Terbium oxychloride, TbOCl, was synthesized via the simple heat-treatment of TbCl3·6H2O and its structure was determined by refinement against X-ray powder diffraction data. TbOCl crystallizes with the matlockite (PbFCl) structure in the tetragonal space group P4/nmm and is composed of alternating (001) layers of (TbO)n and n Cl−.
Keywords: oxychloride, rare-earth oxyhalide, powder diffraction
Abstract
Terbium oxychloride, TbOCl, was synthesized via the simple heat-treatment of TbCl3·6H2O and its structure was determined by refinement against X-ray powder diffraction data. TbOCl crystallizes with the matlockite (PbFCl) structure in the tetragonal space group P4/nmm and is composed of alternating (001) layers of (TbO)n and n Cl−. The unit-cell parameters, unit-cell volume, and density were compared to the literature data of other isostructural rare-earth oxychlorides in the same space group and showed good agreement when compared to the calculated trendlines.
Chemical context
Rare-earth oxychlorides, REOCl, are promising materials for various applications including use as catalysts, sensors, and phosphors (Podkolzin et al., 2007 ▸; Au et al., 1997 ▸; Peringer et al., 2009 ▸; Marsal et al., 2005 ▸,; Kim et al., 2019 ▸; Berdowski et al., 1984 ▸; Imanaka et al., 2001a ▸,b ▸; Okamoto et al., 2002 ▸; Kim et al., 2014 ▸). LaOCl is a stable catalyst for converting methane to methyl chloride (Podkolzin et al., 2007 ▸) and can be used as a sensor material to detect CO2 and Cl2 gases (Marsal et al., 2005 ▸; Imanaka et al., 2001b ▸). The EuOCl catalyst showed high efficiency in converting ethylene to vinyl chloride (Scharfe et al., 2016 ▸). The luminescent properties of REOX (RE = La, Eu; X = F, Cl, Br, I) can be controlled to emit a wide range of visible light from blue to red by changing the crystal symmetries and compositions (Kim et al., 2014 ▸, 2019 ▸). As part of our studies in this area, we now describe the dehydration synthesis and structure of the title compound.
Structural commentary
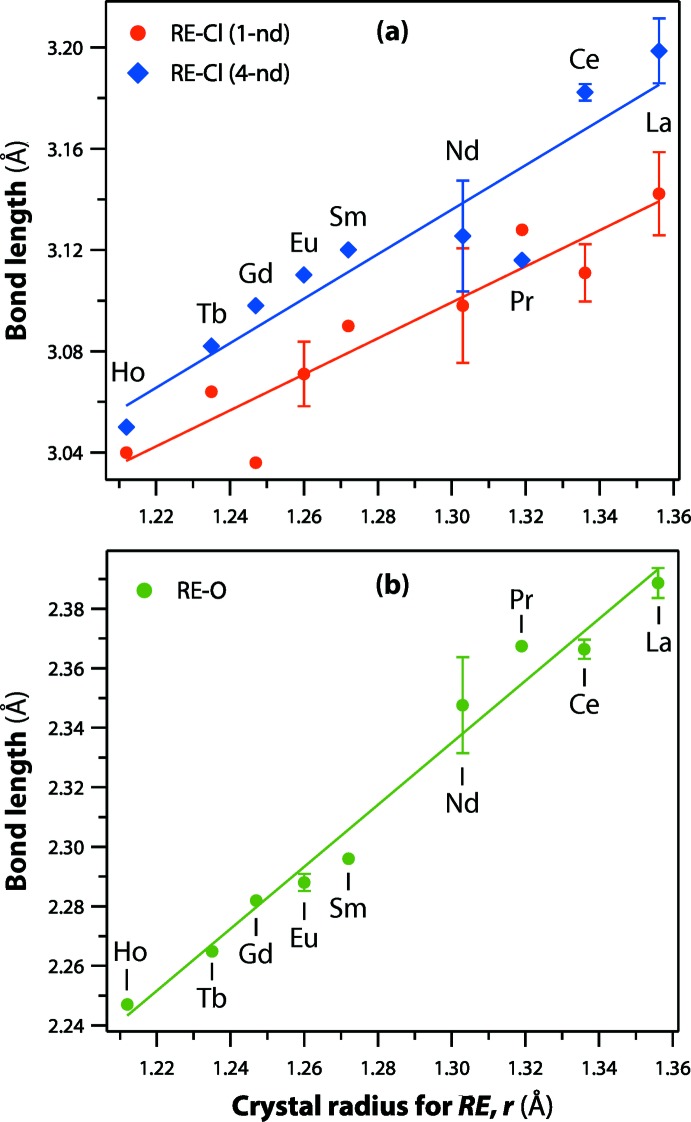
