Although a number of transcription factors have been reported to be involved in HPV gene expression, little is known about the cofactors that support HPV transcription. In this study, we demonstrate that the transcriptional cofactor VGLL1 plays a prominent role in HPV early gene expression, dependent on its association with the transcription factor TEAD1. Whereas TEAD1 is ubiquitously expressed in a variety of tissues, VGLL1 displays tissue-specific expression and is implicated in the development and differentiation of epithelial lineage tissues, where HPV gene expression occurs. Our results suggest that VGLL1 may contribute to the epithelial specificity of HPV gene expression, providing new insights into the mechanisms that regulate HPV infection. Further, VGLL1 is also critical for the growth of cervical cancer cells and may represent a novel therapeutic target for HPV-associated cancers.
KEYWORDS: HPV, gene expression, transcription cofactor, transcription factor
ABSTRACT
The TEAD family of transcription factors requires associating cofactors to induce gene expression. TEAD1 is known to activate the early promoter of human papillomavirus (HPV), but the precise mechanisms of TEAD1-mediated transactivation of the HPV promoter, including its relevant cofactors, remain unexplored. Here, we reveal that VGLL1, a TEAD-interacting cofactor, contributes to HPV early gene expression. Knockdown of VGLL1 and/or TEAD1 led to a decrease in viral early gene expression in human cervical keratinocytes and cervical cancer cell lines. We identified 11 TEAD1 target sites in the HPV16 long control region (LCR) by in vitro DNA pulldown assays; 8 of these sites contributed to the transcriptional activation of the early promoter in luciferase reporter assays. VGLL1 bound to the HPV16 LCR via its interaction with TEAD1 both in vitro and in vivo. Furthermore, introducing HPV16 and HPV18 whole genomes into primary human keratinocytes led to increased levels of VGLL1, due in part to the upregulation of TEADs. These results suggest that multiple VGLL1/TEAD1 complexes are recruited to the LCR to support the efficient transcription of HPV early genes.
IMPORTANCE Although a number of transcription factors have been reported to be involved in HPV gene expression, little is known about the cofactors that support HPV transcription. In this study, we demonstrate that the transcriptional cofactor VGLL1 plays a prominent role in HPV early gene expression, dependent on its association with the transcription factor TEAD1. Whereas TEAD1 is ubiquitously expressed in a variety of tissues, VGLL1 displays tissue-specific expression and is implicated in the development and differentiation of epithelial lineage tissues, where HPV gene expression occurs. Our results suggest that VGLL1 may contribute to the epithelial specificity of HPV gene expression, providing new insights into the mechanisms that regulate HPV infection. Further, VGLL1 is also critical for the growth of cervical cancer cells and may represent a novel therapeutic target for HPV-associated cancers.
INTRODUCTION
Human papillomaviruses (HPVs) are a family of nonenveloped DNA viruses having an approximately 8,000-bp, circular double-stranded DNA genome (1, 2). Some HPVs are high-risk genotypes, including HPV16 and HPV18, and are the primary cause of cervical, vulvar, vaginal, anal, penile, and oropharyngeal cancers (1). The life cycle of HPV is initiated by infection of epithelial basal cells, followed by the establishment of viral genomes as extrachromosomal episomes in undifferentiated cells. Upon differentiation of the infected epithelial cells, HPV undergoes vegetative replication and capsid production, generating progeny virions (1–3). In high-risk HPV infection, viral genomes often become integrated into the host genome during persistent infection, leading to the high-level expression of the viral oncoproteins E6 and E7 (1, 4). The deregulated expression of E6 and E7 inactivates the p53 and pRb tumor suppressor pathways, respectively, driving cell immortalization and carcinogenesis (1, 4).
Expression of early genes during the HPV life cycle is regulated in an epithelial cell-specific manner by the long control region (LCR), which encompasses a region of about 750 bp between the L1 and E6 genes (3, 5). The LCR harbors a number of binding sequences for cellular transcription factors (TFs), including the transcriptional activators AP1, NF1, Oct1, and Skn-1a and the transcriptional repressors YY1 and CDP (5–15). These host TFs regulate the viral early promoter (P97 for HPV16 and P105 for HPV18), which is located at the 3′ end of the LCR and which drives the expression of early genes, including E6 and E7. The early promoter is also regulated by the viral E2 protein, which recognizes specific sequences in the LCR, and E2 transcriptional activity is further modulated by its spliced isoform, E8̂E2 (16). The central portion of the LCR also contains enhancer elements that dictate the specificity of viral gene expression in epithelial cells (3, 5, 8, 17), and studies suggest that NF1 and AP1 contribute to the enhancer function (5–10). Nevertheless, the mechanisms underlying epithelial cell-specific viral gene expression are incompletely understood. The viral promoter remains active in HPV-positive cancer cells, leading to the continuous expression of E6 and E7. Therefore, the mechanisms that regulate the HPV early promoter are of particular importance in the context of tumor biology.
TEAD-family proteins are evolutionarily conserved, ubiquitously expressed TFs that bind to DNA via the consensus sequence AGGAATG (18–20) and include four member proteins in mammals: TEAD1 (originally referred to as TEF-1), TEAD2, TEAD3, and TEAD4. The TEADs themselves show no transcriptional activity and require cofactors to exert transactivation potential (20). Several cofactors directly interact with TEADs, including yes-associated protein (YAP), transcriptional coactivator with PDZ-binding motif (TAZ), vestigial-like proteins (VGLL1, VGLL2, VGLL3, VGLL4), and the p160 family of nuclear receptor coactivators (20).
Human TEAD1 was originally identified as a TF that binds to and activates the simian virus 40 enhancer (21, 22), and in the early 1990s, it was reported to be involved in the regulation of the HPV early promoter (23). In that study, TEAD1 was shown to bind to the HPV16 LCR, and mutations within the binding sites abrogated the activity of the HPV16 early promoter (23). However, no further progress has been made in deciphering the detailed mechanisms of action of TEAD1 on the LCR and its relevant cofactors for HPV gene regulation.
In this study, by employing small interfering RNA (siRNA) knockdown techniques, we demonstrate that TEAD1 is essential for the induction of early gene expression from the HPV16 and HPV18 genomes in human cervical keratinocytes and cervical cancer cell lines. A total of 11 TEAD1 target sites, including 3 previously identified sites (23), were found to be widely distributed throughout the central LCR. Eight of the 11 sites contributed to maintaining the full activity of the early promoter. Intriguingly, an epithelial cell-related transcriptional cofactor, VGLL1, was critical for the transcription of viral early genes, and this activity depended on its association with TEAD1. VGLL1 may partly explain the epithelial cell specificity of HPV gene expression.
RESULTS
TEAD1 and TEAD4 regulate HPV early gene expression.
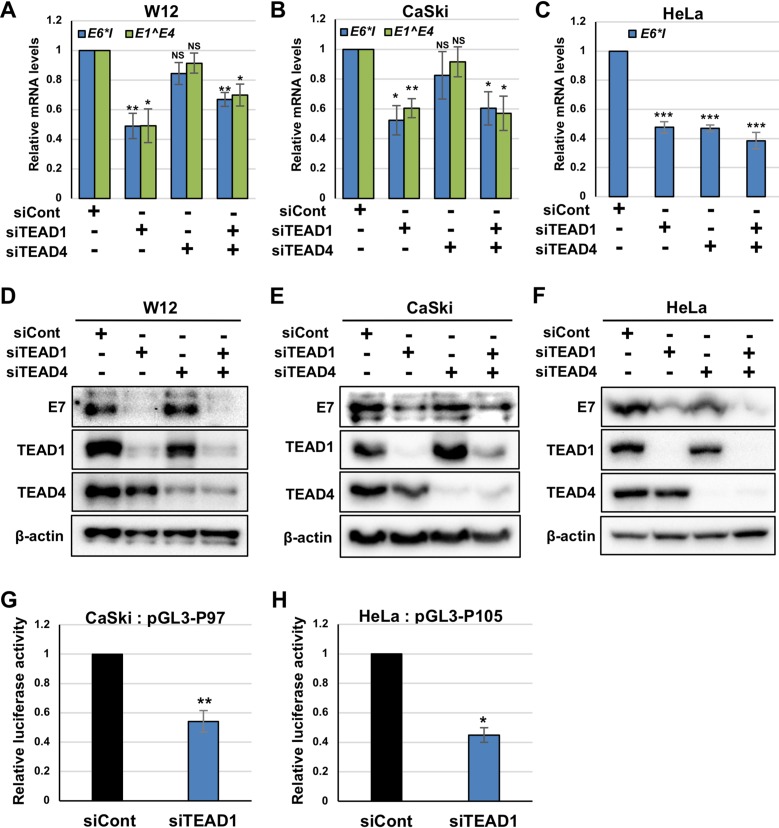
A previous study using plasmid reporter assays demonstrated that TEAD1 is involved in HPV16 gene expression (23), but its role in the transcriptional regulation of authentic HPV genomes has not been investigated. We previously showed that TEAD1 and TEAD4 are expressed in human keratinocytes and are upregulated by HPV E6/E7 (24). To investigate whether TEAD1 and TEAD4 are required for HPV gene expression, we performed siRNA knockdown experiments in W12 cells. W12 cells are human cervical keratinocytes that contain HPV16 episomes and that are derived from cervical intraepithelial neoplasia grade I (CIN1) lesions. We cultured W12 cells under undifferentiated conditions and transfected them with siRNA against TEAD1 (siTEAD1) and/or TEAD4 (siTEAD4). At 2 days after transfection, we quantified the levels of E6*I and E1̂E4 mRNAs by reverse transcription-quantitative PCR (RT-qPCR). E6*I mRNA is a spliced isoform of E6 transcripts expressed from the early promoter (25). Although E1̂E4 mRNA can be transcribed from the viral late promoter, located in the E7 gene, the majority (95%) of the transcripts detected in undifferentiated W12 cells arise from the early promoter (26). The knockdown of TEAD1 but not that of TEAD4 led to significantly reduced levels of E6*I and E1̂E4 mRNAs in W12 cells (Fig. 1A). The simultaneous knockdown of TEAD1 and TEAD4 decreased early gene transcription to levels that were almost comparable to those observed with TEAD1 knockdown alone, suggesting a negligible role of TEAD4 in transcriptional regulation.
FIG 1.
TEAD1 and TEAD4 regulate HPV early gene expression. (A to F) W12 (A and D), CaSki (B and E), and HeLa (C and F) cells were transfected with the indicated siRNA. At 2 days after transfection, the levels of HPV16 E6*I and E1̂E4 mRNAs (A and B) and HPV18 E6*I mRNA (C) were quantified by RT-qPCR and normalized to the level of GAPDH mRNA. The HPV16 E7 (D and E) and HPV18 E7 (F) proteins were detected by immunoblotting with anti-HPV16 and anti-HPV18 E7 antibodies, respectively. The effects of siRNA were verified by immunoblotting with anti-TEAD1 and anti-TEAD4 antibodies. β-Actin was used as the loading control. (G and H) CaSki (G) and HeLa (H) cells were transfected with the indicated siRNAs. Six hours later, the transfected CaSki and HeLa cells were further transfected with pGL3-P97 and pGL3-P105, respectively, together with the Renilla luciferase plasmid. At 2 days after transfection, firefly luciferase activity was measured and normalized to the Renilla luciferase activity after background subtraction. The quantitative data are the averages from three independent experiments, with the error bars representing the standard deviations. P values were determined by Student’s t test. NS, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Similarly, the knockdown of TEAD1 but not TEAD4 reduced the levels of E6*I and E1̂E4 mRNAs in CaSki cells, a cervical cancer cell line bearing integrated HPV16 genomes (Fig. 1B). In contrast, transfection of siTEAD1 and siTEAD4 similarly decreased the level of E6*I mRNA in HeLa cells, a cervical cancer cell line with integrated HPV18 genomes (Fig. 1C). Further, cotransfection of siTEAD1 and siTEAD4 did not enhance the reducing effect of siTEAD1 in CaSki and HeLa cells, suggesting no redundancy between TEAD1 and TEAD4. Western blot analyses confirmed the efficient and specific depletion of TEAD1 and TEAD4 by transfection of siTEAD1 and siTEAD4, respectively (Fig. 1D to F).
