Summary
Background
Whole-breast irradiation after breast-conserving surgery for patients with early-stage breast cancer decreases ipsilateral breast-tumour recurrence (IBTR), yielding comparable results to mastectomy. It is unknown whether accelerated partial breast irradiation (APBI) to only the tumour-bearing quadrant, which shortens treatment duration, is equally effective. In our trial, we investigated whether APBI provides equivalent local tumour control after lumpectomy compared with whole-breast irradiation.
Methods
We did this randomised, phase 3, equivalence trial (NSABP B-39/RTOG 0413) in 154 clinical centres in the USA, Canada, Ireland, and Israel. Adult women (>18 years) with early-stage (0, I, or II; no evidence of distant metastases, but up to three axillary nodes could be positive) breast cancer (tumour size ≤3 cm; including all histologies and multifocal breast cancers), who had had lumpectomy with negative (ie, no detectable cancer cells) surgical margins, were randomly assigned (1:1) using a biased-coin-based minimisation algorithm to receive either whole-breast irradiation (whole-breast irradiation group) or APBI (APBI group). Whole-breast irradiation was delivered in 25 daily fractions of 50 Gy over 5 weeks, with or without a supplemental boost to the tumour bed, and APBI was delivered as 34 Gy of brachytherapy or 38·5 Gy of external bream radiation therapy in 10 fractions, over 5 treatment days within an 8-day period. Randomisation was stratified by disease stage, menopausal status, hormone-receptor status, and intention to receive chemotherapy. Patients, investigators, and statisticians could not be masked to treatment allocation. The primary outcome of invasive and non-invasive IBTR as a first recurrence was analysed in the intention-to-treat population, excluding those patients who were lost to follow-up, with an equivalency test on the basis of a 50% margin increase in the hazard ratio (90% CI for the observed HR between 0·667 and 1·5 for equivalence) and a Cox proportional hazard model. Survival was assessed by intention to treat, and sensitivity analyses were done in the per-protocol population. This trial is registered with ClinicalTrials.gov, NCT00103181.
Findings
Between March 21, 2005, and April 16, 2013, 4216 women were enrolled. 2109 were assigned to the whole-breast irradiation group and 2107 were assigned to the APBI group. 70 patients from the whole-breast irradiation group and 14 from the APBI group withdrew consent or were lost to follow-up at this stage, so 2039 and 2093 patients respectively were available for survival analysis. Further, three and four patients respectively were lost to clinical follow-up (ie, survival status was assessed by phone but no physical examination was done), leaving 2036 patients in the whole-breast irradiation group and 2089 in the APBI group evaluable for the primary outcome. At a median follow-up of 10·2 years (IQR 7·5–11·5), 90 (4%) of 2089 women eligible for the primary outcome in the APBI group and 71 (3%) of 2036 women in the whole-breast irradiation group had an IBTR (HR 1·22, 90% CI 0·94–1·58). The 10-year cumulative incidence of IBTR was 4·6% (95% CI 3·7–5·7) in the APBI group versus 3·9% (3·1–5·0) in the whole-breast irradiation group. 44 (2%) of 2039 patients in the whole-breast irradiation group and 49 (2%) of 2093 patients in the APBI group died from recurring breast cancer. There were no treatment-related deaths. Second cancers and treatment-related toxicities were similar between the two groups. 2020 patients in the whole-breast irradiation group and 2089 in APBI group had available data on adverse events. The highest toxicity grade reported was: grade 1 in 845 (40%), grade 2 in 921 (44%), and grade 3 in 201 (10%) patients in the APBI group, compared with grade 1 in 626 (31%), grade 2 in 1193 (59%), and grade 3 in 143 (7%) in the whole-breast irradiation group.
Interpretation
APBI did not meet the criteria for equivalence to whole-breast irradiation in controlling IBTR for breast-conserving therapy. Our trial had broad eligibility criteria, leading to a large, heterogeneous pool of patients and sufficient power to detect treatment equivalence, but was not designed to test equivalence in patient subgroups or outcomes from different APBI techniques. For patients with early-stage breast cancer, our findings support whole-breast irradiation following lumpectomy; however, with an absolute difference of less than 1% in the 10-year cumulative incidence of IBTR, APBI might be an acceptable alternative for some women.
Introduction
Breast-conserving therapy for patients with early-stage breast cancer conventionally includes adjuvant whole-breast irradiation after lumpectomy to eliminate potential residual microscopic disease in the breast and to yield cancer outcomes comparable to mastectomy.1,2 Whole-breast irradiation has been traditionally delivered over several consecutive weeks, making access to effective breast-conserving therapy problematic for women who work, live far from a radiotherapy facility, care for children, or have other socioeconomic barriers.3–6 The preference of patients to receive short-course radiation has become evident since the introduction of hypofractionated whole-breast irradiation, which reduces treatment duration to 3–4 consecutive weeks.7–9 Omitting radiotherapy might be an option for some older (≥75 years) patients with breast cancer who are at low risk of recurrence10,11 but generally this practice results in increased ipsilateral breast-tumour recurrence (IBTR), which in some cases is associated with worse breast-cancer mortality than in patients with recurrence-free disease.12 Recurrence patterns after breast conservation suggest that adjuvant whole-breast irradiation could be most beneficial in the breast tissue adjacent to the post-excision lumpectomy cavity because IBTRs occur predominantly at this site.13 As an alternative, accelerated partial breast irradiation (APBI) delivers radiotherapy to only the tumour-bearing quadrant over 5 treatment days within an 8-day period, a treatment interval that further reduces the burden of care and potentially improves access to breast-conserving therapy. In the NRG Oncology/NSABP B-39/RTOG 0413 trial, we compared APBI to whole-breast irradiation following lumpectomy for patients with early-stage breast cancer to determine if APBI provided equivalent local tumour control.
