Abstract
Maturation is the last phase of heart development that prepares the organ for strong, efficient, and persistent pumping throughout the mammal’s lifespan. This process is characterized by structural, gene expression, metabolic, and functional specializations in cardiomyocytes (CMs) as the heart transits from fetal to adult states. CM maturation gained increased attention recently due to the maturation defects in pluripotent stem cell-derived CMs (PSC-CMs), its antagonistic effect on myocardial regeneration, and its potential contribution to cardiac disease. Here we review the major hallmarks of ventricular CM maturation and summarize key regulatory mechanisms that promote and coordinate these cellular events. With advances in the technical platforms used for CM maturation research, we expect significant progress in the future that will deepen our understanding of this process and lead to better maturation of PSC-CMs and novel therapeutic strategies for heart disease.
Keywords: cardiomyocyte maturation, cardiac development, regulatory mechanism, regenerative medicin, stem cell, heart regeneration
Subject Terms: Stem Cells
1. Background and Significance
Mammalian heart development is a highly dynamic process that can be conceptually divided into specification, morphogenesis, and maturation (Fig. 1A). Specification refers to the differentiation of the major cardiac lineages from uncommitted mesodermal progenitors. Morphogenesis includes the events that spatially organize cardiac cells, create the structural components of the heart, and properly connect them together. Maturation encompasses the cell- and tissue-level changes that optimize the heart for strong and efficient pumping throughout the animal’s lifespan. While the first two phases have been focal points for developmental cardiology, heart maturation has been less studied until recently.
Figure 1. Heart maturation and its implication in translational medicine.
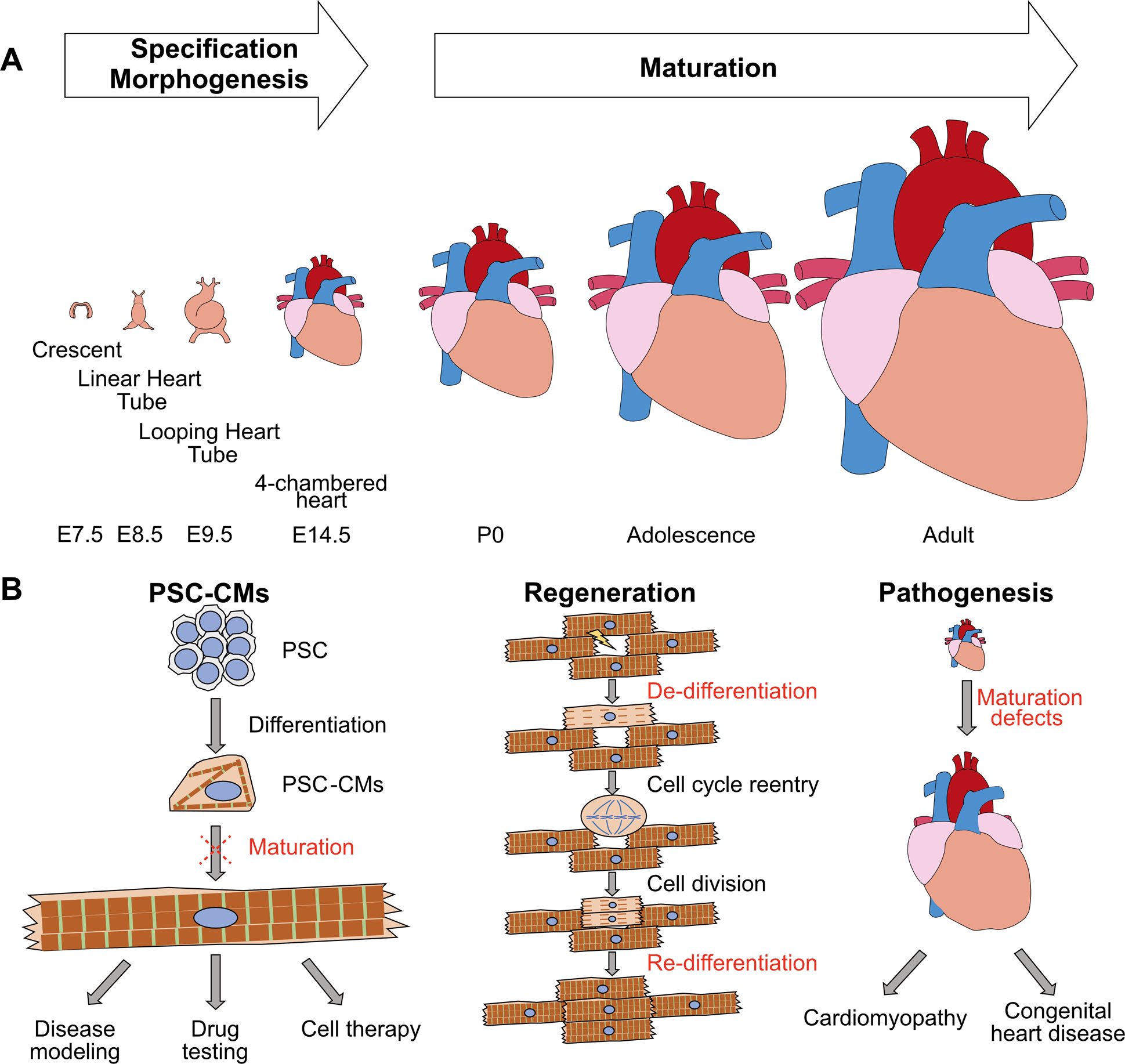
(A) Conceptual scheme of the maturation phase of heart development. Mouse stages are labeled at bottom. (B) Major applications of CM maturation studies. Left: to promote the maturation of PSC-CMs. Mid: to optimize CM regeneration conditions. Right: to better understand cardiac pathogenesis.