We verified the role of TEAD1 in viral early gene expression by assessing the protein levels of E7. The levels of E7 were greatly reduced in TEAD1-knockdown cells, whereas TEAD4 knockdown only slightly decreased the levels of E7 in HeLa cells (Fig. 1D to F). Overall, our data suggest that TEAD1 and TEAD4 regulate viral gene expression in undifferentiated keratinocytes and cancer cell lines, with a greater contribution from TEAD1.
Next, we performed luciferase reporter assays to examine how TEAD1 knockdown affects HPV early promoter activity. We cotransfected CaSki cells with siTEAD1 and a reporter plasmid containing the HPV16 LCR upstream of the luciferase gene (pGL3-P97) and measured luciferase activity 2 days after transfection. We found that the HPV16 P97 promoter activity was reduced in CaSki cells with TEAD1 knockdown relative to that in the controls (Fig. 1G). Moreover, the knockdown of TEAD1 also decreased the HPV18 P105 promoter activity in HeLa cells (Fig. 1H). These results suggest that TEAD1 is required for efficient transcription from the HPV early promoter in cervical cancer cells.
TEAD1 binds to the HPV16 LCR.
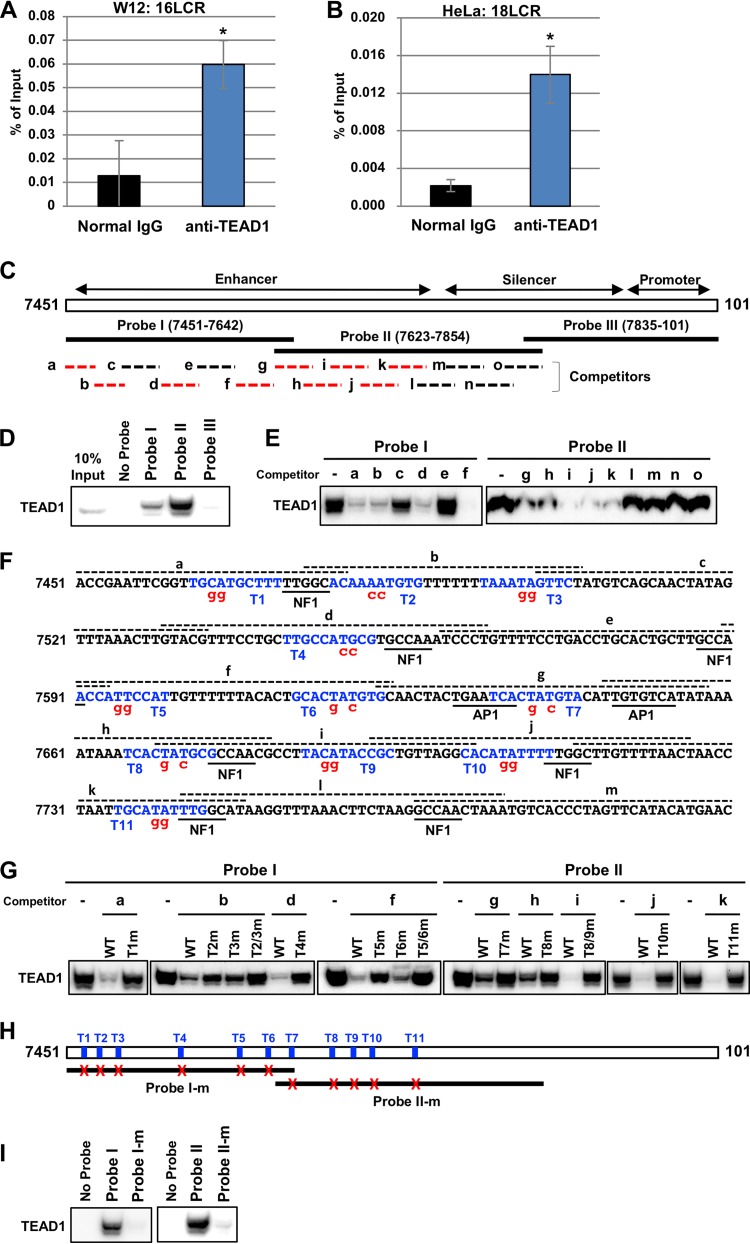
Previous studies showed that TEAD1 binds to the LCRs of HPV16 and HPV31 in vitro in electrophoretic mobility shift assays (23, 27). However, it remained unknown whether TEAD1 binds to the HPV LCR in cells. To address the in vivo association of TEAD1 with the LCR, we performed chromatin immunoprecipitation (ChIP). ChIP revealed that TEAD1 bound to the HPV16 LCR in W12 cells (Fig. 2A) and the HPV18 LCR in HeLa cells (Fig. 2B). Thus, endogenous TEAD1 binds to the HPV LCR within the cell.
FIG 2.
TEAD1 binds to the HPV LCRs. (A and B) Cross-linked chromatin prepared from W12 (A) and HeLa (B) cells was immunoprecipitated with an anti-TEAD1 antibody or normal rabbit IgG, and the recovered DNA was quantified by real-time PCR with primers for the HPV16 (A) and HPV18 (B) LCRs, respectively (16LCR and 18LCR, respectively). The levels of TEAD1 binding to the HPV16 or HPV18 genome are shown as percentages of the amount of input DNA. The data are averages from three experiments performed using independent chromatin preparations, with the error bars representing standard deviations. *, P < 0.05 (Student’s t test). (C) Schematic representation of the HPV16 LCR (nt 7451 to 101). The enhancer, silencer, and promoter regions are defined according to a previous study (52). The biotinylated DNA probes (probes I, II, and III) used in the DNA pulldown assays are indicated by solid lines. Oligonucleotide competitors (competitors a to o) are indicated by dashed lines. The competitors indicated by the red dashed lines inhibited TEAD1 binding to the probes (data shown in panel E). (D) The indicated biotinylated DNA probes were coupled to Dynabeads/M-280 streptavidin and incubated with HeLa nuclear extract; 10% of the input volume and the entire precipitated fractions were analyzed by immunoblotting using anti-TEAD1 antibody. (E) Unlabeled oligonucleotide competitors (a to o) were added to the binding reaction mixture, and the competition of TEAD1 binding against probe I or II was examined by DNA pulldown assays, as described in the legend to panel D. (F) Nucleotide sequence spanning the region from nt 7451 to 7800 of the HPV16 LCR. The TEAD-binding motifs (T1 to T11) are indicated in blue. The regions corresponding to the competitors (competitors a to m) are indicated by dashed lines over the nucleotide sequence. The nucleotide sequences in the mutant competitors and probes are denoted in red. The previously identified binding motifs for NF1 and AP1 are underlined. (G) Unlabeled oligonucleotide competitors or those having mutations were added to the binding reaction mixtures, and inhibition of TEAD1 binding to probe I or II was examined by DNA pulldown assays as described in the legend to panel D. (H) Schematic representation of the 11 TEAD1 target sites in the HPV16 LCR and the mutant probes (probes I-m and II-m). The TEAD1 target sites are indicated by blue bars (T1 to T11), and the mutated target sites in probes I-m and II-m are indicated by red crosses. (I) TEAD1 binding to the mutant probes was examined by DNA pulldown assays.
Next, we performed in vitro DNA pulldown assays to find TEAD-binding sites in the HPV16 LCR. Three biotinylated DNA probes (probes I, II, and III, shown in Fig. 2C), spanning nucleotides (nt) 7451 to 101 of the HPV16 genome, were incubated with the HeLa cell nuclear extract and affinity purified, and bound TEAD1 was detected by Western blot analysis. We found that TEAD1 bound to probes I (nt 7451 to 7642) and II (nt 7623 to 7854) but not to probe III (nt 7835 to 101) (Fig. 2D). To decipher the regions containing TEAD1-binding sites, we performed pulldown assays with oligonucleotide competitors (competitors a to o in Fig. 2C). We found that competitors a, b, d, and f but not competitors c and e effectively blocked TEAD1 binding to probe I and that competitors g, h, i, j, and k but not competitors l, m, n, and o suppressed the binding to probe II (Fig. 2E). Thus, TEAD1-binding sites are distributed throughout the enhancer region of the HPV16 LCR (Fig. 2C, red) but not in the silencer and promoter regions.
We used the JASPAR database (http://jaspar.genereg.net) (28) to search for TF binding sites and identified a number of putative TEAD-binding motifs within the oligonucleotide competitors spanning the enhancer region of the HPV16 LCR. To uncover TEAD target sites in the LCR, we performed DNA pulldown assays using competitors with mutations in the predicted motifs. We found that mutating CA to GG in the TEAD-binding motif designated “T1” restored TEAD1 binding to probe I (Fig. 2F and G), demonstrating that TEAD1 recognizes T1. Mutating either T2 or T3 partially recovered TEAD1 binding to probe I, and mutating both T2 and T3 together completely restored the binding (Fig. 2F and G), revealing that TEAD1 recognizes both T2 and T3. Using this approach, we uncovered a total of 11 potential TEAD1-binding sites (sites T1 to T11) in the enhancer region of the HPV16 LCR (Fig. 2F and G). Furthermore, introducing mutations into all target sites in probes I and II (probes I-m and II-m, respectively) greatly reduced TEAD1 binding to the probes (Fig. 2H and I). Of these 11 TEAD1 target sites, 3 sites (T9, T10, and T11) were previously demonstrated to be bound by TEAD1 (23). Thus, eight TEAD1 target sites (T1 to T8) were additionally identified in this study. Interestingly, several sites (T1, T2, T4, T5, T7, T8, T10, and T11) are flanked by or partially overlapped the binding sites for NF1 or AP1 (Fig. 2F), both of which play important roles in HPV early gene expression (5–10).
TEAD1 target sites control the activity of the HPV early promoter.
