PRRS, caused by PRRSV, is an economically critical factor in pig farming worldwide. As PRRSV is a lipid membrane-wrapped virus, merging of the PRRSV envelope with the host cell membrane is indispensable for viral infection. However, there is a lack of knowledge on its membrane fusion. Here, we first explored when and where PRRSV membrane fusion occurs. Furthermore, we determined which host cell factors were involved in the process. Importantly, PRRSV GP5 is shown to be cleaved by cathepsin E during membrane fusion. Our work not only provides information on PRRSV membrane fusion for the first time but also deepens our understanding of the molecular mechanisms of PRRSV infection, which provides a foundation for future applications in the prevention and control of PRRS.
KEYWORDS: PRRSV, membrane fusion, host cell protease, GP5, cathepsin E
ABSTRACT
Porcine reproductive and respiratory syndrome (PRRS) is a serious viral disease affecting the global swine industry. Its causative agent, PRRS virus (PRRSV), is an enveloped virus, and therefore membrane fusion between its envelope and host cell target membrane is critical for viral infection. Though much research has focused on PRRSV infection, the detailed mechanisms involved in its membrane fusion remain to be elucidated. In the present study, we performed confocal microscopy in combination with a constitutively active (CA) or dominant negative (DN) mutant, specific inhibitors, and small interfering RNAs (siRNAs), as well as multiple other approaches, to explore PRRSV membrane fusion. We first observed that PRRSV membrane fusion occurred in Rab11-recycling endosomes during early infection using labeled virions and subcellular markers. We further demonstrated that low pH and cathepsin E in Rab11-recycling endosomes are critical for PRRSV membrane fusion. Moreover, PRRSV glycoprotein 5 (GP5) is identified as being cleaved by cathepsin E during this process. Taken together, our findings provide in-depth information regarding PRRSV pathogenesis, which support a novel basis for the development of antiviral drugs and vaccines.
IMPORTANCE PRRS, caused by PRRSV, is an economically critical factor in pig farming worldwide. As PRRSV is a lipid membrane-wrapped virus, merging of the PRRSV envelope with the host cell membrane is indispensable for viral infection. However, there is a lack of knowledge on its membrane fusion. Here, we first explored when and where PRRSV membrane fusion occurs. Furthermore, we determined which host cell factors were involved in the process. Importantly, PRRSV GP5 is shown to be cleaved by cathepsin E during membrane fusion. Our work not only provides information on PRRSV membrane fusion for the first time but also deepens our understanding of the molecular mechanisms of PRRSV infection, which provides a foundation for future applications in the prevention and control of PRRS.
INTRODUCTION
As intracellular obligate pathogens, viruses deliver genetic materials into the host cytoplasm to establish infection (1). In particular, merging of the viral envelope with the host cell target membrane, that is, membrane fusion, is an essential step for enveloped viruses to release their genomes (2, 3). This complicated process is mediated by viral fusion protein(s), together with host cell receptor(s), proteases, and other factors (4). In-depth studies of viral membrane fusion will provide a novel basis for the prevention and control of enveloped viral diseases. For example, efficient broad-spectrum vaccines can be developed against viral fusion protein(s), and potent antiviral drugs may be designed to specifically disrupt the membrane fusion process (5–8).
Porcine reproductive and respiratory syndrome (PRRS), characterized by reproductive failures and respiratory symptoms, has led to huge economic losses to the global swine industry (9–11). The annual loss due to PRRS is estimated at $664 million in the United States alone (12). Due to the lack of effective vaccines, PRRS still circulates worldwide, especially in China (13). The causative agent of this disease, PRRS virus (PRRSV), is a member of the Porartevirus genus of the Arteriviridae family and the order Nidovirales (14). All PRRSV isolates are classified as either PRRSV-1 or PRRSV-2 (15).
PRRSV is an enveloped, positive-sense, single-stranded RNA virus with a genome size of ∼15 kb (16, 17). It encodes and incorporates glycoprotein 2 (GP2), GP3, GP4, GP5, GP5a, and membrane proteins M and E into its envelope (18, 19). PPRSV specifically infects swine and limited cells, such as its primary in vivo target, pulmonary alveolar macrophages (PAMs), and the African green monkey kidney epithelial cell line MA-104 and its derivative, MARC-145 cells (20, 21). Previous studies have shown that PRRSV infection is via low pH-dependent clathrin-mediated endocytosis (22, 23). Despite that numerous studies have focused on PRRSV infection (24–26), its membrane fusion has not yet been elucidated.
Here, we explored this issue through several approaches. First, we monitored the time point and location of PRRSV membrane fusion in MARC-145 cells and PAMs. Subsequently, we identified which host cell factors and viral envelope proteins were involved in this process and how they took effect.
RESULTS
PRRSV membrane fusion occurs during early infection.
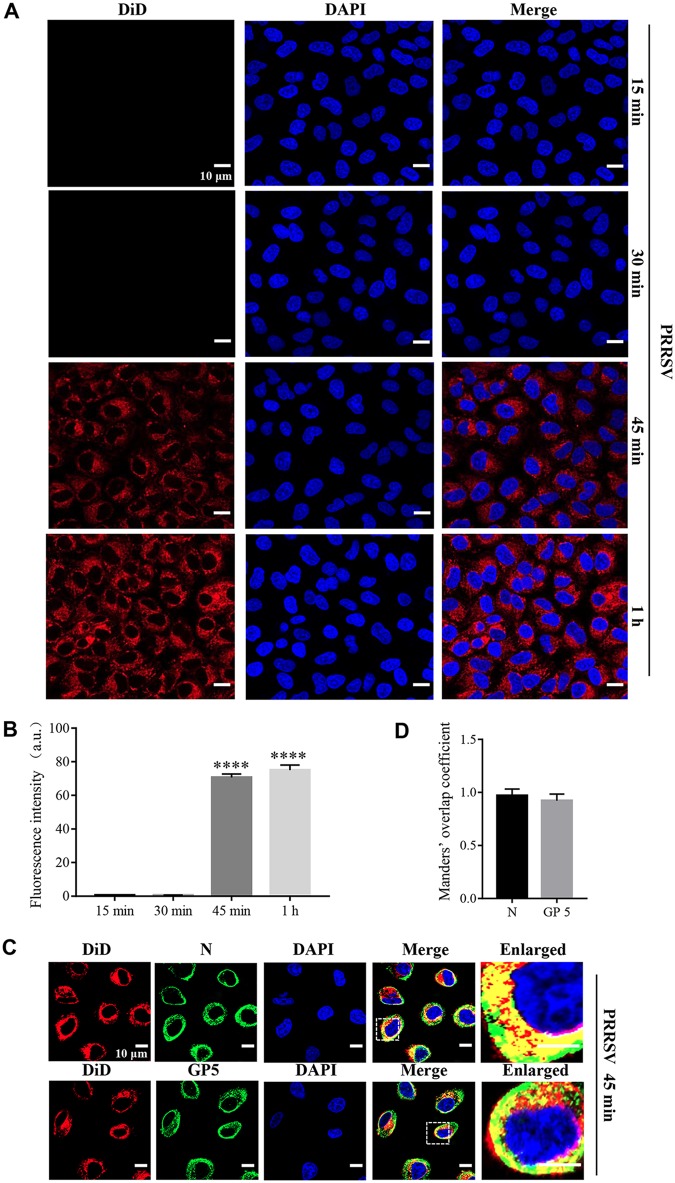
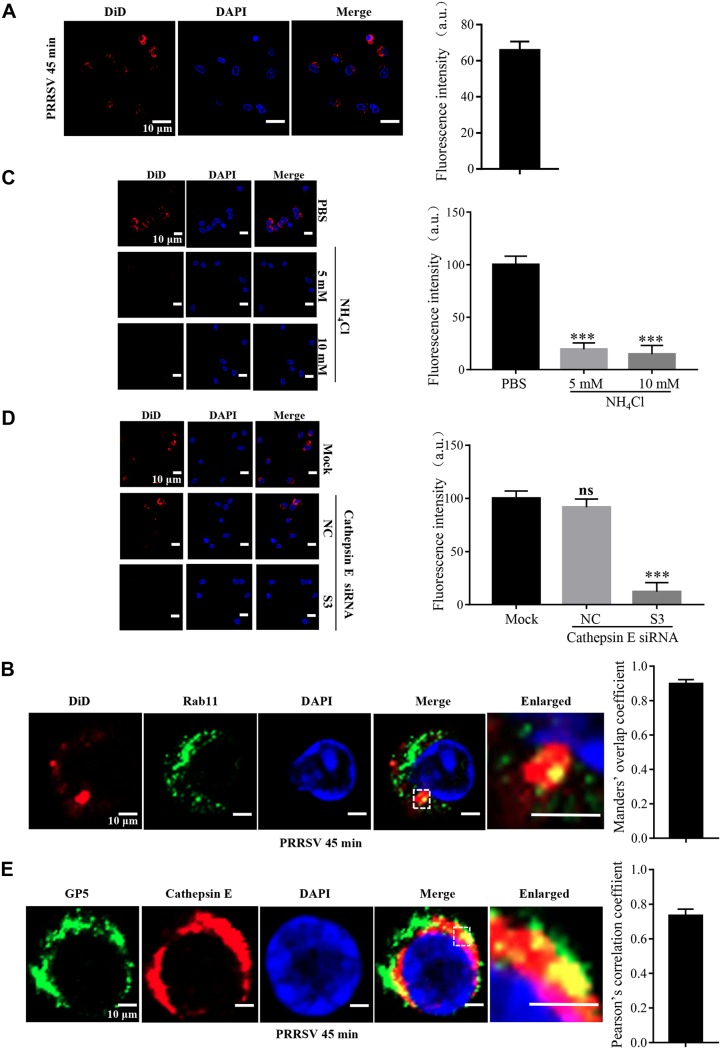
In order to visualize PRRSV membrane fusion, we labeled a typical PRRSV-2 strain, BJ-4, with a saturating amount of the lipophilic dye DiD in the envelope. DiD is applicable in membrane fusion research since the fluorescence emission from saturating DiD is low due to a self-quenching effect between neighboring dyes, while the fluorescence intensity increases (dequenching) when the molecules diffuse from the viral envelope into the host cell membrane (27, 28). We inoculated MARC-145 cells with labeled PRRSV virions at 37°C and carried out confocal microscopy for different time periods. As shown in Fig. 1A and B, we observed an increase in DiD fluorescence as early as 45 min postinfection (mpi), suggesting that PRRSV membrane fusion occurred during the early phase. To validate visualization of the labeled virions, we performed this analysis once again with specific antibodies against PRRSV nucleocapsid (N) protein and GP5 at 45 mpi, respectively, and analyzed the results using Manders’ overlap coefficient. Figure 1C and D show that DiD fluorescence did indicate the virions because of their colocalization (the value was >0.6). These results show that PRRSV membrane fusion occurs during early infection in MARC-145 cells.