Methods
Study design and participants
NSABP B-39/RTOG 0413 was a randomised, phase 3, equivalence trial done in 154 clinical centres in the USA, Canada, Ireland, and Israel. Eligible patients had to be older than 18 years and to have received lumpectomy for stage 0 cancer (ie, ductal carcinoma in situ [DCIS]) or stage I or II (tumour size ≤3 cm) invasive adenocarcinoma of the breast with no evidence of distant metastases. Their life expectancy had to be at least 10 years, excluding the breast cancer diagnosis but including any comorbidities. Surgical resection margins needed to be free of cancer, including DCIS. The primary tumour must have been tested for oestrogen receptor and, in some cases, for progesterone receptor. Up to three axillary lymph nodes could be positive for metastases. Patients with all histologies and multifocal breast cancers were eligible and had to be randomly assigned to groups within 42 days of the most recent surgery.
Approximately 20 months after the study opened, recruitment was closed in two low-risk groups because of high accrual: patients with DCIS who were aged 50 years and older, regardless of hormone-receptor status, and patients with negative lymph nodes, invasive disease, and positive for hormone receptor who were aged 50 years and older. Accrual was continued for all other patients. This study was approved by institutional review boards at the participating clinical centres, and all participants provided written informed consent.
Randomisation and masking
Patients were randomly assigned (1:1) to receive either whole-breast irradiation (whole-breast irradiation group) or APBI (APBI group) using a biased-coin-based minimisation algorithm14 and were stratified according to disease stage (DCIS only, invasive disease with negative axillary nodes, invasive disease with 1–3 positive nodes), menopausal status, hormone-receptor status (oestrogen-receptor-positive or progesterone-receptor-positive vs negative for both), and intention to receive chemotherapy. Randomisation was done centrally by the statistical and data management centre (Pittsburgh, PA, USA). Online patient entry was done through a National Surgical Adjuvant Breast and Bowel Project server, which provided treatment assignment to the investigator for enrolment. Patients, investigators, and statisticians could not be masked to treatment allocation.
Procedures
External beam radiotherapy was used to deliver whole-breast irradiation doses of 50 Gy per day in 25 total fractions spread over 5 weeks. Boost therapy was permitted and was left to the discretion of the radiation oncologist. For APBI, delivery was 34 Gy with brachytherapy or 38·5 Gy with external beam, three-dimensional conformal radiation therapy (3DCRT) in 10 fractions, given twice daily at least 6 h apart, on 5 treatment days within an 8-day period. Brachytherapy included high-dose-rate (HDR) multi-catheter or HDR single-entry (MammoSite single-lumen, MammoSite multi-lumen, Contura multi-lumen balloon [all three from Hologic, USA], and SAVI Brachy [Cianna Medical, USA]) methods. The APBI method was selected by the treating radiation oncologist. Every institution’s radio¬therapy facilities were quality-assessed and each case of APBI was centrally reviewed for radiotherapy quality. The use of chemotherapy, hormonal therapy, or both was at the discretion of the treating medical oncologist. Chemo¬therapy was delivered before radiotherapy for patients in the whole-breast irradiation group and after radiotherapy for patients in the APBI group. Patients were followed up by physical examination or phone contact every 6 months for years 1–5 and every 12 months thereafter or at the occurrence of a protocol event. They were assessed for all local, regional, or distant recurrences and new primary cancers. Bilateral mammograms were required annually. Outcomes were diagnosed and reported by the study sites and were confirmed by a central medical review of supporting documentation.
Outcomes
The primary endpoint for analysis was IBTR (invasive and non-invasive) as a first recurrence. Other local (ipsilateral chest wall), regional, or distant breast cancer recurrences and death before IBTR were treated as competing risks. Secondary endpoints were recurrence-free interval, distant disease-free interval, overall sur¬vival, quality of life, and any treatment toxicities. The definition for recurrence-free interval was the time from randomisation to first diagnosis of a local, regional, or distant recurrence; for distant disease-free interval, it was the time to first diagnosis of distant disease; and for overall survival it was deaths due to all causes. Although it was not a protocol-specified secondary endpoint, for completeness, the endpoint of disease-free survival, define as the time from randomisation to breast cancer recurrence, second primary malignancy, or death, was also assessed.
Adverse events as assessed by treating physician were reported on the basis of the descriptions and grading scales from the revised National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. They were documented at baseline, end of radiotherapy, 1 month, 6 months, and 12 months after completion of radiotherapy, and every 12 months thereafter.
Statistical analysis
The primary analysis of IBTR was through an equivalence test. A margin of 50% increase in the relative risk (RR) was selected as acceptable. Formally, we defined ABPI as inferior to whole-breast irradiation if the ratio of the risk of IBTR after ABPI relative to the risk of IBTR after whole-breast irradiation was equivalent to or greater than 1·5, and we defined whole-breast irradiation as inferior to ABPI if this RR (≤1:1·5) was 0·667. We defined APBI as equivalent to whole-breast irradiation if neither of these conditions were true. We estimated the risk of IBTR after APBI versus after whole-breast irradiation using a Cox proportional hazard model stratified for disease stage, menopausal status, hormone-receptor status, and intention to receive chemotherapy.
