasic, bimodal waveform at 2-Hz stimulation rate. Scale bars are 200 μm. Mean and SE are plotted. *P < 0.05.
We repeated this experiment using 3D NRVM spheroids with similar cellular compositions: 1) 1,000 NRVMs per spheroid, 2) 500 NRVMs plus 500 fibroblasts per spheroid, and 3) 1,000 NRVMs plus 1,000 fibroblasts per spheroid. The three spheroid groups did not significantly differ in their diameters after seeding, and we did not observe significant differences in the strength-duration relationships across the three groups (Fig. 5D). This may have been due to fibroblasts’ ability to migrate out of the nonmotile cardiomyocyte cluster, decreasing the effective fibroblast content difference between our groups at the time of threshold measurement (Fig. 5E).
To study the effect of fibroblasts in combination with anisotropy, we seeded NRVMs on abraded coverslips at the usual density (200,000 cells/cm2) mixed with the same number of fibroblasts (200,000 fibroblast/cm2; Fig. 6, A and B). When aligned perpendicular to the electrical field, the stimulation thresholds compared with the control group (NRVM only, parallel aligned) were significantly increased, with an effect size larger than that of anisotropy or fibroblasts independently (Figs. 4 and 5).
Fig. 6.
Cell alignment (anisotropy) and fibroblasts synergistically impact electrical excitability. A and B: differential interference contrast (DIC) imaging (A) and immunostaining for α-sarcomeric actinin (α-SA), vimentin, and 4′,6′-diamidino-2-phenylindole (DAPI; B) of neonatal rat ventricular myocyte (NRVM) monolayers on abraded substrate with and without added fibroblasts (FBs). k, Thousand. C, top: strength-duration relationships for NRVM monolayer cultures with added fibroblasts and oriented perpendicular to the electrical field vs. cultures without added fibroblasts and oriented parallel to the electrical field. C, bottom: data replotted on a log-log scale, n = 3–5 monolayers per group. Electrical stimulation was performed using a voltage source with a biphasic, bimodal waveform at 2-Hz stimulation rate. Mean and SE are plotted. *P < 0.05.
We hypothesized that the strength-duration relationship shifts in the presence of fibroblasts and/or anisotropic alignment may be at least in part due to changes in cell shape and sarcomere structure or cell-to-cell junctions.
We measured cell area, perimeter, and the ratio of major to minor axis of the best-fit ellipse (Fig. 7). We found that both added fibroblast configurations resulted in larger cells but did not increase elongation. However, added fibroblasts in the presence of an abraded substrate (anisotropic alignment) led to larger and elongated cells (Fig. 7, A–C). We also investigated sarcomere structure in the presence of fibroblasts and/or anisotropy. We found that replacing half of the NRVMs with fibroblasts (100,000 cardiomyocytes/cm2 plus 100,000 fibroblasts/cm2) led to more pronounced sarcomeres and α-sarcomeric actinin on immunofluorescence but not adding fibroblasts to NRVMs (200,000 cardiomyocytes/cm2 plus 200,000 fibroblasts/cm2). Similarly, an abraded substrate (anisotropic alignment) led to higher α-sarcomeric actinin and clearer sarcomeres, which was abrogated with the addition of fibroblasts (200,000 cardiomyocytes/cm2 plus 200,000 fibroblasts/cm2; Fig. 7, D–F).
Fig. 7.
Neonatal rat ventricular myocyte (NRVM) hypertrophy in the presence of fibroblasts (FB) and on abraded substrates. A: cardiomyocyte area per cell. B: cell perimeter. C: ratio of major to minor axis length of a fitted ellipse normalized to control. A/B, long/short axis. D: example line plots of α-sarcomeric actinin intensity to demonstrate sarcomere structure. E and F: immunostaining for (E) and mean fluorescence intensity per cell in (F) the α-sarcomeric actinin channel. Number of cells quantified: n = 122–124 cells per group. Scale bars are 15 μm. Mean and SE are plotted. ANOVA with Tukey multiple-comparison correction was used. *P < 0.05. k, Thousand.
