Abstract
An epidemic of extreme respiratory deterrence, pneumonia and shortness of breath, the SARS-CoV-2 viral infection began in Wuhan, Hubei Province, China in December 2019, and rapidly spread across China and beyond, with human to human transmission. On February 12, 2020, World Health Organization officially named the new coronavirus disease as coronavirus disease 19 (COVID-19). Most COVID-19 patients were diagnosed with pneumonia and many were treated using Chinese medicines and other secondary therapies. As of April 22, 2020, the total figure of infected patients has crossed 2.6 million people worldwide with over 180,000 deaths and 700,000 patients that have recovered. Preliminary reports suggest that certain drugs, such as chloroquine and antiviral nucleotide analogues such as remdesivir, which inhibit viral replication, can target the new coronavirus, although their usefulness in the clinic is still under debate. An expert US committee developed the US NIH guidelines for COVID-19 treatment, which was just released and will be regularly updated. This manuscript reviews the epidemiology, etiology, mortality, COVID-19 clinical symptoms, and potential therapeutic drugs, while highlighting the seriousness and damage-induced by SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, MERS, Morbidity, Chloroquine, Rendesivir.
SUMMARY
1. Introduction
2. Etiology
3. Epidemiology and recent figures
4. Incubation period for COVID-19
5. COVID-19 mortality rate
6. COVID-19 symptoms
7. Treatment options for SARS-CoV-2 infection
8. Conclusion
1. Introduction
In December 2019, Wuhan in the Hubei province of China announced an active epidemic of pneumonia associated with a novel coronavirus, identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1,2. Over the next few weeks, infections spread throughout China and elsewhere in the world3,4. Chinese media, clinical and science institutions have reacted swiftly, such that the latest virus is identified and the viral genome sequence is shared quickly worldwide2. On January 30, 2020 the World Health Organization (WHO) announced an outbreak of Public Health Emergency of International Concern (PHEIC)5.
On February 12, 2020, WHO named the disease coronavirus disease 2019 (COVID-19) caused by the novel coronavirus5. A consortium of foreign experts collaborating with Chinese colleagues of various specializations has sought to hold on this outbreak6. The first infected cases were associated with a food market in Wuhan1. Coronaviruses have enveloped, positive sense single-stranded RNA genome, infecting humans and a wide range of animals. They were for the first time characterized and cultured by Tyrell and Bynoe in 1966, from patients with flu and common cold7. They were known as coronaviruses, based on their morphology as spherical virions with a central shell and surface projections identical to a solar corona (Latin: corona means crown)8. There are four separate subfamilies, the alpha, beta, gamma, and delta coronaviruses. Alpha and beta coronaviruses appear to come from mammals, especially from bats, whereas gamma and delta coronaviruses derive from pigs and birds9. The genome length ranges from 26kb to 32kb. Among the seven subtypes of coronavirus that may have the ability to infect humans the beta-coronavirus is considered the most dangerous; this is the one that causes significant morbidity and mortality in humans. SARS-CoV-2 virus belongs to the genus beta-coronavirus9,10.
The four main operational genes encodes for spike protein (S), nucleocapsid protein (N), membrane glycoprotein (M) and a small membrane protein (SM), with an additional membrane glycoprotein (HE) occurring in the HCoV-OC43 and HKU1 beta-coronaviruses9. Figure 1 presents the SARS-CoV-2 structure. The virus sequence is 96% similar with a bat-related coronavirus throughout the entire genome10. According to WHO, no specific medicine or antiviral was found to treat or prevent novel coronavirus until now11. It has been noted that Chinese medication (CM), including oral administration of protective herbal formulae, the usage of CM sachets and herbal medicine fumigations, etc., is typically used to deter and manage novice coronavirus in China when the epidemic begins11,12. Chinese herbal and traditional medicines were also used in 2003 to combat SARS, which was the most severe infectious disease epidemic in China before COVID-1911,13.
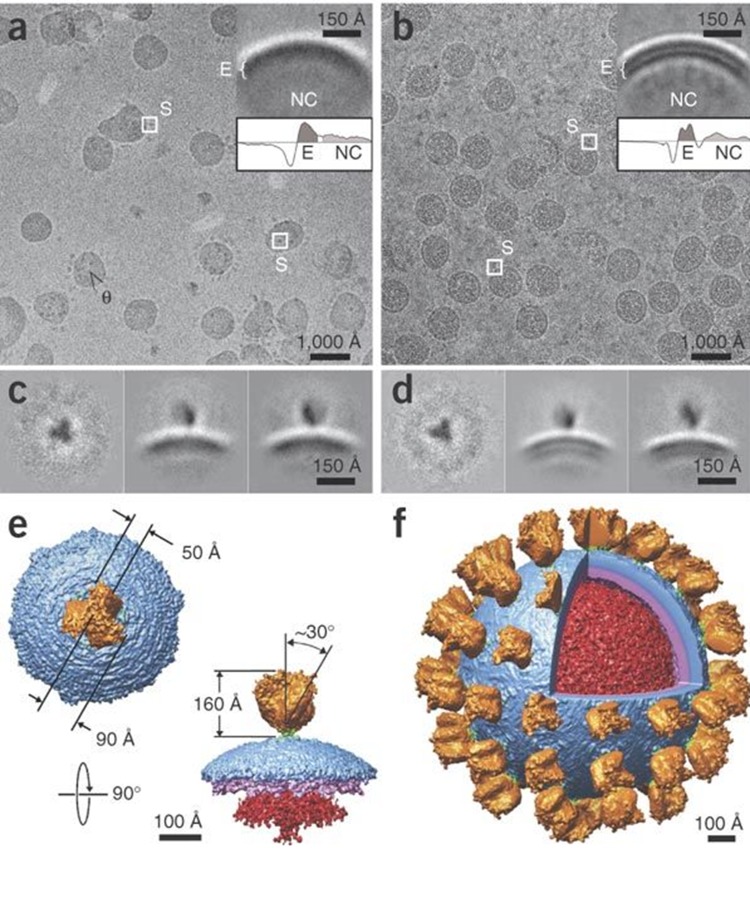
Figure 1. Figure 1: SARS-CoV structure.
(a, b) Microscopic images at 5 micron indicate NC for nucleocapsid, S for spikes and E for envelope (c, d) Two dimensional class averages data from a and b (e) Spike attachment with outer capsid, (f) SARS-CoV with spikes red nucleocapsid and yellow spikes21; This figure was reproduced from Beniac et al.21, with permission; “This article is made available via the PMC Open Access Subset for unrestricted research re-use and secondary analysis in any form or by any means with acknowledgement of the original source”.