FIG 1.
PRRSV membrane fusion occurs during early infection in MARC-145 cells. (A) PRRSV membrane fusion was visualized using labeled PRRSV. An evident increase of DiD fluorescence (red) was observed at as early as 45 mpi. MARC-145 cells were inoculated with labeled PRRSV virions (MOI = 10) at 37°C for the indicated time periods (15 min, 30 min, 45 min, and 1 h) and analyzed by confocal microscopy. (B) The total fluorescence intensity of DiD was calculated using ImageJ software. ****, P < 0.0001. (C) The labeled PRRSV was validated with the specific antibodies against N protein and GP5. MARC-145 cells were inoculated with labeled PRRSV virions (MOI = 10) at 37°C for 45 min and analyzed by confocal microscopy. DiD fluorescence (red) colocalized with that of PRRSV GP5 or N protein (green) at the time point. (D) The colocalization of DiD and PRRSV GP5 or N protein was assessed by determination of Manders’ overlap coefficient using Image-Pro Plus software. The mean Manders’ overlap coefficient ± the SD is representative of three individual enlarged pictures. Images were taken at a 400× or 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm.
PRRSV membrane fusion occurs in Rab11-recycling endosomes.
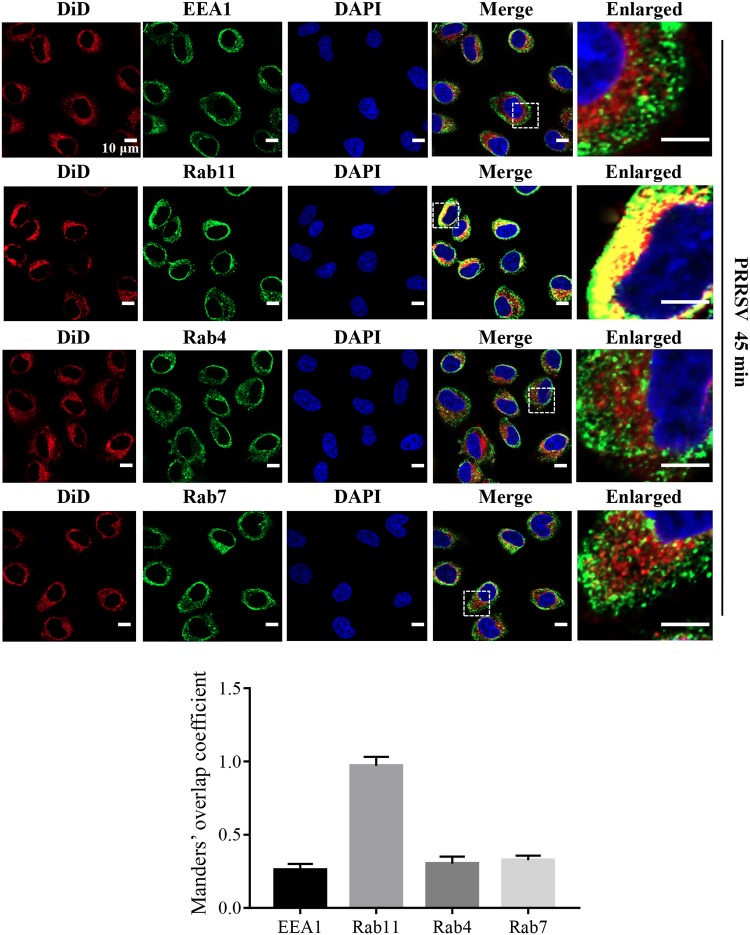
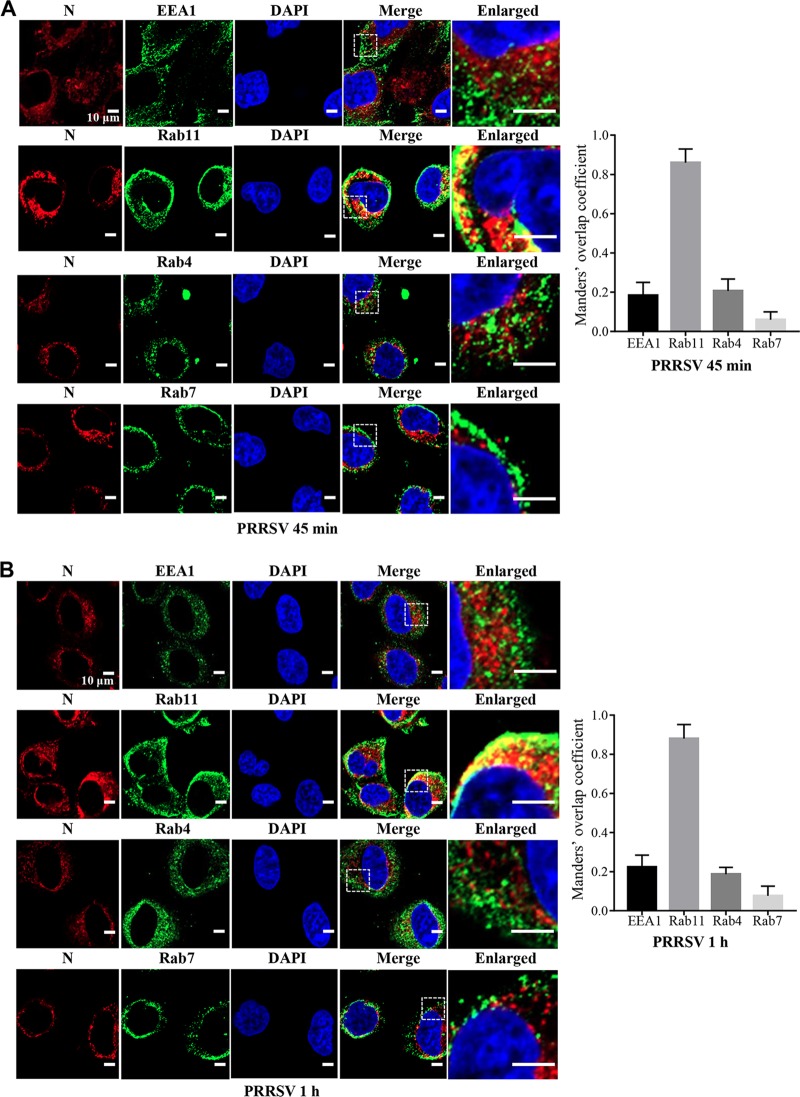
Next, we investigated where PRRSV membrane fusion occurs in MARC-145 cells using DiD-labeled PRRSV and subcellular markers through confocal microscopy (Fig. 2). We found that DiD fluorescence colocalized with Rab11-marked recycling endosomes at 45 mpi when PRRSV membrane fusion occurred (Fig. 1), rather than early endosomes (early endosome antigen 1 [EEA1]) or late endosomes (Rab7) (29, 30). Interestingly, the DiD fluorescence did not colocalize with Rab4, which mediates fast endocytic recycling directly from early endosomes (29). We further demonstrated that PRRSV was only in Rab11-recycling endosomes at 45 mpi and 1 h postinfection (hpi) during membrane fusion with the specific antibody against N protein (Fig. 3).
FIG 2.
PRRSV membrane fusion occurs in Rab11-recycling endosomes in MARC-145 cells. Colocalization of DiD fluorescence (red) and Rab11-recycling endosomes (green) was observed. MARC-145 cells were inoculated with labeled PRRSV virions (MOI = 10) at 37°C for 45 min and analyzed by confocal microscopy. Images were taken at a 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. The colocalization was assessed by determination of Manders’ overlap coefficient using Image-Pro Plus software. The mean Manders’ overlap coefficient ± the SD is representative of three individual enlarged pictures.
FIG 3.
