Abstract
Background
Epithelial ovarian cancer (EOC) is one of the most prevalent malignancies affecting females worldwide; however, its etiology mechanism remains unclear. In various malignancies, miR-145-5p is a widely accepted and versatile miRNA. Therefore, our research focused on exploring the activity and etiology of miR-145-5p in the modulation of metastasis, migration, and proliferation of EOC cells. The direct reactions between the 3′UTRs of SMAD4 mRNA and miR-145-5p were verified using dual luciferase reporter test. SKOV-3 cells were subsequently transfected using miR-145-5p mimics. Cell migration, death, and proliferation were evaluated using MTT, flow cytometry, and Transwell test. In addition, SMAD4 transcription and translation were evaluated using qRT-PCR and Western blot.
Results
We found that miR-145-5p expression was repressed prevalently in EOC tissues, apart from SMAD4 upregulation. Excessive miR-145-5p expression remarkably reinforced EOC cell death and repressed EOC cell proliferation. Furthermore, upregulated miR-145-5p expression noticeably repressed migration via MMP-2 and MMP-9 downregulation. Moreover, SMAD4 was downregulated via miR-145-5p transfection. The dual luciferase test revealed that miR-145-5p directly targeted SMAD4.
Conclusions
Our research suggests that miR-145-5p serves as a malignancy repressor and exerts an essential impact on inhibiting malignancy generation and reinforcing EOC death via targeting SMAD4. MiR-145-5p application could serve as a promising strategy to treat EOC.
Keywords: MiR-145-5p, Epithelial ovarian cancer, SMAD4, Migration, Apoptosis, Proliferation
Introduction
Epithelial ovarian cancer (EOC) is the dominant contributor to gynecologic malignancy-related death in females with poor prognosis, with an annual mortality of approximately 125,000 [1]. Unfortunately, only 19% of the total ovarian malignancy cases are identified early. In most women, it is diagnosed at an advanced stage, which largely explains the poor prognosis of this malignancy. Germline mutations of the genes BRCA1 and BRCA2, which encode proteins essential for the repair of double-strand DNA breaks through homologous recombination, lead to increased cancer predisposition. BRCA mutations are present in approximately 14% of epithelial ovarian cancers. The poor mortality of this illness is attributable to diagnosis made at the terminal stage, accounting for approximately 70% of the total ovarian malignancies [2, 3]. Contemporary research suggests that every histologic type of EOC is related to different morphologic and molecular mutations, such as endometroid, clear-cell, mucinous, and serous carcinomas [4–6]. Consequently, further research on the etiology and mechanism that reinforce ovarian malignancies are required to explore reliable predictors as well as innovative drugs to develop an efficient personalized treatment.
MicroRNAs (miRNAs) are a group of small single-stranded RNAs consisting of 22 nucleotides with a typical hairpin secondary structure [7]. They modulate gene silencing via directly targeting at an mRNA for degeneration or repressing translation [8]. A change in miRNA expression is related to different kinds of malignancies, such as EOC [9–11]. MiRNAs serve as the essential modulators of multiple fundamental biological reactions, such as those associated with malignancy generation [12]. Reportedly, miRNAs exert an essential impact on cell proliferation, apoptosis, and differentiation [13–15]. Primary malignancies, as well as cell lines, display a considerable expression of multiple malfunctioning miRNAs in comparison with normal tissues [16]. For example, miR-34 located at the downstream of p53 could modulate proliferation repression, cell death, and senescence stimulation in multiple cell types [17, 18]. Numerous miRNAs display malignancy-repressing capabilities where an abnormal expression of miRNAs in malignancies could offer a promising treatment strategy [19]. Particularly, excessive miR-145-5p expression can repress serous EOC progression [20]. Nevertheless, we need to further elucidate the functions and mechanism of miR-145-5p.
In the present study, we explored the expression and activity of miR-145-5p in EOC. We revealed that EOC was possibly modulated via miR-145-5p. When compared with that in normal ovarian tissues (NOT), miR-145-5p was constantly repressed in EOC tissues. However, excessive miR-145-5p expression repressed EOC cell proliferation and migration as well as triggered EOC cell death. Such activities are related to the inhibition of SMAD4 expression.
Materials and methods
EOC cell lines and tissues
We acquired 18 samples of EOC tissues and another 18 samples of NOT (surrounding the malignancies) from the Xi’an Gaoxin Hospital. The diagnosis of every participant was histopathologically verified. None of the patients underwent previous malignancy-counteracting treatment or displayed distant metastasis. Every specimen was obtained between 2015 and 2017, and it was fixed using formalin with the approval from the local ethics committee. The study was approved by the Ethics Committee of the Xi’an Gaoxin Hospital. Fully informed written consent was acquired from every patient.