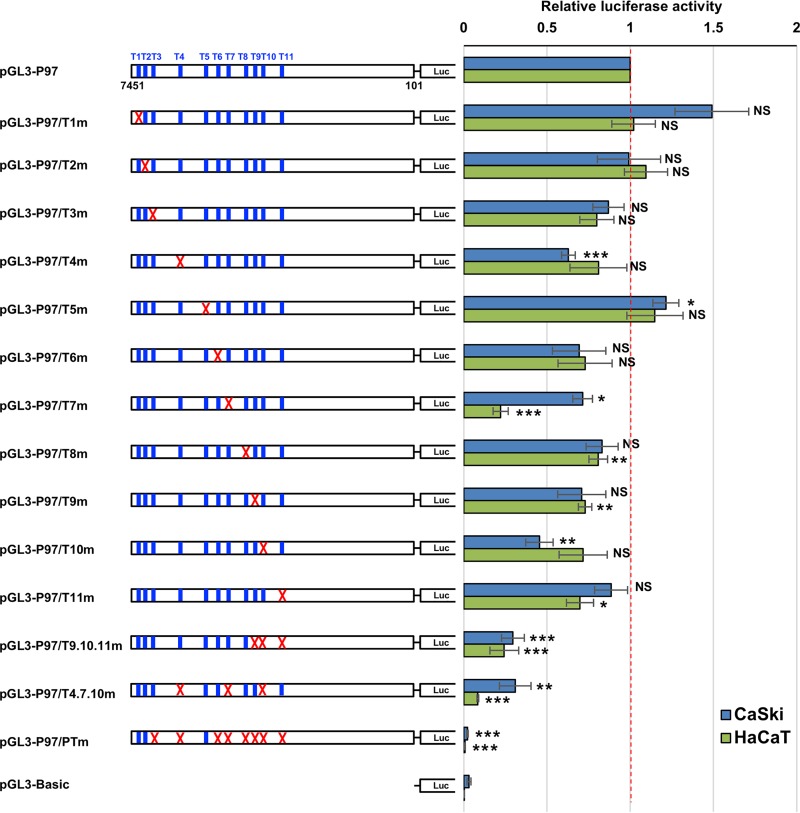
To assess how each TEAD1 target site contributes to viral early promoter activity, we introduced individual mutations (T1m to T11m) into the HPV16 LCR in the reporter plasmid (pGL3-P97/T1m to pGL3-P97/T11m) and measured the promoter activity in CaSki cells. Mutating T4, T7, or T10 significantly reduced the promoter activity, whereas mutating T3, T6, T8, T9, or T11 slightly downregulated the activity, although the differences were not statistically significant (Fig. 3). In contrast, introducing mutations into T1 or T5 increased the promoter activity, whereas mutations in T2 did not affect the promoter activity. These results indicate that each target site, except for T1, T2, and T5, contributes to transcriptional activation of the early promoter. Furthermore, mutating all TEAD1 target sites other than T1, T2, and T5 (pGL3-P97/PTm) completely abolished the promoter activity, ruling out an involvement of T1, T2, and T5 in promoter activation.
FIG 3.
TEAD1 target sites regulate HPV early promoter activity. CaSki or HaCaT cells were transfected with the indicated firefly luciferase (Luc) reporter plasmid, together with the Renilla luciferase plasmid. At 2 days after transfection, the firefly luciferase activity was measured and normalized to the Renilla luciferase activity after background subtraction. The mutated TEAD1 target sites are indicated by red crosses. The data are averages from three independent experiments, with the error bars representing standard deviations. P values were determined by Student’s t test. NS, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.005.
To evaluate the relative contribution of the remaining eight sites to the early promoter activity, we constructed two reporter plasmids having triple-site mutations in T4, T7, and T10 (pGL3-P97/T4.7.10m), each of which significantly contributed to the promoter activity, or in T9, T10, and T11 (pGL3-P97/T9.10.11m), which were previously reported to be involved in the early promoter activity (23). Both triple-site-mutated plasmids showed further reduced promoter activity compared to that of each single-site-mutated plasmid but retained some promoter activity compared to that of pGL3-P97/PTm. These results suggest that the five sites (T4, T7, T9, T10, and T11) play dominant roles in regulating the early promoter.
Similar results were also obtained with cells of the HaCaT cell line, an HPV-negative, immortalized human keratinocyte line (Fig. 3), although introducing mutations into T7 decreased the promoter activity in HaCaT cells more markedly than in CaSki cells. Collectively, these results indicate that TEAD1 plays a substantial role in the direct regulation of HPV early gene transcription.
Identification of TEAD cofactors involved in HPV gene expression.
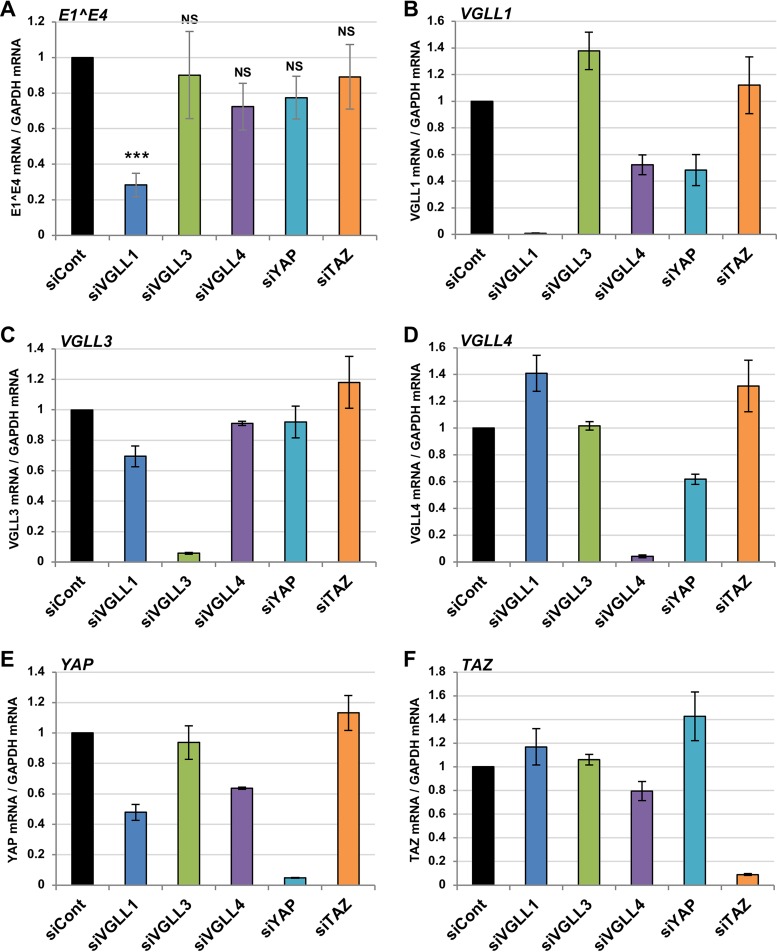
TEADs have no transcriptional activity and require cofactors to regulate transcription (20). Indeed, Ishiji et al. suggested that the TEAD1-mediated transcription of the HPV16 early genes requires an unknown epithelial cell-specific coactivator(s) (23). To explore the TEAD1 cofactor(s) required for HPV gene regulation, we investigated five TEAD cofactors (YAP, TAZ, VGLL1, VGLL3, and VGLL4) that are expressed in W12 cells. We used siRNAs to individually deplete each cofactor and quantified the level of E1̂E4 mRNA by RT-qPCR. We found that VGLL1 knockdown significantly reduced the levels of E1̂E4 mRNA (Fig. 4A). The specific and efficient knockdown of each cofactor was confirmed by real-time PCR (Fig. 4B to F). VGLL1 has been implicated in the development and differentiation of epithelial lineage tissues (29–32), where HPV gene expression occurs; therefore, VGLL1 is a likely candidate for the TEAD cofactor involved in HPV early gene expression.
FIG 4.
Identification of TEAD cofactors involved in HPV gene expression. W12 cells were transfected with the indicated siRNA. At 2 days after transfection, the levels of HPV16 E1̂E4 (A), VGLL1 (B), VGLL3 (C), VGLL4 (D), YAP (E), and TAZ (F) mRNAs were determined by RT-qPCR, with normalization to the level of GAPDH mRNA. The data are averages from three independent experiments, with error bars representing standard deviations. P values were determined by Student’s t test. NS, not significant (P > 0.05); ***, P < 0.005. siYAP, siRNA against YAP; siTAZ, siRNA against TAZ.
VGLL1 is required for HPV gene expression.
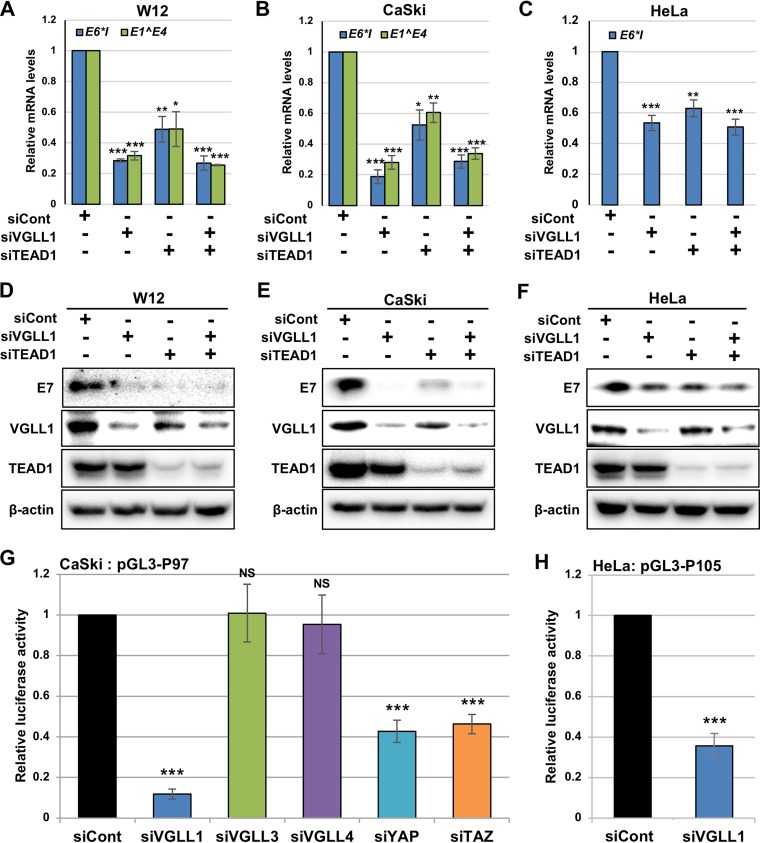
To further examine the role of VGLL1 in HPV gene expression, we performed VGLL1 knockdown alone or with TEAD1 knockdown in W12, CaSki, and HeLa cells. As shown in Fig. 5A to C, VGLL1 depletion markedly reduced the mRNA levels of viral early genes, and the simultaneous depletion of VGLL1 and TEAD1 led to mRNA levels comparable to those observed with VGLL1 depletion alone, suggesting that VGLL1 and TEAD1 cooperatively regulate HPV early gene transcription and that VGLL1 is essential for HPV gene expression. Western blot analyses showed that the transfection of siRNA against VGLL1 (siVGLL1) effectively depleted VGLL1 without affecting the protein levels of TEAD1, whereas TEAD1 knockdown slightly reduced VGLL1 protein levels in W12 and CaSki cells (Fig. 5D and E). As expected, the E7 protein levels were reduced in W12, CaSki, and HeLa cells that had been depleted of VGLL1 and/or TEAD1 (Fig. 5D to F). Taken together, these results reveal that both TEAD1 and VGLL1 are crucial for HPV early gene expression.
FIG 5.
