Efforts to cure HIV infection have focused primarily on the elimination of latently infected CD4+ T cells. Few studies have addressed the unique reservoir of infectious HIV that exists on follicular dendritic cells (FDCs), persists in vivo during antiretroviral therapy, and likely contributes to viral rebound upon cessation of antiretroviral therapy. We assessed the efficacy of a novel HIV-specific chimeric antigen receptor (CAR) T cell to target both HIV-infected CD4+ T cells and the FDC reservoir in vitro. Although CAR-T cells eliminated CD4+ T cells that express HIV, they did not respond to or eliminate FDC bound to HIV. These findings reveal a fundamental limitation to CAR-T cell therapy to eradicate HIV.
KEYWORDS: HIV-1, chimeric antigen receptor T cells, follicular dendritic cells
ABSTRACT
The major obstacle to a cure for HIV infection is the persistence of replication-competent viral reservoirs during antiretroviral therapy. HIV-specific chimeric antigen receptor (CAR) T cells have been developed to target latently infected CD4+ T cells that express virus either spontaneously or after intentional latency reversal. Whether HIV-specific CAR-T cells can recognize and eliminate the follicular dendritic cell (FDC) reservoir of HIV-bound immune complexes (ICs) is unknown. We created HIV-specific CAR-T cells using human peripheral blood mononuclear cells (PBMCs) and a CAR construct that enables the expression of CD4 (domains 1 and 2) and the carbohydrate recognition domain of mannose binding lectin (MBL) to target native HIV Env (CD4-MBL CAR). We assessed CAR-T cell cytotoxicity using a carboxyfluorescein succinimidyl ester (CFSE) release assay and evaluated CAR-T cell activation through interferon gamma (IFN-γ) production and CD107a membrane accumulation by flow cytometry. CD4-MBL CAR-T cells displayed potent lytic and functional responses to Env-expressing cell lines and HIV-infected CD4+ T cells but were ineffective at targeting FDC bearing HIV-ICs. CD4-MBL CAR-T cells were unresponsive to cell-free HIV or concentrated, immobilized HIV-ICs in cell-free experiments. Blocking intercellular adhesion molecule-1 (ICAM-1) inhibited the cytolytic response of CD4-MBL CAR-T cells to Env-expressing cell lines and HIV-infected CD4+ T cells, suggesting that factors such as adhesion molecules are necessary for the stabilization of the CAR-Env interaction to elicit a cytotoxic response. Thus, CD4-MBL CAR-T cells are unable to eliminate the FDC-associated HIV reservoir, and alternative strategies to eradicate this reservoir must be sought.
IMPORTANCE Efforts to cure HIV infection have focused primarily on the elimination of latently infected CD4+ T cells. Few studies have addressed the unique reservoir of infectious HIV that exists on follicular dendritic cells (FDCs), persists in vivo during antiretroviral therapy, and likely contributes to viral rebound upon cessation of antiretroviral therapy. We assessed the efficacy of a novel HIV-specific chimeric antigen receptor (CAR) T cell to target both HIV-infected CD4+ T cells and the FDC reservoir in vitro. Although CAR-T cells eliminated CD4+ T cells that express HIV, they did not respond to or eliminate FDC bound to HIV. These findings reveal a fundamental limitation to CAR-T cell therapy to eradicate HIV.
INTRODUCTION
The major barrier to a cure for HIV is the persistence of replication-competent virus despite a sustained suppression of virus replication in the context of antiretroviral therapy (ART) (1). Most strategies to eliminate HIV infection have targeted the latently infected CD4+ T cell reservoir (2). Another important, but understudied source of replication-competent HIV during ART is the follicular dendritic cell (FDC) reservoir. FDCs reside in the germinal centers of secondary lymphoid tissues (e.g., lymph nodes, spleen, and gut-associated lymphoid tissue [GALT]) and are not permissive to HIV (3). Instead, they trap virus in the form of an immune complex (IC) on their surface (3–6). Virus bound to FDCs is potently infectious to CD4+ T cells and much more resistant to neutralizing antibodies than soluble virus (7). In untreated chronic HIV infection, the amount of virus found bound to FDCs is approximately 10-fold more than cell-associated virus in lymph nodes (8, 9). During ART, the FDC reservoir decays, but it still remains detectable in individuals on ART (9). Although the level of infectivity of virus bound to FDCs after years of ART is unknown, replication-competent virus was readily detected in a nonpermissive mouse model 9 months after virus was bound to the FDCs (5).
Chimeric antigen receptor (CAR) T cells have demonstrated remarkable effectiveness in the treatment of lymphoma and other hematologic malignancies, and several groups are pursuing CAR-T cells as a strategy to eliminate HIV infection either alone or in conjunction with latency reversal agents (10–14). HIV-specific CARs recognize the native HIV envelope glycoprotein (Env) on the infected cell surface. This is in stark contrast to natural cytotoxic T lymphocytes (CTLs) which detect processed and presented peptides in the context of self, major histocompatibility (MHC) class I molecules. Prior studies have demonstrated that HIV-specific CAR-T cells are effective in targeting HIV-expressing cells (10–16), but whether they can recognize and eliminate FDC that bear HIV-ICs is unknown.
We evaluated the ability of CAR-T cells to recognize and eliminate FDC bound to HIV-IC in vitro. We utilized a novel expanded-spectrum bispecific CAR, the CD4-MBL CAR (13), which contains as the targeting motif the extracellular domains of human CD4 (D1D2) and the carbohydrate recognition domain (CRD) of human mannose binding lectin (MBL); the targeting motif is linked to the CD28 transmembrane region, followed by the intracellular signaling domains of CD28 and CD3ζ. The CD4-MBL CAR recognizes two distinct highly conserved elements of native gp120 and previously has been shown to potently suppress in vitro-spreading infection in PBMCs infected with genetically diverse HIV-1 primary isolates (13). The work reported herein confirmed the ability of PBMCs transduced with the CD4-MBL CAR to lyse HIV-infected CD4+ T cells and demonstrated that this was dependent upon ICAM-1 expression. CAR-T cells were unable, however, to recognize or lyse FDCs associated with HIV-IC, illustrating a fundamental limitation to CAR-T cell therapy for HIV infection.
RESULTS
Evaluation of CAR expression and function.
