The title compound is a 6-chloronicotinate salt of a one-dimensional cationic nickel(II) coordination polymer with 4,4′-bipyridine. The nickel(II) ion in the polymeric cation is octahedrally coordinated by four water molecule O atoms and by two 4,4′-bipyridine N atoms. The 4,4′-bipyridine ligands act as bridges, connecting the symmetry-related nickel(II) ions into polymeric chains along the b-axis direction. In the extended structure, these chains, the anions and the water molecules of crystallization are assembled into a three-dimensional network via strong O—H⋯O and O—H⋯N hydrogen bonds
Keywords: nickel(II); 6-chloronicotinic acid; 4,4′-bipyridine; coordination polymer; hydrogen-bond motif; crystal structure
Abstract
A 6-chloronicotinate (6-Clnic) salt of a one-dimensional cationic nickel(II) coordination polymer with 4,4′-bipyridine (4,4′-bpy), namely, catena-poly[[[tetraaquanickel(II)]-μ-4,4′-bipyridine-κ2 N:N′] bis(6-chloronicotinate) tetrahydrate], {[Ni(C10H8N2)(H2O)4](C6H3ClNO2)2·4H2O}n or {[Ni(4,4′-bpy)(H2O)4](6-Clnic)2·4H2O}n, (1), was prepared by the reaction of nickel(II) sulfate heptahydrate, 6-chloronicotinic acid and 4,4′-bipyridine in a mixture of water and ethanol. The molecular structure of 1 comprises a one-dimensional polymeric {[Ni(4,4′-bpy)(H2O)4]2+}n cation, two 6-chloronicotinate anions and four water molecules of crystallization per repeating polymeric unit. The nickel(II) ion in the polymeric cation is octahedrally coordinated by four water molecule O atoms and by two 4,4′-bipyridine N atoms in the trans position. The 4,4′-bipyridine ligands act as bridges and, thus, connect the symmetry-related nickel(II) ions into an infinite one-dimensional polymeric chain extending along the b-axis direction. In the extended structure of 1, the polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n, the 6-chloronicotinate anions and the water molecules of crystallization are assembled into an infinite three-dimensional hydrogen-bonded network via strong O—H⋯O and O—H⋯N hydrogen bonds, leading to the formation of the representative hydrogen-bonded ring motifs: tetrameric R 2 4(8) and R 4 4(10) loops, a dimeric R 2 2(8) loop and a pentameric R 4 5(16) loop.
Chemical context
Functional coordination polymers have attracted great interest in recent years, mostly due to their aesthetics and many interesting properties such as catalytic, magnetic and luminescent, potential for use in gas storage and separation, molecular sensing (Mueller et al., 2006 ▸; Bosch et al., 2017 ▸; Zhang et al., 2015 ▸; Zeng et al., 2014 ▸, 2016 ▸; Douvali et al., 2015 ▸; Xu et al., 2017 ▸; Zhou et al., 2017 ▸).
The organic ligands, used as building blocks in the construction of coordination polymers, need to be multifunctional, which is evident from the position, coordination ability and steric hindrance of their donor atoms and/or groups. The design of functional coordination polymers with the desired structures is not always straightforward and is strongly dependent on the experimental conditions including the type of solvents, starting metal salts, additional ligands, temperature, hydrothermal conditions and pH value (Li et al., 2016 ▸; Zhou et al., 2016 ▸; Gu et al., 2016 ▸). Aromatic carboxylic acids with additional functional groups have become popular in the design of coordination polymers. The main reasons are the many possible and unpredictable coordination modes of this type of ligand and their affinity for participation in supramolecular interactions (Gu et al., 2016 ▸, 2017 ▸, 2018 ▸; Wang et al., 2016 ▸; Zhang et al., 2019 ▸).
The metal complexes of chlorinated analogues of the nicotinate anion (e.g. 2-chloronicotinate and 5-chloronicotinate) have not been particularly well-studied [as of March 2020, there are around 20 crystal structures in the CSD (Groom et al., 2016 ▸) for each ligand]. Furthermore, no metal complexes of the 4-chloronicotinate anion have been reported. The crystal structures of only three metal complexes of 6-chloronicotinate (6-Clnic) are known so far (Xia et al., 2012a