VGLL1 regulates HPV early gene expression. (A to F) W12 (A and D), CaSki (B and E), and HeLa (C and F) cells were transfected with the indicated siRNA. At 2 days after transfection, the levels of HPV16 E6*I and E1̂E4 mRNAs (A and B) and HPV18 E6*I mRNA (C) were quantified by RT-qPCR and normalized to the level of GAPDH mRNA. The HPV16 E7 (D and E) and HPV18 E7 (F) proteins were detected by immunoblotting with anti-HPV16 and anti-HPV18 E7 antibodies, respectively. The effects of siRNA were verified by immunoblotting with an anti-VGLL1 and anti-TEAD1 antibodies. β-Actin was used as the loading control. (G and H) CaSki (G) and HeLa (H) cells were transfected with the indicated siRNAs. Six hours later, the cells were further transfected with the indicated reporter plasmids together with the Renilla luciferase plasmid. At 2 days after transfection, firefly luciferase activity was measured and normalized to the Renilla luciferase activity after background subtraction. The quantitative data are averages from three independent experiments, with error bars representing standard deviations. P values were determined by Student’s t test. NS, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Next, we examined how VGLL1 knockdown affects early promoter activity in luciferase reporter assays. We found that the transfection of siVGLL1, but not siRNA against VGLL3 (siVGLL3) and siRNA against VGLL4 (siVGLL4), significantly decreased the promoter activities of HPV16 P97 in CaSki cells (Fig. 5G). In contrast, knockdown of YAP or TAZ moderately decreased the HPV16 P97 promoter activity, suggesting that YAP and TAZ might be involved in HPV transcription to a lesser extent. The knockdown of VGLL1 also significantly reduced the HPV18 P105 promoter activity in HeLa cells (Fig. 5H), showing that VGLL1 is required for transcription from the HPV early promoter.
Notably, we were not able to detect the VGLL1 protein by immunoblotting without prior enrichment of VGLL1 by immunoprecipitation (data not shown), suggesting that the expression of VGLL1 is considerably low. Interestingly, previous work showed that the HPV16 P97 promoter activity is decreased upon exogenous expression of TEAD1, possibly due to a decrease in the endogenous TEAD coactivator(s) at the HPV promoter (23). Overall, these data suggest that the amount of TEAD cofactor(s) within the cell is limited and a determinant for transcriptional activation.
VGLL1 displays TEAD-dependent binding to the HPV16 LCR.
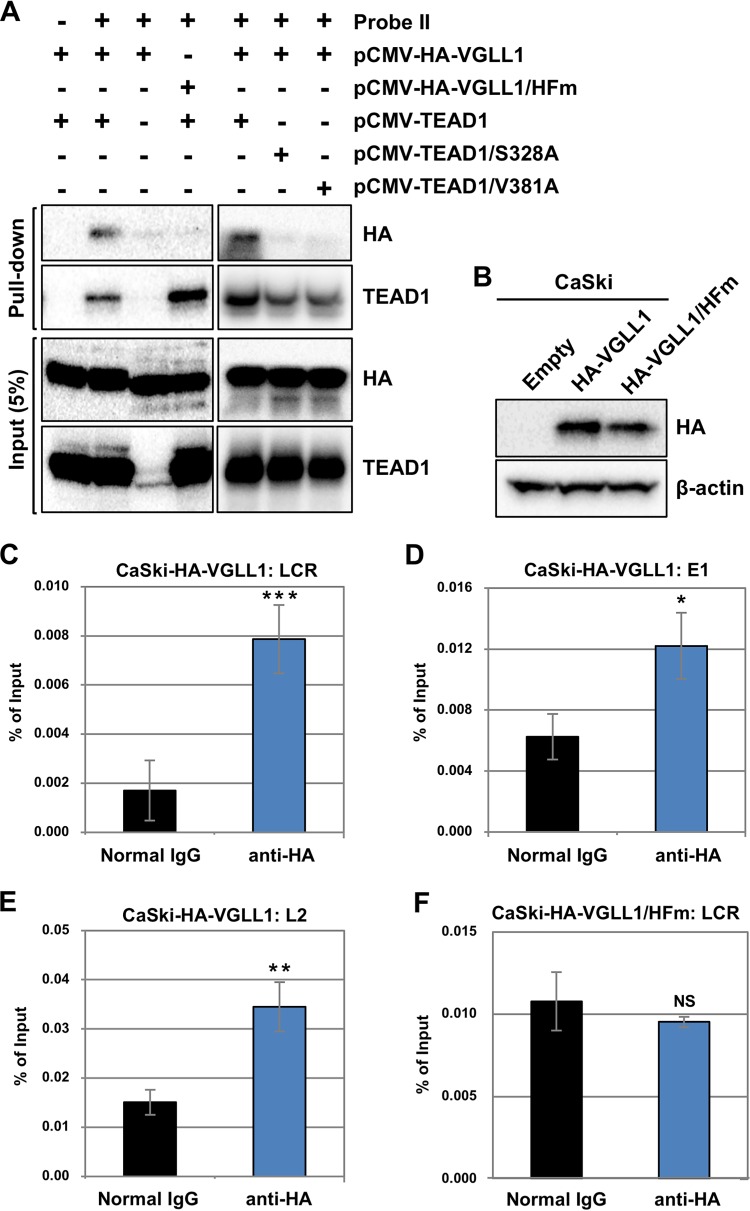
To investigate whether VGLL1 binds to the HPV16 LCR, we performed in vitro DNA pulldown assays. We transfected HEK293 cells with an expression plasmid for hemagglutinin (HA)-VGLL1 alone or together with one for TEAD1, lysed the cells, and examined protein binding to probe II (Fig. 2C) by immunoblotting. We detected HA-VGLL1 in the bound fraction together with TEAD1 but not without TEAD1 (Fig. 6A). Furthermore, HA-VGLL1/HFm, a mutant of VGLL1 with H44A and F45A substitutions in its TEAD-binding domain that cannot associate with TEAD (33), failed to bind probe II in the presence of TEAD1. Two TEAD1 mutants with the S328A or V381A substitution, which were assumed from structural analysis to be defective in VGLL1 binding (33), also failed to recruit VGLL1 to probe II (Fig. 6A). These data strongly suggest that VGLL1 must form a complex with TEAD1 in order to bind to the HPV LCR.
FIG 6.
VGLL1 binds to the HPV LCR via TEADs. (A) Biotinylated DNA probe II (Fig. 2C) was coupled to Dynabeads/M-280 streptavidin and incubated with cell extract from HEK293 cells that had been transfected with the indicated expression plasmids. Five percent of the input volume (Input) and the entire precipitated fractions (Pull-down) were analyzed by immunoblotting with anti-HA and anti-TEAD1 antibodies. (B) CaSki cells stably expressing HA-VGLL1 (CaSki-HA-VGLL1) or HA-VGLL1/HFm (CaSki-HA-VGLL1/HFm) were analyzed for expression of HA-VGLL1 by immunoprecipitation, followed by immunoblotting with an anti-HA antibody. β-Actin was used as the loading control. (C to F) Cross-linked chromatin prepared from CaSki-HA-VGLL1 (C to E) or CaSki-HA-VGLL1/HFm (F) cells were immunoprecipitated with an anti-HA antibody or normal rabbit IgG, and the recovered DNA was quantified by real-time PCR with primers for the HPV16 LCR (C and F), E1 (D), and L2 (E). The levels of HA-VGLL1 binding to the HPV16 genomes are shown as percentages of the amount of input DNA. The data are averages from three experiments performed using independent chromatin preparations, with the error bars representing standard deviations. P values were determined by Student’s t test. NS, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Next, we performed ChIP assays to investigate whether VGLL1 binds to HPV16 genomes in vivo. We stably transduced CaSki cells with retroviral vectors expressing HA-VGLL1 or HA-VGLL1/HFm and confirmed their expression by immunoprecipitation followed by immunoblotting (Fig. 6B). We utilized a two-step, cross-linking method that enables the detection of indirect associations of transcriptional cofactors with DNA (34). Briefly, the cells were fixed with disuccinimidyl glutarate to cross-link protein-protein interactions, followed by fixation with formaldehyde for DNA-protein cross-linking. We found that HA-VGLL1 bound to HPV16 DNA within CaSki cells expressing HA-VGLL1 (Fig. 6C to E). The LCR showed an approximately 4-fold enrichment over the background, whereas the E1 and L2 regions exhibited an approximately 2-fold enrichment over the background (Fig. 6C to E), suggesting that VGLL1 primarily associates with the LCR. Importantly, our ChIP analysis revealed that HA-VGLL1/HFm did not bind to the HPV LCR DNA in CaSki cells (Fig. 6F), suggesting that VGLL1 binding to the LCR in cells depends on an interaction with TEADs.
VGLL1 knockdown inhibits cervical cancer cell growth.
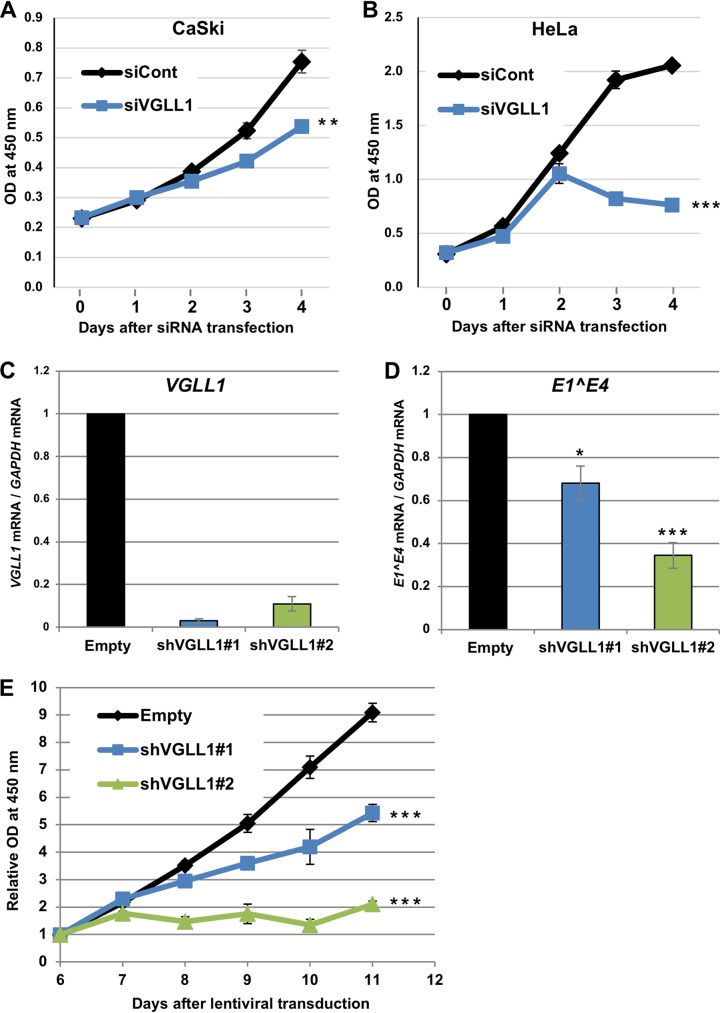
Sustained expression of E6 and E7 is critical for the growth of CaSki and HeLa cells (35, 36); therefore, we examined whether VGLL1 knockdown inhibits the growth of these cell lines. We found that VGLL1 knockdown significantly suppressed the growth of CaSki and HeLa cells, although the effect of siVGLL1 was substantially stronger in HeLa cells than in CaSki cells (Fig. 7A and B). To assess the long-term effects of VGLL1 depletion, we transduced CaSki cells with lentiviral vectors expressing short hairpin RNAs (shRNAs) targeting VGLL1 (shVGLL1). We found that CaSki cells stably expressing shVGLL1#1 (CaSki-shVGLL1#1) or shVGLL1#2 (CaSki-shVGLL1#2) showed reduced levels of VGLL1 and E1̂E4 mRNAs (Fig. 7C and D). In addition, CaSki-shVGLL1#1 and CaSki-shVGLL1#2 markedly impaired cell growth starting at 8 days postransduction (Fig. 7E). Thus, VGLL1 contributes to the growth of cancer cell lines infected with HPV.