Although the overall area of connexin-43 was mostly unchanged across groups compared with the control (Fig. 8, A and B), we observed differences in connexin-43 immunofluorescence spot size and number. When fibroblasts were added (200,000 cardiomyocytes/cm2 plus 200,000 fibroblasts/cm2), connexin-43 immunofluorescence spots were more numerous and smaller in size. A similar trend was observed when fibroblasts were added to anisotropic cultures. However, the opposite trend was observed in anisotropic cultures without added fibroblasts (Fig. 8, A and C). We did not observe any differences in connexin-43 localization to different parts of the cell membrane across groups. As a control, we performed the same measurements with N-cadherin, but we observed no significant differences across groups (Fig. 8D). These data indicate that connexin-43 distribution is impacted by fibroblast content and anisotropic alignment.
Fig. 8.
Added fibroblasts lead to connexin-43 cluster fragmentation but do not impact N-cadherin. A: immunostaining for α-sarcomeric actinin (αSA), connexin-43 (Cx43), and 4′,6′-diamidino-2-phenylindole (DAPI) of neonatal rat ventricular myocyte (NRVM) monolayers on smooth (top) or abraded (bottom) substrate with and without added fibroblasts. B and C: quantification of connexin-43 total fluorescence area (B) and spot number and size (C) for the different images in A. D: immunostaining for αSA, N-cadherin (Ncad), and DAPI of NRVM monolayers on smooth (top) or abraded (bottom) substrate with and without added fibroblasts. Number of areas quantified: n = 3–4 per group. Error bars are 50 μm. Mean and SE are plotted. ANOVA with Tukey multiple-comparison correction was used. *P < 0.05. FB, fibroblasts; k, thousand; Mrg, merged.
TBX18 reprogramming raises strength-duration thresholds of induced pacemaker cells.
The mammalian sinoatrial node is only a few square millimeters in area with ~5,000 pacemaker cells that initiate the spontaneous action potentials (4). At the core of the sinoatrial node, cells are particularly disorganized and the cell-to-cell electrical coupling is weaker compared with the surrounding atrial myocardium (3). We speculate that these properties would prevent the sinoatrial node from being driven by external electrical stimuli such as atrial tachyarrhythmias so that the sinus rhythm could resume with minimal recovery time on self-termination of the tachycardia. We (16–18) have previously shown that re-expression of an embryonic transcription factor, TBX18, directly reprograms ventricular cardiomyocytes into induced pacemaker cells that recapitulate electrophysiological and morphological hallmarks of native pacemaker cells. We hypothesized that 3D spheroids consisting of TBX18-induced pacemaker cells would require higher electrical stimulation thresholds compared with control spheroids, reflecting the disorganized cellular orientation and weak electrical coupling of the native sinoatrial node. NRVMs were transduced in suspension with adenoviral vectors expressing either human TBX18 or GFP, then aggregated into spheroids over 4 days, and moved to a fibronectin-coated, flat substrate for 48 h before stimulation (Fig. 9A). The strength of the applied electrical field was increased for each stimulation duration until the spheroid in the field of view started contracting synchronously with the 1-Hz pacing frequency. We selected GFP and TBX18 spheroids with similar diameters to avoid confounding by discrepancies in size, as described below. The threshold electrical field strength at which the spheroids were captured (contracted synchronously) at 1-Hz pacing frequency was recorded against the stimulation duration (Fig. 9B). The threshold electrical field strength required for pacing was higher for TBX18 spheroids across the measured stimulation durations (2–20 ms). We measured the dimensions of the spheroids at day 4 of aggregation and 2 days postplating (Fig. 9C). Interestingly, the average diameter of TBX18-induced pacemaker spheroids was larger than control spheroids, which would positively impact excitability (Fig. 3). Since the volume of TBX18-reprogrammed pacemaker cells is smaller than of ventricular cardiomyocytes (18), which is a shared phenotype with native pacemaker cells (4), the larger diameter of TBX18-induced pacemaker spheroids is likely due to non-cell-autonomous effects of the induced pacemaker cells.
Fig. 9.
TBX18 reprogramming negatively impacts electrical excitability. A: timeline of experiment. B: strength-duration relationships for diameter-matched TBX18 (TBX) vs. green fluorescent protein (GFP) control neonatal rat ventricular myocyte spheroids under electrical field stimulation at 1-Hz frequency, n = 6 per group. Inset is diameters of spheroids for which we recorded strength-duration curves. C: comparison of the diameter of 3-dimensional spheroids composed of TBX18 vs. GFP control cardiomyocytes at day 4 of aggregation, n = 155 and 183, respectively, or at day 2 postplating of spheroids on a flat substrate (day 6 overall), n = 34 and 32, respectively. All electrical stimulation was performed a 1 Hz using a voltage source with a biphasic, bimodal waveform. Mean and SE are plotted. Two-way ANOVA with Tukey multiple-comparison correction was used. *P < 0.05. ns, Not significant.