▸,b
▸; Li et al., 2006 ▸). Recently, we have reported the synthesis, crystal structure and properties of a one-dimensional nickel(II) coordination polymer with mixed ligands: 6-fluoronicotinate as the main ligand and 4,4′-bipyridine (4,4′-bpy) as the supporting ligand (Politeo et al., 2020 ▸).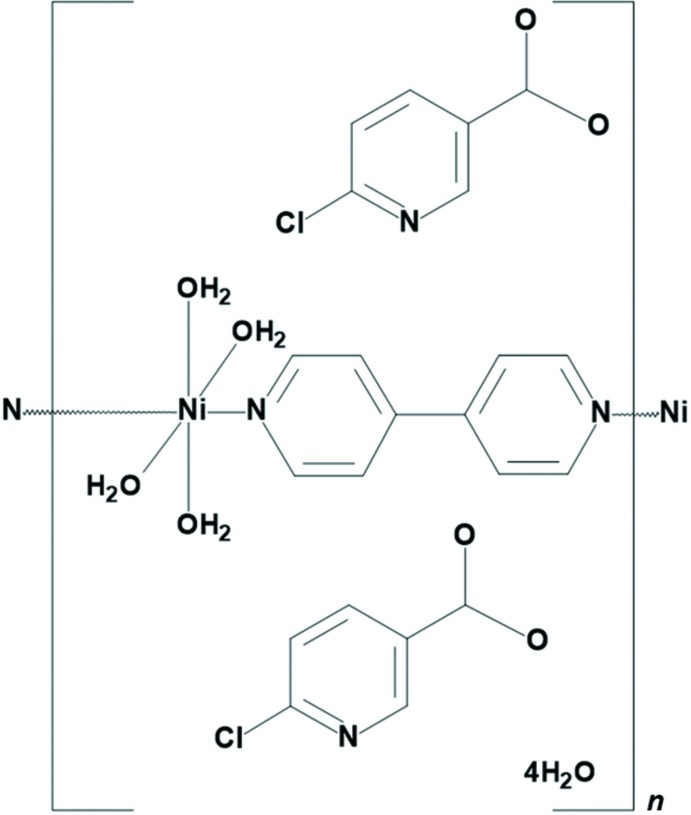
In a continuation of our work on coordination polymers with mixed ligands, we set out to prepare a similar coordination polymer with 6-chlorinicotinate and 4,4′-bipyridine, as we did with 6-fluoronicotinate (Politeo et al., 2020 ▸). Therefore, we carried out the synthesis and crystallization under the same experimental conditions (in a mixture of water and ethanol and with the same molar ratios of the nickel(II) sulfate and ligands), in hope that the analogous nickel(II) coordination polymer could be obtained. We also wanted to examine the influence of the possible weak intermolecular interactions involving the chlorine atoms (e.g. C—H⋯Cl interactions) on the assembly of the polymeric chains in the crystal packing, especially since the analogous C—H⋯F interactions were not found in the crystal packing of the nickel(II) coordination polymer with 6-fluoronicotinate (Politeo et al., 2020 ▸). Unfortunately, we were not able to prepare the desired nickel(II) coordination polymer under these experimental conditions, but instead we obtained a 6-chloronicotinate salt of a one-dimensional cationic nickel(II) coordination polymer with 4,4′-bipyridine, namely the title compound, {[Ni(4,4′-bpy)(H2O)4](6-Clnic)2·4H2O}n, (1).
Structural commentary
As the nickel(II) ion is situated on an inversion center, the asymmetric unit of 1 contains one half of a nickel(II) ion, two coordinated water molecules, one 6-chloronicotinate ligand, one half of a 4,4′-bipyridine ligand and two water molecules of crystallization (Fig. 1 ▸). Therefore, the molecular structure of 1 comprises a one-dimensional polymeric {[Ni(4,4′-bpy)(H2O)4]2+}n cation and two 6-chloronicotinate anions and four uncoordinated water molecules per repeating polymeric unit. The nickel(II) ion in the polymeric {[Ni(4,4′-bpy)(H2O)4]2+}n cation is octahedrally coordinated by four water molecule O atoms (O1, O2, O1i and O2i) [symmetry code: (i) −x + 1, −y + 1, −z + 1] and by two 4,4′-bipyridine N atoms (N1 and N1i) in the trans position (N1i—Ni1—N1 = 180°). The 4,4′-bipyridine ligands act as bridges and, thus, connect the symmetry-related nickel(II) ions into infinite one-dimensional polymeric chains extending along the b-axis direction (Fig. 2 ▸).
Figure 1.
The molecular structure of 1, comprising a {[Ni(4,4′-bpy)(H2O)4]2+}n cation, 6-chloronicotinate anion and water molecules of crystallization. The atomic numbering scheme of the asymmetric unit is shown and displacement ellipsoids are drawn at the 40% probability level.
Figure 2.
The infinite one-dimensional polymeric chain of {[Ni(4,4′-bpy)(H2O)4]2+}n cations in 1, extending along the b-axis direction.
The octahedral coordination environment around the nickel(II) ion is only slightly distorted, as indicated by the angles for the cis pairs of the ligating atoms [89.00 (5)–91.00 (5)°]. The Ni1—O1 and Ni1—O2 bond lengths [2.0643 (15) Å and 2.0850 (13) Å, respectively] are very similar to each other and comparable to those seen in the related structures containing {[Ni(4,4′-bpy)(H2O)4]2+}n cation. The Ni—N1 bond length [2.0715 (14) Å] is also in agreement with those reported for the structures containing the {[Ni(4,4′-bpy)(H2O)4]2+}n cation (Zheng et al., 2002 ▸; Gong et al., 2009 ▸; Li, 2011 ▸; Gao et al., 2016 ▸; Sun et al., 2013 ▸; Wang et al., 2006 ▸; Sanram et al., 2016 ▸; Hu & Zhang, 2010 ▸).
The 4,4′-bypyridine ring is not coplanar with either coordinated water molecule O1 or O2 atoms and is rotated about the Ni1—N1 bond (by approximately 2°), as is evident from the torsion angles Ni1—N1—C5—C4 and Ni1—N1—C1—C2 [177.75 (16) and −177.83 (16)°, respectively].