EOC cell lines (SKOV-3) were purchased from the Chinese Academy of Science and were cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) medium supplemented with 10% or 20% fetal bovine serum (FBS, Gibco) and 1% penicillin (Sigma-Aldrich, Inc., St-Louis, MO, USA) in a humidified 5% CO2 incubator at 37 °C.
Cell transfection
SKOV-3 cells were transfected using mature miR-145-5p mimics to explore the modulating impact of miR-145-5p. Lipofectamine 3000 reagent was applied to the transfection, as per the manufacturer’s instructions. Nonhomologous miRNA mimics served as the negative control (NC). Cells underwent trypsinization, and 24 h after transfection, they were harvested for cell death and proliferation test. MiR-145-5p mimics and NC were purchased from RiboBio Co., Ltd. (Guangzhou, China).
RNA extraction and real-time PCR
We isolated total RNA via TRIzol and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). We used Superscript III Kit (Life Technologies) to perform reverse transcription. Thereafter, complementary DNAs were assessed using qRT-PCR. Related transcriptions were quantified and assessed with qRT-PCR using the SYBR Green PCR Supermix Kit. Procedures were performed at least thrice for every specimen.
Cell viability assay
Cell survival was assessed using MTT test. Plates containing 96 wells were used to plant the cells (5 × 104 cells/mL) that were cultivated under the conditions of 5% CO2 and 37 °C. MTT test was conducted at 48 h after transfection. Cell survival was assessed by supplementing 10 μL of MTT to every well. Cells were incubated at 37 °C for 4 h, following which they were evaluated using a microplate reader at 570 nm (Thermo Scientific). Procedures were performed thrice independently.
Assessment of cell death
Apoptosis was evaluated using flow cytometry (FC). After the cells were acquired, they were washed twice using cold PBS. The supernatant was discarded after 5 min of centrifugation at 100 rpm. The pellet was re-suspended in a binding buffer. FITC-Annexin V and propidium iodide (PI) were used for supplementation. The mixture was incubated at room temperature for 10 min. Fluorescence signals were evaluated using FACScan flow cytometer.
TUNEL test
Using 4% paraformaldehyde, SKOV-3 cells were fixed on the slide. Dead cells were labeled using TUNEL.
Cell migration test
Cell migration was evaluated via the Transwell test. Briefly, 50,000 cells in DMEM containing 1 μg/mL of mitomycin C without serum were planted on the top well of 24-well poly-carbonate Transwell filters (Millipore, Bedford, MA, USA), whereas the bottom well was supplemented with DMEM with 10% fetal bovine serum. After 48 h of incubation, we removed the cells in the top well, whereas those on the bottom surface were fixed, stained, and quantified.
Dual luciferase reporter test
Our research examined whether excessive miR-145-5p expression repressed the firefly luciferase function in SKOV-3 cells. Such cells were previously transducted using luciferase plasmids with SMAD4 3′UTR to examine whether miR-145-5p could directly modulate SMAD4 expression. Briefly, pGL3-SMAD4 was cotransfected using miR-145-5p mimics or a repressor into cultivated SKOV-3 cells using the Neon Transfection Kit (Lonza). Luciferase function was assessed using the Dual-Luciferase Reporter Assay System (Promega).
Western blot
Proteins were quantified by Bradford test (Bio-Rad, Hercules, CA, USA) and standard SDS–PAGE. These proteins were then isolated on 8–15% Tris–HCl polyacrylamide gels (Bio-Rad), following which they were transferred to polyvinylidene difluoride. Thereafter, the blots were incubated overnight using particular primary antibodies (anti-p21, anti-cyclinD, anti-MMP-9, anti-SMAD4, anti-p27, anti-MMP-2, and anti-β-actin from Cell Signaling Technology, USA) within TBST at 4 °C. Re-incubation was performed using secondary antibodies in conjugation with horseradish peroxidase. Enhanced chemiluminescence plus a detection reagent (Pierce, Rockford, USA) was employed to evaluate immunoreactive signals.
Statistical analysis
The outcome is presented as mean ± SEM. Difference between various groups was evaluated via unequal-variance Student’s t-test (two-tailed) or ANOVA, followed by Tukey’s post hoc test. P < 0.05 is considered significant.
