Abstract
Background
Chronic plaque psoriasis is an immune‐mediated, chronic, inflammatory skin disease, which can impair quality of life and social interaction. Disease severity can be classified by the psoriasis area and severity index (PASI) score ranging from 0 to 72 points. Indoor artificial salt bath with or without artificial ultraviolet B (UVB) light is used to treat psoriasis, simulating sea bathing and sunlight exposure; however, the evidence base needs clear evaluation.
Objectives
To assess the effects of indoor (artificial) salt water baths followed by exposure to artificial UVB for treating chronic plaque psoriasis in adults.
Search methods
We searched the following databases up to June 2019: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trial registers, and checked the reference lists of included studies, recent reviews, and relevant papers for further references to relevant trials.
Selection criteria
Randomised controlled trials (RCTs) of salt bath indoors followed by exposure to artificial UVB in adults who have been diagnosed with chronic plaque type psoriasis. We included studies reporting between‐participant data and within‐participant data. We evaluated two different comparisons: 1) salt bath + UVB versus other treatment without UVB; eligible comparators were exposure to psoralen bath, psoralen bath + artificial ultraviolet A UVA) light, topical treatment, systemic treatment, or placebo, and 2) salt bath + UVB versus other treatment + UVB or UVB only; eligible comparators were exposure to bath containing other compositions or concentrations + UVB or UVB only.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We used GRADE to assess the certainty of the evidence.
The primary efficacy outcome was PASI‐75, to detect people with a 75% or more reduction in PASI score from baseline. The primary adverse outcome was treatment‐related adverse events requiring withdrawal. For the dichotomous variables PASI‐75 and treatment‐related adverse events requiring withdrawal, we estimated the proportion of events among the assessed participants.
The secondary outcomes were health‐related quality of life using the Dermatology Life Quality Index, (DLQI) pruritus severity measured using a visual analogue scale, time to relapse, and secondary malignancies.
Main results
We included eight RCTs: six reported between‐participant data (2035 participants; 1908 analysed), and two reported within‐participant data (70 participants, 68 analysed; 140 limbs; 136 analysed). One study reported data for the comparison salt bath with UVB versus other treatment without UVB; and eight studies reported data for salt bath with UVB versus other treatment with UVB or UVB only. Of these eight studies, only five reported any of our pre‐specified outcomes and assessed the comparison of salt bath with UVB versus UVB only. The one included trial that assessed salt bath plus UVB versus other treatment without UVB (psoralen bath + UVA) did not report any of our primary outcomes. The mean age of the participants ranged from 41 to 50 years of age in 75% of the studies. None of the included studies reported on the predefined secondary outcomes of this review. We judged seven of the eight studies as at high risk of bias in at least one domain, most commonly performance bias. Total trial duration ranged between at least two months and up to 13 months.
In five studies, the median participant PASI score at baseline ranged from 15 to 18 and was balanced between treatment arms. Three studies did not report PASI score. Most studies were conducted in Germany; all were set in Europe. Half of the studies were multi‐centred (set in spa centres or outpatient clinics); half were set in a single centre in either an unspecified settings, a psoriasis daycare centre, or a spa centre. Commercial spa or salt companies sponsored three of eight studies, health insurance companies funded another, the association of dermatologists funded another, and three did not report on funding.
When comparing salt bath plus UVB versus UVB only, two between‐participant studies found that salt bath plus UVB may improve psoriasis when measured using PASI 75 (achieving a 75% or more reduction in PASI score from baseline) (risk ratio (RR) 1.71, 95% confidence interval (CI) 1.24 to 2.35; 278 participants; low‐certainty evidence). Assessment was conducted at the end of treatment, which was equivalent to six to eight weeks after start of treatment. The two trials which contributed data for the primary efficacy outcome were conducted by the same group, and did not blind outcome assessors. The German Spas Association funded one of the trials and the funding source was not stated for the other trial.
Two other between‐participant studies found salt bath plus UVB may make little to no difference to outcome treatment‐related adverse events requiring withdrawal compared with UVB only (RR 0.96, 95% CI 0.35 to 2.64; 404 participants; low‐certainty evidence). One of the studies reported adverse events, but did not specify the type of events; the other study reported skin irritation. One within‐participant study found similar results, with one participant reporting severe itch immediately after Dead Sea salt soak in the salt bath and UVB group and two instances of inadequate response to phototherapy and conversion to psoralen bath + UVA reported in the UVB only group (low‐certainty evidence).
Authors' conclusions
Salt bath with artificial ultraviolet B (UVB) light may improve psoriasis in people with chronic plaque psoriasis compared with UVB light treatment alone, and there may be no difference in the occurrence of treatment‐related adverse events requiring withdrawal. Both results are based on data from a limited number of studies, which provided low‐certainty evidence, so we cannot draw any clear conclusions.
The reporting of our pre‐specified outcomes was either non‐existent or limited, with a maximum of two studies reporting a given outcome.
The same group conducted the two trials which contributed data for the primary efficacy outcome, and the German Spas Association funded one of these trials. We recommend further RCTs that assess PASI‐75, with detailed reporting of the outcome and time point, as well as treatment‐related adverse events. Risk of bias was an issue; future studies should ensure blinding of outcome assessors and full reporting.
Plain language summary
Indoor bathing in salt water followed by exposure to artificial ultraviolet B light for chronic plaque psoriasis
Review question
We reviewed the evidence about the effect of indoor bathing in salt water for adults with chronic plaque psoriasis followed by artificial ultraviolet B (UVB) light treatment. We evaluated two different comparisons: 1) Salt bath with UVB versus other treatment without UVB; eligible comparators were exposure to psoralen bath, psoralen bath + artificial ultraviolet A (UVA) light, topical treatment, systemic treatment (oral or injected medicines that work throughout the entire body), or placebo (an inactive substance). 2) Salt bath with UVB versus other treatment with UVB or UVB only; eligible comparators were exposure to bath containing other compositions or concentrations + UVB or UVB only. The degree of severity of psoriasis can be measured by the psoriasis area and severity index (PASI). Improvement can be indicated by a reduction of PASI. We requested at least a 75% reduction in PASI‐75 score to evaluate a potential beneficial effect. To evaluate a potential harmful effect, we chose treatment‐related side effects severe enough to stop treatment.
Background
Chronic plaque psoriasis is a skin disease characterised by red‐coloured lesions with silvery scales. Bathing in the Dead Sea and exposure to the sun may improve the lesions but may not be practical for most patients. Artificial salt bath and exposure to UVB could simulate the natural exposure.
Study characteristics
The evidence is current to June 2019. We included eight trials (1976 analysed participants). One included trial assessed salt bath plus UVB versus other treatment without UVB (psoralen bath + UVA), but it did not report our primary outcomes for this comparison. Eight trials assessed our second comparison of interest: salt bath with UVB versus other treatment with UVB or UVB only, and those that reported any outcomes of interest (only five studies) specifically compared salt bath with UVB versus UVB only.
No study reported our secondary outcomes. The duration of trials in total ranged between at least two months and up to 13 months. Outcomes were assessed at the end of treatment.
We analysed trials in which different treatments were applied to different participants, and we separately analysed trials in which different treatments were applied to the same participant, but to different body parts. Participants were male or female, and their ages mostly ranged from 41 to 50 years. In five studies, the median PASI score at baseline ranged from 15 to 18 and was balanced between treatment arms. Three studies did not report PASI score. Three studies were sponsored by commercial spa or salt companies, one by health insurance companies, one by an association of dermatologists, and three did not report on funding. Half of the studies were conducted in multiple centres; the remainder were conducted in single centres. Most of the studies were conducted in Germany; all were based in Europe.
Key results
No study reported primary outcome data for the comparison salt bath with UVB versus other treatment without UVB; five studies reported primary outcome data for salt bath with UVB versus UVB only. With respect to achieving PASI‐75, the pooled data of two studies indicated that salt bath + UVB may reduce psoriasis severity compared to UVB only (low‐certainty evidence); however, these two studies were conducted by the same group, and the German Spas Association funded one of the trials (the other trial did not report any funding source). Neither of the studies hid the treatment allocation from the outcome assessors.
When assessing treatment‐related adverse events requiring withdrawal, data from three other studies showed there may be little to no difference between salt bath plus UVB and the comparator UVB only (low‐certainty evidence). The adverse events included skin irritation and severe itch immediately after Dead Sea salt soaks (salt bath + UVB group), and inadequate response to phototherapy and conversion to psoralen bath + UVA (UVB only group).
Certainty of the evidence
We judged the evidence for ‘PASI‐75’ and ‘treatment‐related adverse events requiring withdrawal’ as low certainty. Our confidence was affected by limitations, such as risk of bias (examples being inadequate blinding and high probability of publication bias). The reporting of our outcomes was non‐existent or limited.
Summary of findings
Summary of findings 1. Salt bath + UVB compared with UVB alone for chronic plaque psoriasis.
| Salt bath + UVB compared with UVB alone for chronic plaque psoriasis | ||||||
| Patient or population: patients with chronic plaque psoriasis Setting: indoor clinic or spa Intervention: salt bath + UVB Comparison: UVB alone | ||||||
| Outcomes | Illustrative comparative risks (95% CI)* | Relative effect (95% CI) | Number of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Assumed riskc | Corresponding risk | |||||
| PASI‐75 (number of participants with event) 6 to 8 weeks after start of treatment | Study population | RR 1.71 (1.24 to 2.35) |
278 (2 studies) |
⊕⊕⊝⊝ LOWa | The event was defined as achieving a 75% or more reduction in PASI score from baseline. | |
| 285 per 1000 | 487 per 1000 (353 to 669) |
|||||
| Treatment‐related adverse events requiring withdrawal (number of participants withdrawing) 3 to 8 weeks after the start of treatmentd | Study population | RR 0.96 (0.35 to 2.64) | 404 (2 studies) |
⊕⊕⊝⊝ LOWb | The event was defined as dropping out of the study because of treatment‐related complications. | |
| 35 per 1000 | 34 per 1000 (12 to 92) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PASI: Psoriasis Area and Severity Index. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a We downgraded the certainty of evidence by two levels for this outcome. We downgraded one level because of study limitations (risk of bias). Due to lack of blinding, we judged a high bias of performance bias. We downgraded one level because of high probability of publication bias. The only two between‐participant studies that did contribute data on salt bath + UVB versus UVB alone (Brockow 2007a; Brockow 2007b) to this outcome were conducted by the same sponsor.
b We downgraded the certainty of evidence by two levels for this outcome. We downgraded one level because of study limitations (risk of bias). Due to lack of blinding, we judged a high risk of performance bias. We downgraded one level because of high probability of publication bias. There were only two between‐participant studies (Klein 2011; Leaute‐Labreze 2001) that contributed data on salt bath + UVB versus UVB alone to this outcome.
c The assumed risk is based on the mean number of events across the control groups. Calculation regarding PASI‐75 response: Number of events: 22 + 14 = 36, total number of participants: 66 + 60 = 126, risk per 1000: 36 / 126 * 1000 = 285.
d Two between‐participant studies (Klein 2011, Leaute‐Labreze 2001) reported seven events with salt bath + UVB and seven events with UVB only. One additional within‐participant study (Dawe 2005) reported one event with salt bath + UVB and two events with UVB only.
Background
Description of the condition
Prevalence
Psoriasis is a multifactorial, immune‐mediated chronic inflammatory skin disease (Nestle 2009), affecting roughly 2% of the world population (Christophers 2001). In population‐based surveys, the prevalence of psoriasis is 2.2% in the continental USA (Stern 2004), and it is 1.5% in the UK (Gelfand 2005). In adults, prevalence ranges from 0.9% to 8.5% and incidence ranges from 78.9 to 230 per 100,000 person‐years on a global scale (Parisi 2013). The occurrence of psoriasis increases with age and with distance of geographic regions from the equator; there is no clear difference in the prevalence between men and women (Parisi 2013).
Clinical signs and course
Clinically, chronic plaque psoriasis, which is also known as psoriasis vulgaris, is the most common type of psoriasis, affecting 80% to 90% of people with psoriasis (Griffiths 2007; Lebwohl 2003). It is characterised by easily defined red‐coloured lesions (Nestle 2009). The plaques are a result of an inflammatory and hyperproliferative (growing faster than usual) epidermis (Nestle 2009). They are typically found on the outer sides of the elbow and knee joints as well as on the scalp and back. A genetic predisposition may be the most important basis for an onset of psoriasis (Nestle 2009). A dysregulated immune system may explain the pathogenesis of inflammation and keratinocyte activation and proliferation (Nestle 2009). The disease severity can change with time, sometimes with long periods of calm and sometimes with severe exacerbation (Parisi 2013), with or without recognised trigger factors, such as infection and other environmental factors as referred to in section 'Risk factors' in Description of the condition. The extent of the affected body surface area may be used to classify the severity as mild (less than 5%), moderate (5% to 10%), or severe (greater than 10%) (Menter 2007). Henseler suggested two forms of nonpustular psoriasis: the early onset form was associated with familial inheritance, and the late onset form occurred predominantly sporadically (Henseler 1985).
Symptoms
The main symptoms are itching and pain associated with uncomfortable scaling as a result of loss of cells from the epidermal layer of the skin (Martin 2015).
Diagnosis
A medical doctor can suspect the diagnosis on the appearance of the skin and a dermatologist may confirm the diagnosis. Scaly and erythematous plaques that may be painful and itching are typical skin characteristics (Nestle 2009). A skin biopsy may be helpful to clarify the clinical diagnosis.
Prognosis
The majority of patients experience mild skin lesions (Nestle 2009). Severe lesions may impair quality of life and social interaction.
Severity indices
Physician‐reported severity indices
Fredriksson 1978 developed the Psoriasis Area and Severity Index (PASI). It is a measure of average redness, thickness, and scaliness of skin lesions weighted by the area of involvement. Table 2 displays characteristics of the PASI. Response to therapy may be assessed by the percentage of people who have achieved a 75% or more reduction in their PASI score from baseline, which is referred to as PASI‐75. Itching is a substantial symptom of psoriasis, which may be assessed by a visual analogue scale (VAS) from 0 ('no itching') to 100 ('severe itching') as proposed by Zhu 2014.
1. Psoriasis Area and Severity Index (PASI).
| Section | Area involved | Severity | ||||
| Item | Factor | Item | Factor | Item | Clinical signs | Factor |
| Head | 0.1 | ‐ | 0, 1, 2, 3, 4, 5, or 6 | Sum of clinical signs | ‐ | 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 |
| ‐ | ‐ | 0% | 0 | ‐ | Erythema (redness) | 0, 1, 2, 3, or 4 |
| ‐ | ‐ | < 10% | 1 | ‐ | Induration (thickness) | 0, 1, 2, 3, or 4 |
| ‐ | ‐ | 10% to < 30% | 2 | ‐ | Desquamation (scaling) | 0, 1, 2, 3, or 4 |
| ‐ | ‐ | 30% to < 50% | 3 | ‐ | ‐ | ‐ |
| ‐ | ‐ | 50% to < 70% | 4 | ‐ | ‐ | ‐ |
| ‐ | ‐ | 70% to < 90% | 5 | ‐ | ‐ | ‐ |
| ‐ | ‐ | 90% to 100% | 6 | ‐ | ‐ | ‐ |
| Arms | 0.2 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Trunk | 0.3 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Legs | 0.4 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Subtotal | Section factor *area factor* sum of clinical signs factor | |||||
| Total | Sum of subtotals; range 0 to 72 | |||||
According to Fredriksson 1978. Online calculator available (Corti 2009). Instructions: multiply section factor with area involved factor and multiply with sum of clinical signs factor; repeat for all four sections and add up all four subtotals. The items and corresponding factors regarding the area involved and the severity are exemplified for the section item 'head' and are not shown for the other section items 'arms', 'trunk', and 'legs'. Severity of clinical signs range from none (zero) to maximum (four).
Patient‐reported severity indices
Finlay 1994 developed the Dermatology Life Quality Index (DLQI). The following website provides further information and interpretation: www.dermatology.org.uk. The aim of this questionnaire is to measure how much the skin problem of a person has affected his or her life over the previous week (see Table 3).
2. Dermatology Life Quality Index (DLQI) questionnaire.
| Number | Question | Answer options |
| 1 | Over the last week, how itchy, sore, painful, or stinging has your skin been? | Very much A lot A little Not at all |
| 2 | Over the last week, how embarrassed or self‐conscious have you been because of your skin? | Very much A lot A little Not at all |
| 3 | Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? | Very much A lot A little Not at all Not relevant |
| 4 | Over the last week, how much has your skin influenced the clothes you wear? | Very much A lot A little Not at all Not relevant |
| 5 | Over the last week, how much has your skin affected any social or leisure activities? | Very much A lot A little Not at all Not relevant |
| 6 | Over the last week, how much has your skin made it difficult for you to do any sport? | Very much A lot A little Not at all Not relevant |
| 7 | Over the last week, has your skin prevented you from working or studying? (If "No", over the last week, how much has your skin been a problem at work or studying?) |
Yes No Not relevant |
| 8 | Over the last week, how much has your skin created problems with your partner or any of your close friends? | Very much A lot A little Not at all Not relevant |
| 9 | Over the last week, how much has your skin caused any sexual difficulties? | Very much A lot A little Not at all Not relevant |
| 10 | Over the last week, how much of a problem has the treatment for your skin been, for example, by making your home messy or by taking up time? | Very much A lot A little Not at all Not relevant |
The aim of the Dermatology Life Quality Index (DLQI) questionnaire is to measure how much the life of adults has been affected by their skin problem over the last week. The DLQI is calculated by summing the score of each question, resulting in a maximum of 30 and a minimum of zero. The higher the score, the more quality of life is impaired. The scoring of each question is as follows: Very much (three), A lot (two), A little (one), Not at all (zero), Not relevant (zero); question seven = Yes (three) to unanswered (zero).
Meaning of the total DLQI score: zero to one = no effect at all on patient's life; two to five = small effect on patient's life; six to 10 = moderate effect on patient's life; 11 to 20 = very large effect on patient's life; 21 to 30 = extremely large effect on patient's life. Minimal Clinically Important Difference (MCID) of the DLQI: a change in DLQI score of at least four points is considered clinically important (Basra 2008).
Finlay 1994 developed the DLQI. The following website provides further information and interpretation: www.dermatology.org.uk.
Risk factors
The process by which psoriasis arises is not fully understood. Population, family, and twin studies have shown a genetic contribution to psoriasis (Griffiths 2007; Lønnberg 2013; Rahman 2005), which includes the association of psoriasis with certain human leukocyte antigen (HLA) alleles (Gupta 2014). An allele may be described of either the part of a gene that was inherited from the father, or the other part of a gene that was inherited from the mother. HLA genes encode for certain proteins on the surface of cells that help control the immune system. Currently, it is estimated that genetic data explain only 30% of psoriasis (Barker 2014). Prieto‐Perez 2013 stated that "the many genes associated with psoriasis and the immune response include tumour necrosis factor alpha, interleukin 23, and interleukin 12."
Prieto‐Perez 2013 also referred to the development of new drugs (e.g. etanercept, adalimumab, infliximab, ustekinumab, secukinumab) that target cytokines (e.g. tumour necrosis factor alpha, p40 subunit of interleukin 23 and interleukin 12) and, as a result, suppress the unwanted dysregulated and more than usual immune response. Bachelez 2013 also addressed the involvement of the immune system in the pathogenesis of psoriasis and stated, for example, that interleukin 36 is strongly elevated in the keratinocytes of psoriatic tissue.
Huerta 2007 conducted a prospective cohort study with nested case‐control analysis, in which a group of people were followed over a period of time. A random sample of controls was matched to people who developed psoriasis by age, sex, and calendar year. Odds ratios were adjusted for age and other risk factors. The study found an association between previous skin disorders, infectious disorders, obesity, and smoking with the onset of psoriasis. The authors did not find an association with other potential risk factors, such as stress, diabetes (high blood sugar), hypertension (high blood pressure), hyperlipidaemia (high blood fat), cardiovascular disease, or rheumatoid arthritis.