2. Etiology
Preliminary studies show that this virus shares very high genome resemblance with 2002 bat-derived SARS coronaviruses14. Therefore, this virus was initially named 2019 novel coronavirus (2019-nCoV). Coronavirus is a RNA based enveloped entity named for its 9-12 nm long surface spikes for solar corona presence15. The coronaviral genome enclosed in the envelope bears four main structural proteins, including the spike protein (S), which binds to the Angiotensin Converting Enzyme 2 (ACE2) receptor and then mediates a fusion between the envelope and the cell membranes in the host cell to enable the virus reach to host cell16,17. On February 11, 2020 focused on phylogeny, taxonomy, and defining procedure, by the International Committee for Taxonomy of viruses a Coronavirus Study Group officially classified it as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)18. Shortly, WHO named the disease caused by this virus coronavirus disease 2019 (COVID-19)19. According to the current evidence, COVID-19 could initially be transmitted by bats and could be transferred to humans by means of pangolins20, or other wildlife sold on the maritime market in Huanan, but later propagated by human to human transmission14 , although other possibilities of transmission are not yet excluded.
3. Epidemiology and recent figures
The first reported patients happened in December 201915. At the beginning, morbidity was very low. However, in January 2020 it hit a turning point. A significant rise in infected patients occurred in cities outside of Hubei Province during the second half of this month, due to the movement of people before the Chinese lunar New Year22. After an exponential rise until January 23, 2020 the infection traveled through countries and draws significant global interest. The occurrence of human-to-human infection was reported in the clusters of affected family members and medical staff23, by contact, droplets and fomite10,24. So far, there is no proof of intrauterine infection documented25.
WHO reported over 10,000 cases of COVID-19 infections across China in late January 202015. On 13 February 2020, 13332 new cases were registered from Hubei for the first time. National Health Commission of China in its diagnosis and treatment program (trial fifth version) recommended chest CT diagnosis for clinical confirmation of infection in suspected cases15. By February 19, 2020, overall reported cases were 74,280 in China and 924 in other 24 countries, with 2009 worldwide deaths23. On April 17, 2020, a total of 2,200,358 infected cases were reported all over the world with 1,494,415 active cases and 705,907 closed cases. Of the total active cases, 1,437,938 (96%) were foundto have mild symptoms, and 56,477 (4%) were tagged as serious or critical cases. Among closed cases, 558,168 (79%) are recovered to their normal conditions and discharged, while 147,787 (21%) deaths occurred (Table 1 and Figure 2)26.
Table 1. Statistics by WHO; accessed on April 17, 2020.
| Active cases | Closed cases |
|---|---|
| 1,494,415 currently infected patients | 705,907 cases which had an outcome |
| 1,437, 938 (96%) mild conditions | 558,168 (79%) recovered cases |
| 56,477 (4%) critical cases | 147,787 (21%) deaths occurred |
Figure 2. Recorded total infected people, total deaths and total recovered cases in top 10 prevalent countries.
Adapted from26.
4. Incubation period for COVID-19 patients
The virus is believed to have incubation periods ranging between approximately 2-14 days (time from infection to symptoms) on the basis of the following sources:
1. Incubation time for COVID-19 between 2 and 10 days has been recorded by the WHO27.
2. The Chinese National Health Commission initially expected a 10 to 14 days incubation period28.
3. The Centers for Disease Control and Prevention (CDC) from United States of America assumes the incubation period of 2 to 14 days for COVID-1929.
4. Doctors and health-care practitioners, of leading Chinese group DXY.cn predicts an incubation period of "3 to 7 days, and up to 14 days."
In a report released on February 9, 2020, the incubation time was observed as long as 24 days (range from 0-24 days; average of 3 days)30. The WHO stated on February 10, 2020, at its press conference, that a very long duration of incubation can represent double exposure, 24 days was a specified point to be regarded in the sense of the study's key results31. Nevertheless, more recently, in a JAMA report released on February 21, 2020, a 19-day incubation period in infected patient has been identified30, Hubei Province registered another case with an incubation time of 27 days, on February 22, 202032. The incubation time period has averaged 5.2 days. However, it greatly differs between the patients, according to a Chinese article reported by New England Journal of Medicine on January 30, 202028. An analysis sponsored by the Holland Ministry of Health and released by Eurosurveillance examined data for 88 identified travelers to and from Wuhan, which were identified as COVID-19 infected patients between January 20 and 28, 2020. It was calculated that the mean incubation period was 6.4 days. The incubation duration varied from 2.1 to 11.1 days. The 11.1-day maximum limit may be known as conservative28. A comparison with other viruses can be found in Table 2.
Table 2. Comparison with other viruses.
Adapted from33
| Serial no. | Virus | Incubation period |
|---|---|---|
| 1 | Novel Coronavirus COVID-19 | 2-14 or 0-24 |
| 2 | SARS | 2-7 days, as long as 10 days |
| 3 | MERS | 5 days, range 2-14 |
| 4 | Swine Flu | 1-4 days, as long as 7 days |
| 5 | Seasonal Flu | 2 days, 1-4 range |
5. COVID-19 Mortality rate
WHO Director, general dr. Tedros Adhanom Ghebreyesus, said in his inaugural remarks at the COVID-19 press briefing on March 3, 2020, that there is a 3.4% mortality rate globally. In comparison, seasonal influenza normally kills fewer than 1% of affected individuals34. Initially, at a press conference on Wednesday, January 29, 2020, and again on February 10, 2020, the WHO listed 2% as a mortality rate estimation. However, on January 29, 2020, WHO specified that this was a very early and provisional estimate, that might have changed28. On February 20, out of 55,924 laboratory reported cases, were 2,114 reported deaths, with a case fatality rate of 3.8%35. National Health Commission in China released on February 4, 2020, the following figures36.
1. Mortality rate was found 4% in Wuhan.
2. Fatality rate in other provinces was 0.16%
3. Mortality rate was 3.1% in the Hubei province
4. 97% of the China total death by COVID-19 were in the Hubei province.