Results
MiR-145-5p was downregulated in EOC tissues
The qRT-PCR that was performed to explore miR-145-5p in EOC tissues revealed that the transcription of miR-145-5p was repressed in EOC tissues compared with nonmalignant ones (Fig. 1). Moreover, SMAD4 transcription and translation were noticeably upregulated in EOC tissues compared with nonmalignant ones. In addition, the expression of p53 was also increased in EOC tissues. Overall, miR-145-5p was often repressed in EOC and remarkably modulated SMAD4.
Fig. 1.
MiR-145-5p expression was inhibited in epithelial ovarian cancer (EOC). a-b, Expression of miR-145-5p (a) and SMAD4 (b) in EOC and healthy ovarian tissues (surrounding the malignancies; Con) was assessed using qRT-PCR. c-e, Western blots (c) and the quantitative evaluation of SMAD4 (d) and p53 (e) in EOC and tumor adjacent tissues. Data are presented as means ± SEM, n = 18. **P < 0.01, compared with the NC group
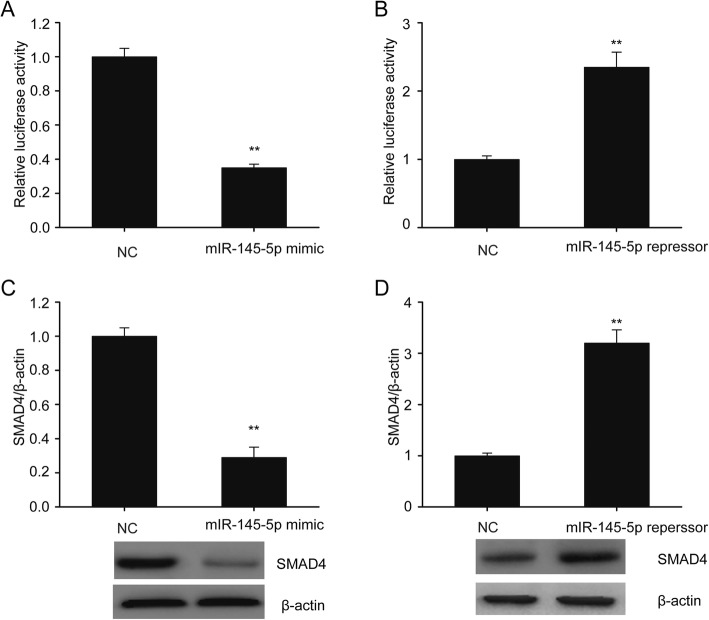
MiR-145-5p targeted SMAD4 in SKOV-3 cells
Bioinformatics analysis indicated that SMAD4 could be targeted via miR-145-5p. The SMAD4 3′UTR function of SKOV-3 cells was inhibited via excessive miR-145-5p expression but was enhanced via miR-145-5p downregulation (Fig. 2a-b). Therefore, miR-145-5p probably regulated SMAD4 directly via 3′UTR binding. Moreover, excessive miR-145-5p expression repressed SMAD4 translation. However, miR-145-5p repression reinforced SMAD4 translation of SKOV-3 cells (Fig. 2c-d).
Fig. 2.
MiR-145-5p targeted SMAD4. a-b, Associations between the 3′UTRs of SMAD4 transcripts and miR-145-5p were assessed using dual luciferase test. c-d, Immunoblots and the quantitative evaluation of SMAD4 in SKOV-3 cells that were transfected for 24 h using miR-145-5p mimic (c) or miR-145-5p repressor (d). Data are presented as means ± SEM, n = 3. **P < 0.01, compared with the NC group
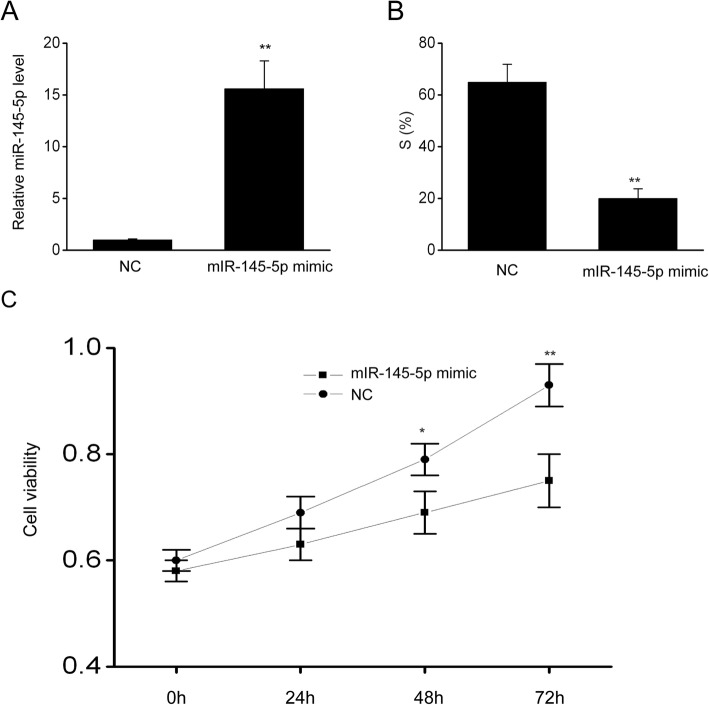
MiR-145-5p repressed SKOV-3 cell proliferation
SKOV-3 cells were transfected using miR-145-5p mimics, and their proliferation was assessed using MTT and FC to explore the contribution of miR-145-5p to EOC cell proliferation. MiR-145-5p concentration was remarkably elevated in SKOV-3 cells in comparison with that in NC (Fig. 3a). Further, FC revealed that the S phase of SKOV-3 cells was repressed in specimens supplemented with miR-145-5p mimic compared with that of NC (Fig. 3b). Moreover, MTT test proved that SKOV-3 cell proliferation was remarkably repressed following an excessive miR-145-5p expression in comparison with NC cell proliferation (Fig. 3c), suggesting that miR-145-5p repressed EOC cell proliferation.