Comorbidity
Psoriasis may be associated with other diseases, such as arthritis, depression, inflammatory bowel disease, and cardiovascular diseases (Oliveira 2015). As inflammation is involved in some of the associated diseases, it can be speculated whether these diseases may be connected with psoriasis on the ground of common genetic and immune‐related factors.
Health‐related quality of life
Psoriasis causes considerable psychological disability and has a major impact on a person's quality of life (Rapp 1999). Thus, perception of psychosocial disability and quality of life may be paramount when assessing the importance of the condition to an individual and the subsequent treatment of the disease (Menter 2007). A patient‐reported index of disease severity is described above (DLQI). Wahl interviewed 22 hospitalised patients with psoriasis and found that bodily suffering was a core variable with regard to the patients' experience of living with psoriasis (Wahl 2002). For people with psoriasis, itching is a measure of the effect the disease has on quality of life (Zhu 2014).
Cost
The financial burden on people with this disease and on healthcare providers is considerable and was about 35.2 billion dollars in the USA in 2013 (Vanderpuye‐Orgle 2015). It was estimated that incremental medical costs contributed 34.7%; reduced health‐related quality of life, 33.5%; and productivity losses, 31.8% (Vanderpuye‐Orgle 2015).
Description of the intervention
Salt bath followed by artificial ultraviolet B light was developed to simulate exposure to salt and sunlight delivered during climatotherapy (relocation to a region with a climate more favourable to the outcome) at the Dead Sea (Huang 2018). For example, the whole body is soaked in salt water for 15 to 30 minutes, which may have various concentrations ranging from 1 g to 250 g sodium chloride dissolved in one litre water. The soaking is repeated several times a week for a maximum of 20 to 30 applications within a time period of eight weeks. After bathing, various doses of ultraviolet B (UVB) light are applied to the whole body (Gambichler 2000a). The combination of bathing in salt water with UVB bathing thereafter may be called balneophototherapy (Halverstam 2008). Patients with psoriasis could improve with balneophototherapy (Harari 2007).
Treatments for people with chronic plaque psoriasis include a variety of alternatives such as:
topical therapy including steroidal and non‐steroidal agents;
systemic medications (Sbidian 2017) including various biological drugs (Nunez 2019), and additional investigational agents being studied (Ellis 2019);
phototherapy including UVB irradiation alone without bathing in salt water, and
photochemotherapy including ultraviolet A (UVA) irradiation plus psoralen, a compound that aids the absorption of UVA irradiation by making the skin more susceptible to the effects of light rays.
Ultraviolet radiation and its wavelength is defined by the International Commission on Illumination CIE 2011: "radiation for which the wavelengths are shorter than those for visible radiation; the range between 100 nm and 400 nm is commonly subdivided into: UVA: 315 nm to 400 nm; UVB: 280 nm to 315 nm; and UVC: 100 nm to 280 nm". UVB may be subdivided by some authors in broad band (280 nm to 315 nm), narrow band (311), or selective band (300 nm to 315 nm).
The choice of an appropriate therapy is associated with the grade of severity and may be used as comparators with salt baths.
Topical therapy including steroidal and non‐steroidal agents may be mainly used for mild or localised disease (Lebwohl 2005; Menter 2007).
Systemic medications are mainly used for severe or refractory disease (Lebwohl 2005; Menter 2007), which include various oral agents as well as injectable biological agents.
Phototherapy including UVB irradiation alone without bathing in salt water may be used predominantly for moderate or more extensive disease (Lebwohl 2005; Menter 2007).
Phototherapy and photochemotherapy may be also used for those not responding sufficiently to topical treatment (Singh 2016; van de Kerkhof 2004); psoralen plus UVA (PUVA) light photochemotherapy may be used for moderate or severe disease (Lebwohl 2005; Menter 2007). For people with widespread chronic plaque psoriasis, phototherapy may be regarded as the first‐choice treatment because it may have fewer adverse events than systemic or biological agents (Nguyen 2009). Psoralen is a photosensitising compound that aids the absorption of UVA light and increases the skin's sensitivity to light, but subsequently may have carcinogenic properties (Archier 2012). It is administered either orally, in bath water, as a cream, or as a gel. Thus, PUVA is divided into oral PUVA, bath PUVA, and topical (cream) PUVA (Chen 2013). Momtaz 1998 reported that PUVA photochemotherapy has been used in psoriasis and 23 other skin disorders. The risk of development of squamous cell carcinomas may be high if the patients have received ionising radiation or inorganic arsenic, or if patients require continuous treatment for many years. Melanomas may also develop in a few patients. Stern 1998 warned that the use of PUVA should be weighed against the persistent, dose‐related increase in the risk of squamous cell cancer.
How the intervention might work
Salt bath, with or without phototherapy, is being widely used to treat moderate‐to‐severe psoriasis. The mechanism of action of UVB is not completely clear (Weatherhead 2013). UVB phototherapy for treating psoriasis may result in the induction of apoptosis (programmed cell death) of keratinocytes, which are the main cell types in the skin, as well as epidermal and dermal T lymphocytes (cells within the skin that are involved in inflammation) (Weatherhead 2011; Wong 2013). The minimum amount of UVB that produces redness 24 hours after exposure is used as the starting dose for UVB light treatments; this 'minimal erythema dose' decreases after salt water bathing (Gambichler 1998).
There may be increased UV transmission to the skin after soaking in salt water (Gambichler 2011), which may lead to stronger inflammation (e.g. UV‐induced erythema) and also to increased apoptosis of keratinocytes and T lymphocytes, and thus improved clearance of psoriasis (Gambichler 2011; Wong 2013). The mechanism of action of low concentrations of sodium chloride in water used in indoor salt water baths followed by artificial UVB light is unknown. Speculative mechanisms have been proposed, for example, salt solutions might wash out unfavourable substances from the skin lesions, salt water bathing might increase skin sensitivity to UVB light and thereby increasing its action on the lesion, single salt components could act favourably on the cells of the skin lesions, the intervention might inhibit cell proliferation (Schempp 2000).
Why it is important to do this review
Studies suggest that indoor salt water baths with (Brockow 2007), or without exposure to artificial UVB (Schiener 2007), may benefit patients with psoriasis. However, the evidence underlying clinical efficacy, such as longer remission from disease and higher dermatology‐related quality of life, has not yet been clearly evaluated. It should be pointed out that the use of high concentrations of salt is cumbersome due to the large amounts of salt needed. Although indoor salt water baths are widely used in practice to treat chronic plaque psoriasis, no systematic review has been conducted to assess its effectiveness for reducing skin lesions and improving quality of life. Therefore, the aim of this review is to evaluate the efficacy and any severe adverse events of indoor salt water baths by focusing on patient‐centred outcomes. The methods planned for this review were published as a protocol: Indoor salt water baths followed by artificial UVB light for chronic plaque psoriasis (Peinemann 2015).
Objectives
To assess the effects of indoor salt water baths followed by exposure to artificial ultraviolet B (UVB) light for treating chronic plaque psoriasis in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that randomised people or limbs to either the test intervention or a control intervention.
We did not find a reason to exclude right‐left comparative studies. Nevertheless, pretreatment of the skin of a body part investigated in a cross‐over design may influence the results as opposed to no pretreatment. To prevent the concerning additional bias, we excluded cross‐over designs. We suggested that simultaneous application of the intervention of interest on multiple body parts of each participant may distract the scoring of the Psoriasis Area and Severity Index (PASI) response and other subjective assessments. We are positive that right‐left comparative study should not include comparing different body parts. Different body parts may be affected differently and the assessment may introduce bias. We did not find a reason to exclude cluster‐randomised trials. Throughout the review, we used the term 'people or limbs' to accommodate people who were randomised, as well as randomised legs, arms, and elbows.
We planned to include the following designs.
Simple parallel group design according to the Cochrane Handbook for Systematic Reviews of Interventions, section 9.3.1, quote: "participants are individually randomised to one of two intervention groups, and a single measurement for each outcome from each participant is collected and analyzed" (Higgins 2011).
Multiple observations for the same outcome, specifically repeated measurements. If the data were to be included in a meta‐analysis, we planned to select a single time point and to analyse only data at this time point for studies in which it was presented.
Multiple observations for the same outcome, specifically, multiple body parts receive different interventions according to the Handbook section 9.3.8, quote: "These trials have similarities to cross‐over trials: whereas in cross‐over trials individuals receive multiple treatments at different times, in these trials they receive multiple treatments at different sites." (Higgins 2011). In the present review, we considered paired comparisons within participants, such as comparisons of right versus left arm, leg, elbow, or knee. We planned to separately analyse the within‐participant data and the between‐participant data.
Cluster‐randomised trials according to the Cochrane Handbook section 9.3.2, quote: "groups of participants, such as schools or families are randomized to different interventions. [...] Participants within any cluster often tend to respond in a similar manner, and thus their data can no longer be assumed to be independent of one another" (Higgins 2011).
We did not include the following study designs.
Cross‐over design according to the Handbook section 9.3.3, quote: "all participants receive all interventions in sequence: they are randomized to an ordering of interventions, and participants act as their own control.". We expected a high risk of carryover according to the Handbook section 16.4.2, quote: "Carryover is the situation in which the effects of an intervention given in one period persist into a subsequent period, thus interfering with the effects of different subsequent intervention" (Higgins 2011).
Multiple observations for the same outcome, specifically, multiple body parts receive the same intervention according to the Handbook section 9.3.7, quote: "people are randomized, but multiple parts (or sites) of the body receive the same intervention, a separate outcome judgement being made for each body part, and the number of body parts is used as the denominator in the analysis. [...] This is similar to the situation in cluster‐randomized trials, except that participants are the 'clusters" (Higgins 2011).
Comparison of different body parts within participants, such as comparing arm versus leg.
Types of participants
Adults (i.e. 18 years of age or older) of any ethnic background or gender who have been diagnosed with chronic plaque type psoriasis by a dermatologist. We excluded people with pustular psoriasis, guttate psoriasis, or inverse psoriasis.
Types of interventions
The aim of this review was to assess the efficacy of salt bath added to ultraviolet B (UVB) light. We did not seek to assess efficacy of salt bath only.
Comparison 1: salt bath + UVB versus other treatment without UVB
Test intervention
Exposure to indoor salt water bath followed by artificial UVB light for chronic plaque psoriasis. We included studies where bathing in salt water was performed indoors during or prior to exposure to UVB. We excluded studies where bathing in salt water was performed outdoors and for leisure purposes, such as bathing in geothermal sea water, bathing in a thermal lagoon, or bathing in a salty lake.
Control intervention
Exposure to psoralen bath, psoralen bath + artificial ultraviolet A (UVA) light, topical treatment, systemic treatment, or placebo.
Comparison 2: salt bath+ UVB versus other treatment + UVB or UVB only
Test intervention
Exposure to indoor salt water bath followed by artificial UVB for chronic plaque psoriasis. We included studies where bathing in salt water is performed indoors during or prior to exposure to UVB. We excluded studies where bathing in salt water is performed outdoors and for leisure purposes, such as bathing in geothermal sea water, bathing in a thermal lagoon, or bathing in a salty lake.
Control intervention
Exposure to bath containing other compositions or concentrations + UVB or UVB only.
Types of outcome measures
We did not use the outcomes listed here as criteria for including studies; they are the outcomes of interest within studies identified for inclusion.
Primary outcomes
Between‐participant data as well as within‐participant data:
physician‐assessed outcome: psoriasis area and severity index (PASI)‐75;
physician‐assessed outcome: treatment‐related adverse events requiring withdrawal.
Secondary outcomes
Between‐participant as well as within‐participant data:
participant‐reported outcome: dermatology life quality index (DLQI);
participant‐reported outcome: pruritus severity using a visual analogue scale (VAS) from 0 ('no itching') to 100 ('severe itching');
physician‐reported outcome: time to relapse;
physician‐assessed outcome: secondary malignancies.
We did not consider using the Physician Global Assessment (PGA), because we favoured PASI, which is reportedly validated and preferred for use in clinical trials (Robinson 2012).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 4 June 2019:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2019, Issue 6 in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Online trials registers
We (FP, SP) searched the following online trials registers up to 6 June 2019 using the term 'psoriasis' in the field 'condition' and 'phototherapy' in the field 'intervention':
the ISRCTN registry (www.isrctn.com) (called 'The metaRegister of Controlled Trials' in the protocol);
ClinicalTrials.gov (www.clinicaltrials.gov) (called 'The US National Institutes of Health Ongoing Trials Register' in the protocol);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from bibliographies
We checked the bibliographies of included studies, relevant articles, and review articles for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. However, we examined data on adverse effects from the included studies that we identified.
Data collection and analysis
Some parts of the methods section of this Cochrane Review used text that was originally published in other Cochrane publications co‐authored by FP and protocol contributor Doreen Tushabe (predominantly Peinemann 2013a and Peinemann 2013b), as well as using text that was originally published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
While preparing this systematic review, we endorsed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement, adhered to its principles and conformed to its checklist (Moher 2009). We downloaded all titles and abstracts retrieved by electronic searching to an Excel spreadsheet (Microsoft Corp 2011), and removed any duplicates. Two review authors (FP, SP) independently examined any remaining references. We included a study selection flow chart in the review (Figure 1).
1.
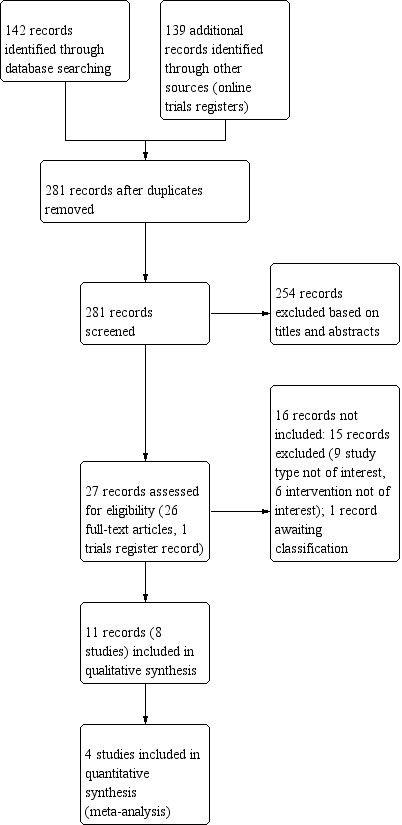
Study flow diagram.
We excluded those studies that clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (FP, SP) independently assessed the eligibility of retrieved papers. We resolved any disagreements by discussion between the two review authors; no third party arbitration was necessary. We documented reasons for exclusion in the Characteristics of excluded studies tables. If we identified multiple reports of one study we used the most‐up‐to‐date full‐text results. We checked the multiple reports for possible duplicate data, addressed the issue and did not include duplicate data in the analysis.
Data extraction and management
For each included study, two review authors (FP, SP) extracted study characteristics and outcomes independently onto a data extraction form. The data included: information on study design, participant characteristics (such as inclusion criteria, age, disease severity, comorbidity, previous treatment, number enrolled in each arm), interventions (such as type of irradiation, type of bath, dose applied, duration of therapy, control treatment), risk of bias, follow‐up duration, outcome measures, and deviations from the study protocol. We pilot tested The Cochrane Editorial Resources Committee's data collection form for intervention reviews (for RCTs only) available from the Cochrane Skin Group website: www.skin.cochrane.org/resources, then used this for data extraction. We resolved differences between review authors by discussion.
Assessment of risk of bias in included studies
Two review authors (FP, SP) independently appraised the risk of bias in the included studies. We resolved differences between review authors by discussion. We used the items listed within Cochrane's tool for assessing risk of bias (Higgins 2011):
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data such as missing data (attrition bias);
selective reporting such as not reporting pre‐specified outcomes (reporting bias); and
other sources of bias such as bias related to the specific study design (other bias).
In general, a judgement of "low risk" of bias was given if plausible bias is unlikely to seriously alter the results, for example, if the participants and investigators enrolling those participants could not foresee the assignment. A judgement of "high risk" of bias was given if plausible bias seriously weakens confidence in the results, for example, if the participants or investigators enrolling those participants could possibly foresee the assignments. A judgement of "unclear" risk of bias was given if plausible bias raises some doubt about the results, for example, the method of concealment is not described or not described in sufficient detail to allow a definite judgement.
To draw conclusions about the overall risk of bias for an outcome, it was necessary to summarise the results of the 'Risk of bias' assessments performed for each individual study. We assessed the risk of bias at study level, not by outcome. We aligned the summary assessment to the Cochrane 'Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies', as shown in Table 8.7a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed whether the risk of bias for a single outcome was low, unclear, or high, then we interpreted whether the plausible bias was unlikely to seriously alter the results, raises some doubts about the results, or seriously weakened the confidence in the results. We assessed within a study whether there was a low risk of bias for all key domains, an unclear risk of bias for one or more domains, or a high risk of bias for one or more key domains. We assessed across studies whether most information was from studies at low risk of bias or at high or unclear risk of bias, and we assessed across studies whether the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of results.
Measures of treatment effect
For time‐to‐event data such as survival, we planned to extract the hazard ratio (HR) and its standard error or confidence interval (CI) from trial reports; if these were not reported, we planned to estimate the logHR and its standard error using the methods of Parmar 1998 and using the tool provided by Tierney 2007.
For dichotomous data, such as PASI‐75 and treatment‐related adverse events requiring withdrawal, we estimated the proportion of events among the assessed participants. We pooled the data and estimated a risk ratio (RR) and a P value for overall effect by using the Mantel‐Haenszel statistic and a fixed‐effect model. If the P value was not reported by the study, then we estimated the P value by using the web‐based Easy Fisher Exact Test Calculator (Social Science Statistics 2018). In case of rare events, we planned to use Peto odds ratios instead, but this was not applied.
For continuous outcomes (e.g. PASI), we planned to extract the final value or change from baseline and corresponding standard deviation (SD) of the outcome of interest and the number of people or limbs assessed at endpoint in each treatment arm at the end of follow‐up. This was in order to estimate the mean difference (MD) or standardised mean difference (SMD) between treatment arms. We planned to analyse and present continuous data using the MD if all results were measured on the same scale (e.g. PASI). If this was not the case (e.g. fear, or quality of life), we planned to use the SMD. Eligible continuous outcomes were not reported.
We planned to extract the proportion of participants who reached the 50% reduction of the respective psoriatic lesion score compared to the baseline value at a fixed time point to allow pooling of data. If this was not possible, we planned to extract the time necessary to reach 50% reduction compared to baseline value. To calculate a mean difference, the number of people at risk and the probability of an event at a given time is required. Where studies did not provide that information, we chose to describe the individual results and not to pool the data. If data were given in a chart, we deduced the numbers from the chart.
Where possible, all data that we extracted were those relevant to an intention‐to‐treat (ITT) analysis in which all data were analysed in groups to which they were assigned. We stated if this was not possible. We noted the time points at which outcomes were collected and reported. We reported the 95% CIs for all analyses.
Unit of analysis issues
We included between‐participant data as well as within‐participant data. In general, within‐participant studies apply the intervention to a body part such as a limb and the comparator to a different body part such as the opposite limb (e.g. right arm versus left arm). Those data are distinct. Consequently, we separately analysed and reported information on between‐participant data and within‐participant data. PASI‐75 is the primary efficacy outcome for both data types.
If studies reported multiple intervention groups, we designated the intervention group and each different comparator group.
Dealing with missing data
We conformed to Cochrane's principal options for dealing with missing data (Higgins 2011). If data were missing, or only imputed data were reported, we planned to contact trial authors to request data on the outcomes of the people or limbs of the study. When relevant data regarding study selection, data extraction, and 'Risk of bias' assessment were missing, we planned to contact study authors to retrieve the missing data. If data remained missing after contact with authors, we analysed only the available data and addressed the potential impact on the findings in the Discussion.