5. Mortality rate nationwide was 2.1%.
Elderly patients with specific illnesses are considered at higher risk, regardless of whether they have a coronavirus or not36. Patients with heart disease, high blood pressure or diabetes treated with ACE2-increasing drugs may have a high risk for serious COVID-19 infection37. A mortality rate comparison with other viruses is shown in Table 3, while the COVID-19 fatality rate by age, sex and with comorbidity is shown in Table 4, Table 5 and Table 6, respectively.
Table 3. Mortality rate comparison with other viruses.
Adapted from36.
| Serial no. | Virus | Death Rate |
|---|---|---|
| 1 | SARS-CoV-2 | 2% |
| 2 | SARS | 9.6% |
| 3 | MERS | 34% |
| 4 | Swine Flu | 0.02% |
Table 4. COVID-19 fatality rate by age.
Adapted from38.
| Serial.no. | Age | Death rate confirmed cases | Death rate all cases |
|---|---|---|---|
| 1 | 80+ years old | 21.9% | 14.8% |
| 2 | 70-79 years old | - | 8.0% |
| 3 | 60-69 years old | - | 3.6% |
| 4 | 50-59 years old | - | 1.3% |
| 5 | 40-49 years old | - | 0.4% |
| 6 | 30-39 years old | - | 0.2% |
| 7 | 20-29 years old | - | 0.2% |
| 8 | 10-19 years old | - | 0.2% |
| 9 | 0-9 years old | - | No fatalities |
Table 5. COVID-19 fatality rate by sex.
Adopted from38.
| Serial.no. | Sex | Death rate confirmed cases | Death rate all cases |
|---|---|---|---|
| 1 | Male | 4.7% | 2.8% |
| 2 | Female | 2.8% | 1.7% |
Table 6. COVID-19 fatality rate with comorbidity.
Adopted from38.
| Serial.no. | Pre-existing conditions | Death rate confirmed cases | Death rate all cases |
|---|---|---|---|
| 1 | Cardiovascular disease | 13.2% | 10.5% |
| 2 | Diabetes | 9.2% | 7.3% |
| 3 | Hypertension | 8.4% | 6.0% |
| 4 | Chronic respiratory disease | 8.0% | 6.3% |
| 5 | Cancer | 7.6% | 5.6% |
| 6 | No pre-existing conditions | - | 0.9% |
6. COVID-19 symptoms
Typically, COVID-19 triggers flu-like symptoms, such as fever and cough. These symptoms can develop into pneumonia, chest strain, chest pain, and difficulty to breath in elderly people and other patients who have other chronic health conditions. It looks like it begins with a fever and leads to dry coughing. One week after infection the conditions become harsh and lead to breath shortening with approximately 20% of patients requiring hospital treatment and medication29. The COVID-19 illness appears to seldom induce runny nose, sneezing or sore throat (only around 5% of patients have such symptoms). Painful throat sneezing, and stuffy nose are typically symptoms of seasonal flu or cold39,40. A few patients may develop pain or hemoptysis and many may also be completely asymptomatic24,41. Older people with comorbidity and serious alveolar injury are more prone to experience respiratory failure42. The onset of disease will demonstrate rapid progression to organ dysfunction (e.g., acute kidney injury, shock, acute cardiac injury, acute respiratory distress syndrome ARDS) and death in severe cases41. Sometime patients might develop lower or normal white blood cell count, thrombocytopenia or lymphopenia, with increased C-reactive protein level and extended activated thromboplastin time24,41,42. 80% of infected cases are found to be mild, with normal fever and flu and the patients can recover at home. In short, a patient having upper respiratory tract symptoms and fever with leukopenia or lymphopenia should be suspected (Table 7, Table 8, Table 9).
Table 7. COVID-19 common symptoms.
Adapted from41.
| Serial.no | Symptoms | % |
|---|---|---|
| 1 | Fever | 98.6% |
| 2 | Fatigue | 69.6% |
| 3 | Dry Cough | 59.4% |
Table 8. COVID-19 symptoms from study of Huang et al.1.
Adapted from1.
| Serial no. | Common symptoms | % |
|---|---|---|
| 1 | Fever | 98% |
| 2 | Cough | 76% |
| 3 | Muscle pain or Fatigue | 44% |
| Serial no. | Less common symptoms | % |
| 1 | Sputum production | 28% |
| 2 | Headache | 8% |
| 3 | Hemoptysis | 5% |
| 4 | Diarrhea | 3% |
Table 9. COVID-19 symptoms from study of Chen et al.42.
Reproduced from42 with permission.
| Serial no. | Signs and Symptoms | % |
|---|---|---|
| 1 | Fever | 83% |
| 2 | Cough | 82% |
| 3 | Shortness of breath | 31% |
| 4 | Muscle ache | 11% |
| 5 | Confusion | 9% |
| 6 | Headache | 8% |
| 7 | Sore throat | 5% |
| 8 | Runny nose | 4% |
| 9 | Chest pain | 2% |
| 10 | Diarrhea | 2% |
| 11 | Vomiting | 1% |
| 12 | More than one symptom | 90% |
| 13 | Fever, cough and shortness of breath | 15% |
7. Treatment options for SARS-CoV-2 infection
To date, there are no proven effective antiviral therapies for the infection caused by SARS-CoV-2. Treatment in several hospitals includes the use of prophylactic antibiotics to prevent secondary infection. Initial reports have shown that some antivirals with antibiotics combination can be given orally with benefits1. On January 25, 2020 a joint research team from the Shanghai Institute of Materia Medica and Shanghai Tech University conducted a silicon drug screening and enzyme activity testing and reported 30 agents with significant antiviral activity against SARS-CoV-243. The following are some drugs which have been used against COVID-19 in-vitro. Their use in the clinic is still under debate at this time.
7.1. Chloroquine as a general antiviral agent
Chloroquine, a commonly used anti-malarial and autoimmune medication, has recently been identified as a possible broad spectrum antiviral drug44,45. In vitro, chloroquine is reported to be a variable bioactive agent that has antiviral activity against RNA viruses, such as rabies virus46 poliovirus47, HIV48,49 and hepatitis C virus50.
Chloroquine is known to prevent infection of the cells by the virus through increasing the endosomal pH needed for virus for cell fusion, and interfering with the glycosylation of SARS-CoV cell receptors51.