Cardiomyocytes (CMs) drive heart contraction. In maturation, CMs undergo changes that permit the cells to sustain billions of cycles of forceful contraction and relaxation. The term “CM maturation” refers to the constellation of changes to cell structure, metabolism, function, and gene expression that convert fetal CMs to adult CMs. This term also refers to the overarching developmental program that drives and coordinates the wide spectrum of phenotypic changes.
The recent attention to CM maturation has been driven by a surging interest in cardiac regenerative medicine (Fig. 1B). Although current technology allows for efficient differentiation of human pluripotent stem cells (PSCs) into CMs, these PSC-CMs exhibit immature phenotypes that resemble fetal CMs1, 2. Despite tremendous progress in promoting PSC-CM maturation by tissue engineering-based methods3, 4, recently reviewed in references5 and6, complete maturation of PSC-CMs has yet to be achieved. This maturation bottleneck severely impairs the use of PSC-CMs in in vitro modeling for pathological, pharmacological, or therapeutic purposes. Electrophysiological maturation defects of PSC-CMs also result in arrhythmogenic risk from cell replacement therapy7. New knowledge in the developmental biology of maturation is essential for tissue engineers to rationally design better approaches to promote the maturation of PSC-CMs.
CM maturation research is also significant due to its connection to CM regeneration. Natural CM regeneration occurs through proliferation of existing CMs8–10. While CMs exhibit proliferative capacity in the fetus, they quickly lose this potential after birth11, concurring with changes characteristic of CM maturation. Factors that promote CM maturation, such as thyroid hormone12, 13 and oxygen14, are antagonistic to CM proliferation. On the other hand, proliferative CMs undergo “dedifferentiation” that includes sarcomere disassembly and upregulation of genes characteristic of fetal CMs15–17. Forced proliferation of adult CMs by over-expression of activated Yap18 or miR199a19 adversely impacts heart function and causes lethality. Therefore, understanding the Yin and Yang between maturation and proliferation is essential to design strategies to stimulate CM regeneration while minimizing its side effects.
Defective CM maturation could also contribute to heart diseases. For example, sarcomere gene mutations that cause cardiomyopathy have largely been studied for their impact on sarcomere function and Ca2+ sensitivity20. However, sarcomere assembly is a key driver of CM maturation that not only organizes intracellular structures21, but also modulates signal transduction22. Thus, sarcomere mutations could cause cardiomyopathy by impairing the programs that coordinate CM maturation. As another example, a subset of congenital heart disease patients develops late heart failure. Although this has been attributed to complications of cardiac surgery or the longstanding impact of aberrant hemodynamic loads, some congenital heart disease mutations could affect genes that regulate CM maturation22–25 and thereby predispose to late myocardial dysfunction.
In this review, we first describe the phenotypic hallmarks of CM maturation and next summarize regulatory mechanisms that trigger and coordinate CM maturation. Ventricular, atrial, and nodal CMs undergo distinct changes during maturation. Most research to date has focused on ventricular CMs, and accordingly we restrict the scope of this review to ventricular CMs.
2. Major Hallmarks of CM Maturation
Major biological processes in CM maturation are described below. Experientially measurable parameters are summarized in Table 1. Selected recent efforts to mature PSC-derived CMs using a combination of three dimensional culture and physical and biological stimuli are summarized in Table 2.
Table 1.