Assessment of heterogeneity
We planned to assess heterogeneity (composed of dissimilar parts) between studies by visual inspection of forest plots; by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (I² statistic) (Higgins 2003); and if possible, by subgroup analyses. If there was evidence of substantial heterogeneity, we planned to investigate and report the possible reasons for this. An I² statistic greater than 50% was considered to indicate substantial heterogeneity, demonstrating a considerable variation in results. In this case, we planned to present the graphical display of a forest plot, but we did not plan to report an average value for the intervention effect.
Assessment of reporting biases
We planned to assess study protocols to see if the planned outcomes have been reported, but did not identify any. We planned to assess reporting bias, such as publication bias, by constructing a funnel plot if there were a sufficient number of included studies for a particular outcome (that is, at least 10 studies included in a meta‐analysis).
Data synthesis
We analysed the data using the Review Manager 5.3 software (Review Manager 2014); one review author entered the data (FP) and another review author checked it (SP). If sufficient clinically similar studies were available, we deemed it feasible to pool data. We planned to consider clinical homogeneity and statistical heterogeneity before pooling the data. If the I² statistic value was greater than 50%, we did not combine studies in order to report an average value for the intervention effect. We planned to use random‐effects models with inverse variance weighting for all analyses (DerSimonian 1986).
Concerning the dichotomous events of PASI‐75 and treatment‐related adverse events requiring withdrawal, we pooled the data and estimated a risk ratio and a P value for overall effect by using the Mantel‐Haenszel statistic and a fixed‐effect model.
Where possible, all data that we extracted were those relevant to an intention‐to‐treat (ITT) analysis in which all data were analysed in groups to which they were assigned. We stated if this was possible. We noted the time points at which outcomes were collected and reported. We reported the 95% confidence intervals (CIs) for all analyses.
We planned to use ordinal data from people who answered the Dermatology Life Quality Index (DLQI) questionnaire (Table 3) (Melilli 2006) or the visual analogue scale (VAS) on pruritus (itching), as well as from people who achieved a remission and people who experienced secondary malignancy. However, these data were not reported.
In the protocol, we addressed a problem that might arise if we included only a single study in an analysis of dichotomous data. In this instance, the confidence intervals around risk ratios calculated in Review Manager 2014 are unreliable. Where results were estimated for individual studies with low numbers of outcomes (less than 10 in total), or where the total sample size was less than 30 people, or limbs and a risk ratio was used, we planned to report the proportion of outcomes in each treatment group together with a P value from a Fisher's exact test. But this problem did not arise.
The analysis should be in intention‐to‐treat and all missing data were considered as failure.
Results on outcomes that were not predefined were also reported in Effects of interventions as additional information, though, the results were not relevant for the conclusion of the present review.
'Summary of findings' tables and GRADE assessments
We prepared one 'Summary of findings' table using the GRADE profiler software (GRADEpro GDT 2014) concerning the comparison of between‐participant data: salt bath + UVB compared with other treatment + UVB. We listed the predefined two primary outcomes: PASI‐75 and treatment‐related adverse events requiring withdrawal. For each outcome, two review authors (FP, SP) independently assessed the certainty of the evidence by using the five GRADE considerations, that is, study limitations, inconsistency, indirectness, imprecision, and publication bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). Due to lack of data, we did not prepare other 'Summary of findings' tables, which might be possible for comparison type one regarding between‐participant data and for both comparison types regarding within‐participant data.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We planned to conduct a sensitivity analysis removing studies at high or uncertain risk of bias. However, we did not undertake any sensitivity analyses due to the limited number of studies included in this review.
Results
Description of studies
Results of the search
The Electronic searches of databases and online trials registers retrieved 281 records (142 from databases and 139 from online trials registries) once duplicates were removed (Figure 1). We screened the title and abstract of 281 records and excluded 254 records. We screened the full texts of the remaining 27 records and included 11 records and did not include 16 records. The exclusion reasons for 15 records (Excluded studies) are shown in the Characteristics of excluded studies. One record is listed in Studies awaiting classification, and described in Characteristics of studies awaiting classification. The latter record (NCT02713711) could be an eligible study for inclusion in a future update. The results and full description of the methods of the study were not available. We included eight studies (Arnold 2001; Brockow 2007a; Brockow 2007b; Dawe 2005; Gambichler 2001; Klein 2011; Leaute‐Labreze 2001; Schiener 2007) associated with 11 records. For a full description of our screening process, see Figure 1.
Included studies
Of the 27 potentially relevant records, we included 11 records (Included studies), which are associated with eight different studies (Figure 1). The characteristics of the eight included studies are described in the Characteristics of included studies tables.
Design and sample sizes
We included eight randomised controlled trials (RCTs) (2105 participants; 1976 analysed) in this review. Six studies were parallel RCTs (2035 participants; 1908 analysed) that randomised patients to an intervention or comparator group (Arnold 2001; Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). Leaute‐Labreze 2001 randomised participants to an intervention group (24 analysed), or two different comparator groups (22 and 21 analysed). Schiener 2007 randomised participants to an intervention group (299 analysed), or three different comparator groups (270, 285, and 305 analysed). Two studies (70 participants; 68 analysed) were within‐participants studies (140 limbs; 136 analysed) (Dawe 2005; Gambichler 2001). Dawe 2005 randomised arms or legs to an intervention or comparator group (120 limbs; 116 analysed), and Gambichler 2001 randomised elbows to an intervention or comparator group (20 limbs; 20 analysed). The majority of studies did not report the recruitment periods. The earliest reported recruitment happened in the year 2001.
Assessment at the end of treatment was reported by seven studies: up to eight weeks (Arnold 2001), six weeks (Brockow 2007a), six weeks (Brockow 2007b), eight weeks (Gambichler 2001), seven weeks (Klein 2011), three weeks (Leaute‐Labreze 2001), and eight weeks (Schiener 2007) after start of treatment. In one study (Dawe 2005), we did not identify a clear reporting of treatment duration. Assessment at the end of follow‐up was reported by six studies: up to eight months (Arnold 2001), six months (Brockow 2007a), six months (Brockow 2007b), 12 months (Dawe 2005), six months (Klein 2011), 12 months (Leaute‐Labreze 2001) after end of treatment. In two studies (Gambichler 2001; Schiener 2007), we did not identify a clear reporting of follow‐up duration. The duration of trials in total (start of treatment to end of follow‐up) was roughly: up to four months (Arnold 2001), up to eight months (Brockow 2007a), up to eight months (Brockow 2007b), at least 12 months (Dawe 2005), at least two months (Gambichler 2001), up to eight months (Klein 2011), up to 13 months (Leaute‐Labreze 2001), and at least two months (Schiener 2007).
Setting
Five of the eight included studies were set in Germany (Brockow 2007a; Brockow 2007b; Gambichler 2001; Klein 2011; Schiener 2007), and a single study was set in the Netherlands (Arnold 2001), the UK (Dawe 2005), and France (Leaute‐Labreze 2001), respectively. Four studies were conducted in a single centre (Arnold 2001; Dawe 2005; Gambichler 2001; Leaute‐Labreze 2001), and the remaining four studies (Brockow 2007a; Brockow 2007b; Klein 2011; Schiener 2007) were conducted in more than one centre. Five studies reported the recruitment of outpatients (Arnold 2001; Brockow 2007a; Brockow 2007b; Gambichler 2001; Schiener 2007), and the other three studies did not report any information on recruitment (Dawe 2005; Klein 2011; Leaute‐Labreze 2001). One single‐centre study was conducted in the Psoriasis Day Care Centre at Ede, the Netherlands. Another single‐centre study was conducted in a spa centre at Salies‐de‐Bearn, France. Two other single‐centre studies were conducted; one in Germany and one in the UK. Four multi‐centre studies were conducted in spa centres or outpatient clinics in Germany. Three of eight studies were sponsored by commercial spa or salt companies (German Spas Association (Deutscher Heilbäderverband); Mavena Healthcare AG, Switzerland, the commercial spa facility La Compagnie Fermiere de Salies de Bearn), one by health insurance companies ("primary" health insurance companies in Bavaria, Germany), one by an association of dermatologists (Berufsverband der Deutschen Dermatologen, Germany), and three did not report on funding.
Participants
The study participants were diagnosed with psoriasis by a dermatologist. Six studies reported a mean age range from 41 to 49 years of age in the intervention group, and from 45 to 50 years of age in the control group (Arnold 2001; Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). One study reported a mean age of 36 years in the intervention group and 46 years of age in the comparator group (Gambichler 2001). One study did not report information on age (Dawe 2005). Four studies reported male gender from 56% to 74% in the intervention group and from 60% to 62% in the control group (Brockow 2007a; Brockow 2007b; Klein 2011; Schiener 2007). Two studies reported male gender in 40% and 57% for all people or limbs, respectively (Arnold 2001; Gambichler 2001). Two studies did not report information on gender (Dawe 2005; Leaute‐Labreze 2001).
All eight included studies reported on assessing the skin type by using the Fitzpatrick phototyping scale (Fitzpatrick 1988), which results in six categories. Most participants were categorised type III, in some studies, a considerable fraction of participants were also categorised II and IV. According to the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA): "The Fitzpatrick skin phototype is a commonly used system to describe a person's skin type in terms of response to ultraviolet radiation (UVR) exposure." The various types correspond to pale white skin (I), white skin (II), light brown skin (III), moderate brown skin (IV), dark brown skin (V), and deeply pigmented dark brown to black skin (VI).
As a measure of severity of disease, the PASI score ranging from 0 to 72 points was assessed at the beginning of the study in five studies (Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). In the test arm versus control arms, the median PASI score was 17 versus 16 (Brockow 2007a), 17 versus 18 (Brockow 2007b), 15.1 versus 15.3 (Klein 2011), 15.0 versus 15.7 (Leaute‐Labreze 2001), and 16 versus 16 versus 17 versus 17 (Schiener 2007). Concerning baseline PASI score, there was no imbalance between treatment arms. Three studies (Arnold 2001; Dawe 2005; Gambichler 2001) did not report the PASI score.
Four of the studies reporting between‐participant data (Brockow 2007a; Brockow 2007b; Klein 2011; Schiener 2007) stated the proportion of participants who had any experience of former phototherapy, which was balanced across the treatment groups. Three studies (Brockow 2007a; Brockow 2007b; Schiener 2007) reported that between 80% and 90% of included participants had previous phototherapy, while one study (Klein 2011) reported a smaller proportion between 10% and 15%. One of the studies reporting within‐participant data (Dawe 2005) reported a proportion of 75%.
Four of the studies reporting between‐participant data (Brockow 2007a; Brockow 2007b; Klein 2011; Schiener 2007) stated the proportion of participants who had previous systematic therapy due to psoriasis, which was balanced across the treatment groups. Three studies (Brockow 2007a; Brockow 2007b; Schiener 2007) reported that between 20% and 40% of included participants had previous systematic therapy, while one study (Klein 2011) reported a smaller proportion between 1% and 3%. One of the studies reporting within‐participant data (Dawe 2005) reported a proportion of 12%.
Three of the studies reporting between‐participant data (Brockow 2007a; Brockow 2007b; Schiener 2007) stated the proportion of participants who had previous inpatient care due to psoriasis, which was balanced across the treatment groups. These studies reported that between 40% and 60% of included participants had previous inpatient care. Klein 2011 reported that about 55% of participants had previous topical treatment due to psoriasis.
Three studies reported on the duration of disease. Two of the studies reporting between‐participant data (Leaute‐Labreze 2001; Schiener 2007) reported a mean or median duration of 15 to 20 years. One of the studies reporting within‐participant data (Gambichler 2001) reported a mean duration of 2.5 years.
Interventions and comparisons
To make various salt concentrations comparable, we converted the information on salt solutions into the unit g/L (Table 4).
3. Salt concentrations.
| Study | Intervention | Comparator |
| Arnold 2001 | 6.7 g/L NaCl dissolved in tap water | 5.7 mg/L psoralen dissolved in tap water |
| Brockow 2007a | 250 to 270 g/L NaCl present in natural spring water | no bath |
| Brockow 2007b | 45 to 120 g/L NaCl present in natural spring water | no bath |
| Dawe 2005 | 150 g/L Dead Sea salt dissolved in tap water | no bath |
| Gambichler 2001 | 240 g/L NaCl dissolved in tap water | 0.2 g/L present in tap water |
| Klein 2011 | 100 g/L Dead Sea salt dissolved in tap water | no bath |
| Leaute‐Labreze 2001 | 250 g/L NaCl present in natural sping water | no bath |
| Schiener 2007 | 250 g/L NaCL dissolved in tap water | 0.5 mg/L psoralen dissolved in tap water |
NaCl: sodium chloride
Comparison 1: salt bath + UVB versus other treatment without UVB
We identified one comparison in one of the studies reporting between‐participant data (Schiener 2007). Schiener 2007 randomised 310 participants to the intervention group salt water + UVB and randomised 321 participants to the comparator group psoralen bath + UVA.
Schiener 2007: salt bath + UVB versus psoralen bath + UVA
We did not identify comparisons in the studies reporting within‐participants data
Comparison 2: salt bath + UVB versus other treatment + UVB or UVB only
We identified seven comparisons in the six studies reporting between‐participant data (Arnold 2001; Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). UVB was applied in addition to another treatment, such as psoralen bath or tap water bath in two comparator groups. UVB only was applied in five comparator groups. Arnold 2001 randomised 20 participants to the intervention group salt water + UVB and 20 participants to the comparator group psoralen bath + UVB. Schiener 2007 randomised 310 participants to the intervention group salt bath + UVB, randomised 301 participants to the comparator group UVB only, and randomised 301 participants to tap water + UVB. The other studies (Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001) randomised participants to the intervention group salt bath + UVB, and to the comparator group UVB only.
Arnold 2001: salt bath + UVB versus psoralen bath + UVB
Brockow 2007a: salt bath + UVB versus UVB only
Brockow 2007b: salt bath + UVB versus UVB only
Klein 2011: salt bath + UVB versus UVB only
Leaute‐Labreze 2001: salt bath + UVB versus UVB only
Schiener 2007: salt bath + UVB versus UVB only
Schiener 2007: salt bath + UVB versus tap water + UVB
We identified two comparisons in the two studies reporting within‐participants data (Dawe 2005; Gambichler 2001). Both studies randomised body parts to salt water + UVB versus UVB only.
Dawe 2005: salt bath + UVB versus UVB only
Gambichler 2001: salt bath + UVB versus UVB only
Salt bath
People, legs, arms, or elbows were soaked for 15 to 30 minutes in salt water with a sodium chloride salt concentration ranging from 0.8 g/L to 250 g/L. The sources were natural springs in three studies (Brockow 2007a; Brockow 2007b; Leaute‐Labreze 2001), dissolved sodium chloride in tap water in three studies (Arnold 2001; Gambichler 2001; Schiener 2007), and dissolved commercial Dead Sea salt in tap water in two studies (Dawe 2005; Klein 2011). In general, salt bath + UVB was applied once a day, three to five days a week, for up to eight weeks, and reaching a maximum number of 15 to 35 applications.
UVB
For irradiation with UVB light, the studies used devices such as Philips (Eindhoven, the Netherlands) 100 Watts TL‐01 lamps. Four studies used only 311 nm narrowband UVB (Arnold 2001; Dawe 2005; Klein 2011; Leaute‐Labreze 2001), two studies used only 280 nm to 320 nm broadband UVB (Brockow 2007b; Gambichler 2001), and two studies used both (Brockow 2007a; Schiener 2007). Schiener 2007 reported a 300 nm to 320 nm 'selective phototherapy', which represents a part of the broadband UVB and which has been predominantly in German phototherapy centres. Some studies assessed a so‐called minimal erythema dose (MED) before treatment to determine a starting dose, which might be set at 50% of the MED. The MED was defined as the dose to produce a just‐detectable erythema with sharp borders within 24 hours in some uninvolved and untanned skin areas of 2 cm2. The authors of four studies reported mean starting doses from 0.02 J/cm2 to 0.4 J/cm2 (Brockow 2007b; Gambichler 2001; Klein 2011; Leaute‐Labreze 2001). In general, UVB was applied once a day, three to five days a week, for up to eight weeks, and reaching a maximum number of 15 to 35 applications.
Cumulative UVB doses
Regarding the intervention (salt water bath + UVB), the authors of five studies (Brockow 2007a; Dawe 2005; Klein 2011; Leaute‐Labreze 2001; Schiener 2007) reported mean cumulative doses for primarily or only using narrowband UVB from 11.8 J/cm2 to 50.7 J/cm2 and the authors of two studies (Brockow 2007b; Gambichler 2001) reported mean cumulative doses for only using broadband UVB from 2.7 J/cm2 to 7.2 J/cm2. Regarding the comparator (UVB alone), the authors of seven studies reported mean cumulative doses for narrowband UVB from 12.5 J/cm2 to 41.3 J/cm2and for broadband UVB from 2.8 J/cm2 to 7.2 J/cm2 (Brockow 2007a; Brockow 2007b; Dawe 2005; Gambichler 2001; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). One study did not report UVB doses (Arnold 2001).
Outcomes
Primary outcomes
Psoriasis area and severity index (PASI)‐75
Between‐participant data
Two of six studies (Brockow 2007a; Brockow 2007b) reported PASI‐75.
Four of six studies (Arnold 2001; Klein 2011; Leaute‐Labreze 2001; Schiener 2007) reported aggregate data on PASI, such as mean PASI and PASI‐50, but did not report PASI‐75 as defined in the inclusion criteria. It is not possible to deduce PASI‐75 from mean PASI or PASI‐50, but it is possible to calculate PASI‐75 if individual data are available. Therefore, we sent e‐mail requests to the authors of the respective studies to send us individual data on PASI. We used the e‐mail addresses provided by recent papers of the authors. If an e‐mail address was no longer active, we sent the request to an e‐mail address provided by the institution that was involved in the study. We sent enquiries to authors of all included studies. Detailed information is provided in Appendix 6. We asked for individual patient data on PASI of the studies by Arnold 2001, Brockow 2007a, Brockow 2007b, Klein 2011, Leaute‐Labreze 2001, and Schiener 2007 to enable a calculation of PASI‐75. We did not receive the requested information.
Within‐participant data
Two of two studies (Dawe 2005, Gambichler 2001) reported individual severity scores which are different from PASI, and they consequently did not report PASI‐75. We sent enquiries to authors of all included studies. Detailed information is provided in Appendix 6. We asked for individual patient data on PASI of the studies by Dawe 2005 and Gambichler 2001 to enable a calculation of PASI‐75. We did not receive the requested information.
Treatment‐related adverse events requiring withdrawal
Between‐participant data
Two of six studies reported treatment‐related adverse events requiring withdrawal (Klein 2011; Leaute‐Labreze 2001). Four of six studies did not report this outcome.
Within‐participant data
One study (Dawe 2005) reported this outcome.
Secondary outcomes
The included studies did not report outcomes that were predefined as secondary by the present review including Dermatology Life Quality Index (DLQI), Pruritus severity using a visual analogue scale (VAS) from 0 ('no itching') to 100 ('severe itching'), Time to relapse, and Secondary malignancies.
Excluded studies
Of 25 potentially relevant records, we excluded 15 records (Figure 1). One record (Studies awaiting classification) is awaiting classification and is described in the Characteristics of studies awaiting classification table. The reason for exclusion of 15 records (Excluded studies) are described in the Characteristics of excluded studies table and are based on:
not intervention of interest (n = 6): Dead Sea bathing and sun exposure; geothermal sea bathing + UVB; sulphurous thermal spring water, bath not followed by UVB consistently; lagoon bathing followed by UVB; oral retinoid added to bath and UVB; and UVB but not salt water baths;
not study type of interest (n = 9): systematic review (n = 5); single‐arm study (n = 2); nonsystematic review (n = 2).