DISCUSSION
Our data underline the importance of inherent properties of cardiomyocytes and engineered cardiac tissue constructs on their electrical excitability by external electrical fields. The quasihyperbolic strength-duration relationship for single cells, cell aggregates, and cardiac tissue is well-characterized (39) and provides a macroscopic view of the electrical excitability at a range of stimulation durations and strengths. It is common to compare the stimulation threshold of single cardiomyocytes or engineered cardiac tissues at a single chosen stimulation duration or frequency (6, 36). Our data indicate that geometric dimension or cellular alignment has a similar effect size across the strength-duration curve (Figs. 3 and 4), whereas pacing frequency and fibroblasts impacted the strength-duration curves only toward longer stimulation durations (Figs. 2 and 5). Therefore, it is important to scan the whole range of stimulation strengths and durations when determining the appropriate stimulation parameters for a given culture or tissue to avoid confounding factors related to the ability to capture. It is equally important to study the range of excitation parameters when determining excitation thresholds after experimental manipulation.
Pacing at higher frequencies led to right-shifted strength-duration curves in both monolayers and 3D spheroids of NRVMs. This could be due to a voltage pulse attempting to depolarize the membrane before it is fully recovered from depolarization from a previous voltage pulse. At higher pacing frequencies, the voltage pulses would arrive at a more depolarized state of the relative refractory period. Similarly, TBX18-induced pacemaker cells have intrinsic automaticity, which results in a higher probability of a stimulation signal to fall within a refractory period. In addition, TBX18-induced pacemaker cells, akin to native pacemaker cells, exhibit a more depolarized maximum diastolic potential (18), which further contributes to the higher external stimulation thresholds.
Cardiac fibroblasts are known to modulate electrical excitability and conduction in myocardial tissue via gap-junctional coupling among fibroblasts and between fibroblasts and cardiomyocytes (20, 26, 38). Addition of fibroblasts to cardiomyocytes of the usual density negatively impacted excitability. Interestingly, however, replacing 50% of cardiomyocytes with the same number of fibroblasts did not impact the strength-duration relationship of 100% cardiomyocytes. This is in line with direct electrical coupling between fibroblasts and cardiomyocytes, with or without concurrent electrical field excitation of the fibroblasts. On the other hand, any negative effect of replacing the fibroblasts could have been abrogated by a more easily excitable, less dense monolayer. Similarly, the greater excitability of anisotropic NRVM monolayers oriented parallel to the electrical field could be due to the previously demonstrated cell-autonomous effect of orientation (39) or the superior coupling and resulting increasing conduction velocity of uniformly oriented cardiomyocytes (19). It is interesting to note that the reverse relationship has been observed in studies of isolated whole tissue (8).
The cardiac tissue parameters that affect excitability exhibited both synergistic and antagonistic effects when combined. For example, adding fibroblasts led to a more pronounced effect on anisotropy-patterned monolayers (Fig. 4). On the other hand, the larger dimension of TBX18-induced pacemaker spheroids was not sufficient to abrogate the high stimulation threshold of TBX18-induced pacemaker spheroids presumably benefiting due to their disorganized architecture and weak cell-to-cell electrical coupling.
Although the Lapicque-Hill model was theoretically derived for a transmembrane current source, we employed a voltage source in our experiments. However, this model has been successfully used in the past with the assumption that once an infinitesimally small area of the cellular membrane is sufficiently charged to activate depolarizing sodium channels, the voltage source can be considered equivalent to a current source (39). In most of our experiments, stimulation duration was measured as a function of field strength. This led to an earlier detection of an effect in the low-duration part of the spectrum for strength-duration curves with an apparently homogeneous effect size (e.g., Fig. 4), where the variance is smaller. However, some of the measurements were made while holding the stimulation duration constant and varying the field strength. It is unlikely that this would impact power when the effect size is large (Fig. 2, A and B, and Fig. 9A).