PRRSV is present in Rab11-recycling endosomes during membrane fusion in MARC-145 cells. (A and B) PRRSV located in Rab11-recycling endosomes during membrane fusion at 45 mpi (A) and 1 hpi (B). MARC-145 cells were inoculated with labeled PRRSV virions (MOI = 10) at 37°C for 45 min and 1 h, and colocalization of PRRSV N protein (red) with Rab11-recycling endosomes (green) was observed. Images were taken at a 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. The colocalization was assessed by determination of Manders’ overlap coefficient using Image-Pro Plus software. The mean Manders’ overlap coefficient ± the SD is representative of three individual enlarged pictures.
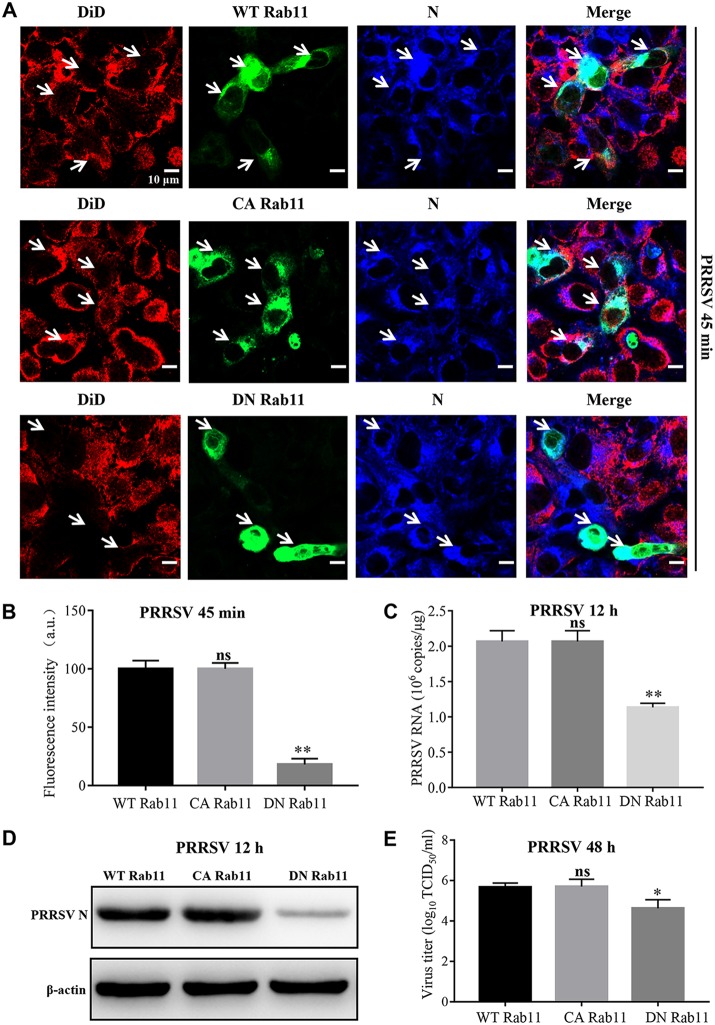
To confirm the importance of Rab 11 during PRRSV membrane fusion, confocal microscopy was conducted in the MARC-145 cells transfected with wild-type (WT), constitutive active (CA), or dominant negative (DN) Rab11. CA Rab11 is a construct with a single point mutation (Q70L) to keep constitutive activity of recycling endosomes, while DN Rab11 is a construct with a single point mutation (S25N) to confer defective function of recycling endosomes (31, 32). As shown in Fig. 4A and B, PRRSV membrane fusion was similar in CA Rab11-transfected cells to that in WT Rab11-transfected ones, but it was significantly hampered in DN Rab11-transfected ones. Subsequent PRRSV infection was inhibited by DN Rab11, as indicated by decreased open reading frame 7 (ORF7) replication (Fig. 4C) and N protein expression (Fig. 4D). Viral titration using a 50% tissue culture infective dose (TCID50) assay further showed that the progeny viral titers were reduced by ∼10-fold (∼1 log10 TCID50/ml; P < 0.05; Fig. 4E).
FIG 4.
Importance of Rab 11 for PRRSV membrane fusion. (A) PRRSV membrane fusion was hampered in DN Rab11 (green)-transfected cells, as shown by decreased DiD fluorescence (red). PRRSV was detected with the specific antibody against N protein (blue). The white arrows indicate the WT, CA, or DN Rab11-expressing MARC-145 cells. MARC-145 cells were transfected with the construct expressing WT, CA, or DN Rab11 (2.5 μg) and infected with an MOI of 10 labeled PRRSV virions at 37°C for 45 min. Images were taken at a 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. (B) The fluorescence intensity of DiD in WT, CA, or DN Rab11-expressing region was calculated using ImageJ software. (C to E) PRRSV ORF7 replication (C), N protein expression (D), and PRRSV progeny viral titers (E) were reduced in the DN Rab11-transfected cells. The infected cells were collected for PRRSV ORF7 replication by RT-qPCR at 12 hpi, N protein expression by IB at 12 hpi, or viral titers using TCID50 at 48 hpi. ns, not significant; *, P < 0.05; **, P < 0.01.
These data provide evidence that the location of PRRSV membrane fusion is the Rab11-recycling endosomes in MARC-145 cells.
Low pH and cathepsin E are critical for PRRSV membrane fusion.
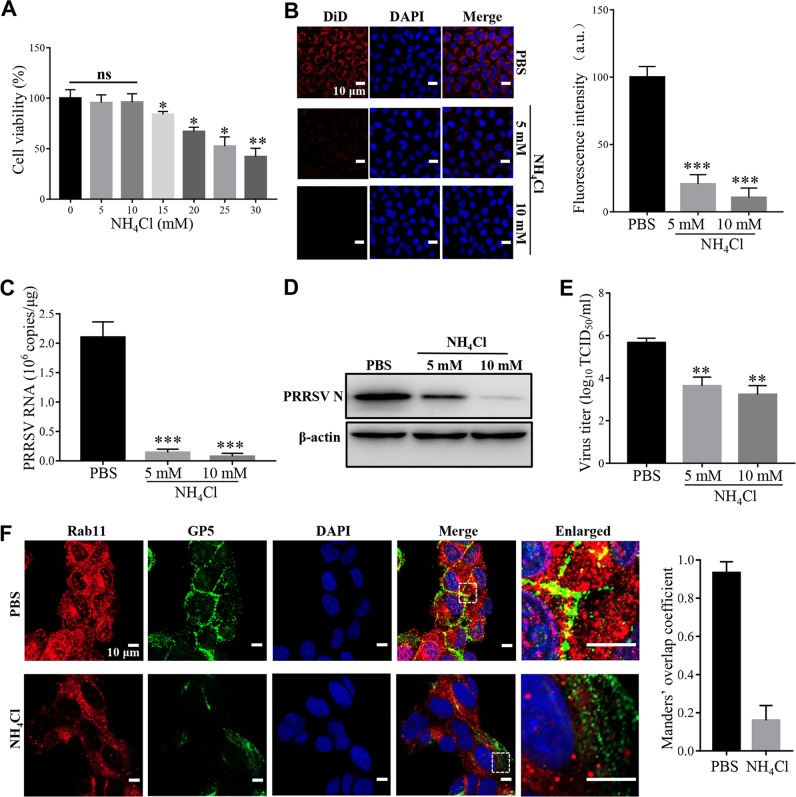
Rab11-recycling endosomes are mildly acidic (∼pH 6.5), with the presence of the aspartic proteases cathepsins D and E (33, 34). Therefore, we first studied the effect of low pH on PRRSV membrane fusion in MARC-145 cells using a noncytotoxic selective inhibitor (ammonium chloride [NH4Cl]; Fig. 5A) at 5 and 10 mM. These two doses of NH4Cl have been demonstrated to prevent endosomal acidification and have been utilized in previous studies (22, 23). Figure 5B to E show that NH4Cl at both doses significantly prevented PRRSV membrane fusion and infection, as shown by reduced DiD fluorescence, ORF7 replication, N protein expression, and progeny viral titers, respectively. The inhibition effect was probably due to the disturbance of cellular traffic since PRRSV was not present in Rab11-recycling endosomes (Fig. 5F), a finding consistent with previous studies (22, 23).
FIG 5.