Using PBMCs from four HIV-uninfected donors, we generated HIV Env-specific CD4-MBL CAR-T cells and control 139 CAR-T cells (13) with the corresponding constructs (Fig. 1A). The transduction efficiency, as assessed by the percentage of CD8+ cells that were also CD4+, was >95% for all donors (median, 98%; range, 95.5% to 98.9%). Additionally, CD4 median fluorescence intensity (MFI) on CD4+CD8− T cells from PBMCs transduced with the CD4-MBL CAR was 2.6-fold higher (median, 22,256) than PBMCs transduced with the 139 CAR retrovirus (median, 8,544) as shown in a representative example (Fig. 1B).
FIG 1.
CAR expression and function in transduced PBMCs. (A) Schematic diagram of expression constructs of the 139 and CD4-MBL CARs. (B) Top, representative flow cytometry plot of CD4 and CD8 expression 7 days after CAR transduction of PBMCs after gating on single-cell lymphocytes. Bottom, histogram illustrates overlay comparison of CD4 expression on the CD4+CD8− population. (C) Representative flow diagrams of 6-h activation assay depicting IFN-γ and CD107a expression in CD8+ population of transduced 139 and CD4-MBL CAR-T cells cocultured at a 1:1 ratio alone or with BJAB (Env−) or TF228 (Env+) cells. (D) Percent lysis, as determined by CFSE release assay, of 2 × 104 BJAB or TF228 target cells cultured 4 h with control 139 or CD4-MBL CAR-T cells at the indicated effector:target ratio. Data shown are 1 of 3 independent experiments run in duplicate.
We next determined the activation state of the CAR-T cells alone or in the presence of TF228 (Env+) or BJAB (Env−) target cells using CD107a and interferon gamma (IFN-γ) expression (Fig. 1C). A median of 70% (range, 68% to 82%) CD4-MBL CAR-T cells expressed CD107a and a median of 20% (range, 19% to 35%) CD4-MBL CAR-T cells were CD107a+ IFN-γ+ when cultured with Env− expressing TF228. In contrast, very low levels of CD107a+ or CD107a+IFN-γ+ cells were observed when CD4-MBL CAR-T cells were cultured alone (median, 3.1% CD107a+) or with Env− BJAB (median, 6.5% CD107a+). As expected, CD107a expression was minimal in 139-CAR-T cells cultured alone (median, 3.2%) or with either BJAB (median, 4.3% CD107a+) or TF228 cells (median, 1.7% CD107a+).
We then measured lysis of target cells after a 4-hour coculture with CAR-transduced cells from 4 different donors (Fig. 1D). At a 3:1 effector: target ratio, CD4-MBL CAR-T cells lysed TF228 cells (20% to 56% lysis), whereas BJAB cells were relatively spared (7.4% to 7.6% lysis). In contrast, 139 CAR-T cells generated from the same donors mediated only low levels of killing of TF228 (1.5% to 5.1% lysis) and BJAB cells (2.0% to 8.1% lysis). These data demonstrated the specificity of the CD4-based CAR-T cells and their cytotoxicity toward Env-expressing cells.
FDC bind infectious HIV ex vivo.
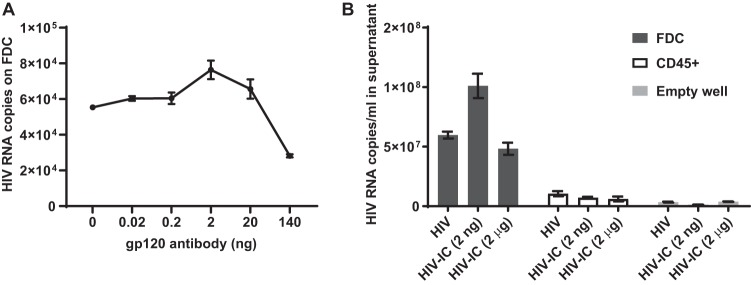
Because FDCs trap HIV as immune complexes (ICs) (4), we first optimized in vitro HIV trapping using a nonneutralizing monoclonal gp120-specific antibody, Chessie13, over a range of 0.02 ng to 140 ng and 1 × 108 viral RNA copies of HIVIIIB viral stock, as described in the Materials and Methods. HIV-ICs were added to 2 × 104 FDCs and cultured for 1 h prior to quantitation of trapped HIV (Fig. 2A). Increasing quantities of anti-gp120 up to 2 ng enhanced HIV binding to FDC, whereas higher concentrations decreased binding. We determined that binding of HIV to FDC was optimal with 1 × 108 viral RNA copies and 2 ng of antibody (Fig. 2A).
FIG 2.
Formation of infectious HIV-ICs on FDCs. (A) HIV-IC binding to FDCs is dependent on gp120 antibody concentration. HIV-ICs were preformed using 1 × 108 viral RNA copies and a range of nonneutralizing gp120 antibody and added to 2 × 104 FDCs isolated from tonsils. Cells were washed twice and lysed. Viral RNA was purified and quantified using RT-qPCR. The mean and standard error of the mean (SEM) of triplicate samples from a representative of 3 experiments is shown. (B) HIV bound to FDCs is infectious. HIV or HIV-ICs prepared with 1 × 108 viral RNA copies and 2 ng or 2 μg of gp120 antibody in 60 μl were added to irradiated FDCs, CD45+ tonsil cells, or empty wells. Samples were washed twice and cultured with H9 cells for 3 days. Viral RNA was purified and quantified from the cell culture supernatant using RT-qPCR. The mean and SEM from one of two independent experiments in triplicate are shown.
We then established whether optimal virus trapping correlated with increased rescue of infectious virus from FDCs or controls. FDCs or control cells were irradiated to prevent HIV replication from any permissive tonsil cells and were loaded with HIV-ICs using two antibody concentrations. A viral rescue assay was performed as described in the Materials and Methods (Fig. 2B). Nearly a 6-fold increase in viral production was detected when cultured with FDCs compared with tonsillar CD45+ cells. Viral production from H9 cells cultured with FDCs and optimal HIV-ICs was 1.6- and 2-fold higher than cultures with no antibody and 2 μg of gp120 antibody, respectively. In contrast, the addition of the gp120 antibody to cocultures with CD45+ cells did not increase viral production. Based on these studies, all other experiments containing HIV-ICs were formed using 2 ng of the gp120 antibody and 1 × 108 viral RNA copies of HIVIIIB viral stock.