We followed an intention-to-treat principle. However, as a sensitivity analysis, we tested a per-protocol population for equivalence of IBTR that excluded patients who did not receive any of their randomly assigned treatment. We also estimated the cumulative incidence of IBTR accounting for competing risks.
The definitive analysis was planned after 175 IBTR events had been reported in patients receiving their randomly assigned treatment to provide a statistical power of 85% to reject either inferiority hypothesis if the risk of IBTR after APBI versus after whole-breast irradiation was 1. Assuming a 10-year cumulative incidence of IBTR of 4·3% in the whole-breast irradiation group, we estimated that sufficient power could be achieved by enrolling 4214 patients equally allocated among the radiotherapy groups. Because we observed that the overall hazard for IBTR as a first event was not constant as anticipated at trial design and decreased over time, the protocol was amended to allow reporting either when the target number of events was achieved or when the median time of follow-up of patients for vital status was 10 years, whichever came first. Study accrual, adverse events, and interim results were monitored by an independent data monitoring committee. Three formal interim analyses were prespecified in the statistical analysis plan when 44, 88, and 132 IBTR events were observed. For all interim analyses, each one-sided hypo¬thesis was done with an alpha of 0·001. The alpha for final analysis was 0·0493, adjusted to control for interim analysis testing.
For the secondary endpoints of distant disease-free interval, recurrence-free interval, overall survival, and disease-free survival, distributions of time-to-event for each treatment group were estimated by the Kaplan-Meier method and were compared between treatments by stratified log-rank tests. Hazard ratios (HRs) and 95% CIs were calculated from stratified Cox proportional hazards models. Forest plots were used to summarise the results of various exploratory subgroup analyses. Ten characteristics were examined for IBTR and recurrence-free interval outcomes, which would result in up to one significant interaction test (p<0·05) expected by chance alone. Secondary analyses followed an intention-to-treat principle and used a significance level of 0·05. The frequencies of adverse events were reported only for patients for whom such data were available. Analyses were done using SAS version 9.4. The trial was registered with ClinicalTrials.gov, NCT00103181.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 21, 2005, and April 16, 2013, 4216 patients were enrolled in the trial, with 2109 randomly assigned to the whole-breast irradiation group and 2107 to the APBI group (figure 1). As of July 31, 2018, the median time of follow-up was 10·2 years (IQR 7·5–11·5), initiating the reporting of primary results. At that time, 4132 (98%) of all randomly assigned patients had follow-up or contact information available to establish survival status and 4125 (98%) had follow-up information available on recurrence and disease-free endpoints, and so were evaluable for the primary outcome (2036 in the whole-breast irradiation group and 2089 in the APBI group).
Figure 1: Trial profile.
*Clinical follow-up required a physical examination, whereas follow-up to assess survival status was permitted via phone contact.
Patient and tumour characteristics of all randomly assigned patients at baseline were similar in the two treatment groups (table). The median age of patients was 54 years (IQR 47–64), 2587 (61%) were post¬menopausal, 3788 (90%) were white, and 299 (7%) were black. 3185 (76%) patients had invasive breast cancer and 1031 (24%) had DCIS. 2518 (79%) of patients with invasive breast cancers and 908 (88%) with DCIS were positive for oestrogen receptor, progesterone receptor, or both. In patients with invasive cancers, the histological grade was 1 (low) in 1039 (33%), 2 (intermediate) in 1247 (39%), and 3 (high) in 839 (26%). In patients with DCIS, the histological grade was 1 in 148 (14%), 2 in 260 (25%), and 3 in 289 (28%). The grade was unknown in 60 (2%) of patients with invasive cancers and 334 (32%) of those with DCIS.
Table:
Patient, treatment, and tumour-related characteristics for all enrolled patients
| Characteristics | WBI group (N=2109) | PBI group (N=2107) | ||
|---|---|---|---|---|
| Age at entry, years | ||||
| <50 | 810 | (38%) | 811 | (38%) |
| 50–70 | 1,054 | (50%) | 1,026 | (49%) |
| >70 | 245 | (12%) | 270 | (13%) |