The structural parameters of REOCl (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho) in the literature and current study are summarized in Table 1 ▸. All these REOCl compounds crystallize in the matlockite (PbFCl; Bannister, 1934 ▸) structure within the tetragonal P4/nmm space group. The crystal structure of TbOCl contains alternating (001) layers of (TbO)n and n Cl− (Fig. 1 ▸ a). The Tb cation is coordinated by five chloride ions and four oxygen atoms, forming a mono-capped TbO4Cl5 square antiprism (Fig. 1 ▸ b and 1c). The RE—Cl and RE—O bond lengths in the REOCl compounds are provided in Table 1 ▸. With larger RE cations in the structures, the RE—Cl and RE—O bond lengths increase (Fig. 2 ▸).
Table 1. Structural parameters of REOCl compounds.
All compounds crystallize in the P4/nmm space group. For the RE—Cl bond lengths, the first value refers to one neighboring Cl atom, and the second number refers to four neighboring Cl atoms. Densities are calculated from crystallographic data.
| RE | a(Å) | c(Å) | V (Å3) | Density(g cm−3) | RE—O(Å) | RE—Cl(Å) | Cl⋯Cl(Å) | Cl⋯O(Å) | O⋯O (Å) | ICSD/PDF |
|---|---|---|---|---|---|---|---|---|---|---|
| Ho | 3.893 | 6.602 | 100.1 | 7.182 | 2.247 | 3.04, 3.05 | 3.24 | 3.12 | 2.753 | 76171 (Templeton & Dauben, 1953 ▸) |
| Dy | 3.91 | 6.62 | 101.2 | 7.023 | 00–047-1725 (Kirik et al., 1996 ▸) | |||||
| Tb | 3.9269 | 6.648 | 102.5 | 6.815 | 00–048-1648 (Kirik et al., 1996 ▸) | |||||
| Tb | 3.9279 | 6.6556 | 102.7 | 6.804 | 2.2649 | 3.064, 3.082 | 3.271 | 3.151 | 2.7774 | Current study |
| Gd | 3.9495 | 6.6708 | 104.1 | 6.661 | 2.2839 | 3.036, 3.098 | 3.267 | 3.176 | 2.7927 | 59232 (Meyer & Schleid, 1986 ▸) |
| Gd | 3.9698 | 6.7008 | 105.6 | 6.564 | 2.28 | 3.212, 3.071 | 3.428 | 3.089 | 2.8071 | 77820 (Hölsä et al., 1996 ▸) |
| Eu | 3.9646 | 6.695 | 105.2 | 6.42 | 2.286 | 3.08, 3.11 | 3.3 | 3.17 | 2.8034 | 28529 (Bärnighausen et al., 1965 ▸) |
| Eu | 3.9668 | 6.6955 | 105.4 | 6.412 | 2.2901 | 3.062, 3.1103 | 3.289 | 3.183 | 2.80492 | 54682 (Schnick, 2004 ▸) |
| Sm | 3.982 | 6.721 | 106.6 | 6.289 | 2.296 | 3.09, 3.12 | 3.31 | 3.19 | 2.8157 | 26581 (Templeton & Dauben, 1953 ▸) |
| Nd | 4.04 | 6.77 | 110.5 | 5.882 | 2.359 | 3.114, 3.11 | 3.428 | 3.165 | 2.86 | 31665 (Zachariasen, 1949 ▸) |
| Nd | 4.0249 | 6.7837 | 109.9 | 5.914 | 2.3362 | 3.082, 3.141 | 3.343 | 3.221 | 2.84603 | 59231 (Meyer & Schleid, 1986 ▸) |
| Pr | 4.053 | 6.799 | 111.7 | 5.723 | 2.3674 | 3.128, 3.116 | 3.441 | 3.178 | 2.866 | 31664 (Zachariasen, 1949 ▸) |
| Ce | 4.0866 | 6.8538 | 114.5 | 5.558 | 2.3687 | 3.1190, 3.1846 | 3.3942 | 3.2572 | 2.8897 | 412069 (Schnick, 2004 ▸) |