Major Parameters of CM maturation
| Gene Expression | Morphology | Functional Readouts | |
|---|---|---|---|
| Myofibril | Overall increase of mature sarcomere components Isoform switching: MYH6 to MYH7 (hs) MYH7 to MYH6 (mm) TNNI1 to TNNI3 TTN-N2BA to TTN-N2B MYL7 to MYL2 |
Sarcomere assembly and expansion Improved sarcomere alignment Increased sarcomere length (~2.2 μm) M-line formation |
Sarcomere contraction: Diastolic sarcomere length Fractional shortening Shortening velocity Contractile Force |
|
Electrophysiology & Ca2+ handling |
Increase of ventricular ion channels, e.g. KCNJ2 Decrease of automaticity ion channels, e.g. HCN4 Increase of Ca2+ handling molecules, e.g. LTCC, RYR2 and SERCA2 |
T-tubule formation and organization SR expansion and organization Dyad formation and distribution |
Action potential: Resting Vm (~−85 mV) Max dVm/dt (~200 V/s) Duration and shape Ca2+ transient: Peak amplitude Time to peak Decay time Diastolic Ca2+ |
| Metabolism | Glycolysis decrease Mitochondria biogenesis increase FAO increase Oxidative phosphorylation increase Energy transfer system increase |
Mitochondria # and size incr. (up to 40% cell volume) Cristae formation and organization Inter-myofibrillar localization |
Oxygen consumption rate Electron transport chain activity IMM electrochemical gradient Extracellular acidification rate |
| Other | Cell cycle gene silencing Hypertrophy gene upregulation Changes of cell adhesion genes, e.g. ICD and costamere components |
Polyploidization Binucleation in >80% rodent CMs but only ~25% human CMs Maturational hypertrophy (~30 fold) ICD formation |
Abbreviations: FAO, fatty acid oxidation; Vm, membrane potential; ICD, intercalated disk; hs, Homo sapiens; mm, Mus musculus; IMM, inner mitochondrial membrane
Table 2.
Efforts to promote hiPSC-CM maturation by 3D tissue engineering
| Reference | Huang et al.26 | Ronaldson-Bouchard et al.4, 27 | Shadrin et al.28 | Mills et al.29 | Ruan et al.30 | Hirt et al., Mannhardt et al., Lemoine et al.31–33 | Nunes et al.34 | |
|---|---|---|---|---|---|---|---|---|
| Engineered tissue size and treatments | 0.5 mm × 0.2 mm, T3 + Dex + IGF1 for 1 wk | 6 mm × 2 mm, early ramped field stim. 2–6 Hz | 7 mm × 7 mm, RPMI + B27 + insulin for 1wk, 5% FBS for 2 wks | 1 mm × 0.5 mm, low glucose, high palmitate, no insulin | 20 mm × 0.3 mm, static stress for 2 wks + electrical stim. for 1 wk | 8 mm × 0.2–1.3 mm, ± pacing | ~600 μm wide gel on inelastic silk core; ramped field stim. 1–6 Hz | |
| Myofibril assembly | isoform switching | ↑MYH6, ↑MLC2v; ↓MYH7, ↓MLC2a, ↓ TNNI1 | ↑MYH7, ↑TNNI3 | ↑MLC2V, ↑TNNI3, ↑MYH7; ↓MLC2a | ↑MLC2v; ↑TTN N2B, ↑MYH7/6, ↑TNNI3/1 | not described | MLC2v detectable | ↓MYH6 |
| Sarcomere organization | Orderly register of A-bands, I-bands, H-zone and Z-lines; no M-lines. | Orderly register of A-bands, I-bands, Z-lines and M-lines. | Orderly register of A-bands, I-bands, H-zone and Z-lines; no M-lines. | Clear Z-lines, I-bands and A-bands; no M-line | Improved; lack detailed analysis of TEM | Regular Z-lines; inconsistent I- and A-bands; no M-line | Regular Z-lines; I-band and H-zone detectable; no M-line | |
| sarcomere length | 2 μm | 2.2 μm | 2.1 μm | 2.3 μm | not described | 1.6 μm | not described | |
| Electrophysiology and Ca2+ handling | expression of channels & regulators | ↑KCNJ2, ↑RYR2, ↑SERCA, ↑NCX1 | ↑RYR2, ↑SERCA; ↓HCN4 | ↑CASQ2, ↑S100A1 | not described | ↑SERCA,↑RYR2 | no detectable changes | ↑KCNJ2 |
| T-tubule | adjacent to sarcomeres; unclear alignment | well developed and aligned | not detectable | adjacent to sarcomeres; unclear alignment | not detectable | not detectable | not detectable | |
| resting Vm | not quantified | –70 mV | –71 mV | –60 mV | nd | –73.5 mV | –80 mV | |
| Max dV/dt | not quantified | 23 V/s | 38 V/s | 148 V/s | nd | 219 V/s | 125 V/s | |