Studies awaiting classification
NCT02713711 randomised 24 adult participants with psoriasis in a single‐centre study in Chile. The number of participants correspond to the two treatment groups of interest. The intervention was salt bath followed by artificial UVB. The comparator was artificial UVB only. The study was sponsored by Universidad Catolica del Maule, Chile. This study is completed and the authors have submitted the manuscript. At the present time, the manuscript has not been accepted for publication. Thus, the results of the outcomes are not available yet (correspondence with study author shown in Appendix 6).
Risk of bias in included studies
The risk of bias table in Characteristics of included studies provides details of each item of the risk of bias tool for randomised controlled trials. Figure 2 and Figure 3 provide an overview.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The authors of six studies clearly described an adequate random sequence generation and an adequate concealment of the allocation, which was judged as low risk of bias (Brockow 2007a; Brockow 2007b; Dawe 2005; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). In the other two studies, selection bias from random sequence generation and an adequate concealment of the allocation was judged as unclear risk of bias (Arnold 2001; Gambichler 2001).
Blinding
The authors of five studies reported that they did not blind investigators and patients and we judged a high risk of performance bias (Brockow 2007a; Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007). A further study did not report this topic so we also judged it as high risk of performance bias (Arnold 2001). One study reported the issue of blinding, but patients and nurses were not blinded (Dawe 2005). As physicians may have been blinded, we judged an unclear risk of performance bias. One other study reported partial blinding of people or limbs, and we judged an unclear risk of bias (Gambichler 2001).
Most of the studies tried to blind the persons involved in outcome assessment, but this could not be achieved in all cases. We judged a low risk of detection bias in four studies as the assessors were presumably unaware of patients' treatment assignments (Brockow 2007a; Gambichler 2001; Leaute‐Labreze 2001; Schiener 2007). We judged an unclear risk in two studies as the blinding was tried but was not achieved (Brockow 2007b; Dawe 2005). The authors of one study did not report this topic so we considered it high risk of detection bias (Arnold 2001) . In addition, one study was considered at high risk as blinding was not implemented (Klein 2011).
Incomplete outcome data
Concerning three studies, we were unsure whether a rather low proportion of dropouts or a high proportion of dropouts but the application of an intention‐to‐treat analysis may have affected the outcome (Klein 2011; Leaute‐Labreze 2001; Schiener 2007). Therefore, we judged them at unclear risk of attrition bias. We judged attrition bias as high risk in three studies (Arnold 2001; Brockow 2007b; Dawe 2005) as a considerable number of patient data could not be used in the analysis. In two studies, we did not identify attrition bias and they were judged low risk of bias (Brockow 2007a; Gambichler 2001).
Selective reporting
No protocol was available for any included study. Thus, we searched for inconsistencies within the reporting of the article. We did not identify a selective reporting issue and judged all eight studies at unclear risk of reporting bias (Arnold 2001; Brockow 2007a; Brockow 2007b; Dawe 2005; Gambichler 2001; Klein 2011; Leaute‐Labreze 2001; Schiener 2007).
Other potential sources of bias
We did not identify a substantial source of other bias from the information available in the reports; hence, we judged all eight included studies at unclear risk of other sources of bias due to insufficient information available to make a full assessment (Arnold 2001; Brockow 2007a; Brockow 2007b; Dawe 2005; Gambichler 2001; Klein 2011; Leaute‐Labreze 2001; Schiener 2007).
Effects of interventions
See: Table 1
Comparison 1: Salt bath plus UVB versus other treatment without UVB
Primary outcomes
PASI‐75 response
We did not identify appropriate data.
Treatment‐related adverse events requiring withdrawal
We did not identify appropriate data.
Secondary outcomes
Dermatology Life Quality Index (DLQI)
We did not identify appropriate data.
Pruritus severity using a visual analogue scale (VAS) from 0 ('no itching') to 100 ('severe itching')
We did not identify appropriate data.
Time to relapse
We did not identify appropriate data.
Secondary malignancies
We did not identify appropriate data.
Not predefined outcomes
We identified outcomes reported in the eight included studies that were not predefined by the current review. These outcomes should not have an impact on the conclusion. Nevertheless, the respective results should be reported to provide a broader picture of the results reported in the various studies.
PASI‐50 response (between‐participant data)
Schiener 2007 reported the proportion of participants who achieved a 50% or more reduction in their Psoriasis Area and Severity Index score (PASI‐50) from baseline (Table 5). Schiener 2007 reported proportions of PASI‐50 of the intervention group salt bath + UVB that were similar to those of the comparator group psoralen bath + UVA (Table 5). The difference between treatment groups was not statistically significant.
4. PASI‐50 (between‐participant data).
| Study | Time point | Intervention | Comparators | P value1 | ||||
| Description | A/R | Proportion | Description | A/R | Proportion | |||
| Brockow 2007a | Session 18 (six weeks) | Salt bath + UVB | 79/81 | 86% (68 of 79) | UVB only | 71/79 | 54% (38 of 71) | < 0.001 |
| Brockow 2007b | Session 18 (six weeks) | Salt bath + UVB | 79/81 | 73% (58 of 79) | UVB only | 64/83 | 50% (32 of 64) | 0.0053 |
| Schiener 2007 | Eight weeks | Salt bath + UVB | 299/310 | 74.9% (224 of 299) | Tap water + UVB | 285/301 | 60.7% (173 of 285) | 0.0003 |
| Psoralen bath + UVA | 305/321 | 78.4% (239 of 305) | 0.3369 |
1: Estimated by review authors using Easy Fisher Exact Test Calculator (Social Science Statistics 2018); statistically signifiant P values (P < 0.05) in bold font.
A/R: number of analysed/randomised people; n.r.: not reported; PASI‐50: Participants have achieved a 50% or more reduction in their Psoriasis Area and Severity Index score from baseline; UVB: artificial ultraviolet B light
PASI (between‐participant data)
We did not identify appropriate data.
Clearance of psoriatic lesions (within‐participant data)
We did not identify appropriate data.
Treatment‐related adverse events
All eight included studies reported on treatment‐related adverse events (Table 6) such as dermatitis solaris, phototoxic reaction, erythema, burning, stinging, itching, itchy papular eruption, edema, and blisters. The relationship of adverse events with study interventions was classified as definitive, possible, or unlikely by the study authors. We extracted only adverse events which were classified as definitive or possible. We limited the reporting on statistically significant results.
5. Treatment‐related adverse events.
| Study | Intervention | Comparators | P value1 | ||||||
| Description | A/R | Type | Events | Description | A/R | Type | Events | ||
| Arnold 2001 | Salt bath + UVB | 16/20 | Burning erythema | 2 | Psoralen bath + UVA | 17/20 | Burning erythema | 2 | 1.0 |
| Brockow 2007a | Salt bath + UVB | 79/81 | Dermatitis solaris | 2 | UVB | 71/79 | Dermatitis solaris | 1 | 1.0 |
| Brockow 2007b | Salt bath + UVB | 76/81 | 3 x phototoxic reaction 1 × burning/stinging |
4 | UVB | 53/83 | 2x phototoxic reaction 3x dermatitis solaris |
5 | 0.4861 |
| Dawe 2005 | Salt bath + UVB | 41/60 arms or legs | 3 x itching, 2 x stinging 1 x itchy papular eruption |
6 | UVB | 41/60 arms or legs | n.a. | 0 | < 0.0257 |
| Gambichler 2001 | Salt bath + UVB | 10/10 elbows | n.a. | 0 | Tap water + UVB | 10/10 elbows | n.a. | 0 | 1.0 |
| Klein 2011 | Salt bath + UVB | 180/183 | 'Definite' or 'probable' events | 21 | UVB | 179/184 | 'Definite' or 'probable' events | 11 | 0.0943 |
| Leaute‐Labreze 2001 | Salt bath + UVB | 24/24 | Itching and burning in most cases | 12 | UVB | 21/21 | Itching and burning in most cases | 7 | 0.3661 |
| Schiener 2007 | Salt bath + UVB | 284/310 | Phototoxic reactions | 33 | UVB | 252/301 | Phototoxic reactions | 31 | 0.894 |
| Tap water + UVB | 266/301 | Phototoxic reactions | 19 | 0.0809 | |||||
| Psoralen bath + UVA | 297/321 | Phototoxic reactions | 16 | 0.0072 | |||||
| Grade IV or V erythemas | 3 | UVB | 252/301 | Grade IV or V erythemas | 1 | 0.6263 | |||
| Tap water + UVB | 266/301 | Grade IV or V erythemas | 2 | 1.0 | |||||
| Psoralen bath + UVA | 297/321 | Grade IV or V erythemas | 5 | 0.7252 | |||||
| Oedema2 | n.r. | n.r. | n.r. | Oedema2 | n.r. | n.a. | |||
| Blisters2 | n.r. | n.r. | n.r. | Blisters2 | n.r. | n.a. |
1: Estimated by review authors using Easy Fisher Exact Test Calculator (Social Science Statistics 2018); statistically signifiant P values (P < 0.05) in bold font.
2: Schiener 2007 reported seven patients who developed edema and four who developed blisters, but the adverse events were not linked to a treatment group.
A/R: number of analyzed/randomized people or limbs; n.a.: not applicable; n.r.: not reported; UVA: artivicial ultraviolet A light; UVB: artificial ultraviolet B light
Between‐participant data
Schiener 2007 reported 33 events in 284 assessed participants after exposure of salt water + UVB versus 16 events in 297 participants after psoralen bath + UVA. The difference between the treatment groups was statistically significant (P = 0.0072) in favour of psoralen bath + UVA. The 49 events were phototoxic reactions.
Within‐participant data
We did not identify statistically significant results.
Treatment‐related severe adverse events
Between‐participant data
Schiener 2007 reported zero events in the intervention group salt bath + UVB and three events (photodermatitis, photodermatitis, and malignant melanoma) and three events in the comparator group psoralen bath + UVA (Table 7). The difference between the treatment groups was not statistically significant.
6. Treatment‐related serious adverse events.
| Study | Intervention | Comparators | P value1 | ||||||
| Description | A/R | Type | Events | Description | A/R | Type | Events | ||
| Schiener 2007 | Salt bath + UVB | 284/310 | n.a. | 0 | UVB only | 252/301 | n.a. | 0 | 1.0 |
| Tap water + UVB | 266/301 | 1 x photodermatitis; 1 x exacerbation as guttate psoriasis; 1 alcohol problem |
3 | 0.1125 | |||||
| Psoralen bath + UVA | 297/321 | 2 x photodermatitis; 1 x malignant melanoma2 |
3 | 0.2491 |
1: Estimated by review authors using Easy Fisher Exact Test Calculator (Social Science Statistics 2018); statistically signifiant P values (P < 0.05) in bold font.
2: Schiener 2007 provided detailed information on the malignant melanoma case: "A malignant melanoma was diagnosed after the eighth irradiation. Queries at the trial site disclosed that an 'atypical nevus' had already been detected by a dermatologist 17 months before study enrollment. Furthermore, it is very unlikely that the tumor had progressed during the short period of 2 weeks." Though, a relationship between study intervention and event was judged as possible, we did not separately include the case as a secondary malignancy.
A/R: number of analysed/randomised people; n.a.: not applicable; UVA: artificial ultraviolet A light; UVB: artificial ultraviolet B light
Within‐participant data
We did not identify appropriate data.
Patient‐reported outcomes
The authors of four studies (Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007) reported patient‐reported outcomes (Table 8). We did not consider the self‐administered Psoriasis Area and Severity Index (S‐PASI), the patients' self‐rated analogue to the clinician‐rated PASI, which is scored and interpreted as the PASI (Brockow 2007a). We did not consider the short form of the questionnaire on experience with skin complaints (SF‐QES) reported by Schiener 2007, because the test of statistical significance on change scores were applied across four different treatment groups only.
7. Patient‐reported outcomes.
| Study | HRQL | Intervention | Comparators | P value1 | ||||
| Measure | Description | A/R | Change of index | Description | A/R | Change of index | ||
| Brockow 2007b2 | Global rating of disease severity by patients. Mean change of score (standard deviation) baseline to end of treatment (maximum 6 weeks). | Salt bath + UVB | n.r./81 | 29.9 (24.7) | UVB only | n.r./83 | 12.4 (24.6) | n.r. |
| Brockow 2007b2 | " baseline to 3 months | Salt bath + UVB | n.r./81 | 32.2 (27.6) | UVB only | n.r./83 | 22.5 (25.5) | n.r. |
| Brockow 2007b2 | " baseline to 6 months | Salt bath + UVB | n.r./81 | 22.5 (27.9) | UVB only | n.r./83 | 24.3 (27.6) | n.r. |
| Brockow 2007b2 | Global rating of treatment effect by patients. Mean (standard deviation) score at end of treatment (maximum 6 weeks). | Salt bath + UVB | n.r./81 | 81.2 (19.2) | UVB only | n.r./83 | 64.5 (27.7) | n.r. |
| Brockow 2007b2 | Global rating of tolerability by patients. Mean (standard deviation) score at end of treatment (maximum 6 weeks). | Salt bath + UVB | n.r./81 | 87.4 (12.3) | UVB only | n.r./83 | 87.4 (12.3) | n.r. |
| Brockow 2007b2 | Self‐administered PASI (S‐PASI). Number of participants with reduction of S‐PASI by 50% or more (%) at end of treatment (maximum 6 weeks). | Salt bath + UVB | 77/81 | 45 (58) | UVB only | 61/83 | 24 (39) | 0.04 |
| Brockow 2007b2 | " at 3 months. | Salt bath + UVB | 72/81 | 40 (56) | UVB only | 54/83 | 24 (44) | 0.28 |
| Brockow 2007b2 | " at 6 months. | Salt bath + UVB | 67/81 | 30 (45) | UVB only | 46/83 | 24 (50) | 0.70 |
| Klein 2011 | Psoriasis disability index at eight weeks on session 35 | Salt bath + UVB | 144/1833 | ‐18.3% ((22.3 ‐ 27.3) / 27.3)5 | UVB only | 106/1844 | ‐15.2%% ((25.6 ‐ 30.2) / 30.2)5 |
n.r. |
| Klein 2011 | " at one month | Salt bath + UVB | n.r./183 | ‐26.1% ((20.2 ‐ 27.3) / 27.3)5 | UVB only | n.r./184 | ‐20.5%% ((24.0 ‐ 30.2) / 30.2)5 |
n.r. |
| Klein 2011 | " at six months | Salt bath + UVB | n.r./183 | ‐30.0% ((19.1 ‐ 27.3) / 27.3)5 | UVB only | n.r./184 | ‐23.5%% ((23.1 ‐ 30.2) / 30.2)5 |
n.r. |
| Klein 2011 | Sickness impact profile at eight weeks on session 35 | Salt bath + UVB | 144/183 | ‐19.0% ((4.7 ‐ 5.8) / 5.8)5 |
UVB only | 106/184 | ‐10.7% ((5.0 ‐ 5.6) / 5.6)5 |
n.r. |
| Klein 2011 | " at one month | Salt bath + UVB | n.r./183 | ‐20.7% ((4.6 ‐ 5.8) / 5.8)5 |
UVB only | n.r./184 | ‐8.9% ((5.1 ‐ 5.6) / 5.6)5 |
n.r. |
| Klein 2011 | " at six months | Salt bath + UVB | n.r./183 | ‐31.0% ((4.0 ‐ 5.8) / 5.8)5 |
UVB only | n.r./184 | ‐21.4% ((4.4 ‐ 5.6) / 5.6)5 |
n.r. |
| Klein 2011 | Improvement of physical complaints of Freiburg Life Quality Assessment on session 35 (eight weeks) | Salt bath + UVB | 144/183 | +16.7% ((2.8 ‐ 2.4) / 2.4) | UVB only | 106/184 | +8.3% ((2.6 ‐ 2.4) / 2.4) | <0.001 |
| Klein 2011 | Global health total of Freiburg Life Quality Assessment on session 35 (eight weeks) | Salt bath + UVB | 144/183 | +37.5% ((3.3 ‐ 2.4) / 2.4) | UVB only | 106/184 | +8.3% ((2.6 ‐ 2.4) / 2.4) | 0.007 |
| Klein 2011 | Global health skin only of Freiburg Life Quality Assessment on session 35 (eight weeks) | Salt bath + UVB | 144/183 | +100.0% ((4.8 ‐ 2.4) / 2.4) | UVB only | 106/184 | +54.2% ((3.7 ‐ 2.4) / 2.4) | 0.001 |
| Klein 2011 | Global impression of therapy: very good or good on session 35 (eight weeks) | Salt bath + UVB | 144/183 | 64.8% | UVB only | 106/184 | 32.8% | <0.001 |
| Klein 2011 | Global impression of therapy: very bad or bad on session 35 (eight weeks) | Salt bath + UVB | 144/183 | 5.3% | UVB only | 106/184 | 30.4% | <0.001 |
| Leaute‐Labreze 2001 | Quality of life index determined on a 10‐cm analog scale on day 21 (three weeks) | Salt bath + UVB | 24/24 | ‐60%6 | UVB only | 21/21 | ‐50%6 | n.r. |
| Schiener 20072 | Global rating of disease severity by patients. Mean change of score (standard deviation) baseline to 2, 4, and 6 weeks or to end of treatment (maximum 8 weeks). | Salt bath + UVB | 299/310 | 36.3 (26.4) | UVB only | 270/301 | 21.5 (26.3) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 36.3 (26.4) | Tap water + UVB | 285/301 | 28.1 (29.9) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 36.3 (26.4) | Psoralen bath + UVA | 305/321 | 43.2 (27.5) | n.r. |
| Schiener 20072 | Global rating of treatment effect by patients. Mean (standard deviation) score at 2, 4, and 6 weeks or at end of treatment (maximum 8 weeks). | Salt bath + UVB | 299/310 | 77.8 (21.3) | UVB only | 270/301 | 59.0 (27.0) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 77.8 (21.3) | Tap water + UVB | 285/301 | 65.3 (29.4) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 77.8 (21.3) | Psoralen bath + UVA | 305/321 | 82.9 (22.5) | n.r. |
| Schiener 20072 | Global rating of tolerability by patients. Mean (standard deviation) score at 2, 4, and 6 weeks or at end of treatment (maximum 8 weeks). | Salt bath + UVB | 299/310 | 85.1 (16.7) | UVB only | 270/301 | 75.8 (21.3) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 85.1 (16.7) | Tap water + UVB | 285/301 | 82.3 (19.6) | n.r. |
| Schiener 20072 | " | Salt bath + UVB | 299/310 | 85.1 (16.7) | Psoralen bath + UVA | 305/321 | 88.6 (16.1) | n.r. |
| Schiener 20077 | Short Form of the Questionnaire on Experience with Skin complaints (SF‐QES). Change scores did not differ significantly between groups (P = 0.47). Overall median improvement was ‐0.14 (25th to 75th percentile, ‐1.27 to 0.00; n = 1107 participants). Assessment at 2, 4, and 6 weeks or at end of treatment (maximum 8 weeks). | no data | no data | no data | no data | no data | no data | 0.47 |
| Schiener 20078 | Self‐administered PASI (S‐PASI). Number of participants with reduction of S‐PASI by 50% or more (%) at 2, 4, and 6 weeks or at end of treatment (maximum 8 weeks). | Salt bath + UVB | 286/310 | 217 (75.9) | UVB | 258/301 | 134 (51.9) | <0.001 |
| Schiener 20078 | " | Salt bath + UVB | 286/310 | 217 (75.9) | Tap water + UVB | 266/301 | 174 (65.4) | 0.009 |
| Schiener 20078 | " | Salt bath + UVB | 286/310 | 217 (75.9) | Psoralen bath + UVA | 292/321 | 237 (81.2) | 0.13 |
1: P values reported by study
2: Brockow 2007b and Schiener 2007. Global ratings on on disease severity, treatment effect, and tolerability using 100‐mm visual analog scales. Positive change scores indicated improvement, higher scores at the end of treatment indicated better effectivenes or tolerability.