| Ce | 4.0785 | 6.8346 | 113.7 | 5.596 | 2.36413 | 3.103, 3.180 | 3.38 | 3.254 | 2.88393 | 72154 (Wołcyrz & Kepinski, 1992 ▸) |
| La | 4.109 | 6.865 | 115.9 | 5.454 | 2.39 | 3.14, 3.18 | 3.45 | 3.24 | 2.9055 | 24611 (Sillen & Nylander, 1941 ▸) |
| La | 4.117 | 6.881 | 116.6 | 5.42 | 2.3866 | 3.126, 3.2046 | 3.416 | 3.2751 | 2.9112 | 40297 (Brixner & Moore, 1983 ▸) |
| La | 4.1351 | 6.904 | 118.1 | 5.355 | 2.395 | 3.165, 3.209 | 3.457 | 3.268 | 2.92397 | 77815 (Hölsä et al., 1996 ▸) |
| La | 4.1162 | 6.8746 | 116.5 | 5.428 | 2.3832 | 3.138, 3.201 | 3.425 | 3.265 | 2.9106 | 84330 (Hölsä et al., 1997 ▸) |
| La | 4.12 | 6.882 | 116.8 | 5.412 | 00–008-0477 (Swanson et al., 1957 ▸) |
Figure 1.

(a) Crystal structure of TbOCl, (b) the coordination environment of Tb, and (c) polyhedron representation of the Tb environment.
Figure 2.
The RE—Cl and RE—O bond lengths in the REOCl compounds listed in Table 1 ▸ as a function of RE crystal radius (coordination = 9) according to Shannon (1976 ▸). Where multiple values were available, averages and standard deviations are included for the datapoints. For (a), 1-nd and 4-nd denote 1 and 4 neighbor distances, respectively
The shortest Cl⋯Cl separation in TbOCl is 3.271 (4) Å, which compares with the van der Waals diameter of a Cl− ion of about 3.62 Å. The Cl⋯Cl distances of other REOCl compounds are also short, ranging from 3.24 to 3.46 Å on going from Ho3+ to La3+. With non-bonded vectors shorter than the van der Waals separation, strong interactions between atoms are expected in the structure (Maslen et al., 1996 ▸). Templeton & Dauben (1953 ▸) mention the presence of weaker anion–anion repulsion between Cl atoms in REOCl structures. The structural parameters of TbOCl were compared with the trendlines calculated using the values from Table 1 ▸ (Fig. 3 ▸). The unit-cell parameters and volumes increase linearly with the larger RE cations (Shannon, 1976 ▸) whereas the densities decrease non-linearly, fitting well to a 2nd order polynomial trend.
Figure 3.
(a, b) Unit-cell parameters (a and c, respectively), (c) unit-cell volumes, and calculated unit-cell densities as a function of the crystal radius of the RE (coordination = 9) according to Shannon (1976 ▸) compared to literature values provided in Table 1 ▸.