| APD | APD80 1000 ms at 0.5 Hz pacing | APD90 500 ms | ADP80 450 ms | APD90 110 ms, APD50 60 ms | nd | nd | APD90 120 ms | |
| AP “notch” | not detectable | Yes | not described | Yes | nd | Yes | nd | |
| Ca2+ transient | enhanced | enhanced | visible | enhanced | nd | nd | nd | |
| Ca2+ storage & SR release | nd | enhanced | nd | nd | nd | nd | enhanced | |
| metabolism | metabolic gene expr. | ↑PPARA, ↑PGC1a | ↑TFAM, ↑PGC1a | ↑COX6A2, ↑CKMT2, ↑CKM | ↑redox and FAO genes | nd | nd | nd |
| mitochondria amount | increase by TEM | increase by TEM | increase by TEM | mtDNA increase | nd | nd | nd | |
| mitochondria alignment | close to sarcomeres | close to sarcomeres | nd | close to sarcomeres | nd | nd | close to sarcomeres | |
| mitochondria cristae | nd | well developed | well developed | nd | nd | immature | nd | |
| mitochondria functions | nd | OCR and ECAR increased | nd | Incr. maximal OCR and OCR reserve | nd | nd | nd | |
| Proliferation & hypertrophy | cell-cycle gene expr. | nd | nd | nd | cell cycle gene downregulation | nd | nd | nd |
| proliferation rate | nd | nd | decrease | decrease | nd | nd | decrease | |
| CM size | Incr. to 735 μm2 | Incr. to 1500 μm2 | nd | nd | Incr. to 795 μm2 | nd | Incr. to 917 μm2 | |
| Tissue integration and physiology | ICD | ICD on TEM, Cx43 at cell poles | ICD on TEM, Cx43 at cell poles | NCad at cell poles; Cx43 mislocalized | ICD on TEM; Cx43 and NCad mislocalized | Primitive ICD on TEM | Cx43 mislocalized, | nascent ICD |
| contractility | 2.1–4.4 mN/mm2 | 3 mN/mm2 | 23 mN/mm2 | 0.3 mN | 1.3 mN/mm2 | up to 0.15 mN | nd | |
| Frank-Starling relationship | nd | nd | detectable | nd | detectable | detectable | nd | |
| Force-freq. relationship | flat | positive | flat or slightly negative | nd | positive | flat | nd | |
| Response to β-agonists | Incr. contraction rate & amplitude | Incr. contraction rate & amplitude | nd | Incr. contraction rate & amplitude | Incr. contraction rate but not force amplitude | Incr. force amplitude; rate not described | Incr. rate, force not described | |
| Post-pause potentiation | nd | present | nd | nd | nd | present | nd | |
| Conduction vel. (cm/s) | up to 40 | 25 | 25.1 | nd | 2.76 | nd | 15 | |
| inotropic response to extracellular Ca2+ (EC50) | nd | ~0.4 mM | nd | 1 mM | nd | 0.6 mM | nd | |
Abbreviations: Dex, dexamethasone; stim, stimulation; Vm, membrane voltage, APD, action potential duration; AP, action potential; nd, not described; ICD, intercalated disc; TEM, transmission electron microscopy; NCad, N-cadherin
2.a. Myofibril Maturation
Myofibrils are specialized cytoskeletal structures that serve as the contractile apparatuses of CMs35, 36. Sarcomeres are longitudinally repeated subunits of myofibrils. A mature sarcomere comprises thin filaments (sarcomeric actin, troponins, tropomyosin), thick filaments (myosin heavy and light chains and their associated proteins, such as myosin binding protein C), titin filaments, Z-lines (actinin and its interacting proteins), and M-lines (myomesin, and its interacting proteins) (Fig. 2A). In a process powered by ATP hydrolysis, myosin complexes exert “power strokes” on thin filaments that slide thick filaments toward the barbed end of sarcomeric actin filaments, which are anchored at Z-lines. This action shortens the distance between Z-lines and results in muscle contraction. Z-lines and M-lines cross-link thin and thick filaments respectively and ensure their alignment. Titin is a gigantic protein with N- and C-termini anchored to Z- and M- lines, respectively. Z-lines are also attached with other cytoskeletal components such as desmin (a type of intermediate filament), microtubules, and the non-sarcomeric actomyosin system, which mechanically integrates these cytoskeletal structures.
Figure 2. Structural maturation of CMs.
