The NF-κB transcription factor is activated via different key inflammatory pathways and typically results in the fast expression of several proinflammatory genes as well as negative feedback loop genes to prevent excessive inflammation. In the current report, we describe that infection of cells with the porcine alphaherpesvirus pseudorabies virus (PRV) triggers a gradual and persistent aberrant activation of NF-κB, which does not result in expression of hallmark proinflammatory or negative feedback loop genes. In addition, although PRV-induced NF-κB activation shares some mechanistic features with canonical NF-κB activation, it also shows remarkable differences; e.g., it is largely independent of the canonical IκB kinase (IKK) and even renders infected cells resistant to canonical NF-κB activation by the inflammatory cytokine TNF-α. Aberrant PRV-induced NF-κB activation may therefore paradoxically serve as a viral immune evasion strategy and may represent an important tool to unravel currently unknown mechanisms and consequences of NF-κB activation.
KEYWORDS: NF-κB, evasion, herpes, innate, pseudorabies virus
ABSTRACT
The nuclear factor kappa B (NF-κB) is a potent transcription factor, activation of which typically results in robust proinflammatory signaling and triggering of fast negative feedback modulators to avoid excessive inflammatory responses. Here, we report that infection of epithelial cells, including primary porcine respiratory epithelial cells, with the porcine alphaherpesvirus pseudorabies virus (PRV) results in the gradual and persistent activation of NF-κB, illustrated by proteasome-dependent degradation of the inhibitory NF-κB regulator IκB and nuclear translocation and phosphorylation of the NF-κB subunit p65. PRV-induced persistent activation of NF-κB does not result in expression of negative feedback loop genes, like the gene for IκBα or A20, and does not trigger expression of prototypical proinflammatory genes, like the gene for tumor necrosis factor alpha (TNF-α) or interleukin-6 (IL-6). In addition, PRV infection inhibits TNF-α-induced canonical NF-κB activation. Hence, PRV infection triggers persistent NF-κB activation in an unorthodox way and dramatically modulates the NF-κB signaling axis, preventing typical proinflammatory gene expression and the responsiveness of cells to canonical NF-κB signaling, which may aid the virus in modulating early proinflammatory responses in the infected host.
IMPORTANCE The NF-κB transcription factor is activated via different key inflammatory pathways and typically results in the fast expression of several proinflammatory genes as well as negative feedback loop genes to prevent excessive inflammation. In the current report, we describe that infection of cells with the porcine alphaherpesvirus pseudorabies virus (PRV) triggers a gradual and persistent aberrant activation of NF-κB, which does not result in expression of hallmark proinflammatory or negative feedback loop genes. In addition, although PRV-induced NF-κB activation shares some mechanistic features with canonical NF-κB activation, it also shows remarkable differences; e.g., it is largely independent of the canonical IκB kinase (IKK) and even renders infected cells resistant to canonical NF-κB activation by the inflammatory cytokine TNF-α. Aberrant PRV-induced NF-κB activation may therefore paradoxically serve as a viral immune evasion strategy and may represent an important tool to unravel currently unknown mechanisms and consequences of NF-κB activation.
INTRODUCTION
The porcine pseudorabies virus (PRV) belongs to the largest subfamily of the Herpesviridae family, the alphaherpesviruses, which includes highly prevalent human herpes simplex viruses 1 and 2 (HSV-1 and HSV-2, respectively) and varicella-zoster virus (VZV), as well as pathogens with a profound impact on animal health and production, such as equine herpesvirus 1 (EHV-1) in horses, bovine herpesvirus 1 (BoHV-1) in cattle, or Marek’s disease virus (MDV) in chickens. PRV displays strong functional and genetic similarities with other representatives of the taxon and is therefore frequently used as a model system to study general aspects of alphaherpesvirus biology, including viral interactions with the immune system and host cell signaling pathways (1).
The nuclear factor kappa B (NF-κB) pathway represents one of the most potent signaling nodules in the early immune response, playing a pivotal role in the coordination and regulation of a wide variety of immune system defensive mechanisms upon the appearance of threatening stimuli, including viral infections (2, 3). This is accomplished through NF-κB-mediated transactivation of a subset of genes principally involved in key regulatory steps of inflammatory and cell survival events. The direct sensing of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) and prototypical proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), is a powerful activator of the canonical NF-κB signaling cascade (4–6). Briefly, NF-κB-activating signaling typically converges in the activation of the inhibitory κB (IκB) kinase (IKK) signalosome. In the unstimulated state, NF-κB dimers are associated with inhibitory IκB proteins, which prevent the nuclear import of NF-κB. The activated IKK signalosome drives the phosphorylation of IκB, which is followed by its proteasomal degradation and uncoupling of the NF-κB dimers (e.g., p65/RelA-p50, the most abundant and important in canonical activation). Uncoupled NF-κB dimers become targets for phosphorylation, which facilitates nuclear migration, ultimately resulting in NF-κB-dependent gene transactivation (7).
As part of the first line of defense, the NF-κB pathway triggers a potent and acute immune response. To avoid hyperactivation of the immune system, proper and fast negative regulation of NF-κB transcriptional activity is crucial (8–10). Thus, NF-κB pathway dysregulation is closely linked with allergies (11), autoimmune disorders (12), and neurodegenerative diseases (13), as well as with the development of several types of cancer (14), among other pathologies (15). Hence, in addition to several proinflammatory and cell survival-related genes, activated NF-κB typically also triggers the potent expression of negative feedback loop proteins, like IκBα and A20. IκBα is the main factor responsible for the cytoplasmic retention of NF-κB (p65-p50) dimers in the unstimulated situation but is also responsible for the recruitment of DNA-bound NF-κB subunits from the cell nucleus to the cytoplasm, in this way inhibiting the transcriptional response and restoring the resting state of the pathway (16–19). A20 is a deubiquitin ligase (DUB) that negatively regulates NF-κB activation by targeting the signal transducers RIP-1 and TRAF6 upstream of the IKK signalosome (20, 21).
Many different types of viruses have been reported to trigger NF-κB pathway activation, including human immunodeficiency virus type 1 (HIV-1) (22), rabies virus (23), Ebola virus (24), influenza virus (25), Newcastle disease virus (NDV) (26), and porcine reproductive and respiratory syndrome virus (PRRSV) (27). In the case of the Herpesviridae family, viral species of the three subfamilies have been reported to activate NF-κB signaling (3), including the alphaherpesviruses herpes simplex viruses 1 and 2 (HSV-1 and HSV-2, respectively) (28, 29), the betaherpesviruses human cytomegalovirus (HCMV) and human herpesvirus 6 (HHV-6) (30, 31), and the gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi’s sarcoma herpesvirus (KSHV) (32, 33). Interestingly, herpesviruses often appear to trigger a persistent NF-κB activation (3), suggesting that the negative feedback loop may be compromised, although this has not yet been addressed in detail. In this study, we describe that the porcine alphaherpesvirus pseudorabies virus (PRV) triggers the persistent activation of NF-κB but that the mode and consequences of activation divert substantially from canonical NF-κB activation, being substantially slower, without the detectable expression of either negative feedback loop genes or proinflammatory genes, and rendering the infected cells resistant to TNF-α-induced canonical NF-κB activation. Our data therefore show that PRV profoundly manipulates the central NF-κB signaling axis in infected cells, preventing its normal activation mode and proinflammatory consequences, which may represent a powerful immune evasion strategy.
RESULTS
PRV infection leads to progressive and persistent IκBα degradation.
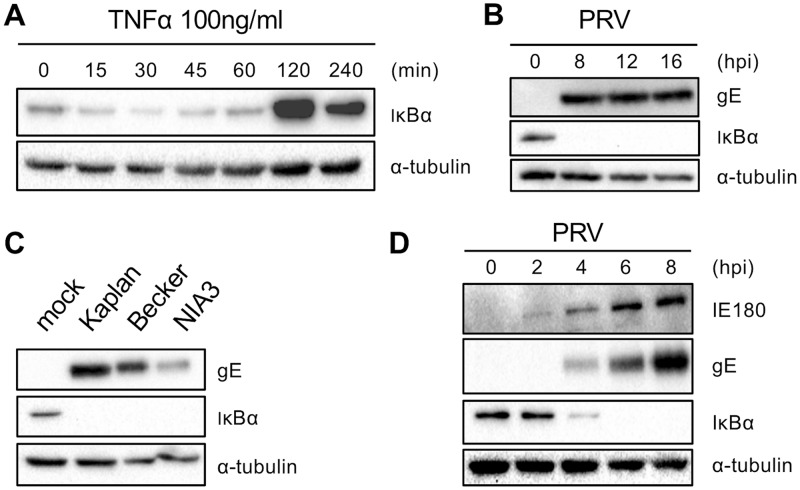
NF-κB activation typically occurs via degradation of the NF-κB inhibitory protein IκBα, followed by translocation of the NF-κB p65-p50 dimer to the nucleus. Activators of NF-κB signaling, like TNF-α, trigger rapid IκBα degradation, followed by a negative feedback loop consisting of NF-κB-induced (over)expression of inhibitory proteins, including IκBα (8, 9, 34, 35). In line with this, we found that addition of porcine TNF-α to mock-infected porcine swine testicle (ST) cells drives fast IκBα degradation within approximately 30 min, followed by a very substantial upregulation of IκBα, reaching a peak in protein accumulation at 2 h after stimulation (Fig. 1A). Quite to the contrary, PRV-infected ST cells showed a complete and persistent IκBα degradation at all the analyzed time points of infection (8, 12, and 16 h postinfection [hpi]; multiplicity of infection [MOI], 10 PFU/cell) (Fig. 1B). Hence, PRV-mediated NF-κB activation continues until late time points of infection, without any indication of IκBα protein restoration. This suggests that the typical NF-κB inhibitory feedback mechanism is impaired in PRV-infected cells. Degradation of IκBα by PRV did not appear to be virus strain dependent, since it was observed for all three PRV strains tested (Kaplan, NIA-3, and Becker) (Fig. 1C).
FIG 1.
(A) Western blot analysis of IκBα protein levels at different time points posttreatment of ST cells with porcine TNF-α (100 ng/ml). (B) Western blot analysis of IκBα protein levels at different time points postinoculation of ST cells with PRV strain Kaplan (MOI, 10 PFU/cell). (C) Western blot analysis of IκBα protein levels in ST cells at 8 hpi with PRV strain Kaplan, Becker, or NIA-3 (MOI, 10 PFU/cell). (D) Western blot analysis of IκBα protein levels at different time points postinoculation of ST cells with PRV strain Kaplan (MOI, 10 PFU/cell). For each panel, representative blots out of three independent repeats are shown.
To determine from which time point onwards PRV triggers IκBα degradation, IκBα dynamics in infected ST cells were assessed in a time frame ranging from 0 to 8 hpi. This revealed that PRV infection triggers progressive IκBα degradation, initiating at between 2 hpi and 4 hpi and resulting in the entire degradation of the protein at 6 hpi (Fig. 1D), indicating that PRV-mediated IκBα degradation proceeds much slower than the degradation observed with a typical stimulus of NF-κB signaling, like TNF-α (Fig. 1A).
PRV infection leads to the progressive and persistent nuclear translocation of NF-κB p65.