| Race | ||||
| White | 1,886 | (89%) | 1,902 | (90%) |
| Black | 154 | (7%) | 145 | (7%) |
| Asian | 39 | (2%) | 24 | (1%) |
| Other | 9 | (<1%) | 11 | (1%) |
| Unknown | 20 | (1%) | 23 | (1%) |
| Multi-racial | 1 | (<1%) | 2 | (<1%) |
| Ethnicity | ||||
| Non-Hispanic | 1,912 | (91%) | 1,912 | (91%) |
| Hispanic or Latino | 80 | (4%) | 92 | (4%) |
| Unknown | 117 | (6%) | 103 | (5%) |
| Intent to receive chemotherapy | ||||
| No | 1,498 | (71%) | 1,498 | (71%) |
| Yes | 611 | (29%) | 609 | (29%) |
| Menopausal Status | ||||
| Pre-menopausal | 813 | (39%) | 816 | (39%) |
| Post-menopausal | 1,296 | (61%) | 1,291 | (61%) |
| Hormone receptor status | ||||
| Positive ER, PR or both | 1,716 | (81%) | 1,710 | (81%) |
| Negative for ER and PR | 393 | (19%) | 397 | (19%) |
| Disease Stage | ||||
| DCIS | 513 | (24%) | 518 | (25%) |
| Invasive node-negative | 1,376 | (65%) | 1,371 | (65%) |
| Invasive node-positive | 220 | (10%) | 218 | (10%) |
| Number of positive nodes | ||||
| DCIS/Invasive N0 | 1,889 | (90%) | 1,889 | (90%) |
| 1 | 166 | (8%) | 160 | (8%) |
| 2 | 33 | (2%) | 38 | (2%) |
| 3 | 9 | (<1%) | 12 | (1%) |
| Unknown | 12 | (1%) | 8 | (<1%) |
| Histologic grade | ||||
| Grade I (low) | 601 | (28%) | 586 | (28%) |
| Grade II (intermediate) | 734 | (35%) | 773 | (37%) |
| Grade III (high) | 570 | (27%) | 558 | (26%) |
| Unknown | 204 | (10%) | 190 | (9%) |
| Index tumor focality | ||||
| Unifocal | 1913 | (91%) | 1935 | (92%) |
| Multifocal | 176 | (8%) | 160 | (8%) |
| Unknown | 20 | (1%) | 12 | (1%) |
| Invasive histologic type | ||||
| DCIS | 513 | (24%) | 518 | (25%) |
| Ductal | 1289 | (61%) | 1276 | (61%) |
| Lobular | 89 | (4%) | 101 | (5%) |
| Other | 43 | (2%) | 42 | (2%) |
| Unknown | 175 | (8%) | 170 | (8%) |
| Invasive pathologic tumor size | ||||
| DCIS | 513 | (24%) | 518 | (25%) |
| ≤10 mm | 584 | (28%) | 586 | (28%) |
| 11–20 mm | 637 | (30%) | 644 | (31%) |
| >20 mm | 199 | (9%) | 186 | (9%) |
| Unknown* | 176 | (8%) | 173 | (8%) |
| Risk Group† | ||||
| DCIS | 513 | (24%) | 518 | (25%) |
| Low-risk invasive | 389 | (18%) | 378 | (18%) |
| Other invasive | 1028 | (49%) | 1032 | (49%) |
| Unknown | 179 | (8%) | 179 | (8%) |
Data are n (%). Percentages might not add up because of rounding. APBI=accelerated partial breast irradiation. ER=oestrogen receptor. DCIS=ductal carcinoma in situ. PR=progesterone receptor. WBI=whole-breast irradiation.
Exact pathological tumour size is unknown but was required to be ≤3 cm for eligibility.
Risk group was patterned from the American Society of Radiation Oncology’s consensus guidelines for APBI.15
Of patients enrolled in the APBI group, 1536 (73%) indicated 3DCRT as their intended APBI tech nique, 451 (21%) indicated single-entry brachytherapy, and 120 (6%) indicated multi-catheter brachytherapy. Of patients enrolled in the whole-breast irradiation group, 1697 (80%) received an optional 1-week sequential surgical-cavity boost to at least 60 Gy. Treatment adher¬ence was high in both groups, with 4006 (95%) randomly assigned patients completing their assigned radiotherapy per protocol. 1149 (27%) patients received chemotherapy. In 3426 patients with oestrogen-receptor-positive or progesterone-receptor-positive cancers, 1399 (82%) of 1716 in the whole-breast irradiation group underwent adjuvant hormonal therapy, as did 1458 (85%) of 1710 patients in the APBI group.
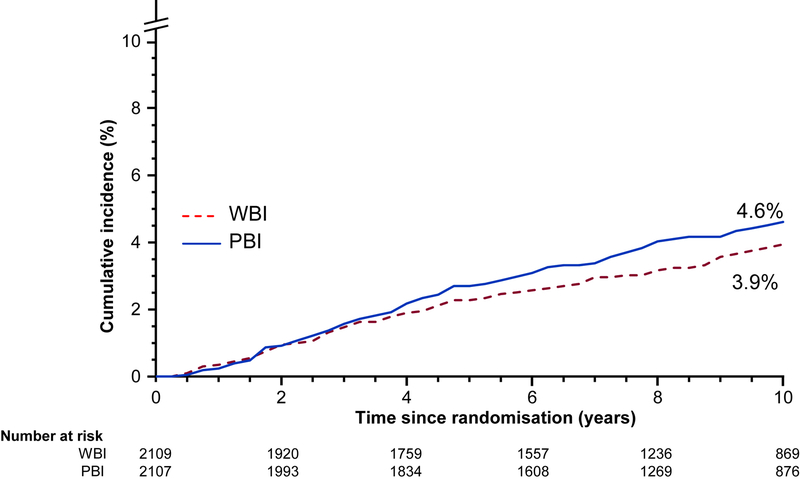
71 (3%) of 2036 patients in the whole-breast irradiation group and 90 (4%) of 2089 in the APBI group who were evaluable for the primary outcome had IBTR. To declare APBI and whole-breast irradiation equivalent regarding the risk of IBTR, the 90% CI for the observed HR comparing APBI to whole-breast irradiation had to be entirely between 0·667 and 1·5. We observed an HR of 1·22 with a 90% CI of 0·94–1·58, which did not meet the equivalence criteria and favoured whole-breast irradiation. The 10-year cumulative incidence of IBTR was 3·9% (95% CI 3·1–5·0) in the whole-breast irradiation group and 4·6% (3·7–5·7) in the APBI group for an absolute difference of 0·7% (figure 2). The primary equivalence analysis was repeated using the per-protocol population of 4023 patients and yielded consistent findings. 67 (3%) of 2004 patients in the whole-breast irradiation group and 88 (4%) of 2019 in the APBI group in the per-protocol population had IBTR. The HR was 1·29 (90% CI 0·99–1·69), again not meeting the criteria for equivalence and favouring whole-breast irradiation.
Figure 2: Cumulative incidence of in-breast tumour recurrence.
