The bovine respiratory disease complex (BRDC) causes high mortality and morbidity in cattle, causing economic losses worldwide. Influenza D virus (IDV) is considered to be a causative agent of the BRDC. Here, we developed a reverse genetics system that allows for the generation of IDV from cloned cDNAs and the introduction of mutations into the IDV genome. This reverse genetics system will become a powerful tool for use in studies related to understanding the molecular mechanisms of viral replication and pathogenicity and will also lead to the development of new countermeasures against the BRDC.
KEYWORDS: bovine respiratory disease complex, influenza D virus, mutant, recombinant virus, reverse genetics, transfection
ABSTRACT
Influenza D virus (IDV) was initially isolated in the United States in 2011. IDV is distributed worldwide and is one of the causative agents of the bovine respiratory disease complex (BRDC), which causes high morbidity and mortality in feedlot cattle. The molecular mechanisms of IDV pathogenicity are still unknown. Reverse genetics systems are vital tools not only for studying the biology of viruses, but also for use in applications such as recombinant vaccine viruses. Here, we report the establishment of a plasmid-based reverse genetics system for IDV. We first verified that the 3′-terminal nucleotide of each 7-segmented genomic RNA contained uracil (U), contrary to previous reports, and we were then able to successfully generate recombinant IDV by cotransfecting 7 plasmids containing these genomic RNAs along with 4 plasmids expressing polymerase proteins and nucleoprotein into human rectal tumor 18G (HRT-18G) cells. The recombinant virus had a growth deficit compared to the wild-type virus, and we determined the reason for this growth difference by examining the genomic RNA content of the viral particles. We found that the recombinant virus incorporated an unbalanced ratio of viral RNA segments into particles compared to that of the wild-type virus, and thus we adjusted the amount of each plasmid used in transfection to obtain a recombinant virus with the same replicative capacity as the wild-type virus. Our work here in establishing a reverse genetics system for IDV will have a broad range of applications, including uses in studies focused on better understanding IDV replication and pathogenicity, as well as in those contributing to the development of BRDC countermeasures.
IMPORTANCE The bovine respiratory disease complex (BRDC) causes high mortality and morbidity in cattle, causing economic losses worldwide. Influenza D virus (IDV) is considered to be a causative agent of the BRDC. Here, we developed a reverse genetics system that allows for the generation of IDV from cloned cDNAs and the introduction of mutations into the IDV genome. This reverse genetics system will become a powerful tool for use in studies related to understanding the molecular mechanisms of viral replication and pathogenicity and will also lead to the development of new countermeasures against the BRDC.
INTRODUCTION
Influenza D virus (IDV), a member of the family Orthomyxoviridae, was first isolated from pigs with respiratory illness in Oklahoma, USA, in 2011 (1, 2). Epidemiological analyses revealed, based on their high seroprevalence for IDV, that cattle are the main host of the virus (2, 3). Further epidemiological studies revealed that IDVs circulate in cattle in many countries, including the United States (2–4), Mexico (5), China (6), Japan (7, 8), France (9), Italy (10), Ireland (11), Luxembourg (12), and African countries (13). Furthermore, serological studies showed that IDV antibodies are found in pigs (14), sheep (15, 16), goats (15, 16), dromedary camels (13, 17), horses (18), and humans (19). These findings imply that IDVs are globally distributed in several animal hosts.
IDV is one of the causative agents of the bovine respiratory disease complex (BRDC) (5, 20). The BRDC causes high morbidity and mortality in feedlot cattle, and over 40% of cattle death in the United States is due to the BRDC, which causes severe economic losses (21). To control the BRDC, combined vaccines consisting of several known causative viral (and bacterial) agents have been employed, but their efficacies are limited (21–23). One possible reason for this reduced efficacy is that the vaccines did not contain all causative agents of the BRDC. Recent metagenomic analyses indicate that IDVs are found in cattle with BRDC (5). Therefore, addition of IDV to vaccines may help to control the BRDC.
Influenza A and B viruses (IAV and IBV, respectively) possess 8-segmented negative-sense RNA segments (PB2, PB1, PA, hemagglutinin [HA], nucleoprotein [NP], NA, M, and NS) as genomes, whereas influenza C virus (ICV) and IDV possess 7-segmented segments (PB2, PB1, P3, HEF, NP, M, and NS) as genomes. The viral RNA (vRNA) of influenza virus forms the ribonucleoprotein complex together with three polymerase subunits, PB2, PB1, and PA/P3, in addition to NP. The HEF of IDV is a spike protein on the viral envelope that binds the cell surface via terminal 9-O-acetylated sialic acid (24), which is also known to be a receptor of ICV (25), while the M2 protein of IDV acts as an ion channel protein (26). Although the functions of the M1, NS1, and NS2 proteins of IDV are unknown, we speculate that their functions are similar to those of the ICV counterparts (27–30).
Reverse genetics systems are vital tools not only for studying the biology of viruses but also for use in applications such as recombinant vaccine viruses. While reverse genetics systems for IAV (31–33), IBV (34, 35), and ICV (28, 36) exist, a reverse genetics system for IDV has not been established yet. Such a system could aid in understanding the molecular properties of IDV, in addition to improving methods of disease control. In reverse genetics systems for IAV, IBV, or ICV, RNA polymerase I (PolI) promoter and terminator were used to construct plasmids expressing viral RNAs. These plasmids were cotransfected with plasmids expressing viral-polymerase proteins (PB2, PB1, and PA/P3) and NP into cells, and recombinant viruses were rescued from the supernatants of transfected cells (28, 32, 33, 35, 36). Other systems have also been developed for IAV. These used RNA polymerase I and II transcriptional units, allowing the generation of both vRNA and mRNA from one viral cDNA template (32), or used vRNA transcription with ribozyme-mediated generation of the exact ends of the segments (37). Here, we established a plasmid-based reverse genetics system with IDV using the RNA PolI system, which is similar to that used in reverse genetics systems for other influenza-type viruses.
RESULTS
Determination of the 3′-terminal sequences of genomic RNA segments of IDV.