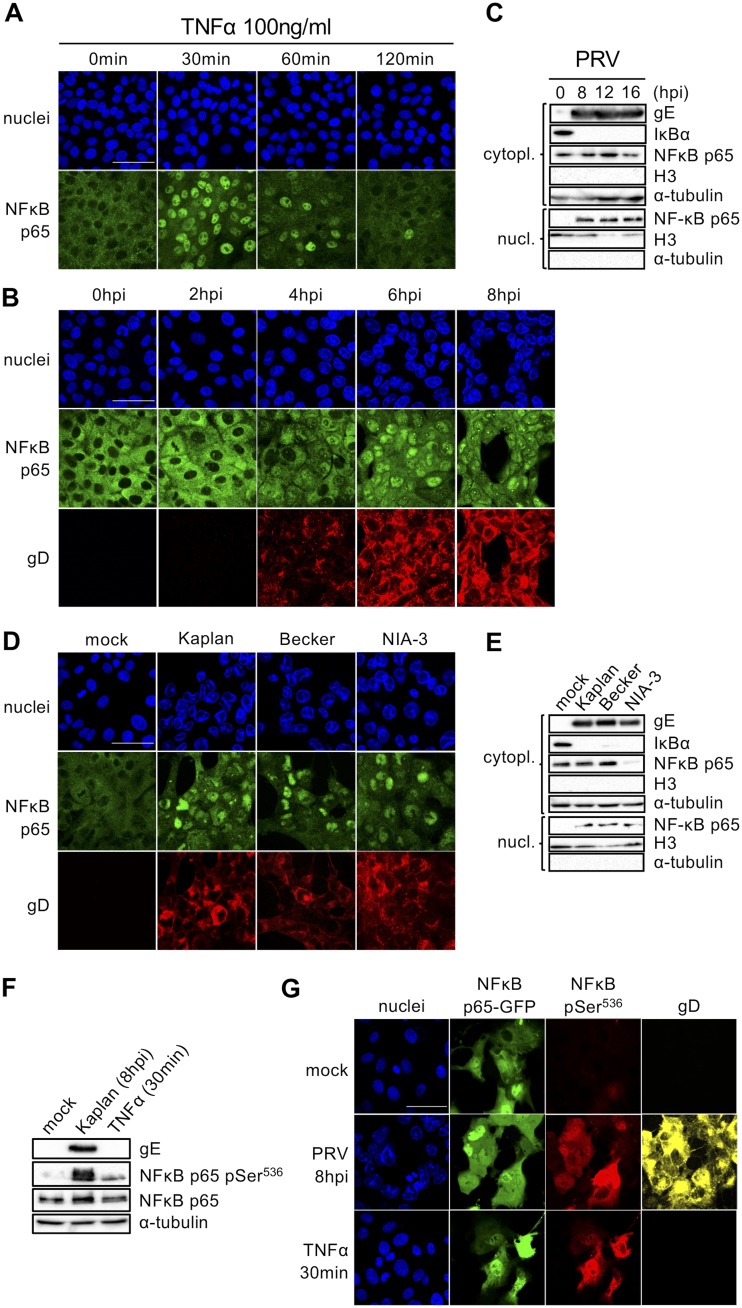
IκBα degradation is typically followed by the nuclear import of NF-κB p65 (8, 36, 37). When ST cells were treated with TNF-α, as expected, the NF-κB p65 subcellular distribution radically switched from cytoplasmic to nuclear shortly after stimulation (30 min) in almost the entire cell population. As a result of the fast inhibitory regulation of the NF-κB pathway, the cytoplasmic distribution of p65 was gradually restored at 1 and 2 h after treatment with TNF-α (Fig. 2A).
FIG 2.
(A) Confocal microscopy of NF-κB p65 at different time points posttreatment of ST cells with porcine TNF-α (100 ng/ml). NF-κB p65 is shown in green, and nuclei are shown in blue. Bar, 50 μm. (B) Confocal microscopy of NF-κB p65 at different time points postinoculation of ST cells with PRV strain Kaplan (MOI, 10 PFU/cell). NF-κB p65 is shown in green, PRV gD is shown in red, and cell nuclei are shown in blue. Bar, 50 μm. (C) Western blot analysis of NF-κB p65 in the nuclear (nucl.) and cytoplasmic (cytopl.) fractions of PRV Kaplan-infected ST cells at 8, 12, and 16 hpi (MOI, 10 PFU/cell). Histone 3 (H3) and α-tubulin served as nuclear and cytoplasmic reference markers, respectively. (D) Confocal microscopy of NF-κB p65 in ST cells at 8 hpi with PRV strain Kaplan, Becker, or NIA-3 (MOI, 10 PFU/cell). NF-κB p65 is shown in green, PRV gD is shown in red, and nuclei are shown in blue. Bar, 50 μm. (E) Western blot analysis of NF-κB p65 in the nuclear and cytoplasmic fractions of ST cells at 8 hpi with PRV strain Kaplan, Becker, or NIA-3 (MOI, 10 PFU/cell). Histone 3 (H3) and α-tubulin served as nuclear and cytoplasmic markers, respectively. (F) Western blot analysis of total NF-κB p65 and phospho-Ser536-NF-κB p65 in mock-infected, PRV Kaplan-infected (8 hpi; MOI, 10 PFU/cell), and TNF-α-stimulated (30 min, 100 ng/ml) ST cells. (G) Confocal microscopy of ST cells transfected with GFP-p65 and subsequently infected with PRV Kaplan (8 hpi; MOI, 10 PFU/cell) or treated with TNF-α (30 min, 100 ng/ml) or left untreated and uninfected (mock). NF-κB p65-GFP is shown in green, phospho-Ser536-NF-κB p65 is shown in red, gB is shown in yellow, and nuclei are shown in blue. Bar, 50 μm. The confocal images and Western blots shown in this figure are representative examples from three independent repeats of the experiments.
In line with the kinetics of PRV-induced IκBα protein degradation, p65 nuclear translocation assays confirmed that, in comparison with TNF-α treatment, NF-κB activation occurred much more slowly in PRV-infected cells, without an obvious negative feedback loop. Indeed, the nuclear translocation of p65 could be observed starting at between 2 hpi and 4 hpi and was virtually complete at 6 hpi, coinciding with the time of IκBα degradation (Fig. 2B). However, the nuclear translocation of p65 was persistent and continued until very late time points of infection (16 hpi) without an indication of the gradual restoration of cytoplasmic p65 localization, as confirmed by cell fractionation assays (the infection-induced detachment of cells prevented optimal immunofluorescence assays [IF] from 12 hpi onwards) (Fig. 2C). Similar to the observations for IκBα degradation, the nuclear translocation of p65 was observed for all three PRV strains tested (Fig. 2D and E).
The release of NF-κB from the IκBα inhibitor is followed by cytoplasmic regulatory phosphorylations prior to nuclear import (38, 39). Particularly, the phosphorylation of NF-κB p65 at serine residue 536 is habitually used as a hallmark of NF-κB activation, as this phosphorylation event plays a key role in optimal gene transactivation (39, 40). We therefore analyzed the phosphorylation status of NF-κB p65 at this residue in PRV-infected or TNF-α-treated ST cells. Western blot assays (WB) showed that PRV infection triggered the very robust phosphorylation of p65 at serine 536 (Fig. 2F). To assess this in individual cells by immunofluorescence, ST cells were transfected with a plasmid harboring the gene for green fluorescent protein (GFP)-tagged p65, since the signal of endogenous p65 was too weak to pick up by immunofluorescence staining in individual cells. Figure 2G shows substantial levels of Ser536-phosphorylated p65 in PRV-infected and TNF-α-treated GFP-p65-expressing ST cells but only low levels of phosphorylated p65 in mock-treated cells.
Thus, the subcellular localization and phosphorylation status of NF-κB p65 confirm that the NF-κB pathway is persistently activated by PRV, and the lack of restoration of cytoplasmic p65 localization further points to a strong dysregulation of the inhibitory feedback mechanisms of the pathway.
PRV-induced persistent IκBα degradation and p65 nuclear translocation are also observed in infected primary PoRECs and epithelial and neuronal cells of other species.
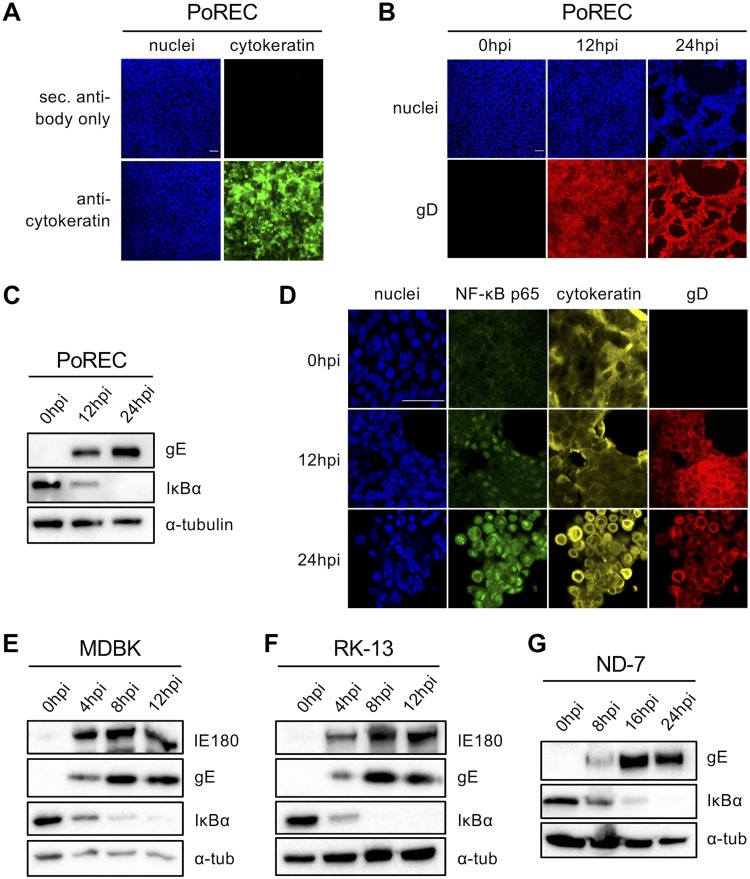
For PRV, as well as for other alphaherpesviruses, host invasion occurs via epithelial mucosae, including the mucosae of the oral and respiratory tract (1). To determine whether PRV-induced IκBα degradation and p65 nuclear translocation also occur in biologically important primary epithelial cells, primary respiratory epithelial cells were isolated from the tracheas of 7-week-old piglets (see the isolation procedure in Materials and Methods). The isolated cell population showed protein expression of the epithelium-specific marker cytokeratin in over 90% of cells (Fig. 3A). In addition, the specificity of the cytokeratin antibody was tested and confirmed on tissue sections of porcine trachea, where the positive signal was limited to epithelial cells (data not shown). Primary porcine respiratory epithelial cells (PoRECs) were cultured in a transwell system, allowing the generation of an air-liquid interface that mimics the natural environment found in respiratory tract epithelium. In vivo, PRV particles infect the respiratory tract from the apical side (lumen), and from there, virus spreads to other host body tissues (41). Immunofluorescence stainings showed successful apical PRV infection of PoRECs, with infection of the majority of the cell monolayer being seen at 12 hpi and complete infection and a very obvious cytopathic effect (CPE) being seen at 24 hpi (Fig. 3B). As shown in Fig. 3C, PRV infection also induced progressive IκBα degradation in primary tracheal cells, with partial IκBα degradation being seen at 12 hpi and complete degradation being seen at 24 hpi. Furthermore, the nuclear translocation of NF-κB p65 was observed in the majority of infected primary tracheal cells at 12 and 24 hpi (Fig. 3D). Hence, persistent activation of the NF-κB pathway was also observed in PRV-infected primary porcine respiratory epithelial cells.
FIG 3.
(A) Confocal microscopy of cytokeratin staining (and the corresponding secondary [sec.] antibody negative control) in tracheal porcine respiratory epithelial cells (PoRECs). Cytokeratin is shown in green, and nuclei are shown in blue. Bar, 50 μm. (B) Confocal microscopy of PRV gD expression in PoRECs infected with PRV Kaplan at 12 and 24 hpi (MOI, 10 PFU/cell; apical inoculation). PRV gD is shown in red, and nuclei are shown in blue. Bar, 50 μm. (C) Western blot analysis of IκBα in PRV Kaplan-infected PoRECs at 0, 12, and 24 hpi (MOI, 10 PFU/cell; apical inoculation). (D) Confocal microscopy of NF-κB p65 in PoRECs infected with PRV Kaplan at 0, 12, and 24 hpi (MOI, 10 PFU/cell; apical inoculation). NF-κB p65 is shown in green, cytokeratin is shown in yellow, gD is shown in red, and nuclei are shown in blue. Bar, 50 μm. The images in panels A to D are representative examples of the results obtained on PoRECs isolated from five different piglets. (E) Western blot analysis of IκBα in PRV Kaplan-infected bovine MDBK cells at 0, 4, 8, and 12 hpi (MOI, 10 PFU/cell). (F) Western blot analysis of IκBα in PRV Kaplan-infected rabbit kidney 13 (RK-13) cells at 0, 4, 8, and 12 hpi (MOI, 10 PFU/cell). (G) Western blot analysis of IκBα in PRV Kaplan-infected rat ND-7 cells at 0, 8, 16, and 24 hpi (MOI, 10 PFU/cell). The Western blots shown in panels E to G are representative examples from three independent repeats of the experiments.
Furthermore, to test whether the PRV-induced activation of NF-κB is restricted to porcine cells or can also be observed in epithelial cells from other species that are susceptible to PRV infection, we analyzed the bovine MDBK and rabbit kidney 13 (RK-13) epithelial cell lines. Both cell lines show high levels of susceptibility to PRV infection and have frequently been used to study PRV biology (1, 42, 43). As shown in Fig. 3E and F, PRV triggered progressive IκBα degradation in MDBK and RK-13 cells similar to that observed in porcine ST cells. Thus, NF-κB activation can be induced by PRV in cells from different species. PRV, like most alphaherpesviruses, is a neurotropic virus and particularly targets sensory neurons (1). Therefore, we also wanted to determine whether virus-induced NF-κB activation occurs in sensory neurons. To this end, ND-7 cells, which are immortalized primary sensory neurons of rat dorsal root ganglia that are susceptible to PRV infection, were used (44). Figure 3G shows that PRV-induced IκBα degradation could also be observed in ND-7 cells. In ND-7 cells, IκBα degradation appeared to occur more slowly, reflecting the slower replication kinetics of PRV in this cell type. In conclusion, PRV-induced NF-κB activation also occurs in primary porcine epithelial cells, in epithelial cell lines of different species, and in rat sensory neuronal cells.