We posit that these properties are reflective of the structure of the endogenous pacemaking tissue, the sinoatrial node, and contribute to its protection from extranodal electrical potentials in the heart either physiological or pathological in nature (22). These protective properties should be considered in engineered biological pacemakers that aim to pace and entrain the heart (7), limiting negative impact from an electrically active, dimensionally overwhelming myocardium. An ideal, electrically insulated cardiac tissue structure should be small in size, containing nonmyocytes such as fibroblasts and isotropic in the core. These elements would intrinsically protect the cardiac tissue from arrhythmic events especially at higher oscillation frequencies. The sinoatrial node or a biological pacemaker would require another design element, in which the cells that populate the exit pathways (10, 22) would be oriented parallel to the direction of the electrical field.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant R01-HL-111646-01A1 and National Science Foundation Grant 1609831 to H. C. Cho.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N.S., N.F., and H.C.C. conceived and designed research; M.N.S. and N.F. performed experiments; M.N.S. analyzed data; M.N.S. and H.C.C. interpreted results of experiments; M.N.S. and H.C.C. prepared figures; M.N.S. and H.C.C. drafted manuscript; M.N.S. and H.C.C. edited and revised manuscript; M.N.S., N.F., and H.C.C. approved final version of manuscript.
REFERENCES
- 1.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol 293: H1883–H1891, 2007. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of cell generation and turnover in the human heart. Cell 161: 1566–1575, 2015. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Bleeker WK, Mackaay AJ, Masson-Pévet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res 46: 11–22, 1980. doi: 10.1161/01.RES.46.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 47: 658–687, 2000. doi: 10.1016/S0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 5.Buruljanowa I, Wassilew G, Radanov S. [Age-related structural changes in the stimuli-conducting system of the human heart]. Zentralbl Allg Pathol 133: 433–438, 1987. [PubMed] [Google Scholar]
- 6.Cashman TJ, Josowitz R, Johnson BV, Gelb BD, Costa KD. Human engineered cardiac tissues created using induced pluripotent stem cells reveal functional characteristics of BRAF-mediated hypertrophic cardiomyopathy. PLoS One 11: e0146697, 2016. doi: 10.1371/journal.pone.0146697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HC, Marbán E. Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res 106: 674–685, 2010. doi: 10.1161/CIRCRESAHA.109.212936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado C, Steinhaus B, Delmar M, Chialvo DR, Jalife J. Directional differences in excitability and margin of safety for propagation in sheep ventricular epicardial muscle. Circ Res 67: 97–110, 1990. doi: 10.1161/01.RES.67.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Eng G, Lee BW, Protas L, Gagliardi M, Brown K, Kass RS, Keller G, Robinson RB, Vunjak-Novakovic G. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun 7: 10312, 2016. doi: 10.1038/ncomms10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res 104: 915–923, 2009. doi: 10.1161/CIRCRESAHA.108.193193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillis AM, Fast VG, Rohr S, Kléber AG. Spatial changes in transmembrane potential during extracellular electrical shocks in cultured monolayers of neonatal rat ventricular myocytes. Circ Res 79: 676–690, 1996. doi: 10.1161/01.RES.79.4.676. [DOI] [PubMed] [Google Scholar]
- 12.Gluck JM, Herren AW, Yechikov S, Kao HK, Khan A, Phinney BS, Chiamvimonvat N, Chan JW, Lieu DK. Biochemical and biomechanical properties of the pacemaking sinoatrial node extracellular matrix are distinct from contractile left ventricular matrix. PLoS One 12: e0185125, 2017. doi: 10.1371/journal.pone.0185125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol 7: 204–214, 2015. doi: 10.4330/wjc.v7.i4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res 107: 35–44, 2010. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 15.Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Börnchen C, Müller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 74: 151–161, 2014. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Hu YF, Dawkins JF, Cho HC, Marbán E, Cingolani E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med 6: 245ra94, 2014. doi: 10.1126/scitranslmed.3008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor N, Galang G, Marbán E, Cho HC. Transcriptional suppression of connexin43 by TBX18 undermines cell-cell electrical coupling in postnatal cardiomyocytes. J Biol Chem 286: 14073–14079, 2011. doi: 10.1074/jbc.M110.185298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol 31: 54–62, 2013. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci USA 107: 565–570, 2010. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kofron CM, Kim TY, King ME, Xie A, Feng F, Park E, Qu Z, Choi BR, Mende U. Gq-activated fibroblasts induce cardiomyocyte action potential prolongation and automaticity in a three-dimensional microtissue environment. Am J Physiol Heart Circ Physiol 313: H810–H827, 2017. doi: 10.1152/ajpheart.00181.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci USA 106: 10097–10102, 2009. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Hansen BJ, Csepe TA, Zhao J, Ignozzi AJ, Sul LV, Zakharkin SO, Kalyanasundaram A, Davis JP, Biesiadecki BJ, Kilic A, Janssen PM, Mohler PJ, Weiss R, Hummel JD, Fedorov VV. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci Transl Med 9: eaam5607, 2017. doi: 10.1126/scitranslmed.aam5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ZQ. Scale space approach to directional analysis of images. Appl Opt 30: 1369–1373, 1991. doi: 10.1364/AO.30.001369. [DOI] [PubMed] [Google Scholar]
- 24.Lokuta A, Kirby MS, Gaa ST, Lederer WJ, Rogers TB. On establishing primary cultures of neonatal rat ventricular myocytes for analysis over long periods. J Cardiovasc Electrophysiol 5: 50–62, 1994. doi: 10.1111/j.1540-8167.1994.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 4: 2307, 2013. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney VM, Mezzano V, Morley GE. A review of the literature on cardiac electrical activity between fibroblasts and myocytes. Prog Biophys Mol Biol 120: 128–133, 2016. doi: 10.1016/j.pbiomolbio.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 6: e12–e23, 2012. doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxwell JT, Wagner MB, Davis ME. Electrically induced calcium handling in cardiac progenitor cells. Stem Cells Int 2016: 8917380, 2016. doi: 10.1155/2016/8917380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol 479: 265–279, 1994. doi: 10.1113/jphysiol.1994.sp020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opthof T, de Jonge B, Jongsma HJ, Bouman LN. Functional morphology of the mammalian sinuatrial node. Eur Heart J 8: 1249–1259, 1987. doi: 10.1093/oxfordjournals.eurheartj.a062200. [DOI] [PubMed] [Google Scholar]
- 31.Opthof T, de Jonge B, Masson-Pevet M, Jongsma HJ, Bouman LN. Functional and morphological organization of the cat sinoatrial node. J Mol Cell Cardiol 18: 1015–1031, 1986. doi: 10.1016/S0022-2828(86)80290-5. [DOI] [PubMed] [Google Scholar]
- 32.Park J, Ryu J, Choi SK, Seo E, Cha JM, Ryu S, Kim J, Kim B, Lee SH. Real-time measurement of the contractile forces of self-organized cardiomyocytes on hybrid biopolymer microcantilevers. Anal Chem 77: 6571–6580, 2005. doi: 10.1021/ac0507800. [DOI] [PubMed] [Google Scholar]
- 33.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res 118: 400–409, 2016. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plonsey R, Barr RC. Bioelectricity: A Quantitative Approach. New York: Springer, 2007. [Google Scholar]
- 35.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA 101: 18129–18134, 2004. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med 5: e115–e125, 2011. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tandon N, Taubman A, Cimetta E, Saccenti L, Vunjak-Novakovic G. Portable bioreactor for perfusion and electrical stimulation of engineered cardiac tissue. Conf Proc IEEE Eng Med Biol Soc 2013: 6219–6223, 2013. doi: 10.1109/EMBC.2013.6610974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson SA, Copeland CR, Reich DH, Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation 123: 2083–2093, 2011. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tung L, Sliz N, Mulligan MR. Influence of electrical axis of stimulation on excitation of cardiac muscle cells. Circ Res 69: 722–730, 1991. doi: 10.1161/01.RES.69.3.722. [DOI] [PubMed] [Google Scholar]
- 40.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell 8: 252–261, 2011. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng 13: 245–267, 2011. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA 98: 11295–11300, 2001. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 14: 869–882, 2014. doi: 10.1039/C3LC51123E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zack GW, Rogers WE, Latt SA. Automatic measurement of sister chromatid exchange frequency. J Histochem Cytochem 25: 741–753, 1977. doi: 10.1177/25.7.70454. [DOI] [PubMed] [Google Scholar]