FIG 7.
VGLL1 knockdown inhibits cervical cancer cell growth. (A and B) CaSki (A) and HeLa (B) cells transfected with scrambled siRNA (siCont) or siRNA targeting VGLL1 (siVGLL1) were examined for cell viability using a Cell Counting Kit-8 (Dojindo) on the indicated days after transfection. The data are the average optical density (OD) values (450 nm) obtained from triplicate experiments, with error bars representing standard deviations. P values were determined by Student’s t test. **, P < 0.01; ***, P < 0.005. (C and D) CaSki cells were transduced with a lentiviral vector expressing an shRNA targeting VGLL1 (shVGLL1#1 or shVGLL1#2) or an empty vector (Empty) and selected with puromycin for 2 days. At 5 days after transduction, cells were analyzed for the expression of VGLL1 (C) or E1̂E4 (D) mRNA by RT-qPCR. (E) The viability of the transduced CaSki cells was measured on the indicated days after transduction, as described above. The data are the average relative optical density values (450 nm) obtained from triplicate experiments, with the error bars representing standard deviations. ***, P < 0.005 (Student's t test).
VGLL1 is upregulated by HPV infection.
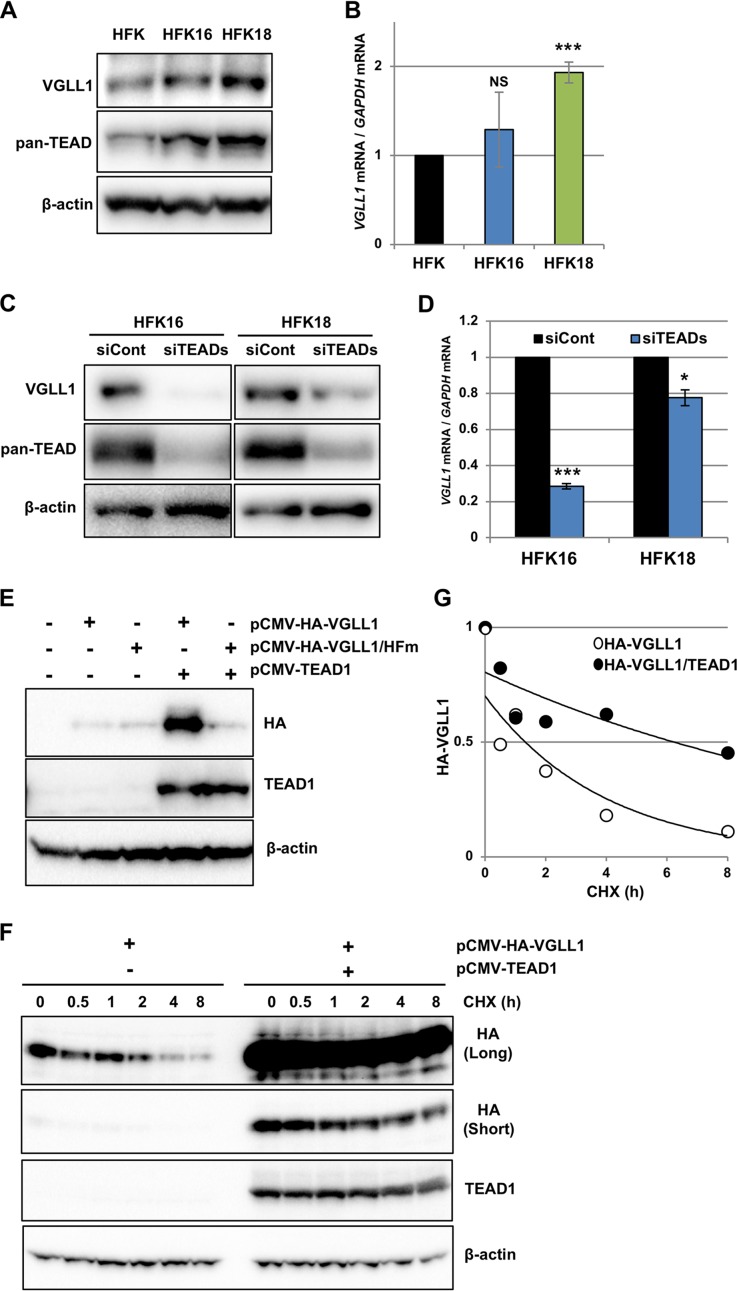
We hypothesized that if the level of VGLL1 in infected cells is critical for HPV gene expression, then HPV may have evolved a strategy to upregulate VGLL1 expression and, in turn, early gene expression. To test this hypothesis, we compared the levels of VGLL1 in primary human foreskin keratinocytes (HFK) containing HPV16 (HFK16) or HPV18 (HFK18) whole genomes to those in parental HFKs. We observed increased levels of VGLL1 protein and mRNA in HFK16 and HFK18 compared to those in HFK, although the difference in the mRNA levels between HFK and HFK16 was not statistically significant (Fig. 8A and B). As previously reported (24), HFK16 and HFK18 also showed increased levels of TEADs (Fig. 8A). TEADs contribute to the HPV-induced upregulation of VGLL1 expression, given that the knockdown of all four TEADs in HFK16 and HFK18 reduced the protein (Fig. 8C) and mRNA (Fig. 8D) levels of VGLL1. We found two typical TEAD-binding motifs [CATTC(C/T)] within the 160-bp region upstream of the transcription start site for the VGLL1 gene (data not shown), which strongly suggests that TEAD directly binds to and activates the VGLL1 promoter.
FIG 8.
HPV infection leads to the upregulation of VGLL1. (A) Primary human keratinocytes (HFK) and HFK containing HPV16 (HFK16) or HPV18 (HFK18) genomes were analyzed for expression of VGLL1 by immunoprecipitation, followed by immunoblotting with an anti-VGLL1 antibody. The TEADs and β-actin in the input fraction were detected by immunoblotting with anti-pan-TEAD and anti-β-actin antibodies, respectively. (B) The levels of VGLL1 mRNA in HFK, HFK16, and HFK18 were determined by RT-qPCR and normalized to the level of GAPDH mRNA. The levels of VGLL1 mRNA are presented as relative levels compared to those in HFK. The data are averages from three independent experiments, with error bars representing standard deviations. P values were determined by Student's t test. NS, not significant (P > 0.05); ***, P < 0.005. (C) HFK16 and HFK18 were transfected with scrambled siRNA (siCont) or a mixture of TEAD1/2/3/4 siRNAs (siTEADs). At 2 days after transfection, the cells were analyzed for the expression of VGLL1 by immunoprecipitation, followed by immunoblotting with an anti-VGLL1 antibody. The TEADs and β-actin in the input fraction were detected by immunoblotting with anti-pan-TEAD and anti-β-actin antibodies, respectively. β-Actin was used as the loading control. (D) The levels of VGLL1 mRNA in HFK16 and HFK18 transfected with siCont or siTEADs were determined by RT-qPCR, with normalization to the level of GAPDH mRNA. The quantitative data are averages from three independent experiments, with error bars representing standard deviations. P values were determined by Student's t test. *, P < 0.05; ***, P < 0.005. (E) HEK293 cells transfected with the indicated expression plasmids were analyzed for the expression of HA-VGLL1 (or HA-VGLL1/HFm) and TEAD1 by immunoblotting with anti-HA and anti-TEAD1 antibodies. β-Actin was used as the loading control. (F) HEK293 cells that had been transfected with the indicated expression plasmids were cultured in medium containing cycloheximide (CHX). The cells were collected at the indicated time points, and the levels of HA-VGLL1 and TEAD1 proteins were detected by immunoblotting with anti-HA and anti-TEAD1 antibodies, respectively. β-Actin was used as the loading control. Long- and short-exposure images of the same blot are shown for HA-VGLL1. (G) The levels of HA-VGLL1 in panel F were quantified with Image Lab software (Bio-Rad) and plotted on the graph. The data are presented as the relative level of HA-VGLL1 compared to that in cells without cycloheximide treatment (0 h).
Finally, prompted by the observation that TEAD1 knockdown reduced the protein levels of VGLL1 (Fig. 5D to E), we examined whether TEAD1 stabilizes the VGLL1 protein within the cell. We transfected HEK293 cells with the expression plasmid for HA-VGLL1 alone or together with that for TEAD1 and detected HA-VGLL1 by immunoblotting. We found that the coexpression of TEAD1 dramatically increased the level of VGLL1 but not that of HA-VGLL1/HFm (Fig. 8E), suggesting that VGLL1 is stabilized by the direct association with TEAD1. Furthermore, cycloheximide chase assays showed that TEAD1 coexpression prolonged the half-life of the VGLL1 protein (Fig. 8F) from 1.3 h in the absence of TEAD1 to 6.2 h in the presence of TEAD1 (Fig. 8G). Thus, TEADs upregulate VGLL1 expression by both increasing mRNA and stabilizing protein through a direct interaction. Taken together, these results suggest that HPV infection increases VGLL1 expression via the upregulation of TEADs.
DISCUSSION
Eukaryotic transcriptional complexes are composed of multiple proteins, including TFs and associated regulatory cofactors. In general, TFs directly bind to DNA and regulate transcription, while cofactors fine-tune gene expression through interactions with TFs. Although a number of TFs have been reported to be involved in HPV gene expression, little is known about relevant cofactors. In this study, we have demonstrated that a less-studied cofactor, VGLL1, plays a prominent role in HPV early gene transcription, dependent on its association with TEAD1.
VGLL1 was originally identified as an ortholog of Drosophila Vestigial (Vg), which regulates wing development as a cofactor for Scalloped (Sd), a Drosophila ortholog of TEAD (29). In humans, VGLL1 mRNA is detectable in the placenta, kidney, and lung but not in the liver or brain (29, 37). Intriguingly, the VGLL1 protein is expressed in placental trophoblasts (32), which are an in vivo target for HPV infection (38, 39) and which support the complete life cycle of HPV (40, 41). In Xenopus, the expression of VGLL1 is restricted to skin epidermal cells (30), which is consistent with the epithelial tropism of HPV. These data suggest that VGLL1 is mainly expressed in the epithelial lineage tissues, whereas TEAD is ubiquitously expressed in a variety of tissues (20). Therefore, our data, together with the previous findings, suggest that VGLL1 may contribute to the epithelial specificity of HPV gene expression, in collaboration with the ubiquitous transcription factor TEAD.