Low pH is critical for PRRSV membrane fusion. (A) Cytotoxicity of the low-pH inhibitor NH4Cl. MARC-145 cells were pretreated with NH4Cl (5, 10, 15, 20, 25, or 30 mM) at 37°C for 4 h. The cell viability was then measured by using a CellTiter 96 AQueous One Solution cell proliferation assay. (B) PRRSV membrane fusion was hampered by NH4Cl as decreased DiD fluorescence (red). The cells were pretreated with NH4Cl (5 and 10 mM) at 37°C for 1 h and then inoculated with an MOI of 10 labeled PRRSV virions at 37°C for 45 min. Confocal microscopy was carried out to analyze the membrane fusion. The total fluorescence intensity of DiD was calculated using ImageJ software. (C to E) PRRSV ORF7 replication (C), N protein expression (D), and progeny viral titers (E) were reduced in the cells treated with NH4Cl (5 and 10 mM). The cells were collected for PRRSV ORF7 replication by RT-qPCR at 12 hpi, N protein expression by IB at 12 hpi, or viral titers using TCID50 at 48 hpi. (F) The inhibitor of low pH disturbed the location of PRRSV in Rab11-recycling endosomes (red). MARC-145 cells were pretreated with NH4Cl (10 mM) at 37°C for 1 h and then inoculated with an MOI of 10 PRRSV (visualized with the specific antibody against GP5, green) at 37°C for 45 min, and confocal microscopy was performed to detect the location. The colocalization was assessed by determination of Manders’ overlap coefficient using Image-Pro Plus software. The mean Manders’ overlap coefficient ± the SD is representative of three individual enlarged pictures. Images were taken at a 400× or 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
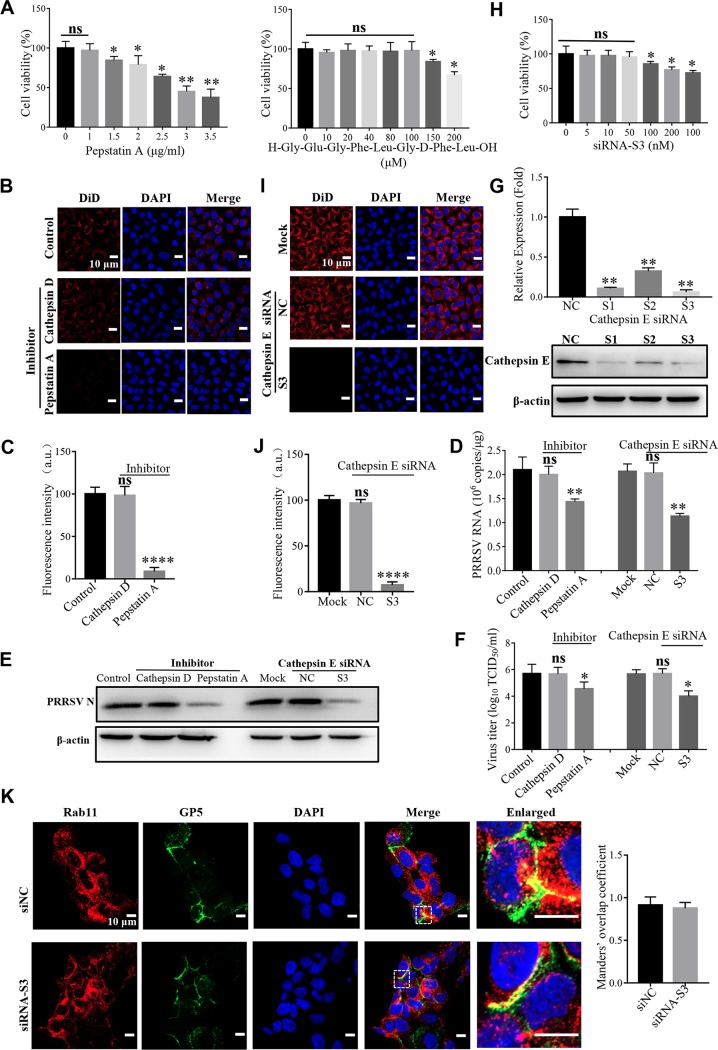
Next, we studied the effect of aspartic proteases on PRRSV membrane fusion. As shown in Fig. 6A to F, the noncytotoxic pan-aspartic protease inhibitor (pepstatin A, Fig. 6A) significantly prevented PRRSV membrane fusion, as indicated by reduced DiD fluorescence (Fig. 6B and C). PRRSV infection was also inhibited by pepstatin A, as indicated by decreased ORF7 replication (Fig. 6D), N protein expression (Fig. 6E), and progeny viral titers (Fig. 6F), respectively. Subsequently, we identified whether cathepsin D or cathepsin E took effect. A noncytotoxic specific cathepsin D inhibitor (H-Gly-Glu-Gly-Phe-Leu-Gly-d-Phe-Leu-OH) did not influence PRRSV membrane fusion and infection as pepstatin A (Fig. 6A to F). We further utilized small interference RNAs (siRNAs) targeting cathepsin E to measure its function. All the three cathepsin E-specific siRNAs (S1, S2, and S3) significantly decreased its endogenous expression at the both the mRNA level and the protein level (Fig. 6G). We exploited the noncytotoxic siRNA-S3 with the most efficient knockdown efficiency in the following assays (Fig. 6G and H). The siRNA-S3 significantly prevented PRRSV membrane fusion, as shown by reduced DiD fluorescence (Fig. 6I and J) and PRRSV infection, as indicated by decreased ORF7 replication (Fig. 6D), N protein expression (Fig. 6E), and progeny viral titers (Fig. 6F), respectively. The inhibition effect was probably due to the disturbance of the cathepsin E catalytic activity instead of cellular traffic since PRRSV was still in Rab11-recycling endosomes (Fig. 6K).
FIG 6.
Cathepsin E is critical for PRRSV membrane fusion. (A) Cytotoxicity of the pan-aspartic protease inhibitor (pepstatin A) or cathepsin D inhibitor (H-Gly-Glu-Gly-Phe-Leu-Gly-d-Phe-Leu-OH). MARC-145 cells were pretreated with pan-aspartic protease inhibitor (1, 1.5, 2, 2.5, 3, or 3.5 μg/ml) or cathepsin D inhibitor (10, 20, 40, 80, 100, 150, or 200 μM) at 37°C for 4 h. The cell viability was then measured by using a CellTiter 96 AQueous One Solution cell proliferation assay. (B and I) PRRSV membrane fusion was hampered by the inhibitor of pan-aspartic proteases or siRNA targeting cathepsin E (S3) as decreased DiD fluorescence (red). The cells were pretreated with pan-aspartic protease inhibitor (1 μg/ml) or cathepsin D inhibitor (100 μM) or transfected with siRNA-S3 or siNC (50 nM) at 37°C for 1 h and then inoculated with an MOI of 10 labeled PRRSV virions at 37°C for 45 min. Confocal microscopy was carried out to analyze membrane fusion. (C and J) Total fluorescence intensity of DiD was calculated using ImageJ software. (D to F) PRRSV ORF7 replication (D), N protein expression (E), and progeny viral titers (F) were reduced in the cells treated with pepstatin A (1 μg/ml) or transfected with siRNA-S3 (50 nM). The cells were collected for PRRSV ORF7 replication by RT-qPCR at 12 hpi, N protein expression by IB at 12 hpi, or viral titers using TCID50 at 48 hpi. (G and H) MARC-145 cells were transfected with siRNA-S3 (50 nM) at 37°C for 36 h and then lysed for RT-qPCR to examine the cathepsin E mRNA level or IB to examine the cathepsin E protein level or cytotoxicity. (K) Knockdown of cathepsin E did not affect the location of PRRSV in Rab11-recycling endosomes (red). MARC-145 cells were transfected with siRNA-S3 (50 nM) at 37°C and then inoculated with an MOI of 10 PRRSV (visualized with the specific antibody against GP5, green) at 37°C for 45 min, and confocal microscopy was performed to detect the location. Manders’ overlap coefficient was determined using Image-pro Plus software. The mean Manders’ overlap coefficient ± the SD is representative of three individual enlarged pictures. Images were taken at a 400× or 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. ns, not significant; *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
Cathepsin E interacts with PRRSV GP5.
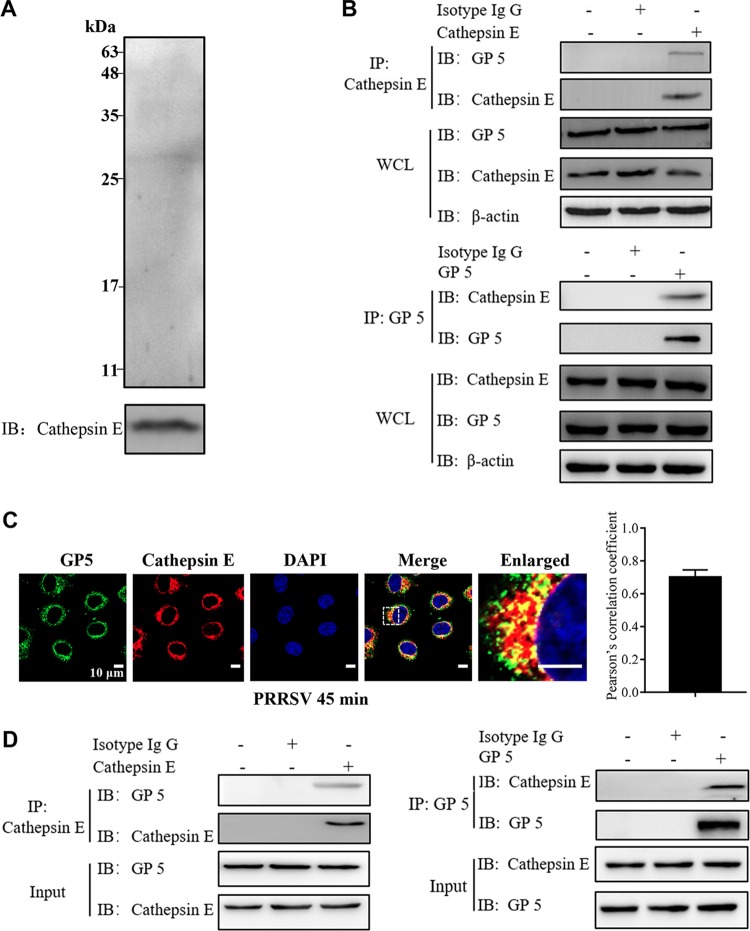
We initially performed an immunoprecipitation (IP) assay using cathepsin E as bait to identify which PRRSV structural proteins were its target in PRRSV-infected MARC-145 cells. As shown in Fig. 7A, the positive serum against PRRSV detected one band precipitated by cathepsin E in the infected cell lysates. The molecular mass of the band was between 25 and 35 kDa, presumably corresponding to PRRSV GP5. As a consequence, we utilized the specific antibody against PRRSV GP5 and detected the target protein (data not shown). We further found by IP that endogenous cathepsin E and PRRSV GP5 bound to each other in the PRRSV-infected cell lysates (Fig. 7B). We also verified their endogenous colocalization in the infected cells (Fig. 7C). The colocalization coefficient was expressed as Pearson’s correlation coefficient, and the value was >0.5, suggesting that there existed an interaction. Moreover, we performed the in vitro pulldown assay using exogenous cathepsin E and GP5 and found that cathepsin E and GP5 interacted with each other (Fig. 7D). All of these results show that cathepsin E strongly interacts with PRRSV GP5.
