Abstract
Purpose:
We assessed the relevance of Slug (SNAI2) for apoptosis resistance and invasion potential of neuroblastoma (NB) cells in vitro and in vivo.
Experimental Design:
We evaluated the effect of Imatinib Mesylate (IM) on invasion and analyzed the genes modulated by IM treatment in NB cells. Slug expression, inhibited by IM treatment, was knocked-down in NB cells by RNA interference and the effects on invasion and apoptosis were evaluated in vitro. A pseudometastatic model of NB in SCID mice was used to assess the effects of Slug-silencing alone or in combination with IM treatment on metastasis development.
Results:
Microarray analysis revealed that several genes, including Slug, were down-regulated by IM. Slug expression was detectable in 8 of 10 human NB cell lines. Two Slug-expressing cell lines were infected with a vector encoding a miRNA to Slug mRNA. Infected cells with reduced levels of Slug were tested for the expression of apoptosis-related genes (p53, Bax, Bcl-2), previously identified as Slug targets. Bcl-2 was down-regulated in Slug-interfered cells. Slug down-regulation increased sensitivity to apoptosis induced by IM, etoposide or doxorubicin. Invasion of Slug-silenced cells was reduced in vitro. Animals injected with Slug-silenced cells had fewer tumors than controls and the inhibition of tumor growth was even higher in animals treated with IM.
Conclusions:
Slug down-regulation facilitates apoptosis induced by pro-apoptotic drugs in NB cells and decreases their invasion capability in vitro and in vivo. Slug inhibition, possibly combined with IM, may represent a novel strategy for treatment of metastatic NB.
Keywords: Slug, metastasis, neuroblastoma, Imatinib Mesylate, apoptosis
Introduction
Formation of secondary tumors (metastasis) in organs distant from the primary neoplasm is the leading cause of death in cancer patients (1). Along the metastatic route, tumor cells invade the tissues surrounding the primary tumor, enter either the lymphatics or the bloodstream, survive and eventually arrest in the circulation, extravasate into a tissue and grow at the distant site (2). To fulfil these tasks, cancer cells must undergo profound and interconnected changes that modify their morphology and function. At molecular level, these changes are driven by the modulation of several genes whose identity is not yet completely known. Schematically, the picture emerging from recent data suggests that tumor cells transit to mesenchymal phenotype (3, 4), upregulate specialized proteases (5) and expose membrane receptors that allow them to home to permissive locations (6). In addition, metastatic cells also need to activate angiogenesis at distant sites (7) by releasing angiogenic factors. A growing body of evidence has pointed out to the relevance of several distinct families of transcription factors able to mediate the changes required for local invasion, the initial and critical step to metastasis (4).
Neuroblastoma (NB) is the most common extra-cranial childhood tumor with a prevalence of one case in 7,000 live births (8). NB derives from cells of the sympatoadrenal lineage of the neural crest that arrest at some stage of their differentiation (8). Approximately 40% of newly diagnosed patients present with localized disease that, in most cases, is easily curable or regresses spontaneously (9). By contrast, about 50% of patients present with metastatic disease associated with very poor outcome despite intensive therapeutic protocols including high-dose chemotherapy and myeloablative regimens followed by hemopoietic rescue (10). A peculiar type of NB, so called stage 4S, characterized by a small primary tumor and a widespread involvement of liver, skin, or bone marrow that almost always spontaneously regress (11), accounts for approximately 5–7% of the cases. The clinical heterogeneity of NB is mirrored by the complexity of the genetics features that characterize this tumor (8). Apart from MYCN genomic amplification, the alteration more consistently associated with poor outcome (12), several other genetic changes are currently used to better define prognosis (9).
The neuroectodermal origin of NB, suggests that this tumor can spread from the primary site using mechanisms similar to those operating during the formation and delamination of the embryonic neural crest (13). Thus, the same gene(s) that are relevant for the migration of neural crest cells may also play a role in the acquisition of the invasive phenotype of NB cells.
Imatinib Mesylate (Gleevec, Glivec, STI-571) is a soluble small molecule successfully used to treat Chronic Myelogenous Leukemia (CML) for its inhibitory activity of the BCR-ABL tyrosine kinase (14). Subsequently, the use of Imatinib Mesylate (IM) has been extended to gastrointestinal stromal tumors (GIST) where it acts by inhibiting c-Kit (15). We and others demonstrated that IM is also active in inhibiting NB growth in vitro and in vivo (16–18). Of interest, other studies confirming the anti-proliferative activity of IM in NB have suggested that the drug’s activity is not strictly dependent on c-Kit inhibition (19, 20). It should be also noted that a recent clinical trial on refractory or relapsed childhood solid tumors including NB showed that IM as a single agent at a dose of 440 mg/m2/day demonstrates little or no activity (21).
In this study, we evaluated the effect of IM in reducing invasion and metastasis in NB cells. By applying microarray analysis, we identified the transcription factor Slug (SNAI2) (4) as a target of IM. By inhibiting Slug expression by RNA interference, we found that reduced Slug levels decreased survival and invasion in NB. Finally, in a pseudometastatic model of NB in mice, we demonstrated an additive effect of Slug-silencing and IM treatment in reducing the metastatic burden. These data suggest that Slug is a relevant gene for regulation of the metastatic potential of NB cells and that strategies combining IM treatment and Slug inhibition may be suitable as a treatment of metastatic NB.
Materials and Methods
Cell lines.
Human NB cell lines SK-N-BE2c, SK-N-BE, SK-N-AS, SH-EP, KCNR, ACN, RN-GA, GI-CA-N, LAN-5, HTLA-230 were kept in culture as previously described (16).
Reagents.
IM was provided by Novartis Pharma AG (Basel, Switzerland), Etoposide by Bristol-Myers Squibb (Latina, Italy) and Doxorubicin by Pharmacia (Milan, Italy).
Microarray analysis.
Total RNA was extracted from cells seeded on Matrigel®-coated dishes (BD Biosciences, Bedford, MA) using RNeasy spin columns (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Double-stranded cDNA was synthesized starting with 5 μg of total RNA using the SuperScript System (Invitrogen). The cDNA was purified by filtration through Multiscreen filter plate (Millipore), and transcribed in vitro using T7 RNA polymerase (Epicenter, Madison, WI) and biotinylated nucleotides (PerkinElmer Life Sciences). Hybridization buffer containing the spike pool reagent was added to each of the fragmented cRNA mixtures and each sample was hybridized to the Human Genome U133 2.0 array (Affymetrix, Santa Clara, CA) at 45 °C for 18 h as recommended by the manufacturer. The hybridized chips were washed and stained using Affymetrix Fluidics Station 450 and the EukGE-WS2v4_450 protocol as recommended by the manufacturer. The staining was performed using streptavidin-phycoerythrin conjugate (Molecular Probes, Eugene, OR), followed by biotinylated antibody against streptavidin (Vector Laboratories, Burlingame, CA), and then streptavidin-phycoerythrin. The chips were scanned using an Affymetrix Genechip Scanner and .cel files were generated with Affymetrix Microarray Suite 5.0 (MAS 5.0) software. Using normalized data, after removing lowly expressed genes (those with a signal value below 43), and those that were consistently called absent “A”, we imposed a non-stringent 1.1 fold change between IM-treated and untreated samples. A t-test was performed, and qualifiers with a p-Value less than or equal to 0.05 were then subjected to false discovery testing (FDR) to select qualifiers with an FDR of less than or equal to 25%. These analyses were independently performed on expression data from both the GI-CA-N and HTLA-230. Using these criteria, a total of 21 qualifiers were regulated by IM in both cell lines. Slug was represented by one of these qualifiers.
Quantitative real-time PCR.
Total RNA was extracted by RNeasy extraction kit (QIAGEN Inc. Valencia, CA) and reverse transcribed according to standard protocols. Real-time PCR was carried out using an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). TAQMAN® technology; the Assays-On-Demand kit (Hs00161904_m1) for human Slug was used. TAQMAN® pre-developed kit part #4326315E for the human β-actin was used to normalize. Reactions were run in triplicate in two independent experiments.
Retrovirus/Lentivirus vectors and infections.