Supramolecular features
The extended structure of 1 features strong O—H⋯O and O—H⋯N hydrogen bonds, weak C—H⋯O hydrogen bonds (Table 1 ▸) and π–π interactions [Cg2⋯Cg2; where Cg2 is the centroid of the 6-chloronicotinate pyridine ring N2/C6–C10; Cg2⋯Cg2 distance = 3.6769 (12) Å; dihedral angle between the planes = 0.00 (10)°; slippage = 1.085 Å]. The strong hydrogen bonds link the polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n, the 6-chloronicotinate anions and the water molecules of crystallization into an infinite three-dimensional network. The structure can be better analyzed if viewed down the b-axis direction (the direction along which the polymeric chain of {[Ni(4,4′-bpy)(H2O)4]2+}n runs). In that projection, the polymeric chains can be regarded as monomeric molecules that are interconnected with the 6-chloronicotinate anions and water molecules of crystallization into a hydrogen-bonded framework (Fig. 3 ▸). The polymeric chains are exclusively hydrogen-bonded to 6-chloronicotinate anions and water molecules, whilst the 6-chloronicotinate anions are additionally assembled by π–π interactions between symmetry-related 6-chloronicotinate pyridine rings.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H11⋯O3i | 0.81 (1) | 1.95 (1) | 2.756 (2) | 175 (2) |
| O1—H12⋯O5 | 0.82 (1) | 1.90 (1) | 2.715 (2) | 175 (2) |
| O2—H21⋯N2ii | 0.81 (1) | 2.08 (1) | 2.885 (2) | 172 (2) |
| O2—H22⋯O4 | 0.81 (1) | 1.96 (1) | 2.757 (2) | 169 (2) |
| O5—H51⋯O3iii | 0.82 (1) | 1.96 (1) | 2.776 (2) | 172 (3) |
| O5—H52⋯O6iv | 0.82 (1) | 2.01 (1) | 2.790 (3) | 160 (3) |
| O6—H61⋯O4 | 0.82 (1) | 1.94 (1) | 2.753 (2) | 177 (3) |
| O6—H62⋯O4v | 0.81 (1) | 2.23 (1) | 3.035 (3) | 174 (3) |
| C4—H4⋯O6ii | 0.93 | 2.40 | 3.288 (3) | 160 |
| C9—H9⋯O5vi | 0.93 | 2.53 | 3.447 (3) | 169 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  .
.
Figure 3.
A fragment of the infinite hydrogen-bonded network of 1 viewed along the b-axis direction. The polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n (represented as monomeric molecules in this projection), 6-chloronicotinate anions and water molecules of crystallization are connected by O—H⋯O and O—H⋯N hydrogen bonds (represented by dotted lines) within the hydrogen-bonded framework.
There are some representative supramolecular ring motifs within the hydrogen-bonded framework of 1: tetrameric  (8) and
(8) and  (10) motifs, a dimeric
(10) motifs, a dimeric  (8) motif and a pentameric
(8) motif and a pentameric  (16) motif (Fig. 4 ▸). The tetrameric
(16) motif (Fig. 4 ▸). The tetrameric  (8) motif is formed between two water molecules of crystallization and two 6-chloronicotinate anions (indicated in blue and green); each 6-chloronicotinate anion is linked via a single carboxylate O atom. The tetrameric
(8) motif is formed between two water molecules of crystallization and two 6-chloronicotinate anions (indicated in blue and green); each 6-chloronicotinate anion is linked via a single carboxylate O atom. The tetrameric  (10) motif is formed between the [Ni(4,4′-bpy)(H2O)4]2+}n cation, a 6-chloronicotinate anion (indicated in red and green, respectively) and two water molecules of crystallization; the cation participates in this motif via a coordinated water O atom and the 6-chloronicotinate anion via both carboxylate O atoms. The dimeric
(10) motif is formed between the [Ni(4,4′-bpy)(H2O)4]2+}n cation, a 6-chloronicotinate anion (indicated in red and green, respectively) and two water molecules of crystallization; the cation participates in this motif via a coordinated water O atom and the 6-chloronicotinate anion via both carboxylate O atoms. The dimeric  (8) motif is formed between the {[Ni(4,4′-bpy)(H2O)4]2+}n cation and the 6-chloronicotinate anion (indicated in red and brown, respectively); the cation is involved in this motif via two coordinated water O atoms and the 6-chloronicotinate anion via both carboxylate O atoms. Finally, the pentameric
(8) motif is formed between the {[Ni(4,4′-bpy)(H2O)4]2+}n cation and the 6-chloronicotinate anion (indicated in red and brown, respectively); the cation is involved in this motif via two coordinated water O atoms and the 6-chloronicotinate anion via both carboxylate O atoms. Finally, the pentameric  (16) motif is composed of the {[Ni(4,4′-bpy)(H2O)4]2+}n cation, two 6-chloronicotinate anions (indicated in red, green and pink) and two water molecules of crystallization; the cation participates in this motif via two coordinated water O atoms, one 6-chloronicotinate anion (shown in green) via both carboxylate O atoms and the pyridine N atom and the other 6-chloronicotinate anion (shown in pink) via its carboxylate O atom only (Fig. 4 ▸). Both coordinated water molecules and water molecules of crystallization participate in the formation of motifs as single- and double-proton donors [coordinated water molecules as single-proton donors in the
(16) motif is composed of the {[Ni(4,4′-bpy)(H2O)4]2+}n cation, two 6-chloronicotinate anions (indicated in red, green and pink) and two water molecules of crystallization; the cation participates in this motif via two coordinated water O atoms, one 6-chloronicotinate anion (shown in green) via both carboxylate O atoms and the pyridine N atom and the other 6-chloronicotinate anion (shown in pink) via its carboxylate O atom only (Fig. 4 ▸). Both coordinated water molecules and water molecules of crystallization participate in the formation of motifs as single- and double-proton donors [coordinated water molecules as single-proton donors in the  (16) and
(16) and  (8) motifs and double-proton donors in the
(8) motifs and double-proton donors in the  (10) motif only; water molecules of crystallization as single-proton donors in the
(10) motif only; water molecules of crystallization as single-proton donors in the  (16) motifs and
(16) motifs and  (10) motifs and double-proton donors in the
(10) motifs and double-proton donors in the  (16) and
(16) and  (8) motifs]. The water molecules of crystallization also participate in some of these motifs [
(8) motifs]. The water molecules of crystallization also participate in some of these motifs [ (16) and
(16) and  (10)] as single-proton acceptors. The 6-chloronicotinate pyridine N atoms act as single-proton acceptors in the
(10)] as single-proton acceptors. The 6-chloronicotinate pyridine N atoms act as single-proton acceptors in the  (16) motif only, whilst the carboxylate O atoms act as both single- and double-proton acceptors [single in the
(16) motif only, whilst the carboxylate O atoms act as both single- and double-proton acceptors [single in the  (16),
(16),  (8) and
(8) and  (10) motifs and double in the
(10) motifs and double in the  (16) and
(16) and  (8) motifs]. Two weak C—H⋯O interactions are also observed (Table 1 ▸).