CD4-MBL CAR-T cells do not lyse cells bearing HIV-ICs.
We next determined whether CD4-MBL CAR-T cells could lyse FDCs bearing HIV-ICs. FDCs were incubated alone, with HIV, HIV-IC, or an irrelevant IC consisting of ovalbumin (OVA) anti-OVA. After target cells were washed to remove unbound ICs or free virus, they were cultured with CD4-MBL CAR-T cells at the effector to target (E:T) ratios indicated (Fig. 3A). Cell lysis above background Env− BJAB cells was only detected when CD4-MBL CAR-T cells were incubated with Env+ TF228 cells. Surprisingly, CD4-MBL CAR-T cells did not kill FDCs bearing HIV or HIV-IC, although FDCs bound >2 × 105 viral RNA copies in the form of HIV-IC (Fig. 3B). To determine whether CD4-MBL CAR-T cells were unresponsive to HIV-ICs, we repeated this assay using BJAB or TF228 cells in the presence of free HIV or HIV-ICs. These cells were selected because BJAB and TF228 cells express CD32, an important receptor used by FDCs to trap HIV-ICs, and are not resistant to CAR-mediated killing. CD4-MBL CAR-T cells exhibited low levels of lysis of BJAB cells in the absence (lysis mean, 7.5%; standard error of the mean [SEM], 2.1%) or presence of HIV (lysis mean, 5.6%; SEM, 2.0%) or HIV-IC (lysis mean, 3.5%; SEM, 1.0%). The results also demonstrate that the absence of killing was not due to an inhibitory effect of HIV-ICs on CAR-mediated killing since HIV-ICs did not affect the lysis of Env-expressing TF228 (lysis mean of 56.4% and SEM of 2.1% for TF228 alone compared to lysis mean of 52.7% and SEM of 3.6% for TF228 with HIV-IC) (Fig. 3C).
FIG 3.
CD4-MBL CAR-T cells do not lyse cells bearing HIV-ICs in a 4-hour CFSE release assay. (A) Percent lysis of TF228 (Env+), BJAB (Env−), and FDCs alone, or bearing HIV, HIV-ICs, or an irrelevant OVA-IC. Unbound ICs were washed twice prior to the addition of CD4-MBL CAR-T cells at the indicated E:T ratios. The graph is a representative of 3 independent experiments performed in triplicate. (B) Corresponding HIV RNA quantitation from indicated cell pellets shown in (A). (C) Percent lysis of TF228 or BJAB cells cultured at the indicated E:T ratios with CD4-MBL CAR-T cells. Target cells were incubated alone or with HIV or HIV-ICs. Unbound virus was either washed from targets or kept in culture upon the addition of effector cells and for the duration of the CFSE release assay. Graph shows one of two independent experiments run in triplicate. (D) gp120 antibody does not inhibit killing of TF228 (Env+) by CD4-MBL CAR-T cells. TF228 were incubated alone or with 10 μg of gp120 antibody and cultured with CD4-MBL CAR-T cells at the indicated ratios. Percent lysis was calculated after a 4-hour CFSE release assay. Graph shows one of two independent experiments in duplicate.
Because CD4-MBL CAR-T cells did not lyse cells bearing HIV-ICs, we investigated whether the antibody used to form ICs blocked CAR recognition of the virus. We incubated TF228 cells with excess gp120 antibody prior to and during a CFSE release assay with CD4-MBL CAR-T cells. There was no evidence of inhibition of CAR-mediated cell lysis in cells treated with excess gp120 antibody compared with controls (Fig. 3D).
CD4-MBL CAR-T cells are unresponsive to cell-free virus.
Because FDCs are neither permissive to HIV infection (17) nor express gp120 on their surface as do infected cells, we sought to determine whether HIV or HIV-ICs were sufficient to induce CD4-MBL CAR-T cell activation, as assessed by CD107a and IFN-γ accumulation. HIV or HIV-ICs representing ∼1 × 108 viral RNA copies were added to CD4-MBL CAR-T cells in the presence or absence of BJABs or TF228 cells. CD4-MBL CAR-T cells were not activated in the presence of HIV (median, 0.4% CD107a+IFN-γ+) or HIV-IC (median, 0.5% CD107a+IFNγ+). As a positive control, CAR activation occurred in the presence of membrane-expressed gp160 on TF228 (median, 20.6% CD107a+IFN-γ+) irrespective of the presence of HIV (median, 24.1% CD107a+IFN-γ+) or HIV-IC (median, 23.2% CD107a+IFN-γ+) (Fig. 4A).
FIG 4.
Cell-free HIV and HIV-ICs are both unable to activate CD4-MBL CAR T cells. (A) Flow cytometry plots showing the accumulation of IFN-γ and CD107a in CD4-MBL CAR-T cells cultured alone, with BJAB (Env−), or with TF228 (Env+) in the presence or absence of HIV or HIV-ICs. Target cells were incubated with HIV or HIV-ICs, and effector cells were added at indicated ratios in the presence of brefeldin A, monensin, and CD107a antibody for 6 h. Cells were labeled with CD4 and CD8 antibody, fixed, permeablized, and labeled with IFN-γ antibody. Flow plots are of CD4+CD8+ single cells and are representative plots of one of two experiments performed in triplicate. (B) Beads bind HIV-ICs but not HIV alone. Magnetic beads were incubated with gp120 antibody or alone and washed to remove unbound antibody. Beads were incubated alone or with HIV and washed to remove unbound virus. Western blot was used to detect p24 from proteins extracted from beads. (C) CD4-MBL CAR activation as assessed by the accumulation of IFN-γ and CD107a as described in (A) incubated alone or in the presence of beads or TF228 in the absence or presence of HIV, HIV-ICs, or CD4 antibody.