FIG 7.
Cathepsin E interacts with PRRSV GP5. (A) MARC-145 cells were infected with PRRSV (MOI = 10) for 45 min and then harvested. Using cathepsin E as bait, IP was conducted to identify the associated protein in WCLs with positive serum against PRRSV. (B) Endogenous cathepsin E interacted with PRRSV GP5. MARC-145 cells were infected with PRRSV (MOI = 10) for 45 min and then harvested. Using cathepsin E or PRRSV GP5 as bait, IP was conducted to identify the associated protein in WCLs with the indicated antibodies. (C) Confocal microscopy was carried out to analyze the colocalization between cathepsin E (red) and PRRSV GP5 (green) in infected MARC-145 cells. Images were taken at a 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm. Pearson’s correlation coefficient was determined using Image-pro Plus software. The mean Pearson’s correlation coefficient ± the SD is representative of three individual enlarged pictures. (D) Exogenous cathepsin E interacted with PRRSV GP5. An in vitro pulldown assay was carried out, and the target proteins were eluted and subjected to IB with the indicated antibodies.
PRRSV GP5 is cleaved by cathepsin E.
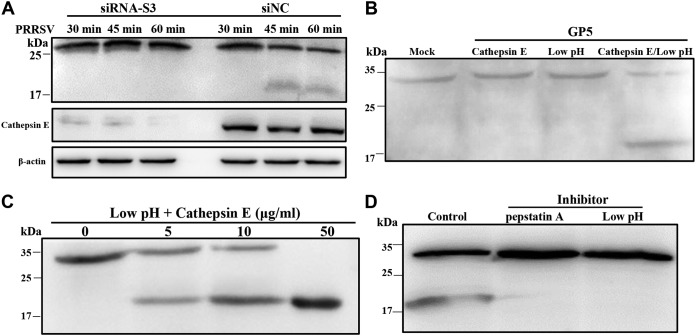
We analyzed whether PRRSV GP5 was the target cleaved by cathepsin E in PRRSV-infected MARC-145 cells. First, we observed a reduction in GP5 abundance and the appearance of a smaller band (between 17 and 25 kDa) at 45 mpi in the infected cells transfected with siRNA-negative control (NC; Fig. 8A). The time point of cleavage was coincided with that of PRRSV membrane fusion (Fig. 1). Mass spectrometry analysis confirmed the cleaved PRRSV GP5 product (data not shown). However, with the transfection of siRNA targeting cathepsin E (S3), there was no proteolytic cleavage of GP5 (Fig. 8A). Furthermore, we evaluated in vitro cleavage using exogenous cathepsin E and GP5. As illustrated in Fig. 8B, PRRSV GP5 was not cleaved by acidic buffer (pH ∼6.5) or cathepsin E alone, whereas it was cleaved by cathepsin E at approximately pH 6.5. Moreover, cathepsin E cleaved PRRSV GP5 in a dose-dependent manner, and the low pH and pan-aspartic protease inhibitors prevented the in vitro cleavage (Fig. 8C and D). These results demonstrate that PRRSV GP5 is cleaved by cathepsin E during membrane fusion.
FIG 8.
Cathepsin E cleaves PRRSV GP5. (A) PRRSV GP5 was cleaved by cathepsin E in infected MARC-145 cells. Knockdown of cathepsin E was performed in MARC-145 cells with siRNA-S3 or siRNA-NC (50 nM) and inoculated with PRRSV (MOI = 10) at 37°C for different time periods (30 min, 45 min, and 1 h). The cells were collected and subjected to IB with mouse anti-GP5 antibody or rabbit anti-cathepsin E antibody. Recombinant PRRSV GP5 was cleaved by cathepsin E under different conditions (B), with different doses (C), or with addition of the inhibitors NH4Cl and pepstatin A (D). Purified GP5 was incubated with different concentrations (0, 5, 10, or 50 μg/ml) of cathepsin E with or without the indicated inhibitors (10 mM NH4Cl and 1 μg/ml pepstatin A) for 15 min at room temperature. The samples were subjected to IB with mouse anti-PRRSV GP5 antibody.
PRRSV membrane fusion in PAMs.
Since PAMs are the PRRSV primary in vivo targets (21), we explored the viral membrane fusion in the cells. We monitored the event at the identical time point in Rab11-recycling endosomes (Fig. 9A and B). The impacts of low pH and cathepsin E on PRRSV membrane fusion were also demonstrated (Fig. 9C and D). We further found the endogenous colocalization between PRRSV GP5 and cathepsin E in the infected PAMs (Fig. 9E). These results show that the membrane fusion mechanisms we found are generic for PRRSV in both PAMs and MARC-145 cells.
FIG 9.
PRRSV membrane fusion in PAMs. PRRSV membrane fusion occurred in Rab11-recycling endosomes (B) during early infection (A). (C and D) PRRSV membrane fusion was hampered by the inhibitors of low pH and siRNA targeting cathepsin E (siRNA-S3) indicated as decreased DiD fluorescence (red). PAMs were pretreated with or without the low pH inhibitor (NH4Cl, 10 mM) or siRNA-S3 (50 nM) at 37°C for 1 h and then inoculated with an MOI of 10 labeled PRRSV virions at 37°C for 45 min. Confocal microscopy was carried out to analyze membrane fusion. The total fluorescence intensity of DiD was calculated using ImageJ software. ns, not significant; ***, P < 0.001. (E) PRRSV GP5 colocalized with cathepsin E in infected PAMs. Confocal microscopy was performed to analyze colocalization between cathepsin E (red) and PRRSV GP5 (green). Manders’ overlap coefficient or Pearson’s correlation coefficient was determined using Image-pro Plus software. The mean Manders’ overlap coefficient or Pearson’s correlation coefficient ± the SD is representative of three individual enlarged pictures. Images were taken at a 400× or 630× magnification and are representative as a single slice of a stack from three independent experiments. Representative images are shown. Scale bars, 10 μm.
DISCUSSION
To our knowledge, we observed here for the first time that PRRSV membrane fusion occurred in Rab11-recycling endosomes during early infection (Fig. 1–3 and 9). Overall, the time point of PRRSV membrane fusion that we observed is consistent with other enveloped viruses (35, 36). PRRSV was previously reported to enter CD163-positive early endosomes but not continue to late endosomes (37). In detail, PRRSV was shown to be present in EEA1-marked early endosomes at 20 and 30 mpi but absent at 45 mpi (37). We assumed that PRRSV might enter into recycling endosomes from then on, where its membrane fusion occurred (Fig. 2, 3, and 9). We further demonstrated that Rab11 was of great importance in PRRSV membrane fusion and subsequent infection (Fig. 4). Rab11 is localized primarily to perinuclear recycling endosomes and regulates a slow recycling pathway. It is associated with several steps of viral life cycles. In particular, the Rab11-mediated endocytic recycling compartment (ERC) is emerging as a critical factor in an increasing number of viral infections (38, 39). Rab11a has been recently reported to be required for PRRSV-induced autophagy to promote viral replication (40). Moreover, low pH and cathepsin E in Rab11 endosomes were shown to be essential for PRRSV membrane fusion and infection (Fig. 5, 6, and 9). As previously described (41), low pH was also crucial for the catalytic activity of cathepsin E (Fig. 8).
Proteolytic cleavage (priming) is common in controlling the activation of membrane fusion mediated by viral glycoproteins (42–44). Involvement of proteases in PRRSV infection was previously reported in PAMs, and cathepsin E especially took effect on uncoating of internalized virions and during subsequent infection (45). Here, we showed that cathepsin E interacted with PRRSV GP5 and cleaved it during viral membrane fusion (Fig. 7 and 8). As cases documented for coronaviruses in the same Nidovirales order (46), whether other host cell proteases are involved in PRRSV membrane fusion would be another intriguing issue to be studied. In fact, related work is being carried out in our laboratory.
The characterized viral fusion proteins usually contain hydrophobic regions, and their postfusion conformations are trimer-of-hairpins (2–4, 43). PRRSV GP5 is a major glycoprotein in the envelope, which consists of an N-terminal cleavable signal peptide, a long hydrophobic region, and a C-terminal hydrophilic part. Its long hydrophobic region is usually assumed to span the membrane three times (18). However, a recent review predicts that only the last part of the hydrophobic region is transmembrane, while other hydrophobic part may form a hairpin structure (47). We hypothesize that GP5, since it is similar to other viral fusion proteins in composition, might function as a viral membrane fusion protein. In the present study, we unraveled GP5 cleavage during PRRSV membrane fusion (Fig. 8). We attempted to identify the specific cleavage site(s) through site-directed mutational studies. Unfortunately, all of the chosen residues might not be involved in cleavage (data not shown). The spike glycoprotein of coronaviruses has been reported to be cleaved at multiple sites (48–50). We speculate that the cleavage sites on PRRSV GP5 may also comprise several residues instead of a just one. Simultaneous mutagenesis of the residues needs to be performed. Investigation on the membrane topology of GP5 will also contribute to testing this hypothesis.