Retroviral constructs for human SLUG were generated by cloning the full-length cDNA of human SLUG obtained by reverse transcriptase-PCR into the pBabe-puro vector system using standard techniques. Slug sequence was confirmed by dideoxysequencing.
Amphotropic retroviruses were created by transient cotransfection of vector DNA into Phoenix cells (22). Briefly, 4×106 cells were seeded in 10 cm plates and the plates were cotransfected with 1.5 μg of packaging plasmid pCMV-VSVG and 13.5μg of pBabe-PURO-SLUG or pBabe-PURO vector by calcium phosphate precipitation. Transfection was carried out in the presence of 25 μM Chloroquine (Sigma, St. Louis, MO). Viral supernatants were harvested 24 hours post-transfection by centrifugation at 3,000 rpm for 5 min, and supplemented with 5 μg/ml polybrene (Sigma) prior to infection. Viral supernatants were used to infected neuroblastoma cell line LAN-5. Forty-eight hours after infection, cells were selected by puromycin (Sigma).
For microRNA-mediated inhibition, we used lentiviral vector pLKO-Slug3 obtained from Addgene (Cambridge, MA) which encodes a microRNA directed against human Slug (23). In control infections, we used lentiviral vector pLKO-GFP that encodes a microRNA directed to GFP (23).
Lentiviruses were generated by transient cotransfection of DNA into 293FT cells. Briefly, 2.5×106 293FT cells were seeded in 10 cm plates and cotransfected with 21 μg of appropriate packaging plasmids pCMVd8.2: pCMV-VSVG (2.5:1) and 20μg of pLKO-SLUG or pLKO-GFP vectors using Calcium Phosphate Transfection Kit (Invitrogen, Carlsbad, CA). After 6–8 hours medium was replaced with 6,0 ml of complete medium supplemented with 1,0 mM of Sodium Pyruvate (Gibco-Brl). Recombinant lentivirus vectors were harvested 48h later, centrifuge 5 min at 3,000 RPM, aliquoted and stored at −80 C. Viral supernatants supplemented with 8 μg/ml polybrene (Sigma) were then used to infected neuroblastoma cell lines LAN-5 and HTLA-230 and 18 hours after infection the cells were selected by Puromycin (Sigma).
Protein analysis.
Cellular proteins were extracted, separated on SDS-polyacrylamide gels and Western blot analyses were carried out as previously described (24). Antibodies used were: anti-SLUG (G-18), anti-p53 (DO-1), anti-Bcl-2 (N-19), anti-Bax (N-20) all from Santa Cruz Biotechnology Inc. (Santa Cruz, CA.); anti-β actin (AC-15) from Sigma-Aldrich (St. Louis, MO).
Tumor invasion in Matrigel-coated chambers.
Cells were pretreated in complete medium supplemented with 10 μM of Imatinib mesylate for 24 hours before plating (1.25 × 105 cell/well) in the BD Matrigel® invasion chambers (BD Biosciences). Mock treatments were carried out pre-treating the cells in the same medium without IM. Medium in the upper chamber was supplemented with 5% FCS. In the lower chamber, FCS concentration was 10%. After 24 hours, cells migrated into the lower chamber were stained and counted. Experiments were carried out in triplicate and repeated twice.
Flow cytometry:
1 × 106 cells were harvested and the pellets were washed twice with PBS. Cells were then fixed in cold 70% ethanol added dropwise while vortexing gently. Fixed cells were kept overnight at 4° C. Cells were centrifuged and pellets were resuspended in 1 ml of PI/RNase Staining Buffer (BD Biosciences). Reactions were incubated for 20 min at 4°C, protected from the light. Samples were analyzed by flow cytometry using a FACScalibur flow cytometer (BD Biosciences). For each sample, at least 2×104 cells were analyzed. Cell cycle distribution was calculated by Cell Quest software (BD Biosciences).
In vivo studies.
For in vivo studies of metastasis suppression, four-week-old CB-17/IcrHsd, severe combined immunodeficient mice (Harlan Italy S.r.l., San Pietro al Natisone, UD, Italy) were fed ad libitum and kept in optimal hygienic conditions in a 12 hours light/12 hours dark cycle. Upon arrival, animals were kept in the animal facility for 1 week before starting the experiments. Animals were injected in the tail vein with 3 × 106 HTLA-230-pLKO-GFP or HTLA-230-pLKO-SLUG cells. They were treated by oral gavage twice daily, 7 d/wk either with Imatinib mesylate (200 mg/kg/day) dissolved in water (treated group) or with water (control group). The experiments were terminated 35 days after day 0. Animals were sacrificed by CO2 inhalation, autopsy was carried out for macroscopic assessment of metastases and organs were collected for histologic analysis. In vivo studies were approved by the Animal Care and Use Committee of the Department BAS-ENEA, Rome, and animal care was in accordance with local institutional guidelines. For histological evaluation, paraffin-embedded tissues were cut in 7 μm sections and processed for hematoxylin/eosin staining according to standard techniques. Evaluation of the percent of invaded areas was carried out utilizing the software Image Tools for Windows version 3 (University of Texas Health Science Center, San Antonio, TX).
Statistical analysis.
Wilcoxon-Mann-Whitney exact test was applied to evaluate the statistical significance between the differences in the metastatic areas in different animal’s groups using GraphPad Prism 4 for Windows (GraphPad Software Inc. San Diego, CA). Results are presented as mean ± standard error of the mean (SEM). The level of significance was set at p < 0.05.
Results
IM inhibits invasion of NB cells in vitro.
Previous studies from our laboratory have shown that NB cells are sensitive to IM most likely through inhibition of c-Kit-regulated survival and proliferative signals (16). In this study, we assessed the ability of IM to inhibit invasion of NB cells in vitro. Human NB cell lines (HTLA-230, LAN-5, SK-N-BE2c and GI-CA-N) were seeded in Matrigel-coated invasion chambers in the absence or in the presence of IM (10 μM). After 24 hours, cells migrated through the Matrigel barrier were stained and counted. Invasion was significantly inhibited by IM in all cell lines, although to different extents (Fig. 1). In an attempt to elucidate the mechanisms by which IM suppresses invasiveness of NB cells, we compared the transcriptional profile of NB cells plated in Matrigel before and after treatment with IM (10 μM for 6 hours), by probing high-density microarrays with RNA from GI-CA-N and HTLA-230 cells. We only focused on differences in expression levels concordantly present in both cell lines. By setting a >1.1-fold variation threshold, we identified 21 qualifiers up-or down-modulated by IM that correspond to the 19 known genes indicated in Table 1. Among these genes, Slug was selected for further studies because of its involvement in cell movement and survival of embryonic neural crest cells as well as in the epithelial-mesenchymal transition (EMT) that occurs at the beginning of the metastatic process (13).
Fig. 1.