After several failed attempts to activate CD4-MBL CAR-T cells in the presence of HIV-ICs bound to FDCs and BJABs, we postulated that insufficient quantities of HIV-ICs were bound to the cell surface to elicit a CAR response. To test this hypothesis, we immobilized HIV-ICs onto beads to determine whether CAR-T cells could respond to immobilized HIV-ICs. Using 4.5-μm magnetic beads, we bound HIV in the presence of gp120 antibody, as determined by Western blot (Fig. 4B). However, CD4-MBL CAR-T cells in the presence of bead-immobilized HIV induced minimal accumulation of CD107a and IFN-γ (median, 1.0% CD107a+IFN-γ+) (Fig. 4C). As a control, we bound CD4 antibody to the beads to test whether targeting the CAR directly would lead to CAR activation (Fig. 4C). Although both immobilized HIV-ICs and free virus were unable to induce CAR activation (median, 1.0% and 1.0% CD107a+IFN-γ+), immobilized anti-CD4-coated beads induced potent CD107a and IFN-γ responses in CD4+CD8+ CD4-MBL CAR-T cells. Remarkably, soluble anti-CD4 antibody induced IFN-γ but not CD107a accumulation (Fig. 4C).
ICAM-1 is required for CD4-MBL CAR-T cell activation.
Because concentrated, immobilized HIV was unable to induce CAR activation, we reasoned that additional interactions at the cell interface were required for CD4-MBL CAR-T cell activation and killing of target cells. Because immune cells, including FDCs, form immunological synapses that stabilize cell:cell interactions, we reasoned that adhesion molecules essential to immune synapse stabilization might positively affect CAR activation. We, therefore, tested whether ICAM-1 and/or LFA-1 was necessary for CD4-MBL CAR-T cell activation and killing (Fig. 5A). Culturing TF228 cells with CAR-T cells in the presence of LFA-1-specific blocking antibody had no effect on TF228 cell lysis, while an ICAM-1-specific blocking antibody reduced TF228 lysis by 50%. We next evaluated the role of ICAM-1 in CD4-MBL CAR-T cell response to autologous HIV-infected CD4+ T cells (Fig. 5B). Again, the addition of LFA-1 blocking antibody had no effect, while ICAM-1-specific antibody reduced CAR-T cell dual expression of CD107a and IFN-γ by 75%.
FIG 5.
Blocking ICAM-1 inhibits CD4-MBL CAR activity. (A) Percent lysis of TF228 in 4-h CFSE release assay in the presence of 10-μg irrelevant antibody or blocking antibody to ICAM-1, LFA-1, or both. Both target and effector cells were incubated with an antibody 30 minutes prior to combination at indicated E:T ratios. Median and range of a representative experiment performed in triplicate are shown. (B) CD4-MBL CAR T cells were incubated alone or with uninfected or HIV-infected autologous CD4+ T cells at 1:1 ratios for 6 h in the presence of brefeldin A, monensin, and CD107a antibody. Cells were labeled with CD4 and CD8 antibody, fixed, and permeabilized for intracellular staining of IFN-γ. Flow cytometry diagrams show IFN-γ and CD107a expression in CD4+CD8+ single cells in a representative of one of three independent experiments.
DISCUSSION
The FDC reservoir of HIV is an important but understudied source of replication-competent virus that presents a significant challenge to eradicating HIV infection. HIV on FDCs is displayed in a manner that is markedly different than CD4+ T cell reservoirs, where newly formed virus buds from the cell membrane and viral peptide is presented by MHC molecules. HIV-specific CAR-T cells are designed to recognize HIV-1 gp120 and theoretically should be able to bind to HIV on FDC. Ours is the first study to evaluate the ability of an HIV-specific CAR-T cell to recognize and kill FDCs that harbor cell-surface HIV. We confirmed that HIV-specific CAR-T cells recognize and lyse cell lines that express cell surface gp160, as well as CD4+ T cells that express HIV. Nevertheless, CD4-MBL CAR-T cells did not recognize nor lyse FDCs that bound HIV, although FDC-bound HIV was highly infectious and facilitated infection of bystander CD4+ T cells. Furthermore, neither soluble nor bead-bound HIV or HIV-IC was recognized by CAR-T cells. CAR-T cell recognition of target cells was significantly impaired by ICAM-1 blockade, suggesting that accessory interactions are key to stabilizing contact between the CAR-T cell and target cells. Collectively, these data suggest that CD4-MBL CAR-T cell therapy is unlikely to directly reduce the FDC reservoir of HIV.
We confirmed that FDCs trap both IC-free virus as well as HIV-ICs in vitro. IC-free HIV on FDCs has been shown to be more labile than HIV-ICs in vitro (6) and may be less suitable for long-term preservation on FDCs, although its presence cannot be ruled out in vivo. It is important to note that our experiments were performed immediately after HIV or HIV-IC binding to FDCs, thus allowing CD4-MBL CAR-T cells to be exposed to FDCs bearing both IC-free HIV and HIV-ICs.
It is known that FDC-associated HIV decays during ART (9, 18). In these studies, both FDC-associated virus and productively infected mononuclear cells appeared to decay in parallel, indicating intricate interactions between the two. Whether the virus on FDCs was preserved during ART, and/or replenished by newly replicated virus, is unknown (9). FDCs isolated from HIV-positive patients on and off ART were able to transmit infection to CD4+ T cells ex vivo (4, 19). In a nonpermissive murine model, infectious virus was readily recovered from FDCs 9 months after passive immunization and HIV challenge (5). The contribution of the FDC-bound HIV reservoir to HIV replication in lymphoid tissues during ART or to rapid rebound following ART interruption remains to be determined.
We determined the effect of antibody concentrations on HIV binding to FDCs. In our experiments, 2 ng of antibody incubated with viral supernatant containing 1 × 108 viral RNA copies was optimal for HIV-IC formation. Assuming 72 Env spikes per virus particle (20) and 2 copies of viral RNA per particle (5 × 107 virus particles), 2 ng of antibody resulted in a 1:1.5 ratio of antibody recognition domains to Env binding sites. Interestingly, binding of HIV to FDCs decreased in the presence of excess gp120 antibody. Saturation of Fc receptors on FDCs, limited bivalent antibody binding to HIV, and prevention of HIV-IC cluster formation are all plausible mechanisms for this decrease and support the hypothesis that HIV maintenance on FDCs is facilitated by multipoint attachment (21).