(8) motifs]. Two weak C—H⋯O interactions are also observed (Table 1 ▸).
Figure 4.
The representative hydrogen-bonded ring motifs (shown by dotted lines) found within the hydrogen-bonded framework of 1, viz. the tetrameric  (8) and
(8) and  (10) motifs, a dimeric
(10) motifs, a dimeric  (8) motif and a pentameric
(8) motif and a pentameric  (16) motif. The polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n are represented as momomeric molecules and shown in red, and various symmetry-related 6-chloronicotinate anions are shown in brown, green, blue and pink (see text).
(16) motif. The polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n are represented as momomeric molecules and shown in red, and various symmetry-related 6-chloronicotinate anions are shown in brown, green, blue and pink (see text).
There are no weak C—H⋯Cl interactions in the extended structure of 1; we hoped that these interactions could have an impact on the assembly of the polymeric chains within the hydrogen-bonding framework of 1: the polymeric chains do not contain the 6-chloronicotinate ligands, but the uncoordinated 6-chloronicotinate anions could still participate in these interactions. However, the possible C—H⋯Cl interactions are most probably hindered by the extensive hydrogen bonding, involving strong O—H⋯O and O—H⋯N hydrogen bonds, which is reflected in the formation of various hydrogen-bonded motifs. This was expected because of the participation of the water molecules of crystallization in the crystal packing of 1, since the compound was crystallized from a mixed water–ethanol solution.
Database survey
Our aim in this work was to prepare a nickel(II) coordination polymer with the mixed ligands 6-chloronicotinate and 4,4′-bipyridine. However, we obtained a cationic nickel(II) coordination polymer with 4,4′-bipyridine, {[Ni(4,4′-bpy)(H2O)4]2+}n. The 6-chloronicotinate is not coordinated to the metal ion, but acts as a counter-ion. This was surprising, as we expected to obtain a coordination polymer similar to the one obtained with the closely related 6-fluoronicotinate anion under the same experimental conditions (Politeo et al., 2020 ▸). The polymeric {[Ni(4,4′-bpy)(H2O)4]2+}n cation is already well known from the literature, as it crystallizes with various carboxylate anions such as fumarate (Zheng et al., 2002 ▸), 3-[4-(carboxymethoxy) phenyl]propanoate (Gong et al., 2009 ▸), 3,3′-(p-phenylene)diacrylate (Li, 2011 ▸), 2-carboxy-4-[4-(3-carboxy-4-carboxylatophenoxy)phenoxy]benzoate (Gao et al., 2016 ▸), 3-(4-carboxyphenyl)propanoate (Sun et al., 2013 ▸), 1,2,4,5-benzenetetracarboxylate (Wang et al., 2006 ▸), 1,4-phenylenedipropanoate (Sanram et al., 2016 ▸) and 2,3-naphthalenedicarboxylate (Hu & Zhang, 2010 ▸).
PXRD and thermal analysis
The experimental and calculated PXRD traces of 1 (Fig. 5 ▸) match nicely, indicating the phase purity of the bulk of 1.
Figure 5.
Experimental (bottom) and calculated (top) PXRD traces for 1.
Compound 1 is thermally stable only up to 40°C (Fig. S1 in the supporting information). Both the coordinated (four) and uncoordinated (four) water molecules were released in the same step (observed mass loss 20.3%, calculated 21.4%), with a pronounced endothermic peak in the DSC curve at 90°C. The thermal decomposition of 1 continues in a broad step (observed mass loss 55.2%) in the wide temperature range of 145–590°C (with two small peaks in the DSC curve at 216 and 480°C), which probably corresponds to the complete degradation of 1. The remaining residue at 600°C is most probably NiO.
Materials and methods
All chemicals for the synthesis were purchased from commercial sources (Merck) and used as received without further purification. The IR spectrum was obtained in the range 4000–400 cm−1 on a Perkin–Elmer Spectrum TwoTM FTIR spectrometer in the ATR mode. The PXRD trace was recorded on a Philips PW 1850 diffractometer, Cu Kα radiation, voltage 40 kV, current 40 mA, in the angle range 5–50° (2θ) with a step size of 0.02°. Simultaneous TGA/DSC measurements were performed at a heating rate of 10°C min−1 in the temperature range 25–600°C, under a nitrogen flow of 50 ml min−1 on a Mettler–Toledo TGA/DSC 3+ instrument. Approximately 2 mg of sample was placed in a standard alumina crucible (70 µl).
Synthesis and crystallization
6-Chloronicotinic acid (0.0525 g, 0.3332 mmol) was dissolved in distilled water (5 ml) using an ultrasonic water bath, 4,4′-bipyridine (0.0244 g, 0.1562 mmol) was dissolved in ethanol (2 ml) and nickel(II) sulfate heptahydrate (0.0446 g, 0.1588 mmol) was dissolved in distilled water (2 ml). The solutions of the two ligands were first mixed together under stirring. The resulting solution was then slowly added to the nickel(II) sulfate solution under stirring. The pH of the final solution was adjusted to 7 by adding an ammonia solution dropwise. The obtained, clear solution was left to slowly evaporate at room temperature for approximately three weeks until light-green crystals of 1, suitable for X-ray diffraction measurements, were obtained, which were collected by filtration, washed with their mother liquor and dried in vacuo. Yield: 0.0496 g (46%). Selected IR bands (ATR) (ν, cm−1): 3376 [ν(O—H)], 3078, 3059 [ν(C—H)], 1615 [ν(C=O)], 1579, 1539, 1419, 1388, 1360 [ν(C—C), ν(C—N)] (Fig. S2, Table S1 in the supporting information).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. C-bound H atoms were positioned geometrically and refined using riding model [C—H = 0.93 Å, U iso(H) = 1.2U eq(C) for the aromatic H atoms]. The H atoms belonging to the water molecules were found in the difference-Fourier maps. The O—H distance was restrained to an average value of 0.82 Å using DFIX and DANG instructions. The isotropic U iso(H) values were also fixed [U iso(H) = 1.2U eq(O)].