3: 183 patients were randomised to Salt bath + UVB. Data from 144 people were analysed and the data from the following 39 patients were not analysed: Three people did not start treatment; one person was not available for second PASI; early withdrawal concerned 35 people; see figure 1 of the article by Klein 2011.
4: 184 patients were randomised to UVB only. Data from 106 patients were analysed and the data from the following 78 patients were not analysed: Five people did not start treatment; two people were not available for second PASI; early withdrawal concerned 71 patients; see figure 1 of the article by Klein 2011. This figure states that 137 patients participated in the follow‐up six months after treatment. However, the same figure also states that only 106 participants were treated through to the end of treatment at session 35 which equals eight weeks after start of treatment. We suppose that this might be a spelling error.
5: Psoriasis disability index or Sickness impact profile change from baseline as a proportion from baseline as reported by Klein 2011 and calculated by review authors using the following formula: ((session 35 ‐ baseline) / baseline). The data show a better improvement in the Salt bath + UVB group compared to the UVB group for all six analyses, though, it is not clear whether the differences are statistically significant.
6: Quality of life index change from baseline as a proportion from baseline as reported and calculated by Leaute‐Labreze 2001 using the following formula: ((day 21 ‐ day 0) / day 0). The data show a decrease of quality of life index for both treatment groups, however, the quality of life index was not defined by the study and it is not clear whether the changes actually mean impairment or improvement.
7: Schiener 2007: The Short Form of the Questionnaire on Experience with Skin complaints (SF‐QES) consists of 4 subscales scored from 0 to 4. One scale has an inverse effect on the underlying construct. Total score, defined as the sum of all subscales, can range from −4 to +12. Higher scores indicate a higher level of stigmatisation feelings.
8: Schiener 2007: The Self‐administered PASI (S‐PASI) was operationalized in the same way as the PASI (reduction of S‐PASI or involved body surface area by 50% or more). The S‐PASI proved to be a reliable, valid, and responsive outcome measure. The S‐PASI is scored and interpreted in the same way as the PASI.
A/R: number of analysed/randomised people; n.r.: not reported; not stat signif: not statistically significant; PUVA: psoralen and ultraviolet A light; UVB: artificial ultraviolet B light
Between‐participant data
Schiener 2007 reported global ratings on disease severity, treatment effect, and tolerability using 100‐mm visual analogue scales (VAS) (Table 8). The numbers of assessed participants in the intervention group salt bath + UVB versus the comparator group psoralen bath + UVA were not specified. Statistical significance was not tested.
Within‐participant data
We did not identify appropriate data.
Comparison 2: Salt bath plus UVB versus other treatment with UVB or UVB only
Please see Table 1.
Primary outcomes
PASI‐75 response
Between‐participant data
Brockow 2007a and Brockow 2007b stated the proportion of participants who achieved PASI‐75 at time points between six to eight weeks after start of treatment (Table 9). Brockow 2007a found a higher proportion of PASI‐75 in the intervention group salt bath + UVB versus the comparator group UVB only. The difference between treatment groups was statistically significant (P = 0.0044) in favour of salt bath. Brockow 2007b also found a higher proportion of PASI‐75 with salt bath + UVB versus UVB only. However, the difference between treatment groups was not statistically significant (P = 0.0632). We pooled the data of both studies and estimated a risk ratio of 1.71 (95% CI 1.24 to 2.35; P = 0.0009; 278 participants; two studies; low‐certainty evidence); Analysis 1.1; Figure 4) which favours salt bath + UVB. Please note that due to the nature of this measurement (that is, the number of patients with a PASI‐75), a high event rate is favourable.
8. PASI‐75 (between‐participant data).
| Study | Time point | Intervention | Comparators | P value1 | ||||
| Description | A/R | Achieved; Failed | Description | A/R | Achieved; Failed | |||
| Brockow 2007a2,3 | session 18 (six weeks) | Salt bath + UVB | 78/81 | 58% (45 of 78); 42% (33 of 78) | UVB only | 66/79 | 33% (22 of 66); 67% (44 of 66) | 0.0044 |
| Brockow 2007b2 | session 18 (six weeks) | Salt bath + UVB | 74/81 | 39% (29 of 74); 1% (45 of 74) | UVB only | 60/83 | 23% (14 of 60); 77% (46 of 60) | 0.0632 |
1: Estimated by review authors using Easy Fisher Exact Test Calculator (Social Science Statistics 2018); statistically signifiant P values (P < 0.05) in bold font.
2: Brockow 2007a and Brockow 2007b classified the data as results from secondary analysis.
3: We identified a spelling error Brockow 2007a: the spelling concerning the heading 'PASI‐75' in table 2 should be 'PASI‐75' instead of 'PASI‐50'.
A/R: number of analysed/randomised people or limbs; UVA: artificial ultraviolet A light; UVB: artificial ultraviolet B light
1.1. Analysis.

Comparison 1: Saline+UVB versus UVB, Outcome 1: PASI‐75 response
4.

Forest plot of comparison: 1 Saline+UVB versus UVB, outcome: 1.1 PASI‐75 response.
Within‐participant data
We did not identify appropriate data.
Treatment‐related adverse events requiring withdrawal
Between‐participant data
Klein 2011 and Leaute‐Labreze 2001 stated the proportion of participants who had treatment‐related adverse events requiring withdrawal (Table 10). The differences between treatment groups in both studies were not statistically significant. We pooled the data of the two studies and calculated a risk ratio of 0.96 (95% CI 0.35 to 2.64; P = 0.94; 404 participants; two studies; low‐certainty evidence); Analysis 1.2; Figure 5). The result did not favour any treatment.
9. Treatment‐related adverse events requiring withdrawal.
| Study | Time point | Intervention | Comparators | P value1 | ||||
| Description | A/R | Type and (number) of events | Description | A/R | Type and (number) of events | |||
| Dawe 2005 | eight weeks | Salt bath + UVB | 58/60 | severe itch immediately after Dead Sea salt soaks (1) | UVB only | 58/60 | inadequate response to phototherapy and conversion to psoralen bath + UVA (2) | 1.0 |
| Klein 2011 | session 35 (eight weeks) | Salt bath + UVB | 180/183 | n.sp. (4) | UVB only | 179/184 | n.sp. (7) | 0.3795 |
| Leaute‐Labreze 2001 | three weeks | Salt bath + UVB | 24/24 | skin irritation (3) | UVB only | 21/21 | skin irritation (0) | 0.2364 |
1: Estimated by review authors using Easy Fisher Exact Test Calculator (Social Science Statistics 2018); statistically signifiant P values (P < 0.05) in bold font.
A/R: number of analysed/randomised people or limbs; n.r.: not reported; n.sp.: not specified; UVA: artificial ultraviolet A light; UVB: artificial ultraviolet B light
1.2. Analysis.

Comparison 1: Saline+UVB versus UVB, Outcome 2: Treatment‐related adverse events requiring withdrawal (between participant)
5.

Forest plot of comparison: 1 Saline + UVB versus UVB, outcome: 1.2 Treatment‐related adverse events requiring withdrawal (between participants).
Within‐participant data
Dawe 2005 reported one event with salt bath + UVB and two events with UVB only (Analysis 1.3; Figure 6). The result did not favour any treatment; low‐certainty evidence.
1.3. Analysis.

Comparison 1: Saline+UVB versus UVB, Outcome 3: Treatment‐related adverse events requiring withdrawal (within participant)
6.

Forest plot of comparison: 1 Saline + UVB versus UVB, outcome: 1.3 Treatment‐related adverse events requiring withdrawal (within participants).
Secondary outcomes
Dermatology Life Quality Index (DLQI)
We did not identify appropriate data.
Pruritus severity using a visual analogue scale (VAS) from 0 ('no itching') to 100 ('severe itching')
We did not identify appropriate data.
Time to relapse
We did not identify appropriate data.
Secondary malignancies
We did not identify appropriate data.
Not predefined outcomes
We identified outcomes reported in the eight included studies that were not predefined by the current review. These outcomes should not have an impact on the conclusion. Nevertheless, the respective results should be reported to provide a broader picture of the results reported in the various studies.
PASI‐50 response (between‐participant data)
Brockow 2007a, Brockow 2007b and Schiener 2007 reported PASI‐50 (Table 5). Brockow 2007a found a higher proportion of PASI‐50 in the intervention group bath + UVB versus the comparator group UVB only. The difference between treatment groups was statistically significant (P < 0.001) in favour of salt bath + UVB. Brockow 2007b found also a higher proportion of PASI‐50 in the intervention group salt bath + UVB versus the comparator group UVB only. The difference between treatment groups was also statistically significant (P < 0.0053) in favour of salt bath + UVB. Schiener 2007 found a higher proportion of PASI‐50 in the intervention group salt bath + UVB versus the comparator group tap water + UVB. The difference between treatment groups was again statistically significant (P < 0.0003) in favour of salt bath + UVB.
PASI (between‐participant data)
Arnold 2001, Klein 2011, Leaute‐Labreze 2001 reported the change of PASI score from baseline ((day 21 to day 0)/day 0) (Table 11). Klein 2011 found a greater reduction of PASI in the intervention group salt bath + UVB versus the comparator group UVB only. The difference between treatment groups was statistically significant (P < 0.0001) in favour of salt bath + UVB. Arnold 2001 and Leaute‐Labreze 2001 reported data on percentage change in PASI for each group with both groups showing a reduction in PASI; however, no formal statistical comparison of the two groups were presented.
10. PASI (between‐participant data).
| Study | Time point | Intervention | Comparators | P value1 | ||||
| Description | A/R | Change of PASI2 | Description | A/R | Change PASI2 | |||
| Arnold 2001 | Session 24 (eight weeks) | Salt bath + UVB | 16/20 | ‐90% ((1.5 to 14.6)/14.6) | Psoralen bath + UVA | 17/20 | ‐74% ((3.1 to 11.9)/11.9) | n.r. |
| Klein 2011 | Session 35 (eight weeks) | Salt bath + UVB | 179/183 | ‐82% ((2.7 to 15.1)/15.1) | UVB only | 177/184 | ‐44% ((8.6 to 15.3)/15.3) | < 0.0001 |
| Leaute‐Labreze 2001 | Session 15 (three weeks) | Salt bath + UVB | 24/24 | ‐55% | UVB only | 21/21 | ‐64% | n.r. |
1: P value calculated by study authors.
2: Calculation takes use of the values measured on the following days: (day 21 ‐ day 0)/day 0.
A/R: number of analysed/randomised people; n.r.: not reported; PASI: Psoriasis Area and Severity Index score; UVA: artificial ultraviolet B light; UVB: artificial ultraviolet B light
Clearance of psoriatic lesions (within‐participant data)
Gambichler 2001 and Dawe 2005 reported various clearance of psoriatic lesions scores (Table 12). In both studies, differences in change of scores from baseline were not statistically significant between treatment groups.
11. Clearance of psoriatic lesions scores (within‐participant data).
| Study | Score | Body parts | Intervention | Comparators | P value1 | ||||
| Description | BL | Change of score2 | Description | BL | Change of score2 | ||||
| Dawe 2005 | scaling, erythema, and induration score measured at 8 months3 | arms or legs | Salt bath + UVB | 6.8 | ‐63% ((2.5 to 6.8)/6.8) | UVB | 6.8 | ‐63% ((2.5 to 6.8)/6.8) | "not significant" |
| Gambichler 2001 | five‐point severity score measured after 30 treatments (time point n.r.) | elbows | Salt bath + UVB | 7.9 | ‐68% ((2.5 to 7.9)/7.9) | Tap water + UVB | 7.8 | ‐69% ((2.4‐7 to 8)/7.8) | "not significant" |
1: statistical significance reported by study authors
2: Calculation takes use of the values measured on the following weeks: (week 8 ‐ week 0)/week 0.
3: Dawe 2005: numerals deduced from the line chart shown in figure 1 of the article.
A/R: number of analysed/randomised units; BL: baseline score; n.r.: not reported
Treatment‐related adverse events
Between‐participant data
We did not identify statistically significant results.
Within‐participant data
Dawe 2005 reported six events in 41 assessed arms or legs after exposure of salt bath + UVB versus zero events after exposure of UVB only. The difference between the treatment groups was statistically significant (P < 0.0257) in favour of no treatment. The six events in the intervention arm included three times itching, two times stinging, and one time itchy papular eruption.
Treatment‐related severe adverse events
Between‐participant data
Schiener 2007 reported zero events in the intervention group salt bath + UVB and three events (photodermatitis, exacerbation as guttate psoriasis, and alcohol problem) in the comparator group tap water + UVB (Table 7). Schiener 2007 also reported zero events in the comparator group UVB only. The difference between the treatment groups was not statistically significant.
Within‐participant data
We did not identify appropriate data.
Patient‐reported outcomes
The authors of four studies (Brockow 2007b; Klein 2011; Leaute‐Labreze 2001; Schiener 2007) reported patient‐reported outcomes (Table 8). We did not consider the self‐administered Psoriasis Area and Severity Index (S‐PASI), the patients' self‐rated analogue to the clinician‐rated PASI, which is scored and interpreted as the PASI (Brockow 2007a). We did not consider the short form of the questionnaire on experience with skin complaints (SF‐QES) reported by Schiener 2007, because the test of statistical significance on change scores were applied across four different treatment groups only.
Between‐participant data
Brockow 2007b reported global ratings on disease severity, treatment effect, and tolerability using 100 mm visual analogue scales (Table 8). The numbers of assessed participants in the intervention group salt water + UVB versus the comparator group UVB only were not specified. Statistical significance was not tested.
Klein 2011 reported a change of the Freiburg Life Quality Assessment for the three parts physical complaints, global health total, and global health skin only (Table 8). The data showed improvement in both treatment groups with analyses conducted at the time point session 35 equivalent to six weeks after start of treatment. The results showed statistically significantly better improvement in the intervention group salt bath + UVB versus the comparator group UVB only, which favours salt bath + UVB.
Klein 2011 reported a change from baseline for the psoriasis disability index and the sickness impact profile for the intervention group salt bath + UVB group versus the comparator group UVB only (Table 8). Statistical significance was not tested.
Klein 2011 reported the proportion of global impression of therapy (very good or good) as well as global impression of therapy (very bad or bad) (Table 8). The differences between the intervention group salt bath + UVB versus the comparator group UVB only were statistically significant, which favours salt bath + UVB.
Leaute‐Labreze 2001 reported a change from baseline for the quality of life index for the intervention group salt bath + UVB versus the comparator group UVB only (Table 8). Statistical significance was not tested.
Schiener 2007 reported global ratings on disease severity, treatment effect, and tolerability using 100‐mm visual analogue scales (Table 8). The numbers of assessed participants in the intervention group salt bath + UVB versus the comparator group tap water + UVB versus the comparator group UVB only were not specified. Statistical significance was not tested.
Within‐participant data
We did not identify appropriate data.
Discussion
Summary of main results
Our review evaluated the current state of evidence on the efficacy of the following:
Indoor salt bath + ultraviolet B (UVB) versus other treatment without UVB (comparison one);
Indoor salt bath + UVB versus other treatment + UVB or UVB only (comparison two).
We included eight randomised controlled trials (RCTs) on chronic plaque psoriasis. With respect to predefined outcomes, comparison one was not reported by any included study. With respect to predefined outcomes, comparison two was reported by five included studies.
Concerning the primary outcome Psoriasis Area and Severity Index (PASI‐75) response, two between‐participant studies randomised participants to the intervention group salt bath + UVB or to the comparator group UVB only and found that salt bath + UVB may improve psoriasis (low‐certainty evidence) (Table 1).
Concerning the primary outcome treatment‐related adverse events requiring withdrawal, two other between‐participant studies also randomised participants to the intervention group salt bath + UVB or to the comparator group UVB only and found that there may be little to no difference between the groups with regard to this outcome (low‐certainty evidence) (Table 1). One of the studies reported skin irritation; the other did not specify the type of adverse events reported by the participants. One within‐participant study (paired right/left comparison) randomised skin areas to the intervention group salt bath + UVB or to the comparator group UVB only and found that there may be little to no difference between the groups with regard to this outcome (low‐certainty evidence). The study reported one event with the salt bath + UVB group (severe itch immediately after Dead Sea salt soaks), and two events with the UVB only group (inadequate response to phototherapy and conversion to psoralen bath + ultraviolet A (UVA)).
None of the predefined secondary outcomes of this review were reported in any of the included studies (Dermatology Life Quality Index (DLQI), pruritus severity using a visual analogue scale (VAS), time to relapse, or secondary malignancies).
The reporting of our pre‐specified outcomes was either non‐existent or limited, with a maximum of two studies reporting a given outcome. The two trials that contributed data for the primary efficacy outcome did not blind their outcome assessors and were conducted by the same group: one of the two trials was funded by the German Spas Association, but the other trial did not state a funding source.
Overall completeness and applicability of evidence
Seven of the eight included studies were published in 2007 or earlier, and we can assume that the majority of patients were treated 10 to 20 years ago. Nonetheless, we presume that the principle treatment procedures might not deviate considerably from the current practice.
The primary outcome PASI‐75 was reported in two out of six between‐participant studies (Brockow 2007a; Brockow 2007b), but not in the two within‐participant studies.
The primary outcome treatment‐related adverse events requiring withdrawal was reported in two other between‐participant studies (Klein 2011; Leaute‐Labreze 2001), and in one within‐participant study (Dawe 2005). Out of eight studies, the primary efficacy outcome (PASI‐75 for two different data types) was reported by only two studies.
Out of eight studies, the primary adverse event outcome (treatment‐related adverse events requiring withdrawal for two different data types) were reported by only two between‐participant studies and only one within‐participant study. None of the predefined secondary outcomes were reported, so there were no patient‐reported outcomes. For one of the two comparisons, none of the predefined outcomes were reported, so there is no evidence to assess at all for half of what was of interest.
Ultraviolet B phototherapy might pose a risk of carcinogenesis, especially of squamous cell carcinoma, and thus the cumulative exposure time should be controlled (Chang 2014; de Gruijl 2002). The studies included in the present review lack long‐term observation and secondary neoplasia was not addressed.
All studies were conducted by non‐academic institutions that build their business on the evaluated treatments. Therefore, a financial conflict of interest might be present in most, if not all of the included studies. Five of the eight studies were conducted in Germany. This preference might be the result of a lasting culture‐specific increase of supply in the past or of a supplier‐induced demand in recent times.
Key issues are that most trials conducted did not contribute data to the primary outcomes and the primary efficacy outcome data all come from two small unblinded trials conducted by a single group of investigators. There was no information for the primary or secondary outcomes on salt water baths + UVB versus no UVB, which is a key comparison.
Quality of the evidence
To accommodate events in the analysis of the primary outcome PASI‐75, we used the number of people that reached PASI‐75. We downgraded the certainty of evidence by two levels for this outcome (low certainty of evidence). We downgraded one level because of study limitations (risk of bias). Due to lack of blinding, we judged a high risk of performance bias. We downgraded one level because of high probability of publication bias. Six studies included in the review did not contribute to the primary outcome. The two studies that did contribute data (Brockow 2007a; Brockow 2007b) were conducted by the same sponsor. It should be acknowledged that some studies tried to blind outcome assessment and assessed if the blinding could be realised. In general, lack of blinding of outcome assessment contributed to a high risk of bias in most studies.
Treatment‐related adverse events requiring withdrawal was also used as primary outcome. We downgraded the certainty of evidence by two levels for this outcome (low certainty of evidence). We downgraded one level because of study limitations (risk of bias). Due to lack of blinding, we judged a high bias of performance bias. We downgraded one level because of high probability of publication bias. Five studies included in the review did not contribute to the primary outcome. Three studies did contribute data, two between‐participant studies (Klein 2011; Leaute‐Labreze 2001) and one within‐participant study (Dawe 2005).