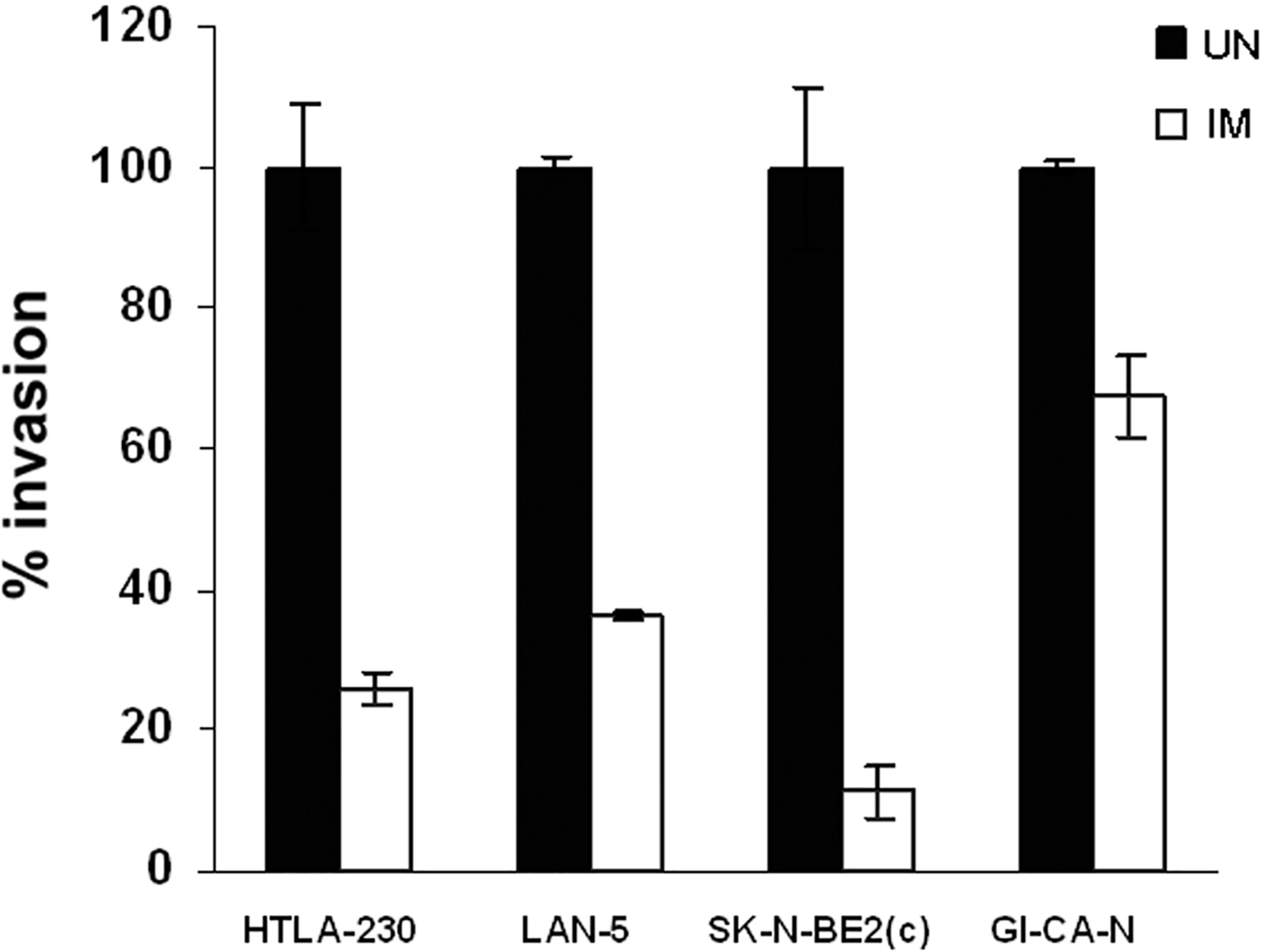
Imatinib Mesylate (IM) treatment inhibits invasion of human NB cells in Matrigel®-coated invasion chambers. Cells were seeded in the upper chamber in medium supplemented with 5% FCS, treated with IM (see details in Materials and Methods) or left untreated (UN). After 22 hours, cells migrated in the lower chamber were stained and counted. In the lower chamber, medium supplemented with 10% FCS was used as chemo-attractant. Invasion of the untreated cells was set to 100. Results are reported as % migration compared to untreated cells ± SD. Experiments were carried out twice in triplicate.
Table 1.
Genes whose expression is concordantly modulated by IM in GI-CA-N and HTLA-230 NB cell lines
| Identifier/descriptor | Symbol | GI-CA-N (IM/UN) | t Test | HTLA-230 (IM/UN) | t Test |
|---|---|---|---|---|---|
| Hypothetical gene supported by BC031266 | na | −1,40 | 0,04 | −1,36 | 0,04 |
| Polo-like kinase 2 | PLK2 | −1,42 | 0,01 | −1,51 | 0,04 |
| Calcitonin gene-related peptide-receptor component protein | RCP9 | −1,48 | 0,04 | −1,23 | 0,02 |
| Embryo brain specific protein | EBSP | −1,31 | 0,02 | −1,35 | 0,01 |
| Activin A receptor, type II | ACVR2 | 1,68 | 0,04 | 1,78 | 0,03 |
| Mab-21-like 2 | MAB21L2 | 1,15 | 0,00 | 1,19 | 0,04 |
| Snail homolog 2 | SLUG (SNAI2) | −1,45 | 0,02 | −1,18 | 0,04 |
| Pleckstrin homology-like domain, family A, member 1 | PHLDA1 | −1,43 | 0,04 | −3,04 | 0,04 |
| Thymocyte protein thy28 | THY28 | −1,12 | 0,02 | −1,28 | 0,01 |
| Collaborates/cooperates with ARF | CARF | −1,76 | 0,01 | −1,37 | 0,04 |
| Phosphatidylinositol 4-kinase type-II beta | PI4K2B | 1,14 | 0,01 | 1,24 | 0,04 |
| SH3 domain protein D19 | EVE1 | −1,33 | 0,00 | −1,46 | 0,03 |
| Tumor protein p53 inducible nuclear protein 1 | TP53INP1 | 1,40 | 0,01 | 1,60 | 0,03 |
| Chromosome 13 open reading frame 11 | C13orf11 | 1,19 | 0,05 | 1,30 | 0,04 |
| FLJ20758 protein | FLJ20758 | −1,54 | 0,02 | −1,38 | 0,00 |
| Two pore segment channel 2 | TPCN2 | −1,47 | 0,04 | −1,40 | 0,02 |
| Anaphase promoting complex subunit 1 | ANAPC1 | 1,18 | 0,02 | 1,19 | 0,05 |
| Chromosome 14 open reading frame 145 | C14orf145 | 1,35 | 0,02 | 1,40 | 0,04 |
| Hypothetical protein DKFZp761I2123 | DKFZp761I2123 | −1,43 | 0,02 | −1,13 | 0,04 |
Slug inhibition by RNA interference in NB cells.
Recent data indicate that Slug expression is relevant for melanoma metastasis (23). Since NB shares the neuroctodermal origin with melanoma, we undertook experiments to assess whether Slug is involved in NB invasion and metastasis. By western blotting, expression of Slug was detected in 8 of 10 human NB cell lines analyzed (Fig. 2A). Two NB cell lines (HTLA-230 and LAN-5) that express Slug were selected for further studies. Slug expression, measured by quantitative real-time PCR, was significantly down-regulated by IM treatment (10 μM for 6 hours) in both cell lines (Fig. 2B).
Fig. 2.