PRV-induced NF-κB activation does not depend on the multiplicity of infection and requires expression of viral late genes.
In a next step, we investigated whether NF-κB activation also occurred in multiple-step infections in which several rounds of virus replication allow the spread of the infection to the entire ST cell population. This type of infection is more reflective of natural virus invasion of the host. As shown in Fig. 4A, while IκBα degradation was fully completed after one round of infection (8 hpi), when the vast majority of the cell population was simultaneously infected (MOI, 3 and 10 PFU/cell), the loss of IκBα was limited when only part of the population was infected (MOI, 0.1, 0.3, and 1 PFU/cell). This suggests that NF-κB activation is restricted to PRV-infected cells and does not involve intercellular communication (e.g., cytokine signaling, direct cell-cell interactions). In line with this, when low-MOI assays were allowed to proceed to full infection of all the cells (24 hpi), this resulted in complete IκBα degradation, like that observed in single-step infections (Fig. 4B).
FIG 4.
(A and B) Western blot analysis of IκBα in PRV Kaplan-infected ST cells at 8 hpi (A) and 24 hpi (B) using an MOI of 0.1, 0.3, 1, 3, and 10 PFU/cell. (C) Western blot analysis of IκBα in ST cells infected with either PRV Kaplan or UV-inactivated PRV Kaplan at 8 hpi (MOI, 10 PFU/cell). (D) Western blot analysis of IκBα in PRV-infected ST cells (MOI, 10 PFU/cell) treated or not treated with phosphonoacetic acid (PAA; 200 or 400 μg/ml) from 30 min prior to infection onwards and collected at 4, 6, and 8 hpi. As a control for successful PAA treatment, expression of US3 (early [E]) and gE (late [L]) was analyzed. (E) Western blot analysis of IκBα in PRV-infected ST cells (MOI, 10 PFU/cell; 8 hpi) untreated or treated with PAA (400 μg/ml) starting at 30 min before inoculation or starting at 1, 2, 3, 4, and 6 hpi. All samples were analyzed at 8 hpi. As a control for successful PAA treatment, expression of IE180 (immediate early [IE]), US3 (E), and gE, gD, and gC (L) were analyzed. The Western blots shown are representative examples from three independent repeats of the experiments.
Further, UV light-inactivated PRV was unable to trigger IκBα degradation (Fig. 4C). This indicates that the initial steps of infection, such as virus entry, the nuclear delivery of nucleocapsids, or the release of viral genomes, are insufficient and that active virus replication is required to trigger persistent NF-κB activation.
To determine whether viral late gene expression is required for NF-κB pathway activation, ST cells were (pre)treated with phosphonoacetic acid (PAA), a DNA polymerase inhibitor (45). The expression of late (L) genes is dramatically reduced by PAA, since their expression highly depends on viral genome replication. As shown in Fig. 4D, PAA (at 200 or 400 μg/ml) efficiently inhibited PRV-induced IκBα degradation at 6 hpi and 8 hpi, indicating that a late viral protein(s) is involved in IκBα degradation. Starting PAA treatment at different time points of infection (from 30 min before inoculation to 6 hpi) showed that addition of PAA starting at 30 min before inoculation or at 1 hpi completely inhibited PRV-induced IκBα degradation. Addition of PAA at 2 hpi, 3 hpi, or 4 hpi resulted in partial IκBα degradation, and addition at 6 hpi could not prevent PRV-induced IκBα degradation (Fig. 4E). Assessing the expression levels of different viral late proteins, including the true late protein gC, indicated that expression of these proteins is efficiently inhibited when PAA treatment is initiated at up to 2 hpi but is less efficiently inhibited when PAA is added later. Taking all these results into account, the viral activator(s) of NF-κB appears to be expressed at between 1 hpi and 6 hpi, which fits quite well with the kinetics of IκBα degradation and the nuclear import of NF-κB p65 (Fig. 1D and 2B).
PRV-induced degradation of IκBα is proteasome dependent and involves (inefficient) IκBα phosphorylation.
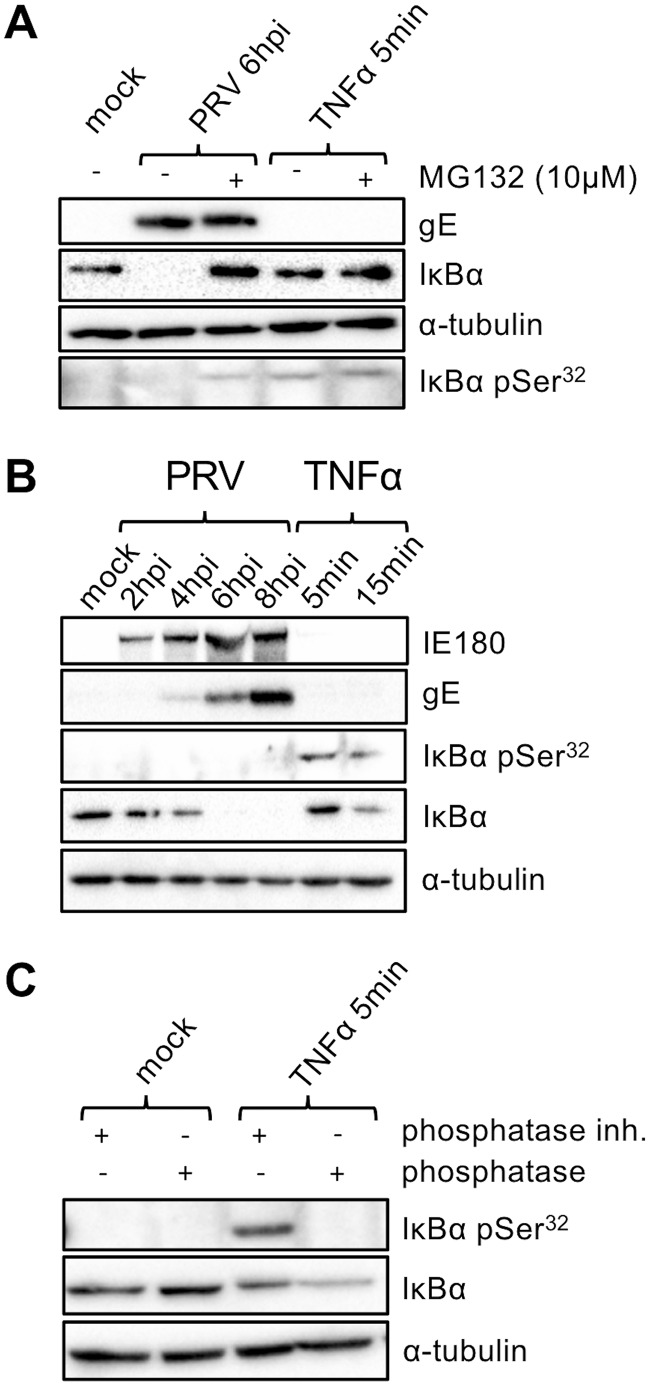
IκBα degradation upon canonical NF-κB activation, e.g., via addition of TNF-α, occurs via the 26S proteasome (46, 47). To assess whether the IκBα degradation observed in PRV-infected cells is also proteasome dependent, cells were treated with the proteasome inhibitor MG132. As can be seen in Fig. 5A, as expected, addition of MG132 impaired the ability of TNF-α to trigger IκBα degradation in mock-infected ST cells. Importantly, addition of MG132 at 2 hpi also protected IκBα from PRV-induced degradation at 6 hpi. Hence, the PRV-induced degradation of IκBα occurs via the proteasome.
FIG 5.
(A) Western blot analysis of total IκBα and IκBα phosphorylated at serine 32 (pSer32) either in PRV Kaplan-infected ST cells (6 hpi; MOI, 10 PFU/cell) or in TNF-α-treated ST cells (5 min posttreatment, 100 ng/ml) in the presence or absence of the proteasome inhibitor MG132 (10 μM; which was added for 4 h starting at 2 hpi in PRV-infected cells and which was used for preincubation for 4 h in TNF-α-stimulated cells). (B) Western blot analysis of total IκBα and IκBα phosphorylated at serine 32 either in PRV Kaplan-infected ST cells (0, 2, 4, 6, and 8 hpi; MOI, 10 PFU/cell) or in TNF-α-treated ST cells (5 and 15 min posttreatment, 100 ng/ml). (C) Western blot analysis of total IκBα and IκBα phosphorylated at serine 32 in mock-treated or TNF-α-treated ST cells (5 min, 100 ng/ml) incubated or not with bacteriophage lambda protein phosphatase. The Western blots shown are representative examples from three independent repeats of the experiments.
During canonical NF-κB signaling, IκBα degradation is triggered via phosphorylation of IκBα at Ser32 and Ser36 by the IκBα kinase (IKK) complex (48–52). To address if a similar process underlies IκBα degradation in PRV-infected cells, the IκBα phosphorylation status at Ser32 was assessed in the first 8 h of infection, when progressive PRV-induced IκBα degradation occurs. As shown in Fig. 5B, while TNF-α treatment led to fast and noticeable IκBα Ser32 phosphorylation (at 5 and 15 min after TNF-α treatment), phosphorylated Ser32 of IκBα was not detected in PRV-infected ST cells, including at 4 hpi, when IκBα degradation had started. From this result, we concluded either that phosphorylation on the Ser32 residue does not precede PRV-mediated IκBα degradation or that the gradual nature of IκBα degradation observed in PRV-infected cells (versus the rapid degradation observed with TNF-α treatment) involves reduced phosphorylation or a reduced efficiency of phosphorylation below the detection limit for the phosphorylation signal. To further address this, IκBα degradation in PRV-infected ST cells was blocked by addition of the proteasome inhibitor MG132 at 2 hpi, enabling the analysis of a possibly accumulated phosphorylation status of IκBα in the first 6 h of infection. As shown in Fig. 5A (bottom row), IκBα retention (by proteasome inhibition) resulted in a weak but detectable phosphorylation of IκBα at Ser32, suggesting that PRV infection still targets IκBα for phosphorylation but that this is not detectable without proteasome inhibition. Since the observed phospho-Ser32 IκBα protein bands were weak, we validated the specificity of the antibody by bacteriophage lambda protein phosphatase (λ PP) treatment. As shown in Fig. 5C, as expected, λ PP treatment abolished the phospho-Ser32 IκBα signal.
In conclusion, the PRV-induced degradation of IκBα depends on proteasomal activity and involves the (possibly inefficient) phosphorylation of IκBα Ser32.
PRV infection blocks the prototypical NF-κB-induced gene expression profile.
As shown in Fig. 1, canonical NF-κB activation, e.g., via TNF-α treatment, triggers a potent negative feedback loop, consisting of NF-κB-dependent expression of negative regulators of NF-κB signaling, including IκBα (8, 9, 34, 35). Our data suggest that this negative feedback loop is impaired in PRV-infected cells, since PRV-induced IκBα degradation was not followed by an obvious increase in IκBα or the nuclear export of p65 (Fig. 1 and 2). One possible explanation for this result may be that PRV-induced NF-κB activation in fact does trigger a negative feedback response but that the newly produced IκBα is constitutively degraded. To assess this, we again treated ST cells with the proteasome inhibitor MG132, but at different time intervals postinfection (4 h of treatment starting at either 2 hpi, 4 hpi, or 8 hpi). Figure 6A shows that, like in our previous assay, addition of MG132 at 2 hpi and analysis at 6 hpi restored the IκBα band. However, addition of MG132 at 4 hpi (when virus-induced IκBα degradation has initiated; Fig. 1D) and analysis at 8 hpi did not show the full restoration of the IκBα band, and addition of MG132 at 8 hpi (when virus-induced IκBα degradation is complete; Fig. 1D) and analysis at 12 hpi did not show the restoration of IκBα degradation at all. These data suggest that the lack of the IκBα protein beyond initial virus-induced IκBα degradation is not due to a continued IκBα degradation process but is likely due to a lack of IκBα gene expression/protein production and, hence, a distorted NF-κB feedback loop.
FIG 6.
(A) Western blot analysis of IκBα in PRV Kaplan-infected ST cells (MOI, 10 PFU/cell) analyzed at 6, 8, and 12 hpi and treated or not with the proteasome inhibitor MG132 (10 μM) for 4 h starting at 2, 4, or 8 hpi. (B to E) RT-qPCR analysis of NFKBIA (IκBα) (B), TNFAIP3 (A20) (C), TNFA (TNF-α) (D), and IL-6 (IL-6) (E) gene transcription in PRV Kaplan-infected ST cells (4, 8, and 12 hpi; MOI, 10 PFU/cell) or TNF-α-treated ST cells (30 min, 1 h, 2 h, and 4 h; 100 ng/ml). Graphs represent the mean and standard deviation values of relative mRNA expression obtained from three independent repeats of the experiment. (F) Schematic representation of the NF-κB–luciferase reporter assay (pNiFty-Luc plasmid; Invivogen). The graph shows the mean and standard deviation values of relative light units registered in three independent NF-κB–luciferase reporter assays in mock-infected, PRV-infected (16 hpi; MOI, 10 PFU/cell), and TNF-α-stimulated (16 h; 100 ng/ml) ST cells that had previously been transfected with the pNiFty-Luc (5×NF-κB–luciferase) plasmid. The pCDNA3-eGFP plasmid was cotransfected to assess for a similar transfection efficiency under all analyzed conditions, as illustrated by the Western blot. Asterisks indicate statistically significant differences. **, P < 0.01; ***, P < 0.001.