In general, the reporting did not facilitate a clear and instant understanding. The variety in outcome reporting reduced the pooling of data considerably. Many data are not eligible for meta‐analysis, which may create a selection bias within the review. The authors of four studies (Arnold 2001; Dawe 2005; Gambichler 2001; Leaute‐Labreze 2001) did not report a participant flow diagram. Acknowledging the study limitations in the reporting of the two primary outcomes, we judge an unclear internal validity. Secondary outcomes of this review were not measured by any of the included studies; therefore, we were unable to determine the certainty of evidence for these outcomes.
Potential biases in the review process
We conducted a comprehensive search for studies including a search for ongoing studies. The search was broad and sensitive; thus, the risk of not detecting a relevant study is very small. We did not make any post‐hoc analysis decisions after seeing the data. Only two studies reported PASI‐75, and only two studies reported treatment‐related adverse events requiring withdrawal, but we retained the predefined primary outcomes. We tried to access individual study data to complement missing information. Although, we rephrased the name of both outcomes, this did not actually affect the planned concept, but enabled clarification. Subsequently, we assume that there were no relevant departures from protocol that could be potential sources of bias. The restriction to treatment‐related adverse events instead of extracting all adverse events probably reduced the number of events included in the analyses. It is not fully clear if this modification caused or prevented bias.
Agreements and disagreements with other studies or reviews
The Ontario Health Technology Assessment (Health Quality Ontario 2009) included four RCTs in a systematic review that are also included in the present review (Brockow 2007a; Brockow 2007b; Leaute‐Labreze 2001; Schiener 2007): quote "The purpose of this evidence based analysis was to determine the effectiveness and safety of ultraviolet phototherapy for moderate‐to‐severe plaque psoriasis. " The authors concluded that quote "Spa salt water baths prior to phototherapy did increase short term clinical response of moderate‐to‐severe plaque psoriasis but did not decrease cumulative ultraviolet irradiation dose" and judged that there was high‐quality and adequate study evidence for this statement. Clinical response was defined as an improvement in physical signs and secondary psychological effects as well as reduction in inflammation and control of skin shedding.
In 2004, the Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG) was commissioned by the Federal Joint Committee (Gemeinsamer Bundesausschuss; G‐BA) to conduct an evaluation of the benefits and harms of different types of balneophototherapy. The systematic literature search was conducted separately for asynchronous and synchronous balneophototherapy in the databases MEDLINE, Embase and CENTRAL (in each case: coverage until March 2006). In the final report (full report in German: IQWiG 2006a; executive summary in English: IQWiG 2006b), three types of balneophototherapy were evaluated.
1. Asynchronous bath‐PUVA (psoralen plus UVA) compared with UVB only (or tap water bath + UVB)
Intervention and comparator do not match the inclusion criteria of the present review (not intervention of interest)
2. Asynchronous salt bath + UVB compared with UVB only (or tap water bath + UVB)
Intervention and comparator match comparison 2
Three included RCTs: the so‐called "BP‐BVDD‐Studie 2004", Dawe 2005, and Leaute‐Labreze 2001
Conclusion: Salt bath + UVB has an additional benefit compared to UVB only (and also compared to tap water bath + UVB)
3. Synchronous balneophototherapy (Dead Sea salt bath + UVB) compared with UVB only
Intervention and comparator match comparison 2
A single RCT: the so‐called "TOMESA_PV‐Studie 2006"
Conclusion: Dead Sea salt bath + UVB has an additional benefit compared to UVB only
The two studies BP‐BVDD‐Studie 2004 and TOMESA_PV‐Studie 2006 are not publicly available. Thus, of the eight studies included in the present review, only two published studies were considered by IQWiG 2006a. The Federal Joint Committee (Gemeinsamer Bundesausschuss, G‐BA), the highest decision‐making body concerning the distribution of the Statutory Health Insurance funds in Germany, decided that indoor salt water baths followed by artificial ultraviolet B (UVB) light for patients with severe and medium severe chronic plaque psoriasis can be reimbursed not only by hospitals, but also by practices (G‐BA 2008).
Roos 2010 suggested in a nonsystematic review to offer also natural balneophototherapy (Dead Sea climatotherapy) to patients though it would include additional travel and accommodation costs. Chen 2013 did not detect a difference in the effect between those phototherapy variations in a Cochrane Review. These results supported our decision that we did not differentiate between broad‐band ultraviolet B, narrow‐band ultraviolet B, mixed type irradiations, and psoralen bath + UVA in the present review. In a systematic review on ultraviolet based therapy for psoriasis, Almutawa 2013, in contrast, did report some differences among the phototherapy variations: quote "As a monotherapy, PUVA was more effective than NB‐UVB, and NB‐UVB was more effective than BB‐UVB and bath PUVA in the treatment of adults with moderate to severe plaque‐type psoriasis, based on clearance as an end point."
Authors' conclusions
Implications for practice.
Low‐certainty evidence indicates that people with chronic plaque psoriasis may see a reduction in psoriasis severity after treatment with indoor salt bath + ultraviolet B light (UVB) compared against UVB alone.
Low‐certainty evidence for the same comparison indicates that people with chronic plaque psoriasis may experience little to no difference in risk of treatment‐related adverse events requiring withdrawal.
As the evidence is based on data from a limited number of studies, which provided low‐certainty evidence, we cannot draw a clear conclusion regarding the benefit or harm of indoor (artificial) salt water baths followed by exposure to artificial UVB for treating chronic plaque psoriasis in adults. The two trials which contributed data for the primary efficacy outcome were conducted by the same group, and one of the two trials was funded by the German Spas Association, although the other trial did not report any funding source. Neither trial blinded the outcome assessors.
Of our two comparisons of interest, one (Indoor salt bath + UVB versus other treatment without UVB) was assessed by a single study, which did not report any of our pre‐specified outcomes. The other comparison (Indoor salt bath + UVB versus other treatment + UVB or UVB only) was assessed by all eight included studies, but their reporting of our pre‐specified outcomes was either non‐existent or limited, with a maximum of two studies reporting a given outcome.
Implications for research.
We recommend further randomised controlled trials (RCTs) that assess Psoriasis Area and Severity Index (PASI‐75), with detailed reporting of the outcome, as well as treatment‐related adverse events requiring withdrawal. Future studies should be independently funded, and should ensure blinding of assessment and should enable blinding of performance. The time points of assessing any outcome should be specified. We think that 'end of treatment' might not be sufficiently clear. Several time points should be used to allow matching with other studies. The number of people or limbs available for analysis should be given for every time point. The included studies lacked data on all secondary outcomes. These outcomes should be considered in future studies.
The limited number of trials and centres suggest a need for increased generalisability in the evidence base for this treatment. Future studies should consider potential harm by UVB exposure. Thus, it is important to keep contact with patients, general practitioners, and dermatologists and inform about the well‐being of the participants on a regular basis. In this context it seems meaningful to develop core outcomes for psoriasis treatment trials through the Cochrane Skin Core Outcome Set Initiative (CS‐COUSIN; http://cs-cousin.org). To consider any potential harm by UVB exposure, future study protocols should include long‐term observations. Future studies should clarify the reporting according to the CONSORT statement (Schulz 2010).
Future comparisons should include less logistically‐complicated types of phototherapy, such as oral psoralen plus UVA (PUVA). Secondary malignancies are uncommon, and differences very unlikely to be detected in future RCTs. Observational studies are more likely to provide evidence on this topic.
History
Protocol first published: Issue 11, 2015 Review first published: Issue 5, 2020
Acknowledgements
The review authors appreciate the kind help given by Hywel Williams, Bob Boyle, Robert Dellavalle, Laura Prescott, Elizabeth Doney, Emma Axon, Helen Scott, and Finola Delamere from the Cochrane Skin editorial base during the process of drafting the review. We thank Doreen Tushabe for participating in the protocol.
The Cochrane Skin editorial base wishes to thank Laurence Le Cleach, Cochrane Dermatology Editor for this review; the clinical referee Ignacio Garcia Doval; the consumer referee Liz Dale; and Heather Maxwell, who copy‐edited the review.
Appendices
Appendix 1. Skin Group Specialised Register (CRS) search strategy
Psoria* and (((balneotherapy or balneo‐therapy or soak* or bath* or salt* or dead sea or sole$ or saline) and (phototherapy* or ultraviolet or UVB or uv‐b or uv light)) or balneophototherapy or balneo‐phototherapy)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Psoriasis] explode all trees #2 psoria*:ti,ab,kw #3 #1 or #2 #4 soak*:ti,ab,kw #5 (balneotherapy or balneo‐therapy):ti,ab,kw #6 bath*:ti,ab,kw #7 (salt* or dead sea or saltwater or sole* or saline):ti,ab,kw #8 MeSH descriptor: [Baths] explode all trees #9 {or #4‐#8} #10 MeSH descriptor: [Phototherapy] explode all trees #11 phototherap*:ti,ab,kw #12 MeSH descriptor: [Ultraviolet Therapy] explode all trees #13 (ultraviolet or UVB or uv‐b):ti,ab,kw #14 uv light:ti,ab,kw #15 MeSH descriptor: [Ultraviolet Rays] explode all trees #16 (TL01 or TL‐01 or 311‐nm):ti,ab,kw #17{ or #10‐#16} #18 #9 and #17 #19 balneophototherapy:ti,ab,kw #20 balneo‐phototherapy:ti,ab,kw #21 #18 or #19 or #20 #22 #3 and #21
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Psoriasis/ 2. psoria$.mp. 3. 1 or 2 4. soak$.mp. 5. (balneotherapy or balneo‐therapy).mp. 6. bath$.mp. 7. (salt$ or dead sea or saltwater or sole$ or saline).mp. 8. Baths/ 9. or/4‐8 10. exp Phototherapy/ 11. phototherap$.mp. 12. exp Ultraviolet Therapy/ 13. (ultraviolet or UVB or uv‐b).mp. 14. uv light.mp. 15. Ultraviolet Rays/ 16. (TL01 or TL‐01 or 311‐nm).mp. 17. or/10‐16 18. 9 and 17 19. balneophototherapy.mp. 20. balneo‐phototherapy.mp. 21. 18 or 19 or 20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. clinical trials as topic.sh. 27. randomly.ab. 28. trial.ti. 29. 22 or 23 or 24 or 25 or 26 or 27 or 28 30. exp animals/ not humans.sh. 31. 29 not 30 32. 3 and 21 and 31
[22‐31: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. exp psoriasis/ 2. psoria$.mp. 3. 1 or 2 4. soak$.mp. 5. (balneotherapy or balneo‐therapy).mp. 6. exp balneotherapy/ 7. exp bath/ 8. bath$.mp. 9. (salt$ or dead sea or saltwater or sole$ or saline).mp. 10. or/4‐9 11. exp phototherapy/ 12. phototherap$.mp. 13. (ultraviolet or UVB or uv‐b).mp. 14. uv light.mp. 15. exp ultraviolet radiation/ 16. (TL01 or TL‐01 or 311‐nm).mp. 17. or/11‐16 18. 10 and 17 19. balneophototherapy.mp. 20. balneo‐phototherapy.mp. 21. 18 or 19 or 20 22. crossover procedure.sh. 23. double‐blind procedure.sh. 24. single‐blind procedure.sh. 25. (crossover$ or cross over$).tw. 26. placebo$.tw. 27. (doubl$ adj blind$).tw. 28. allocat$.tw. 29. trial.ti. 30. randomized controlled trial.sh. 31. random$.tw. 32. or/22‐31 33. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 34. human/ or normal human/ 35. 33 and 34 36. 33 not 35 37. 32 not 36 38. 3 and 21 and 37
Appendix 5. LILACS search strategy
psoria$ and (balneotherapy or balneo‐therapy or balneophototherapy or balneo‐phototherapy or salt$ or saline or bath$ or bano) These terms searched with the Controlled clinical trials topic‐specific query filter.
Appendix 6. Inquiries on missing data and inclusion criteria
We sent an e‐mail to Karl Ludwig Resch <k.l.resch@d‐i‐g.org>, sponsor of the so‐called 'BD‐BVDD 2004' study included by IQWiG 2006b to see if it is linked to the results published by Karl Ludwig Resch as a co‐author of Brockow 2007a, Brockow 2007b, and Schiener 2007. We also asked for individual patient data on PASI to enable a calculation of PASI‐75. The address was presumed active as we communicated before but we did not receive a response to our inquiry.
We sent an e‐mail to Annette Klein <annette.klein@klinik.uni‐regensburg.de> and <sekretariat.derma@ukr.de>, first author of Klein 2011. We inquired whether the so‐called 'TOMESA‐PV 2006' study included by IQWiG 2006b is linked to the results published by the TOMESA‐study group of Klein 2011. We also asked for individual patient data on PASI to enable a calculation of PASI‐75. TOMESA stands for Dead Sea salt in German language using the first two letters of each of the following three words 'Totes Meer Salz'. PV stands for Psoriasis vulgaris. We received an automatic reply from <Sekretariat.Derma@klinik.uni‐regensburg.de> but did not receive a response to our inquiry.
We sent e‐mails to W.P. Arnold <arnoldp@zgu.nl>, Alain Taieb <alain.taieb@chu‐bordeaux.fr>, R.S. Dawe <r.s.dawe@dundee.ac.uk>, and Thilo Gambichler <t.gambichler@klinikum‐bochum.de> and asked for individual patient data on PASI of the studies by Arnold 2001, Dawe 2005, Gambichler 2001, and Leaute‐Labreze 2001 to enable a calculation of PASI‐75. We received automatic replies from Ziekenhuis Gelderse Vallei <communicatie@zgv.nl> and <no‐reply@chu‐bordeaux.fr> but did not receive a response to our inquiry. We received a response from Robert Dawe (Staff) <r.s.dawe@dundee.ac.uk> who kindly reminded us that he did not measure PASI. We received a response from Prof. Dr. med. Thilo Gambichler <t.gambichler@klinikum‐bochum.de>, the author of the present review, that study data were no more available.
We contacted Gabriel Nasri Marzuca‐Nassr on 16 November 2016 by using the social networking site ResearchGate. His name was posted by ClinicalTrials.gov to indicate as the responsible person of the completed study NCT02713711. This RCT is included in the present review but results are not yet available (08 March 2017). We asked for the proposed publication date and for sharing the data. He replied quickly and told us that the manuscript of the study is submitted but not yet accepted for publication. He confirmed that the patients bathed in the salt water. On 10 May 2018, we sent a request to Gabriel Nasri Marzuca‐Nassr, again using ResearchGate. He replied that he is writing the final manuscript and will submit it in a few months. On 7 June 2019, we sent a request to Gabriel Nasri Marzuca‐Nassr, again using ResearchGate.
Data and analyses
Comparison 1. Saline+UVB versus UVB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 PASI‐75 response | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.24, 2.35] |
| 1.2 Treatment‐related adverse events requiring withdrawal (between participant) | 2 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.35, 2.64] |
| 1.3 Treatment‐related adverse events requiring withdrawal (within participant) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.36] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnold 2001.
| Study characteristics | ||
| Methods | Two‐group parallel, phase III randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control)
|
|
| Interventions |
Intervention (n = 20)
Control intervention (n = 20)
The interventions were applied once a day, three days a week, for up to eight weeks, and reaching a maximum number of 24 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Publication type is a letter to the editor. Conflicts of interest: issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, so assume not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, so assume not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 353): "In the salt‐UVB group, four patients dropped out: one had been included while on methotrexate medication, one had been given a prescription for calcipotriol ointment during the study and in two cases the PASI was lost or incorrectly entered into the computer." Quote (page 353): "In the psoralen‐UVB group, three patients dropped out: one had to be hospitalized during the study for a non‐psoriasis‐related disease, for unknown reasons one did not show up after six treatments and in one the PASI was lost (as above)." Comment: we judged a high risk of bias as a considerable number of patient data could not be used in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify a selective reporting issue and judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. Publication type is a letter to the editor. As a result, the description of patients and methods, especially statistical approaches is limited. Tables and figures are completely absent. The number of enrolled patients is unclear. The type of analysis, such as per protocol, as treated, or per intention to treat is not reported. |
Brockow 2007a.
| Study characteristics | ||
| Methods | Two‐group parallel, phase III pragmatic randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control): balanced
|
|
| Interventions |
Intervention (n = 81)
Control intervention (n = 79)
The interventions were applied once a day, three days a week, for up to six weeks, and reaching a maximum number of 18 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Quote (page 731): "This study was funded by the German Spas Association (Deutscher Heilbäderverband)." Conflicts of interest: issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 726): "Study participants were externally randomized [...] via a central telephone hotline. One computer‐generated randomization list with blocks of 12 masked to the site investigators was prepared for each of the participating spa centres." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 726): "Allocation concealment was broken just before the first intervention was administered." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 727): "Blinding of the participants was not possible due to the nature of the interventions." Comment: the authors reported that they did not blind investigators and patients and we judged a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 727; page 729): "Blinding of PASI raters was intended. The study centre did not inform the dermatological clinics about treatment allocation. Only the phototherapists at the spa centres knew the treatment allocation. Both patients and phototherapists were instructed not to inform the PASI raters about treatment allocation. Success of observer blinding was evaluated at the end of the intervention period by a questionnaire. [...] PASI raters stated that they knew the treatment allocation in 42% of cases (60/142)." Comment: we judged a low risk of detection bias in four studies as the assessors were presumably unaware of patients' treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 81 patients of the intervention group withdrew early and did not attend follow‐up visits. 8 of 79 patients of the control group withdrew early and did not attend follow‐up visits; 3 further patients were lost to follow‐up but were included in the analysis. Comment: we think that the proportion of dropouts is low and may not affect the outcome. Thus, we judged a low risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify a selective reporting issue and judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. |
Brockow 2007b.
| Study characteristics | ||
| Methods | Two‐group parallel, phase III pragmatic randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control): balanced except gender
|
|
| Interventions |
Intervention (n = 81)
Control intervention (n = 83)
The interventions were applied once a day, three days a week, for up to six weeks, and reaching a maximum number of 18 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
|
| Notes | Funding not reported Conflicts of interest: issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1028): "Study participants were externally randomized [...] via a central telephone hotline. One computer‐generated randomization list with blocks of 12 masked to the site investigators was prepared for each of the participating spa centres by the responsible biometrician of the study." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 1028): "Allocation concealment was broken shortly before the first intervention was administered by the responsible phototherapists at the spa centres." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 1029): "Blinding of the participants was not possible due to the nature of the interventions." Comment: the authors reported that they did not blind investigators and patients and we judged a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 1029; page 1030): "Blinding of PASI raters was intended. The study center did not inform the trial sites (dermatological clinics) about treatment allocation. Only the phototherapists at the spa centers knew the treatment allocation. Both patients and phototherapists were instructed not to inform the PASI raters or other staff at the trial sites about treatment allo‐ cation. Success of observer blinding was evaluated at the end of the intervention period. A questionnaire asked the PASI raters to state whether they knew treatment allocation or not. [...] At the end of the intervention period PASI raters stated that they knew the treatment allocation in 50% of cases (65/129)." Comment: we judged an unclear risk as blinding was tried but was not achieved. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 of 81 patients of the intervention group withdrew early and did not attend follow‐up visits. 19 of 83 patients of the control group withdrew early and did not attend follow‐up visits. Comment: we think that the proportion of dropouts is high and may affect the outcome. Thus, we judged a high risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify a selective reporting issue and judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. |
Dawe 2005.
| Study characteristics | ||
| Methods | Two‐group parallel split‐body, phase III randomised controlled trial with paired comparison of right versus left limb
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics
|
|
| Interventions |
Intervention (n = 60 lower extremity)
Control intervention (n = 60 lower extremity other side)
Both both legs received the same number of treatments with a mean of 25 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
Scaling, erythema, and induration score measured at 8 months after end of treatment. |
|
| Notes | Quote (page 615): "The sum of Scaling, Erythema and Induration (SEI) scores, each on a 0–4 scale, was used as a psoriasis severity measure for each of the selected site symmetrical plaques. This is a modification of the Psoriasis Area and Severity Index we used in other studies."