Synthesis and crystallization
The title compound was synthesized by a simple heat treatment of TbCl3·6H2O (Alfa Aesar, 99.99%). About 0.5 g of TbCl3·6H2O was placed in an alumina crucible, heated to 400°C at 5°C min−1, held for 8 h, and then cooled to room temperature at 5°C min−1. This synthesis method was used in our previous study (Riley et al., 2018 ▸). The resulting product was a light-brown powder, which was ground in a mortar and pestle for X-ray powder diffraction analysis.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The unit-cell parameters were obtained using TOPAS (version 4.2; Bruker, 2009 ▸) by refining the GdOCl pattern (ICSD 77820) with geometrical and chemical resemblance as a starting model. The Rietveld refinement was performed using JANA2006 (Petříček et al., 2014 ▸) with the obtained unit-cell parameters as initial values. A pseudo-Voigt function with other peak-shape parameters were used to fit peaks, and the background was modeled with a Chebychev polynomial. The plot of the Rietveld refinement result is shown in Fig. 4 ▸. The final refinement converged at R wp = 3.22%.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | TbOCl |
| M r | 210.4 |
| Crystal system, space group | Tetragonal, P4/n m m |
| Temperature (K) | 293 |
| a, c (Å) | 3.9279 (2), 6.6556 (5) |
| V (Å3) | 102.68 (1) |
| Z | 2 |
| Radiation type | Cu Kα, λ = 1.54188 Å |
| Specimen shape, size (mm) | Cylinder, 25 × 25 |
| Data collection | |
| Diffractometer | Bruker D8 Advance |
| Specimen mounting | Packed powder pellet |
| Data collection mode | Reflection |
| Scan method | Step |
| 2θ values (°) | 2θmin = 5, 2θmax = 68.977, 2θstep = 0.019 |
| Refinement | |
| R factors and goodness of fit | R p = 0.020, R wp = 0.032, R exp = 0.009, R(F) = 0.033, χ2 = 13.690 |
| No. of parameters | 17 |
Figure 4.
Measured, calculated, and difference XRD patterns of TbOCl.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989020004387/hb7896sup1.cif
Rietveld powder data: contains datablock(s) I. DOI: 10.1107/S2056989020004387/hb7896Isup2.rtv
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020004387/hb7896Isup3.hkl
CCDC reference: 1993793
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The Pacific Northwest National Laboratory is operated by Battelle under Contract Number DE-AC05–76RL01830.
supplementary crystallographic information
Crystal data
| TbOCl | Z = 2 |
| Mr = 210.4 | Dx = 6.804 Mg m−3 |
| Tetragonal, P4/nmm | Cu Kα radiation, λ = 1.54188 Å |
| a = 3.9279 (2) Å | T = 293 K |
| c = 6.6556 (5) Å | light brown |
| V = 102.68 (1) Å3 | cylinder, 25 × 25 mm |
Data collection
| Bruker D8 Advance diffractometer | Data collection mode: reflection |
| Radiation source: sealed X-ray tube | Scan method: step |