APBI=accelerated partial breast irradiation. WBI=whole-breast irradiation.
Significantly more evaluable patients in the APBI group had recurrence-free interval events than patients in the whole-breast irradiation group (figure 3). The 10-year point estimate of recurrence-free interval for the whole-breast irradiation group was 93·4% (95% CI 92·1–94·6), and in the APBI group it was 91·8% (90·4–93·0; figure 3). There were no significant differences between APBI and whole-breast irradiation for distant disease-free interval or overall sur vival (figure 3). The 10-year point estimate for distant disease-free interval in the whole-breast irradiation group was 97·1% (96·2–97·8), and in the APBI group it was 96·7% (95·7–97·4). There were 174 deaths reported in the whole-breast irradiation group and 199 in the APBI group. The 10-year point estimate for overall survival was 91·3% (89·8–92·6) in the whole-breast irradiation group and 90·6% (89·1–91·9) in the APBI group. There were 93 (2%) deaths due to breast cancer (n=4132), with 44 (2%) of 2039 patients in the whole-breast irradiation group and 49 (2%) of 2093 patients in the APBI group. There was no significant difference between radiotherapy groups for disease-free survival. 376 (18%) of 2036 patients in the whole-breast irradiation group and 435 (21%) of 2089 patients in the APBI group had a disease-free survival event (HR 1·12, 95% CI 0·98–1·29, p=0·10), with 10-year point estimates of 79·7% (77·7–81·6) for the whole-breast irradiation group and 78·1% (76·0–80·0) for the APBI group. The number of participants by the site of first disease-free survival event is shown in the appendix (p 2).
Figure 3:
Kaplan-Meier estimates of (A) recurrence-free interval, (B) distant disease-free interval, and (C) overall survival HRs, 95% CIs, and p values are based on the Cox proportional hazards model, stratified on disease stage, menopausal status, hormone receptor status, and intention to receive chemotherapy.
APBI=accelerated partial breast irradiation. HR=hazard ratio.
We did exploratory analyses in the intention-to-treat population to determine if there were any variations in treatment effects for whole-breast irradiation and APBI between subgroups previously identified as prognostic for IBTR. In addition to the stratification factors, we included subgroups defined by invasive tumour size and risk group. Low-risk inva sive disease was defined as unifocal, hormone-receptor positive, with tumours 2 cm in diameter or smaller, infiltrating ductal, node-negative, and affecting patients aged 50 years and older, on the basis of the American Society of Radiation Oncology’s consensus guidelines for APBI.15 There were no differences in the treatment effects between any of the subgroups except invasive pathological tumour size, for which APBI was favourable in patients with invasive tumours sized 10 mm or smaller (figure 4). There was an increase in the point estimates of the HRs for severity of disease stage but they were not significantly different from each other, as shown by the overlapping CIs. Results were similar when considering recurrence-free interval events; however, the interaction between treatment and invasive pathological tumour size was not significant (appendix p 3).
Figure 4: Exploratory post-hoc analysis using forest plots for IBTR.
DCIS=ductal carcinoma in situ. IBTR=ipsilateral breast-tumour recurrence. APBI=partial breast irradiation. WBI=whole-breast irradiation.
Adverse event information was available for 4109 (97%) of all enrolled patients, with 2020 in the whole-breast irradiation group and 2089 in APBI group. The highest CTCAE toxicity grade reported from APBI was grade 1 in 845 (40%), grade 2 in 921 (44%), and grade 3 in 201 (10%) patients. The highest toxicity reported from whole-breast irradiation was grade 1 in 626 (31%), grade 2 in 1193 (59%), and grade 3 in 143 (7%) patients. Grades 4 and 5 toxicities were low, in ten (<1%) patients in the APBI group and six (<1%) in the whole-breast irra diation group. There were no significant differences in the number of patients with second primary cancers reported between the two groups. Of the 4125 patients with clinical follow-up data available, there were 392 (10%) with at least one second primary cancer reported, 200 (10%) of 2036 in the whole-breast irradiation group and 192 (9%) of 2089 in the APBI group (HR 0·93, 95% CI 0·76–1·13, p=0·46). Of the 392 patients with at least one second primary cancer, 135 (34%) reported contralateral breast cancers and ipsilateral breast sarcomas, 72 (36%) in the whole-breast irradiation group and 63 (33%) in the APBI group (HR 0·83, 0·59–1·17, p=0·29).
Discussion
In this trial, we investigated whether a several-day course of radiotherapy (APBI) to the surgical cavity region was equivalent to several weeks of radio therapy to the entire breast (whole-breast irradiation) in preventing IBTR after lumpectomy for patients with early-stage breast cancer. APBI did not meet the criteria for equivalence to whole-breast irradiation in controlling IBTR on the basis of the upper limit of the hazard ratio’s CI. However, the absolute difference in the 10-year cumulative incidence of IBTR was less than 1%. The risk of a recurrence-free interval event was significantly higher for APBI than whole-breast irradiation but the absolute difference between 10-year recurrence-free estimates was also small (<1·6%). Distant disease-free interval, overall survival, and disease-free survival were not different for APBI versus whole-breast irradiation.