In addition to GP5, we considered the possibility of other envelope proteins involved in PRRSV membrane fusion. PRRSV minor envelope proteins (GP2, GP3, and GP4) have been demonstrated to be a major determinant of viral tropism in cell culture (51, 52). PRRSV GP2 contains an N-terminal signal peptide, a large ectodomain, one hydrophobic transmembrane region, and a short cytoplasmic tail (53). Previous evidence shows that GP4 protein is a glycosyl-phosphatidylinositol (GPI)-modified membrane-associated protein with two predicted hydrophobic domains: an N-terminal domain as the signal peptide and a C-terminal domain to anchor the protein to membrane (54). A recent work reports that PRRSV GP3 consists of a cleaved signal peptide, a highly glycosylated domain, an unglycosylated C-terminal domain, and a hydrophobic region exhibiting an unusual hairpin-like membrane topology (amphiphilic helix) (55). Although these proteins do not comply with the properties of typical viral fusion proteins, we cannot preclude their involvement in PRRSV membrane fusion, which needs to be clarified in the future.
In conclusion, we observed that PRRSV membrane fusion occurred in Rab11-recycling endosomes during early infection. We further demonstrated that low pH and cathepsin E were crucial for PRRSV membrane fusion. Moreover, PRRSV GP5 was cleaved by cathepsin E during this process. These results deepen our understanding of PRRSV pathogenesis, and the findings with GP5 and cathepsin E may be helpful in the development of drugs and vaccines against PRRSV infection.
MATERIALS AND METHODS
Cells and viruses.
PAMs were collected from lung lavage samples obtained from 4-week-old specific-pathogen-free pigs. The collection procedure was authorized and supervised by the Ethical and Animal Welfare Committee of Key Laboratory of Animal Immunology of the Ministry of Agriculture of China (permit no. 2017006). The experiment was performed according to the Chinese Regulations of Laboratory Animals, The Guidelines for the Care of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China). PAMs were routinely maintained in Roswell Park Memorial Institute 1640 medium (RPMI 1640) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified 37°C and 5% CO2 incubator. MARC-145 and human embryonic kidney-293T (HEK293T) cell lines were kept in our laboratory and maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated FBS and antibiotics.
A typical PRRSV-2 strain BJ-4 (GenBank accession no. AF331831, isolated in 1996 in China and kindly provided by Hanchun Yang of China Agricultural University) was prepared as described in our previous study (56).
Antibodies.
Rabbit anti-EEA1 antibody (catalog no. 2411S) was purchased from Cell Signaling Technology (Boston, MA). Rabbit anti-Rab4 antibody (catalog no. ab13252), mouse anti-Rab7 antibody (catalog no. ab50533), rabbit anti-Rab11 antibody (catalog no. ab3612), rabbit anti-cathepsin E antibody (catalog no. ab36996), and horseradish peroxidase (HRP)-labeled goat anti-rabbit (catalog no. ab6721) or mouse (catalog no. ab6789) IgG antibody were purchased from Abcam (Cambridge, United Kingdom). Positive sera against PRRSV, mouse or rabbit anti-PRRSV N, and GP5 antibodies were kept in our laboratory (57). Alexa Fluor 488-goat anti-rabbit (catalog no. A-11034) or mouse (catalog no. A-11029), Alexa Fluor 647-goat anti-rabbit (catalog no. A-21245) or mouse (catalog no. A-21236), and Alexa Fluor 405-goat anti-mouse (catalog no. A-21245) antibodies were purchased from Invitrogen (Carlsbad, CA).
Reagents.
DiD (catalog no. D7757), Lipofectamine 2000 reagent (catalog no. 2066194), and Lipofectamine RNAiMAX transfection reagent (catalog no. 13778150) were purchased from Invitrogen. NAP-5 gel filtration columns (catalog no. 17-0853-02) were purchased from GE Healthcare (Boston, MA). Paraformaldehyde (PFA; catalog no. P1110) and Triton X-100 (catalog no. T8200) were purchased from Solarbio (Beijing, China). Cathepsin E (catalog no. 1294-AS) was purchased from R&D Systems (Minneapolis, MN). Low-pH inhibitor NH4Cl (catalog no. A9434) and pan-aspartic protease inhibitor pepstatin A (catalog no. EI10) were purchased from Sigma-Aldrich (St. Louis, MO). Cathepsin D inhibitor (catalog no. N-1075.0005) was purchased from Bachem (Bubendorf, Switzerland). A CellTiter 96 AQueous one solution cell proliferation kit (catalog no. G3582) was purchased from Promega (Madison, WI). Radioimmunoprecipitation assay (RIPA) lysis buffer (catalog no. P0013B) and DAPI (4′,6′-diamidino-2-phenylindole; catalog no. C1006) were purchased from Beyotime Biotechnology (Shanghai, China). Complete EDTA-free protease inhibitor cocktail (catalog no. 04693116001) and Universal SYBR green Master (catalog no. 04913914001) were purchased from Roche (Mannheim, Germany). Enhanced chemiluminescence (ECL) reagent (catalog no. P0013B) was purchased from NCM Biotechnology (Suzhou, China). Protein A/G beads (catalog no. 88802) and TRIzol reagent were purchased from Thermo Fisher Scientific (Waltham, MA).
Plasmids.
The constructs for WT (catalog no. 12674), DN (catalog no. 12678), and CA Rab11 (catalog no. 49553) were kindly provided by Richard Pagano and Marci Scidmore, respectively, via Addgene (Watertown, MA) (31, 32). The PCAGGS expression vector was kept in our laboratory.
PRRSV labeling.
PRRSV BJ-4 was labeled with lipophilic dye DiD according to the manufacturer’s instructions. Briefly, 100 ml of the PRRSV stock (2 mg/ml protein concentration) was incubated with 3 ml of 25 mM DiD dissolved in dimethyl sulfoxide for 2 h with gentle vortexing in dark at room temperature. Unincorporated dye was removed by buffer exchange into the HEPES 145 buffer (50 mM HEPES [pH 7.4], 145 mM NaCl) by using NAP-5 gel filtration columns. The labeled virions were aliquoted, snap-frozen in liquid nitrogen, and stored at –80°C. Immediately before use, the labeled virions were thawed and filtered through a 0.22-μm-pore-size syringe filter (Millipore, Boston, MA) to remove viral aggregates. The infectivity of labeled PRRSV was comparable to that of naive ones (data not shown).
Confocal microscopy.
MARC-145 cells or PAMs grown on glass coverslips in six-well plates were infected with DiD-labeled PRRSV at a multiplicity of infection (MOI) of 10 at 37°C for different time periods (15 min, 30 min, 45 min, and 1 h). At the end of each time point, cells were washed with PBS, fixed with 4% PFA for 20 min, and permeabilized with 0.1% Triton X-100 for 5 min at room temperature. For visualization of the labeled virions, the infected cells were stained with mouse or rabbit anti-PRRSV N and GP5 antibodies, respectively, followed by staining with Alexa Fluor 488-goat anti-mouse or rabbit and Alexa Fluor 405-goat anti-mouse antibodies, respectively. For visualization of intracellular markers, the infected cells were stained with rabbit anti-EEA1, rabbit anti-Rab4, rabbit anti-Rab11, or mouse anti-Rab7 antibody, followed by staining with Alexa Fluor 488-goat anti-rabbit or anti-mouse antibody. For visualization of cathepsin E, the infected cells were stained with rabbit anti-cathepsin E antibody, followed by staining with Alexa Fluor 647-goat anti-rabbit antibody. The cells were examined by a confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany). Images were taken at a 400× or 630× magnification (40× or 63× objective) and were representative as a single slice of a stack from three independent experiments (58, 59). The numerical aperture (NA) of the 40× objective is 0.95, and the NA of the 63× objective is 1.4. Quantitative analyses of the DiD fluorescence were performed using ImageJ software (60, 61). The quantitative colocalization analyses were carried out according to the method of Zinchuk and Grossenbacher-Zinchuk (62). Manders’ overlap coefficient (>0.6) indicates an actual overlap of the signals and is considered to represent the true degree of colocalization. Pearson’s correlation coefficient (>0.5) describes the correlation of the intensity distribution between channels.
Cell transfection.
MARC-145 cells were seeded at a density of 4 × 105 cells/ml culture medium overnight. The cells were then transfected with WT, CA, or DN Rab11 construct (2.5 μg) using Lipofectamine 2000 reagent according to the manufacturer’s instructions.
Quantitative real-time PCR.
Total RNAs were extracted using TRIzol reagent from the indicated cells and reverse transcribed into cDNAs by PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China) according to the manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) was performed using Universal SYBR green Master on a 7500 Fast RT-PCR system (Applied Biosystems, Foster City). PCR was conducted with 1 μl of cDNAs with primers specific for PRRSV ORF7 (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set as the endogenous control. A plasmid containing PRRSV ORF7 was used as the template to generate a standard curve, and the actual viral RNA copies were calculated based on it.