All influenza viruses possess complementary sequences between the 5′ and 3′ terminal regions of each genome segment. The 5′- and 3′-terminal nucleotides of each segment are adenine (A) and uracil (U), respectively, which are conserved in IAV, IBV, and ICV. However, a previous study reported that, although the 3′-terminal nucleotides of the HEF, M, and NS segments of the IDV genome are U, those of the PB2, PB1, P3, and NP segments are cytosine (C), which is not complementary to the A of 5′-terminal nucleotides (1). To confirm these previous findings, we reassessed 3′- and 5′-terminal sequence regions of each segment of D/swine/Oklahoma/1334/2011 (D/OK) through the 3′ or 5′ rapid amplification of cDNA ends (RACE) method. Our results confirmed that the 5′-terminal nucleotides of every segment of IDV were A (Fig. 1A). However, we found that 3′-terminal nucleotides of all segments were U in our D/OK stock, in contrast to those in a previous report (1) (Fig. 1B). We next determined 3′-terminal sequences of genome segments from 10 plaque-cloned D/OK stocks and two other IDV stocks of D/bovine/Yamagata/10710/2016 and D/bovine/Nebraska/9-5/2012 and found that segments from all IDVs tested contained U. These data verify that the 3′-terminal sequence of each IDV segment is U, as is seen for other influenza viruses.
FIG 1.
Sequences of 5′- and 3′-terminal regions of viral RNA segments of influenza D virus. The 5′ and 3′ rapid amplification of cDNA ends (RACE) method was performed with D/swine/Oklahoma/1334/2011 (D/OK). (A) Genomic RNA sequences at 5′-terminal regions of RNA segments of D/OK are shown, in which each number indicates the nucleotide position from the 5′ end. (B) Genomic RNA sequences at 3′-terminal regions of RNA segments of D/OK are shown, in which each number indicates the nucleotide position from the 3′ end.
Generation of recombinant D/OK virus by reverse genetics.
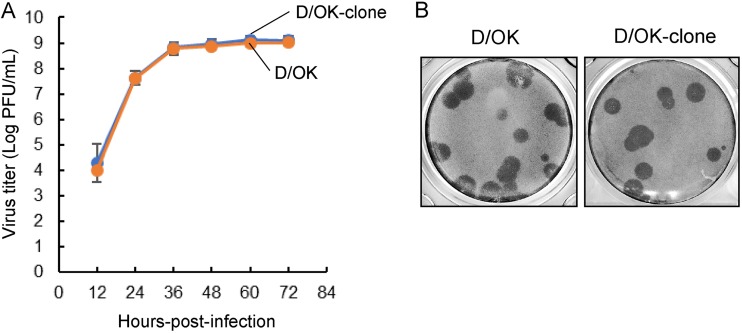
Prior to development of a reverse genetics system for IDV, we characterized the D/OK-clone, which was obtained by biological cloning through plaque purification. The growth kinetics and plaque phenotype of the clone were similar to those of the original D/OK stock (Fig. 2). Genome sequences of the D/OK-clone showed two synonymous substitutions in the PB2 and P3 segments and one nonsynonymous substitution (Ala to Thr) at amino acid position 204 of PB2, compared to D/OK sequences deposited in the NCBI database (Table 1).
FIG 2.
Growth properties of cloned D/OK. (A) Growth kinetics of cloned D/OK (D/OK-clone) were examined in swine testis (ST) cells. D/OK-clone and original D/OK were inoculated onto cells at a multiplicity of infection (MOI) of 0.01. Virus titers were determined at 12-h intervals postinfection by plaque assay and reported as the mean titer with standard deviations (n = 3). (B) Representative plaque morphology of D/OK-clone in ST cells is shown. The plaques were immunologically stained with mouse anti-D/OK polyclonal antibody.
TABLE 1.
Sequence differences observed between D/swine/Oklahoma/1334/2011 and its plaque-purified virus
| Segment | Amino acid position | Amino acid in strain: |
Nucleotide sequence in strain: |
||
|---|---|---|---|---|---|
| D/OKa | D/OK-cloneb | D/oK | D/OK-clone | ||
| PB2 | 204 | Ala | Thr | GCA | ACA |
| 601 | Ala | Ala | GCG | GCA | |
| P3 | 520 | Val | Val | GTC | GTT |
D/OK, wild-type D/swine/Oklahoma/1334/2011.
D/OK-clone, a single clone obtained by three consecutive plaque purifications of D/OK on ST cells.
We cloned cDNAs of 7 vRNA segments of the D/OK-clone into a plasmid for vRNA synthesis (referred to as pPolI-D/OK-PB2, pPolI-D/OK-PB1, pPolI-D/OK-P3, pPolI-D/OK-HEF, pPolI-D/OK-NP, pPolI-D/OK-M, and pPolI-D/OK-NS, respectively) and generated 4 plasmids for vRNA synthesis of PB2, PB1, P3, and NP that possessed C at their 3′ terminal nucleotides (referred to as pPolI-D/OK-PB2/C, pPolI-D/OK-PB1/C, pPolI-D/OK-P3/C, and pPolI-D/OK-NP/C). We additionally prepared 4 plasmids expressing the PB2, PB1, P3, and NP proteins of D/OK (referred to as pCAGGS-PB2, pCAGGS-PB1, pCAGGS-P3, and pCAGGS-NP). We used two sets of vRNA expression plasmids for reverse genetics; one set consisted of pPolI-D/OK-PB2, -PB1, -P3, -HEF, -NP, -M, and -NS, and the other set consisted of pPolI-D/OK-PB2/C, -PB1/C, -P3/C, -HEF, -NP/C, -M, and -NS. We then transfected human rectal tumor 18G (HRT-18G) cells with 0.2 μg of each plasmid from each set and 4 protein expression plasmids, pCAGGS-PB2, -PB1, -P3, and -NP. After incubation for 5 days, the supernatant was transferred to fresh swine testis (ST) cells and incubated for 2 to 3 additional days. Virus rescue was checked by assessing the cytopathic effect (CPE) and performing a hemagglutination test, as well as reverse transcriptase PCR (RT-PCR). We successfully rescued a virus (OK-RG) with the first set of plasmids but not with the second set, and we verified that the OK-RG sequence had no mutations in the genome, including in the 3′ terminal nucleotides of all segments. There was no amplification by a control PCR without reverse transcriptase of any sequences from the supernatants containing the rescued virus (Fig. 3A and B) and thus no carryover of the plasmids for transfection. These data demonstrated the successful generation of recombinant D/OK virus through reverse genetics, but only for instances where the 3′ terminal sequences of vRNAs contained U.
FIG 3.