To further assess this, expression of the IκBα-encoding gene (the NFKBIA gene) was assessed by reverse transcription (RT)-quantitative PCR (qPCR) analysis. The RT-qPCR assay revealed that while TNF-α, as expected, potently induced NFKBIA gene expression, PRV infection did not trigger such upregulation, and NFKBIA transcript levels remained similar to those observed in mock-infected cells (Fig. 6B), whereas at all the time points of the analysis (4, 8, and 12 hpi), NF-κB activation was demonstrated via IκBα degradation and NF-κB p65 nuclear translocation (Fig. 1 and 2). Hence, we confirmed that PRV blocks the replenishment of the IκBα protein by inhibiting the transcription of the IκBα-encoding gene.
This prompted us to analyze the expression of other prototypical NF-κB-stimulated genes in order to address whether the inhibition of gene expression was restricted to the NFKBIA gene. To avoid a possible bias, we chose three NF-κB-stimulated genes, belonging to nonrelated phylogenetic clusters of genes, that are located on different chromosomes. Nonetheless, all three encode polypeptides that play determinant roles in NF-κB pathway physiology and were as follows: the TNFAIP3 (encoding the A20 deubiquitin ligase), TNFA (encoding TNF-α), and IL-6 (encoding interleukin-6 [IL-6]) (53–56) genes. The A20 deubiquitin ligase is an important player in the NF-κB inhibitory feedback loop and blocks the activating signaling coming from diverse receptors upstream of the IKK signalosome (20, 21). Both TNF-α and IL-6 are hallmark proinflammatory cytokines (57, 58). RT-qPCR analysis showed that in PRV-infected ST cells, none of these three genes were upregulated at any of the analyzed time points postinfection. In contrast, as expected, TNF-α treatment led to a substantial increase in the transcription of all three genes (Fig. 6C to E). To further broaden the analysis of the effect of PRV infection on typical NF-κB-induced gene expression, an NF-κB–luciferase reporter assay was performed. To this end, an NF-κB-responsive plasmid containing five repeats of NF-κB binding sites (5′-GGGGACTTTCC-3′) within a minimal promoter that controls firefly luciferase gene expression was used. As shown in Fig. 6F, although addition of TNF-α triggered significant NF-κB-driven luciferase expression, PRV infection repressed NF-κB-induced luciferase expression, even below the levels observed for mock-treated cells. The cotransfection of pcDNA3 harboring enhanced green fluorescent protein (pcDNA3-eGFP) allowed confirmation of the equal transfection efficiency under all three conditions analyzed (Fig. 6F). In summary, gene expression analyses revealed that although PRV infection leads to persistent NF-κB activation, it does not allow the proper expression of prototypical NF-κB-responsive genes, including genes encoding hallmark proinflammatory proteins TNF-α and IL-6 or negative feedback loop protein IκBα or A20.
PRV-infected cells do not respond to TNF-α stimulation.
Since we found that PRV triggers persistent NF-κB activation and apparently prevents an NF-κB-induced negative feedback loop (e.g., a lack of restoration of IκBα protein levels), we wondered whether this inhibition of negative feedback could be overcome by treating PRV-infected cells with a classical NF-κB stimulant like TNF-α.
We chose to analyze this at a rather early time point, 4 hpi, since PRV-induced NF-κB activation could be observed at this time point but was not yet complete (e.g., IκBα protein degradation [Fig. 1D] and p65 nuclear translocation [Fig. 2B] were incomplete).
First, we added TNF-α at 4 hpi to PRV- or mock-infected ST cells and analyzed IκBα protein levels by Western blotting at 0, 0.5, 1, 2, and 4 h posttreatment. In the case of a functional NF-κB-induced negative feedback loop, the later time points would be expected to show the restoration of the IκBα protein levels. As shown in Fig. 7A, TNF-α treatment did not trigger the detectable upregulation of IκBα protein expression in PRV-infected cells at any of the time points tested (rather, IκBα degradation gradually proceeded, as was observed in the absence of TNF-α), whereas IκBα protein expression was obviously restored at 2 h after TNF-α treatment in mock-infected cells. These results indicate that PRV-infected cells are not sensitive to TNF-α stimulation. To corroborate this observation, we tested NF-κB p65 nuclear import in PRV-infected ST cells at 4 hpi after a 30-min treatment with TNF-α. As demonstrated in Fig. 7B, a 30-min treatment with TNF-α triggered a massive nuclear redistribution of NF-κB p65 in mock-infected cells, whereas this was not the case in PRV-infected cells. To further address at which stage in the signaling axis that PRV-mediated inhibition of TNF-α-induced NF-κB occurs, the phosphorylation of IκBα on Ser32 upon TNF-α stimulation was assessed (Fig. 7C). Western blotting indicated that while TNF-α treatment triggered IκBα phosphorylation in mock-infected cells, PRV-infected cells were also refractory to this phosphorylation, indicating that TNF-α-induced NF-κB activation is blocked by the virus at or upstream of the IKK signalosome. Hence, whereas PRV triggers a persistent but aberrant NF-κB activation without the expression of prototypical proinflammatory genes, at the same time, the virus renders the infected cell nonresponsive to TNF-α-induced NF-κB activation.
FIG 7.
(A) Western blot analysis of IκBα in either TNF-α-stimulated ST cells (100 ng/ml; left), PRV Kaplan-infected ST cells challenged at 4 hpi with TNF-α (MOI, 10 PFU/cell; 100 ng/ml; middle), or PRV Kaplan-infected ST cells that were left untreated (MOI, 10 PFU/cell; right), analyzed at the indicated time points. (B) Confocal microscopy of NF-κB p65 in mock-infected or PRV-infected ST cells (4 hpi; MOI, 10 PFU/cell) treated or not treated with TNF-α for 30 min (100 ng/ml). NF-κB p65 is shown in green, PRV gB is shown in red, and nuclei are shown in blue. Bar, 50 μm. (C) Western blot analysis of total and Ser32-phosphorylated IκBα protein in either mock-infected or PRV-infected cells (4 hpi; MOI, 10 PFU/cell) treated or not treated with TNF-α for 5 min (100 ng/ml). The Western blots and the confocal microscopy images shown are representative examples from three independent experimental repeats.
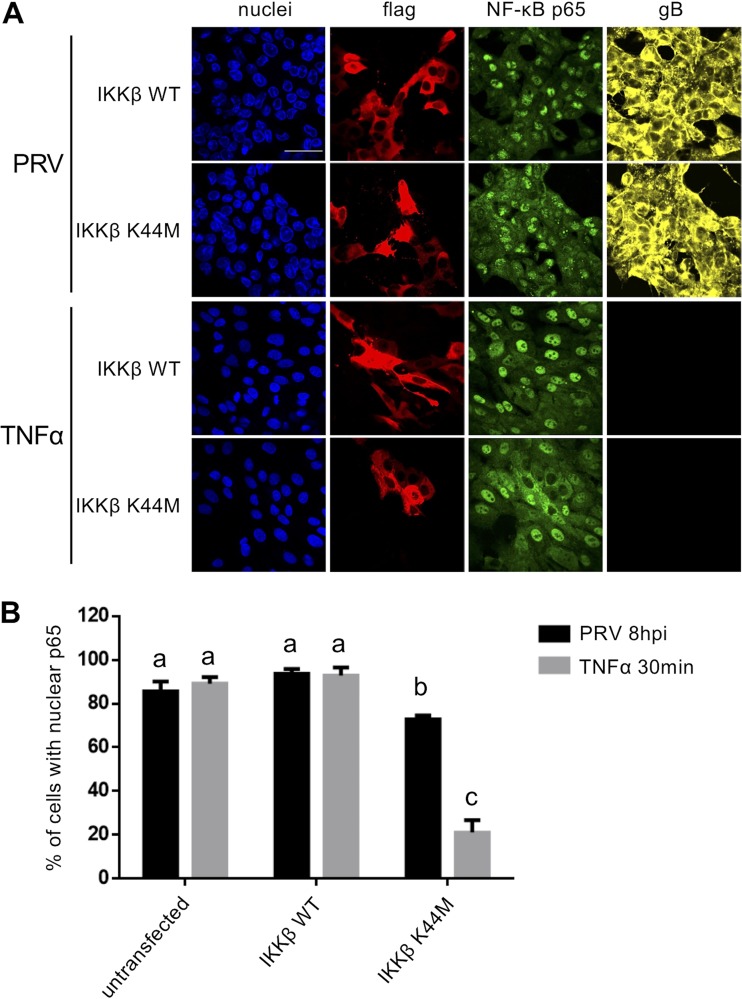
IKKβ kinase activity is not essential for the PRV-mediated nuclear import of NF-κB p65.
The lack of a response of PRV-infected cells to TNF-α suggests that the virus follows an alternative route to trigger NF-κB activation. To further address this, we assessed the involvement of the IκB kinase (IKK) signalosome in PRV-induced NF-κB activation in ST cells. The IKK signalosome consists of three subunits (IKKα, IKKβ, and the regulatory factor IKKγ/NEMO) (59). The IKKβ subunit is of particular importance, as it provides the catalytic activity required for the phosphorylation of IκBα at Ser32 and Ser36 (allowing its subsequent proteasomal degradation) (52, 60). To assess the involvement of IKKβ activity in PRV-mediated NF-κB activation, we overexpressed either a wild-type (WT) or kinase-dead variant of IKKβ. The latter harbors a replacement of the lysine amino acid residue at position 44 (ATP-binding site) by a methionine (IKKβ K44M) (60). We examined NF-κB activation by determining the nuclear translocation of p65. Whereas overexpression of the kinase-dead variant of IKKβ virtually abolished TNF-α-driven NF-κB p65 nuclear translocation in mock-infected ST cells, it significantly but only scantly reduced PRV-induced p65 nuclear translocation (Fig. 8A and B). This indicates that the PRV-induced nuclear translocation of NF-κB p65 does not predominantly depend on (the IKKβ subunit of) the IKK signalosome.
FIG 8.
(A) Confocal microscopy of NF-κB p65 in PRV-infected (8 hpi; MOI, 10 PFU/cell) or TNF-α-treated (30 min, 100 ng/ml) ST cells that had previously been transfected with either the pCMV2-IKK-2 WT plasmid (encoding Flag-tagged WT IKKβ) or the pCMV2-IKK-2 K44M plasmid (encoding kinase-inactive Flag-tagged IKKβ). NF-κB p65 is shown in green, PRV gB is shown in yellow, the Flag tag is shown in red, and nuclei are shown in blue. Bar, 50 μm. (B) The graph represents the mean and standard deviation of the percentage of WT IKKβ- or kinase-inactive IKKβ-transfected cells with a predominant nuclear localization of NF-κB p65, based on three independent repeats of the experiment. Significantly different (P < 0.05) results are indicated with different letters.
Activation of NF-κB does not appear to affect PRV replication efficiency.
Finally, we assessed whether the inhibition of NF-κB activation affected virus replication efficiency. To this end, we inhibited the nuclear import of NF-κB p65 in ST cells by overexpression of an IκBα superrepressor (SR), consisting of a mutated, nondegradable version of the inhibitory IκBα protein in which the residues at the serine 32 and serine 36 phosphorylation sites that control IκBα degradation are replaced by alanines (IκBα S32A/S36A) (48, 61). This nondegradable IκBα mutant provides a persistent negative regulation of the NF-κB pathway, ensuring the cytoplasmic retention of NF-κB dimers. As shown in Fig. 9A and B, both the TNF-α- and PRV-induced nuclear import of NF-κB p65 was efficiently inhibited in cells expressing the IκBα superrepressor (SR) S32A/S36A. When looking at the single-cell level, no obvious differences in viral gB protein expression between IκBα S32A/S36A-transfected cells and nontransfected cells could be observed (Fig. 9A). To confirm this observation, we performed a quantitative flow cytometric analysis of virus replication using a PRV recombinant virus (PRV-GS443) that expresses the capsid protein VP26 fused to GFP (VP26-GFP) (62). Figure 9C and D show that at 8 hpi of ST cells with PRV-GS443 (MOI, 10 PFU/cell), VP26-GFP expression was very much like that in IκBα SR S32A/S36A-transfected (hemagglutinin [HA]-positive) cells and nontransfected cells. This result suggests that the nuclear import of NF-κB p65 is not required for optimal expression of the capsid protein VP26.
FIG 9.