In the three decades since the 1991 National Cancer Institute Consensus Statement supporting breast conservation for patients with early-stage disease,16 numerous clinical trials and meta-analyses have confirmed the importance of adjuvant radiotherapy for tumour control in the treated breast.12,17 These data reflect outcomes from adjuvant whole-breast irradiation. Because more than 50% of patients with breast cancer each year are diagnosed with early-stage disease,18 approximately 100 000 women annually would have to consider radio-the rapy for breast conservation in the USA alone. It is important that women know whether new radiotherapy approaches that might be more convenient are equally effective as whole-breast irradiation. The primary endpoint of the NSABP B-39/RTOG 0413 trial was, therefore, to ensure that the outcomes with short-course APBI would be equivalent to those with whole-breast irradiation for women who want breast-conserving therapy. To that end, the trial was designed to include a representative population of patients with early-stage breast cancer undergoing breast conservation and was powered to detect whether APBI could become an equivalent standard approach for all patients.
Our finding that APBI was not equivalent to whole-breast irradiation for in-breast tumour control contrasts with results from other randomised trials that enrolled more narrowly selected patient populations. The Ontario Clinical Oncology Group RAPID trial,19 for example, sought to show whether APBI was non-inferior to whole-breast irradiation, enrolling 2135 patients who had received lumpectomy and had node-negative breast cancers smaller than 3 cm. They excluded women who were younger than 40 years or who had lobular or multifocal breast cancer. With 84% of these patients having hormone-sensitive breast cancers and their median age being 61 years, the IBTR at 8 years from APBI was 3% versus 2·8% from whole-breast irradiation, thus APBI was non-inferior. Two other randomised trials20,21 with similar non-inferiority designs in comparable populations (mostly patients with node-negative breast cancer, at least 95% of whom had hormone-sensitive disease, and with median age >60 years), also showed that APBI was non-inferior to whole-breast irradiation when measuring IBTR incidence at 5 years post-lumpectomy. Likewise, the UK IMPORT LOW trial22 found that protracted APBI (delivered over 15 treatments daily for 3 weeks) was also non-inferior to whole-breast irradiation with the same delivery schedule when the outcome was IBTR. The trial recruited patients with invasive, mostly node-negative (97%) breast cancer, a median age of 62 years, and tumours that were 90% grade I–II and 95% hormone-sensitive. By comparison, the large population enrolled in our study had a median age of 54 years and included subgroups known to have both worse and better incidence of IBTR that is more broadly representative of all patients with breast cancer who undergo breast radiotherapy after lumpectomy. However, our exploratory post-hoc analysis showed no difference in the treatment effect in any of the risk categories or stratification factors, except for invasive pathological tumours 10 mm in diameter or smaller, for which APBI was favourable. This interaction with treatment was not significant in any of the other subgroups. Our findings, therefore, support whole-breast irradiation post-lumpectomy for all patients who have breast conservation.
When we designed our trial, the influence of breast cancer subtype on IBTR after breast-conserving therapy was unknown. One of the limitations of our trial is the absence of human epidermal growth factor receptor 2 (HER2) information for the enrolled patients with invasive breast cancer. Furthermore, our study was not designed to assess whether whole-breast irradiation and APBI following breast-conserving surgery are equivalent in different subgroups of patients, or to test for differences in outcomes from the various APBI techniques. Therefore, even the predefined subgroups included in the exploratory analyses were not adequately powered to draw definitive conclusions regarding the treatment effect. Differences in outcomes among the various forms of APBI, as well as quality of life (including patient-reported outcomes and cosmetic results), and quality assurance analyses are reported in separate works.19, 23
The protracted time required to deliver whole-breast irradiation has been associated with greater use of mastectomy or underuse of radiotherapy after lumpectomy.5,6 Hypofractionated whole-breast irradiation has reduced treatment duration to 3–4 weeks but still re quires daily commuting, days off work, child care, or other arrangements, so patients would prefer an even shorter radiation course.7,8 APBI was developed as an alternative to potentially improve access to effective breast conservation therapy by reducing treatment duration to several days to further lessen burden of care. Although equivalence to whole-breast irradiation was not shown in our study, the small difference in 10-year IBTR and recurrence-free interval without a significant difference in distant disease-free interval, disease-free survival, and overall survival might be acceptable to small breast cancer populations similar to those enrolled in other trials.19,20 The safety of APBI might consequently become an important factor for selecting radiotherapy methods. The RAPID trial19 reported worse grade 2 acute toxicity with whole-breast irradiation and worse grade 2–3 late toxicity from APBI. A similar pattern was not seen in our trial. Other trials evaluating different partial breast irradiation methods have reported similar toxicity to that of whole-breast irradiation.20, 23
To our knowledge, our study represents the largest and most comprehensive trial investigating the efficacy of APBI compared with whole-breast irradiation after lumpectomy in a broad population of patients with breast cancer. Our findings support whole-breast irradiation but the absolute outcome difference compared with APBI is small, so partial breast irradiation might also be an acceptable treatment for some patients.
Supplementary Material
Research in context.
Evidence before this study
We did a comprehensive literature search for articles published in any language between Jan 1, 1990, and Dec 31, 2003, using PubMed and MEDLINE for any prospective studies and ClinicalTrials.gov for ongoing or completed trials, in which accelerated partial breast irradiation (APBI) alone was investigated as an adjuvant radiotherapy modality after breast-conserving surgery, compared with whole-breast irradiation. Search terms included: “partial breast irradiation”, “early breast cancer”, “breast-conserving therapy”, “radiation therapy”, “accelerated partial breast irradiation”, “brachytherapy”, “multicatheter brachytherapy”, “balloon-based brachytherapy”, “intraoperative irradiation”, “IORT”, and “adjuvant therapy”. At that time, nearly all evidence was from prospective phase 2, single-institution, and some multi-institution trials that showed low cancer recurrence after breast-conserving therapy and APBI compared with historical whole-breast irradiation data. These studies were done in highly specific patient populations with low-risk, short follow-up, and using only a single irradiation technique. One ongoing phase 3 trial was studying whether brachytherapy APBI in a similarly well defined, low-risk population and found that it was non-inferior to whole-breast irradiation. Questions remained, particularly on the applicability of APBI to a broader patient population undergoing breast-conserving therapy and whether recurrence equivalent to whole-breast irradiation would be seen with modern adjuvant systemic and local therapy techniques and in a more diverse patient population. Additional uncertainty remained about the other techniques of APBI.