7.1.1 Specific antiviral activity of Chloroquine against COVID-19
The China National Center for Biotechnology Development recently reported that chloroquine is one of the three drugs with a promising profile against the current SARS-CoV-2. Chloroquine remodeling was explored in hospitals in Beijing, in central China’s Hunan Province and South China’s Guangdong Province52. The drug that could potentially prevent SARS-COV-2 infection at low molecular concentrations is chloroquine, with a half-maximal effective concentration (EC50) of 1.13 μM and a half-cytotoxic concentration (CC50) greater than 100 μm53. However, in treating malaria, there are minor risks for adverse events, such as macular retinopathy and cardiomyopathy, whichare side effects caused by long term use of chloroquine54-56. Evidently, chloroquine could be used for the treatment of COVID-19 because of its effectiveness and safety for long term use57. A narrative letter by Chinese authors reported that chloroquine phosphate used in many clinical trials has marked efficacy against COVID-19. After oral administration, chloroquine is distributed widely across the body, including the lung. The EC90 value in Vero E6 cells was 6.90 μM, that can be clinically attainable in patients with rheumatoid arthritis who received 500 mg administration, which is demonstrated in their plasma53. Hydroxychloroquine, which is a derivative of chloroquine, may have lower adverse effects than chloroquine58.
7.1.2 Ethical issues regarding the use of Chloroquine against the COVID-19
Administration of chloroquine against COVID-19 is experimental. Therefore, ethical trial approval is necessary, and ethically justifiable as the best treatment available (i.e. off-label). Additional information on chloroquine’s effects and usefulness for the treatment of COVID-19 patients will soon be published due to its experimental use in the emerging outbreak. Due to the high number of patients infected and the lack of approved drugs, timely release of this information may be critical. The WHO confirms that there is currently no evidence from randomized control trials to warn about specific COVID-19 drug treatments and that unlicensed therapies can only be performed in the sense of ethically-approved clinical trials or under strict supervision of the Controlled Emergency Use of Unregistered Procedures System (MEURI). Meanwhile, the recommendations for “Clinical management of severe acute respiratory infection when novel coronavirus (SARS-COV-2) infection is suspected”59. The authors tend to agree with this viewpoint of WHO to view chloroquine as experimental. The outbreak is not the perfect environment for doing so, but even the use of chloroquine off-label can be followed by many concerns; the first being the health of patients, which should be followed by close supervision. The ethical approach to off-label drug use also varies from country to country, raising concerns regarding equity. Off-label drug use could cause severe medication shortages when needed for malaria, since chloroquine remains a crucial medication in the treatment of malaria in many parts of the world60.
7.1.3 Specific precautions before using chloroquine against COVID-19
For the use of chloroquine phosphate an expert consensus was published on February 20, 2020, by a multicenter collaboration group of the Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province. Preliminary measures recommended by the panel include blood tests to rule out the risk of anemia, thrombocytopenia or leukopenia, as well as serum electrolyte abnormalities and/or hepatic and renal function dysfunctions. It is recommended to rule out the development of QT interval prolongation or bradycardia and to perform patient interviews to find out visual and/or mental disturbance. Electrocardiography was routinely recommended and quinolones, macrolides, ondansetron as well as various antiarrhythmic, antidepressant and antipsychotic drugs which prolong the QT interval should be avoided as recommended by the panel61.
7.2. Remdesivir
Remedsivir is an analogue of adenosine, which incorporates into nascent viral RNA chains resulting in pre-mature termination. Recently, it was recognized as a potential antiviral medication against a broad variety of RNA viruses (including SARS / MERS-CoV) in cultivated cells, mice and non-human primates (NHP) models62-64. The EC90 value of remdesivir in Vero E6 cells against SARS-CoV-2 was 1.76 μM, indicating that its working concentration is likely to be attained in NHP models. The remdesivir has also effectively inhibited virus infection in a human cell line (Huh-7 cells of human liver cancer) that is susceptible to SARS-CoV-265. Animal experiments showed that remdesivir can significantly reduce the viral load of MERS-CoV in the lung tissue of infected mice, enhance lung function and minimize pathological damage to the lung tissue66.
7.3. Favipiravir
On February 15, 2020, in China, favipiravir was approved for the treatment of the 2019 novel influenza. Currently, this drug undergoes clinical trials to treat COVID-19. Favipiravir is an inhibitor of a new type of RNA-dependent polymerase RNA (RdRp)67. The value of Favipiravir EC50 in Vero E6 cells (cells used in the novel coronavirus study) was as high as 67 μM. Although more in vivo research is required to test this antiviral nucleoside, it has also been shown to be 100% effective in protecting mice from the Ebola virus68. Preliminary results from a total of 80 patients (including the experimental group and the control group) have shown that favipiravir has a more potent antiviral effect than lopinavir / ritonavir43.
7.4. Convalescent plasma and monoclonal antibodies
Convalescent plasma and monoclonal antibodies are suggested therapies for the treatment of COVID-19 patients. Convalescent plasma or immunoglobulins are used as a last resort to improve the survival rate of patients with SARS, whose condition continued to deteriorate after pulsed methylprednisolone therapy69. In addition, some studies found a shorter hospital stay and lower mortality in patients treated with convalescent plasma70. Further probability includes Leronlimab, a humanized monoclonal antibody (CCR5 antagonist), and a nucleoside RNA polymerase inhibitor galidesivir, both of which have shown survival benefits in many deadly virus infections and are considered potential useful candidates for treatment71,72.
7.5. HIV protease inhibitors as potent antiviral against the SARS-CoV-2
Lopinavir and Ritonavir are both HIV protease inhibitors that suppress the cleavage of a polyprotein into multiple functional proteins. At the Rajavithi Hospital in Thailand, the infectious disease team used a combination of oseltamivir (anti-influenza agent) and lopinavir/ritonavir to successfully improve the status of patients with severe conditions73.
7.6. Baricitinib as suggested antiviral against SARS-CoV-2
Baricitinib, used for the treatment of rheumatoid arthritis, is an inhibitor of AAK1 and Janus kinase and is recommended to control viral replication74. Machine learning models predicted that AP2-associated protein kinase 1 (AAK1) drugs that disrupt these proteins can inhibit viral entry into the target cells75. The use of baricitinib in susceptible COVID-19-associated patients with ongoing pneumonia should be taken with strict caution76.