Characterization of recombinant D/OK. RNA was extracted from the culture supernatant of ST cells inoculated with the transfectant. RT-PCR protocol was performed with primers specific for (A) the full length or (B) a partial length of each segment with (+) or without (−) reverse transcriptase (RT). The amplified products were subjected to 1% agarose gel electrophoresis. (C) Growth kinetics of recombinant D/OK (OK-RG) generated by reverse genetics were examined in ST cells. OK-RG and D/OK-clone were inoculated onto cells at an MOI of 0.01. Virus titers were determined at 12-h intervals postinfection by plaque assay and reported as the mean titer with standard deviations (n = 3). Representative plaque morphologies of OK-RG (D), passaged OK-RGs (P1 to P6) and regenerated virus of the 6th passaged virus by reverse genetics (rP6) (E), and plaque-purified OK-RG clones (1–3) in ST cells (F) were used for comparison to the other plaque morphologies in each panel. The plaques were immunologically stained with mouse anti-D/OK polyclonal antibody.
We next compared growth kinetics of the D/OK-clone and the OK-RG virus in ST cells (Fig. 3C). Surprisingly, viral titers of OK-RG were 3 to 4 log lower than those of the D/OK-clone, even though the genome sequences of both viruses were identical. Plaque size for the OK-RG virus was smaller than that for the D/OK-clone (Fig. 3D). OK-RG passaged at a low multiplicity of infection exhibited large plaques after the 6th passage (Fig. 3E). This passaged virus with large plaques contained a PB2-M55I mutation; however, the recombinant virus rP6 containing this mutation generated by reverse genetics again formed small plaques. In another experimental trial, we purified the virus with small plaques by plaque picking but could not obtain the virus with large plaques (Fig. 3F). Taken together, these findings suggest the presence of an unknown factor contributing to the inhibition of virus growth in the rescued OK-RG preparation.
Verification of genomic RNA content packing in OK-RG particles.
To gain insight into the mechanism underlying the OK-RG growth deficit, we first confirmed vRNA expression in the plasmid-transfected cells. We extracted intracellular vRNAs from the cells and examined content of each RNA segment by Northern blot analysis (Fig. 4A). vRNA expression varied between segments even though equal amounts of each plasmid were used for transfection, whereas similar amounts of each vRNAs were detected in D/OK-clone-infected cells.
FIG 4.
vRNAs in plasmid-transfected cells and in OK-RG particles. (A) Intracellular vRNAs extracted from plasmid-transfected HRT-18G cells at 24, 48, 72, and 96 h posttransfection were examined by Northern blot analysis with segment-specific riboprobes. vRNAs extracted from D/OK-clone-infected cells at 48 h postinfection (p.i.) were also examined. (B) RNAs extracted from the purified D/OK-clone, OK-RG, OK-RG2, OK-RG3, and plaque-purified OK-RG (OK-RG/14p) were subjected to Northern blot analysis with segment-specific and 18S rRNA-specific riboprobes. (C) Genomic RNAs extracted from the purified D/OK-clone, OK-RG, and OK-RG/14p were subjected to urea-polyacrylamide gel electrophoresis. Each RNA was run on a 4% urea-polyacrylamide gel for 10 h at 20 mA. The gel was stained with SYBR Gold nucleic acid gel stain.
We next examined and compared vRNA content packaged into the D/OK-clone and OK-RGs, including two additional recombinant OK-RG-2 and OK-RG-3 viruses, which had been rescued independently, by Northern blot analysis (Fig. 4B). Interestingly, levels of PB2, P3, and NP RNAs were lower in sucrose gradient-purified OK-RG particles than in purified D/OK-clone particles, whereas levels of HEF, M, and NS RNAs were slightly lower in OK-RG particles than in D/OK-clone particles. Levels of PB1 RNA were comparable between OK-RG and D/OK-clone particles. These results suggest that vRNA content packaged into OK-RG particles does not reflect vRNA content in the transfected cells.
In our previous report, 7-segmented (hemagglutinin [HA] segment-deficient) IAV incorporated ribosomal RNAs into its virion (38). Thus, we examined the presence of 18S rRNA in purified recombinant virus, and we found a greater amount of 18S rRNAs in OK-RG particles compared to that in D/OK-clone particles (Fig. 4B). Taken together, these data demonstrate that OK-RG disproportionally incorporates genomic RNA segments, as well as rRNA, into viral particles. This result suggests that a considerable number of noninfectious particles, such as defective interference particles, are generated in the supernatant through reverse genetics procedures, which may lead to the growth deficit of OK-RG compared to the D/OK-clone.
To confirm this hypothesis, we directly examined the content of RNA segments packaged into viral particles. We extracted vRNAs from purified OK-RG and separated them through urea-polyacrylamide gel electrophoresis (PAGE), which revealed different profiles of RNA segments for the D/OK-clone and OK-RG (Fig. 4C). P3 and NP RNA levels were substantially lower in OK-RG than in the D/OK-clone, and abundant 18S rRNA, whose band likely covered that of HEF, was detected in OK-RG through Northern blot analysis (Fig. 4B). We found a few extra bands between HEF and NP and between NP and M, suggesting the presence of undefined, deleted forms of RNA segments that were not detected through Northern blot analysis. These data also suggest that OK-RG disproportionally incorporates genomic RNA segments during particle formation.
Optimization of the reverse genetics system.
Based on our Northern blot (Fig. 4B) and urea-PAGE (Fig. 4C) analyses, we reconsidered transfection conditions for reverse genetics in order to package similar amounts of each RNA segment into viral particles. We tested an additional 17 transfection conditions by increasing and/or decreasing each vRNA- or protein-expressing plasmid (Table 2). Although viruses were rescued under all conditions, most plaque sizes were smaller than those of the D/OK-clone (Fig. 5A). However, viruses rescued under condition 14 (OK-RG/14) included a high population of those forming large plaques (Fig. 5A). We then plaque isolated this virus and propagated it once in ST cells (referred to as OK-RG/14p). When growth kinetics of OK-RG/14 and OK-RG/14p were tested in ST cells (Fig. 5B), we found that OK-RG/14p grew to a titer similar to that of the D/OK-clone, which was approximately 10-fold higher than that of OK-RG/14, suggesting that OK-RG/14p possessed a similar infectivity to that of wild-type D/OK. In addition, we confirmed that the entire genome sequence of OK-RG/14p was identical to that of the D/OK-clone. To rule out crosscontamination with the parent virus and to confirm the optimality for transfection condition 14, we produced a virus with an artificial, synonymous mutation by creating a new EcoRI recognition sequence in the HEF segment using reverse genetics. We rescued transfectant viruses (OK-EcoRI-RGs) under the several selected conditions (conditions 12 to 17), resulting in a large population of those forming large plaques under condition 14 (Fig. 5C) possessing an EcoRI recognition site in the HEF segment (Fig. 5D).