In contrast to limited CAR responses to soluble and immobilized HIV, CAR-T cells were activated in the presence of soluble and immobilized CD4 antibody. Interestingly, soluble CD4 antibody was able to induce IFN-γ expression but minimal CD107a expression, while immobilized CD4 induced the expression of both. The antibody clone used in our experiments was Leu3a and was calculated in one report to bind to CD4 with a dissociation constant (Kd) of 1.8 × 10−10 (22). This clone was previously shown to prevent gp120 binding to CD4, implying it had a higher affinity for CD4 than gp120 (23). Although the affinity of the CD4-MBL CAR for gp120 has not been determined, we postulate that the affinity is below the threshold limit necessary for CD4-MBL CAR signaling, thereby rendering HIV alone insufficient for CD4-MBL CAR-mediated signaling and cytotoxicity.
Recent reports suggest that CAR-T cells can form either CTL-like immune synapses (24) or nonclassical immune synapses (25) upon antigen recognition. Similar to findings by Davenport et al. (25), blocking LFA-1 did not affect CAR-T cell-mediated cytotoxicity in our experiments. However, ICAM-1, an integrin involved in cell adhesion and immune synapse stabilization (26), was essential for CAR-T cell activity; to our knowledge this is the first report of the requirement of ICAM-1 for CAR-T cell activation. Whether an immune synapse was required for CAR-T cell activation was not determined in our experiments, although it seems plausible based upon the known role of ICAM-1 in the formation of immune synapses and the prior studies of CAR-T cells noted above. Although FDCs express ICAM-1 (27–29), it is unclear whether CAR-T cells are capable of forming immune synapses with FDCs bearing HIV-ICs. In contrast to infected cells that bud virus from the plasma membrane, whole virus particles are held on the surface of the FDC. The intact virus particle, roughly 120 to 140 nm in diameter, bound to an antibody of approximately 10 nm in size, is spatially separated from the FDC cell surface where ICAM-1 and other molecules critical for synapse formation and stabilization are located. Thus, we postulate that gp120 on the virus and ICAM-1 receptors on FDCs are likely incapable of an arrangement that resembles an immune synapse necessary for CTL signaling and sustained contact (30) and that this accounts for the failure of CD4-MBL CAR-T cells to recognize and eliminate HIV-ICs on FDCs.
There are several limitations to our studies. The major limitation is that this work was performed in vitro with FDCs isolated from uninfected tonsils. HIV-ICs used in this study were devoid of complement or other factors described to assist binding of HIV to FDCs (19, 31–33). Relative concentrations of gp160 on Env-expressing cell lines, infected CD4+ T cells, magnetic bead-bound HIV, and FDC-bound HIV were not compared and may have impacted responses by CD4-MBL CAR-T cells. Whether these contributions enhance HIV stabilization on FDCs and influence CAR-T cell-mediated killing remain to be determined. Additionally, FDCs reside in an activated environment, and it is unknown whether dynamics within germinal centers might affect CAR-T cell activity in vivo. Ultimately, studies of HIV-specific CAR-T cells in vivo would be necessary to corroborate whether they are unable to eliminate the FDC reservoir. It would be essential for these studies to be performed in the absence of active replication in CD4+ T cells, as the production of virus particles by these cells is in direct equilibrium with the FDC reservoir (9).
CAR-T cells are a promising therapy to achieve sustained HIV remission, given their long-term persistence in vivo (34, 35), which should render them capable of providing a response as latently infected CD4+ T cells activate HIV expression. Nevertheless, the inability of the CD4-MBL CAR-T cells to reduce HIV bound to FDCs in vitro suggests that other strategies must be sought to eliminate the FDC reservoir. Designs that improve CAR affinity and reactivity to native virus could be therapeutically detrimental. Detectable levels of plasma viral loads could result in cytokine release syndrome, a common and potentially lethal symptom of CAR activation in recipients with cancer (36). Furthermore, it is not clear that enhanced CAR affinity for native virus would improve the development of an immune synapse. Thus, other strategies in conjunction with CAR therapy should be considered. Alternative strategies to eliminate the viral reservoir on FDCs have been proposed, including CD21 decoy receptor, HIV-targeting immunotoxins, and rituximab (19, 37). The only strategy shown by immunohistochemistry to deplete simian immunodeficiency virus (SIV) within germinal centers of lymphoid tissue was administration of rituximab (38). Whether rituximab treatment eliminated FDCs or reduced HIV trapping below the limit of detection on FDCs was not evaluated. Strategies that deplete FDCs or remove HIV from FDCs, in combination with CAR-T cell therapy, may lead to novel approaches to treat and cure HIV-1 infection.
MATERIALS AND METHODS
Studies using human cells and tissues.
Studies using blood or tonsil cells obtained from humans were reviewed and approved by Brigham Young University’s institutional review board. Informed consent was received from all donors from whom peripheral blood was obtained. The use of tonsils removed from patients undergoing routine tonsillectomy was deemed exempt as per number 4.
Cell lines and virus stock.
The Env-expressing B cell line TF228 (39) was obtained from the NIH AIDS Reagent Program. BJAB, the Env-deficient parental B cell line to TF228, was a gift from Bradford Berges, Brigham Young University (BYU), Provo, UT. Cells were cultured in 10% complete medium (CM) consisting of RPMI 1640 (Gibco) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals), 1× glutamax (ThermoFisher Scientific), 1 mM HEPES (HyClone), and 50 μg/ml gentamicin (Lonza).
HIVIIIB (40, 41) and the H9 cell line (40, 42, 43) were both obtained from Robert Gallo through the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID). HIVIIIB stock was prepared by infecting H9 cells for 2 h with viral supernatant at 37°C, after which the cells were washed in phosphate-buffered saline (PBS) to remove infecting inoculum and were cultured at 2 × 105 cells/ml in 20% CM. Posttinfection, the viral supernatant was collected on days 9 and 12 by centrifugation at 300 × g for 10 min, filtered through a 0.45-μm syringe filter, and stored in 1.8-ml aliquots in liquid nitrogen. The reverse transcriptase mRNA transcript was used to quantify viral stocks via reverse transcription-quantitative PCR (RT-qPCR) (44).
gp120 monoclonal antibody purification from hybridoma culture.