(A) Confocal microscopy of NF-κB p65 in PRV-infected (8 hpi; MOI, 10 PFU/cell) or TNF-α-stimulated (30 min, 100 ng/ml) ST cells that had previously been transfected with the pCMV4-3×HA-IκBα SS32,36AA plasmid encoding an IκBα superrepressor (SR; S32A/S36A) fused to a 3×HA tag. NF-κB p65 is shown in green, PRV gB is shown in yellow, the HA tag is shown in red, and cell nuclei are shown in blue. (B) The mean and standard deviation of the percentage of IκBα S32A/S36A (SR)-transfected cells and nontransfected cells with a predominant nuclear localization of NF-κB p65, based on three independent repeats of the experiment. Significantly different (P < 0.05) results are indicated with different letters. (C) Flow cytometric contour plots of ST cells that were either not transfected and not infected (mock, upper left), transfected with IκBα S32A/S36A-HA and not infected (SR, upper right), not transfected and infected with PRV-GS443 (VP26-GFP; MOI, 10 PFU/cell; 8 hpi) (PRV, lower left), and transfected with IκBα S32A/S36A-HA and infected with PRV-GS443 (MOI, 10 PFU/cell; 8 hpi) (PRV+SR, lower right). The y axis represents the fluorescence intensity registered for VP26-GFP, and the x axis shows the fluorescence intensity obtained for the APC channel (stained HA tag). (D) The mean and standard deviation of the median fluorescence intensity (MFI) ratios of VP26-GFP in nontransfected or IκBα S32A/S36A (SR)-transfected cells based on three independent repeats of the assay. ns, no significant difference. (E) Western blot analysis of IE180 (IE), US3 (E), gE (L), and VP5 (L) expression in mock-infected and PRV Kaplan-infected (MOI, 10 PFU/cell; 8 hpi) ST cells that had previously been transfected (or not) with scrambled or NF-κB p65-targeting silencing RNAs (siRNA). NF-κB p65 protein levels were determined to assess efficient knockdown. (F) Extracellular virus yields (log10 number of PFU per milliliter) determined at 8, 16, and 24 hpi in ST cells transfected with scrambled siRNA or NF-κB p65-targeting siRNA and infected at an MOI of 10 PFU/cell. (G) Extracellular virus yields (log10 number of PFU per milliliter) determined at 24 and 48 hpi in ST cells transfected with scrambled siRNA or NF-κB p65-targeting siRNA and infected at an MOI of 0.01 PFU/cell.
To further assess a possible role for NF-κB p65 in PRV replication, we designed specific silencing RNAs targeting porcine NF-κB p65, which resulted in the efficient knockdown (KD) of NF-κB p65 protein levels in ST cells (Fig. 9E). The knockdown of p65 in ST cells did not noticeably affect the protein expression of either immediate early or α (IE180), early or β (US3), and late or γ (VP5, gE) genes upon PRV infection (MOI, 10 PFU/cell). In addition, virus growth curves, consisting of both single-step growth curves at an MOI of 10 PFU/cell and multistep growth curves at an MOI of 0.01 PFU/cell, did not show noticeable differences in PRV titers between ST cells treated with scrambled small interfering RNA (siRNA) and ST cells treated with p65 siRNA (Fig. 9F and G).
In conclusion, activation of NF-κB and expression of the p65 protein are not required for optimal virus replication in PRV-infected ST cells.
DISCUSSION
In the current report, we show that infection of epithelial cells with the porcine alphaherpesvirus pseudorabies virus (PRV) results in the gradual and persistent degradation of IκBα, the inhibitory partner protein of the NF-κB complex. In addition, the degradation of IκBα is accompanied by a concomitant nuclear translocation of the p65 subunit of NF-κB and Ser536 phosphorylation of p65, which are all hallmarks of NF-κB activation. NF-κB activation and p65 protein expression do not appear to be required for PRV replication in cell culture. However, PRV-induced NF-κB activation did not result in the expression of prototypical NF-κB-controlled genes, like those for TNF-α, IL-6, IκBα, and A20, or expression of a NF-κB-driven reporter gene. Moreover, we found that PRV-infected cells are resistant to TNF-α-induced canonical NF-κB activation, indicating that PRV profoundly modulates and manipulates NF-κB signaling, interfering with the proinflammatory consequences of this signaling axis (Fig. 10).
FIG 10.
Schematic representation of a hypothetical model of how PRV modulates the NF-κB signaling axis, based on the findings obtained in the current work.
Our finding that PRV triggers persistent NF-κB activation in infected cells—in porcine, bovine, and rabbit epithelial cell lines as well as in primary porcine respiratory epithelial cells—indicates that this may be a common feature of several herpesviruses. Indeed, persistent NF-κB activation has been reported for HSV-1 (28, 63), another alphaherpesvirus, and for members of other herpesvirus subfamilies, including the betaherpesvirus HCMV (64) and the gammaherpesvirus EBV (65). Despite the apparent conservation of persistent NF-κB activation among different herpesviruses, the function of NF-κB in virus replication is still incompletely understood. In HSV-1, inhibition of NF-κB either by overexpression of a dominant active IκBα superrepressor or via addition of broad anti-inflammatory molecules like prostaglandin A1 resulted in reduced HSV-1 progeny yields (63). Patel and colleagues also found that overexpression of a dominant active IκBα superrepressor led to lower HSV-1 titers but that viral protein synthesis was not affected (28). In line with this, the single or double knockout of p65 and p50 did not affect viral protein expression, viral genome synthesis, or virus morphology but reduced extracellular virus titers (66). For PRV, we found that inhibition of NF-κB either by overexpression of a dominant active IκBα superrepressor or by the knockdown of NF-κB p65 protein expression did not result in noticeable changes in the expression of representative α, β, or γ gene products, in line with the observations for HSV-1 (28, 66). However, in contrast to what was found in HSV-1 infection, the extracellular titers of virus released from ST cells in which p65 was knocked down were similar to those of virus released from normal cells. Zhao et al. reported earlier that addition of the anti-inflammatory molecule resveratrol resulted in the downregulation of PRV gene expression and genome replication and claimed that this could be due to resveratrol-mediated inhibition of NF-κB (67). However, resveratrol has been shown to affect several other key stress-signaling pathways, like the phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase, or mTOR pathway (68), which may possibly contribute to the observed reduced virus fitness. Although the possibility of cell type-dependent differences in virus replication and/or a potential contribution of particular subsets of NF-κB complexes to virus replication cannot be excluded at present, our data suggest that NF-κB does not directly contribute to PRV replication in cell culture.
Canonical activation of the NF-κB complex occurs via the fast IKK-mediated phosphorylation of IκBα at serine residues 32 and 36, followed by IκBα ubiquitination and degradation via the proteasome (46–49). Although we found that in PRV-infected cells IκBα degradation is also proteasome dependent, the PRV-induced process is considerably slower than that observed for TNF-α, taking several hours to completion at about 6 hpi. Also, although IκBα Ser32 phosphorylation could be observed in PRV-infected cells, this was detectable only in cells in which the IκBα protein was allowed to accumulate via the addition of a proteasome inhibitor (Fig. 5A and B), indicating that PRV-induced IκBα degradation occurs considerably less efficiently than that observed with an extracellular NF-κB activator like TNF-α. One possible explanation may be that PRV does not depend on the canonical IKK signalosome to trigger IκBα degradation. In support of this hypothesis, we found that transfection of an inactive K44M mutant of IKKβ (60), an essential component of the IKK signalosome, only slightly reduced PRV-induced p65 nuclear translocation. These results are different from what has been reported for HSV-1, where the IKK complex, including IKKα and/or IKKβ, has been reported to contribute to NF-κB activation (63, 69). Notwithstanding, another report indicated that protein kinase R activation is necessary for NF-κB activation in HSV-1-infected cells, pointing to an alternative source of stimulation different from the canonical pathway (70). Since we also observed that PRV-infected cells are resistant to TNF-α-induced IκBα Ser32 phosphorylation and NF-κB activation, we speculate that PRV inactivates one or more signaling components at or upstream of the IKK signalosome. It will be interesting in future research to further pinpoint how PRV disrupts canonical NF-κB activation and which alternative pathway(s) may be used by PRV to trigger IκBα degradation.
The UV inactivation of PRV and the treatment of infected cells with PAA abolished the ability of PRV to trigger persistent NF-κB activation, indicating that viral (late) gene expression is required for NF-κB activation. This is again different from reports for HSV, which point to several (immediate) early viral proteins that contribute to NF-κB activation (28, 69, 71, 72). Although PAA addition blocked PRV-induced NF-κB activation, the timing of IκBα degradation and p65 nuclear translocation (mainly occurring between 2 hpi and 6 hpi) indicates that the viral factor(s) involved is likely not a true late protein but may be expressed with early-late gene expression characteristics.
The PRV-induced persistent activation of NF-κB is characterized by the irreversible loss of IκBα and the constant nuclear distribution of NF-κB p65 and is in line with the findings obtained with HSV-1 (28, 63). The major explanation behind this persistent NF-κB activation appears to be the lack of a negative feedback loop by a viral shutoff of prototypical NF-κB-induced gene expression, including expression of genes encoding negative feedback loop proteins IκBα and A20. In line with this, PRV also prevents the NF-κB-induced expression of genes encoding proinflammatory cytokines TNF-α and IL-6. Since TNF-α and IL-6 display protective activity against acute infections with alphaherpesviruses, like HSV-1 (73–75), inhibition of TNF-α and IL-6 expression in PRV-infected cells might represent an advantage for the virus in its interaction with the host immune response. An NF-κB reporter assay suggests that PRV infection in fact results in a general transcriptional shutoff of NF-κB-targeted genes, in line with earlier findings for HSV-1 (28). For HSV-1, it has been suggested that nuclear NF-κB p65 may be recruited to viral promoters (e.g., the ICP0 promoter) rather than cellular promoters (76, 77), and it has been suggested that HSV-1 proteins like VP16 may interact with p65 and thereby perturb normal NF-κB-dependent transactivation (78). Also, ICP27 of HSV-1 has been reported to inhibit Daxx sumoylation, thereby repressing p65 acetylation, which is required for the correct transcriptional activity of NF-κB (79). In PRV infection, we did see the (persistent) nuclear import of NF-κB p65, indicating that the functional inhibition of NF-κB should happen in the nucleus. Although the causes of the lack of prototypical NF-κB-induced gene expression in PRV-infected cells are unclear at present and will require additional studies, we hypothesize that PRV may alter the NF-κB signalosome, e.g., via the interaction of one or more viral proteins with nuclear NF-κB, in this way restricting its activity.
In summary, although PRV does not appear to need NF-κB for replication in cell culture, it has a dramatic impact on the NF-κB pathway in infected cells, resulting in persistent NF-κB activation without the detectable expression of prototypical NF-κB-induced genes, including genes encoding negative feedback loop proteins and proinflammatory cytokines TNF-α and IL-6. In addition, PRV-infected cells are resistant to TNF-α-induced canonical NF-κB activation, indicating that PRV-mediated interference with NF-κB signaling may contribute to viral modulation of early proinflammatory responses in the infected host.
MATERIALS AND METHODS
Cell cultures and viruses.
Swine testicle (ST) cells (ATCC CRL-1746; Sus scrofa, pig) were cultured in modified Eagle’s medium (MEM) supplemented with 10% inactivated fetal bovine serum (FBS), 100-U/ml penicillin, 0.1-mg/ml streptomycin, 50-μg/ml gentamicin, and 1 mM sodium pyruvate (all from Gibco, Thermo Fisher Scientific) (ST medium). Cells of the rabbit kidney 13 (RK-13) cell line (ATCC CCL-37; Oryctolagus cuniculus, rabbit) were cultured in MEM supplemented with 10% inactivated FBS, 100-U/ml penicillin, 0.1-mg/ml streptomycin, and 50-μg/ml gentamicin. Bovine kidney (MDBK) cells (ATCC CCL-22; Bos taurus, cow) were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco) supplemented with 5% inactivated FBS, 100-U/ml penicillin, 0.1-mg/ml streptomycin, and 1 mM sodium pyruvate. Finally, the sensory neuronal cell line ND7 (44) was cultured with DMEM (high glucose) supplemented with 10% inactivated FBS, 50-U/ml penicillin, and 50-μg/ml streptomycin. For neuronal differentiation, cells were incubated with DMEM–Ham’s F-12 medium (1:1), 0.5% inactivated FBS, 50-U/ml penicillin, 50-μg/ml streptomycin, and 1 mM dibutyryl cyclic AMP (db cAMP; Sigma-Aldrich) for 48 h prior to infection. The three wild-type (WT) PRV strains used in this study were WT PRV Kaplan (80), WT PRV Becker (81), and WT PRV NIA-3 (82). Mutant virus PRV-GS443, encoding the green fluorescent protein (GFP) fused to the PRV UL35 gene product (VP26 capsid protein) cloned in the WT PRV Becker backbone, was obtained from Gregory A. Smith (Northwestern University Feinberg School of Medicine, Chicago, IL, USA) (62). PRV stocks were titrated by serial dilution assays on ST cells. Unless the multiplicity of infection (MOI) is specifically mentioned, all the PRV infections performed in this work were done at an MOI of 10 PFU/cell on confluent cell monolayers.