TABLE 2.
Transfection conditions used for reverse genetics
| Plasmid function | Segment | Amt of plasmid (μg) under condition: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| Protein expression | PB2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| PB1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| P3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| NP | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| vRNA synthesis | PB2 | 0.2 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 |
| PB1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.02 | 0.1 | 0.1 | 0.1 | 0.02 | 0.02 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.02 | |
| P3 | 0.2 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | |
| HEF | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | |
| NP | 0.2 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | 0.2 | 1.0 | 0.6 | 0.2 | 0.2 | 0.5 | |
| M | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | |
| NS | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.02 | 0.1 | |
FIG 5.
Growth properties of recombinant viruses generated under different conditions in reverse genetics. (A) Representative plaque morphologies in ST cells of the viruses rescued under both original and 17 different conditions (shown in Table 2), as well as that of the D/OK-clone, are shown. The plaques were immunologically stained with mouse anti-D/OK polyclonal antibody. (B) Growth kinetics of recombinant OK-RG/14 and plaque-purified OK-RG/14p generated under transfection condition 14 were examined. OK-RG/14, OK-RG/14p, OK-RG, and the D/OK-clone were inoculated onto ST cells at an MOI of 0.01. Virus titers were determined at 12-h intervals postinfection by plaque assay and reported as the mean titer with standard deviation (n = 3). (C) Representative plaque morphologies of D/OK-EcoRI-RGs under transfection conditions 12 to 17 (as shown in Table 2) in ST cells. Three independent experiments (no. 1 to 3) were performed for the generation of D/OK-EcoRI-RG. (D) The HEF gene of D/OK-RG, D/OK-EcoRI-RG/14p, or D/OK-clone was amplified by RT-PCR with primers that yielded a full-length 2,049-bp fragment, followed by digestion with the restriction enzyme EcoRI. The presence of EcoRI sites in the PCR products yielded both 1,307- and 738-bp fragments.
We next assessed the content of RNA segments packaged into purified OK-RG/14p particles. In a Northern blot analysis, the RNA segment content of OK-RG/14p was similar to that of the D/OK-clone, and 18S rRNA was not detected in OK-RG/14p particles (Fig. 4B). In a urea-PAGE analysis, the profiles of RNA segments were similar between D/OK-clone and OK-RG/14p, except for a greater amount of PB2 and/or PB1 RNA packaging in OK-RG/14p particles (Fig. 4C). No RNA content was detected in the control material prepared from mock-infected cells using the same procedures. These results suggest that a well-balanced packaging of RNA segments can be achieved under transfection condition 14, leading to similar growth properties for OK-RG/14p and the D/OK-clone.
DISCUSSION
Reverse genetics systems, which are used to generate recombinant viruses, are vital tools not only to study the biology of influenza viruses but also for developing antiviral strategies such as the generation of recombinant vaccine viruses. Here, we established a plasmid-based reverse genetics system for IDV.
For previously published reverse genetics systems with IAV, IBV, and ICV, human embryonic kidney 293T (HEK293T) cells were mainly used for virus rescue due to their high transfection efficiency and the presence of T antigen required for replication of pCAGGS-based vectors (28, 32, 33, 35, 36). In this study, we first tested this cell line for potential use in an IDV reverse genetics system. Although recombinant D/OKs were rescued after several attempts, their infectivities (PFU titers) were low, despite high hemagglutination titers in the supernatant of transfected cells. Their plaque sizes were also small, suggesting the presence of abundant noninfectious particles. Therefore, we next tested HRT-18G cells, which have been widely used for IDV isolation, in our IDV reverse genetics system. Although this cell line showed a much lower transfection efficiency than that of HEK293T cells, recombinant D/OKs with high infectivity were rescued, as revealed by higher PFU/HA ratios than those seen with HEK293T cells. These findings imply that support of viral replication is a more important factor to consider than transfection efficiency when choosing cells for use in an IDV reverse genetics system. Therefore, a possible system in which the bovine (or swine) PolI vector is utilized as a vRNA synthetic plasmid for transfection in bovine (or swine) cells may enhance the rescue efficiency of recombinant IDVs.
IAV, IBV, and ICV contain adenine (A) and uracil (U) at the 5′- and 3′-terminal ends, respectively, of every RNA segment, and complementary sequences of both terminal regions, including these two nucleotides, form panhandle structures of viral ribonucleoprotein (RNP), as well as transcription/replication promoter structures for the viral genome in IAV. Here, we found that all RNA segments of IDV also possess U at their 3′-terminal ends, in contrast to a previous report showing that PB2, PB1, P3, and NP RNAs of D/OK possessed cytosine (C) (1). Although we were not able to rescue recombinant D/OK with RNA segments possessing C at the 3′-terminal ends, this does not eliminate the possibility that the original D/OK may possess C at the 3′-terminal ends of these RNA segments. However, it is likely that viral passages in the laboratory would result in the selection of D/OK with U at the 3′-terminal ends of all RNA segments. Alternatively, C at the 3′-terminal end may be incorrectly read due to an undefined artifact resulting from the RACE method (1). Further sequence analyses of the 3′-terminal ends of RNA segments in other multiple-IDV isolates will clarify this point.