7.7. Drugs under clinical trials for COVID-19
Clinical trials presently focus on the efficacy of different drugs, such as immunoglobulins, arbidol hydrochloride combined with interferon atomization, ritonavir plus oseltamivir, ASC09F plus oseltamivir, mesenchymal stem cell treatment, lopinavir plus ritonavir, hydroxychloroquine, darunavir plus cobicistat, methylprednisolone and washed microbiota transplantation65,77. Repurposing these available drugs for immediate use in treatment of SARS-CoV-2 infections could improve the available clinical management78. Study of Xiaoling Xu et al. suggests that tocilizumab is an important therapy in serious COVID-19 patients, which offered a new therapeutic approach to this deadly infectious disease79.
8. Conclusion
The virus SARS-CoV-2 is a fatal disease having a high mortality rate with a total of 2,200,358 certified cases and 147,787 deaths recorded all over the world. To date, there is no efficacious, proven medicinal therapy. A committee of US experts has developed treatment guidelines for COVID-19 patients which are regularly updated80.
A model drug, chloroquine, proposed by WHO could potentially work against the novel coronavirus. Remdesvir, a nucleoside analogue, it also holds promise for the use in treating COVID-19 patients. There are additional proposed drugs against COVID-19, such as drugs approved by FDA for the treatment of other pathologies, including ribavirin, penciclovir, nitazoxanide, nafamostat, chloroquine and two well-known drugs having broad spectrum activity i.e. remdesivir (GS5734) and favipiravir (T-705). Leronlimab, a humanised monoclonal antibody (CCR5 antagonist) arbidol hydrochloride combined with interferon atomisation, lopinavir plus ritonavir, ritonavir plus oseltamivir, ASC09F plus oseltamivir, mesenchymal stem cell treatment could also be a choice for the treatment of COVID-19 patients. Lopinavir and Ritonavir are both HIV protease inhibitors that suppress the cleavage of a polyprotein into multiple functional proteins. Based on historical records and human reports of SARS and prevention of H1N1 influenza, Chinese herbal formula may be an effective solution to COVID-19 prevention in high-risk populations, although their usefulness in the clinic for this purpose still has to be demonstrated. In short, SARS-CoV-2 is a highly transmissible virus and clinical trials are required for finding and confirming promising drug candidates and effective vaccines.
Acknowledgments
We are grateful to Quaid-i-Azam University, Islamabad, Pakistan for its support.
Footnotes
Conflict of interests: The authors declare no conflict of interest.
Coronavirus disease 19 (COVID-19); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); World Health Organization (WHO); Public Health Emergency of International Concern (PHEIC); spike protein (S); nucleocapsid protein (N); membrane glycoprotein (M); small membrane protein (SM); Chinese medication (CM); 2019 novel coronavirus (2019-nCoV); Centers for Disease Control and Prevention (CDC); non-human primates (NHP).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang Chaolin, Wang Yeming, Li Xingwang, Ren Lili, Zhao Jianping, Hu Yi, Zhang Li, Fan Guohui, Xu Jiuyang, Gu Xiaoying, Cheng Zhenshun, Yu Ting, Xia Jiaan, Wei Yuan, Wu Wenjuan, Xie Xuelei, Yin Wen, Li Hui, Liu Min, Xiao Yan, Gao Hong, Guo Li, Xie Jungang, Wang Guangfa, Jiang Rongmeng, Gao Zhancheng, Jin Qi, Wang Jianwei, Cao Bin. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A Novel Coronavirus from Patients with Pneumonia in China, 2019. Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F., Tan Wenjie. New England Journal of Medicine. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. Phan Lan T., Nguyen Thuong V., Luong Quang C., Nguyen Thinh V., Nguyen Hieu T., Le Hung Q., Nguyen Thuc T., Cao Thang M., Pham Quang D. New England Journal of Medicine. 2020;382(9):872-874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The first two cases of 2019‐nCoV in Italy: Where they come from? Giovanetti Marta, Benvenuto Domenico, Angeletti Silvia, Ciccozzi Massimo. Journal of Medical Virology. 2020;92(5):518-521. doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) World Health Organization; Accessed in April 2020. 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 6.Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. Wu Zunyou, McGoogan Jennifer M. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.CULTIVATION OF VIRUSES FROM A HIGH PROPORTION OF PATIENTS WITH COLDS. Tyrrell D.A.J, Bynoe M.L. The Lancet. 1966;287(7428):76-77. doi: 10.1016/s0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- 8.Literature on Severe Acute Respiratory Syndrome (SARS) (2003-2011): A Bibliometric Study. Zehra F. Aligarh Muslim University. 2012 [Google Scholar]
- 9.The COVID‐19 epidemic. Velavan Thirumalaisamy P., Meyer Christian G. Tropical Medicine & International Health. 2020;25(3):278-280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A pneumonia outbreak associated with a new coronavirus of probable bat origin. Zhou Peng, Yang Xing-Lou, Wang Xian-Guang, Hu Ben, Zhang Lei, Zhang Wei, Si Hao-Rui, Zhu Yan, Li Bei, Huang Chao-Lin, Chen Hui-Dong, Chen Jing, Luo Yun, Guo Hua, Jiang Ren-Di, Liu Mei-Qin, Chen Ying, Shen Xu-Rui, Wang Xi, Zheng Xiao-Shuang, Zhao Kai, Chen Quan-Jiao, Deng Fei, Liu Lin-Lin, Yan Bing, Zhan Fa-Xian, Wang Yan-Yi, Xiao Geng-Fu, Shi Zheng-Li. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Luo Hui, Tang Qiao-Ling, Shang Ya-Xi, Liang Shi-Bing, Yang Ming, Robinson Nicola, Liu Jian-Ping. Chinese Journal of Integrative Medicine. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hygiene and preventive medicine in ancient China. Needham J, Gwei-Djen L. Journal of the History of Medicine and Allied Sciences. 1962;17:429–78. doi: 10.1093/jhmas/xvii.4.429. [DOI] [PubMed] [Google Scholar]