Anti-HIV-1 gp120 hybridoma (Chessie 13-39.1) from George Lewis (45) was obtained through the NIH AIDS Reagent Program. FBS used for hybridoma culture was depleted of immunoglobulin via passage through a 1-ml Hi-Trap protein G column (GE Healthcare). Cells were grown in T175 flasks (Corning) at an initial density of 2 × 105 cells/ml in ∼100 ml 10% CM for 2 to 3 weeks. Cell-free supernatant was collected, filtered through a 0.22-μm cellulose acetate membrane (ThermoFisher), and added to a 1-ml HiTrap protein G column at a flow rate of 1 ml/min. The column was washed with 10 ml PBS, and antibody was eluted with 0.1 M glycine (pH 2.7). Collection tubes containing 60 μl 1 M Tris-HCl (pH 9.5) were used to collect a total volume of 1 ml of eluent. Fractions were analyzed via SDS-PAGE and bicinchoninic acid (BCA) assays for protein purity and concentration, respectively.
HIV immune complex formation with gp120 monoclonal antibody.
A total of 50 μl of viral supernatant, containing 1 × 108 viral RNA copies, was thawed and incubated with nonneutralizing gp120 antibody at various concentrations. Immune complexes were incubated at 4°C for 1 h, then added to 2 × 104 nonpermissive target cells (e.g., FDCs and BJABs) or sheep anti-rat Dynabeads (ThermoFisher), and incubated for 45 minutes at room temperature. Following binding, the cells or beads were washed in phosphate-buffered saline (PBS) twice to remove unbound HIV and were used for subsequent experiments.
For quantification of bound HIV-ICs, samples were washed in PBS, and viral RNA was isolated using the QIAamp viral RNA isolation kit (Qiagen). cDNA was synthesized from an equal volume of purified viral RNA using Superscript IV (ThermoFisher) according to manufacturer’s instructions and quantified using qPCR as described previously (44).
HIV infection.
A total of 1 × 108 HIVIIIB copies were used to infect target cells. Target cells were washed in PBS and suspended in thawed HIVIIIB stock. Cells were incubated in 12- by 75-mm fluorescence-activated cell sorter (FACS) tubes at 37°C for 2 h, washed twice in PBS, and suspended in CM.
Isolation of follicular dendritic cells from tonsils.
FDCs were isolated as described (5, 46) with minor modifications. Tonsils were cut into 2-mm pieces in 5 ml of tonsil buffer (RPMI 1640, 50 μg/ml gentamicin, and 10 mM HEPES). One Wünsch unit of Liberase TM (Roche) and 175 IU of DNase I (Sigma) were added and incubated in a 37°C water bath for 45 to 60 minutes with occasional mixing. Tissue-free cells were collected, and remaining tissue was subjected to an additional digestion. Remaining tissue was triturated gently with a 25-ml and then a 10-ml serological pipette. The tissue-free cells were passed through a 100-μm nylon filter into complete medium and centrifuged for 6 minutes at 350 × g. The cell pellet was suspended in complete medium, layered on top of a 25% to 43% discontinuous Percoll gradient, and centrifuged at 2,000 × g for 30 minutes at room temperature. Cells were collected at the 25% to 43% interface, washed in complete medium to remove the Percoll solution, and suspended in 3 ml of CM containing 20 μg unlabeled mouse IgM (Southern Biotech) and 6 μg of FDC-specific biotin-CNA.42 antibody (ThermoFisher Scientific). Samples were incubated at 4°C for at least 2 h to overnight, washed in PBS, and suspended in 100 μl FACS buffer (2% FBS in PBS) containing APC-streptavidin (Jackson Immunoresearch) and phycoerythrin (PE)-anti-CD45 (Beckman Coulter). Samples were incubated for 45 minutes at 4°C, washed in FACS buffer, filtered through a 30-μm filter-capped tube (Corning), and labeled with 7-aminoactinomycin D (7-AAD) prior to sorting. FDC (7-AAD−CD45−CNA.42+) and CD45+ cells (7-AAD−CD45+CNA.42−) were sorted on the BD FACSAria Fusion instrument. Sorted cells were washed and irradiated at 12 Gy and used for further experiments.
Rescue infection assay.
H9 cells were added at a 1:10 ratio to irradiated FDCs or CD45+ cells bearing HIV or HIV-ICs, as described above, and cultured for 3 days in CM. The supernatant was collected, and viral RNA was isolated using the QIAamp viral RNA isolation kit (Qiagen). Viral RNA was reverse transcribed using Superscript IV (Invitrogen), and the reverse transcriptase transcript was quantified using qPCR as described previously (44).
CAR-T cell production.
CAR retrovirus was prepared as described previously (47) using plasmids characterized by Ghanem et al. (13). The transduction of PBMCs isolated from buffy coats of fresh heparinized blood was prepared as described to produce HIV-specific CAR-T cells (CD4-MBL CAR) and the control CAR-T cells (139 CAR) (10).
CFSE release assay.
Target cells used in cytotoxicity assays were labeled with carboxyfluorescein succinimidyl ester (CFSE) (ThermoFisher) according to specifications recommended by the manufacturer. Cells were washed twice and suspended in CFSE buffer (PBS supplemented with 10% FBS, 1× glutamax, 1 mM HEPES, 1× nonessential amino acids, and 1× sodium pyruvate). Target cells, as indicated in each experiment, were added to a 96-well round-bottom plate. For spontaneous release of CFSE, target cells were placed in wells without effector cells and the final volume adjusted to 200 μl. For 100% lysed controls, wells were prepared as described for the spontaneous release samples with the addition of 100 μg/ml digitonin.
Effector cells were added at the ratios indicated in each experiment. After the addition of effectors, the final volume in each well was adjusted to 200 μl. The plates were centrifuged at 400 × g for 2 minutes and placed in a 37°C incubator. After 4 h, plates were centrifuged at 325 × g for 5 minutes and 100 μl of the cell-free supernatant was transferred into a 96-well black plate and read on a Synergy high-throughput (HT) plate reader using the fluorescent settings with the filters 485/20 for excitation and 528/20 for emission. Percent lysis was quantified with the following equation: (sample – spontaneous release)/(100% lysed – spontaneous release) × 100.
Western blot.
To verify HIV-IC binding to magnetic Dynabeads (ThermoFisher), samples were diluted in 4× loading buffer (50 mM Tris-HCl [pH 6.8], 4% SDS, 4% [vol/vol] β-mercaptoethanol, 40% glycerol, and bromophenol blue), heated to 95°C for 10 minutes, and run on a Tris-tricine SDS-PAGE. The protein was transferred to a nitrocellulose membrane (Bio-Rad) using a Mini Trans-Blot cell (Bio-Rad) in cold transfer buffer at 20 V for 10 minutes, followed by 100 V for 60 minutes.