In this study, we successfully established a reverse genetics system for IDV by fine adjustment of quantitative transfection conditions for viral RNA synthetic and protein expression plasmids. This adjustment resulted in the balanced packaging of 7 RNA segments in viral particles, leading to the rescue of recombinant D/OK, with growth properties similar to the D/OK-clone. Interestingly, unlike in reverse genetics with IAV, such recombinant D/OK viruses have not been selected in other methodological trials such as high-dilution passages or plaque picking of the rescued viruses with low infectivity, suggesting that noninfectious interfering particles would be consecutively cosegregated once the imbalanced contents of genome segments in the infected cells are maintained. This would lead to a small-plaque phenotype, even though whole-genome sequences are identical to that of the original D/OK-clone with a large-plaque phenotype. This finding suggests an intrinsically different mechanism for genome packaging between IAV and IDV. Two models for genome packaging of influenza virions have been proposed, random and selective packaging (39). Previous studies support the hypothesis that IAV most likely contains a selective packaging mechanism (39–42). IAV and IBV incorporate eight RNPs arranged in a specific “1 + 7” pattern, in which seven RNPs surround a central RNP (40, 43, 44). Surprisingly, artificially generated 7-segmented IAV, which lacks an HA segment, incorporated rRNA as the eighth RNP instead of an HA segment (38). Interestingly, ICV and IDV both possess a 7-segmented genome, yet also incorporated 8 RNPs, although the inclusion of rRNA in virions was not assessed (45). These findings imply that all influenza viruses may incorporate 8 RNPs, suggesting the presence of an undefined random packaging mechanism in addition to a selective packaging one, especially for ICV and IDV. Several studies also support the interpretation that the genome packaging mechanisms of IAV and ICV are different. In one study, artificially generated virus-like particles (VLPs) of IAV with fewer than 8 RNA segments were less efficiently produced than those with one complete set of 8 RNA segments (46, 47), whereas in a different study, VLPs of ICV with single RNA segments were produced more efficiently than those with a complete set of 7 RNA segments (27, 48). Our present study also supports such a difference, in which OK-RG incorporated unbalanced ratios of RNA segments and rRNA into particles when equal amounts of plasmids were used for transfection. This imbalance may have resulted in the generation of abundant noninfectious particles in the supernatant of transfected cells, which may have interfered with virion production. However, this transfection condition is regularly used for reverse genetics with IAV, suggesting a difference in genome packaging between IAV and IDV. Further studies are required to elucidate differences in the genome packaging mechanism between 8-segmented and 7-segmented viruses.
While our article was pending review for publication, another research group reported development of a reverse genetics system for IDV (49). They used an alternative system, in which seven plasmids with RNA PolI-PolII transcription units were used for transfection to rescue the virus, and also used a different strain, D/swine/Oklahoma/1314/2011, from the strain used in our study. Notably, this strain seems to originally possess U at the 3′-terminal nucleotide of all 7 RNA segments, according to the reported primer sequences for cDNA amplification, being the same sequence as the 3′-terminal nucleotide of PB2, PB1, PA, and NP segments of our strain. However, their strain grew to a lower titer than that of our strain in Madin-Darby canine kidney (MDCK) cells. Our strain grew to a titer of 108 PFU/ml in this cell line, which was over 100 times higher than the titer of their strain. We are planning to develop a similar PolI-PolII-based system with our strain and to compare it with our PolI-based system in the context of accuracy and utility for generation of IDVs.
In conclusion, this study established a PolI-based reverse genetics system for IDV. However, transfection procedures must be carefully observed, since we determined that only one transfection condition (condition 14) can efficiently generate virions with original infectivity. Nonetheless, this system will allow us to produce recombinant viruses with viable mutation(s) in the IDV genome and will aid in the study of molecular mechanisms of virus replication, including genome packaging and pathogenicity. Based on such knowledge, we will be able to generate live attenuated IDVs, which could be an effective control measure against the BRDC.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic kidney 293T cells (obtained from Riken BRC, RCB2202), human rectal tumor 18G (HRT-18G) cells (CRL-11663; obtained from ATCC), and swine testis (ST) cells (CRL-1746; obtained from ATCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Fujifilm Wako Pure Chemical, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS) at 37°C. D/swine/Oklahoma/1334/2011 (D/OK) (GenBank accession no. JQ922305 to JQ922311) (1) and D/bovine/Nebraska/9-5/2012 (accession no. KM392468 to KM392474) (4) were kindly provided by B. Hause (Kansas State University). Our isolate of D/bovine/Yamagata/10710/2016 (accession no. LC318665to LC318671) (45) was also used. These IDVs were propagated in ST cells in Eagle’s minimum essential medium (MEM; Life Technologies/Gibco, Paisley, UK) containing 0.3% bovine serum albumin (MEM/BSA) and supplemented with 0.5 μg/ml l-1-tosylamide-2-phenylmethyl chloromethyl ketone (TPCK)-trypsin (Worthington, Lakewood, NJ) and stocked at −80°C.
Construction of plasmids for reverse genetics.
RNA was extracted from culture supernatant of plaque-cloned D/OK (D/OK-clone)-infected ST cells using Isogen-LS reagent (Nippon Gene, Tokyo, Japan). The extracted RNA was reverse transcribed using PrimeScript reverse transcriptase (TaKaRa Bio, Shiga, Japan), and the cDNAs were amplified by PCR using KOD FX Neo (Toyobo, Osaka, Japan) and a set of segment-specific primers containing 15-nucleotide-overlapped sequences to the cloning site of the pHH21 plasmid, which contains human RNA polymerase I (PolI) promoter and murine PolI terminator sequences (33). The PCR products were purified with a FastGene gel/PCR extraction kit (Nippon Genetics, Tokyo, Japan) and cloned into BsmBI-digested pHH21 using a Gibson assembly master mix (New England BioLabs [NEB] Japan, Tokyo, Japan), resulting in pPolI-D/OK-PB2, -PB1, -P3, -HEF, -NP, -M, and -NS plasmids. pPolI-D/OK-PB2/C, -PB1/C, -P3/C, and -NP/C plasmids possessing C at the 3′-terminal ends of viral RNAs were also generated using a set of segment-specific primers containing C at the 3′-terminal end, and 15-nucleotide-overlapped sequences to the cloning site of pHH21. Coding regions of PB2, PB1, P3, and NP segments were PCR amplified using KOD FX Neo and 15-nucleotide-overlapped sequences to the cloning site of pCAGGS plasmid (50). The PCR products were cloned into the EcoRI- and XhoI-digested pCAGGS using a Gibson assembly master mix, resulting in pCAGGS-D/OK-PB2, -PB1, -P3, and -NP plasmids.
Generation of recombinant IDV by reverse genetics.
Sixty-percent-confluent HRT-18G cells, seeded on a 6-well plate, were transfected with 7 viral RNA-synthetic plasmids and 4 protein (PB2, PB1, P3, and NP) expression plasmids using 0.2 μg of each plasmid with 11 μl of 1 μg/μl PEI Max (Polysciences, Warrington, PA). Prior to transfection, the plasmid DNAs and transfection reagent were mixed and incubated at 23°C for 20 min. The mixtures were then added to the cells and incubated at 37°C. At 2 days posttransfection, the supernatants were removed, and cells were washed twice with MEM before the addition of 2 ml of MEM/BSA containing 0.5 μg/ml TPCK-trypsin. After 3 days of incubation at 37°C, the supernatants were collected, diluted 10-fold with MEM/BSA, and inoculated on ST cells for 1 h. The cells were washed twice in MEM before the addition of 2 ml of MEM/BSA containing 0.5 μg/ml TPCK-trypsin, and then incubated at 37°C. At 2 to 3 days postinfection, supernatants were collected, and virus titers were determined by plaque assay in ST cells. The supernatant stock was directly utilized as single-passage viruses for each experiment to characterize growth properties of the viruses. In addition, second-passage viruses in ST cells were used for virus purification for urea-PAGE and Northern blot analyses.