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | {[Ni(C10H8N2)(H2O)4](C6H3ClNO2)2·4H2O}n |
| M r | 672.11 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 296 |
| a, b, c (Å) | 10.7997 (3), 11.2319 (2), 12.0225 (3) |
| β (°) | 95.184 (2) |
| V (Å3) | 1452.38 (6) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.92 |
| Crystal size (mm) | 0.24 × 0.18 × 0.16 |
| Data collection | |
| Diffractometer | Oxford Diffraction Xcalibur2 diffractometer with Sapphire 3 CCD detector |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2018 ▸) |
| T min, T max | 0.927, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 11778, 2541, 2144 |
| R int | 0.025 |
| (sin θ/λ)max (Å−1) | 0.595 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.029, 0.074, 1.07 |
| No. of reflections | 2541 |
| No. of parameters | 211 |
| No. of restraints | 12 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.23, −0.23 |
The highest difference peak is 0.86 Å away from the O4 atom and the deepest difference hole is 0.84 Å away from the Cl1 atom.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020004193/hb7900sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020004193/hb7900Isup2.hkl
IR, TGA and DSC data. DOI: 10.1107/S2056989020004193/hb7900sup3.docx
CCDC reference: 1992951
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [Ni(C10H8N2)(H2O)4](C6H3ClNO2)2·4H2O | F(000) = 696 |
| Mr = 672.11 | Dx = 1.537 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.7997 (3) Å | Cell parameters from 6296 reflections |
| b = 11.2319 (2) Å | θ = 4.4–32.2° |
| c = 12.0225 (3) Å | µ = 0.92 mm−1 |
| β = 95.184 (2)° | T = 296 K |
| V = 1452.38 (6) Å3 | Prism, light-green |
| Z = 2 | 0.24 × 0.18 × 0.16 mm |
Data collection
| Oxford Diffraction Xcalibur2 diffractometer with Sapphire 3 CCD detector | 2144 reflections with I > 2σ(I) |
| ω–scan | Rint = 0.025 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2018) | θmax = 25.0°, θmin = 4.2° |
| Tmin = 0.927, Tmax = 1.000 | h = −12→12 |
| 11778 measured reflections | k = −13→13 |
| 2541 independent reflections | l = −14→14 |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.029 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.074 | w = 1/[σ2(Fo2) + (0.0367P)2 + 0.3591P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max < 0.001 |
| 2541 reflections | Δρmax = 0.23 e Å−3 |
| 211 parameters | Δρmin = −0.23 e Å−3 |
| 12 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ni1 | 0.500000 | 0.500000 | 0.500000 | 0.02724 (12) | |
| Cl1 | 0.14959 (7) | −0.20644 (6) | 0.64253 (6) | 0.0683 (2) | |
| N1 | 0.49579 (14) | 0.68437 (13) | 0.49747 (12) | 0.0302 (4) | |
| N2 | 0.14853 (18) | 0.02547 (18) | 0.65643 (15) | 0.0489 (5) | |
| O1 | 0.63069 (14) | 0.50565 (11) | 0.63625 (12) | 0.0365 (3) | |
| H11 | 0.6796 (16) | 0.5604 (14) | 0.6359 (19) | 0.044* | |
| H12 | 0.6766 (17) | 0.4477 (13) | 0.6434 (18) | 0.044* | |
| O2 | 0.35666 (14) | 0.50182 (12) | 0.60512 (12) | 0.0381 (3) | |
| H21 | 0.363 (2) | 0.5067 (18) | 0.6729 (8) | 0.046* | |
| H22 | 0.2918 (13) | 0.4706 (19) | 0.5828 (17) | 0.046* | |
| O3 | 0.21575 (15) | 0.29848 (13) | 0.36561 (13) | 0.0513 (4) | |
| O4 | 0.15477 (15) | 0.36711 (14) | 0.52437 (14) | 0.0577 (5) | |
| O5 | 0.77684 (18) | 0.31004 (15) | 0.67043 (15) | 0.0578 (5) | |
| H51 | 0.765 (2) | 0.280 (2) | 0.7309 (13) | 0.069* | |
| H52 | 0.8451 (14) | 0.341 (2) | 0.670 (2) | 0.069* | |
| O6 | −0.02489 (19) | 0.4641 (2) | 0.64545 (15) | 0.0691 (5) | |