| Specimen mounting: packed powder pellet | 2θmin = 5°, 2θmax = 68.977°, 2θstep = 0.019° |
Refinement
| Rp = 0.020 | 17 parameters |
| Rwp = 0.032 | Weighting scheme based on measured s.u.'s |
| Rexp = 0.009 | (Δ/σ)max = 0.030 |
| R(F) = 0.033 | Background function: 8 Chebyshev polynoms |
| 3292 data points | Preferred orientation correction: March & Dollase |
| Profile function: Pseudo-Voigt |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Tb1 | 0.5 | 0 | 0.3305 (2) | 0.002 | |
| Cl1 | 0 | 0.5 | 0.1298 (9) | 0.002 | |
| O1 | 1 | 0 | 0.5 | 0.002 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Tb1 | 0.002 | 0.002 | 0.002 | 0 | 0 | 0 |
| Cl1 | 0.002 | 0.002 | 0.002 | 0 | 0 | 0 |
| O1 | 0.002 | 0.002 | 0.002 | 0 | 0 | 0 |
Geometric parameters (Å, º)
| Tb1—Tb1i | 3.5784 (13) | Tb1—O1v | 2.2649 (7) |
| Tb1—Tb1ii | 3.5784 (13) | Tb1—O1 | 2.2649 (7) |
| Tb1—Tb1iii | 3.5784 (13) | Tb1—O1vi | 2.2649 (7) |
| Tb1—Tb1iv | 3.5784 (13) | Tb1—O1vii | 2.2649 (7) |
| Tb1i—Tb1—Tb1ii | 66.57 (2) | Tb1iii—Tb1—O1vii | 99.31 (4) |
| Tb1i—Tb1—Tb1iii | 66.57 (2) | Tb1iv—Tb1—O1v | 99.31 (4) |
| Tb1i—Tb1—Tb1iv | 101.82 (4) | Tb1iv—Tb1—O1 | 37.817 (14) |
| Tb1i—Tb1—O1v | 37.817 (14) | Tb1iv—Tb1—O1vi | 99.31 (4) |
| Tb1i—Tb1—O1 | 99.31 (4) | Tb1iv—Tb1—O1vii | 37.817 (14) |
| Tb1i—Tb1—O1vi | 37.817 (14) | O1v—Tb1—O1 | 120.25 (6) |
| Tb1i—Tb1—O1vii | 99.31 (4) | O1v—Tb1—O1vi | 75.63 (3) |
| Tb1ii—Tb1—Tb1iii | 101.82 (4) | O1v—Tb1—O1vii | 75.63 (3) |
| Tb1ii—Tb1—Tb1iv | 66.57 (2) | O1—Tb1—O1vi | 75.63 (3) |
| Tb1ii—Tb1—O1v | 37.817 (14) | O1—Tb1—O1vii | 75.63 (3) |
| Tb1ii—Tb1—O1 | 99.31 (4) | O1vi—Tb1—O1vii | 120.25 (6) |
| Tb1ii—Tb1—O1vi | 99.31 (4) | Tb1—O1—Tb1viii | 120.25 (4) |
| Tb1ii—Tb1—O1vii | 37.817 (14) | Tb1—O1—Tb1iii | 104.37 (2) |
| Tb1iii—Tb1—Tb1iv | 66.57 (2) | Tb1—O1—Tb1iv | 104.37 (2) |
| Tb1iii—Tb1—O1v | 99.31 (4) | Tb1viii—O1—Tb1iii | 104.37 (2) |
| Tb1iii—Tb1—O1 | 37.817 (14) | Tb1viii—O1—Tb1iv | 104.37 (2) |
| Tb1iii—Tb1—O1vi | 37.817 (14) | Tb1iii—O1—Tb1iv | 120.25 (4) |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1; (ii) −x+1/2, y+1/2, −z+1; (iii) −x+3/2, y−1/2, −z+1; (iv) −x+3/2, y+1/2, −z+1; (v) x−1, y, z; (vi) −y+1/2, x−3/2, z; (vii) −y+1/2, x−1/2, z; (viii) x+1, y, z.
References
- Au, C. T., He, H., Lai, S. Y. & Ng, C. F. (1997). Appl. Catal. Gen. 159, 133–145.
- Bannister, F. A. (1934). Miner. Mag. j. Miner. Soc. 23, 587–597.
- Bärnighausen, H., Brauer, G. & Schultz, N. (1965). Z. Anorg. Allg. Chem. 338, 250–265.
- Berdowski, P. A. M., van Herk, J., Jansen, L. & Blasse, G. (1984). Phys. Status Solidi B, 125, 387–391.
- Brixner, L. H. & Moore, E. P. (1983). Acta Cryst. C39, 1316.
- Bruker (2009). TOPAS. Bruker AXS, Karlsruhe, Germany.