- 13.Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. Liu Jianping, Manheimer Eric, Shi Yi, Gluud Christian. Journal of alternative and complementary medicine (New York, N.Y.) 2004;10(6):1041–51. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 14.Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lu Roujian, Zhao Xiang, Li Juan, Niu Peihua, Yang Bo, Wu Honglong, Wang Wenling, Song Hao, Huang Baoying, Zhu Na, Bi Yuhai, Ma Xuejun, Zhan Faxian, Wang Liang, Hu Tao, Zhou Hong, Hu Zhenhong, Zhou Weimin, Zhao Li, Chen Jing, Meng Yao, Wang Ji, Lin Yang, Yuan Jianying, Xie Zhihao, Ma Jinmin, Liu William J, Wang Dayan, Xu Wenbo, Holmes Edward C, Gao George F, Wu Guizhen, Chen Weijun, Shi Weifeng, Tan Wenjie. Lancet (London, England) 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronavirus Disease 2019 (COVID-19): A Perspective from China. Zu Zi Yue, Jiang Meng Di, Xu Peng Peng, Chen Wen, Ni Qian Qian, Lu Guang Ming, Zhang Long Jiang. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pre-fusion structure of a human coronavirus spike protein. Kirchdoerfer Robert N, Cottrell Christopher A, Wang Nianshuang, Pallesen Jesper, Yassine Hadi M, Turner Hannah L, Corbett Kizzmekia S, Graham Barney S, McLellan Jason S, Ward Andrew B. Nature. 2016;531(7592):118–21. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Xu Xintian, Chen Ping, Wang Jingfang, Feng Jiannan, Zhou Hui, Li Xuan, Zhong Wu, Hao Pei. Science China. Life sciences. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. Gorbalenya Alexander E., Baker Susan C., Baric Ralph S., de Groot Raoul J., Drosten Christian, Gulyaeva Anastasia A., Haagmans Bart L., Lauber Chris, Leontovich Andrey M, Neuman Benjamin W., Penzar Dmitry, Perlman Stanley, Poon Leo L.M., Samborskiy Dmitry, Sidorov Igor A., Sola Isabel, Ziebuhr John. 2020 [Google Scholar]
- 19.WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization; Accessed in April 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 20.Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. Lam Tommy Tsan-Yuk, Shum Marcus Ho-Hin, Zhu Hua-Chen, Tong Yi-Gang, Ni Xue-Bing, Liao Yun-Shi, Wei Wei, Cheung William Yiu-Man, Li Wen-Juan, Li Lian-Feng, Leung Gabriel M, Holmes Edward C., Hu Yan-Ling, Guan Yi. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 21.Architecture of the SARS coronavirus prefusion spike. Beniac Daniel R, Andonov Anton, Grudeski Elsie, Booth Tim F. Nature structural & molecular biology. 2006;13(8):751–2. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epidemiological characteristics of 1212 COVID-19 patients in Henan, China. Wang Pei, Lu Junan, Jin Yanyu, Zhu Mengfan, Wang Lingling, Chen Shunjie. 2020 doi: 10.1016/j.ijid.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Chan Jasper Fuk-Woo, Yuan Shuofeng, Kok Kin-Hang, To Kelvin Kai-Wang, Chu Hin, Yang Jin, Xing Fanfan, Liu Jieling, Yip Cyril Chik-Yan, Poon Rosana Wing-Shan, Tsoi Hoi-Wah, Lo Simon Kam-Fai, Chan Kwok-Hung, Poon Vincent Kwok-Man, Chan Wan-Mui, Ip Jonathan Daniel, Cai Jian-Piao, Cheng Vincent Chi-Chung, Chen Honglin, Hui Christopher Kim-Ming, Yuen Kwok-Yung. Lancet (London, England) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Characteristics of Coronavirus Disease 2019 in China. Guan Wei-Jie, Ni Zheng-Yi, Hu Yu, Liang Wen-Hua, Ou Chun-Quan, He Jian-Xing, Liu Lei, Shan Hong, Lei Chun-Liang, Hui David S C, Du Bin, Li Lan-Juan, Zeng Guang, Yuen Kwok-Yung, Chen Ru-Chong, Tang Chun-Li, Wang Tao, Chen Ping-Yan, Xiang Jie, Li Shi-Yue, Wang Jin-Lin, Liang Zi-Jing, Peng Yi-Xiang, Wei Li, Liu Yong, Hu Ya-Hua, Peng Peng, Wang Jian-Ming, Liu Ji-Yang, Chen Zhong, Li Gang, Zheng Zhi-Jian, Qiu Shao-Qin, Luo Jie, Ye Chang-Jiang, Zhu Shao-Yong, Zhong Nan-Shan. The New England journal of medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Chen Huijun, Guo Juanjuan, Wang Chen, Luo Fan, Yu Xuechen, Zhang Wei, Li Jiafu, Zhao Dongchi, Xu Dan, Gong Qing, Liao Jing, Yang Huixia, Hou Wei, Zhang Yuanzhen. Lancet (London, England) 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coronavirus disease 2019 (COVID-19)Situation Report –51. World Health Organization; Accessed in April 2020. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10
- 27.Coronavirus disease 2019 (COVID-19)Situation Report –23. World Health Organization; Accessed in April 2020. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200212-sitrep-23-ncov.pdf?sfvrsn=41e9fb78_4 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200212-sitrep-23-ncov.pdf?sfvrsn=41e9fb78_4
- 28.Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. Li Qun, Guan Xuhua, Wu Peng, Wang Xiaoye, Zhou Lei, Tong Yeqing, Ren Ruiqi, Leung Kathy S M, Lau Eric H Y, Wong Jessica Y, Xing Xuesen, Xiang Nijuan, Wu Yang, Li Chao, Chen Qi, Li Dan, Liu Tian, Zhao Jing, Liu Man, Tu Wenxiao, Chen Chuding, Jin Lianmei, Yang Rui, Wang Qi, Zhou Suhua, Wang Rui, Liu Hui, Luo Yinbo, Liu Yuan, Shao Ge, Li Huan, Tao Zhongfa, Yang Yang, Deng Zhiqiang, Liu Boxi, Ma Zhitao, Zhang Yanping, Shi Guoqing, Lam Tommy T Y, Wu Joseph T, Gao George F, Cowling Benjamin J, Yang Bo, Leung Gabriel M, Feng Zijian. The New England journal of medicine. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novel Wuhan (2019-nCoV) Coronavirus. Carlos W Graham, Dela Cruz Charles S, Cao Bin, Pasnick Susan, Jamil Shazia. American journal of respiratory and critical care medicine. 2020;201(4):P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 30.Presumed Asymptomatic Carrier Transmission of COVID-19. Bai Yan, Yao Lingsheng, Wei Tao, Tian Fei, Jin Dong-Yan, Chen Lijuan, Wang Meiyun. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novel Coronavirus Disease 2019 (COVID-19): An Emerging Infectious Disease in the 21st Century. Tavakoli Ahmad, Vahdat Katayon, Keshavarz Mohsen. Iranian South Medical Journal. 2020;22(6):432-450. [Google Scholar]