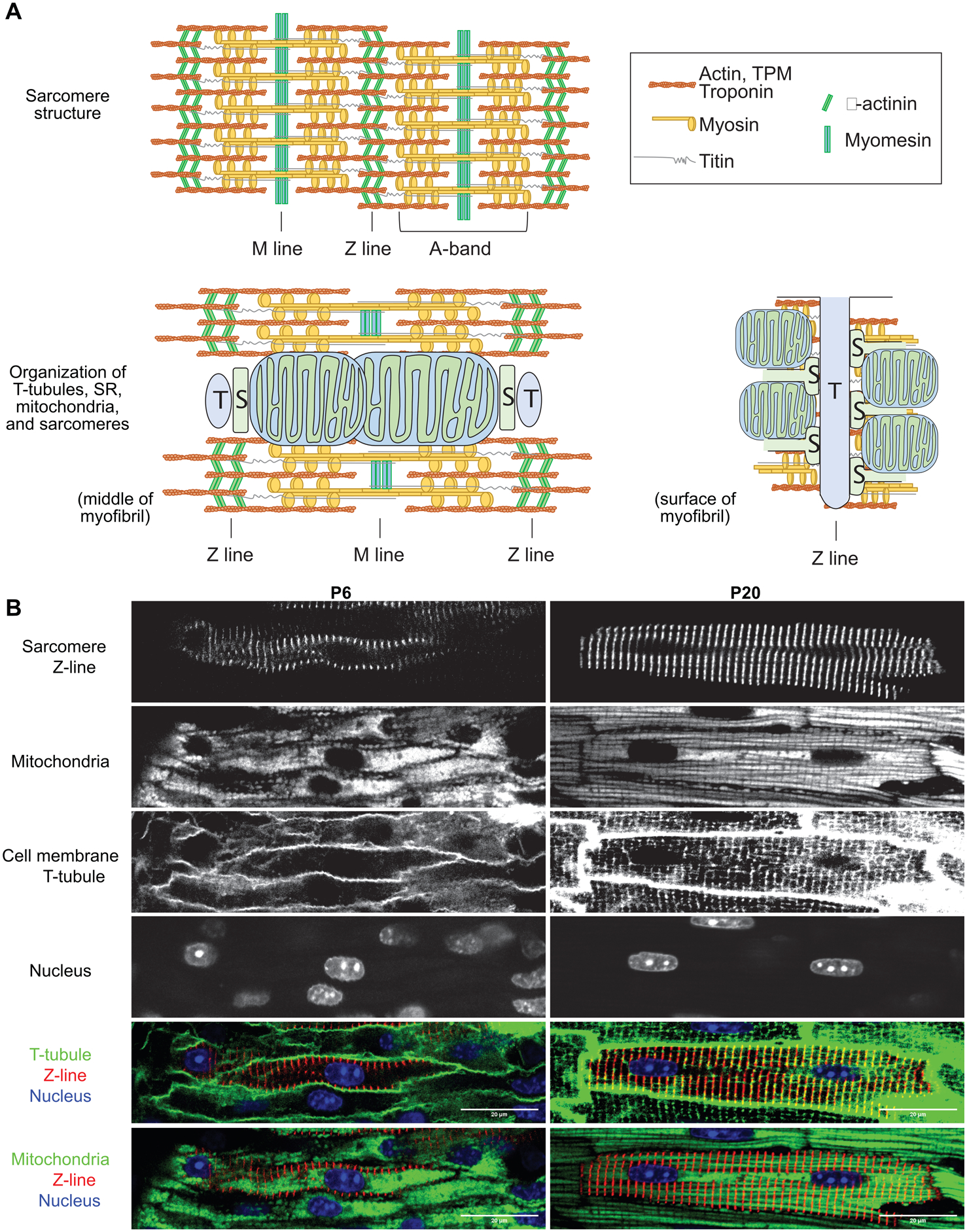
(A) A schematic view of sarcomere components in mature CMs (top) and spatial relationship between sarcomeres and T-tubule (T), SR (S) and mitochondria in mature CMs (bottom). Bottom left: a view across the middle of a myofibril. Bottom right: a view on the cytoplasmic surface of a myofibril. (B) In situ confocal images of murine myocardium at postnatal day 6 (P6) and P20. Sarcomere Z-lines were labeled by AAV-Actn2-GFP infection. Mitochondria, T-tubules and nuclei were stained by TMRM (polarized mitochondria), FM 4–64 (plasma membrane), and Hoechst (DNA), respectively, through Langendoff perfusion. Merged images highlight T-tubule-sarcomere and mitochondria-sarcomere associations that are established during postnatal maturation.
Sarcomere assembly initiates at cardiac specification, and continuously occurs in both immature and mature CMs. Thus, the emergence of sarcomeres should be treated as a marker of CM identity, but not maturation. However, CM maturation is characterized by massive expansion of myofibrils (Fig. 2B), as new sarcomeres are continuously added in alignment with pre-existing myofibrils both longitudinally and laterally. Very little is known about the molecular mechanisms that drive sarcomere expansion.
Sarcomere maturation also features changes in ultrastructural organization. When observed by transmission electron microscopy (TEM), mature sarcomeres exhibit more clear banding as compared to immature sarcomeres, suggesting improved alignment of sarcomere filaments. Z-lines increase in width and alignment, and the distance between Z-lines (often called sarcomere length) also increases to ~2.2 μm in diastole in mature, loaded CMs. Although the M-line protein myomesin is present in fetal sarcomeres, the M-line is difficult to visualize by TEM in fetal heart. With maturation, the M-line becomes distinct, likely due to increased thick filament alignment37.