- Hölsä, J., Lastusaari, M. & Valkonen, J. (1997). J. Alloys Compd, 262, 299–304.
- Hölsä, J., Säilynoja, E., Koski, K., Rahiala, H. & Valkonen, J. (1996). Powder Diffr. 11, 129–133.
- Imanaka, N., Okamoto, K. & Adachi, G. (2001a). Chem. Lett. 30, 130–131.
- Imanaka, N., Okamoto, K. & Adachi, G. (2001b). Electrochem. Commun. 3, 49–51.
- Kienle, M. & Jacob, M. (2003). XRD Commander. Bruker AXS GmbH, Karlsruhe, Germany.
- Kim, D., Jeong, J. R., Jang, Y., Bae, J.-S., Chung, I., Liang, R., Seo, D.-K., Kim, S.-J. & Park, J.-C. (2019). Phys. Chem. Chem. Phys. 21, 1737–1749. [DOI] [PubMed]
- Kim, D., Park, S., Kim, S., Kang, S.-G. & Park, J.-C. (2014). Inorg. Chem. 53, 11966–11973. [DOI] [PubMed]
- Kirik, S., Yakimov, I., Blochin, A. & Soloyov, L. (1996). ICDD Grant-in-Aid, Institute of Chemistry, Krasnoyarsk, Russia.
- Marsal, A., Centeno, M. A., Odriozola, J. A., Cornet, A. & Morante, J. R. (2005). Sens. Actuators B Chem. 108, 484–489.
- Maslen, E. N., Streltsov, V. A., Streltsova, N. R. & Ishizawa, N. (1996). Acta Cryst. B52, 576–579.
- Meyer, G. & Schleid, T. (1986). Z. Anorg. Allg. Chem. 533, 181–185.
- Momma, K. & Izumi, F. (2011). J. Appl. Cryst. 44, 1272–1276.
- Okamoto, K., Imanaka, N. & Adachi, G. (2002). Solid State Ionics, 154–155, 577–580.
- Palatinus, L. & Chapuis, G. (2007). J. Appl. Cryst. 40, 786–790.
- Peringer, E., Salzinger, M., Hutt, M., Lemonidou, A. A. & Lercher, J. A. (2009). Top. Catal. 52, 1220–1231.
- Petříček, V., Dusek, M. & Palatinus, L. (2014). Z. Kristallogr. 229, 345–352.
- Podkolzin, S. G., Stangland, E. E., Jones, M. E., Peringer, E. & Lercher, J. A. (2007). J. Am. Chem. Soc. 129, 2569–2576. [DOI] [PubMed]
- Riley, B. J., Pierce, D. A., Crum, J. V., Williams, B. D., Snyder, M. M. V. & Peterson, J. A. (2018). Prog. Nucl. Energy, 104, 102–108.
- Scharfe, M., Lira-Parada, P. A., Amrute, A. P., Mitchell, S. & Pérez-Ramírez, J. (2016). J. Catal. 344, 524–534.
- Schnick, W. (2004). Private Communication.
- Shannon, R. D. (1976). Acta Cryst. A32, 751–767.
- Sillen, L. G. & Nylander, A. L. (1941). Svensk Kemisk Tidskrift, 53, 367–372.
- Swanson, H. E., Gilfrich, N. T. & Cook, M. I. (1957). Circ. Bur. Stand. pp. 539.
- Templeton, D. H. & Dauben, C. H. (1953). J. Am. Chem. Soc. 75, 6069–6070.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Wołcyrz, M. & Kepinski, L. (1992). J. Solid State Chem. 99, 409–413.
- Zachariasen, W. H. (1949). Acta Cryst. 2, 388–390.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989020004387/hb7896sup1.cif
Rietveld powder data: contains datablock(s) I. DOI: 10.1107/S2056989020004387/hb7896Isup2.rtv
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020004387/hb7896Isup3.hkl
CCDC reference: 1993793
Additional supporting information: crystallographic information; 3D view; checkCIF report