TABLE 1.
Primers for PCR, RT-qPCR, and siRNAs
| Target gene | Sequence (5′–3′) |
|
|---|---|---|
| Sense | Antisense | |
| GP5 | CACTCGGAGTGCTGGGGAAGTGCCGGAC | TCGAGATCTCTTATCGTCGTCATCCTTGTAATCCAGC |
| PRRSV-ORF7 | AAACCAGTCCAGAGGCAAGG | GCAAACTAAACTCCACAGTGTAA |
| GAPDH | CCTTCCGTGTCCCTACTGCCAAC | GACGCCTGCTTCACCACCTTCT |
| siCathepsin E-S1 | GCCCUUCCGACAAGAUUAATT | UUAAUCUUGUCGGAAGGGCTT |
| siCathepsin E-S2 | CCCAUAAUUUGGACAUGAUTT | AUCAUGUCCAAAUUAUGGGTT |
| siCathepsin E-S3 | GCUACGACCACUCCCAUUUTT | AAAUGGGAGUGGUCGUAGCTT |
| siCathepsin E-NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Immunoblotting.
The infected cells were harvested and lysed in RIPA lysis buffer containing a cocktail of protease inhibitors. Whole-cell lysates (WCLs) were normalized to equal amounts of β-actin, separated by 15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrotransferred onto Immobilon-P membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked in 5% skimmed milk for 1 h and probed with the indicated primary antibodies. After incubation with HRP-labeled goat anti-mouse or rabbit IgG antibody as the secondary antibody, the indicated proteins were visualized with ECL reagent.
PRRSV titration assay.
The treated MARC-145 cells were inoculated with PRRSV BJ-4 (MOI = 1) at 37°C for 3 h. The viruses not entering into the cells were then washed away. At 48 hpi, the virus yields were measured by a TCID50 assay in MARC-145 cells (63).
Inhibitor treatments.
MARC-145 cells or PAMs were seeded onto 24-well plates and treated with inhibitors of low pH at different concentrations (5, 10, 15, 20, 25, or 30 mM), pan-aspartic proteases at different concentrations (1, 1.5, 2, 2.5, 3, or 3.5 μg/ml), and cathepsin D at different concentrations (10, 20, 40, 80, 100, 150, or 200 μM) for 1 h at 37°C before subsequent experiments.
Cell viability assay.
The cytotoxic effect of the inhibitors for 4 h on MARC-145 cells or PAMs was evaluated with a CellTiter 96 AQueous One Solution cell proliferation assay. Briefly, the assay was performed by adding a small amount of the CellTiter 96 AQueous One Solution reagent directly to the wells, incubating for 4 h and then recording the absorbance at 490 nm with a microplate reader (Thermo Fisher Scientific).
RNA interference.
Three siRNAs targeting cathepsin E and siRNA-NC were designed and synthesized by GenePharma (Shanghai, China). In knockdown experiments, MARC-145 cells were transfected with the indicated siRNAs at a final concentration of 50 nM using Lipofectamine RNAiMAX according to the manufacturer’s instructions for 36 h. RT-qPCR and immunoblotting (IB) were used to determine the knockdown efficiencies. The cells were transfected with a potent siRNA targeting cathepsin E (S3) and then applied for subsequent experiments. The indicated siRNAs were listed in Table 1.
IP.
For IP, the indicated primary antibodies were first bound to protein A/G beads at 4°C for 4 h. The samples were subsequently incubated with the beads at 4°C overnight, and the associated proteins were tested by IB as described above.
Construction of the eukaryotic expression plasmids.
The optimized cDNA encoding GP5 was cloned into PCAGGS expression vector with the insertion of a Flag tag sequence at the C terminus of GP5 (64). This construct was verified by Sangon (Shanghai, China).
Protein expression and purification.
Eukaryotic expression was conducted by transfection of GP5 expression vector into HEK-293T cells for 48 h using Lipofectamine 2000 reagent. The transfected cells were lysed in RIPA lysis buffer supplemented with protease inhibitors and clarified by centrifugation at 12,000 rpm at 4°C for 15 min to collect supernatants. Protein A/G beads were incubated with mouse anti-GP5 antibody and WCLs at 4°C, eluted by 0.05 M glycine-HCl buffer (pH 2.2; 0.2 M glycine, 0.2 M HCl), and neutralized by 1 M Tris buffer (pH 10.4).
In vitro pulldown assay.
The primary antibodies were first bound to protein A/G beads at 4°C for 4 h and then incubated with cathepsin E or purified GP5 at 4°C for 2 h. The beads were next incubated with purified GP5 or cathepsin E at 4°C overnight. After extensive washing with Tris-buffered saline, the target proteins were eluted and subjected to IB with the indicated antibodies.
In vitro cleavage assay.
Purified GP5 was incubated with cathepsin E (10 μg/ml) under pH 6.5 for 15 min at room temperature with NH4Cl (10 mM) or pepstatin A (1 μg/ml). The samples were analyzed by IB using mouse anti-PRRSV GP5 antibody.
Statistical analysis.
Three replicates were included in all experiments, and each experiment was independently repeated at least three times. The experimental data are presented as group means and standard deviations (SD) and were analyzed by two-way analyses of variance using GraphPad software (San Diego, CA). Asterisks indicate statistical significance as follows (ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
ACKNOWLEDGMENTS
We thank Hanchun Yang from the China Agricultural University for providing PRRSV-2 strain BJ-4.
This study was supported by grants from the National Natural Science Foundation of China (31490601, 31972690, and 31602036), the Science-Technology Foundation for Outstanding Young Scientists of Henan Academy of Agricultural Sciences (2020YQ01 and 2019ZC60), the Earmarked Fund for Modern Agro-industry Technology Research System of China (CARS-35), and the Special Fund for the Henan Agriculture Research System (S2012-06). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
The authors declare there are no conflicts of interest.
REFERENCES
- 1.Chazal N, Gerlier D. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev 67:226–237. doi: 10.1128/mmbr.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison SC. 2008. Viral membrane fusion. Nat Struct Mol Biol 15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison SC. 2015. Viral membrane fusion. Virology 479–480:498–507. doi: 10.1016/j.virol.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White JM, Whittaker GR. 2016. Fusion of enveloped viruses in endosomes. Traffic 17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzin L, Alvarez M. 2003. Enfuvirtide for prophylaxis against HIV infection. N Engl J Med 349:2169–2170. doi: 10.1056/NEJM200311273492218. [DOI] [PubMed] [Google Scholar]
- 6.Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, Ploquin A, Doria-Rose NA, Staupe RP, Bailey M, Shi W, Choe M, Marcus H, Thompson EA, Cagigi A, Silacci C, Fernandez-Rodriguez B, Perez L, Sallusto F, Vanzetta F, Agatic G, Cameroni E, Kisalu N, Gordon I, Ledgerwood JE, Mascola JR, Graham BS, Muyembe-Tamfun JJ, Trefry JC, Lanzavecchia A, Sullivan NJ. 2016. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351:1339–1342. doi: 10.1126/science.aad5224. [DOI] [PubMed] [Google Scholar]
- 7.Misasi J, Gilman MS, Kanekiyo M, Gui M, Cagigi A, Mulangu S, Corti D, Ledgerwood JE, Lanzavecchia A, Cunningham J, Muyembe-Tamfun JJ, Baxa U, Graham BS, Xiang Y, Sullivan NJ, McLellan JS. 2016. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science 351:1343–1346. doi: 10.1126/science.aad6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan L, Zhang S, Wang Y, Li Y, Wang X, Yang Q. 2018. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J Virol 92:e00809-18. doi: 10.1128/JVI.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Done SH, Paton DJ. 1995. Porcine reproductive and respiratory syndrome: clinical disease, pathology, and immunosuppression. Vet Rec 136:32–35. doi: 10.1136/vr.136.2.32. [DOI] [PubMed] [Google Scholar]
- 10.Rossow KD. 1998. Porcine reproductive and respiratory syndrome. Vet Pathol 35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- 11.Lunney JK, Benfield DA, Rowland RR. 2010. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res 154:1–6. doi: 10.1016/j.virusres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtkamp DJ, Kliebenstein JB, Neumann EJ. 2013. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod 21:72–84. [Google Scholar]
- 13.Guo Z, Chen XX, Li R, Qiao S, Zhang G. 2018. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: a molecular epidemiological perspective. Virol J 15:2. doi: 10.1186/s12985-017-0910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Mushegian AR, Nibert M, Sabanadzovic S, Sanfacon H, Siddell SG, Simmonds P, Varsani A, Zerbini FM, Gorbalenya AE, Davison AJ. 2017. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch Virol 162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 15.Shi M, Lam TT, Hon CC, Hui RK, Faaberg KS, Wennblom T, Murtaugh MP, Stadejek T, Leung FC. 2010. Molecular epidemiology of PRRSV: a phylogenetic perspective. Virus Res 154:7–17. doi: 10.1016/j.virusres.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Conzelmann KK, Visser N, Van Woensel P, Thiel HJ. 1993. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology 193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijder EJ, Meulenberg JJ. 1998. The molecular biology of arteriviruses. J Gen Virol 79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- 18.Dokland T. 2010. The structural biology of PRRSV. Virus Res 154:86–97. doi: 10.1016/j.virusres.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snijder EJ, Kikkert M, Fang Y. 2013. Arterivirus molecular biology and pathogenesis. J Gen Virol 94:2141–2163. doi: 10.1099/vir.0.056341-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Kwang J, Yoon IJ, Joo HS, Frey ML. 1993. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol 133:477–483. doi: 10.1007/bf01313785. [DOI] [PubMed] [Google Scholar]