Comment: not defined as outcome. Quote (page 615): "Adverse effects were documented as in standard practice. Erythema was recorded as grade 1 (mild and asymptomatic), grade 2 (well‐demarcated, not painful), grade 3 (painful) and grade 4 (severe with blistering)." Comment: not defined as outcome. Quote (page 615): "Blinding was achieved by: (i) randomization as above." Comment: blinding cannot be achieved by randomisation. Conflicts of interest: Quote "We are grateful to Mavena Healthcare AG, Switzerland who funded this study and provided the Dead Sea salt used. Mavena was not involved in the design, conduct or analysis of the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 615): "The study treatment administered to each side was allocated randomly, both as a measure to ensure successful assessor blinding and also to avoid allocation bias. A blocked random allocation list (variably sized blocks) was generated with user‐written software ('ralloc' command) for Stata (Stata 7.0; Stata Corporation, College Station, TX, U.S.A., 2001)." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 615): "Allocated treatments for each successive entrant to the study were placed in sealed, opaque envelopes which were opened by a nonblinded research nurse only after each patient had signed the informed consent form." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 615): "Blinding was achieved by: (i) randomization as above; (ii) ensuring assessments were always performed before soaks; and (iii) reminders by the nonblinded study nurse to patients not to tell the assessing doctor which limb was receiving the soaks. Patients were not blinded." Comment: patients and nurse were not blinded, while physicians appeared to be blinded. Thus, we judged an unclear risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote (page 615): "Those assessing psoriasis severity (SEI) scores were kept unaware of which side was receiving Dead Sea salt soaks. Blinding was achieved by reminders by the nonblinded study nurse to patients not to tell the assessing doctor which limb was receiving the soaks." Comment: we judged an unclear risk as blinding was tried but was not achieved. Blinding effects were not evaluated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 615): "Forty‐one (68%) participants remained in the study throughout their UVB course. The 19 study withdrawals were because of: [...]" Comment: 32% of patients withdrew. We think that the proportion of dropouts is high and may affect the outcome. Thus, we judged a high risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 616): "For the 41 patients who reached clearance or minimal residual activity while participating in the study there was no detectable difference [...]" Comment: we are not positive if this may considerably affect the outcome and we judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. |
Gambichler 2001.
| Study characteristics | ||
| Methods | Two‐group parallel split‐body, randomised controlled trial with paired comparison of right versus left elbow
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics
|
|
| Interventions |
Intervention (n = 10, one elbow)
Control intervention (n = 10, the other elbow)
Both elbows were simultaneously exposed to the solution by bandaging the sites with soaked cotton wool. The interventions were applied to both elbows once a day, three to five days a week, for up to eight weeks, and reaching a maximum number of 30 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Outcomes of the trial not classified as primary or secondary
|
|
| Notes | Funding not reported Conflicts of interest: issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 22): "A prospective, randomized, one‐blind, right/left comparison, investigating the efficacy of BPT in psoriasis with highly concentrated salt water versus tap water, was performed in an out‐patient setting during autumn and winter." Comment: sequence generation not clear |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not clear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 22): "A prospective, randomized, one‐blind, right/left comparison, investigating the efficacy of BPT in psoriasis with highly concentrated salt water versus tap water, was performed in an out‐patient setting during autumn and winter." "Both elbows were simultaneously exposed to these liquids (30°C) by bandaging the sites with soaked cotton wool." Comment: partially blinding of people or limbs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 23): "To avoid interobserver variation, all patients were clinically assessed by one physician who was not informed which pretreatment had been used prior to phototherapy." Comment: blinding of outcome assessment achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: we did not identify a noteworthy proportion of dropouts and judged a low risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify a selective reporting issue and judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. |
Klein 2011.
| Study characteristics | ||
| Methods | Two‐group parallel, phase III randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control): balanced
|
|
| Interventions |
Intervention (n = 183)
Control intervention (n = 184)
The interventions were applied once a day, three to five days a week, and reaching a maximum number of 35 applications. Duration of UV irradiation was not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
The authors reported early withdrawal before treatment session 35, which might be projected to a time point of about eight weeks after start of treatment. The authors also reported that some people or limbs had an early withdrawal because of adverse events. |
|
| Notes | Figure 1 states that 137 patients participated in the follow‐up six months after treatment. However, figure 1 also states that only 106 participants were treated through to the end of treatment at session 35 which equals eight weeks after start of treatment. We suppose that this might be a spelling error. The trial was sponsored by the primary health insurance companies in Bavaria, Germany, and completely independent from the producers of any of the medical devices used. The Bavarian ministry of social affairs approved and supervised all procedures. Conflicts of interest: the authors declared that there are none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 571): "Randomization was performed by an independent organization so that trial physicians had no influence on the allocated treatment strategy." Quote (page 572): "Randomization was performed centrally by an independent organization (central telephone randomization). Randomization was stratified by centre and skin type. Treatments were randomly assigned in a 1: 1 ratio with a block size of 6." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 572): "Allocation concealment was assured until the end of the study phase. Allocation concealment was broken before realizing the statistical analysis." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 571): "Blinding of patients was not possible." Comment: the authors reported that they did not blind investigators and patients and we judged a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (page 577): "This study was not conducted at special skilled PT departments of university institutions, but at private practices of single dermatologists. Patients and evaluator were unblinded. Therefore, we categorised the trial as effectiveness study." Comment: the authors reported that they did not blind outcome assessors and we judged a high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (page 574, figure 1): "Intervention group: 3 did not start treatment; 1 no second PASI available; 35 early withdrawal, 6 with clearance; follow‐up 6 months after treatment: 136 of 183 randomized (179 included in ITT analysis)" Quote (page 574, figure 1): "Control group: 5 did not start treatment; 2 no second PASI available; 71 early withdrawal, 1 with clearance; follow‐up 6 months after treatment: 137 of 184 randomized (177 included in ITT analysis)" Comment: the authors reported a considerable proportion of dropouts. However, they including the data of almost all randomised participants in an intention‐to‐treat analysis. We are unsure if the dropouts may affect the outcome and therefore we judged an unclear risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 576, table 4): "Patients with adverse events with an incidence of ≥5%" Patients with adverse events with an incidence of <5% were not reported. Comment: we are not positive if this may considerably affect the outcome and thus we judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. spelling error. |
Leaute‐Labreze 2001.
| Study characteristics | ||
| Methods | Three‐group parallel, phase III randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control): balanced
|
|
| Interventions |
Intervention (n = 24, group C)
Control intervention (n = 21, group B)
The interventions were applied once a day, five days a week, for up to three weeks, and reaching a maximum number of 15 applications. Duration of UV irradiation was not reported. Group A (n = 22): saline salt water only. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
The patients were evaluated during the treatment at days 0, 7, 14, and 21 (end of treatment). The patients were evaluated 1 year after the treatment. |
|
| Notes | Funding: this study was supported in part by la Compagnie Fermiere de Salies de Bearn. Conflicts of interest: issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1036): "Randomization was centrally controlled by the Dermatology Department in Bordeaux." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 1036): "Randomization was centrally controlled by the Dermatology Department in Bordeaux." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of patients and staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 1036): "A second investigator, blinded for randomization, examined the patient at days 0 and 21. The lesions were photographed before and after the study." Comment: we assume that the assessment of the primary outcome was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (page 1037): "Four patients dropped out early and were excluded from the statistical analysis: 3 had rapidly occurring adverse effects (skin irritation with saline spa water) and 1 had contracted a pulmonary infection." Quote (page 1037): "Only 40 patients (60% of those included) could be reexamined 1 year after their stay." Comment: the authors reported a considerable proportion of dropouts. However, they including the data of almost all randomised participants in an intention‐to‐treat analysis. We are unsure if the dropouts may affect the outcome and therefore we judged an unclear risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 1036): "The statistical analysis was based on the intention‐to‐treat principle." Quote (page 1037): "Data for the 67 remaining patients are summarized in table 2." Data of 4 patients were excluded from the analysis, though, 3 patients had adverse events such as skin irritation. Comment: we are not positive if this affected the outcome, thus, we judged an unclear risk of bias. |
| Other bias | Unclear risk | Quote (page 1036): "A sample containing 90 patients was selected." Quote (page 1036): "The inclusions started January 1996, and an intermediary analysis was conducted in April 1996, as 71 patients were included. The results of this analysis showed that additional inclusions could not add sufficient power to change its conclusions, and the trial's investigators decided to stop the inclusions." Comment: deviation from the protocol and potentially result‐orientated analysis |
Schiener 2007.
| Study characteristics | ||
| Methods | Four‐group parallel, phase III randomised controlled trial
|
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control 1 versus control 2 versus control 3): balanced
|
|
| Interventions |
Intervention (n = 299, SW‐UVB)
Control intervention 1 (n = 270, UVB)
Control intervention 2 (n = 285, TW‐UVB)
Control intervention 3 (n = 305, psoralen bath + UVA)
The UV doses were individually adapted to erythemal response.
The interventions were applied once a day, four days a week, for up to eight weeks, and reaching a maximum number of 32 applications. Duration and mechanism (device) of UV irradiation were not reported. |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
The patients were evaluated during the treatment at baseline and after 2, 4, 6 and 8 (end of treatment) weeks. |
|
| Notes | This study was supported in part by the Berufsverband der Deutschen Dermatologen, a professional, noncommercial association of German dermatologists. Conflicts of interest: Issue not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 587): "One computer‐generated randomization list with block lengths of 12 was prepared for each trial site by the responsible biometrician of the study. Patients were centrally randomized to receive UV‐B, TW UV‐B, SW UV‐B, or psoralen bath + UVA." Comment: the authors clearly described an adequate random sequence generation, which was judged as low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 587): "Allocation was concealed until eligibility was checked and informed consent was given." Comment: the authors clearly described an adequate concealment of the allocation, which was judged as low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 589): "Patients were not told to which intervention they were randomized. Nevertheless, only patients assigned to TW UV‐B or psoralen bath + UVA could in fact be considered to be blinded. The bath solutions of TW UV‐B and psoralen bath + UVA do not differ in physical appearance, taste, or color. Patients assigned to SW UV‐B or UV‐B could not be blinded. A highly concentrated salt‐water bath can be easily identified by taste or buoyancy." Comment: the authors reported that they did not blind patients and we judged a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (page 589): "Blinding of PASI raters was intended. Both the staff and the patients were instructed not to inform PASI raters about the treatment allocation. Success of rater blinding was evaluated at the end of the intervention period. [...] At the end of the intervention period, PASI raters stated that they knew the treatment assignment in 58.2% of cases (587/1008)." Comment: we judged a low risk of detection bias as the assessors were presumably unaware of patients' treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention (SW‐UVB) 310 randomised ‐ 84 not assigned intervention, lost, or discontinued = 226, but 299 included in analysis (11 excluded) Intervention (UVB alone) 301 randomised ‐ 116 not assigned intervention, lost, or discontinued = 185, but 270 included in analysis (31 excluded) Intervention (TW‐UVB) 301 randomised ‐ 123 not assigned intervention, lost, or discontinued = 178, but 285 included in analysis (24 excluded) Intervention (psoralen bath + UVA) 321 randomised ‐ 88 not assigned intervention, lost, or discontinued = 233, but 305 included in analysis (16 excluded) Comment: the authors reported a considerable proportion of dropouts. However, they including the data of almost all randomised participants in an intention‐to‐treat analysis. We are unsure if the dropouts may affect the outcome and therefore we judged an unclear risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not identify a selective reporting issue and judged an unclear risk of bias. |
| Other bias | Unclear risk | Comment: we did not identify other bias such as design‐specific risks of bias, baseline imbalance, blocked randomisation in unblinded trials, and differential diagnostic activity. |
CaCl2: calcium chloride;KCl: potassium chloride; MED: minimal erythema dose; MgCl2: magnesium chloride; MPD: minimal phototoxic dose; NaCl: sodium chloride; PASI: Psoriasis Area and Severity Index; SD: standard deviation; UV: ultra violet.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 2012 | Study type not of interest: systematic review |
| Browne 2017 | Study type not of interest: nonsystematic review |
| Cattaneo 2012 | Study type not of interest: single‐arm study |
| Chen 2013 | Study type not of interest: systematic review |
| Claes 2006 | Study type not of interest: systematic review |
| Even 1996 | Intervention not of interest: Dead Sea bathing and sun exposure |
| Eysteinsdottir 2014 | Intervention not of interest: geothermal sea bathing + UVB |
| Gambichler 2000b | Study type not of interest: systematic review |
| Gawlik 2001 | Study type not of interest: nonsystematic review |
| Guan 2017 | Study type not of interest: systematic review |
| Hollo 2004 | Study type not of interest: single‐arm study |
| Morri 2012 | Intervention not of interest: sulphurous thermal spring water, bath not followed by UVB consistently |
| Olafsson 2002 | Intervention not of interest: lagoon bathing followed by UVB |
| Scholl 1981 | Intervention not of interest: oral retinoid added to bath and UVB |
| Werfel 2015 | Intervention not of interest: UVB but not salt water baths |
UV: ultra violet
Characteristics of studies awaiting classification [ordered by study ID]
NCT02713711.
| Methods | Four‐group parallel, phase III randomised controlled trial
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Characteristics (intervention versus control)
|
| Interventions |
Intervention (artificial balneophototherapy group)
Comparator (artificial balneotherapy group)
Comparator (phototherapy group)
Control (no treatment group)
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
The patients were evaluated during the treatment at days 1, 12, and 26 (end of treatment). |
| Notes | Sponsor: Universidad Catolica del Maule, Chile. Study start date December 2013; study completion data 2014; first registered with ClinicalTrials.gov in March 2016. No study results posted. No publication available. Thus, risk of bias cannot be assessed and results cannot be presented. Official title: "Comparison of the effectiveness of artificial balneotherapy, phototherapy, and artificial balneophototherapy in the treatment of psoriasis: A randomized controlled trial" |
Differences between protocol and review
In section Types of studies, we specified various subtypes of randomised designs and whether they were planned for inclusion.
Under Types of interventions: We acknowledged the two different comparisons we would be including to ensure it was clear to the reader. Comparison one would be salt bath + UVB versus other treatment + UVB. Comparison two would be salt bath + UVB versus other treatment + UVB or UVB only. The results of both comparisons would be reported separately.
In section Primary outcomes, we distinguished between‐participant data from within‐participant data. We rephrased the adverse outcome "treatment‐related adverse events requiring withdrawal" instead of "serious adverse events requiring withdrawal" to make clear that the adverse outcome is linked with the treatment.
In section Types of outcome measures, we added that we used the web‐based Easy Fisher Exact Test Calculator (Social Science Statistics 2018). We also added that we would extract numerical data from graphs where needed.
In section Assessment of risk of bias in included studies, we did not assess detection bias and attrition bias for each outcome separately because of sparse reporting.
In section Unit of analysis issues, we stated that we separately reported the results of between‐participant data and within‐participant data.We also added how we would handle studies with multiple intervention arms.
We added more details regarding the 'Summary of findings' table we created. We also described the GRADE approach.
Only two studies reported the predefined PASI‐75, and six studies reported severity scores which could not be used in the present review. We wanted to report the respective results to provide a broader picture of the results of the various studies. Therefore, we reported outcomes although they were not predefined by the current review and they should not have an impact on the conclusion.
Contributions of authors
FP was the contact person with the editorial base. FP co‐ordinated contributions from the co‐authors and wrote the final draft of the review. FP, MH, SP, TC, TG screened papers against eligibility criteria. FP obtained data on ongoing and unpublished studies. FP, MH, SP, TG appraised the quality of papers. FP, MH, SP, TC, TG extracted data for the review and sought additional information about papers. FP entered data into RevMan. FP, MH, SP, TG analysed and interpreted data. FP, MH, SP, AL, TG worked on the methods sections. FP, MH, SP, TG drafted the clinical sections of the background and responded to the clinical comments of the referees. FP, MH, SP, AL, TG responded to the methodology and statistics comments of the referees. DC checked the Plain language summary section. FP is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
University of Cologne, Germany
Provision of the full texts of articles
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Frank Peinemann: nothing to declare.
Marco Harari: "I am the Director of the DMZ Medical Center, in which patients are treated with climatotherapy (not artificial UV treatment)."
Sandra Peternel: nothing to declare.
Thalia Chan: nothing to declare.
David Chan: nothing to declare.
Alexander Labeit: nothing to declare.
Thilo Gambichler: "Since 1998, I have been performing research in the field of indoor salt water baths followed by artificial ultraviolet B light. I am an author of the following studies Gambichler 2001 (included study) and Gambichler 2000b (excluded study). In this review, I was not involved in extracting data from these studies."