An integral element of myofibril maturation is sarcomeric isoform switching, in which several sarcomere components switch from a fetal to an adult isoform due to transcriptional changes or alternative splicing. In rodents, among the most well-known is the myosin heavy chain switch from fetal Myh7 to adult Myh6. By contrast, MYH7 is the predominant isoform in adult heart of humans, and this isoform preference is already established by 5 weeks of gestation38, 39. Whether a MYH6-to-MYH7 switch occurs at an earlier stage of human cardiogenesis remains undetermined, but this event is suggested by predominant expression of MYH6 in newly differentiated human induced PSC-CMs (hiPSC-CMs)40.
Isoform switching also affects other sarcomere components. For example, the regulatory light chain of myosin was predominantly expressed by the gene MYL7 (often known as MLC-2a) in all early fetal CMs. However, this isoform switches to MYL2 (also known as MLC-2v) as ventricular CMs mature, and MYL7 expression becomes restricted to atrial CMs41, 42. Fetal CMs primarily express slow skeletal troponin I (TNNI1), and this is replaced by cardiac troponin I (TNNI3) in mature CMs43.The more compliant splicing isoform of titin (N2BA isoform) is preferentially expressed in fetal hearts, and after birth the stiffer N2B isoform predominates44. Likewise, the fetal isoform of myomesin (EH-myomesin) is expressed in fetal CMs, and this transits to myomesin isoforms lacking the EH domain in mature CMs. This isoform transition has been associated with the appearance of the M-line37. Cardiac troponin T and tropomyosin also undergo maturationally regulated alternative splicing45.
2.b. Maturation of Electrophysiology and Ca2+ Handling
The strength, speed, and rhythm of CM contraction and relaxation are tightly controlled by electrical impulses and oscillations of cytoplasmic Ca2+ concentration. The electrical signals take the form of the action potential, which is determined by cardiac ion channels. In mature CMs, the resting membrane potential is maintained at ~−85 mV by the inward rectifying current IK146. Potassium channels Kir2.1 and Kir2.2, encoded by genes KCNJ2 and KCNJ12, respectively, are the major channels that establish and maintain the resting membrane potential. The action potential is initiated by rapid opening of voltage-gated sodium channels (mainly Nav1.5; encoded by SCN5A), which permits Na+ influx (INa) and membrane depolarization. Depolarization is followed by the activity of transient outward potassium current (Ito) that results in a unique “notch” shape in the action potential of maturation CMs. Membrane depolarization opens the L-type Ca2+ channels (Cav1.2), which generate the Ca2+ current (ICa,L) responsible for the “plateau” phase of the action potential in human CMs. Action potential of murine CMs do not exhibit a clear plateau phase. The depolarizing effect of ICa,L is counteracted by an array of temporally controlled repolarizing potassium currents, including IKs, IKr, and IK1. Upon Cav1.2 inactivation, the repolarizing potassium currents re-establish the resting membrane potential.
Immature CMs differ in important ways from mature CMs in electrophysiology. First, the resting membrane potential of immature CMs is less negative (~−50 to −60 mV) as a result of insufficient expression of Kir2.1 and Kir2.247. Second, the upstroke velocity of immature CMs (~15–30 V/s) is slower due to lower activity and expression of SCN5A and other sodium channels48, 49. Third, the plateau phase of the action potential is longer in mature CMs, partly due to higher expression of Cav1.2 core component CACNA1C50 and alternative splicing of its auxiliary subunit CACNB251.
Membrane depolarization is coupled to sarcomere contraction through Ca2+-induced Ca2+ release (CICR). In systole, Cav1.2 activation allows a small amount of extracellular Ca2+ to enter cells, where it activates the ryanodine receptor 2 (RYR2) to release Ca2+ from the sarcoplasmic reticulum (SR, specialized endoplasmic reticulum in CMs). In diastole, Ca2+ is cleared from the cytosol to the SR via the sarco/endoplasmic reticulum Ca²⁺-ATPase (SERCA2), and to the extracellular space via the Na+-Ca2+ exchanger (NCX).
CICR occurs in proximity to plasma membrane. In small, immature CMs where sarcomeres are relatively proximal to the cell surface, Ca2+ that is released at the cell periphery is sufficient to trigger sarcomere contraction. However, as CMs enlarge and sarcomeres expand toward the cell interior, Ca2+ that is released at the cell periphery cannot rapidly activate interior sarcomeres. To solve this problem, CMs evolved transverse-tubules (T-tubules; Fig. 2), which are invaginations of plasma membrane that penetrate transversely into the center of mature CMs. This structural specialization juxtaposes the plasma membrane with subdomains of SR to form dyads, where Cav1.2 and RYR2 cluster in proximity to form Ca2+ release units. These structural specializations allow the action potential to travel rapidly along T-tubules to the interior of cells, where they trigger dyads to release Ca2+ in close proximity to sarcomeres.