The membrane was probed for p24 (183-H12-5C, prepared in-house) and detected using IRDye800CW-donkey anti-mouse (Li-Cor) at a concentration of 1:10,000 for 1 h at room temperature. After washing the membrane twice in Tris-buffered saline with Tween 20 (TBST), the blot was imaged on an Odyssey Li-Cor scanner on both the 700- and 800-nm channel to detect the prestained molecular weight ladder and target protein, respectively.
Flow cytometry analysis.
Cells were suspended in 100 μl PBS containing 10 μg Chrompure IgG (Jackson ImmunoResearch) to block irrelevant IgG binding. After 10 minutes, primary antibody was added and incubated at 4°C for 1 h. Samples were washed in 3 ml PBS, suspended in 300 μl PBS, and analyzed on BD FACSAria Fusion instrument. At least 10,000 events were recorded per sample.
CAR-T cell activation assay.
Equal concentrations of target and effector cells were added for each sample in FACS tubes in a total volume of 200 μl of assay media (10% HI human serum, 1× glutamax, 1× NEAA, and 10 mM HEPES, in PBS). A total of 100 μl of assay medium, supplemented with 3× monensin (ThermoFisher Scientific), 3× brefeldin A (Sigma), and 2 μl of APC-anti-CD107a (Biolegend) was added. Cells were incubated for 6 h at 37°C, washed, and incubated for 20 min at room temperature (RT) with 100 μl PBS containing APCFire-750-anti-CD4 (Biolegend), FITC-anti-CD8 (Biolegend), and 10 μg Chrompure mouse IgG (Jackson Immunoresearch). The samples were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) according to the manufacturer’s instructions. The cells were suspended in 100 μl perm/wash buffer with PE-anti-IFN-γ (Biolegend), incubated 20 minutes at RT, washed in perm/wash buffer, suspended in PBS, and analyzed on a BD FACSAria Fusion instrument. Single cells gated on CD4+CD8+ were analyzed for CD107a and IFN-γ expression.
ACKNOWLEDGMENTS
This work was funded by an NIH/NIAID award (UM1AI126617) to the Martin Delaney BELIEVE Collaboratory to E.A.B., E.C., and G.F.B., as well as a fellowship from the Brigham Young University to M.T.O.
We declare no conflicts of interest and that funding sources had no role in the experimental design, data collection, interpretation of data, or decision to submit the manuscript for publication.
REFERENCES
- 1.Eisele E, Siliciano RF. 2012. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sengupta S, Siliciano RF. 2018. Targeting the latent reservoir for HIV-1. Immunity 48:872–895. doi: 10.1016/j.immuni.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz J, van Lunzen J, Tenner-Racz K, Grossschupff G, Racz P, Schmitz H, Dietrich M, Hufert FT. 1994. Follicular dendritic cells retain HIV-1 particles on their plasma membrane, but are not productively infected in asymptomatic patients with follicular hyperplasia. J Immunol 153:1352–1359. [PubMed] [Google Scholar]
- 4.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, Thacker TC, Crandall KA, McArthur JC, Burton GF. 2008. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol 82:5548–5561. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, Burton GF. 2001. Persistence of infectious HIV on follicular dendritic cells. J Immunol 166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Franklin BA, Keele BF, Tew JG, Gartner S, Szakal AK, Estes JD, Thacker TC, Burton GF. 2002. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcgamma receptors. J Immunol 168:2408–2414. doi: 10.4049/jimmunol.168.5.2408. [DOI] [PubMed] [Google Scholar]
- 7.Heath SL, Tew JG, Tew JG, Szakal AK, Burton GF. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 8.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, Dailey PJ, Balfour HH, Erice A, Perelson AS. 1996. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 9.Cavert W, Notermans DW, Staskus K, Wietgrefe SW, Zupancic M, Gebhard K, Henry K, Zhang ZQ, Mills R, McDade H, Schuwirth CM, Goudsmit J, Danner SA, Haase AT. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Patel B, Ghanem MH, Bundoc V, Zheng Z, Morgan RA, Rosenberg SA, Dey B, Berger EA. 2015. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J Virol 89:6685–6694. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibman RS, Richardson MW, Ellebrecht CT, Maldini CR, Glover JA, Secreto AJ, Kulikovskaya I, Lacey SF, Akkina SR, Yi Y, Shaheen F, Wang J, Dufendach KA, Holmes MC, Collman RG, Payne AS, Riley JL. 2017. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog 13:e1006613. doi: 10.1371/journal.ppat.1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony-Gonda K, Bardhi A, Ray A, Flerin N, Li M, Chen W, Ochsenbauer C, Kappes JC, Krueger W, Worden A, Schneider D, Zhu Z, Orentas R, Dimitrov DS, Goldstein H, Dropulić B. 2019. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci Transl Med 11:eaav5685. doi: 10.1126/scitranslmed.aav5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanem MH, Bolivar-Wagers S, Dey B, Hajduczki A, Vargas-Inchaustegui DA, Danielson DT, Bundoc V, Liu L, Berger EA. 2018. Bispecific chimeric antigen receptors targeting the CD4 binding site and high-mannose Glycans of gp120 optimized for anti–human immunodeficiency virus potency and breadth with minimal immunogenicity. Cytotherapy 20:407–419. doi: 10.1016/j.jcyt.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Zou F, Lu L, Chen C, He D, Zhang X, Tang X, Liu C, Li L, Zhang H. 2016. Chimeric antigen receptor t cells guided by the single-chain fv of a broadly neutralizing antibody specifically and effectively eradicate virus reactivated from latency in CD4+ T lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretrovial therapy. J Virol 90:9712–9724. doi: 10.1128/JVI.00852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen IS, Yang OO, Zack JA, Kitchen SG. 2015. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther 23:1358–1367. doi: 10.1038/mt.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. 1994. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 84:2878–2889. doi: 10.1182/blood.V84.9.2878.2878. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda R, Hashimoto K, Baba M, Shigeta S, Sugai N. 1996. Follicular dendritic cells in vitro are not susceptible to infection by HIV-1. AIDS 10:595–602. doi: 10.1097/00002030-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. 2014. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heesters BA, Lindqvist M, Vagefi PA, Scully EP, Schildberg FA, Altfeld M, Walker BD, Kaufmann DE, Carroll MC. 2015. Follicular dendritic cells retain infectious HIV in cycling endosomes. PLoS Pathog 11:e1005285. doi: 10.1371/journal.ppat.1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelderblom HR, Hausmann EH, Ozel M, Pauli G, Koch MA. 1987. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology 156:171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 21.Burton GF, Keele BF, Estes JD, Thacker TC, Gartner S. 2002. Follicular dendritic cell contributions to HIV pathogenesis. Semin Immunol 14:275–284. doi: 10.1016/s1044-5323(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Williams PS, Zborowski M, Chalmers JJ. 2006. Binding affinities/avidities of antibody-antigen interactions: quantification and scale-up implications. Biotechnol Bioeng 95:812–829. doi: 10.1002/bit.21024. [DOI] [PubMed] [Google Scholar]