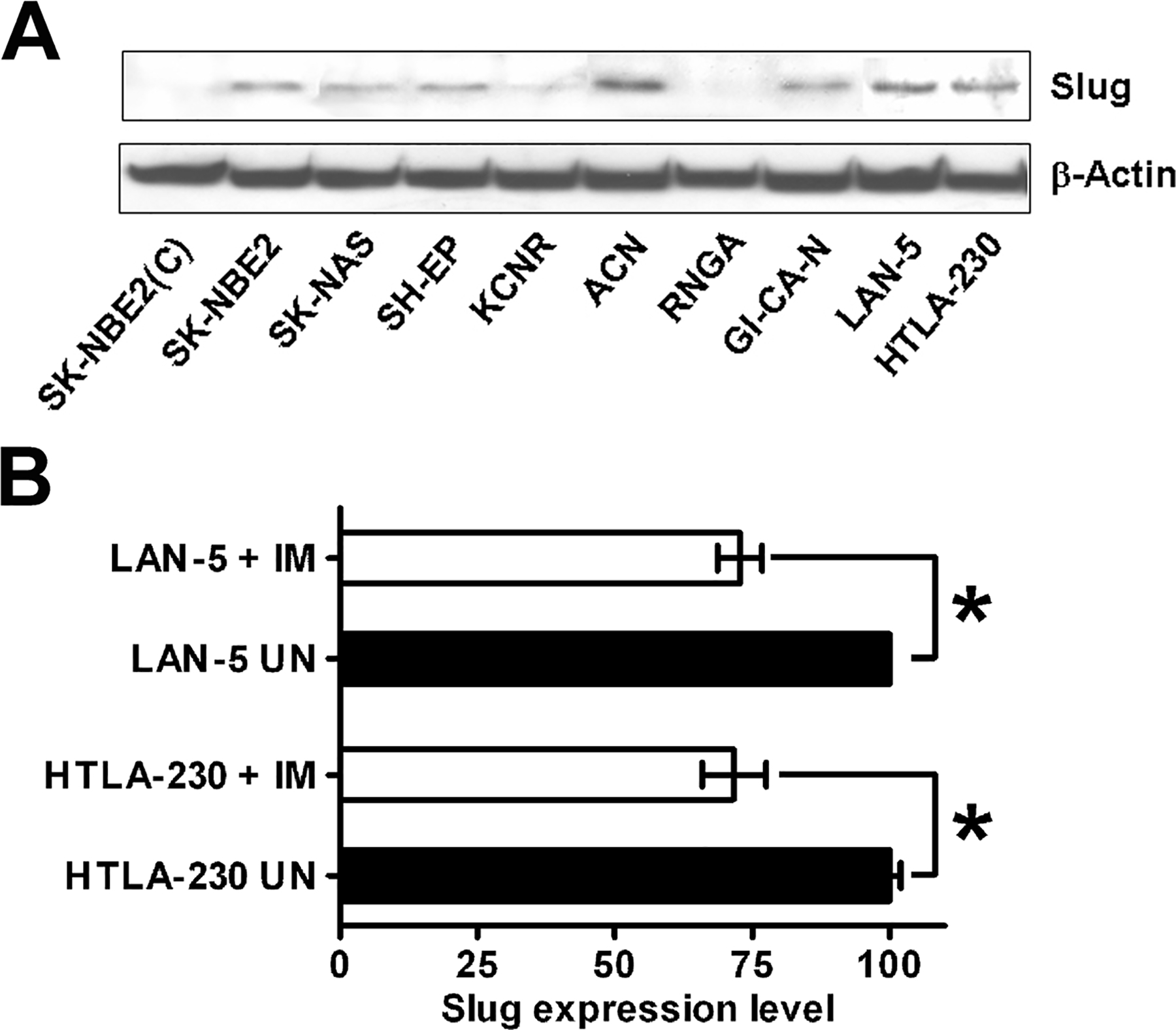
A: Slug is expressed in human NB cell lines. Ten cell lines were analyzed for Slug expression by immunoblotting. Filter was stripped and reprobed with anti-beta-actin antibody for normalization. The experiment was repeated twice with similar results. B: Slug expression measured by quantitative real-time PCR in LAN-5 and HTLA-230 cells, untreated or treated with 10 μM IM for 6 hours. Expression levels in untreated cells were taken as 100. Results are reported ± SD. Asterisks indicate statistically-significant differences (t test, p < 0.01). Experiments were carried out twice in triplicate.
To evaluate whether endogenous Slug plays any role in NB cells, we down-regulated its expression in LAN-5 and HTLA-230 cells upon infection with a lentiviral vector (pLKO-Slug) that encodes a microRNA targeting human Slug mRNA (23). Control infections were carried out with a vector (pLKO-GFP) encoding a microRNA directed against a segment of the coding sequence of Green Fluorescent Protein (GFP) (23). Likewise, the effects of Slug overexpression were analyzed in LAN-5 cells retrovirally transduced with a vector (pBabe-Slug) which encodes human Slug. After puromycin selection, infected cells were tested for Slug expression by western blotting. In pLKO-Slug infected cells, Slug expression was barely detectable both in LAN-5 and HTLA-230 cells; by contrast, pLKO-GFP-infected cells showed Slug levels similar to those in parental cells (Fig. 3A and B). pBabe-Slug cells expressed a higher amount of Slug compared to parental cells (Fig. 3A and B).
Fig. 3.
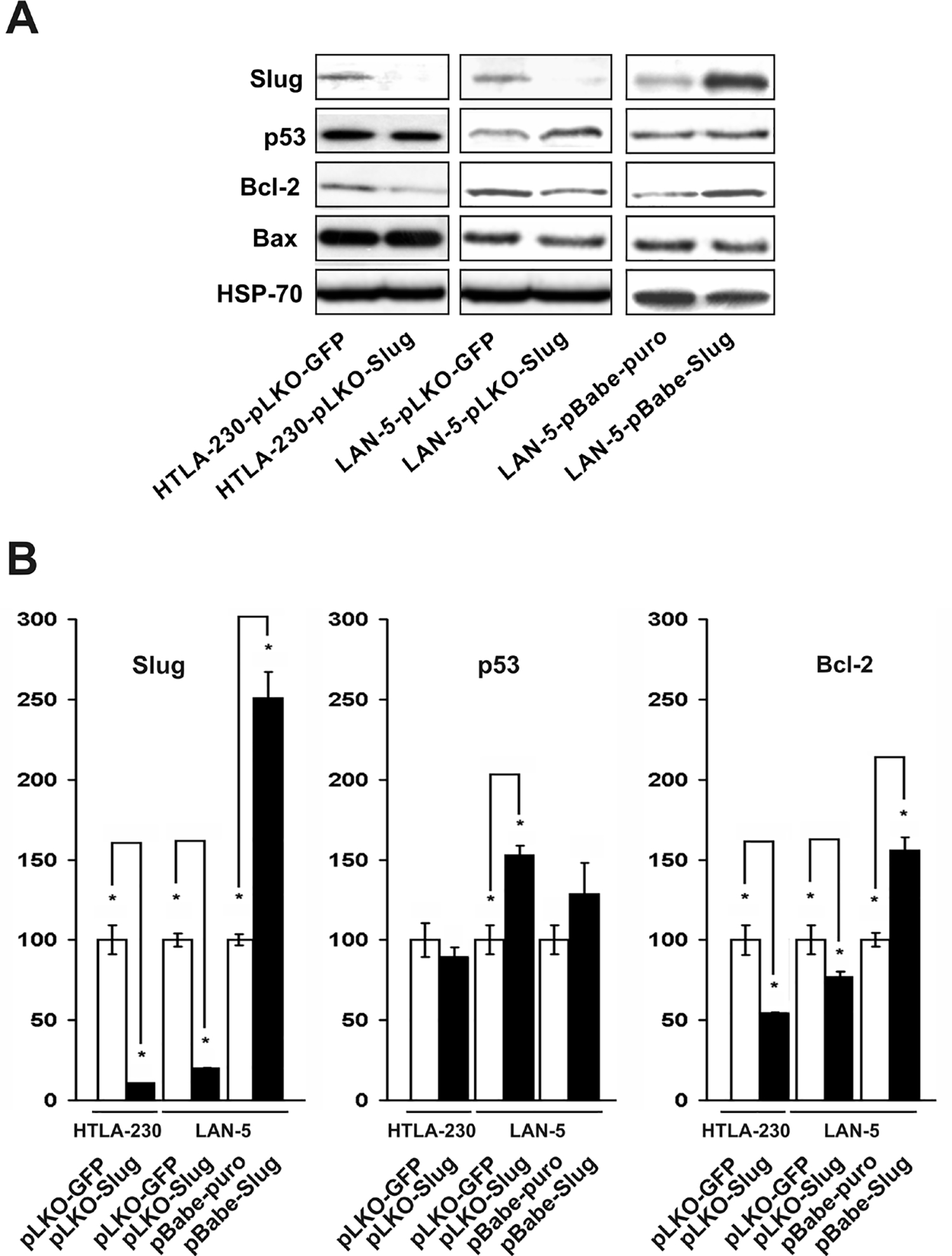
A: Slug, p53, Bcl-2 and Bax immunodetection in Slug-silenced (HTLA-230-pLKO-Slug and LAN-5-pLKO-Slug) and Slug-overexpressing (LAN-5-pBABE-Slug) NB cells. Cell extracts were analyzed for expression of the indicated proteins using specific antibodies. The experiment was repeated twice with similar results. B: Densitometric analysis of Slug, p53 and Bcl-2 levels detected by immunoblotting. Expression level of each protein in controls was set to 100. Levels are reported as % of control ± SD.
Since Slug has been shown to be involved in the control of apoptosis (25) and in the EMT that is linked to the acquisition of the invasive phenotype (4), we analyzed the expression of several Slug targets in Slug-silenced (pLKO-Slug) and Slug-overexpressing (pBabe-Slug) cells by immunoblotting. In LAN-5-pLKO-Slug cells and in HTLA-230-pLKO-Slug cells, antiapoptotic Bcl-2 was down-regulated (Fig. 3A and B). By contrast, in Slug overexpressing LAN-5-pBabe-Slug cells, Bcl-2 was upregulated (Fig. 3A and B). Proapoptotic Bax was reported to be a Slug target in some cellular contexts (26), but in our analyses, its expression did not change in a consistent manner. p53 was upregulated only in LAN-5-pLKO-Slug cells. In all cell lines, E-cadherin, a well known target of Slug (27), was undetectable (not shown).