The structural basis of T-tubule maturation is poorly understood. Caveolin-3 (CAV3) is thought to regulate plasma membrane invagination52, but T-tubules still form in Cav3 knockout mice53. BIN1 increases membrane curvature of T-tubules in mice54, and BIN1 overexpression induces T-tubule-like structures in PSC-CMs55. However, the transverse alignment of T-tubules is preserved in Bin1 knockout CMs in mice54. JPH2 is required to juxtapose T-tubule and SR membranes56, but JPH2 disruption only results in mild cell-autonomous loss of T-tubule organization in murine CMs57. Although ACTN2 is essential for T-tubule organization22, how T-tubules are anchored to Z-lines remains unclear. A recent study identified a Z-line component nexilin (NEXN) as a new regulator of T-tubules58. Whether NEXN mediates Z-line-T-tubule association remains to be determined.
Whereas mature ventricular CMs exhibit low automaticity, immature CMs and PSC-CMs spontaneously beat, a phenotype that likely contributes to arrhythmia when PSC-CMs are transplanted in myocardial infarction models7. Multiple factors contribute to the automaticity of PSC-CMs, including the expression of pacemaker channels such as hyperpolarization activated cyclic nucleotide gated potassium channel 4 (HCN4), the resting membrane potential that is closer to the action potential activation threshold, and spontaneous Ca2+ release, which drives membrane depolarization through the Ca2+-Na+ exchanger59.
2.c. Metabolic Maturation
An adult human heart is estimated to use ~6 kg ATP per day60, with the primary consumers being myosin ATPases, which are needed for sarcomere contraction, and SERCA, which drives Ca2+ clearance and sarcomere relaxation. This ATP is primarily produced through oxidative phosphorylation using lipid substrates61.
In maturation, CMs undergo multiple adaptations to enable a high and sustained rate of ATP production. Chief among them is increased number and size of mitochondria, which occupy up to 40% of cell volume62. The morphology and size of mitochondria is controlled by their fusion and fission. Perturbation of pro-fusion proteins such as mitofusin 1/2 (MFN1/263, 64), or overexpression of pro-fission proteins such as DRP165, resulted in decreased mitochondrial size in maturing CMs. Mitochondria also become associated with sarcomeres during maturation (Fig. 2). Sarcomere disassembly caused decreased mitochondrial size21, suggesting a functional link between sarcomeres and mitochondrial morphology. Mitochondria are also attached to SR, potentially through ER-mitochondria contact sites. This close organization leads to efficient ATP transport from mitochondria to ATPases in sarcomeres and SR66.
Mature mitochondria contain densely organized cristae, the foldings of the inner mitochondrial membranes that house the electron transport chain and ATP synthase. By contrast, in immature CMs, which primarily produce ATP through glycolysis, mitochondria exhibit few and poorly-aligned cristae67. Cristae maturation requires an array of molecules such as OPA168, 69,the MICOS complex70 and cardiolipin-based lipid-protein microdomains71. ATP synthase72 may also drive cristae curvature formation.
The metabolic transition from immature CMs to mature CMs is driven by activation of metabolic transcriptional regulators including Ppargc1a/b, Ppara, Nrf1/2, and Esrra/b/g73, upregulation of genes involved in fatty acid metabolism, oxidative phosphorylation, and mitochondrial biogenesis, and downregulation of glycolytic genes74, 75. Isoform switching also contributes to metabolic maturation. Hexokinase, which executes the first committed step of glycolysis, is predominantly hexokinase 1 (HK1) in fetal and neonatal CMs76. In adult CMs, the predominant isoform is hexokinase 2 (HK2)77, which exhibits less glycolytic activity. Cytochrome c oxidase (COX) subunit 8, a component of complex IV of the electron transport chain, also switches between COX8A and COX8B isoforms in CM maturation78,although the contribution of this switch to CM maturation remains to be determined.
Less is known about anabolic metabolism changes in CM maturation. Immature, proliferative CMs create a high demand for nucleotide biosynthesis, which is suppressed after CMs mature. Conversely, high glucose promotes nucleotide biosynthesis through the pentose phosphate pathway and inhibits CM maturation79. Because CM maturation involves a remarkable increase of protein-built components such as myofibrils, and extensive expansion of lipid bilayers in T-tubules, SR and mitochondria, protein and lipid biosynthesis are also expected to be highly active. However, little work has been done to characterize these two anabolic processes during CM maturation.