| H61 | 0.027 (2) | 0.434 (2) | 0.608 (2) | 0.083* | |
| H62 | −0.063 (2) | 0.511 (2) | 0.604 (2) | 0.083* | |
| C1 | 0.5043 (2) | 0.74728 (16) | 0.40446 (16) | 0.0366 (5) | |
| H1 | 0.509493 | 0.706173 | 0.337893 | 0.044* | |
| C2 | 0.5057 (2) | 0.86964 (16) | 0.40158 (16) | 0.0368 (5) | |
| H2 | 0.511821 | 0.909144 | 0.334314 | 0.044* | |
| C3 | 0.49798 (17) | 0.93401 (15) | 0.49898 (15) | 0.0280 (4) | |
| C4 | 0.48697 (19) | 0.86865 (16) | 0.59518 (16) | 0.0361 (5) | |
| H4 | 0.480202 | 0.907506 | 0.662667 | 0.043* | |
| C5 | 0.48605 (19) | 0.74622 (15) | 0.59102 (16) | 0.0359 (5) | |
| H5 | 0.478228 | 0.704445 | 0.656807 | 0.043* | |
| C6 | 0.1538 (2) | 0.1347 (2) | 0.61298 (18) | 0.0456 (5) | |
| H6 | 0.147862 | 0.199385 | 0.660541 | 0.055* | |
| C7 | 0.16748 (18) | 0.15756 (17) | 0.50216 (16) | 0.0356 (5) | |
| C8 | 0.1733 (2) | 0.06049 (18) | 0.43203 (17) | 0.0414 (5) | |
| H8 | 0.180909 | 0.072105 | 0.356359 | 0.050* | |
| C9 | 0.1679 (2) | −0.05304 (19) | 0.47386 (18) | 0.0439 (5) | |
| H9 | 0.172347 | −0.119476 | 0.428227 | 0.053* | |
| C10 | 0.1557 (2) | −0.06397 (19) | 0.58621 (18) | 0.0429 (5) | |
| C11 | 0.17917 (18) | 0.28366 (18) | 0.46017 (19) | 0.0419 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ni1 | 0.0411 (2) | 0.01267 (17) | 0.02797 (19) | −0.00048 (13) | 0.00315 (14) | 0.00009 (12) |
| Cl1 | 0.0830 (5) | 0.0554 (4) | 0.0687 (4) | 0.0079 (3) | 0.0195 (4) | 0.0253 (3) |
| N1 | 0.0420 (9) | 0.0166 (7) | 0.0319 (8) | 0.0007 (6) | 0.0030 (7) | −0.0009 (6) |
| N2 | 0.0538 (12) | 0.0589 (12) | 0.0347 (10) | −0.0021 (9) | 0.0074 (9) | 0.0018 (9) |
| O1 | 0.0476 (9) | 0.0236 (7) | 0.0370 (8) | −0.0015 (6) | −0.0024 (7) | 0.0019 (6) |
| O2 | 0.0462 (9) | 0.0351 (8) | 0.0340 (7) | −0.0063 (6) | 0.0083 (7) | −0.0039 (6) |
| O3 | 0.0695 (11) | 0.0353 (8) | 0.0500 (10) | −0.0096 (7) | 0.0104 (8) | −0.0005 (7) |
| O4 | 0.0557 (10) | 0.0410 (9) | 0.0795 (12) | −0.0068 (7) | 0.0228 (9) | −0.0210 (8) |
| O5 | 0.0731 (13) | 0.0458 (10) | 0.0537 (10) | 0.0071 (8) | 0.0020 (10) | 0.0065 (8) |
| O6 | 0.0751 (14) | 0.0873 (14) | 0.0481 (10) | 0.0270 (10) | 0.0226 (10) | 0.0193 (9) |
| C1 | 0.0597 (14) | 0.0203 (9) | 0.0305 (10) | −0.0001 (8) | 0.0075 (10) | −0.0023 (8) |
| C2 | 0.0601 (14) | 0.0186 (9) | 0.0325 (11) | −0.0011 (8) | 0.0082 (10) | 0.0023 (8) |
| C3 | 0.0325 (10) | 0.0169 (9) | 0.0346 (10) | 0.0011 (7) | 0.0023 (8) | 0.0008 (7) |
| C4 | 0.0578 (13) | 0.0201 (9) | 0.0308 (10) | 0.0006 (8) | 0.0061 (9) | −0.0036 (8) |
| C5 | 0.0570 (13) | 0.0190 (9) | 0.0321 (11) | −0.0002 (8) | 0.0057 (9) | 0.0042 (8) |
| C6 | 0.0457 (13) | 0.0504 (14) | 0.0410 (12) | −0.0061 (10) | 0.0055 (10) | −0.0107 (10) |
| C7 | 0.0318 (11) | 0.0374 (11) | 0.0376 (11) | −0.0035 (8) | 0.0039 (9) | −0.0055 (9) |
| C8 | 0.0526 (14) | 0.0384 (12) | 0.0340 (11) | −0.0028 (10) | 0.0081 (10) | −0.0004 (9) |
| C9 | 0.0543 (14) | 0.0353 (11) | 0.0432 (13) | 0.0000 (10) | 0.0094 (11) | −0.0036 (10) |
| C10 | 0.0408 (13) | 0.0447 (13) | 0.0440 (13) | 0.0027 (9) | 0.0080 (10) | 0.0089 (10) |
| C11 | 0.0326 (12) | 0.0358 (11) | 0.0570 (14) | −0.0041 (9) | 0.0032 (10) | −0.0103 (10) |
Geometric parameters (Å, º)
| Ni1—O1i | 2.0643 (15) | O6—H61 | 0.818 (10) |
| Ni1—O1 | 2.0643 (15) | O6—H62 | 0.813 (10) |
| Ni1—N1i | 2.0715 (14) | C1—C2 | 1.375 (3) |
| Ni1—N1 | 2.0715 (14) | C1—H1 | 0.9300 |
| Ni1—O2i | 2.0850 (13) | C2—C3 | 1.385 (2) |
| Ni1—O2 | 2.0850 (13) | C2—H2 | 0.9300 |
| Cl1—C10 | 1.741 (2) | C3—C4 | 1.384 (2) |
| N1—C1 | 1.333 (2) | C3—C3ii | 1.483 (3) |