We analyzed whether Slug levels affect cell proliferation by seeding Slug-silenced and Slug-overexpressing cells in normal culture conditions (medium + 10% FCS) and counting the cells at different time points. The growth rate was not significantly different (p > 0.05) in Slug-silenced HTLA-230-pLKO-Slug and LAN-5-pLKO-Slug cells compared to their respective controls (data not shown). Similarly, proliferation of LAN-5-pBABE-Slug cells was undistinguishable from that of control cells (not shown). These results are consistent with recent data indicating that Slug does not affect proliferation of melanoma cells (23).
Slug silencing inhibits invasion of NB cells in vitro.
We tested whether Slug knock down affected the invasion capabilities of NB cells by using an in vitro invasion assay. Cells were seeded in the upper part of a Matrigel®-coated invasion chamber in a reduced (5%) FCS concentration. After 22 hours, cells that migrated in the lower chamber containing a higher (10%) FCS concentration were stained and counted. In both Slug-silenced cell lines invasion was significantly reduced (Fig. 4A and B). We also tested the effects of IM on the invasion capability of HTLA-230-pLKO-Slug and HTLA-230-pLKO-GFP cells. Compared to data obtained using the parental cell lines (see Fig. 1), IM-treated HTLA-230-pLKO-GFP and LAN-5-pLKO-GFP cells exhibited reduced invasion (Fig. 4A and B). Compared to untreated cells, no further decrease in invasion was observed in IM-treated HTLA-230-pLKO-Slug and LAN-5-pLKO-Slug cells (Fig. 4A and B). Of interest, LAN-5-pBABE-Slug cells that overexpress Slug showed an increased invasion capability; IM treatment reduced the invasion of these cells and of the control LAN-5-pBABE-puro cells (Fig 4C). Together, these data demonstrate that Slug modulates invasion of NB cells in vitro.
Fig. 4.
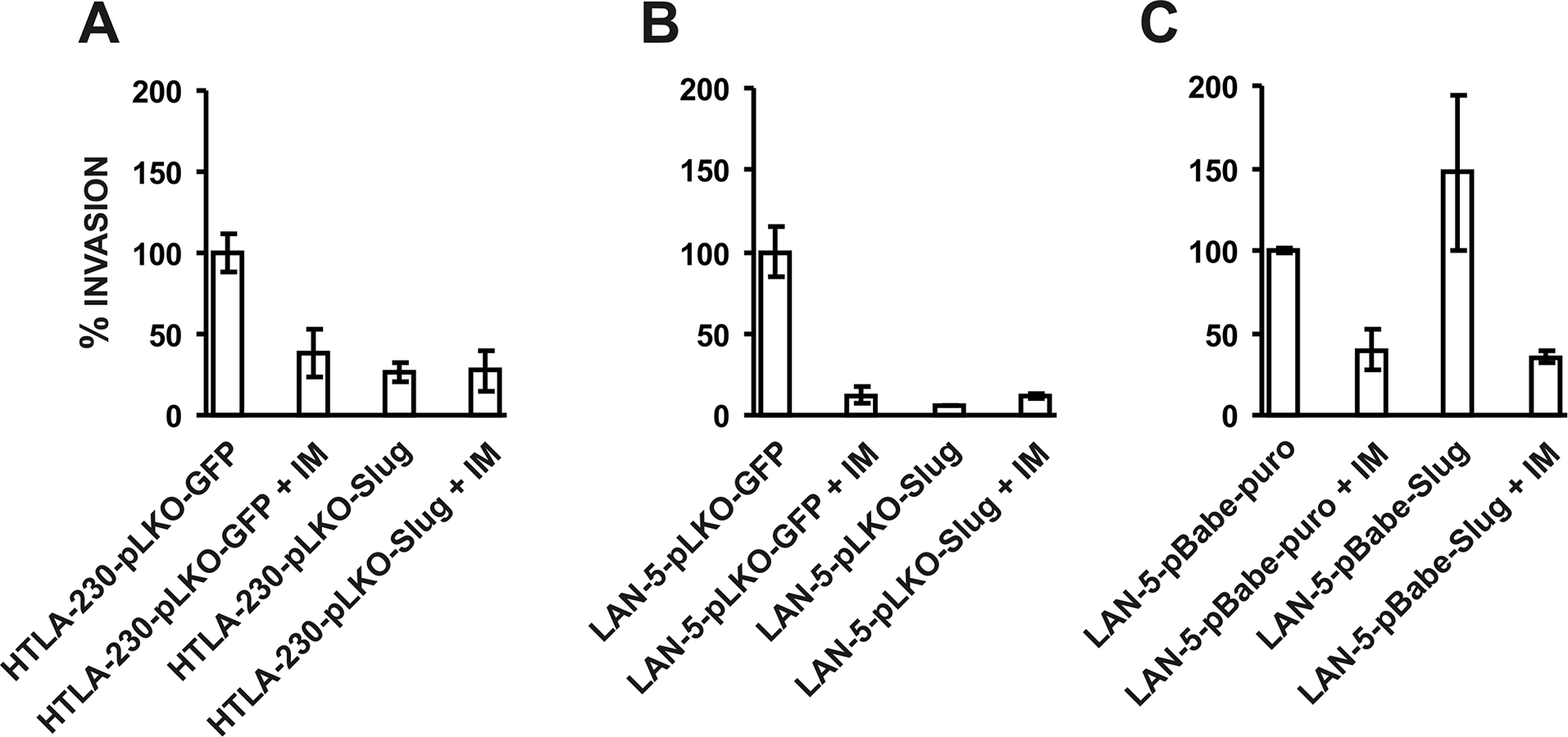
Slug silencing inhibits (A and B) and Slug overexpression promotes (C) invasion of human NB cells in Matrigel®-coated invasion chambers. Cells were seeded in the upper chamber in medium supplemented with 5% FCS. Where indicated, cells were treated with IM. After 22 hours, cells migrated in the lower chamber were stained and counted. In the lower chamber, medium supplemented with 10% FCS was used as chemo-attractant. Invasion of each untreated cell line was set to 100. Results are reported as % migration ± SD compared to untreated cells. Experiments were carried out twice in triplicate.
Slug silencing and pro-apoptotic drug treatment cooperate in inducing apoptosis of NB cells.
Given the activity of Slug in cell survival through regulation of pro-apoptotic and anti-apoptotic factors, we assessed the apoptosis susceptibility of Slug-silenced cells in the absence or in the presence of IM (24 μM). We used this IM concentration since it is near the calculated IC50 for the two NB parental cell lines (LAN-5 and HTLA-230) (17) from which the Slug-silenced cells were derived. HTLA-230-pLKO-Slug, LAN-5-pLKO-Slug cells and their controls were grown in normal growth conditions (medium + 10% FCS) in the presence or in the absence of IM for different times (1, 2 and 3 days). At the end of each treatment, cells were fixed and stained with propidium iodide (PI) for flow cytometric analysis. Spontaneous (without IM) apoptosis (subG1 peak) increased only in HTLA-230-pLKO-Slug cells compared to control HTLA-230-pLKO-GFP (Table 2). In Slug-silenced cell lines, apoptosis induced by IM was markedly increased at each time point (p< 0.001, χ2 test) except after 1 day treatment of LAN-5-pLKO cells (Table 2). Thus, at least in vitro, simultaneous Slug down-regulation by RNA interference and IM treatment cooperate in promoting apoptosis. In addition, we tested whether Slug-silencing could also cooperate in inducing apoptosis with etoposide and doxorubicin, two drugs frequently used in standard therapeutic protocols for NB. Both Slug-silenced cell lines (LAN-5-pLKO-Slug and HTLA-230-pLKO-Slug) showed significantly increased apoptosis (p< 0.001, χ2 test) compared to controls at 1, 2 and 3 days of treatment (LAN-5-pLKO-Slug + doxorubicin) and at 1 and 2 days of treatment (LAN-5-pLKO-Slug + etoposide, HTLA-230-pLKO-Slug + etoposide and HTLA-230-pLKO-Slug + doxorubicin) (Table 2). These data suggest that Slug-silencing can be usefully combined with apoptosis-inducing drugs in order to accelerate and/or maximize anti-tumor effect.