Added value of this study
To our knowledge, NSABP B-39/RTOG 0413 is the first phase 3 trial testing all forms of APBI compared with whole-breast irradiation, enrolling a heterogeneous group of patients with early-stage breast cancer and undergoing breast-conservation therapy, including the largest number of patients, and providing the longest follow-up reported to date. Our trial was designed to include a more representative cross-section of patients than previous trials and to be powered to detect whether APBI could become a standard approach for all patients. We found that our prespecified study criteria for equivalence of APBI and whole-breast irradiation were not met. These findings contrast with those reported in the IMPORT LOW, GEC-ESTRO, and RAPID trials, which showed non-inferiority of APBI versus whole-breast irradiation, but studied narrow patient populations, and used only a single irradiation technique.
Implications of all the available evidence
The findings of NSABP B-39/RTOG 0413 support whole-breast irradiation. However, the absolute differences in ipsilateral breast-tumour recurrence were small, such that APBI might be an acceptable treatment in some patients similar to those enrolled in other trials. The shortened irradiation course reduces the burden of radiotherapy and could therefore improve access for certain patients who choose breast conservation.
Acknowledgments
This trial was funded by the NCI, Department of Health and Human Services, Public Health Service Grants U10-CA-180868, U10-CA-180822, UG1-CA-189867, and U24-CA-19067.
Funding National Cancer Institute, US Department of Health and Human Services.
Declaration of interests
FAV reports consultancy fees from ImpediMed, outside the submitted work, and is an employee of the MHP Radiation Oncology Institute. JRW reports a grant to her institution from IntraOp Medical, which supports a separate clinical trial (OSU 16106), and support for travel from IBA and Qfix, outside the submitted work. RRK reports an unrestricted educational and research grant from Elekta for an interstitial brachytherapy collaborative clinical research study (PROMIS), and minor stock options with Cianna Medical. PAG reports grants from the US National Cancer Institute (NCI), outside the submitted work. KAW reports grants from NCI for NRG Oncology/Radiation Therapy Oncology Group, in support of the submitted work. SP reports consultancy fees from MedPacto to his institution, patents related to Oncotype Dx (all rights transferred to NSABP Foundation), and stock options with ImmuneOncia Therapeutics and Novomics. HMK serves on the advisory boards of Genomic Health, Cardinal Health, and Targeted Medical Education, and has received consultancy fees and grants to his institution from Genomic Health. He is an employee of the New England Journal of Medicine group, and has received an honorarium from Physicians’ Education Resources, and editorial royalties from McGraw-Hill Professional and UpToDate. LJP reports a patent pending with PFS Genomics. JPC reports grants from NCI, during the conduct of the study. EPM serves on the advisory boards of Genomic Health, Genentech, Roche, Biotheranostics, and Daiichi Sankyo, is a consultant for Merck, and is a member of the Speaker’s Bureau with Genentech, Roche, and Genomic Health. HDB sits on the Breast Advisory Board of Merck, is a consultant and lectures with the Speakers’ Bureau of Genomic Health, and has stock with AbbVie and Pfizer, outside the submitted work; and reports grants from the US National Institutes of Health, including support for travel, during the conduct of the study. WJC Jr is a consultant with Bristol-Myers Squibb and is a member of data monitoring committees with AstraZeneca. All other authors declare no competing interests.
Footnotes
Data sharing
The study protocol and informed consent form will be made available. Individual participant data that underlie the results reported in this Article, after de-identification, will be available within 1 year after publication and will be accessible through the NCTN Data Archive.
Contributor Information
Frank A Vicini, NRG Oncology, Pittsburgh, PA, USA; MHP Radiation Oncology Institute, St Joseph Mercy Hospital Campus, Pontiac, MI, USA.
Reena S Cecchini, NRG Oncology, Pittsburgh, PA, USA; University of Pittsburgh, Pittsburgh, PA, USA.
Julia R White, NRG Oncology, Pittsburgh, PA, USA; Ohio State University Comprehensive Cancer Center—Arthur G James Cancer Hospital and Richard J Solove Research Institute, Columbus, OH, USA.
Douglas W Arthur, NRG Oncology, Pittsburgh, PA, USA; Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA.
Thomas B Julian, NRG Oncology, Pittsburgh, PA, USA; Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA.
Rachel A Rabinovitch, NRG Oncology, Pittsburgh, PA, USA; University of Colorado Cancer Center, Aurora, CO, USA.
Robert R Kuske, NRG Oncology, Pittsburgh, PA, USA; Breast Cancer Specialists, Arizona Center for Cancer Care, Scottsdale, AZ, USA.
Patricia A Ganz, NRG Oncology, Pittsburgh, PA, USA; University of California at Los Angeles, Los Angeles, CA, USA.
David S Parda, NRG Oncology, Pittsburgh, PA, USA; Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA.
Michael F Scheier, Carnegie Mellon University, Pittsburgh, PA, USA.