Plaque assay.
Confluent ST cells on a 12-well plate were washed twice with phosphate-buffered saline (PBS), inoculated with 0.1 ml each of serially 10-fold diluted viruses in MEM/BSA, and incubated for 1 h at 37°C. Cell were then washed with MEM/BSA, covered with 1 ml of MEM/BSA containing 1% Seakem GTG agarose (Lonza Japan, Chiba, Japan) and 0.5 μg/ml TPCK-trypsin, and incubated at 37°C for 3 days. One-half ml of 30% formalin in PBS was added to each well for fixation at 4°C overnight. After formalin and agarose were removed, the cells were washed with PBS and permeabilized with 0.1% Triton X-100 in PBS for 15 min at 23°C. After blocking with Block Ace (KAC, Hyogo, Japan), the cells were incubated with anti-IDV mouse immune serum as a primary antibody for 60 min, followed by incubation with biotinylated anti-mouse IgG antibody (catalog no. B7264; Sigma, Kanagawa, Japan) for 30 min, followed by a complex containing 4 μg/ml biotinylated peroxidase (Invitrogen/Thermo Fisher Scientific, Tokyo, Japan) and 8 μg/ml streptavidin (Fujifilm Wako Chemicals) for 30 min. The plaques were visualized by the DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instruction.
Hemagglutination assay.
A hemagglutination assay was performed in U-bottom 96-well microplates as previously described (51). Briefly, serial 2-fold dilutions of the supernatants in 50 μl of PBS were mixed with 50 μl of 0.7% turkey red blood cells and incubated for 30 min at 23°C before reading. The hemagglutination titers were determined as the reciprocal of the highest virus dilution showing complete hemagglutination.
5′ and 3′ RACE.
5′ and 3′ rapid amplification of the cDNA end (RACE) was performed using a 5′/3′ RACE kit (2nd generation; Roche, Basel, Switzerland) with some modifications. Briefly, viral RNAs were extracted from purified virus particles using Isogen-LS. For 5′ RACE, the extracted RNAs were reverse transcribed using segment-specific primers. The cDNA products were then added along with poly(dA) using terminal deoxynucleotidyl transferase (Roche). The cDNAs with added poly(dA) were amplified by segment-specific primers and our oligo-(dT) primer containing a synthetic tag (5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTT-3′) [instead of the oligo(dT)-anchor primer 5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV-3′] provided by the kit), followed by seminested-PCR using segment-specific primers and PCR anchor primer (5′-GACCACGCGTATCGATGTCGAC-3′). For 3′ RACE, a poly(A) tail was added to the viral RNAs using Escherichia coli poly(A) polymerase (NEB). The RNAs with added poly(A) were reverse transcribed using the oligo(dT) primer with added tag, and the cDNAs were amplified by PCR using PCR anchor primer and segment-specific primers. The PCR products were sequenced using a 3130xl Genetic Analyzer (Life Technologies Japan/Applied Biosystems, Tokyo, Japan). The sequences of the segment-specific primers will be provided upon request.
Virus purification.
The virus-containing supernatants from ST cells were clarified by low-speed centrifugation to remove cell debris and concentrated by ultracentrifugation at 40,000 × g for 2 h at 4°C using a P19A rotor (Himac, Tokyo, Japan). The concentrated viruses were resuspended in PBS and layered onto a 20, 30, 50, and 60% discontinuous sucrose gradient and ultracentrifuged at 110,000 × g for 3 h at 4°C using a P32ST rotor (Himac). The virus-containing interface between the 30 and 50% sucrose gradient was collected, diluted in PBS, and ultracentrifuged once more at 110,000 × g for 3 h at 4°C using a P32ST rotor. Purified virus pellets were suspended in a small amount of PBS.
Urea-PAGE.
RNAs of the purified viruses were extracted using an miRNeasy minikit (Qiagen, Tokyo, Japan). To separate RNA segments, urea-PAGE was used as previously described (45), with some modifications. viral RNA (100 ng) was run on a 4% polyacrylamide gel containing 7 M urea. The gel was then stained with SYBR Gold nucleic acid gel stain (Invitrogen). The gel images were obtained by a gel imager (Printgraph; Atto, Tokyo, Japan).
Northern blot analysis.
Northern blot analysis was performed as previously described (38), with some modifications. Briefly, viral RNAs were extracted from purified viruses or from plasmid-transfected or virus-infected HRT-18G cells using Isogen reagents (Nippon Gene). Viral (10 ng) or intracellular (1 μg) RNAs were denatured in denaturing buffer (50% formamide and 17.8% formalin in 1% morpholinepropanesulfonic acid [MOPS] buffer), separated on 1.5% denaturing agarose-formaldehyde gels, and transferred onto a nylon membrane with a molecular weight marker (BioDynamics Laboratory, Tokyo, Japan). Biotin-labeled strand-specific RNA probes for PB2 (hybridizing to nucleotides [nt] 15 to 1257), PB1 (nt 26 to 1260), P3 (nt 23 to 1038), HEF (nt 27 to 1017), NP (nt 28 to 954), M (nt 29 to 1220), NS (to nt 29 to 868) vRNA, and 18S rRNA were synthesized using a biotin-11-UTP (Roche) and a T7 RNA expression kit (Promega, Madison, WI) according to the manufacturer’s instructions. Detection of RNA segments was performed using an ABC kit (Vector Laboratories) and a digoxigenin (DIG) block and wash buffer set (Roche), according to the manufacturer’s instructions. The signals were detected with Clarity ECL substrate (Bio-Rad, Hercules, CA), and blot images were obtained with an Image Quant LAS 4000 Mini system (Fujifilm, Tokyo, Japan).
Data availability.
Sequences were deposited in DDBJ/EMBL/GenBank under accession numbers LC522351 to LC522357.
ACKNOWLEDGMENTS
We thank Ben M. Hause at Kansas State University for providing viruses and Yoshihiro Kawaoka at the University of Tokyo for providing plasmids.