Table 2.
Flow cytometric analysis of untreated, IM-, Etoposide- and Doxorubicin-treated Slug-silenced NB cells
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Cell line / time of treatment | IM | Etoposide | Doxorubicin | |||
| % sub-G1 | p | % sub-G1 | p | % sub-G1 | p | |
| LAN-5-pLKO-GFP | 0.78 | 0.78 | 0.78 | |||
| UN | n.s. | n.s. | n.s. | |||
| LAN-5-pLKO-Slug | 0.79 | 0.79 | 0.79 | |||
| LAN-5-pLKO-GFP | 3.36 | 8.19 | 14.11 | |||
| 1 day | n.s. | <0.001 | <0.001 | |||
| LAN-5-pLKO-Slug | 3.50 | 20.18 | 21.39 | |||
| LAN-5-pLKO-GFP | 29.46 | 17.02 | 14.93 | |||
| 2 days | <0.001 | <0.001 | <0.001 | |||
| LAN-5-pLKO-Slug | 36.68 | 22.21 | 22.65 | |||
| LAN-5-pLKO-GFP | 37.07 | 32.33 | 19.67 | |||
| 3 days | <0.001 | n.s. | <0.001 | |||
| LAN-5-pLKO-Slug | 40.26 | 32.82 | 29.41 | |||
| HTLA-230-pLKO-GFP | 4.86 | 4.86 | 4.86 | |||
| UN | <0.001 | <0.001 | <0.001 | |||
| HTLA-230-pLKO-Slug | 10.91 | 10.91 | 10.91 | |||
| HTLA-230-pLKO-GFP | 3.50 | 5.01 | 5.14 | |||
| 1 day | <0.001 | <0.001 | <0.001 | |||
| HTLA-230-pLKO-Slug | 16.00 | 11.57 | 11.60 | |||
| HTLA-230-pLKO-GFP | 23.34 | 13.27 | 15.89 | |||
| 2 days | <0.001 | <0.001 | <0.001 | |||
| HTLA-230-pLKO-Slug | 43.80 | 15.93 | 18.09 | |||
| HTLA-230-pLKO-GFP | 61.36 | 21.70 | 28.59 | |||
| 3 days | <0.001 | n.s. | n.s. | |||
| HTLA-230-pLKO-Slug | 84.23 | 21.72 | 28.52 | |||
Abbreviations: UN = untreated, IM = Imatinib Mesylate, n.s. = not significant. Treatment concentrations were: IM 24 μM; Etoposide 1 μM (LAN-5) and 5 μM (HTLA-230); Doxorubicin 1 μg/ml. Flow cytometric analysis was carried out on 2 × 104 cells. Statistical significance was calculated by χ2 test.
Effect of Slug silencing in an in vivo pseudometastatic model.
The next step for the characterization of Slug function in NB was to test the effect of Slug knock down in animal models. Tail vein injection in immunodeficient mice (SCID) provides a well known pseudometastatic model for NB (28). Since not all NB cell lines develop tumors after iv injection in mice, we selected HTLA-230 cells because they are the most reliable model of NB metastasis in mice (28). SCID mice were injected with 3 × 106 HTLA-230-pLKO-Slug (20 animals) or HTLA230-pLKO-GFP (20 animals) on day 0. On day 1, mice were divided in 4 groups of 10 animals each (HTLA-230-pLKO-Slug untreated, HTLA-230-pLKO-Slug + IM; HTLA230-pLKO-GFP untreated; HTLA230-pLKO-GFP + IM) and IM treatment was started by oral gavage twice a day (total daily dose: 200 mg/Kg) and continued for 21 days. Animals were sacrificed after the occurrence of the first death (day 35) and autopsy was carried out to remove organs and detect macrometastases. Autopsy demonstrated a reduction of tumor cell burden in certain organs (adrenals, kidneys, liver and lungs) in HTLA-230-pLKO-Slug untreated and HTLA-230-pLKO-Slug + IM groups. The major site for metastases was a pararenal area involved in 100% of the animals in HTLA230-pLKO-GFP untreated and in HTLA230-pLKO-GFP + IM groups. Involvement of this pararenal area was reduced in pLKO-Slug-HTLA-230 untreated and HTLA-230-pLKO-Slug + IM groups. Since the extent of pararenal involvement was correlated to the overall metastatic burden (data not shown), we analyzed the percentage of metastatic invasion in the major sagittal section of the kidney, pararenal area and adrenal of each animal. From each group, histological specimens were prepared and the largest sagittal section of each organ was evaluated using an image analysis software to measure the extent of tumor involvement. We plotted the percentage of the invaded areas to estimate the metastatic burden in each group of animals (Fig. 5A). Differences in metastatic area reached statistical significance (Wilcoxon-Mann-Whitney exact test) in HTLA-230-pLKO-Slug + IM compared to HTLA230-pLKO-GFP untreated (p= 0.045) and in HTLA-230-pLKO-Slug + IM compared to HTLA230-pLKO-GFP + IM (p= 0.013) (Fig 5A). Representative sections of adrenals, kidney and pararenal area of each group are shown in Fig. 5B, C, D and E. The involved area was reduced in the untreated (Fig. 5D) and the IM-treated (Fig. 5E) HTLA-230-pLKO-Slug groups compared to the untreated (Fig. 5B) and the IM-treated (Fig. 5C) HTLA230-pLKO-GFP groups. These findings show that silencing of Slug significantly reduces the metastatic burden in a pseudometastatic model of NB and that this reduction is more evident following IM treatment of Slug-silenced cells.
Fig. 5.