Fig. 3.
MiR-145-5p repressed the proliferation of EOC cells. a, MiR-145-5p expression in SKOV-3 cells following transient transfection using miR-145-5p mimic or NC. b, miR-145-5p prohibited SKOV-3 cell proliferation, as shown using FC. c, Excessive miR-145-5p expression repressed such proliferation, as demonstrated using MTT test. Data are presented as means ± SEM, n = 3. **P < 0.01, *P < 0.05, compared with the NC group
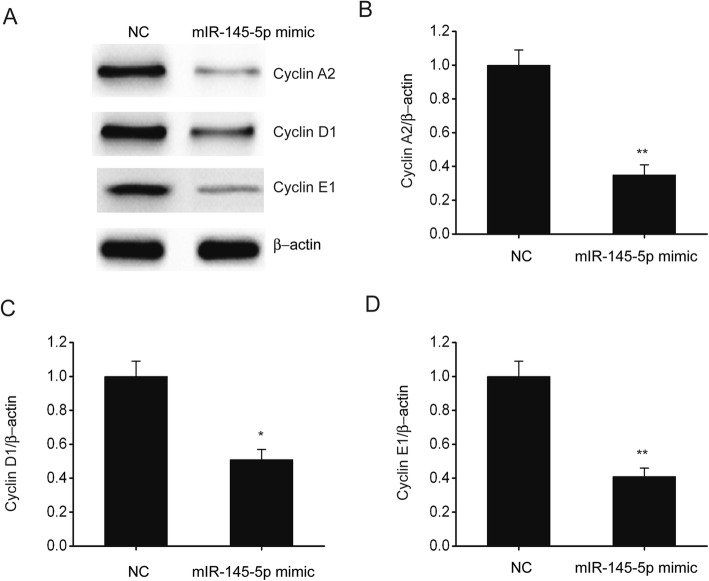
MiR-145-5p repressed the expression of cell cycle-related proteins in SKOV-3 cells
Previous research has demonstrated that cyclins E1, A2, and D1 contributes to the proliferation modulation of EOC [21]. In the current research, we investigated whether miR-145-5p repressed EOC cell proliferation using the abovementioned proteins. We observed that miR-145-5p downregulated the translation of these cell cycle-modulating proteins in SKOV-3 cells (Fig. 4). Therefore, miR-145-5p repressed EOC cell proliferation by repressing the cyclins E1, A2, and D1.
Fig. 4.
MiR-145-5p prohibited the expression of cell cycle-related proteins in SKOV-3 cells. a-d, Immunoblots (a) and the quantitative evaluation of cyclins A2 (b), D1 (c), and E1 (d) in SKOV-3 cells that were transfected for 24 h using miR-145-5p mimic or NC. Data are presented as means ± SEM, n = 3. **P < 0.01, compared with the NC group
MiR-145-5p reinforced EOC cell death
We investigated the contribution of miR-145-5p to EOC cell death through FC using Annexin V/PI staining. Cells that were transfected using miR-145-5p mimics demonstrated a noticeably elevated death rate in comparison with those in NC (Fig. 5a-b). Thereafter, cell death was verified using TUNEL test. Compared with those in NC, cells with positive TUNEL were remarkably reinforced in SKOV-3 cells following an excessive miR-145-5p expression (Fig. 5c-d), proving that miR-145-5p reinforced EOC cell death. In addition, the expression of cleaved capsase-3 was notably upregulated in miR-145-5p mimics transfected SKOV-3 cells (Fig. 5e-f).