The biological functions of VGLL1 in vertebrates are largely elusive, but several studies have implicated its roles in the development and differentiation of epithelial lineage tissues. In Drosophila, VGLL1 can substitute for Vg in wing development (29). A recent comparative transcriptome study has shown that VGLL1 is a specific marker of human undifferentiated trophoblasts and is likely to regulate differentiation in concert with TEAD4 (32). In human and Xenopus embryonic cells, bone morphogenetic protein 4, which can induce keratinocytes from stem cells and regulate epithelial stratification (42, 43), induces VGLL1 expression (30–32). Since HPV gene expression is tightly linked to epithelial differentiation, it is tempting to speculate that VGLL1/TEAD1 modulates the viral early promoter activity during differentiation.
Unlike YAP, VGLL1 has no apparent transactivation domain and shows no transactivation potential even when fused to the Saccharomyces cerevisiae yeast GAL4 DNA binding domain (20, 37). Because the exogenous expression of VGLL1 did not induce robust transactivation of the HPV early promoter in our reporter assays (data not shown), it is unlikely that VGLL1 directly activates HPV transcription. Since the muscle-specific vestigial-like protein VGLL2 has been suggested to induce transcription by recruiting a transcriptional activator(s) or relieving inhibition imposed by a transcriptional repressor(s) (37, 44), we postulate that VGLL1 has similar mechanisms for epithelial cell-specific gene regulation. Further studies are needed to gain mechanistic insights into transcriptional activation by VGLL1.
We discovered that 11 TEAD1 target sites are widely distributed in the enhancer region of the HPV16 LCR. Multiple TEAD-binding motifs were also found in the LCRs of HPV18, HPV31, and HPV45 (data not shown), suggesting that the TEAD1-mediated transcriptional regulation is conserved among high-risk HPVs. Interestingly, 8 of 11 TEAD1 target sites in the HPV16 LCR are flanked by or partially overlapped previously identified binding sites for AP1 or NF1. Of note, mutating the T7 site, which is located between two AP1-binding sites, significantly reduced the promoter activity of the HPV LCR, suggesting that TEAD1 and AP1 cooperatively activate the early promoter. In support of this hypothesis, recent studies have shown that TEADs interact with AP1 and activate various host genes through TEAD/AP1 composite motifs (45–47). In addition, NF1 has been shown to activate HPV gene expression, in synergy with other factors that have not yet been identified (5–8). NF1 binds as a homodimer to a palindromic sequence composed of two NF1-binding motifs, but only the low-affinity-half NF1-binding motifs are found in the HPV LCR (5). Our in vitro DNA pulldown assays showed that 6 out of 7 binding motifs for NF1 are flanked by binding sites for TEAD1, strongly suggesting that NF1 and TEAD1 cooperatively regulate transcription via NF1/TEAD composite motifs. It will be of interest to examine whether TEAD1 interacts with NF1 and, if so, whether NF1 also interacts with VGLL1.
A high level of expression of VGLL1 is observed in basal-like breast cancers and associated with reduced overall survival (48, 49). The VGLL1/TEAD complex induces the expression of insulin-like growth factor binding protein 5 and promotes the anchorage-independent proliferation of prostate and lung cancer cells (33, 50). These observations suggest that VGLL1 plays an important role in cancer progression and raise the question of whether the HPV-induced upregulation of VGLL1 contributes to cervical cancer progression independently of its function in HPV gene expression. Our preliminary analyses showed that VGLL1 knockdown in HPV-negative cervical cancer cells, C33A cells, activates caspases 3/7 (data not shown), raising the possibility that VGLL1 functions as an inhibitor of apoptosis in cancer cells. This function may contribute to the different effects of VGLL1 knockdown on the growth of CaSki and HeLa cells. Further studies are necessary to fully understand the roles of VGLL1 in carcinogenesis. Finally, given that VGLL1 knockdown suppressed the growth of cervical cancer cells, VGLL1 could be a potential therapeutic target for HPV-related cancers.
MATERIALS AND METHODS
Cell culture.
HeLa, CaSki, HaCaT, and HEK293 cells were cultured in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS). W12 (clone 20863) (51) and HFK (Kurabo, Osaka, Japan) cells were maintained with feeder cells as previously described (24). HFK16 and HFK18 were generated in a previous study (24). All experiments using keratinocytes were performed in keratinocyte serum-free medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 30-μg/ml bovine pituitary extract and 1-ng/ml human recombinant epidermal growth factor without feeder cells.
siRNA transfection.
Cells were transfected with siRNA using the Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific). ON-TARGETplus siRNA SMART pools targeting the following genes were purchased from Horizon Discovery (Cambridge, UK): nontargeting control (catalog number D-001810-10), TEAD1 (catalog number L-012603-00), TEAD2 (catalog number L-012611-01), TEAD3 (catalog number L-012604-00), TEAD4 (catalog number L-019570-00), VGLL1 (catalog number L-017939-00), VGLL3 (catalog number L-031975-01), VGLL4 (catalog number L-023447-02), YAP1 (catalog number L-012200-00), and WWT1 (catalog number L-016083-00).
RT-qPCR.
The levels of HPV16 E6*I, HPV16 E1̂E4, HPV18 E6*I, YAP, TAZ, VGLL1, VGLL3, and VGLL4 mRNAs were determined by RT-qPCR as previously described (24). The amounts of cDNA of the target genes were normalized to the amount of the concurrently amplified GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA. The nucleotide sequences of the primers are presented in Table 1.
TABLE 1.
Nucleotide sequences of primers used in RT-qPCR and ChIP assays
| Assay and primer name | Sequence |
Source or reference | |
|---|---|---|---|
| Forward | Reverse | ||
| RT-qPCR | |||
| HPV16 E6*I | 5′-GGAGCGACCCAGAAAGTTAC-3′ | 5′-TGACAGTTAATACACCTCAC-3′ | This study |
| HPV16 E1̂E4 | 5′-GCTGATCCTGCAAGCAACGAAGTATC-3′ | 5′-TTCTTCGGTGCCCAAGGC-3′ | 53 |
| HPV18 E6*I | 5′-TTGGAACTTACAGAGGTGCC-3′ | 5′-TAGTGCCCAGCTATGTTGTG-3′ | This study |
| VGLL1 | 5′-AGCACAGGGTTGCTCTTCA-3′ | 5′-TGGCAGATCCACGAGATACAT-3′ | This study |
| VGLL3 | 5′-TCCCAGTATCTGCCCAACC-3′ | 5′-TGCTGAATACCGCTAACTTCTTC-3′ | 54 |
| VGLL4 | 5′-AACTGCAACCTCTCGCACTG-3′ | 5′-GCTCGGGCTCCTTGTAATTCT-3′ | 50 |
| YAP | 5′-CTCGAACCCCAGATGACTTC-3′ | 5′-CCAGGAATGGCTTCAAGGTA-3′ | 55 |
| TAZ | 5′-TATCCCAGCCAAATCTCGTG-3′ | 5′-TTCTGCTGGCTCAGGGTACT-3′ | 56 |
| GAPDH | 5′-TCACCACCATGGAGAAGGCT-3′ | 5′-CAGGAGGCATTGCTGATGATC-3′ | 57 |
| ChIP assays | |||
| HPV16 LCR | 5′-CACTGCTTGCCAACCATTCC-3′ | 5′-TAAGGCGTTGGCGCATAGTG-3′ | This study |
| HPV16 E1 | 5′-CATGGGGAATGGTTGTGTTA-3′ | 5′-CATACACATTGGAGACACAC-3′ | This study |
| HPV16 L2 | 5′-TATTGCTGATGCAGGTGAC-3′ | 5′-GCAGCCAAAGAGACATCTGA-3′ | This study |
| HPV18 LCR | 5′-ACCTGGTATTAGTCATTTTC-3′ | 5′-TGCCCAACCTATTTCGGTTG-3′ | This study |
Immunoblotting and immunoprecipitation.
Immunoblotting was performed as previously described (24). The primary antibodies used were as follows: anti-TEAD1 (clone 31/TEF-1; BD Transduction Laboratories, San Diego, CA), anti-TEAD4 (clone N-G2; Santa Cruz Biotechnology, Dallas, TX), anti-pan-TEAD (clone D3F7L; Cell Signaling Technology, Danvers, MA), anti-HPV16 E7 (a mixture of clone 8C9 [Thermo Fisher Scientific] and clone ED17 [Santa Cruz Biotechnology]), anti-HPV18 E7 (clone 8E2; Abcam, Cambridge, UK), anti-VGLL1 (a mixture of clones HPA042403 and HPA064616; Merck, Darmstadt, Germany), anti-β-actin (clone C-4; Santa Cruz Biotechnology), and anti-HA tag (clone ab1190; Abcam) antibodies.
Endogenous VGLL1 and ectopically expressed HA-VGLL1 were enriched by immunoprecipitation before immunoblotting. Briefly, 2 × 106 to 3 × 106 cells were lysed in 200 μl of radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology), followed by centrifugation at 10,000 × g for 10 min at 4°C. The supernatant was diluted 3-fold with phosphate-buffered saline containing 1× cOmplete protease inhibitor cocktail (Merck) and incubated with antibodies at 4°C overnight. The antibodies used for immunoprecipitation were as follows: anti-VGLL1 (clone HPA042403; Merck) and anti-HA tag (clone C29F4; Cell Signaling Technology) antibodies. The lysate was further incubated with 30 μl of protein G Dynabeads (Thermo Fisher Scientific) at 4°C for 2 h. The bound proteins were eluted by boiling the beads in SDS sample buffer and detected by immunoblotting. In HeLa cells, endogenous VGLL1 was mainly detected in the insoluble fraction of the cell lysate, for unknown reasons. Endogenous E7 in W12 cells was also enriched by immunoprecipitation with anti-HPV16 E7 (clone 8C9; Thermo Fisher Scientific) before immunoblotting.
ChIP assay.
ChIP assays were performed using SimpleChIP Plus enzymatic chromatin immunoprecipitation kits (Cell Signaling Technology) according to the manufacturer’s instructions. Cross-linked chromatin was prepared from W12 and HeLa cells as previously described (24). For CaSki-HA-VGLL1 or CaSki-HA-VGLL1/HFm cells, cross-linked chromatin was prepared using the two-step cross-linking method as previously described (34). The antibodies used for immunoprecipitation were anti-TEAD1 antibody (clone 31/TEF-1; BD Transduction Laboratories), anti-HA tag antibody (clone C29F4; Cell Signaling Technology), and normal rabbit IgG (Cell Signaling Technology). Precipitated HPV16 or HPV18 DNA fragments were quantified by real-time PCR using the primers shown in Table 1.
Plasmids.