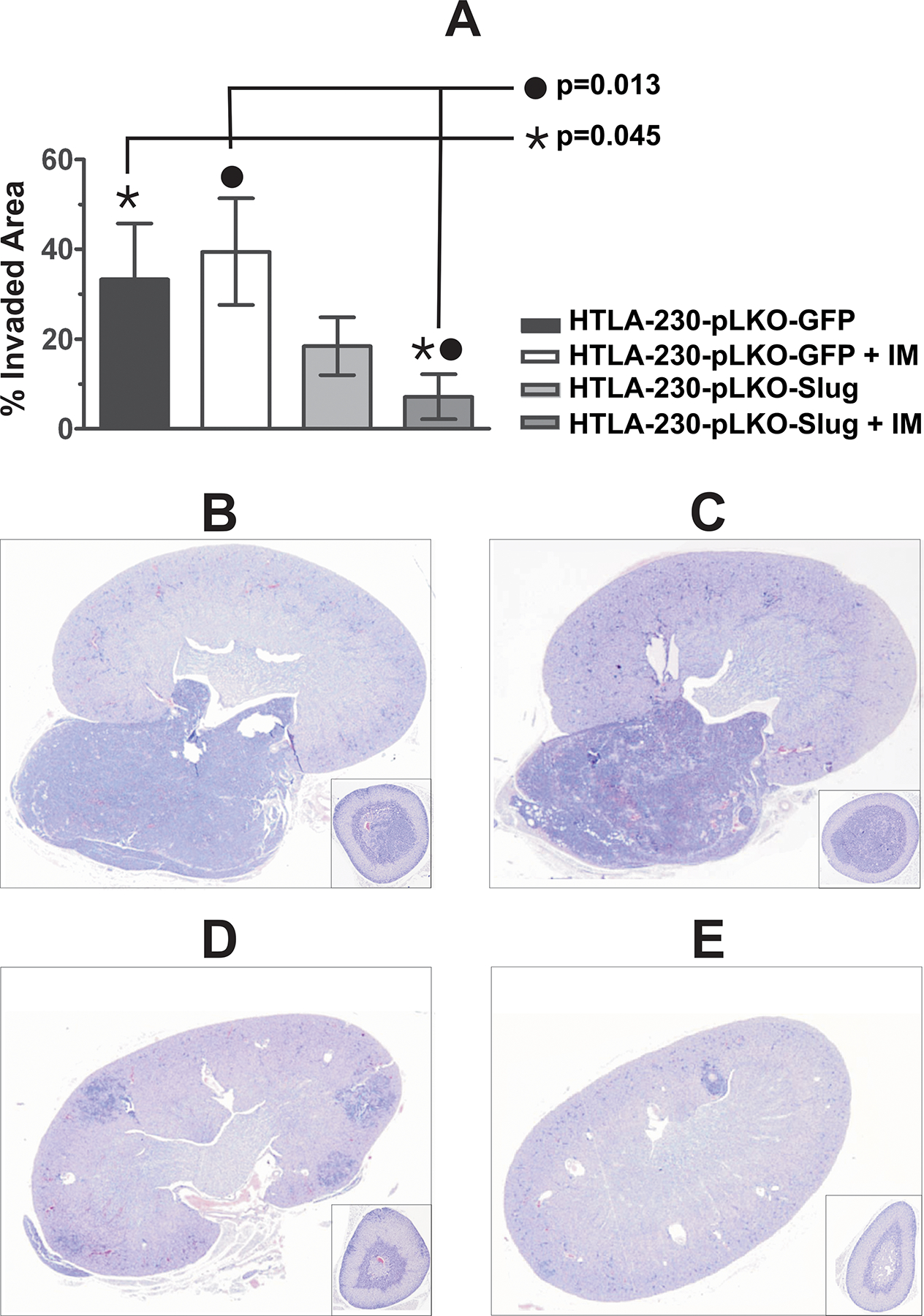
Effect of Slug silencing and IM treatment in a pseudo-metastatic model of NB in vivo. A: The major sagittal section of kidney, pararenal area and adrenals of each animal were evaluated for the extent of metastatic invasion using an image analysis program (see Materials and Methods for details). Data are presented as % of invaded area ± SEM. Significant differences in metastatic burden as compared to the pLKO-GFP untreated group are indicated. Representative histological sections of kidney, pararenal and adrenals of each group of animals. B: HTLA-230-pLKO-GFP untreated; C: HTLA-230-pLKO-GFP + IM; D: HTLA-230-pLKO-Slug untreated; E: HTLA-230-pLKO-Slug + IM.
Discussion
Effective treatment of metastatic NB still remains an unsolved clinical challenge (8). The development of targeted therapies using compounds with a selective mechanism of action and a better toxicity profile compared to conventional cytotoxic drugs paved the way to preclinical studies aimed at using these drugs not only to suppress tumor growth but also to inhibit metastasis. We (16, 17) and others (18–20) reported the activity of IM in inhibiting proliferation of NB cells. These results, together with the role of IM targets in the regulation of cell motility (29, 30), encouraged us to also assess whether IM suppressed NB cell invasion. In vitro assays demonstrated that IM treatment was indeed able to reduce significantly tumor invasiveness. Then, we carried out microarray gene expression analyses of untreated and IM-treated NB cells seeded on a layer of extracellular matrix (Matrigel®) to identify genes possibly involved in modulating the effects of IM on NB invasiveness. The molecular changes necessary for tumor cells to acquire metastatic competence are not yet completely understood. In addition to the important roles during embryonic development, loss of cell adhesion and induction of EMT are also required for tumour progression (3). In fact, induction of EMT represents the first step in the metastatic cascade, allowing cells to delaminate from the primary tumor and to intravasate into lymphatic or blood vessels (13). Activation of Snail, ZEB and basic helix-loop-helix (b-HLH) transcription factors represents one of the molecular switches whereby cells undergoing EMT lose intercellular connections and apico-basal polarity (4). Recently, activation of microRNA-10b by Twist1, an EMT regulator, was also demonstrated to play a role in breast cancer invasion and metastasis (31). Our microarray analysis demonstrated that expression of Slug, a transcriptional repressor whose expression has been associated with mesoderm and migratory neural crest cells undergoing EMT (13), was down-regulated by IM treatment in both cell lines analyzed. Of interest, activation of the c-Kit receptor leads to increased Slug expression in malignant mesothelioma cells (32). c-Kit phosphorylation is down-modulated by IM in NB cells (16) although further data indicate that other receptors can be implicated in the effects of IM (19). Thus, although it is conceivable that the Slug down-regulation in NB cells may be due, at least in part, to the c-Kit inhibition caused by IM treatment, other receptors inhibited by IM could play a role in Slug down-regulation.
Several experimental data have led to the inclusion of Slug into the Snail family of transcription regulators involved in tumor progression and metastasis (23, 33). In our studies, we found that the knock-down of Slug expression, like IM treatment, inhibited the invasion capability of two NB cell lines in vitro. In contrast with the marked effect on invasiveness, neither inhibition nor overexpression of Slug significantly affected cell proliferation of the NB cell lines, in agreement with the data on the proliferation of Slug-silenced melanoma cells (23). Moreover, Slug is also involved in cell survival through the direct or indirect transcriptional regulation of pro-apoptotic (26, 34) and anti-apoptotic (35) genes. We detected a decrease of Bcl-2 expression in Slug-silenced cells, while Bcl-2 levels increased in Slug-overexepressing cells, findings that are in agreement with the anti-apoptotic role of Slug in NB cells. The enhanced propensity of Slug-silenced NB cells to undergo apoptosis is also consistent with the results of IM, etoposide and doxorubicin treatments in vitro, and suggests that Slug inhibition could enhance the beneficial effects of pro-apoptotic drugs even of those (etoposide and doxorubicin) already utilized in therapeutic protocols.
In tumors, migrating cells leaving the primary mass must counteract anoikis (36), a form of apoptotic cell death triggered by the detachment of cells from the extracellular matrix, in order to arrive successfully at the final site of metastasis. Thus, the anti-apoptotic and pro-invasive activities conferred by Slug to NB cells in vitro could cooperate to promote metastasis competence in vivo. Clinical studies have demonstrated that IM treatment is effective in metastatic GIST through its ability to block c-Kit activity (37). A recent study has also reported that Matrigel® invasion of highly aggressive breast carcinoma cells is reduced by IM treatment via the inhibition of activated c-Abl (38). Although it is unknown whether c-Abl is expressed and active in the cell lines utilized in the present study, we cannot exclude that part of the effects induced by IM may be due to c-Abl inhibition, in addition to those dependent on c-Kit (16) or other receptor tyrosine-kinases (19). The inhibition of NB invasion by IM treatment in vitro and the ability of IM to inhibit tyrosine kinases phosphorylation in NB cells (16, 19), suggest that this drug may effectively suppress metastasis formation in NB.
To investigate whether the combination of Slug knock-down and IM treatment can synergistically reduce the metastatic burden in NB, we utilized a well established pseudometastatic model in SCID mice (28). Slug silencing inhibited metastatic growth, although the effects did not reach statistical significance unless Slug-silenced cells were also treated with IM. Surprisingly, in our in vivo model of metastatic NB, IM as a single agent was unable to reduce the metastatic burden. This result is in contrast with the findings of the in vitro assays, in which IM was indeed able to inhibit invasion. Nevertheless, our data on the inefficacy of IM alone in reducing metastasis in vivo, is in keeping with the results of the first clinical trial on IM used as single agent treatment in refractory or relapsed pediatric tumors including NB which reported little or no activity of IM at a dose of 440 mg/m2/day (21). A possible explanation for the apparent discrepancy between in vitro and in vivo results in the present study could be due to inadequate IM concentration at the metastatic sites in the NB-injected animals albeit the dose of IM used in this study (200 mg/Kg/day) was in the range of those inhibiting NB growth in subcutaneous xenografts (17).