- 21.Duan X, Nauwynck HJ, Pensaert MB. 1997. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol 56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- 22.Kreutz LC, Ackermann MR. 1996. Porcine reproductive and respiratory syndrome virus enters cells through a low pH-dependent endocytic pathway. Virus Res 42:137–147. doi: 10.1016/0168-1702(96)01313-5. [DOI] [PubMed] [Google Scholar]
- 23.Nauwynck HJ, Duan X, Favoreel HW, Van Oostveldt P, Pensaert MB. 1999. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J Gen Virol 80:297–305. doi: 10.1099/0022-1317-80-2-297. [DOI] [PubMed] [Google Scholar]
- 24.Van Breedam W, Delputte PL, Van Gorp H, Misinzo G, Vanderheijden N, Duan X, Nauwynck HJ. 2010. Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage. J Gen Virol 91:1659–1667. doi: 10.1099/vir.0.020503-0. [DOI] [PubMed] [Google Scholar]
- 25.Shi C, Liu Y, Ding Y, Zhang Y, Zhang J. 2015. PRRSV receptors and their roles in virus infection. Arch Microbiol 197:503–512. doi: 10.1007/s00203-015-1088-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Yoo D. 2015. PRRS virus receptors and their role for pathogenesis. Vet Microbiol 177:229–241. doi: 10.1016/j.vetmic.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. 2003. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci U S A 100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Sun E, Bujny MV, Kim D, Davidson MW, Zhuang X. 2013. Dual function of CD81 in influenza virus uncoating and budding. PLoS Pathog 9:e1003701. doi: 10.1371/journal.ppat.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 30.Nour AM, Modis Y. 2014. Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol 24:449–454. doi: 10.1016/j.tcb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choudhury A, Dominguez M, Puri V, Sharma DK, Narita K, Wheatley CL, Marks DL, Pagano RE. 2002. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J Clin Invest 109:1541–1550. doi: 10.1172/JCI0215420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortes C, Rzomp KA, Tvinnereim A, Scidmore MA, Wizel B. 2007. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun 75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida-Yamamoto A, Kishibe M, Takahashi H, Iizuka H. 2007. Rab11 is associated with epidermal lamellar granules. J Invest Dermatol 127:2166–2170. doi: 10.1038/sj.jid.5700850. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T. 2013. Emerging roles of recycling endosomes. J Biochem 153:505–510. doi: 10.1093/jb/mvt034. [DOI] [PubMed] [Google Scholar]
- 35.White J, Matlin K, Helenius A. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol 89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A 102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Gorp H, Van Breedam W, Delputte PL, Nauwynck HJ. 2009. The porcine reproductive and respiratory syndrome virus requires trafficking through CD163-positive early endosomes, but not late endosomes, for productive infection. Arch Virol 154:1939–1943. doi: 10.1007/s00705-009-0527-1. [DOI] [PubMed] [Google Scholar]
- 38.Guichard A, Nizet V, Bier E. 2014. RAB11-mediated trafficking in host-pathogen interactions. Nat Rev Microbiol 12:624–634. doi: 10.1038/nrmicro3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vale-Costa S, Amorim MJ. 2016. Recycling endosomes and viral infection. Viruses 8:64. doi: 10.3390/v8030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Li S, Worku T, Hao X, Yang L, Zhang S. 2017. Rab11a is required for porcine reproductive and respiratory syndrome virus induced autophagy to promote viral replication. Biochem Biophys Res Commun 492:236–242. doi: 10.1016/j.bbrc.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 41.Athauda SB, Takahashi T, Inoue H, Ichinose M, Takahashi K. 1991. Proteolytic activity and cleavage specificity of cathepsin E at the physiological pH as examined towards the B chain of oxidized insulin. FEBS Lett 292:53–56. doi: 10.1016/0014-5793(91)80832-n. [DOI] [PubMed] [Google Scholar]
- 42.Heald-Sargent T, Gallagher T. 2012. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses 4:557–580. doi: 10.3390/v4040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kielian M. 2014. Mechanisms of virus membrane fusion proteins. Annu Rev Virol 1:171–189. doi: 10.1146/annurev-virology-031413-085521. [DOI] [PubMed] [Google Scholar]
- 44.Podbilewicz B. 2014. Virus and cell fusion mechanisms. Annu Rev Cell Dev Biol 30:111–139. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- 45.Misinzo GM, Delputte PL, Nauwynck HJ. 2008. Involvement of proteases in porcine reproductive and respiratory syndrome virus uncoating upon internalization in primary macrophages. Vet Res 39:55. doi: 10.1051/vetres:2008031. [DOI] [PubMed] [Google Scholar]
- 46.Millet JK, Whittaker GR. 2015. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res 202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veit M, Matczuk AK, Sinhadri BC, Krause E, Thaa B. 2014. Membrane proteins of arterivirus particles: structure, topology, processing and function. Virus Res 194:16–36. doi: 10.1016/j.virusres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosch BJ, Bartelink W, Rottier PJ. 2008. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol 82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JE, Li K, Barlan A, Fehr AR, Perlman S, McCray PB Jr, Gallagher T. 2016. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc Natl Acad Sci U S A 113:12262–12267. doi: 10.1073/pnas.1608147113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belouzard S, Chu VC, Whittaker GR. 2009. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A 106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das PB, Dinh PX, Ansari IH, de Lima M, Osorio FA, Pattnaik AK. 2010. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J Virol 84:1731–1740. doi: 10.1128/JVI.01774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian D, Wei Z, Zevenhoven-Dobbe JC, Liu R, Tong G, Snijder EJ, Yuan S. 2012. Arterivirus minor envelope proteins are a major determinant of viral tropism in cell culture. J Virol 86:3701–3712. doi: 10.1128/JVI.06836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meulenberg JJ, Petersen-den Besten A. 1996. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology 225:44–51. doi: 10.1006/viro.1996.0573. [DOI] [PubMed] [Google Scholar]
- 54.Du Y, Pattnaik AK, Song C, Yoo D, Li G. 2012. Glycosyl-phosphatidylinositol (GPI)-anchored membrane association of the porcine reproductive and respiratory syndrome virus GP4 glycoprotein and its colocalization with CD163 in lipid rafts. Virology 424:18–32. doi: 10.1016/j.virol.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Krabben L, Wang F, Veit M. 2018. Glycoprotein 3 of porcine reproductive and respiratory syndrome virus exhibits an unusual hairpin-like membrane topology. J Virol 92:e00660-18. doi: 10.1128/JVI.00660-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma H, Jiang L, Qiao S, Zhi Y, Chen XX, Yang Y, Huang X, Huang M, Li R, Zhang GP. 2017. The crystal structure of the fifth scavenger receptor cysteine-rich domain of porcine CD163 reveals an important residue involved in porcine reproductive and respiratory syndrome virus infection. J Virol 91:e01897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Li R, Chen XX, Zhi Y, Deng R, Zhou EM, Qiao S, Zhang G. 2019. Nonmuscle myosin heavy chain IIA recognizes sialic acids on sialylated RNA viruses to suppress proinflammatory responses via the DAP12-Syk pathway. mBio 10:e00574-19. doi: 10.1128/mBio.00574-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C-C, Zhang Y-N, Li Z-Y, Hou J-X, Zhou J, Kan L, Zhou B, Chen P-Y. 2017. Rab5 and Rab11 are required for clathrin-dependent endocytosis of Japanese encephalitis virus in BHK-21 cells. J Virol 91:e01113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y-N, Liu Y-Y, Xiao F-C, Liu C-C, Liang X-D, Chen J, Zhou J, Baloch AS, Kan L, Zhou B, Qiu H-J. 2018. Rab5, Rab7, and Rab11 are required for caveola-dependent endocytosis of classical swine fever virus in porcine alveolar macrophages. J Virol 92:e00797-18. doi: 10.1128/JVI.00797-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen EC. 2013. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 296:378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]
- 61.Wiesmann V, Franz D, Held C, Münzenmayer C, Palmisano R, Wittenberg T. 2015. Review of free software tools for image analysis of fluorescence cell micrographs. J Microsc 257:39–53. doi: 10.1111/jmi.12184. [DOI] [PubMed] [Google Scholar]
- 62.Zinchuk V, Grossenbacher-Zinchuk O. 2009. Recent advances in quantitative colocalization analysis: focus on neuroscience. Prog Histochem Cytochem 44:125–172. doi: 10.1016/j.proghi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints12. Am J Epidemiol 27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 64.Hou YH, Chen J, Tong GZ, Tian ZJ, Zhou YJ, Li GX, Li X, Peng JM, An TQ, Yang HC. 2008. A recombinant plasmid coexpressing swine ubiquitin and the GP5 encoding-gene of porcine reproductive and respiratory syndrome virus induces protective immunity in piglets. Vaccine 26:1438–1449. doi: 10.1016/j.vaccine.2007.12.057. [DOI] [PubMed] [Google Scholar]