Kathryn A Winter, NRG Oncology Statistics and Data Management Center, American College of Radiology, Philadelphia, PA, USA.
Soonmyung Paik, NRG Oncology, Pittsburgh, PA, USA; Yonsei University College of Medicine, Seoul, Korea.
Henry M Kuerer, NRG Oncology, Pittsburgh, PA, USA; MD Anderson Cancer Center, Houston, TX, USA.
Laura A Vallow, Mayo Clinic Florida, Jacksonville, FL, USA.
Lori J Pierce, Southwest Oncology Group Cancer Research Network, Hope Foundation for Cancer Research, Portland, OR, USA; Rogel Cancer Center, University of Michigan, Ann Arbor, MI, USA.
Eleftherios P Mamounas, NRG Oncology, Pittsburgh, PA, USA; Orlando Health, UF Health Cancer Center, Orlando, FL, USA.
Beryl McCormick, NRG Oncology, Pittsburgh, PA, USA; Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Joseph P Costantino, NRG Oncology, Pittsburgh, PA, USA; University of Pittsburgh, Pittsburgh, PA, USA.
Harry D Bear, NRG Oncology, Pittsburgh, PA, USA; Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA.
Isabelle Germain, NRG Oncology, Pittsburgh, PA, USA; Centre Hospitalier Universitaire de Québec—Université Laval, Pavillon Hôtel-Dieu de Québec, Québec City, QC, Canada.
Gregory Gustafson, NRG Oncology, Pittsburgh, PA, USA; Community Clinical Oncology Program, William Beaumont Hospital, Sterling Heights, MI, USA.
Linda Grossheim, NRG Oncology, Pittsburgh, PA, USA; Summit Cancer Center, Post Falls, ID, USA.
Ivy A Petersen, NRG Oncology, Pittsburgh, PA, USA; Mayo Clinic, Rochester, MN, USA.
Richard S Hudes, NRG Oncology, Pittsburgh, PA, USA; Saint Agnes Hospital, Baltimore, MD, USA and Thomas Jefferson University, Baltimore, MD, USA.
Walter J Curran, Jr, NRG Oncology, Pittsburgh, PA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
John L Bryant, NRG Oncology, Pittsburgh, PA, USA; University of Pittsburgh, Pittsburgh, PA, USA.
Norman Wolmark, NRG Oncology, Pittsburgh, PA, USA; University of Pittsburgh, Pittsburgh, PA, USA; Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. [DOI] [PubMed] [Google Scholar]
- 3.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst 2001; 93: 1344–46. [DOI] [PubMed] [Google Scholar]
- 4.Pan IW, Smith BD, Shih YC. Factors contributing to underuse of radiation among younger women with breast cancer. J Natl Cancer Inst 2014; 106: djt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh A, Fu W, Hu C, et al. Impact of race, ethnicity, and socioeconomic factors on receipt of radiation after breast conservation surgery: analysis of the National Cancer Database. Breast Cancer Res Treat 2018; 172: 201–08. [DOI] [PubMed] [Google Scholar]
- 6.Lam J, Cook T, Foster S, et al. Examining determinants of radiotherapy access: do cost and radiotherapy inconvenience affect uptake of breast-conserving treatment for early breast cancer? Clin Oncol 2015; 27: 465–71. [DOI] [PubMed] [Google Scholar]
- 7.Hoopes DJ, Kaziksa D, Chapin P, et al. Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 674–81. [DOI] [PubMed] [Google Scholar]
- 8.Bonin K, McGuffin M, Presutti R. Breast cancer patients’ preferences for adjuvant radiotherapy: whole breast irradiation vs partial breast irradiation—single institutional study. J Canc Educ 2018; 33: 37–43. [DOI] [PubMed] [Google Scholar]
- 9.Rippy EE, Anisworth R, Sathananthan D, et al. Influences on decision for mastectomy in patients eligible for breast conserving surgery. Breast 2014; 23: 273–78. [DOI] [PubMed] [Google Scholar]
- 10.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013; 31: 2382–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015; 16: 266–73. [DOI] [PubMed] [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378: 1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher ER, Anderson S, Redmond C, Fisher B. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol 1992; 8: 161–66. [PubMed] [Google Scholar]
- 14.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 1978; 37: 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa C, Harris EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol 2017; 7: 73–79. [DOI] [PubMed] [Google Scholar]
- 16.NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA 1991; 265: 391–95. [PubMed] [Google Scholar]
- 17.van Maaren MC, de Munck L, de Bock GH, et al. 10-year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016; 17: 1158–70. [DOI] [PubMed] [Google Scholar]
- 18.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017; 67: 439–48. [DOI] [PubMed] [Google Scholar]
- 19.Whelan T, Julian J, Levine M, et al. RAPID: a randomized trial of accelerated partial breast irradiation using 3-dimensional conformal radiation therapy (3D-CRT). San Antonio Breast Cancer Symposium; San Antonio, TX: Dec 4–8, 2018 (abstr GS4–03). [Google Scholar]
- 20.Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016; 387: 229–38. [DOI] [PubMed] [Google Scholar]
- 21.Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015; 51: 451–63. [DOI] [PubMed] [Google Scholar]
- 22.Coles CE, Griffin CL, Kirby AM, et al. Partial breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017; 390: 1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicini FA, Cecchini RS, White JR, et al. Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): A randomized phase III study of conventional whole breast irradiation versus partial breast irradiation for women with stage 0, I, or II breast cancer. San Antonio Breast Cancer Symposium; San Antonio, TX, USA; Dec 4–8, 2018. (abstr GS4-04). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.