To generate pGL3-P97, we used PCR to synthesize a DNA fragment spanning from nt 7451 to 101 of the HPV16 genome (forward primer, 5′-AAG CTT ACC GAA TTC GGT TGC ATG CT-3′; reverse primer, 5′-CCA TGG CAG TTC TCT TTT GGT GCA TA-3′) and cloned this PCR product between the HindIII and NcoI sites of pGL3-Basic (Promega, Madison, WI, USA). pGL3-P105, which contains the HPV18 LCR fragment (from nt 6982 to 102), was similarly constructed using the following primers: forward primer 5′-AAG CTT CTT TAG ACT TAG ATC AAT AT-3′ and reverse primer 5′-CCA TGG TAT TGT GGT GTG TTT CTC ACA-0.3′. The TEAD-binding motifs in the HPV16 LCR were mutated by PCR, and the corresponding region of pGL3-P97 was replaced with the mutated fragments to produce a series of mutant reporter plasmids (Fig. 3). To construct the expression plasmid for HA-VGLL1, VGLL1 cDNA was amplified from HFK cDNAs using PCR with forward primer 5′-GCG GCC GCC ACC ATG TAC CCC TAC GAC GTC CCC GAC TAC GCC GAA GAA ATG AAG AAG ACT GCC-3′ (the HA tag sequence is underlined) and reverse primer 5′-GCG GCC GCT CAA AGA TGC TGC AGG TAT CGA T-3′ and cloned into pCMV, which was created by removing the β-galactosidase gene from pCMV-β (TaKaRa Bio, Shiga, Japan). To generate HA-VGLL1/HFm, we used PCR to change codons 44 (CAC, His) and 45 (TTC, Phe) of the VGLL1 gene to GCA (Ala) and GCA (Ala), respectively. The expression plasmid for TEAD1 (pCMV-TEAD1) was previously described (24). To generate TEAD1/S328A and TEAD1/V381A, we used PCR to change codon 328 of the TEAD1 gene (TCC, Ser) to GCC (Ala) and codon 381 of the TEAD1 gene (GTT, Val) to GCT (Ala), respectively.
Luciferase reporter assay.
CaSki, HeLa, or HaCaT cells (7.5 × 104 cells/well in a 24-well plate) were transfected with 400 ng of the firefly luciferase plasmids indicated above, together with 100 ng of phRG-TK (Promega), which expresses the Renilla luciferase, using X-tremeGENE HP DNA (Merck). When cells were cotransfected with siRNAs and plasmid DNAs, the cells were transfected with 5 pmol of each siRNA 6 h before plasmid transfection. The firefly and Renilla luciferase activities were measured at 2 days posttransfection using a Dual-Glo luciferase assay system (Promega) on an Arvo MX luminescence counter (PerkinElmer, Waltham, MA). The firefly luciferase activities were normalized to the Renilla luciferase activities after subtraction of the background activity, which was obtained from untransfected cells.
DNA pulldown assay.
Biotinylated DNA probes (probes I, II, and III) were prepared by PCR using pGL3-P97 as the template with the following primers: probe I forward primer 5′-biotin-ACC GAA TTC GGT TGC ATG CT-3′, probe I reverse primer 5′-CAT AGT GAT TCA GTA GTT GC-3′, probe II forward primer 5′-biotin-GCA ACT ACT GAA TCA CTA TG-3′, probe II reverse primer 5′-CAC ACA CCC ATG TGC AGT TT-3′, probe III forward primer 5′-biotin-AAA CTG CAC ATG GGT GTG TG-3′, and probe III reverse primer 5′-CAG TTC TCT TTT GGT GCA TA-0.3′. The nuclear extract from HeLa cells was prepared using a nuclear extraction kit (Affymetrix, Santa Clara, CA). Total cell lysates were prepared from HEK293 cells that had been transfected with the expression plasmids. Cells (2 × 106 cells in a 6-cm dish) were transfected with 2.5 μg pCMV-HA-VGLL1 alone or with 0.5 μg pCMV-TEAD1/S328A or pCMV-TEAD1/V381A, cotransfected with 0.125 μg pCMV-HA-VGLL1 and 0.5 μg pCMV-TEAD1 (the coexpression of TEAD1 significantly increased the level of HA-VGLL1, as shown in Fig. 8E), or cotransfected with 2.5 μg pCMV-HA-VGLL1/HFm and 0.5 μg pCMV-TEAD1. The total amount of transfected DNA was adjusted to 3 μg with the empty vector, pCMV. At 2 days after transfection, cells were lysed in 200 μl of RIPA buffer (Santa Cruz Biotechnology), followed by centrifugation at 10,000 × g for 10 min at 4°C, and the supernatant was collected. DNA pulldown assays were performed as described previously (24), except that 150 mM NaCl was used in the binding buffer. For binding competition assays, unlabeled double-stranded oligonucleotides (250 pmol) were added to the binding reaction mixtures. See Table 2 for the nucleotide sequences of the competitors.
TABLE 2.
Nucleotide sequences of competitors used in DNA pulldown assays
| Wild type |
Mutant |
||
|---|---|---|---|
| Competitor | Sequence | Competitor | Sequencea |
| a | 5′-ACCGAATTCGGTTGCATGCTTTTTGGCAC-3′ | a-T1m | 5′-ACCGAATTCGGTTGGGTGCTTTTTGGCAC-3′ |
| b | 5′-GGCACAAAATGTGTTTTTTTAAATAGTTCT-3′ | b-T2m | 5′-GGCACAACCTGTGTTTTTTTAAATAGTTCT-3′ |
| b-T3m | 5′-GGCACAAAATGTGTTTTTTTAAAGGGTTCT-3′ | ||
| b-T2/3m | 5′-GGCACAACCTGTGTTTTTTTAAAGGGTTCT-3′ | ||
| c | 5′-GTTCTATGTCAGCAACTATAGTTTAAACTTGTACG-3′ | ||
| d | 5′-GTACGTTTCCTGCTTGCCATGCGTGCCAAATCCCT-3′ | d-T4m | 5′-GTACGTTTCCTGCTTGCCACCCGTGCCAAATCCCT-3′ |
| e | 5′-TCCCTGTTTTCCTGACCTGCACTGCTTGCCAACCA-3′ | ||
| f | 5′-AACCATTCCATTGTTTTTTACACTGCACTATGTGC-3′ | f-T5m | 5′-AACCATTCCAGGGTTTTTTACACTGCACTATGTGC-3′ |
| f-T6m | 5′-AACCATTCCATTGTTTTTTACACTGCACGACGTGC-3′ | ||
| f-T5/6m | 5′-AACCATTCCAGGGTTTTTTACACTGCACGACGTGC-3′ | ||
| g | 5′-GCAACTACTGAATCACTATGTACATTGTGTCATAT-3′ | g-T7m | 5′-GCAACTACTGAATCACGACGTACATTGTGTCATAT-3′ |
| h | 5′-TTGTGTCATATAAAATAAATCACTATGCGCCAACG-3′ | h-T8m | 5′-TTGTGTCATATAAAATAAATCACGACGCGCCAACG-3′ |
| i | 5′-CTATGCGCCAACGCCTTACATACCGCTGTTAGGCA-3′ | i-T8/9m | 5′-CGACGCGCCAACGCCTTAGGTACCGCTGTTAGGCA-3′ |
| j | 5′-CGCTGTTAGGCACATATTTTTGGCTTGTTTTAACT-3′ | j-T10m | 5′-CGCTGTTAGGCACAGGTTTTTGGCTTGTTTTAACT-3′ |
| k | 5′-GCTTGTTTTAACTAACCTAATTGCATATTTGGCAT-3′ | k-T11m | 5′-GCTTGTTTTAACTAACCTAATTGCAGGTTTGGCAT-3′ |
| l | 5′-TTGGCATAAGGTTTAAACTTCTAAGGCCAACTAAA-3′ | ||
| m | 5′-GCCAACTAAATGTCACCCTAGTTCATACATGAACT-3′ | ||
| n | 5′-CATGAACTGTGTAAAGGTTAGTCATACATTGTTCA-3′ | ||
| o | 5′-CATTGTTCATTTGTAAAACTGCACATGGGTGTGTG-3′ | ||
Underlined nucleotides represent those that were mutated.
Stable transduction of cells.
CaSki cells stably expressing HA-VGLL1 or HA-VGLL1/HFm were produced by transduction with retroviral vectors as previously described (24). CaSki cells expressing shRNA were produced by transduction with lentiviral vectors. The vector plasmids were purchased from Merck (for shVGLL1#1, catalog number TRCN418554; for shVGLL1#2, catalog number TRCN432495) and packaged into lentiviral particles using a lentiviral high-titer packaging mix (TaKaRa Bio). The transduced cells were selected by treatment with 2 μg/ml puromycin, and the surviving cells were pooled and used for experiments.
Cell growth assay.
CaSki (1 × 103 cells/well) and HeLa (5 × 103 cells/well) cells in a 96-well plate were transfected with 1 pmol of control siRNA (siCont) or siVGLL1. CaSki cells stably expressing shVGLL1 or the selection marker alone were seeded at 2 × 103 cells/well in a 96-well plate. Cell viability was measured using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).
Cycloheximide chase assay.
HEK293 cells (2.5 × 105 cells/well) in a 24-well plate were transfected with the expression plasmid for HA-VGLL1 (0.25 μg) alone or together with that for TEAD1 (0.25 μg). The total amount of transfected DNA was adjusted to 0.5 μg with the empty pCMV vector. At 2 days after transfection, cells were treated with 100 μg/ml of cycloheximide. Cells collected at the time points indicated in Fig. 8F were analyzed by immunoblotting.
ACKNOWLEDGMENTS
We thank Paul Lambert and Tomomi Nakahara for providing the W12 cell line. We thank Life Science Editors for editing a draft of the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant number JP18K09244) and a Grant-in-Aid for Cancer Research from the Japan Agency for Medical Research and Development (grant number JP16ck0106178j0102) to S.M.