S.M. received a Grant-in-Aid for Encouragement of Young Scientists (A) (grant number 17H05042) from the Japan Society for the Promotion of Science. T.H. was supported in part by a Grant-in-Aid for Scientific Research (A) (grant number 18H03971) from the Japan Society for the Promotion of Science, and in part by Livestock Promotion Funds from the Japan Racing Association.
REFERENCES
- 1.Hause BM, Ducatez M, Collin EA, Ran Z, Liu R, Sheng Z, Armien A, Kaplan B, Chakravarty S, Hoppe AD, Webby RJ, Simonson RR, Li F. 2013. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog 9:e1003176. doi: 10.1371/journal.ppat.1003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hause BM, Collin EA, Liu R, Huang B, Sheng Z, Lu W, Wang D, Nelson EA, Li F. 2014. Characterization of a novel influenza virus in cattle and swine: proposal for a new genus in the Orthomyxoviridae family. mBio 5:e00031-14. doi: 10.1128/mBio.00031-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson L, Eckard L, Epperson WB, Long LP, Smith D, Huston C, Genova S, Webby R, Wan XF. 2015. Influenza D virus infection in Mississippi beef cattle. Virology 486:28–34. doi: 10.1016/j.virol.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin EA, Sheng Z, Lang Y, Ma W, Hause BM, Li F. 2015. Cocirculation of two distinct genetic and antigenic lineages of proposed influenza D virus in cattle. J Virol 89:1036–1042. doi: 10.1128/JVI.02718-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitra N, Cernicchiaro N, Torres S, Li F, Hause BM. 2016. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for influenza D virus. J Gen Virol 97:1771–1784. doi: 10.1099/jgv.0.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang WM, Wang SC, Peng C, Yu JM, Zhuang QY, Hou GY, Liu S, Li JP, Chen JM. 2014. Identification of a potential novel type of influenza virus in bovine in China. Virus Genes 49:493–496. doi: 10.1007/s11262-014-1107-3. [DOI] [PubMed] [Google Scholar]
- 7.Murakami S, Endoh M, Kobayashi T, Takenaka-Uema A, Chambers JK, Uchida K, Nishihara M, Hause B, Horimoto T. 2016. Influenza D virus infection in herd of cattle, Japan. Emerg Infect Dis 22:1517–1519. doi: 10.3201/eid2208.160362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horimoto T, Hiono T, Mekata H, Odagiri T, Lei Z, Kobayashi T, Norimine J, Inoshima Y, Hikono H, Murakami K, Sato R, Murakami H, Sakaguchi M, Ishii K, Ando T, Otomaru K, Ozawa M, Sakoda Y, Murakami S. 2016. Nationwide distribution of bovine influenza D virus infection in Japan. PLoS One 11:e0163828. doi: 10.1371/journal.pone.0163828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducatez MF, Pelletier C, Meyer G. 2015. Influenza D virus in cattle, France, 2011–2014. Emerg Infect Dis 21:368–371. doi: 10.3201/eid2102.141449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiapponi C, Faccini S, De Mattia A, Baioni L, Barbieri I, Rosignoli C, Nigrelli A, Foni E. 2016. Detection of influenza D virus among swine and cattle, Italy. Emerg Infect Dis 22:352–354. doi: 10.3201/eid2202.151439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn O, Gallagher C, Mooney J, Irvine C, Ducatez M, Hause B, McGrath G, Ryan E. 2018. Influenza D virus in cattle, Ireland. Emerg Infect Dis 24:389–391. doi: 10.3201/eid2402.170759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snoeck CJ, Oliva J, Pauly M, Losch S, Wildschutz F, Muller CP, Hubschen JM, Ducatez MF. 2018. Influenza D virus circulation in cattle and swine, Luxembourg, 2012–2016. Emerg Infect Dis 24:1388–1389. doi: 10.3201/eid2407.171937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem E, Cook EAJ, Lbacha HA, Oliva J, Awoume F, Aplogan GL, Hymann EC, Muloi D, Deem SL, Alali S, Zouagui Z, Fevre EM, Meyer G, Ducatez MF. 2017. Serologic evidence for influenza C and D virus among ruminants and camelids, Africa, 1991–2015. Emerg Infect Dis 23:1556–1559. doi: 10.3201/eid2309.170342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson L, Luo K, Olivier AK, Cunningham FL, Blackmon S, Hanson-Dorr K, Sun H, Baroch J, Lutman MW, Quade B, Epperson W, Webby R, DeLiberto TJ, Wan XF. 2018. Influenza D virus infection in feral swine populations, United States. Emerg Infect Dis 24:1020–1028. doi: 10.3201/eid2406.172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quast M, Sreenivasan C, Sexton G, Nedland H, Singrey A, Fawcett L, Miller G, Lauer D, Voss S, Pollock S, Cunha CW, Christopher-Hennings J, Nelson E, Li F. 2015. Serological evidence for the presence of influenza D virus in small ruminants. Vet Microbiol 180:281–285. doi: 10.1016/j.vetmic.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliva J, Eichenbaum A, Belin J, Gaudino M, Guillotin J, Alzieu JP, Nicollet P, Brugidou R, Gueneau E, Michel E, Meyer G, Ducatez MF. 2019. Serological evidence of influenza D virus circulation among cattle and small ruminants in France. Viruses 11:516–521. doi: 10.3390/v11060516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami S, Odagiri T, Melaku SK, Bazartseren B, Ishida H, Takenaka-Uema A, Muraki Y, Sentsui H, Horimoto T. 2019. Influenza D virus infection in dromedary camels, Ethiopia. Emerg Infect Dis 25:1224–1226. doi: 10.3201/eid2506.181158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedland H, Wollman J, Sreenivasan C, Quast M, Singrey A, Fawcett L, Christopher-Hennings J, Nelson E, Kaushik RS, Wang D, Li F. 2018. Serological evidence for the co-circulation of two lineages of influenza D viruses in equine populations of the Midwest United States. Zoonoses Public Health 65:e148–e154. doi: 10.1111/zph.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White SK, Ma W, McDaniel CJ, Gray GC, Lednicky JA. 2016. Serologic evidence of exposure to influenza D virus among persons with occupational contact with cattle. J Clin Virol 81:31–33. doi: 10.1016/j.jcv.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Ng TF, Kondov NO, Deng X, Van Eenennaam A, Neibergs HL, Delwart E. 2015. A metagenomics and case-control study to identify viruses associated with bovine respiratory disease. J Virol 89:5340–5349. doi: 10.1128/JVI.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilton WM. 2014. BRD in 2014: where have we been, where are we now, and where do we want to go? Anim Health Res Rev 15:120–122. doi: 10.1017/S1466252314000115. [DOI] [PubMed] [Google Scholar]