Isolation of primary PoRECs.
The isolation of porcine respiratory tracheal epithelial cells (PoRECs) was based on procedures described for the isolation of equine and bovine primary respiratory epithelial cells adapted for pig (83–85). Briefly, five 7-week-old piglets (Sus scrofa) were euthanized by intravenous injection of 10 ml sodium pentobarbital (a 20% solution of Nembutal [Sanofi] at 0.125 ml/kg body weight). Animal care and euthanasia were performed according to Federation of European Laboratory Associations (FELASA) guidelines and approved by the Ethical Committee of the Faculty of Veterinary Medicine of Ghent University. Postmortem dissection of the piglets allowed the collection of trachea specimens, which were immediately incubated in ice-cold transport solution (100-U/ml penicillin, 0.1-mg/ml streptomycin, 0.1-mg/ml gentamicin, 0.1-mg/ml kanamycin [Sigma-Aldrich], and 250-ng/ml amphotericin B diluted in phosphate-buffered saline [PBS]). After removal of the surrounding connective tissue and blood vessels tightly associated with the tracheas, the tracheas were washed with PBS and subsequently incubated in a pronase–DNase I solution for 72 h at 4°C. The pronase–DNase I solution consisted of 1.12 mg/ml of pronase (catalog number 11459643001; Roche), 0.08 mg/ml of DNase I (RNase free; catalog number M0303S; New England Biolabs), 4.5 mg/ml of α-d-glucose (Serva), 1 mM sodium pyruvate, 100-U/ml penicillin, 0.1-mg/ml streptomycin, 250-ng/ml amphotericin B (Gibco, Thermo Fisher Scientific), all of which were diluted in PBS. After incubation, trachea-free cells (mainly epithelial cells and fibroblasts) were subjected to plastic adhesion on Nunclon Delta petri dishes (Nunc; Thermo Fisher Scientific) (fibroblasts adhere but epithelial and blood cells do not). Nonadhered cells were harvested from two consecutive rounds of selection (each with a 2-h duration). Then, the cells were counted and 1.2 million cells were plated in type IV collagen (catalog number C5533; Sigma-Aldrich)-precoated 12-well plate transwells with a 0.4-μm pore size (catalog number 3460; Costar; Corning). Cells were incubated in Afi1 medium (DMEM plus Ham’s F-12 medium, 5% FBS, 1% MEM nonessential amino acids [Gibco, Thermo Fisher Scientific], 100-U/ml penicillin, 0.1-mg/ml streptomycin, 1.25 μg/ml amphotericin B). On the day after seeding, nonattached cells were carefully washed away with PBS and Afi1 medium was replaced by Afi2 medium (DMEM plus Ham’s F-12 medium, 2% Ultroser G [catalog number 15950-017; Pall Life Sciences], 100-U/ml penicillin, 0.1-mg/ml streptomycin, 1.25-μg/ml amphotericin B) only in the bottom part of the transwell, generating an air-liquid interface that simulates the natural environment of the respiratory tract. When the cells reached full confluence, they were apically infected with PRV Kaplan. The purity of the isolated cells was assessed by labeling of the epithelial cell-specific marker cytokeratin by immunofluorescence staining (rabbit polyclonal anti-pan-cytokeratin antibody; catalog number ab9377; Abcam). The percentage of cytokeratin-positive cells was always >90%, as shown in Fig. 3A.
Antibodies.
(i) PRV-specific antibodies. For PRV detection, we used the mouse monoclonal antibodies anti-gB antibody (clone 1C11; 1:50 for IF), anti-gD antibody (clone 13D12; 1:50 for IF, 1:100 for WB), anti-gE (clone 18E8; 1:100 for WB) (86), mouse monoclonal anti-US3 antibody (1:50 for IF, 1:100 for WB) (87), mouse monoclonal anti-VP5 antibody (clone 3C10; 1:2,000 for WB) (88), mouse monoclonal anti-gC antibody (clone 8P19; 1:750 for WB) (89), and rabbit polyclonal anti-IE180 antibody (1:1,000 for WB) (90). The US3 and VP5 antibodies were generous gifts from Lynn Enquist (Princeton University, NJ, USA), and the IE180 antibody was kindly provided by Enrique Tabarés (Autónoma University of Madrid, Madrid, Spain). The gC antibody was a kind gift from A. Brun (Mérial, Lyon, France).
(ii) Antibodies for detection of cellular targets or tagged proteins. Mouse anti-IκBα (clone L35A5; 1:1,000 for WB; catalog number 4814), rabbit anti-phospho-IκBα Ser32 (clone 14D4; 1:1,000 for WB; catalog number 2859), mouse anti-NF-κB p65 (clone L8F6; 1:1,000 for WB, 1:400 for IF; catalog number 6956), and rabbit anti-phospho-NF-κB p65 Ser536 (clone 93H1; 1:1,000 for WB, 1:1,000 for IF; catalog number 3033) were obtained from Cell Signaling Technology (CST). Mouse horseradish peroxidase (HRP)-conjugated anti-α-tubulin antibody (clone DM1A; 1:2,000 for WB; catalog number ab40742), rabbit polyclonal anti-GFP antibody (1:1,000 for WB; catalog number ab6556), and rabbit polyclonal anti-pan-cytokeratin antibody (1:200 for IF; catalog number ab9377) were purchased from Abcam. The rabbit polyclonal anti-histone 3 (anti-H3) antibody (1:1,000 for WB; catalog number 17168-1-AP) was obtained from ProteinTech. Mouse monoclonal Flag tag antibody (clone M2; 1:1,000 for IF; catalog number F3165) was obtained from Sigma-Aldrich. The HA tag was detected by the use of the mouse monoclonal anti-HA tag antibody (clone 2-2.2.14), obtained from Thermo Fisher Scientific (1:1,000 for IF and flow cytometry; catalog number 26183).
Plasmids and transfection protocol.
(i) Plasmids. The plasmids used in this study were purchased from the plasmid bank Addgene. The pCDNA3-eGFP plasmid (catalog number 13031) was a contribution of Douglas Golenbock (unpublished). The GFP-RelA (p65) (catalog number 23255) and pCMV4-3×HA-IκBα SS32,36AA (the dominant negative form of IκBα; catalog number 24143) were donated by Warner Greene (61, 91). The pCMV2-IKK-2 WT (IKKβ WT; catalog number 11103) and pCMV2-IKK-2 K44M (IKKβ kinase dead; catalog number 11104) Flag-tagged plasmids were provided by Anjana Rao (60). Competent Escherichia coli DH5α cells were grown in LB medium supplemented with ampicillin (Sigma-Aldrich) overnight at 37°C with gentle shaking. Plasmids were purified using a Pure Link midiprep kit (catalog number K210004; Invitrogen, Thermo Fisher Scientific) following the manufacturer’s protocol. Quantification and quality analysis of plasmid DNA yields were performed with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific).
(ii) Transfection procedure. The transfection of preconfluent ST cells (confluence, 50 to 60%) was performed on 24-well plates using the Lipofectamine reagent (catalog number 18324-012; Invitrogen) as a DNA carrier. For each well, 0.5 μl of the Lipofectamine reagent was mixed with 312 ng of DNA diluted in Opti-MEM medium (Gibco). The volumes of DNA and the Lipofectamine reagent were scaled to 1.25 μg and 2 μl, respectively, for transfections in 6-well plates. The transfection procedure was performed according to the manufacturer’s instructions. At 4 h after addition of the DNA-liposome complexes to ST cells, the cells were washed with PBS and fresh antibiotic-free medium (MEM supplemented with 10% inactivated FBS and 1 mM sodium pyruvate) was added. At 24 h posttransfection, the cells were subjected to infections or treatments.
Cytokines and inhibitors.
Purified recombinant porcine TNF-α was purchased from R&D Systems (catalog number 690-T). The 26S proteasome inhibitor MG132 was obtained from Sigma-Aldrich (MG132 ready-made solution; catalog number M7449). Phosphonoacetic acid (PAA) was purchased from Sigma-Aldrich (catalog number 284270).
Western blotting.
All cell lysates were harvested in ice-cold lysis buffer consisting of TNE (Tris-NaCl-EDTA) buffer, pH 7.5, 1% Nonidet P-40 (catalog number 37129000; Roche), and a protease inhibitor cocktail (cOmplete mini EDTA free; catalog number 11836170001; Roche). In order to detect phosphoproteins, a phosphatase inhibitor cocktail (PhosSTOP; catalog number 04906845001; Roche) was also added to the lysis buffer. Cell lysates were incubated for 20 min at 4°C and immediately frozen. SDS-PAGE and Western blotting were performed as described previously (92). Regular blots were blocked in 5% (wt/vol) nonfat dry milk diluted in 0.1% PBS–Tween 20 (PBS-T) for 1 h at room temperature. When using phosphorylated protein-specific antibodies, 5% (wt/vol) bovine albumin (catalog number 160069; MP Biomedicals) diluted in PBS-T was used for blocking. Primary antibody incubations were performed overnight at 4°C in the corresponding blocking buffers. After 3 washing steps with PBS-T (10 min each), the blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:2,000; catalog number P0447; Dako) or goat anti-rabbit IgG (1:3,000; catalog number P0448; Dako) secondary antibodies for 1 h at room temperature, followed by protein band detection using chemiluminescence with a ChemiDoc MP imaging device (Bio-Rad). Depending on the protein levels and the sensitivity of the antibodies, the Pierce enhanced chemiluminescence (ECL) substrate (Thermo Scientific), ECL Plus substrate (GE Healthcare), or SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific) was used.
Cell fractionation.
Cell fractionation was done as described before (93). Briefly, cell pellets were resuspended in ice-cold lysis buffer (as described above) and incubated at 4°C for 20 min with gentle shaking. The cell lysates were centrifuged for 10 min at 4°C at 10,000 × g. The supernatant was harvested as the cytoplasmic fraction of the lysate. Pellets were washed with 1 ml PBS and again centrifuged for 10 min at 4°C at 10,000 × g. The supernatants were discarded, and the pellets were resuspended in ice-cold radioimmunoprecipitation assay lysis buffer (Abcam) supplemented with a protease inhibitor cocktail (cOmplete mini EDTA free; catalog number 11836170001; Roche).
Immunofluorescence assays.
Cell monolayers were fixed with 4% paraformaldehyde at room temperature for 15 min. Afterwards, the cells were washed three times with PBS and incubated with blocking/permeabilizing buffer (5% inactivated FBS and 0.3% Triton X-100 diluted in PBS) for 1 h at 37°C. Primary antibody incubations were performed overnight at 4°C, diluting the corresponding antibody in the incubation buffer (1% [wt/vol] bovine serum albumin and 0.3% Triton X-100 diluted in PBS). After three washing steps with 1 ml PBS in each step, fluorochrome-linked goat anti-mouse IgG or goat anti-rabbit IgG secondary antibodies or fluorochrome-linked streptavidin (1:200; Invitrogen), depending on the nature of the primary antibody, was incubated for 1 h at 37°C. Hoechst 33342 (1:200; catalog number H3570; Invitrogen) was added at room temperature for 10 min to counterstain the cell nuclei. Fluorescence images were taken using a Leica SPE confocal microscope (Leica) and were analyzed using ImageJ software (NIH, USA).
NF-κB–luciferase reporter assay.
Preconfluent ST cells were transfected with the NF-κB reporter plasmid pNiFty-Luc (Invivogen) following the transfection protocol described above (100 ng of plasmid DNA/well in a 24-well plate). The pNiFty-Luc plasmid harbors five repeats of NF-κB binding sites (5′-GGGGACTTTCC-3′) within the minimal ELAM promoter, which positively controls expression of the firefly luciferase gene. At 4 h after transfection, the cells were either treated with TNF-α or infected with PRV for 16 h. Cell lysates were collected in ice-cold reporter lysis buffer (catalog number E3941; Promega) and were subjected to a single freeze-thaw cycle (−80°C). The luciferase assay was performed using a luciferase assay system from Promega (catalog number E4030) according to the manufacturer’s indications. Detection of luciferase-emitted light was done using a Luminoskan device (Labsystems). pCDNA3-eGFP plasmid transfection was used as transfection control.
Real time-quantitative PCR (RT-qPCR).