- 32.Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. Read Jonathan M, Bridgen Jessica RE, Cummings Derek AT, Ho Antonia, Jewell Chris P. 2020 doi: 10.1098/rstb.2020.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Data-Based Analysis, Modelling and Forecasting of the COVID-19 outbreak. Anastassopoulou Cleo, Russo Lucia, Tsakris Athanasios, Siettos Constantinos. 2020 doi: 10.1371/journal.pone.0230405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.COVID-19 and emergency planning. Winter George. British Journal of Healthcare Management. 2020;26(4):1-3. doi: 10.12968/bjcn.2020.25.4.184. [DOI] [PubMed] [Google Scholar]
- 35.Updates on Wuhan 2019 novel coronavirus epidemic. Kofi Ayittey Foster, Dzuvor Christian, Kormla Ayittey Matthew, Bennita Chiwero Nyasha, Habib Ahmed. Journal of Medical Virology. 2020;92(4):403-407. doi: 10.1002/jmv.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Data-based analysis, modelling and forecasting of the COVID-19 outbreak. Anastassopoulou Cleo, Russo Lucia, Tsakris Athanasios, Siettos Constantinos. PLOS ONE. 2020;15(3):e0230405. doi: 10.1371/journal.pone.0230405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Fang Lei, Karakiulakis George, Roth Michael. The Lancet Respiratory Medicine. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.[The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 39.The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. Yang Yongshi, Peng Fujun, Wang Runsheng, Guan Kai, Jiang Taijiao, Xu Guogang, Sun Jinlyu, Chang Christopher. Journal of autoimmunity. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. Qiu Chengfeng, Xiao Qian, Liao Xin, Deng Ziwei, Liu Huiwen, Shu Yuanlu, Zhou Dinghui, Deng Ye, Wang Hongqiang, Zhao Xiang, Zhou Jianliang, Wang Jin, Shi Zhihua, Da Long. 2020 doi: 10.1002/jmv.25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Wang Dawei, Hu Bo, Hu Chang, Zhu Fangfang, Liu Xing, Zhang Jing, Wang Binbin, Xiang Hui, Cheng Zhenshun, Xiong Yong, Zhao Yan, Li Yirong, Wang Xinghuan, Peng Zhiyong. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Chen Nanshan, Zhou Min, Dong Xuan, Qu Jieming, Gong Fengyun, Han Yang, Qiu Yang, Wang Jingli, Liu Ying, Wei Yuan, Xia Jia'an, Yu Ting, Zhang Xinxin, Zhang Li. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Discovering drugs to treat coronavirus disease 2019 (COVID-19) Dong Liying, Hu Shasha, Gao Jianjun. Drug Discoveries & Therapeutics. 2020;14(1):58-60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 44.New insights into the antiviral effects of chloroquine. Savarino Andrea, Di Trani Livia, Donatelli Isabella, Cauda Roberto, Cassone Antonio. The Lancet Infectious Diseases. 2006;6(2):67-69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Yan Yiwu, Zou Zhen, Sun Yang, Li Xiao, Xu Kai-Feng, Wei Yuquan, Jin Ningyi, Jiang Chengyu. Cell Research. 2012;23(2):300-302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ammonium chloride and chloroquine inhibit rabies virus infection in neuroblastoma cells. Tsiang H., Superti F. Archives of Virology. 1984;81(3-4):377-382. doi: 10.1007/BF01310010. [DOI] [PubMed] [Google Scholar]
- 47.Chloroquine induces empty capsid formation during poliovirus eclipse. Kronenberger P, Vrijsen R, Boeyé A. Journal of Virology. 1991;65(12):7008-7011. doi: 10.1128/jvi.65.12.7008-7011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inhibition of Human Immunodeficiency Virus Infectivity by Chloroquine. TSAI WEN-PO, NARA PETER L., KUNG HSIANG-FU, OROSZLAN STEPHEN. AIDS Research and Human Retroviruses. 1990;6(4):481-489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 49.Chloroquine and Hydroxychloroquine as Inhibitors of Human Immunodeficiency Virus (HIV-1) Activity. Romanelli Frank, Smith Kelly, Hoven Ardis. Current Pharmaceutical Design. 2004;10(21):2643-2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 50.Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. Mizui Tomokazu, Yamashina Shunhei, Tanida Isei, Takei Yoshiyuki, Ueno Takashi, Sakamoto Naoya, Ikejima Kenichi, Kitamura Tsuneo, Enomoto Nobuyuki, Sakai Tatsuo, Kominami Eiki, Watanabe Sumio. Journal of Gastroenterology. 2009;45(2):195-203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Vincent Martin J, Bergeron Eric, Benjannet Suzanne, Erickson Bobbie R, Rollin Pierre E, Ksiazek Thomas G, Seidah Nabil G, Nichol Stuart T. Virology journal. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;43:E019. doi: 10.3760/cma.j.issn.1001-0939.2020.0019. [DOI] [PubMed] [Google Scholar]
- 53.Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Gao Jianjun, Tian Zhenxue, Yang Xu. Bioscience trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 54.Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. Ratliff N B, Estes M L, Myles J L, Shirey E K, McMahon J T. The New England journal of medicine. 1987;316(4):191–3. doi: 10.1056/NEJM198701223160405. [DOI] [PubMed] [Google Scholar]
- 55.Restrictive cardiomyopathy caused by chloroquine. Iglesias Cubero G, Rodriguez Reguero J J, Rojo Ortega J M. British heart journal. 1993;69(5):451–2. doi: 10.1136/hrt.69.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ocular safety of hydroxychloroquine. Bernstein H N. Annals of ophthalmology. 1991;23(8):292–6. [PubMed] [Google Scholar]