Fig. 5.
MiR-145-5p reinforced EOC cell death. a-b, MiR-145-5p reinforced cell death, as demonstrated USING FC. c-d, MiR-145-5p reinforced cell death, as confirmed using TUNEL test. e-f, Immunoblots (e) and the quantitative evaluation of caspase-3 (f) in SKOV-3 cells that were transfected for 24 h using miR-145-5p mimic or NC. Data are presented as means ± SEM, n = 3. **P < 0.01, compared with the NC group
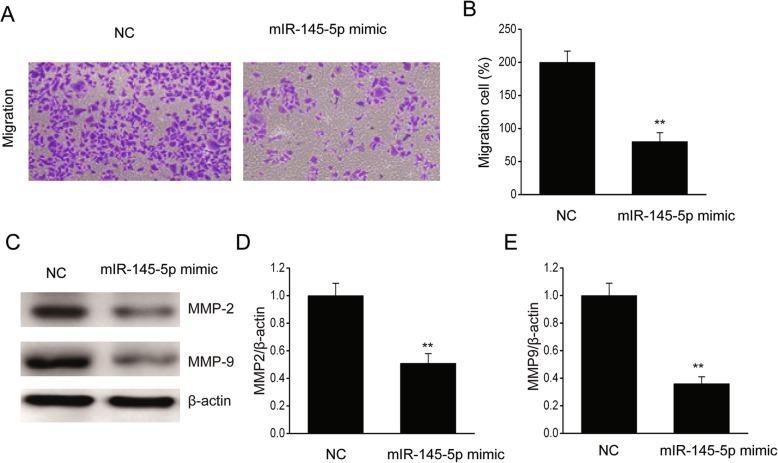
MiR-223 prohibited EOC cell migration
We determined the contribution of miR-145-5p to EOC cell migration using the Transwell test. Migration was noticeably suppressed via transfection using miR-145-5p mimics in SKOV-3 cells (Fig. 6a-b). Matrix metalloproteinases (MMPs) serve as the proteolytic enzymes of ECM relying on zinc, which is commonly used by cells via migration. MMP-2 and MMP-9 have closely been linked to the invasion of various malignancies [22, 23]. Hence, we investigated MMP-2 and MMP-9 translations to assess the contribution of miR-145-5p to EOC invasion. We found that excessive miR-145-5p expression remarkably repressed MMP-2 and MMP-9 translations (Fig. 6c-e), demonstrating that miR-145-5p reinforced EOC cell migration.
Fig. 6.
MiR-145-5p prohibited EOC cell migration. a-b, MiR-145-5p repressed migration, as evaluated via Transwell test. Representative images (a) and the quantitative evaluation of cell migration (b) are displayed. Scale bars = 100 μm. c-e, Immunoblots (c) and the quantitative evaluation of MMP-2 (d) and MMP-9 (e). Data are presented as means ± SEM, n = 3. **P < 0.01, compared with the NC group
Discussion
Our research proved that miR-145-5p was repressed in EOC tissues in comparison with that in the surrounding nonmalignant ones. Moreover, miR-145-5p reinforced EOC cell death and repressed EOC cell proliferation and migration via the direct modulation of SMAD4. Briefly, miR-145-5p could modulate EOC where SMAD4 had a contribution. This research presented an innovative understanding of the contribution of miR-145-5p to EOC etiology and indicated that miR-145-5p could serve as a promising target to treat EOC.
MiRNAs can repress or reinforce human malignancies [24, 25]. In particular, miR-145-5p displays an essential malignancy-repressing impact on malignancy progression [26, 27]. MiR-145-5p expression was repressed in several malignancies, such as non–small-cell lung cancer (NSCLC), ESCC, cervical cancer, and colorectal cancer, wherein it could repress malignancy generation [28–30]. Metadata proved that miR-145-5p is remarkably downregulated in NSCLC compared with that in normal tissues and could assist in a more accurate diagnosis in lung squamous cell carcinoma [31]. MiR-145 can repress various malignancies. Regarding gastric cancer, miR-145 modulates CD44 by directly targeting its 3′UTR [32]. Excessive miR-145 expression represses the migration, invasion, and proliferation of breast malignant cells via the downregulation of transforming growth factor-b expression [33]. In bladder malignancies, miR-145 expression is repressed. Its mimics repress the Warburg effect influenced directly by silencing Kruppel-like factor 4 (KLF4) [34]. Moreover, miR-145 inhibits resistance to oxaliplatin in colorectal cancer via GPR98 downregulation [35]. In ESCC, miR-145 expression is frequently repressed, whereas in esophageal EC9706 and ECA109 cells, its upregulation can prohibit proliferation and trigger cell death [36]. Wang et al. proved that excessive miR-145 expression remarkably represses ECA109 proliferation with the aid of pLVX-IZ-miR-145 vector and enhances the quantity of G2/M cells as well as cell death proportion [37]. In our research, the downregulation of miR-145-5p expression in EOC was verified. The activities and mechanism of the transcription modulation of miR-145-5p were subsequently investigated. Using MTT test, we found that miR-145-5p remarkably repressed EOC cell proliferation and migration. Furthermore, upon assessing the contribution of miR-145-5p to EOC cell death, we observed that miR-145-5p could reinforce EOC cell death. In summary, miR-145-5p could provide an essential target to treat EOC.