RNA isolations were performed using an RNeasy minikit (catalog number 74104; Qiagen) according to the manufacturer’s procedure. RNA was treated with DNase I (RNase free; catalog number M0303S; New England Biolabs) at 37°C for 10 min. DNase I activity was stopped by addition of EDTA (final concentration, 5 mM; Invitrogen) and by incubating the RNAs at 75°C for 10 min. RNA yields were analyzed for quality and quantity using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Five hundred nanograms of DNA-free total RNA was subjected to reverse transcription (RT) by use of an iScript cDNA synthesis kit (catalog number 1708891; Bio-Rad) according to the manufacturer’s instructions to obtain cDNA in a single-step reaction consisting of 5 min at 25°C (priming), 20 min at 46°C (reverse transcription), and 1 min at 95°C (RT inhibition). Primer oligonucleotides were synthesized by Integrated DNA Technologies (IDT). The sequences of the forward and reverse primers used for the amplification of the genes of interest can be found in Table 1. For nonpreexisting primer oligonucleotide sequences, the primer design tool Primer-BLAST (NIH, USA) was used. A SYBR green PCR master mix (catalog number 4309155; Applied Biosystems, Thermo Fisher Scientific) was used for quantitative PCRs (qPCRs) using 1 μl of the cDNA template resulting from RT following the protocol provided by the manufacturer. qPCRs were performed in a final volume of 20 μl on MicroAmp Fast optical 96-well reaction plates (catalog number 4346906; Applied Biosystems, Thermo Fisher Scientific) using a StepOnePlus real-time PCR system (catalog number 4376600; Applied Biosystems, Thermo Fisher Scientific). The relative expression of the target genes (TNFA [encoding TNF-α], IL-6 [encoding IL-6], TNFAIP3 [encoding A20], and NFKBIA [encoding IκBα]) was analyzed by the double delta threshold cycle method and normalized to the level of expression of the 28S rRNA gene, which has been validated as a reference gene in other similar gene expression analyses (94).
TABLE 1.
Forward and reverse primers used for RT-qPCR analyses
| Genea (encoded protein, GenBank accession no.) (reference) | Sequence (5′–3′) |
|
|---|---|---|
| Forward | Reverse | |
| TNFA (TNF-α, NM_214022.1) (97) | 5′-ACT GCA CTT CGA GGT TAT CGG-3′ | 5′-GGC GAC GGG CTT ATC TGA-3′ |
| IL6 (IL-6, NM_214399.1) (98) | 5′-TTC ACC TCT CCG GAC AAA ACT G-3′ | 5′-TCT GCC AGT ACC TCC TTG CTG T-3′ |
| NFKBIA (IκBα, NM_001005150.1) | 5′-AAG CAC TCG GAT ACA GCA GC-3′ | 5′-AGT CGT CAT AGG GCA GCT CA-3′ |
| TNFAIP3 (A20, NM_001267890.1) | 5′-CCT GTT CAG CGA GAC TAC GG-3′ | 5′-AAC GTC CTG GTG ACG TTC TG-3′ |
| 28S rRNA (94) | 5′-GGG CCG AAA CGA TCT CAA CC-3′ | 5′-GCC GGG CTT CTT ACC CAT T-3′ |
All genes are for the pig (Sus scrofa).
Flow cytometry assays.
Cells transfected (or not) with the dominant negative version of IκBα (S32A/S36A; catalog number 24143; Addgene) and subsequently infected (or not) with PRV-GS443 (PRV Becker VP26-GFP) were detached at the time points indicated above using PBS-EDTA plus 0.25% trypsin (Gibco) for 5 min. Trypsin activity was inhibited by adding inactivated FBS (up to 15% FBS). Then, the cells were fixed and permeabilized with BD Cytofix/Cytoperm solution (catalog number 554714BD; BD Biosciences) at 4°C for 20 min. Subsequently, the cells were washed with 1× BD Perm/Wash buffer, made from 10× BD Perm/Wash buffer (catalog number 554714; BD Biosciences) diluted in ultrapure water. Primary and secondary antibodies were diluted in 1× BD Perm/Wash buffer, and each incubation step lasted for 20 min and was carried out at 4°C. Anti-HA tag antibody was used at a dilution of 1:1,000, and allophycocyanin (APC)-conjugated goat anti-mouse IgG antibody was used at a dilution of 1:200. The fluorescence intensity of HA-stained/VP26-GFP-infected cells was quantified by measuring 10,000 gated events with a NovoCyte flow cytometer (ACEA Biosciences). Data analysis was performed using NovoExpress software (ACEA Biosciences).
Knockdown of NF-κB p65 with siRNA.
Silencing RNAs directed toward porcine NF-κB p65 mRNA (GenBank accession number NM_001114281.1) were customized using a design small interfering RNA (siRNA) tool from Integrated DNA Technologies (IDT). Scrambled negative-control siRNAs (catalog number 51-01-19-09; IDT) and NF-κB p65 siRNAs (5′-GCAUCAUGAAGAAGAGUC-3′; 5′-UUGAAAGGACUCUUCUUC) were also synthesized by IDT. The annealing of the two RNA strands was performed by incubating the RNAs at 95°C for 2 min, and then the RNAs were cooled to room temperature to reach the optimal annealing temperature. The transfection of preconfluent ST cells (confluence, 70 to 80%) was done by mixing 2.5 μl of siRNA suspension (10 μM, diluted in nuclease-free duplex buffer [IDT]) and 7.5 μl of the Lipofectamine RNAiMAX reagent (catalog number 13778030; Thermo Fisher Scientific) in Opti-MEM medium per well of a 6-well plate. The siRNA and lipofectamine volumes were reduced one-fourth to adapt the protocol to a 24-well-plate system. The transfection procedure was carried out according to the manufacturer’s indications. Cells were incubated with Lipofectamine reagent-RNA complexes for 48 h, and then they were subjected to virus infections. The effectiveness of NF-κB p65 knockdown was assessed by Western blotting with a specific anti-NF-κB p65 antibody (catalog number 6956; CST).
Virus growth curves.
Confluent ST cell monolayers were infected at a multiplicity of infection of either 10 PFU/cell (one-step infection) or 0.01 PFU/cell (multiple-step infection) in 24-well plates. The virus inoculum was washed away at 2 hpi, and the cell monolayers were washed twice with 1 ml of complete PBS. To ensure that all remaining infectious virus from the input was removed, we treated the cell monolayers with sodium citrate buffer, pH 3.0 (40 mM sodium citrate, 10 mM KCl, 135 mM NaCl) (95), for 2 min at room temperature. Then, the cells were washed again twice with 1 ml of complete PBS, and fresh ST medium was added.
Infectious supernatants were titrated by 1/10 serial dilution assays on ST cells seeded on 96-well plates, with four experimental repeats being performed. The characteristic and strong PRV-derived cytopathic effect served as a readout. Extracellular virus titers were expressed as the number of PFU per milliliter.
Inactivation of PRV infectious particles with UV light.
Inactivation of infectious virus particles (PRV Kaplan) was performed by UV light irradiation (1,000 mJ/cm2) of ice-cold inoculum placed on petri dishes, as described previously (96), using a CL-1000 UV cross-linker (UVP Inc.). The concentration of infectious virus was lowered from an original titer of 109 PFU/ml to 101.63 PFU/ml, that is, a reduction of more than 7 base 10 logarithmic units.
Phosphatase reaction.
Cell lysates were subjected to bacteriophage lambda protein phosphatase (λ PP) treatment (1 μl/reaction mixture; catalog number P0753S; New England BioLabs) at 30°C for 30 min. The reaction was performed in a total volume of 50 μl, according to the manufacturer’s indications.
Statistical analyses.
The statistical significance of the data shown in Fig. 6F, 8B, and 9B and D was calculated by unpaired t tests.
ACKNOWLEDGMENTS
Nicolás Romero is supported by a Ph.D. grant from Research Foundation Flanders (F.W.O.-Vlaanderen; grant number FWO.SPB.2019.0043.01). This research was supported by grants from the F.W.O.-Vlaanderen (grant numbers G017615 and G.019617N) and the Special Research Fund of Ghent University (G.O.A. grant 01G01317 and grant BOFBAS2018000301).
We thank Thomas Mettenleiter (Friedrich Loeffler Institute, Germany), Lynn Enquist (Princeton University, USA), Greg Smith (Northwestern University, USA), and the ID-DLO Institute (The Netherlands) for virus strains. We thank our colleagues Yue Yin, Mizanur Rahman, Mohammad Alkholi, Dayoung Oh, and Jiexiong Xie for the excellent help in experiments related to the primary respiratory cell isolation procedure and to Rudy Cooman for animal caretaking. We also thank Sofie Denaeghel for sharing her expertise in flow cytometry. In addition, we are grateful to Melanie Bauwens and Carine Boone for handling the rabbit and bovine cell lines, kindly provided by the lab of Hans Nauwynck (Ghent University, Ghent, Belgium). We are also very grateful to César de Medina for his contribution to the graphic design of Fig. 10.
We declare no conflict of interest.
N.R. and H.W.F. designed the research. N.R. and C.V.W. performed the experiments. N.R. and H.W.F. analyzed the results, interpreted the data, made the figures, and wrote the manuscript.
REFERENCES
- 1.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurobiology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T, Zhang T, Joo D, Sun SC. 2017. NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoro MG, Rossi A, Amici C. 2003. NF-kappaB and virus infection: who controls whom. EMBO J 22:2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MS, West AP, Ghosh S. 2006. NF-kappaB and the immune response. Oncogene 25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 5.Hayden MS, Ghosh S. 2014. Regulation of NF-κB by TNF family of cytokines. Semin Immunol 26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-kappaB activity. Annu Rev Immunol 18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. 1993. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A 90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun SC, Ganchi PA, Ballard DW, Greene WC. 1993. NF-kappa B controls expression of inhibitor I kappa B-alpha: evidence for an inducible autoregulatory pathway. Science 259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 10.Renner F, Schmitz ML. 2009. Autoregulatory feedback loops terminating the NF-kappaB response. Trends Biochem Sci 34:128–135. doi: 10.1016/j.tibs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Cousins DJ, McDonald J, Lee TH. 2008. Therapeutic approaches for control of transcriptional factors in allergic disease. J Allergy Clin Immunol 121:803–809. doi: 10.1016/j.jaci.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Faustman D. 2000. Defective function of the proteasome in autoimmunity: involvement of impaired NF-κB activation. Diabetes Technol Ther 2:415–428. doi: 10.1089/15209150050194288. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, Camandola S. 2001. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore T, Gapuzan ME, Kalaitzidis D, Starczynowski D. 2002. Rel/NF-kappa B/I kappa B signal transduction in the generation and treatment of human cancer. Cancer Lett 181:1–9. doi: 10.1016/s0304-3835(01)00795-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Lenardo MJ, Baltimore D. 2017. 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeuerle PA, Baltimore D. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 17.Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS Jr. 1992. I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev 6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 18.Arenzana-Seisdedos F, Thompson J, Rodriguez MS, Bachelerie F, Thomas D, Hay RT. 1995. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol 15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay RT, Virelizier JL, Dargemont C. 1997. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J Cell Sci 110:369–378. [DOI] [PubMed] [Google Scholar]
- 20.Shembade N, Harhaj EW. 2012. Regulation of NF-κB signaling by the A20 deubiquitinase. Cell Mol Immunol 9:123–130. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen ZJ, Bhoj V, Seth RB. 2006. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ 13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- 22.Bachelerie F, Alcami J, Arenzana-Seisdedos F, Virelizier JL. 1991. HIV enhancer activity perpetuated by NF-kappa B induction on infection of monocytes. Nature 350:709–712. doi: 10.1038/350709a0. [DOI] [PubMed] [Google Scholar]
- 23.Nakamichi K, Saiki M, Sawada M, Takayama-Ito M, Yamamuro Y, Morimoto K, Kurane I. 2005. Rabies virus-induced activation of mitogen-activated protein kinase and NF-kappaB signaling pathways regulates expression of CXC and CC chemokine ligands in microglia. J Virol 79:11801–11812. doi: 10.1128/JVI.79.18.11801-11812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez O, Valmas C, Basler CF. 2007. Ebola virus-like particle-induced activation of NF-kappaB and Erk signaling in human dendritic cells requires the glycoprotein mucin domain. Virology 364:342–354. doi: 10.1016/j.virol.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronni T, Matikainen S, Sareneva T, Melén K, Pirhonen J, Keskinen P, Julkunen I. 1992. Regulation of IFN-alpha/beta, MxA, 2′-5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J Immunol 158:2363–2374. [PubMed] [Google Scholar]
- 26.Ten RM, Blank V, Le Bail O, Kourilsky P, Israël A. 1993. Two factors, IRF1 and KBF1/NF-kappa B, cooperate during induction of MHC class I gene expression by interferon beta or Newcastle disease virus. C R Hebd Seances Acad Sci III 316:496–501. [PubMed] [Google Scholar]
- 27.Lee SM, Kleiboeker SB. 2005. Porcine arterivirus activates the NF-kappaB pathway through IkappaB degradation. Virology 342:47–59. doi: 10.1016/j.virol.2005.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel A, Hanson J, Mclean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. 1998. Herpes simplex virus type 1 induction of persistent NF-kappaB nuclear translocation increases the efficiency of virus replication. Virology 247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 29.Yedowitz JC, Blaho JA. 2005. Herpes simplex virus 2 modulates apoptosis and stimulates NF-kappaB nuclear translocation during infection in human epithelial HEp-2 cells. Virology 342:297–310. doi: 10.1016/j.virol.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Sambucetti LC, Cherrington JM, Wilkinson GWG, Mocarski ES. 1989. NF-kappa B activation of cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J 8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ensoli B, Lusso P, Schachter F, Josephs SF, Rappaport J, Negro F, Gallo RC, Wong-Staal F. 1989. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factor binding to the HIV-1 enhancer. EMBO J 8:3019–3027. doi: 10.1002/j.1460-2075.1989.tb08452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammarskjöld ML, Simurda MC. 1992. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-kappa B activity. J Virol 66:6496–6501. doi: 10.1128/JVI.66.11.6496-6501.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sgarbanti M, Arguello M, tenOever BR, Battistini A, Lin R, Hiscott J. 2004. A requirement for NF-kappaB induction in the production of replication-competent HHV-8 virions. Oncogene 23:5770–5780. doi: 10.1038/sj.onc.1207707. [DOI] [PubMed] [Google Scholar]