- 23.Wilks D, Walker L, O'Brien J, Habeshaw J, Dalgleish A. 1990. Differences in affinity of anti-CD4 monoclonal antibodies predict their effects on syncytium induction by human immunodeficiency virus. Immunology 71:10–15. [PMC free article] [PubMed] [Google Scholar]
- 24.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, Payne AS. 2016. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davenport AJ, Cross RS, Watson KA, Liao Y, Shi W, Prince HM, Beavis PA, Trapani JA, Kershaw MH, Ritchie DS, Darcy PK, Neeson PJ, Jenkins MR. 2018. Chimeric antigen receptor T cells form non-classical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci U S A 115:E2068–E2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 27.Maeda K, Kosco-Vilbois MH, Burton GF, Szakal AK, Tew JG. 1995. Expression of the intercellular adhesion molecule-1 on high endothelial venules and on non-lymphoid antigen handling cells: interdigitating cells, antigen transporting cells and follicular dendritic cells. Cell Tissue Res 279:47–54. doi: 10.1007/bf00300690. [DOI] [PubMed] [Google Scholar]
- 28.El Shikh ME, El Sayed R, Szakal AK, Tew JG. 2006. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur J Immunol 36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]
- 29.Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. 1991. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med 173:1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg NN, Ostergaard HL. 1995. Characterization of intercellular adhesion molecule-1 (ICAM-1)-augmented degranulation by cytotoxic T cells. ICAM-1 and anti-CD3 must be co-localized for optimal adhesion and stimulation. J Immunol 155:1694–1702. [PubMed] [Google Scholar]
- 31.Fujiwara M, Tsunoda R, Shigeta S, Yokota T, Baba M. 1999. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J Virol 73:3603–3607. doi: 10.1128/JVI.73.5.3603-3607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kacani L, Prodinger WM, Sprinzl GM, Schwendinger MG, Spruth M, Stoiber H, Dopper S, Steinhuber S, Steindl F, Dierich MP. 2000. Detachment of human immunodeficiency virus type 1 from germinal centers by blocking complement receptor type 2. J Virol 74:7997–8002. doi: 10.1128/jvi.74.17.7997-8002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho J, Moir S, Kulik L, Malaspina A, Donoghue ET, Miller NJ, Wang W, Chun TW, Fauci AS, Holers VM. 2007. Role for CD21 in the establishment of an extracellular HIV reservoir in lymphoid tissues. J Immunol 178:6968–6974. doi: 10.4049/jimmunol.178.11.6968. [DOI] [PubMed] [Google Scholar]
- 34.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH. 2015. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. 2012. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey N, Porter D. 2019. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant 25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 37.Bronnimann MP, Skinner PJ, Connick E. 2018. The B-cell follicle in HIV infection: barrier to a cure. Front Immunol 9:20–13. doi: 10.3389/fimmu.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaufin T, Gautam R, Kasheta M, Ribeiro R, Ribka E, Barnes M, Pattison M, Tatum C, MacFarland J, Montefiori D, Kaur A, Pandrea I, Apetrei C. 2009. Limited ability of humoral immune responses in control of viremia during infection with SIVsmmD215 strain. Blood 113:4250–4261. doi: 10.1182/blood-2008-09-177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonak ZL, Clark RK, Matour D, Trulli S, Craig R, Henri E, Lee EV, Greig R, Debouck C. 1993. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res Hum Retroviruses 9:23–32. doi: 10.1089/aid.1993.9.23. [DOI] [PubMed] [Google Scholar]
- 40.Popovic M, Sarngadharan MG, Read E, Gallo RC. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 41.Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, Doran ER, Rafalski JA, Whitehorn EA, Baumeister K, Ivanoff L, Petteway SR, Pearson ML, Lautenberger JA, Papas TS, Ghrayeb J, Chang NT, Gallo RC, Wong-Staal F. 1985. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 42.Mann DL, O'Brien SJ, Gilbert DA, Reid Y, Popovic M, Read-Connole E, Gallo RC, Gazdar AF. 1989. Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retroviruses 5:253–255. doi: 10.1089/aid.1989.5.253. [DOI] [PubMed] [Google Scholar]
- 43.Popovic M, Read-Connole E, Gallo R. 1984. T4 positive human neoplastic cell lines susceptible to and permissive for Htlv-Iii. Lancet 2:1472–1473. doi: 10.1016/s0140-6736(84)91666-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Shapiro L, Fellingham G, Willardson BM, Burton GF. 2011. HIV replication in CD4 + T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by α-1-antitrypsin through altered IκBα ubiquitination. J Immunol 186:3148–3155. doi: 10.4049/jimmunol.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abacioglu YH, Fouts TR, Laman JD, Claassen E, Pincus SH, Moore JP, Roby CA, Kamin-Lewis R, Lewis GK. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses 10:371–381. doi: 10.1089/aid.1994.10.371. [DOI] [PubMed] [Google Scholar]
- 46.Estes JD, Keele BF, Tenner-Racz K, Racz P, Redd MA, Thacker TC, Jiang Y, Lloyd MJ, Gartner S, Burton GF. 2002. Follicular dendritic cell-mediated up-regulation of CXCR4 expression on CD4 T cells and HIV pathogenesis. J Immunol 169:2313–2322. doi: 10.4049/jimmunol.169.5.2313. [DOI] [PubMed] [Google Scholar]
- 47.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, Wilson WH, Rosenberg SA. 2009. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]