MiRNAs act by modulating the expression of target genes [38], and miR-145-5p represses malignancies via targeting some genes, such as mitogen-activated protein kinase 1, specificity protein 1, and nuclear factor-kB [28, 39, 40]. Furthermore, excessive miR-145-5p expression is modulated using circular or long noncoding RNAs [41, 42]. SMAD4 serves as a versatile essential modulator that functions with other transcription factors during TGF-β modulation in malignancy generation [43]. SMAD4 contributes to various cell reactions, and its malfunction is intimately linked to various malignancies, such as thyroid, pancreatic, prostate, and colorectal malignancies [44–46]. Therefore, in our research, miR-145-5p acted on SMAD4 3′UTR transcripts and downregulated SMAD4, thereby participating in the prohibition of EOC cell proliferation and migration as well as reinforcing EOC cell death.
Conclusions
In conclusion, miR-145-5p repressed EOC generation and revealed an miR-145-5p/SMAD4 axis that repressed EOC cell proliferation and migration and reinforced EOC cell death. Therefore, excessive miR-145-5p expression could provide a promising strategy to treat EOC.
Acknowledgements
None.
Authors’ contributions
All authors analyzed and interpreted the patient data regarding the hematological disease and the transplant. All authors performed the histological examination of the kidney, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Xi’an Gaoxin Hospital. Fully informed written consent was acquired from every patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jie Zhou, Email: w84fmpp44e@163.com.
Xiyi Zhang, Email: i95jn7@163.com.
Weiling Li, Email: ztviqkpfl68@163.com.
Yuanyuan Chen, Email: v57s2g@163.com.
References
- 1.Yang H, Dai H, Li L, et al. Age at menarche and epithelial ovarian cancer risk: A meta-analysis and Mendelian randomization study. Cancer Med. 2019;8(8):4012-22. [DOI] [PMC free article] [PubMed]
- 2.Laasik M, Kemppainen J, Auranen A, Hietanen S, Grénman S, Seppänen M, et al. Behavior of FDG-avid supradiaphragmatic lymph nodes in PET/CT throughout primary therapy in advanced serous epithelial ovarian cancer: a prospective study. Cancer Imaging. 2019;19:27. doi: 10.1186/s40644-019-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindgren A, Anttila M, Arponen O, Rautiainen S, Könönen M, Vanninen R, et al. Prognostic value of preoperative dynamic contrast-enhanced magnetic resonance imaging in epithelial ovarian cancer. Eur J Radiol. 2019;115:66–73. doi: 10.1016/j.ejrad.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Lan A, Yang G. Clinicopathological parameters and survival of invasive epithelial ovarian cancer by histotype and disease stage. Future Oncol.2019;15(17):2029-39. [DOI] [PubMed]
- 5.Bodelon C, Killian JK, Sampson JN, et al. Molecular Classification of Epithelial Ovarian Cancer Based on Methylation Profiling: Evidence for Survival Heterogeneity. Clin Cancer Res. 2019;25(19):5937-46. [DOI] [PMC free article] [PubMed]
- 6.Zhang GH, Chen MM, Kai JY, Ma Q, Zhong AL, Xie SH, et al. Molecular profiling of mucinous epithelial ovarian cancer by weighted gene co-expression network analysis. Gene. 2019;709:56–64. doi: 10.1016/j.gene.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Petrescu GE, Sabo AA, Torsin LI, Calin GA, Dragomir MP. MicroRNA based theranostics for brain cancer: basic principles. J Exp Clin Cancer Res. 2019;38:231. doi: 10.1186/s13046-019-1180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian S. miR-4319 inhibited the development of thyroid cancer by modulating FUS-stabilized SMURF1. J Cell Biochem. 2020;121(1):174-82. [DOI] [PubMed]
- 9.Yang C, Kim HS, Song G, Lim W. The potential role of exosomes derived from ovarian cancer cells for diagnostic and therapeutic approaches. J Cell Physiol. 2019;234(12):21493-503. [DOI] [PubMed]
- 10.Li GC, Qin XL, Song HH, et al. Upregulated microRNA-15b alleviates ovarian cancer through inhitbition of the PI3K/Akt pathway by targeting LPAR3. J Cell Physiol. 2019;234(12):22331-42. [DOI] [PubMed]