The additive effect of IM in combination with Slug inhibition is unlikely due to a further reduction of Slug levels, since these levels are already undetectable in Slug-silenced cells. Recent data indicate that IM and other small molecules of the same class bind to and modulate the activity of other targets beyond the tyrosine kinases (39). Thus, it is conceivable that IM treatment may affect the expression/activity of other proteins, regulated by established and/or still unknown IM targets, with a role in modulating the metastatic cascade of NB cells. This hypothesis is consistent with: i) the results of our microarray analysis which shows that IM treatment modulates the expression of several genes, some of which may play a role in NB invasion such as Polo-like kinase 2 (40) and activin receptor type II (41); and ii) the observation that IM treatment suppresses the migration of Slug-overexpressing LAN-5 cells (Fig 4). It should also be taken into account that the in vitro invasion ability of SK-N-BE2c cells that do not express Slug (see Fig. 2), was also reduced by IM treatment, possibly through a modulation of the activity of other unidentified targets. Also of interest is the observation that c-Abl, whose activation is inhibited by IM, appears to promote the in vitro invasion of breast carcinoma cells (38). Even so, in our in vivo model the inhibition of IM targets does not seem sufficient to suppress the development of NB metastasis unless coupled with the complete down-regulation of Slug.
In summary, our data have demonstrated for the first time the relevant role of Slug expression in apoptosis, invasion and metastasis of NB cells in vitro and in vivo. Although metastasis formation in NB as well as in other cancers is a highly complex and organized process that consists of multiple interrelated steps (42), Slug seems to play a central role, so that its inhibition significantly decreases, albeit does not eradicate, metastasis formation in vivo. Furthermore, the present in vivo findings suggest that the combination of Slug down-regulation with IM administration may represent a novel targeted therapy for metastatic NB.
Acknowledgments
This work was partially supported by grants from “Fondazione Italiana per la Lotta al Neuroblastoma” (G.R.), Italian Ministry of Health (C.D), “Io…domani – Associazione per la Lotta contro i Tumori Infantili” (ALTI) (C.D.) and NCI RO1 CA95111 (B.C). R.V. is a fellow of “Fondazione Italiana per la Lotta al Neuroblastoma”.
Abbreviations:
- NB
neuroblastoma
- IM
Imatinib Mesylate
- GIST
Gastro Intestinal Stromal Tumor
- CML
Chronic Myelogenous Leukemia
- FCS
Fetal Calf Serum
References
- 1.Liotta LA. An attractive force in metastasis. Nature 2001;410:24–5. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006;12:895–904. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006;7:131–42. [DOI] [PubMed] [Google Scholar]
- 4.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415–28. [DOI] [PubMed] [Google Scholar]
- 5.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 2006;25:9–34. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev 2006;25:357–71. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis. Annu Rev Med 2006;57:1–18. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003;3:203–16. [DOI] [PubMed] [Google Scholar]
- 9.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007;369:2106–20. [DOI] [PubMed] [Google Scholar]
- 10.De Bernardi B, Nicolas B, Boni L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol 2003;21:1592–601. [DOI] [PubMed] [Google Scholar]
- 11.D’Angio GJ, Evans AE, Koop CE. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet 1971;1:1046–9. [DOI] [PubMed] [Google Scholar]
- 12.Brodeur GM, Seeger RC. Gene amplification in human neuroblastomas: basic mechanisms and clinical implications. Cancer Genet Cytogenet 1986;19:101–11. [DOI] [PubMed] [Google Scholar]
- 13.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 2005;132:3151–61. [DOI] [PubMed] [Google Scholar]
- 14.Druker BJ. Perspectives on the development of a molecularly targeted agent. Cancer Cell 2002;1:31–6. [DOI] [PubMed] [Google Scholar]
- 15.Verweij J, van OA, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 2003;39:2006–11. [PubMed] [Google Scholar]
- 16.Vitali R, Cesi V, Nicotra MR, et al. c-Kit is preferentially expressed in MYCN-amplified neuroblastoma and its effect on cell proliferation is inhibited in vitro by STI-571. Int J Cancer 2003;106:147–52. [DOI] [PubMed] [Google Scholar]
- 17.Meco D, Riccardi A, Servidei T, et al. Antitumor activity of imatinib mesylate in neuroblastoma xenografts. Cancer Lett 2005;228:211–9. [DOI] [PubMed] [Google Scholar]
- 18.Beppu K, Jaboine J, Merchant MS, Mackall CL, Thiele CJ. Effect of imatinib mesylate on neuroblastoma tumorigenesis and vascular endothelial growth factor expression. J Natl Cancer Inst 2004;96:46–55. [DOI] [PubMed] [Google Scholar]
- 19.Te Kronnie G, Timeus F, Rinaldi A, et al. Imatinib mesylate (STI571) interference with growth of neuroectodermal tumour cell lines does not critically involve c-Kit inhibition. Int J Mol Med 2004;14:373–82. [PubMed] [Google Scholar]
- 20.Rossler J, Zambrzycka I, Lagodny J, Kontny U, Niemeyer CM. Effect of STI-571 (imatinib mesylate) in combination with retinoic acid and gamma-irradiation on viability of neuroblastoma cells. Biochem Biophys Res Commun 2006;342:1405–12. [DOI] [PubMed] [Google Scholar]
- 21.Bond M, Bernstein ML, Pappo A, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer 2008;50:254–8. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 1990;18:3587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta PB, Kuperwasser C, Brunet JP, et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet 2005;37:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raschellà G, Tanno B, Bonetto F, et al. The RB-related gene Rb2/p130 in neuroblastoma differentiation and in B-myb promoter down-regulation. Cell Death Differ 1998;5:401–7. [DOI] [PubMed] [Google Scholar]
- 25.Haupt S, Alsheich-Bartok O, Haupt Y. Clues from worms: a Slug at Puma promotes the survival of blood progenitors. Cell Death Differ 2006;13:913–5. [DOI] [PubMed] [Google Scholar]
- 26.Tribulo C, Aybar MJ, Sanchez SS, Mayor R. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev Biol 2004;275:325–42. [DOI] [PubMed] [Google Scholar]
- 27.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci 2003;116:499–511. [DOI] [PubMed] [Google Scholar]
- 28.Bogenmann E. A metastatic neuroblastoma model in SCID mice. Int J Cancer 1996;67:379–85. [DOI] [PubMed] [Google Scholar]
- 29.Bellone G, Ferrero D, Carbone A, et al. Inhibition of cell survival and invasive potential of colorectal carcinoma cells by the tyrosine kinase inhibitor STI571. Cancer Biol Ther 2004;3:385–92. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda A, Sawai H, Takahashi H, et al. The stem cell factor/c-kit receptor pathway enhances proliferation and invasion of pancreatic cancer cells. Mol Cancer 2006;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007;449:682–8. [DOI] [PubMed] [Google Scholar]
- 32.Catalano A, Rodilossi S, Rippo MR, Caprari P, Procopio A. Induction of stem cell factor/c-Kit/slug signal transduction in multidrug-resistant malignant mesothelioma cells. J Biol Chem 2004;279:46706–14. [DOI] [PubMed] [Google Scholar]
- 33.Uchikado Y, Natsugoe S, Okumura H, et al. Slug Expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res 2005;11:1174–80. [PubMed] [Google Scholar]
- 34.Wu WS, Heinrichs S, Xu D, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005;123:641–53. [DOI] [PubMed] [Google Scholar]
- 35.Bermejo-Rodriguez C, Perez-Caro M, Perez-Mancera PA, Sanchez-Beato M, Piris MA, Sanchez-Garcia I. Mouse cDNA microarray analysis uncovers Slug targets in mouse embryonic fibroblasts. Genomics 2006;87:113–8. [DOI] [PubMed] [Google Scholar]
- 36.Gilmore AP. Anoikis. Cell Death Differ 2005;12 Suppl 2:1473–7. [DOI] [PubMed] [Google Scholar]
- 37.Radford IR. Imatinib. Novartis. Curr Opin Investig Drugs 2002;3:492–9. [PubMed] [Google Scholar]
- 38.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res 2006;66:5648–55. [DOI] [PubMed] [Google Scholar]
- 39.Rix U, Hantschel O, Durnberger G, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007;110:4055–63. [DOI] [PubMed] [Google Scholar]
- 40.Wolf G, Elez R, Doermer A, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene 1997;14:543–9. [DOI] [PubMed] [Google Scholar]
- 41.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A 2003;100:8430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ara T, DeClerck YA. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metastasis Rev 2006;25:645–57. [DOI] [PubMed] [Google Scholar]