- 34.de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H, Bach FH. 1993. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J 12:2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bail O, Schmidt-Ullrich R, Israël A. 1993. Promoter analysis of the gene encoding the I kappa B/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J 12:5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiao PJ, Miyamoto S, Verma IM. 1994. Autoregulation of I kappa B alpha activity. Proc Natl Acad Sci U S A 91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. 1993. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappaB. Nature 365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 38.Perkins ND. 2006. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 39.Christian F, Smith EL, Carmody RJ. 2016. The regulation of NF-κB subunits by phosphorylation. Cells 5:E12. doi: 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. 2005. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauwynck H, Glorieux S, Favoreel H, Pensaert M. 2007. Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet Res 38:229–241. doi: 10.1051/vetres:200661. [DOI] [PubMed] [Google Scholar]
- 42.Karger A, Schmidt J, Mettenleiter TC. 1998. Infectivity of a pseudorabies virus mutant lacking attachment glycoproteins C and D. J Virol 72:7341–7348. doi: 10.1128/JVI.72.9.7341-7348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixdorf R, Klupp BG, Mettenleiter TC. 2001. Restoration of function of carboxy-terminally truncated pseudorabies virus glycoprotein B by point mutations in the ectodomain. J Virol 75:11526–11533. doi: 10.1128/JVI.75.23.11526-11533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geenen K, Nauwynck HJ, De Regge N, Braeckmans K, Favoreel HW. 2007. Brn-3a suppresses pseudorabies virus-induced cell death in sensory neurons. J Gen Virol 88:743–747. doi: 10.1099/vir.0.82674-0. [DOI] [PubMed] [Google Scholar]
- 45.Honess RW, Watson DH. 1977. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol 21:584–600. doi: 10.1128/JVI.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. 1995. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A 92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiDonato JA, Mercurio F, Karin M. 1995. Phosphorylation of I kappa B alpha precedes but it is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol 15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev 9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 50.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. 1995. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol 15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. 1996. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 53.Collart MA, Baeuerle P, Vassalli P. 1990. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappaB-like motifs and of constitutive and inducible forms of NF-kappaB. Mol Cell Biol 10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. 1990. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of tumor necrosis factor alpha gene in primary macrophages. J Exp Med 171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krikos A, Laherty CD, Dixit VM. 1992. Transcriptional activation of the tumor necrosis alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem 267:17971–17976. [PubMed] [Google Scholar]
- 56.Libermann TA, Baltimore D. 1990. Activation of interleukin-6 gene expression through NF-kappa B transcription factor. Mol Cell Biol 10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu WM. 2013. Tumor necrosis factor. Cancer Lett 328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka T, Narazaki M, Kishimoto T. 2014. IL-6 in inflammation, immunity and disease. Cold Spring Harb Perspect Biol 6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinz M, Scheidereit C. 2014. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep 15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappa B activation. Science 278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 61.Sun S, Elwood J, Greene WC. 1996. Both amino- and carboxyl-terminal sequences within I kappa B alpha regulate its inducible degradation. Mol Cell Biol 16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith GA, Gross SP, Enquist LW. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A 98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amici C, Belardo G, Rossi A, Santoro MG. 2001. Activation of I kappa B kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J Biol Chem 276:28759–28766. doi: 10.1074/jbc.M103408200. [DOI] [PubMed] [Google Scholar]
- 64.DeMeritt B, Milford LE, Yurochko AD. 2004. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol 78:4498–4507. doi: 10.1128/jvi.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takada H, Imadome KI, Shibayama H, Yoshimori M, Wang L, Saitoh Y, Uota S, Yamaoka S, Koyama T, Shimizu N, Yamamoto K, Fujiwara S, Miura O, Arai O. 2017. EBV induces persistent NF-κB activation and contributes to survival of EBV-positive neoplastic T- or NK-cells. PLoS One 12:e0182682. doi: 10.1371/journal.pone.0182682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taddeo B, Zhang W, Lakeman F, Roizman B. 2004. Cells lacking NF-kappaB or in which NF-kappaB is not activated vary with respect to ability to sustain herpes simplex virus replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J Virol 78:11615–11621. doi: 10.1128/JVI.78.21.11615-11621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Cui Q, Fu Q, Song X, Jia R, Yang Y, Zou Y, Li L, He C, Liang X, Yin L, Lin J, Ye G, Shu G, Zhao L, Shi F, Lv C, Yin Z. 2017. Antiviral properties of resveratrol against pseudorabies virus are associated with the inhibition of IκB kinase activation. Sci Rep 7:8782. doi: 10.1038/s41598-017-09365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulkarni SS, Cantó C. 2015. Molecular targets of resveratrol. Biochim Biophys Acta 1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Hargett D, Rice S, Bachenheimer SL. 2006. Herpes simplex virus type 1 ICP27-dependent activation of NF-kappaB. J Virol 80:10565–10578. doi: 10.1128/JVI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taddeo B, Luo TR, Zhang W, Roizman B. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc Natl Acad Sci U S A 100:12408–12413. doi: 10.1073/pnas.2034952100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Margolis DM, Rabson AB, Straus SE, Ostrove JM. 1992. Transactivation of the HIV-1 TLR by HSV-1 immediate early genes. Virology 186:788–791. doi: 10.1016/0042-6822(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 72.Lu X, Huang C, Zhang Y, Lin Y, Wang X, Li Q, Liu S, Tang J, Zhou L. 2017. The Us2 gene product of herpes simplex virus 2 modulates NF-κB activation by targeting TAK1. Sci Rep 7:8396. doi: 10.1038/s41598-017-08856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minami M, Kita M, Yan XQ, Yamamoto T, Iida T, Sekikawa K, Iwakura Y, Imanishi J. 2002. Role of IFN-gamma and tumor necrosis factor-alpha in herpes simplex virus type 1 infection. J Interferon Cytokine Res 22:671–676. doi: 10.1089/10799900260100150. [DOI] [PubMed] [Google Scholar]
- 74.Feduchi E, Carrasco L. 1991. Mechanism of inhibition of HSV-1 replication by tumor necrosis factor and interferon gamma. Virology 180:822–825. doi: 10.1016/0042-6822(91)90100-p. [DOI] [PubMed] [Google Scholar]
- 75.LeBlanc RA, Pesnicak L, Cabral ES, Godleski M, Straus SE. 1999. Lack of interleukin-6 (IL-6) enhances susceptibility to infection but does not alter latency or reactivation of herpes simplex virus type 1 in IL-6 knockout mice. J Virol 73:8145–8151. doi: 10.1128/JVI.73.10.8145-8151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amici C, Rossi A, Costanzo A, Ciafre S, Marinari B, Balsamo M, Levrero M, Santoro MG. 2006. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment to the IkappaBalpha promoter and directing the factor on viral genes. J Biol Chem 281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 77.Rong BL, Libermann TA, Kogawa K, Ghosh S, Cao LX, Pavan-Langston D, Dunkel EC. 1992. HSV-1 inducible proteins bind to NF-kappaB-like sites in the HSV-1 genome. Virology 189:750–756. doi: 10.1016/0042-6822(92)90599-k. [DOI] [PubMed] [Google Scholar]
- 78.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-κB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JA, Choi MS, Min JS, Kang I, Oh J, Kim JC, Ahn JK. 2017. HSV-1 ICP27 represses NF-κB activity by regulating Daxx sumoylation. BMB Rep 50:275–280. doi: 10.5483/bmbrep.2017.50.5.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan AS, Vatter AE. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- 81.Becker CH. 1967. On primary damage of autonomic ganglia following infection with Herpes suis virus in different animal species. Experientia 23:209–210. (In German.) doi: 10.1007/bf02136291. [DOI] [PubMed] [Google Scholar]
- 82.McFerran JB, Dow C, McCracken RM. 1979. Experimental studies in weaned pigs with three vaccines against Aujeszky’s disease. Comp Immunol Microbiol Infect Dis 2:327–334. doi: 10.1016/0147-9571(79)90020-1. [DOI] [PubMed] [Google Scholar]
- 83.Quintana AM, Landolt GA, Annis KM, Hussey GS. 2011. Immunological characterization of the equine airway epithelium and of a primary equine airway epithelial cell culture model. Vet Immunol Immunopathol 140:226–236. doi: 10.1016/j.vetimm.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Van Cleemput J, Poelaert KCK, Laval K, Maes R, Hussey GS, Van den Broeck W, Nauwynck HJ. 2017. Access to a main alphaherpesvirus receptor, located basolaterally in the respiratory epithelium, is masked by intercellular junctions. Sci Rep 7:16656. doi: 10.1038/s41598-017-16804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang B, Xie J, Van Cleemput J, Wei R, Opsomer G, Nauwynck HJ. 2019. Gammaherpesvirus BoHV-4 infects bovine respiratory epithelial cells mainly at the basolateral side. Vet Res 50:11. doi: 10.1186/s13567-019-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nauwynck HJ, Pensaert MB. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch Virol 140:1137–1146. doi: 10.1007/bf01315422. [DOI] [PubMed] [Google Scholar]
- 87.Olsen LM, Ch'ng TH, Card JP, Enquist LW. 2006. Role of pseudorabies virus Us3 protein kinase during neuronal infection. J Virol 80:6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ch'ng TH, Enquist LW. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J Virol 79:8835–8846. doi: 10.1128/JVI.79.14.8835-8846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Favoreel HW, Nauwynck HJ, Van Oostveldt P, Pensaert MB. 2000. Role of anti-gB and -gD antibodies in antibody-induced endocytosis of viral and cellular cell surface glycoproteins expressed on pseudorabies virus-infected monocytes. Virology 267:151–158. doi: 10.1006/viro.1999.0132. [DOI] [PubMed] [Google Scholar]
- 90.Gómez-Sebastián S, Tabarés E. 2004. Negative regulation of herpes simplex virus type 1 ICP4 promoter by IE180 protein of pseudorabies virus. J Gen Virol 85:2125–2130. doi: 10.1099/vir.0.80119-0. [DOI] [PubMed] [Google Scholar]
- 91.Lf C, Fischle W, Verdin E, Greene WC. 2001. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 92.Deruelle M, Geenen K, Nauwynck HJ, Favoreel HW. 2007. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res 128:65–70. doi: 10.1016/j.virusres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Jacob T, Van den Broeke C, Grauwet K, Baert K, Claessen C, De Pelsmaeker S, Van Waesberghe C, Favoreel HW. 2015. Pseudorabies virus US3 leads to filamentous actin disassembly and contributes to viral genome delivery to the nucleus. Vet Microbiol 177:379–385. doi: 10.1016/j.vetmic.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 94.Tombácz D, Tóth JS, Petrovszki P, Boldogkoi Z. 2009. Whole-genome analysis of pseudorabies virus gene expression by real-time quantitative RT-PCR assay. BMC Genomics 10:491. doi: 10.1186/1471-2164-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piret J, Roy S, Gagnon M, Landry S, Désormeaux A, Omar RF, Bergeron MG. 2002. Comparative study of mechanisms of herpes simplex virus inactivation by sodium lauryl sulfate and n-lauroylsarcosine. Antimicrob Agents Chemother 46:2933–2942. doi: 10.1128/AAC.46.9.2933-2942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deruelle MJ, Van den Broeke C, Nauwynck HJ, Mettenleiter TC, Favoreel HW. 2009. Pseudorabies virus US3- and UL49.5-dependent and -independent downregulation of MHC I cell surface expression in different cell types. Virology 395:172–181. doi: 10.1016/j.virol.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 97.Meissonnier GM, Pinton P, Laffitte J, Cossalter AM, Gong YY, Wild CP, Bertin G, Galtier P, Oswald IP. 2008. Immunotoxicity of aflatoxin B1: impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol Appl Pharmacol 231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Devriendt B, Allois M, Verdonck F, Wache Y, Bimczok D, Oswald IP, Goddeeris BM, Cox E. 2009. The food contaminant fumonisin B(1) reduces the maturation of porcine CD11R1(+) intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet Res 40:40. doi: 10.1051/vetres/2009023. [DOI] [PMC free article] [PubMed] [Google Scholar]