- 57.Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Colson Philippe, Rolain Jean-Marc, Raoult Didier. International journal of antimicrobial agents. 2020;55(3):105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Liu Jia, Cao Ruiyuan, Xu Mingyue, Wang Xi, Zhang Huanyu, Hu Hengrui, Li Yufeng, Hu Zhihong, Zhong Wu, Wang Manli. Cell discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ethical and legal framework and regulation for off-label use: European perspective. Lenk Christian, Duttge Gunnar. Therapeutics and clinical risk management. 2014;10:537–46. doi: 10.2147/TCRM.S40232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Gautret Philippe, Lagier Jean-Christophe, Parola Philippe, Hoang Van Thuan, Meddeb Line, Mailhe Morgane, Doudier Barbara, Courjon Johan, Giordanengo Valérie, Vieira Vera Esteves, Dupont Hervé Tissot, Honoré Stéphane, Colson Philippe, Chabrière Eric, La Scola Bernard, Rolain Jean-Marc, Brouqui Philippe, Raoult Didier. International journal of antimicrobial agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;43(3):185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Remdesivir as a possible therapeutic option for the COVID-19. Al-Tawfiq Jaffar A, Al-Homoud Ali H, Memish Ziad A. Travel medicine and infectious disease. 2020:101615. doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sheahan Timothy P, Sims Amy C, Graham Rachel L, Menachery Vineet D, Gralinski Lisa E, Case James B, Leist Sarah R, Pyrc Krzysztof, Feng Joy Y, Trantcheva Iva, Bannister Roy, Park Yeojin, Babusis Darius, Clarke Michael O, Mackman Richard L, Spahn Jamie E, Palmiotti Christopher A, Siegel Dustin, Ray Adrian S, Cihlar Tomas, Jordan Robert, Denison Mark R, Baric Ralph S. Science translational medicine. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emerging Therapeutic Strategies for COVID-19 patients. Zhu Shudong, Guo Xialing, Geary Kyla, Zhang Dianzheng. Discoveries (Craiova) 2020;8(1):e105. doi: 10.15190/d.2020.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Xu Mingyue, Shi Zhengli, Hu Zhihong, Zhong Wu, Xiao Gengfu. Cell research. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Sheahan Timothy P, Sims Amy C, Leist Sarah R, Schäfer Alexandra, Won John, Brown Ariane J, Montgomery Stephanie A, Hogg Alison, Babusis Darius, Clarke Michael O, Spahn Jamie E, Bauer Laura, Sellers Scott, Porter Danielle, Feng Joy Y, Cihlar Tomas, Jordan Robert, Denison Mark R, Baric Ralph S. Nature communications. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Delang Leen, Abdelnabi Rana, Neyts Johan. Antiviral research. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Warren Travis K, Jordan Robert, Lo Michael K, Ray Adrian S, Mackman Richard L, Soloveva Veronica, Siegel Dustin, Perron Michel, Bannister Roy, Hui Hon C, Larson Nate, Strickley Robert, Wells Jay, Stuthman Kelly S, Van Tongeren Sean A, Garza Nicole L, Donnelly Ginger, Shurtleff Amy C, Retterer Cary J, Gharaibeh Dima, Zamani Rouzbeh, Kenny Tara, Eaton Brett P, Grimes Elizabeth, Welch Lisa S, Gomba Laura, Wilhelmsen Catherine L, Nichols Donald K, Nuss Jonathan E, Nagle Elyse R, Kugelman Jeffrey R, Palacios Gustavo, Doerffler Edward, Neville Sean, Carra Ernest, Clarke Michael O, Zhang Lijun, Lew Willard, Ross Bruce, Wang Queenie, Chun Kwon, Wolfe Lydia, Babusis Darius, Park Yeojin, Stray Kirsten M, Trancheva Iva, Feng Joy Y, Barauskas Ona, Xu Yili, Wong Pamela, Braun Molly R, Flint Mike, McMullan Laura K, Chen Shan-Shan, Fearns Rachel, Swaminathan Swami, Mayers Douglas L, Spiropoulou Christina F, Lee William A, Nichol Stuart T, Cihlar Tomas, Bavari Sina. Nature. 2016;531(7594):381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Treatment With Convalescent Plasma for Critically Ill Patients With SARS-CoV-2 Infection. Zhang Bin, Liu Shuyi, Tan Tan, Huang Wenhui, Dong Yuhao, Chen Luyan, Chen Qiuying, Zhang Lu, Zhong Qingyang, Zhang Xiaoping, Zou Yujian, Zhang Shuixing. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Convalescent plasma as a potential therapy for COVID-19. Chen Long, Xiong Jing, Bao Lei, Shi Yuan. The Lancet. Infectious diseases. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Shanmugaraj Balamurugan, Siriwattananon Konlavat, Wangkanont Kittikhun, Phoolcharoen Waranyoo. Asian Pacific journal of allergy and immunology. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 72.Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Tian Xiaolong, Li Cheng, Huang Ailing, Xia Shuai, Lu Sicong, Shi Zhengli, Lu Lu, Jiang Shibo, Yang Zhenlin, Wu Yanling, Ying Tianlei. Emerging microbes & infections. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potential Therapeutic Agents for COVID-19 Based on the Analysis of Protease and RNA Polymerase Docking. Chang Y, Tung Y Y, Lee K, Chen T, Hsiao Y, Chang H. https://www.preprints.org/manuscript/202002.0242/v1 Preprints. 2020:2020020242. [Google Scholar]
- 74.Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Richardson Peter, Griffin Ivan, Tucker Catherine, Smith Dan, Oechsle Olly, Phelan Anne, Stebbing Justin. Lancet (London, England) 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Family-wide Structural Analysis of Human Numb-Associated Protein Kinases. Sorrell Fiona J., Szklarz Marta, Abdul Azeez Kamal R., Elkins Jon M., Knapp Stefan. Structure. 2016;24(3):401-411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baricitinib for COVID-19: a suitable treatment? Favalli Ennio G, Biggioggero Martina, Maioli Gabriella, Caporali Roberto. The Lancet Infectious Diseases. 2020 doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coronaviruses - drug discovery and therapeutic options. Zumla Alimuddin, Chan Jasper F W, Azhar Esam I, Hui David S C, Yuen Kwok-Yung. Nature reviews. Drug discovery. 2016;15(5):327–47. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Zhou Yadi, Hou Yuan, Shen Jiayu, Huang Yin, Martin William, Cheng Feixiong. Cell discovery. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Effective treatment of severe COVID-19 patients with tocilizumab. Xu Xiaoling, Han Mingfeng, Li Tiantian, Sun Wei, Wang Dongsheng, Fu Binqing, Zhou Yonggang, Zheng Xiaohu, Yang Yun, Li Xiuyong, Zhang Xiaohua, Pan Aijun, Wei Haiming. Proceedings of the National Academy of Sciences of the United States of America. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. US National Institute of Health, Accessed in April 2020. 2020. https://covid19treatmentguidelines.nih.gov/ https://covid19treatmentguidelines.nih.gov/ [PubMed]