New
References
References to studies included in this review
Arnold 2001 {published data only}
- *.Arnold WP, Andel P, Hoop D, Jong-Tieben L, Visser-van Andel M. A comparison of the effect of narrow-band ultraviolet B in the treatment of psoriasis after salt-water baths and after 8-methoxypsoralen baths. British Journal of Dermatology 2001;145(2):352-4. [CENTRAL: CN-00350275] [PMID: ] [DOI] [PubMed]
- Arnold WP, Andel P, Hoop D, Jong-Tieben L, Visser-van Andel M. Difference in effectiveness between salt bath and a bath with 8-methoxypsoralene with small spectrum UVB-therapy. Nederlands tijdschrift voor dermatologie en venereologie 2000;10:106-7. [CENTRAL: CN-00602286] [Google Scholar]
Brockow 2007a {published data only}
- *.Brockow T, Schiener R, Franke A, Resch KL, Peter RU. A pragmatic randomized controlled trial on the effectiveness of highly concentrated saline spa water baths followed by UVB compared to UVB only in moderate to severe psoriasis. Journal of Alternative and Complementary Medicine 2007;13(7):725-32. [CENTRAL: CN-00620063] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brockow 2007b {published data only}
- *.Brockow T, Schiener R, Franke A, Resch KL, Peter RU. A pragmatic randomized controlled trial on the effectiveness of low concentrated saline spa water baths followed by ultraviolet B (UVB) compared to UVB only in moderate to severe psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2007;21(8):1027-37. [CENTRAL: CN-00618957] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dawe 2005 {published data only}
- *.Dawe RS, Yule S, Cameron H, Moseley H, Ibbotson SH, Ferguson J. A randomized controlled comparison of the efficacy of Dead Sea salt balneophototherapy vs. narrowband ultraviolet B monotherapy for chronic plaque psoriasis. British Journal of Dermatology 2005;153(3):613-9. [CENTRAL: CN-00529697] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dawe RS, Yule S, Cameron H, Moseley H, Ibbotson SH, Ferguson J. A randomized controlled study of Dead Sea salt balneophototherapy for psoriasis. British Journal of Dermatology 2004;151(Suppl 68):104. [CENTRAL: CN-00487817] [DOI] [PubMed] [Google Scholar]
Gambichler 2001 {published data only}
- Gambichler T, Rapp S, Senger E, Altmeyer P, Hoffmann K. Balneophototherapy of psoriasis: highly concentrated salt water versus tap water -- a randomized, one-blind, right/left comparative study. Photodermatology, Photoimmunology & Photomedicine 2001;17(1):22-5. [CENTRAL: CN-00327517] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klein 2011 {published data only}
- *.Klein A, Schiffner R, Schiffner-Rohe J, Einsele-Kramer B, Heinlin J, Stolz W, et al. A randomized clinical trial in psoriasis: synchronous balneophototherapy with bathing in Dead Sea salt solution plus narrowband UVB vs. narrowband UVB alone (TOMESA-study group). Journal of the European Academy of Dermatology and Venereology: JEADV 2011;25(5):570-8. [CENTRAL: CN-00802620] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leaute‐Labreze 2001 {published data only}
- Leaute-Labreze C, Saillour F, Chene G, Cazenave C, Luxey-Bellocq ML, Sanciaume C, et al. Saline spa water or combined water and UV-B for psoriasis vs conventional UV-B: Lessons from the Salies de Bearn randomized study. Archives of Dermatology 2001;137(8):1035-9. [CENTRAL: CN-00356070] [PMID: ] [PubMed] [Google Scholar]
Schiener 2007 {published data only}
- Schiener A, Brockow T, Weimer M, Hochdorfer B, Freitag L, Salzer B, et al. Balneo-phototherapy. Superiority can no longer be denied. Journal of Plastic Dermatology 2006;2(2):39-41. [CENTRAL: CN-00613244] [Google Scholar]
- *.Schiener R, Brockow T, Franke A, Salzer B, Peter RU, Resch KL. Bath PUVA and saltwater baths followed by UV-B phototherapy as treatments for psoriasis: a randomized controlled trial. Archives of Dermatology 2007;143(5):586-96. [CENTRAL: CN-00589110] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bailey 2012 {published data only}
- Bailey EE, Ference EH, Alikhan A, Hession MT, Armstrong AW. Combination treatments for psoriasis: A systematic review and meta-analysis. Archives of Dermatology 2012;148(4):511-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Browne 2017 {published data only}
- Browne R, Gee B. The cost-effectiveness of sun, sea and psoriasis. British Journal of Dermatology 2017;177(Suppl 1):38. [Google Scholar]
Cattaneo 2012 {published data only}
- Cattaneo A, Alberti Violetti S, Tavecchio S, Bruni E, Carrera C, Crosti C. Tomesa balneophototherapy in mild to severe psoriasis: A retrospective clinical trial in 174 patients. Photodermatology, Photoimmunology & Photomedicine 2012;28(3):169-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD009481.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Claes 2006 {published data only}
- Claes C, Kulp W, Greiner W, Schulenburg JM, Werfel T. Therapy of moderate and severe psoriasis. GMS Health Technology Assessment 2006;2:Doc07. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Even 1996 {published data only}
- Even-Paz Z, Gumon R, Kipnis V, Abels DJ, Efron D. Dead Sea sun versus Dead Sea water in the treatment of psoriasis. Journal of Dermatological Treatment 1996;7(2):83-6. [CENTRAL: CN-00170079] [Google Scholar]
Eysteinsdottir 2014 {published data only}
- Eysteinsdottir JH, Olafsson JH, Agnarsson BA, Ludviksson BR, Sigurgeirsson B. Psoriasis treatment: Faster and long-standing results after bathing in geothermal seawater. A randomized trial of three UVB phototherapy regimens. Photodermatology, Photoimmunology & Photomedicine 2014;30(1):25-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gambichler 2000b {published data only}
- Gambichler T, Kreuter JA, Altmeyer P, Hoffmann K. Meta-analysis of the efficacy of Balneophototherapy [Meta-Analyse zur Effektivitat der Balneophototherapie]. Aktuelle Dermatologie 2000;26(12):402-6. [Google Scholar]
Gawlik 2001 {published data only}
- Gawlik C, Gibis B, Sander G, Rheinberger P. Usefulness and necessity of unsynchronized photosolotherapy and bath-PUVA--two variants of balneophototherapy--in funded ambulatory health care [Nutzen und Notwendigkeit der nicht-synchronen Photosoletherapie und Bade-PUVA--zweier Varianten der Balneophototherapie--in der ambulanten vertragsarztlichen Versorgung]. Zeitschrift fur arztliche Fortbildung und Qualitatssicherung 2001;95(7):509-12. [PMID: ] [PubMed] [Google Scholar]
Guan 2017 {published data only}
- Guan J, Yuan S, Wu H, Na R, Wu X, Wang X, et al. Effectiveness and safety of traditional Chinese medical bath therapy combined with ultraviolet irradiation in the treatment of psoriasis: A systematic review and metaanalysis of randomized controlled trials. PLOS One 2017;12(3):e0173276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollo 2004 {published data only}
- Hollo P, Bender T, Marschalko M, Gonzalez R, Barna I, Horvath A. No significant change of plasma beta-endorphin levels of psoriasis patients after synchronous balneophototherapy. Photodermatology, Photoimmunology & Photomedicine 2004;20(4):205-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morri 2012 {published data only}
- Morri M, Norat M, Canzi P, Viti S, Mascherpa MA, Angeli P, et al. Balneotherapy and narrow-band phototherapy in the treatment of psoriasis: A comparative study. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2012;171(6):739-48. [CENTRAL: CN-00913067] [Google Scholar]
Olafsson 2002 {published data only}
- Olafsson JH. Psoriasis treatment at the Blue Lagoon in Iceland. Journal of the European Academy of Dermatology and Venereology: JEADV 2002;16(Suppl 1):4-35. [CENTRAL: CN-00478695] [Google Scholar]
Scholl 1981 {published data only}
- Scholl E. Treatment of psoriasis on an outpatient-base using UVB-radiations, oral retinoid and ten percent saline baths (author's transl) [Ambulante Behandlung der Psoriasis mit UVB-Bestrahlungen, aromatischem Retinoid und zehnprozentigen Solbadern]. Schweizerische Rundschau fur Medizin Praxis 1981;70(41):1806-16. [PMID: ] [PubMed] [Google Scholar]
Werfel 2015 {published data only}
- Werfel T, Holiangu F, Niemann KH, Schmerling O, Lullau F, Zedler A, et al. Digital ultraviolet therapy: a novel therapeutic approach for the targeted treatment of psoriasis vulgaris. British Journal of Dermatology 2015;172(3):746-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02713711 {published data only}
- NCT02713711. Effectiveness of artificial balneotherapy, phototherapy and artificial balneophototherapy in the treatment of psoriasis. clinicaltrials.gov/ct2/show/NCT02713711 Date first received: 10 March 2016.
Additional references
Almutawa 2013
- Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. Systematic review of UV-based therapy for psoriasis. American Journal of Clinical Dermatology 2013;14(2):87-109. [PMID: ] [DOI] [PubMed] [Google Scholar]
Archier 2012
- Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. Journal of the European Academy of Dermatology and Venereology: JEADV 2012;26(Suppl 3):22-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bachelez 2013
- Bachelez H, Viguier M, Tebbey PW, Lowes M, Suárez-Fariñas M, Costanzo A, et al. The mechanistic basis for psoriasis immunopathogenesis: translating genotype to phenotype. Report of a workshop, Venice, 2012. British Journal of Dermatology 2013;169(2):283-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barker 2014
- Barker J. Psoriasis heritability: 125 years and counting. British Journal of Dermatology 2014;171(1):3-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Basra 2008
- Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. British Journal of Dermatology 2008;159(5):997-1035. [PMID: ] [DOI] [PubMed] [Google Scholar]
BP‐BVDD‐Studie 2004
- Berufsverband der Deutschen Dermatologen (BVDD). Final report on the clinical efficacy of outpatient balneophototherapy for psoriasis. A multicenter, open, randomized-controlled comparative study. Short title: BP-BVDD [Abschlussbericht zum klinischen Wirksamkeitsnachweis der ambulanten Balneophototherapie für die Psoriasis. Eine multizentrische, offene, randomisiert-kontrollierte Vergleichsstudie. Studienkurztitel: BP-BVDD (Balneo-Phototherapie des Berufs-Verbands der Deutschen Dermatologen)]. Bad Elster: Forschungsinstitut für Balneologie und Kurortwissenschaft 2004.
Brockow 2007
- Brockow T, Schiener R, Franke A, Resch KL, Peter RU. A pragmatic randomized controlled trial on the effectiveness of low concentrated saline spa water baths followed by ultraviolet B (UVB) compared to UVB only in moderate to severe psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV 2007;21(8):1027-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chang 2014
- Chang PL, Hsieh YH, Wang CC, Juliana MM, Tsuruta Y, Timares L, et al. Osteopontin facilitates ultraviolet B-induced squamous cell carcinoma development. Journal of Dermatological Science 2014;75(2):121-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christophers 2001
- Christophers E. Psoriasis--epidemiology and clinical spectrum. Clinical & Experimental Dermatology 2001;26(4):314-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
CIE 2011
- International Commission on Illumination (Commission Internationale de l'Eclairage). eILV online! searchable online database of the International Lighting Vocabulary (ILV) of the current version of the ILV (CIE S 017/E:2011). cie.co.at/index.php/Publications/eILV+online! (accessed 22 October 2016).
Corti 2009
- Corti M, Corti M. Psoriasis Area Severity Index (PASI) Calculator (1.7.1) 2009. pasi.corti.li (accessed 22 July 2014).
de Gruijl 2002
- Gruijl FR. p53 mutations as a marker of skin cancer risk: comparison of UVA and UVB effects. Experimental Dermatology 2002;11(Suppl 1):37-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ellis 2019
- Ellis AG, Flohr C, Drucker AM. Network meta-analyses of systemic treatments for psoriasis: a critical appraisal: Original Articles: Jabbar-Lopez ZK, Yiu ZZN, Ward V et al. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J Invest Dermatol 2017; 137:1646-54. Sbidian E, Chaimani A, Garcia-Doval I et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2017; 12:CD011535. British Journal of Dermatology 2019;180(2):282-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clinical & Experimental Dermatology 1994;19(3):210-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fitzpatrick 1988
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology 1988;124(6):869-71. [DOI] [PubMed] [Google Scholar]
Fredriksson 1978
- Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica 1978;157(4):238-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
G‐BA 2008
- Gemeinsamer Bundesausschuss (G-BA). Richtlinie Methoden vertragsärztliche Versorgung (Balneophototherapie) Beschlussdatum: 13.03.2008. www.g-ba.de/informationen/beschluesse/645 (accessed 1 October 2015).
Gambichler 1998
- Gambichler T, Schröpl F. Changes of minimal erythema dose after water and salt water baths. Photodermatology, Photoimmunology & Photomedicine 1998;14(3-4):109-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gambichler 2000a
- Gambichler T, Küster W, Kreuter A, Altmeyer P, Hoffmann K. Balneophototherapy--combined treatment of psoriasis vulgaris and atopic dermatitis with salt water baths and artificial ultraviolet radiation. Journal of the European Academy of Dermatology and Venereology: JEADV 2000;14(5):425-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gambichler 2011
- Gambichler T, Demetriou C, Terras S, Bechara FG, Skrygan M. The impact of salt water soaks on biophysical and molecular parameters in psoriatic epidermis equivalents. Dermatology 2011;223(3):230-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gelfand 2005
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Archives of Dermatology 2005;141(12):1537-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADEpro GDT. Version (accessed January 2017). Hamilton (ON): Grade Working Group, McMaster University, 2014.
Griffiths 2007
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gupta 2014
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Current Dermatology Reports 2014;3(1):61-78. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halverstam 2008
- Halverstam CP, Lebwohl M. Nonstandard and off-label therapies for psoriasis. Clinics in Dermatology 2008;26(5):546-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harari 2007
- Harari M, Novack L, Barth J, David M, Friger M, Moses SW. The percentage of patients achieving PASI 75 after 1 month and remission time after climatotherapy at the Dead Sea. International Journal of Dermatology 2007;46(10):1087-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Health Quality Ontario 2009
- Health Quality Ontario. Ultraviolet phototherapy management of moderate-to-severe plaque psoriasis: an evidence-based analysis. Ontario Health Technology Assessment Series 2009;9(27):1-66. [PMID: ] [PMC free article] [PubMed]
Henseler 1985
- Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. Journal of the American Academy of Dermatology 1985;13(3):450-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Huang 2018
- Huang A, Seité S, Adar T. The use of balneotherapy in dermatology. Clinics in Dermatology 2018;36(3):363-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Huerta 2007
- Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Archives of Dermatology 2007;143(12):1559-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
IQWiG 2006a
- Institute for Quality and Efficiency in Health Care (IQWiG). Balneo-phototherapy, Final report, Commission N04-04 [Balneophototherapie, Abschlussbericht, Auftrag N04-04, Version 1.0, Stand 21 Dezember 2006, Datum des Auftrags 18 Dezember 2004, veröffentlicht 02 Januar 2007]. www.iqwig.de/de/projekte-ergebnisse/projekte/nichtmedikamentoese-verfahren/n04-04-balneophototherapie.1199.html (accessed 15 June 2019).
IQWiG 2006b
- Institute for Quality and Efficiency in Health Care (IQWiG). Balneo-phototherapy: Executive summary of final report N04-04, Version 1.0, Created: 21 December 2006 (Translation of the executive summary of the final report “Balneophototherapie”). www.ncbi.nlm.nih.gov/books/NBK84168/ (accessed 15 June 2019).
Lebwohl 2003
- Lebwohl M. Psoriasis. Lancet 2003;361(9364):1197–204. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lebwohl 2005
- Lebwohl M, Ting PT, Koo JY. Psoriasis treatment: traditional therapy. Annals of the Rheumatic Diseases 2005;64(Suppl 2):ii83-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lønnberg 2013
- Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Heritability of psoriasis in a large twin sample. British Journal of Dermatology 2013;169(2):412-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martin 2015
- Martin ML, Gordon K, Pinto L, Bushnell DM, Chau D, Viswanathan HN. The experience of pain and redness in patients with moderate to severe plaque psoriasis. Journal of Dermatological Treatment 2015;26(5):401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melilli 2006
- Melilli L, Shikiar R, Thompson C. Minimum clinically important difference in Dermatology Life Quality Index in moderate to severe plaque psoriasis patients treated with adalimumab (P2894). Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB 221. [www.jaad.org/article/S0190-9622(05)04271-4/pdf] [Google Scholar]
Menter 2007
- Menter A, Griffiths CE. Current and future management of psoriasis. Lancet 2007;370(9583):272–84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Microsoft Corp 2011 [Computer program]
- Microsoft Excel. Redmond: Microsoft Corporation, 2011.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6:e1000097. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Momtaz 1998
- Momtaz K, Fitzpatrick TB. The benefits and risks of long-term PUVA photochemotherapy. Dermatologic Clinics 1998;16(2):227-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nestle 2009
- Nestle FO, Kaplan DH, Barker J. Psoriasis. New England Journal of Medicine 2009;361(5):496-509. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nguyen 2009
- Nguyen T, Gattu S, Pugashetti R, Koo J. Practice of phototherapy in the treatment of moderate-to-severe psoriasis. Current Problems in Dermatology 2009;38:59-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nunez 2019
- Núñez M, Huete T, la Cueva P, Sacristán JA, Hartz S, Dilla T. A cost-per-number needed to treat analysis assessing the efficiency of biologic drugs in moderate to severe plaque psoriasis [Evaluación de la eficiencia de los tratamientos biológicos en la psoriasis moderada a grave en España: análisis de coste por número necesario a tratar (NNT)]. Actas Dermo-Sifiliográficas 2019;110(7):546-53. [DOI: 10.1016/j.ad.2018.10.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oliveira 2015
- Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. Anais Brasileiros de Dermatologia 2015;90(1):9-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parisi 2013
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. Journal of Investigative Dermatology 2013;133(2):377-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peinemann 2013a
- Peinemann F, Bartel C, Grouven U, Berthold F. Retinoic acid post consolidation therapy for high-risk neuroblastoma. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010685] [DOI] [PubMed] [Google Scholar]
Peinemann 2013b
- Peinemann F, Tushabe DA, Berthold F. Rapid COJEC versus standard induction therapies for high-risk neuroblastoma. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010774] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prieto‐Perez 2013
- Prieto-Pérez R, Cabaleiro T, Daudén E, Ochoa D, Roman M, Abad-Santos F. Genetics of psoriasis and pharmacogenetics of biological drugs. Autoimmune Diseases 2013;2013:613086. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2005
- Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Annals of the Rheumatic Diseases 2005;64(Suppl 2):ii37-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapp 1999
- Rapp SR, Feldman SR, Exum ML, Fleisher AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2012
- Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2012;66(3):369-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roos 2010
- Roos S, Hammes S, Ockenfels HM. Psoriasis. Natural versus artificial balneophototherapy [Psoriasistherapie. Künstliche versus natürliche Photosole]. Hautarzt 2010;61(8):683-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sbidian 2017
- Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD011535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schempp 2000
- Schempp CM, Ditmar HC, Hummler D, Simon-Haarhaus B, Schulte-Montig J, Schopf E, et al. Magnesium ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase, HLA-DR, B7 molecules, and cytokines. Journal of Investigative Dermatology 2000;115(4):680-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLOS Medicine 2010;7(3):e1000251. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Singh 2016
- Singh RK, Lee KM, Jose MV, Nakamura M, Ucmak D, Farahnik B, et al. The patient's guide to psoriasis treatment. Part 1: UVB phototherapy. Dermatology and Therapy 2016;6(3):307-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Social Science Statistics 2018
- Stangroom J. Easy Fisher Exact Test Calculator for 2 x 2 Contingency Table. www.socscistatistics.com/tests/Default.aspx (accessed 29 June 2018).
Stern 1998
- Stern RS, Liebman EJ, Väkevä L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA Follow-up Study. Journal of the National Cancer Institute 1998;90(17):1278-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stern 2004
- Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. Journal of Investigative Dermatology. Symposium Proceedings 2004;9(2):136-9. [PMID: 15083780 ] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TOMESA_PV‐Studie 2006
- ARGE der KKV Bayern. Final Report: Actively controlled, randomized, multi-center Phase III study demonstrating the efficacy of synchronous balneo-phototherapy (sBPT) in the treatment of psoriasis vulgaris [Abschlussbericht: Aktiv kontrollierte, randomisierte, multi-zentrische Phase III Studie zum Nachweis der Wirksamkeit der synchronen Balneo-Phototherapie (sBPT) bei der Behandlung von Psoriasis vulgaris]. München: Arbeitsgemeinschaft (ARGE) der Krankenkassenverbände (KKV) in Bayern 2006.
van de Kerkhof 2004
- de Kerkhof PC, Vissers WH. Established treatments of psoriasis. Current Drug Targets - Inflammation & Allergy 2004;3(2):145-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vanderpuye‐Orgle 2015
- Vanderpuye-Orgle J, Zhao Y, Lu J, Shrestha A, Sexton A, Seabury S, et al. Evaluating the economic burden of psoriasis in the United States. Journal of the American Academy of Dermatology 2015;72(6):961-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wahl 2002
- Wahl AK, Gjengedal E, Hanestad BR. The bodily suffering of living with severe psoriasis: in-depth interviews with 22 hospitalized patients with psoriasis. Qualitative Health Research 2002;12(2):250-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weatherhead 2011
- Weatherhead SC, Farr PM, Jamieson D, Hallinan JS, Lloyd JJ, Wipat A, et al. Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation. Journal of Investigative Dermatology 2011;131(9):1916-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weatherhead 2013
- Weatherhead SC, Farr PM, Reynolds NJ. Spectral effects of UV on psoriasis. Photochemical & Photobiological Sciences 2013;12(1):47-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2013
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. Journal of Cutaneous Medicine & Surgery 2013;17(1):6-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2014
- Zhu B, Edson-Heredia E, Guo J, Maeda-Chubachi T, Shen W, Kimball AB. Itching is a significant problem and a mediator between disease severity and quality of life for patients with psoriasis: results from a randomized controlled trial. British Journal of Dermatology 2014;171(5):1215-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Peinemann 2015
- Peinemann F, Harari M, Peternel S, Chan T, Gambichler T. Indoor salt water baths followed by artificial ultraviolet B light for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011941] [DOI] [PMC free article] [PubMed] [Google Scholar]