| N1—C5 | 1.334 (2) | C4—C5 | 1.376 (3) |
| N2—C10 | 1.319 (3) | C4—H4 | 0.9300 |
| N2—C6 | 1.337 (3) | C5—H5 | 0.9300 |
| O1—H11 | 0.811 (9) | C6—C7 | 1.378 (3) |
| O1—H12 | 0.818 (9) | C6—H6 | 0.9300 |
| O2—H21 | 0.813 (9) | C7—C8 | 1.383 (3) |
| O2—H22 | 0.807 (10) | C7—C11 | 1.513 (3) |
| O3—C11 | 1.248 (2) | C8—C9 | 1.374 (3) |
| O4—C11 | 1.257 (2) | C8—H8 | 0.9300 |
| O5—H51 | 0.822 (10) | C9—C10 | 1.375 (3) |
| O5—H52 | 0.815 (10) | C9—H9 | 0.9300 |
| O1i—Ni1—O1 | 180.0 | C1—C2—C3 | 119.92 (17) |
| O1i—Ni1—N1i | 89.62 (6) | C1—C2—H2 | 120.0 |
| O1—Ni1—N1i | 90.37 (6) | C3—C2—H2 | 120.0 |
| O1i—Ni1—N1 | 90.38 (6) | C4—C3—C2 | 116.48 (16) |
| O1—Ni1—N1 | 89.62 (6) | C4—C3—C3ii | 121.39 (19) |
| N1i—Ni1—N1 | 180.0 | C2—C3—C3ii | 122.13 (19) |
| O1i—Ni1—O2i | 90.61 (6) | C5—C4—C3 | 120.04 (17) |
| O1—Ni1—O2i | 89.39 (6) | C5—C4—H4 | 120.0 |
| N1i—Ni1—O2i | 89.00 (5) | C3—C4—H4 | 120.0 |
| N1—Ni1—O2i | 91.00 (5) | N1—C5—C4 | 123.37 (17) |
| O1i—Ni1—O2 | 89.39 (6) | N1—C5—H5 | 118.3 |
| O1—Ni1—O2 | 90.61 (6) | C4—C5—H5 | 118.3 |
| N1i—Ni1—O2 | 91.00 (5) | N2—C6—C7 | 124.1 (2) |
| N1—Ni1—O2 | 89.00 (5) | N2—C6—H6 | 117.9 |
| O2i—Ni1—O2 | 180.0 | C7—C6—H6 | 117.9 |
| C1—N1—C5 | 116.61 (16) | C6—C7—C8 | 117.23 (19) |
| C1—N1—Ni1 | 122.63 (12) | C6—C7—C11 | 121.10 (18) |
| C5—N1—Ni1 | 120.76 (12) | C8—C7—C11 | 121.65 (18) |
| C10—N2—C6 | 116.23 (18) | C9—C8—C7 | 120.18 (19) |
| Ni1—O1—H11 | 114.7 (16) | C9—C8—H8 | 119.9 |
| Ni1—O1—H12 | 115.0 (16) | C7—C8—H8 | 119.9 |
| H11—O1—H12 | 102 (2) | C8—C9—C10 | 117.0 (2) |
| Ni1—O2—H21 | 127.6 (17) | C8—C9—H9 | 121.5 |
| Ni1—O2—H22 | 117.6 (16) | C10—C9—H9 | 121.5 |
| H21—O2—H22 | 111 (2) | N2—C10—C9 | 125.3 (2) |
| H51—O5—H52 | 113 (3) | N2—C10—Cl1 | 116.40 (16) |
| H61—O6—H62 | 105 (3) | C9—C10—Cl1 | 118.34 (17) |
| N1—C1—C2 | 123.56 (17) | O3—C11—O4 | 124.1 (2) |
| N1—C1—H1 | 118.2 | O3—C11—C7 | 118.15 (17) |
| C2—C1—H1 | 118.2 | O4—C11—C7 | 117.69 (19) |
| C5—N1—C1—C2 | 1.2 (3) | N2—C6—C7—C11 | −176.9 (2) |
| Ni1—N1—C1—C2 | −177.83 (16) | C6—C7—C8—C9 | −1.2 (3) |
| N1—C1—C2—C3 | 0.0 (3) | C11—C7—C8—C9 | 177.10 (19) |
| C1—C2—C3—C4 | −1.1 (3) | C7—C8—C9—C10 | 0.5 (3) |
| C1—C2—C3—C3ii | 178.6 (2) | C6—N2—C10—C9 | 0.0 (3) |
| C2—C3—C4—C5 | 1.0 (3) | C6—N2—C10—Cl1 | 179.70 (16) |
| C3ii—C3—C4—C5 | −178.7 (2) | C8—C9—C10—N2 | 0.1 (3) |
| C1—N1—C5—C4 | −1.3 (3) | C8—C9—C10—Cl1 | −179.55 (16) |
| Ni1—N1—C5—C4 | 177.75 (16) | C6—C7—C11—O3 | 166.0 (2) |
| C3—C4—C5—N1 | 0.2 (3) | C8—C7—C11—O3 | −12.3 (3) |
| C10—N2—C6—C7 | −0.8 (3) | C6—C7—C11—O4 | −12.0 (3) |
| N2—C6—C7—C8 | 1.4 (3) | C8—C7—C11—O4 | 169.7 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+1, −y+2, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H11···O3i | 0.81 (1) | 1.95 (1) | 2.756 (2) | 175 (2) |
| O1—H12···O5 | 0.82 (1) | 1.90 (1) | 2.715 (2) | 175 (2) |
| O2—H21···N2iii | 0.81 (1) | 2.08 (1) | 2.885 (2) | 172 (2) |
| O2—H22···O4 | 0.81 (1) | 1.96 (1) | 2.757 (2) | 169 (2) |
| O5—H51···O3iv | 0.82 (1) | 1.96 (1) | 2.776 (2) | 172 (3) |
| O5—H52···O6v | 0.82 (1) | 2.01 (1) | 2.790 (3) | 160 (3) |
| O6—H61···O4 | 0.82 (1) | 1.94 (1) | 2.753 (2) | 177 (3) |
| O6—H62···O4vi | 0.81 (1) | 2.23 (1) | 3.035 (3) | 174 (3) |
| C4—H4···O6iii | 0.93 | 2.40 | 3.288 (3) | 160 |
| C9—H9···O5vii | 0.93 | 2.53 | 3.447 (3) | 169 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (iii) −x+1/2, y+1/2, −z+3/2; (iv) x+1/2, −y+1/2, z+1/2; (v) x+1, y, z; (vi) −x, −y+1, −z+1; (vii) −x+1, −y, −z+1.