- 11.Khordadmehr M, Jigari-Asl F, Ezzati H, et al. A comprehensive review on miR-451: A promising cancer biomarker with therapeutic potential. J Cell Physiol. 2019;234(12):21716-31. [DOI] [PubMed]
- 12.Zhang J, Han Z, Dong L, Li Z, Li K, Shi M, et al. MicroRNA-152 and microRNA-448 inhibit proliferation of colorectal cancer cells in vitro by targeting Rictor. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:533–539. doi: 10.12122/j.issn.1673-4254.2019.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Pan Y, Guan P, Li X, You C. Bioinformatics analysis of COL1A1 regulated by miR-129-5p as a potential therapeutic target for gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:540–546. doi: 10.12122/j.issn.1673-4254.2019.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GGu J, Li X, Li H, Jin Z, Jin J. MicroRNA-198 inhibits proliferation and induces apoptosis by directly suppressing FGFR1 in gastric cancer. BiosciRep. 2019;39(6):BSR20181258. Published 2019 Jun 10. [DOI] [PMC free article] [PubMed]
- 15.Li Y, Yan X, Shi J, He Y, Xu J, Lin L, et al. Aberrantly expressed miR-188-5p promotes gastric cancer metastasis by activating Wnt/β-catenin signaling. BMC Cancer. 2019;19:505. doi: 10.1186/s12885-019-5731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Lin J, Jin Y, Chen M, Zheng H, Feng J. The miRNA hsa-miR-6515-3p potentially contributes to lncRNA H19-mediated-lung cancer metastasis. J Cell Biochem. 2019;120(10):17413-21. [DOI] [PMC free article] [PubMed]
- 17.Jun HH, Kwack K, Lee KH, Kim JO, Park HS, Ryu CS, et al. Association between TP53 genetic polymorphisms and the methylation and expression of miR-34a, 34b/c in colorectal cancer tissues. Oncol Lett. 2019;17:4726–4734. doi: 10.3892/ol.2019.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Wang L, Dong D, Wang Z, Ji W, Yu M, et al. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019;52:e12527. doi: 10.1111/cpr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5:10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai J, Zhang P, Liu P, Qu H. Expressions and significance of cyclinD1 in epithelial ovarian cancer cell 3AO. Zhonghua Yi Xue Za Zhi. 2012;92:351–353. [PubMed] [Google Scholar]
- 22.Park Y-J, Kim JY, Lee DY, Zhang X, Bazarsad S, Chung W-Y, et al. PKM2 enhances cancer invasion via ETS-1-dependent induction of matrix metalloproteinase in oral squamous cell carcinoma cells. PLoS One. 2019;14:e0216661. doi: 10.1371/journal.pone.0216661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L, Qian J, Zhu F, Wu F, Zhao H, Zhu H. The prognostic values of matrix metalloproteinases in ovarian cancer. J Int Med Res. 2020;48(1):300060519825983. 10.1177/0300060519825983. [DOI] [PMC free article] [PubMed]
- 24.Yoshii S, Hayashi Y, Iijima H, et al. Exosomal microRNAs derived from colon cancer cells promote tumor progression by suppressing fibroblast TP53 expression. Cancer Sci. 2019;110(8):2396-407. [DOI] [PMC free article] [PubMed]
- 25.Fu Y, Lin L, Xia L. MiR-107 function as a tumor suppressor gene in colorectal cancer by targeting transferrin receptor 1. Cell Mol Biol Lett. 2019;24:31. doi: 10.1186/s11658-019-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang W, Zhang X, Tan W, Gao J, Pan L, Ye X, et al. miR-145-5p suppresses breast Cancer progression by inhibiting SOX2. J Surg Res. 2019;236:278–287. doi: 10.1016/j.jss.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Bellissimo T, Tito C, Ganci F, Sacconi A, Masciarelli S, Di Martino G, et al. Argonaute 2 drives miR-145-5p-dependent gene expression program in breast cancer cells. Cell Death Dis. 2019;10:17. doi: 10.1038/s41419-018-1267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Y, Yan W, Sun C, Liu Q, Wang J, Wang M. miR-145-5p inhibits epithelial-mesenchymal transition via the JNK signaling pathway by targeting MAP 3K1 in non-small cell lung cancer cells. Oncol Lett. 2017;14:6923–6928. doi: 10.3892/ol.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Samowitz WS, Herrick JS. Dysregulated genes and miRNAs in the apoptosis pathway in colorectal cancer patients. Apoptosis. 