- 22.Fulton RW. 2009. Bovine respiratory disease research (1983–2009). Anim Health Res Rev 10:131–139. doi: 10.1017/S146625230999017X. [DOI] [PubMed] [Google Scholar]
- 23.Theurer ME, Larson RL, White BJ. 2015. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J Am Vet Med Assoc 246:126–142. doi: 10.2460/javma.246.1.126. [DOI] [PubMed] [Google Scholar]
- 24.Song H, Qi J, Khedri Z, Diaz S, Yu H, Chen X, Varki A, Shi Y, Gao GF. 2016. An open receptor-binding cavity of hemagglutinin-esterase-fusion glycoprotein from newly-identified influenza D virus: basis for its broad cell tropism. PLoS Pathog 12:e1005411. doi: 10.1371/journal.ppat.1005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers GN, Herrler G, Paulson JC, Klenk HD. 1986. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem 261:5947–5951. [PubMed] [Google Scholar]
- 26.Kesinger E, Liu J, Jensen A, Chia CP, Demers A, Moriyama H. 2018. Influenza D virus M2 protein exhibits ion channel activity in Xenopus laevis oocytes. PLoS One 13:e0199227. doi: 10.1371/journal.pone.0199227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraki Y, Washioka H, Sugawara K, Matsuzaki Y, Takashita E, Hongo S. 2004. Identification of an amino acid residue on influenza C virus M1 protein responsible for formation of the cord-like structures of the virus. J Gen Virol 85:1885–1893. doi: 10.1099/vir.0.79937-0. [DOI] [PubMed] [Google Scholar]
- 28.Muraki Y, Murata T, Takashita E, Matsuzaki Y, Sugawara K, Hongo S. 2007. A mutation on influenza C virus M1 protein affects virion morphology by altering the membrane affinity of the protein. J Virol 81:8766–8773. doi: 10.1128/JVI.00075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pachler K, Vlasak R. 2011. Influenza C virus NS1 protein counteracts RIG-I-mediated IFN signalling. Virol J 8:48. doi: 10.1186/1743-422X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paragas J, Talon J, O’Neill RE, Anderson DK, García-Sastre A, Palese P. 2001. Influenza B and C virus NEP (NS2) proteins possess nuclear export activities. J Virol 75:7375–7383. doi: 10.1128/JVI.75.16.7375-7383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. 1999. Rescue of influenza A virus from recombinant DNA. J Virol 73:9679–9682. doi: 10.1128/JVI.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A 97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann E, Mahmood K, Yang CF, Webster RG, Greenberg HB, Kemble G. 2002. Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci U S A 99:11411–11416. doi: 10.1073/pnas.172393399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D, Cadman A, Zurcher T, Barclay WS. 2002. A reverse genetics approach for recovery of recombinant influenza B viruses entirely from cDNA. J Virol 76:11744–11747. doi: 10.1128/jvi.76.22.11744-11747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crescenzo-Chaigne B, van der Werf S. 2007. Rescue of influenza C virus from recombinant DNA. J Virol 81:11282–11289. doi: 10.1128/JVI.00910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Wit E, Spronken MI, Vervaet G, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2007. A reverse-genetics system for Influenza A virus using T7 RNA polymerase. J Gen Virol 88:1281–1287. doi: 10.1099/vir.0.82452-0. [DOI] [PubMed] [Google Scholar]
- 38.Noda T, Murakami S, Nakatsu S, Imai H, Muramoto Y, Shindo K, Sagara H, Kawaoka Y. 2018. Importance of the 1 + 7 configuration of ribonucleoprotein complexes for influenza A virus genome packaging. Nat Commun 9:54. doi: 10.1038/s41467-017-02517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. 2010. Genome packaging in influenza A virus. J Gen Virol 91:313–328. doi: 10.1099/vir.0.017608-0. [DOI] [PubMed] [Google Scholar]
- 40.Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, Kawaoka Y. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- 41.Chou Y-Y, Vafabakhsh R, Doğanay S, Gao Q, Ha T, Palese P. 2012. One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis. Proc Natl Acad Sci U S A 109:9101–9106. doi: 10.1073/pnas.1206069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber M, Isel C, Moules V, Marquet R. 2014. Selective packaging of the influenza A genome and consequences for genetic reassortment. Trends Microbiol 22:446–455. doi: 10.1016/j.tim.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Noda T, Sugita Y, Aoyama K, Hirase A, Kawakami E, Miyazawa A, Sagara H, Kawaoka Y. 2012. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus. Nat Commun 3:639. doi: 10.1038/ncomms1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsu S, Sagara H, Sakai-Tagawa Y, Sugaya N, Noda T, Kawaoka Y. 2016. Complete and incomplete genome packaging of influenza A and B viruses. mBio 7:e01248-16. doi: 10.1128/mBio.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakatsu S, Murakami S, Shindo K, Horimoto T, Sagara H, Noda T, Kawaoka Y. 2018. Influenza C and D viruses package eight organized ribonucleoprotein complexes. J Virol 92:e02084-17. doi: 10.1128/JVI.02084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann G, Watanabe T, Kawaoka Y. 2000. Plasmid-driven formation of influenza virus-like particles. J Virol 74:547–551. doi: 10.1128/jvi.74.1.547-551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc Natl Acad Sci U S A 100:2002–2007. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muraki Y, Furukawa T, Kohno Y, Matsuzaki Y, Takashita E, Sugawara K, Hongo S. 2010. Influenza C virus NS1 protein upregulates the splicing of viral mRNAs. J Virol 84:1957–1966. doi: 10.1128/JVI.01627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J, Liu R, Zhou B, Chou TW, Ghedin E, Sheng Z, Gao R, Zhai SL, Wang D, Li F. 2019. Development and characterization of a reverse genetics system for influenza D virus. J Virol 93. doi: 10.1128/JVI.01186-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 51.Killian ML. 2014. Hemagglutination assay for influenza virus. Methods Mol Biol 1161:3–9. doi: 10.1007/978-1-4939-0758-8_1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences were deposited in DDBJ/EMBL/GenBank under accession numbers LC522351 to LC522357.