Funding Statement
This work was funded by Foundation of the Croatian Academy of Sciences grant . University of Split grant .
References
- Bosch, M., Yuan, S., Rutledge, W. & Zhou, H.-C. (2017). Acc. Chem. Res. 50, 857–865. [DOI] [PubMed]
- Douvali, A., Tsipis, A. C., Eliseeva, S. V., Petoud, S., Papaefstathiou, G. S., Malliakas, C. D., Papadas, I., Armatas, G. S., Margiolaki, I., Kanatzidis, M. G., Lazarides, T. & Manos, M. J. (2015). Angew. Chem. Int. Ed. 54, 1651–1656. [DOI] [PubMed]
- Gao, P., Bai, H., Bing, Y.-Y. & Hu, M. (2016). Solid State Sci. 52, 118–125.
- Gong, Y.-N., Liu, C.-B., Huang, D.-H. & Xiong, Z.-Q. (2009). Z. Kristallogr. New Cryst. Struct. 224, 421–422.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Gu, J., Cui, Y., Liang, X., Wu, J., Lv, D. & Kirillov, A. M. (2016). Cryst. Growth Des. 16, 4658–4670.
- Gu, J.-Z., Cai, Y., Liang, X.-X., Wu, J., Shi, Z.-F. & Kirillov, A. M. (2018). CrystEngComm, 20, 906–916.
- Gu, J.-Z., Liang, X.-X., Cai, Y., Wu, J., Shi, Z.-F. & Kirillov, A. M. (2017). Dalton Trans. 46, 10908–10925. [DOI] [PubMed]
- Hu, M. & Zhang, Q. (2010). Z. Kristallogr. New Cryst. Struct. 225, 155–156.
- Li, F.-H., Yin, H.-D., Sun, L., Zhao, Q. & Liu, W.-L. (2006). Acta Cryst. E62, m1117–m1118.
- Li, J.-J., Fan, T.-T., Qu, X.-L., Han, H.-L. & Li, X. (2016). Dalton Trans. 45, 2924–2935. [DOI] [PubMed]
- Li, N.-Y. (2011). Acta Cryst. E67, m1397. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Mueller, U., Schubert, M., Teich, F., Puetter, H., Schierle-Arndt, K. & Pastré, J. (2006). J. Mater. Chem. 16, 626–636.
- Politeo, N., Pisačić, M., Đaković, M., Sokol, V. & Kukovec, B.-M. (2020). Acta Cryst. E76, 500–505. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2018). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sanram, S., Boonmak, J. & Youngme, S. (2016). Polyhedron, 119, 151–159.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Sun, C.-Y., Li, W.-J. & Che, P. (2013). Z. Anorg. Allg. Chem. 639, 129–133.
- Wang, H.-H., Yang, H.-Y., Shu, C.-H., Chen, Z.-Y., Hou, L. & Wang, Y.-Y. (2016). Cryst. Growth Des. 16, 5394–5402.
- Wang, X.-L., Qin, C. & Wang, E.-B. (2006). Cryst. Growth Des. 6, 439–443.
- Xia, Q.-H., Guo, Z.-F., Liu, L., Lv, J.-Q. & Li, B. (2012a). Acta Cryst. E68, m1393. [DOI] [PMC free article] [PubMed]
- Xia, Q.-H., Zhang, Y., Liu, L., Shi, L.-F. & Li, B. (2012b). Acta Cryst. E68, m1394. [DOI] [PMC free article] [PubMed]
- Xu, M., Yuan, S., Chen, X.-Y., Chang, Y.-J., Day, G., Gu, Z.-Y. & Zhou, H.-C. (2017). J. Am. Chem. Soc. 139, 8312–8319. [DOI] [PubMed]
- Zeng, M.-H., Yin, Z., Liu, Z.-H., Xu, H.-B., Feng, Y.-C., Hu, Y.-Q., Chang, L.-X., Zhang, Y.-X., Huang, J. & Kurmoo, M. (2016). Angew. Chem. Int. Ed. 55, 11407–11411. [DOI] [PubMed]
- Zeng, M.-H., Yin, Z., Tan, Y.-X., Zhang, W.-X., He, Y.-P. & Kurmoo, M. (2014). J. Am. Chem. Soc. 136, 4680–4688. [DOI] [PubMed]
- Zhang, W.-X., Liao, P.-Q., Lin, R.-B., Wei, Y.-S., Zeng, M.-H. & Chen, X.-M. (2015). Coord. Chem. Rev. 293–294, 263–278.
- Zhang, Y.-X., Lin, H., Wen, Y. & Zhu, Q.-L. (2019). Cryst. Growth Des. 19, 1057–1063.
- Zheng, Y.-Q., Kong, Z.-P. & Lin, J.-L. (2002). Z. Kristallogr. New Cryst. Struct. 217, 195–196.
- Zhou, H.-F., He, T., Yue, K.-F., Liu, Y.-L., Zhou, C.-S., Yan, N. & Wang, Y.-Y. (2016). Cryst. Growth Des. 16, 3961–3968.
- Zhou, Z., He, C., Yang, L., Wang, Y., Liu, T. & Duan, C. (2017). ACS Catal. 7, 2248–2256.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020004193/hb7900sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020004193/hb7900Isup2.hkl
IR, TGA and DSC data. DOI: 10.1107/S2056989020004193/hb7900sup3.docx
CCDC reference: 1992951
Additional supporting information: crystallographic information; 3D view; checkCIF report