2018;23:237–250. doi: 10.1007/s10495-018-1451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Jiang M, Liu Q, Han Z, Zhao Y, Ji S. miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncol Lett. 2018;16:6355–6360. doi: 10.3892/ol.2018.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Chen M, Wu W. Analysis of microRNA (miRNA) expression profiles reveals 11 key biomarkers associated with non-small cell lung cancer. World J Surg Oncol. 2017;15:175. doi: 10.1186/s12957-017-1244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng J-F, Ma X-Q, Wang L-P, Wang W. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol. 2017;23:2337–2345. doi: 10.3748/wjg.v23.i13.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Y, Zhang C, Zhang J, Zhang N, Li T, Fang J, et al. miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression. Int J Oncol. 2017;50:1701–1710. doi: 10.3892/ijo.2017.3945. [DOI] [PubMed] [Google Scholar]
- 34.Minami K, Taniguchi K, Sugito N, Kuranaga Y, Inamoto T, Takahara K, et al. MiR-145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget. 2017;8:33064–33077. doi: 10.18632/oncotarget.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Q, Cheng J, Zhang J, Zhang Y, Chen X, Xie J, et al. miR-145 inhibits drug resistance to Oxaliplatin in colorectal cancer cells through regulating G protein coupled receptor 98. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:566–570. [PubMed] [Google Scholar]
- 36.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, et al. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 37.Wang T-Y, Zhang Q-Q, Zhang X, Sun Q-L, Zhao C-P, Wang X-Y. The effect of recombinant lentiviral vector encoding miR-145 on human esophageal cancer cells. Tumor Biol. 2015;36:9733–9738. doi: 10.1007/s13277-015-3743-1. [DOI] [PubMed] [Google Scholar]
- 38.Moss EG. MicroRNAs: hidden in the genome. Curr Biol. 2002;12:R138–RR40. doi: 10.1016/S0960-9822(02)00708-X. [DOI] [PubMed] [Google Scholar]
- 39.Mei L-L, Wang W-J, Qiu Y-T, Xie X-F, Bai J, Shi Z-Z. miR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-κB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 2017;18:1833. doi: 10.3390/ijms18091833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang S, He X, Xia Y, Hu W, Luo J, Zhang J, et al. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. OncoTargets Ther. 2016;9:2305–2315. doi: 10.2147/OTT.S101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ren K, Li Z, Li Y, Zhang W, Han X. Long noncoding RNA taurine-upregulated gene 1 promotes cell proliferation and invasion in gastric cancer via negatively modulating miRNA-145-5p. Oncol Res Featuring Preclinical Clin Cancer Ther. 2017;25:789–798. doi: 10.3727/096504016X14783677992682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, Huang W, Wang X, Wang T, Chen Y, Chen B, et al. Circular RNA CEP128 acts as a sponge of miR-145-5p in promoting the bladder cancer progression via regulating SOX11. Mol Med. 2018;24:40. doi: 10.1186/s10020-018-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohini M, Arumugam B, Vairamani M, Selvamurugan N. Stimulation of ATF3 interaction with Smad4 via TGF-β1 for matrix metalloproteinase 13 gene activation in human breast cancer cells. Int J Biol Macromol. 2019;134:954–961. doi: 10.1016/j.ijbiomac.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 44.Wang X-L, Huang C. Difference of TGF-β/Smads signaling pathway in epithelial-mesenchymal transition of normal colonic epithelial cells induced by tumor-associated fibroblasts and colon cancer cells. Mol Biol Rep. 2019;46:2749–2759. doi: 10.1007/s11033-019-04719-5. [DOI] [PubMed] [Google Scholar]
- 45.Pu H, Begemann DE, Kyprianou N. Aberrant TGF-β signaling drives castration-resistant prostate cancer in a male mouse model of prostate tumorigenesis. Endocrinology. 2017;158:1612–1622. doi: 10.1210/en.2017-00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y-F, Li T, Jiang F, Ni W-K, Guan C-Q, Liu Z-X, et al. Correlation between S100A11 and the TGF-β 1/SMAD4 pathway and its effects on the proliferation and apoptosis of pancreatic cancer cell line PANC-1. Mol Cell Biochem. 2019;450:53–64. doi: 10.1007/s11010-018-3372-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.