REFERENCES
- 1.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. 2015. Human papillomavirus molecular biology and disease association. Rev Med Virol 25:2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longworth MS, Laimins LA. 2004. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev 68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kajitani N, Satsuka A, Kawate A, Sakai H. 2012. Productive lifecycle of human papillomaviruses that depends upon squamous epithelial differentiation. Front Microbiol 3:152. doi: 10.3389/fmicb.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride AA, Warburton A. 2017. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog 13:e1006211. doi: 10.1371/journal.ppat.1006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard HU. 2013. Regulatory elements in the viral genome. Virology 445:197–204. doi: 10.1016/j.virol.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Gloss B, Yeo-Gloss M, Meisterenst M, Rogge L, Winnacker EL, Bernard HU. 1989. Clusters of nuclear factor I binding sites identify enhancers of several papillomaviruses but alone are not sufficient for enhancer function. Nucleic Acids Res 17:3519–3533. doi: 10.1093/nar/17.9.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong T, Chan WK, Bernard HU. 1990. Transcriptional activation of human papillomavirus 16 by nuclear factor I, AP1, steroid receptors and a possibly novel transcription factor, PVF: a model for the composition of genital papillomavirus enhancers. Nucleic Acids Res 18:465–470. doi: 10.1093/nar/18.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong T, Apt D, Gloss B, Isa M, Bernard HU. 1991. The enhancer of human papillomavirus type 16: binding sites for the ubiquitous transcription factors oct-1, NFA, TEF-2, NF1, and AP-1 participate in epithelial cell-specific transcription. J Virol 65:5933–5943. doi: 10.1128/JVI.65.11.5933-5943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thierry F, Spyrou G, Yaniv M, Howley P. 1992. Two AP1 sites binding JunB are essential for human papillomavirus type 18 transcription in keratinocytes. J Virol 66:3740–3748. doi: 10.1128/JVI.66.6.3740-3748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apt D, Chong T, Liu Y, Bernard HU. 1993. Nuclear factor I and epithelial cell-specific transcription of human papillomavirus type 16. J Virol 67:4455–4463. doi: 10.1128/JVI.67.8.4455-4463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connor M, Bernard HU. 1995. Oct-1 activates the epithelial-specific enhancer of human papillomavirus type 16 via a synergistic interaction with NFI at a conserved composite regulatory element. Virology 207:77–88. doi: 10.1006/viro.1995.1053. [DOI] [PubMed] [Google Scholar]
- 12.Yukawa K, Butz K, Yasui T, Kikutani H, Hoppe-Seyler F. 1996. Regulation of human papillomavirus transcription by the differentiation-dependent epithelial factor Epoc-1/skn-1a. J Virol 70:10–16. doi: 10.1128/JVI.70.1.10-16.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen B, Hariri A, Pittelkow MR, Rosenfeld MG. 1997. Characterization of Skn-1a/i POU domain factors and linkage to papillomavirus gene expression. J Biol Chem 272:15905–15913. doi: 10.1074/jbc.272.25.15905. [DOI] [PubMed] [Google Scholar]
- 14.Bauknecht T, Angel P, Royer HD, Zur Hausen H. 1992. Identification of a negative regulatory domain in the human papillomavirus type 18 promoter: interaction with the transcriptional repressor YY1. EMBO J 11:4607–4617. doi: 10.1002/j.1460-2075.1992.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattison S, Skalnik DG, Roman A. 1997. CCAAT displacement protein, a regulator of differentiation-specific gene expression, binds a negative regulatory element within the 5′ end of the human papillomavirus type 6 long control region. J Virol 71:2013–2022. doi: 10.1128/JVI.71.3.2013-2022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride AA. 2013. The papillomavirus E2 proteins. Virology 445:57–79. doi: 10.1016/j.virol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cripe TP, Haugen TH, Turk JP, Tabatabai F, Schmid PG III, Dürst M, Gissmann L, Roman A, Turek LP. 1987. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J 6:3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquemin P, Hwang JJ, Martial JA, Dolle P, Davidson I. 1996. A novel family of developmentally regulated mammalian transcription factors containing the TEA/ATTS DNA binding domain. J Biol Chem 271:21775–21785. doi: 10.1074/jbc.271.36.21775. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pobbati AV, Hong W. 2013. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther 14:390–398. doi: 10.4161/cbt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson I, Xiao JH, Rosales R, Staub A, Chambon P. 1988. The HeLa cell protein TEF-1 binds specifically and cooperatively to two SV40 enhancer motifs of unrelated sequence. Cell 54:931–942. doi: 10.1016/0092-8674(88)90108-0. [DOI] [PubMed] [Google Scholar]
- 22.Xiao JH, Davidson I, Matthes H, Garnier JM, Chambon P. 1991. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell 65:551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]
- 23.Ishiji T, Lace MJ, Parkkinen S, Anderson RD, Haugen TH, Cripe TP, Xiao JH, Davidson I, Chambon P, Turek LP. 1992. Transcriptional enhancer factor (TEF)-1 and its cell-specific co-activator activate human papillomavirus-16 E6 and E7 oncogene transcription in keratinocytes and cervical carcinoma cells. EMBO J 11:2271–2281. doi: 10.1002/j.1460-2075.1992.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S, Takeuchi T, Ishii Y, Yugawa T, Kiyono T, Nishina H, Kukimoto I. 2017. Human papillomavirus 16 E6 upregulates APOBEC3B via the TEAD transcription factor. J Virol 91:e02413-16. doi: 10.1128/JVI.02413-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olmedo-Nieva L, Muñoz-Bello JO, Contreras-Paredes A, Lizano M. 2018. The role of E6 spliced isoforms (E6*) in human papillomavirus-induced carcinogenesis. Viruses 10:E45. doi: 10.3390/v10010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taguchi A, Nagasaka K, Kawana K, Hashimoto K, Kusumoto-Matsuo R, Plessy C, Thomas M, Nakamura H, Bonetti A, Oda K, Kukimoto I, Carninci P, Banks L, Osuga Y, Fujii T. 2015. Characterization of novel transcripts of human papillomavirus type 16 using cap analysis gene expression technology. J Virol 89:2448–2452. doi: 10.1128/JVI.03433-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanaya T, Kyo S, Laimins LA. 1997. The 5′ region of the human papillomavirus type 31 upstream regulatory region acts as an enhancer which augments viral early expression through the action of YY1. Virology 237:159–169. doi: 10.1006/viro.1997.8771. [DOI] [PubMed] [Google Scholar]
- 28.Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M, Baranašić D, Santana-Garcia W, Tan G, Chèneby J, Ballester B, Parcy F, Sandelin A, Lenhard B, Wasserman WW, Mathelier A. 2019. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 48:D87–D92. doi: 10.1093/nar/gkz1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaudin P, Delanoue R, Davidson I, Silber J, Zider A. 1999. TONDU (TDU), a novel human protein related to the product of vestigial (vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development 126:4807–4816. [DOI] [PubMed] [Google Scholar]
- 30.Faucheux C, Naye F, Tréguer K, Fédou S, Thiébaud P, Théze N. 2010. Vestigial like gene family expression in Xenopus: common and divergent features with other vertebrates. Int J Dev Biol 54:1375–1382. doi: 10.1387/ijdb.103080cf. [DOI] [PubMed] [Google Scholar]
- 31.Krendl C, Shaposhnikov D, Rishko V, Ori C, Ziegenhain C, Sass S, Simon L, Müller NS, Straub T, Brooks KE, Chavez SL, Enard W, Theis FJ, Drukker M. 2017. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc Natl Acad Sci U S A 114:E9579–E9588. doi: 10.1073/pnas.1708341114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soncin F, Khater M, To C, Pizzo D, Farah O, Wakeland A, Arul Nambi Rajan K, Nelson KK, Chang CW, Moretto-Zita M, Natale DR, Laurent LC, Parast MM. 2018. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development 145:dev156273. doi: 10.1242/dev.156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pobbati AV, Chan SW, Lee I, Song H, Hong W. 2012. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure 20:1135–1140. doi: 10.1016/j.str.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Tian B, Yang J, Brasier AR. 2012. Two-step cross-linking for analysis of protein-chromatin interactions. Methods Mol Biol 809:105–120. doi: 10.1007/978-1-61779-376-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis DA, Schmid SI, Howley PM. 2000. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J Virol 74:2679–2686. doi: 10.1128/jvi.74.6.2679-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall AH, Alexander KA. 2003. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol 77:6066–6069. doi: 10.1128/jvi.77.10.6066-6069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda T, Chapman DL, Stewart AF. 2002. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J Biol Chem 277:48889–48898. doi: 10.1074/jbc.M206858200. [DOI] [PubMed] [Google Scholar]
- 38.Hermonat PL, Kechelava S, Lowery CL, Korourian S. 1998. Trophoblasts are the preferential target for human papilloma virus infection in spontaneously aborted products of conception. Hum Pathol 29:170–174. doi: 10.1016/s0046-8177(98)90228-3. [DOI] [PubMed] [Google Scholar]
- 39.Chisanga C, Eggert D, Mitchell CD, Wood C, Angeletti PC. 2015. Evidence for placental HPV infection in both HIV positive and negative women. J Cancer Ther 6:1276–1289. doi: 10.4236/jct.2015.615140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, You H, Chiriva-Internati M, Korourian S, Lowery CL, Carey MJ, Smith CV, Hermonat PL. 2001. Display of complete life cycle of human papillomavirus type 16 in cultured placental trophoblasts. Virology 290:99–105. doi: 10.1006/viro.2001.1135. [DOI] [PubMed] [Google Scholar]
- 41.You H, Liu Y, Agrawal N, Prasad CK, Chiriva-Internati M, Lowery CL, Kay HH, Hermonat PL. 2003. Infection, replication, and cytopathology of human papillomavirus type 31 in trophoblasts. Virology 316:281–289. doi: 10.1016/j.virol.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Zhu XJ, Liu Y, Dai ZM, Zhang X, Yang X, Li Y, Qiu M, Fu J, Hsu W, Chen Y, Zhang Z. 2014. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 10:e1004687. doi: 10.1371/journal.pgen.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattison JM, Melo SP, Piekos SN, Torkelson JL, Bashkirova E, Mumbach MR, Rajasingh C, Zhen HH, Li L, Liaw E, Alber D, Rubin AJ, Shankar G, Bao X, Chang HY, Khavari PA, Oro AE. 2018. Retinoic acid and BMP4 cooperate with p63 to alter chromatin dynamics during surface epithelial commitment. Nat Genet 50:1658–1665. doi: 10.1038/s41588-018-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Günther S, Mielcarek M, Krüger M, Braun T. 2004. VITO-1 is an essential cofactor of TEF1-dependent muscle-specific gene regulation. Nucleic Acids Res 32:791–802. doi: 10.1093/nar/gkh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. 2015. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, Davis RJ, Mao J. 2016. Tead and AP1 coordinate transcription and motility. Cell Rep 14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obier N, Cauchy P, Assi SA, Gilmour J, Lie-A-Ling M, Lichtinger M, Hoogenkamp M, Noailles L, Cockerill PN, Lacaud G, Kouskoff V, Bonifer C. 2016. Cooperative binding of AP-1 and TEAD4 modulates the balance between vascular smooth muscle and hemogenic cell fate. Development 143:4324–4340. doi: 10.1242/dev.139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. 2006. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Castilla MÁ, López-García MÁ, Atienza MR, Rosa-Rosa JM, Díaz-Martín J, Pecero ML, Vieites B, Romero-Pérez L, Benítez J, Calcabrini A, Palacios J. 2014. VGLL1 expression is associated with a triple-negative basal-like phenotype in breast cancer. Endocr Relat Cancer 21:587–599. doi: 10.1530/ERC-13-0485. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, Li F, Chen H, Zhou Z, Zhang L, Ji H. 2014. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res 24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeon S, Allen-Hoffmann BL, Lambert PF. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol 69:2989–2997. doi: 10.1128/JVI.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Connor MJ, Stünkel W, Zimmermann H, Koh CH, Bernard HU. 1998. A novel YY1-independent silencer represses the activity of the human papillomavirus type 16 enhancer. J Virol 72:10083–10092. doi: 10.1128/JVI.72.12.10083-10092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conway MJ, Cruz L, Alam S, Christensen ND, Meyers C. 2011. Cross-neutralization potential of native human papillomavirus N-terminal L2 epitopes. PLoS One 6:e16405. doi: 10.1371/journal.pone.0016405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kooistra SM, Nørgaard LC, Lees MJ, Steinhauer C, Johansen JV, Helin K. 2014. A screen identifies the oncogenic micro-RNA miR-378a-5p as a negative regulator of oncogene-induced senescence. PLoS One 9:e91034. doi: 10.1371/journal.pone.0091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaulk SG, Lattanzi VJ, Hiemer SE, Fahlman RP, Varelas X. 2014. The Hippo pathway effectors TAZ/YAP regulate dicer expression and microRNA biogenesis through Let-7. J Biol Chem 289:1886–1891. doi: 10.1074/jbc.C113.529362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun C, De Mello V, Mohamed A, Ortuste Quiroga HP, Garcia-Munoz A, Al Bloshi A, Tremblay AM, von Kriegsheim A, Collie-Duguid E, Vargesson N, Matallanas D, Wackerhage H, Zammit PS. 2017. Common and distinctive functions of the Hippo effectors Taz and Yap in skeletal muscle stem cell function. Stem Cells 35:1958–1972. doi: 10.1002/stem.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pauklin S, Sernández IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. 2009. Estrogen directly activates AID transcription and function. J Exp Med 206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]