2.d. Proliferation-to-hypertrophy Transition
In mice, CM cell cycle exit occurs within the first postnatal week11. In humans, CM proliferation rate declines rapidly postnatally, but does not reach the steady-state rate of < 1% per year until the second decade of life80, 81. Central cell cycle regulators, such as the cyclin-dependent kinase (CDK) complexes, are tightly repressed during CM maturation82. Recently, it was reported that co-overexpression of CDK1:CCNB and CDK4:CCND complexes, which activate M phase and G1-S phase respectively, was sufficient to reactivate CM proliferation82. This exciting finding awaits confirmation by independent groups. The mechanisms that enforce CM cell cycle exit include the downregulation of mitogenic signals, such as the neuregulin-ErbB axis83, and the inhibition of YAP, a potent activator of CM proliferation84, 85. During postnatal CM maturation, YAP activity is restrained by Hippo kinases84, 85, interactions with cell adhesion complexes86, 87, and nuclear antagonists88.
Despite cell cycle withdrawal, the postnatal heart increases in size by ~30-fold through proportional increase of CM volume, a process called maturational hypertrophy. The liquid-phase cytoplasm is unlikely the major contributor to increased cell volume, as mature CMs are tightly packed and myofibrils and mitochondria occupy most intracellular space. Myofibril expansion is critical for maturational hypertrophy, as the ablation of sarcomeres by Myh6 depletion or Actn2 mutation dramatically decreased CM size during murine CM maturation21, 22. However, whether mitochondria biogenesis and enlargement cell-autonomously contributes to maturational hypertrophy is unclear21, 89.
Another hallmark of CM maturation during the proliferation-to-hypertrophy transition is polyploidization. In murine CMs, the final round of the cell cycle involves karyokinesis without cytokinesis, leading most mature CMs (~90%) to contain two diploid nuclei (“binucleation”)90, 91 (Fig. 2). By contrast, in adult humans, ~75% CMs are mononuclear, but the majority of these nuclei are polyploid due to DNA endoreplication without karyokinesis92, 93. This polyploidization largely develops in the second decade of life80.
CM polyploidization negatively correlates with cell cycle withdrawal94. Residual CM cell cycle activity in adult hearts resides in the mononuclear diploid subset of CMs80, 81, 94. The introduction of a genetic modifier associated with higher mononuclear diploid fraction increased CM cell cycle activity after adult heart injury94. Forced CM polyploidization by ECT2 inhibition, which blocks cytokinesis, is sufficient to suppress the proliferative capacity of CMs in regeneration95, 96. For many cell types, the ploidy of a cell is positively correlated with cell size97, thus CM polyploidization likely promotes maturational hypertrophy. Consistent with this hypothesis, the induction of CM polyploidization was sufficient to increase CM size95, 96. Together, CM polyploidization is partially causative for both CM cell cycle withdrawal and maturational hypertrophy in CM maturation.
2.e. CM Integration into a Mature Tissue
Maturational integration of CMs into cardiac tissues require the formation of specialized CM-CM junctions called intercalated discs (ICDs), which occurs 2–3 weeks after birth in mice. ICDs are hybrid junctions comprising three major types of cell adhesions: fascia adherens, desmosomes, and gap junctions98. Fascia adherens comprise N-cadherin and its associated proteins. Desmosomes comprise desmoglein-2, desmocollin-2, and their anxilary proteins such as plakoglobin, plakophilin-2, and demoplakin. Gap junctions are composed of connexin 43. While fascia adherens and desmosomes mechanically couple the actin cytoskeleton and intermediate filaments of neighboring CMs, gap junctions mediate propagation of electrical and small molecule signals between CMs.
Immature CMs lack ICDs, and ICD components are either not expressed, localized to the interior of cells, or throughout the cell surface. During CM maturation, these molecules redistribute to cell termini to form ICDs. The mechanisms that regulate the targeted localization of ICD components to CM termini are incompletely elucidated, but likely involve protein trafficking along “microtubule highways” extending from the trans-golgi network to cell termini99.
CM integration into tissues also requires attachment to the extracellular matrix (ECM) through specialized focal adhesion-like structures called costameres100. The transmembrane adaptors of costameres include both the integrin complexes and the dystrophin-associated glycoprotein complexes, which anchor to sarcomere Z-lines and non-sarcomere cytoskeleton at the lateral CM membrane.
Beyond tissue integration, ICDs and costameres are likely to play additional roles in CM maturation. For example, both ICDs and costameres harbor vinculin-based actomyosin organizers that are essential for sarcomere assembly101, and potentially mediate longitudinal and lateral sarcomere expansion respectively. ICDs and costameres are also critical sensors of biophysical signals98, 100. Thus, further investigation of ICD and costamere is essential to understand how biophysical signals promote CM maturation (see next section).
3. The Regulation of CM Maturation
CM maturation involves a spectrum of diverse cellular events that occur concurrently. The mechanisms that activate these events and integrate them into a coordinated program is an overarching question for CM maturation research.
3.a. Microenvironmental Instruction
The microenvironment of the maturing myocardium provides necessary and sufficient information to instruct CM maturation. This notion is supported by two lines of evidence. First, in vitro culture of primary mature CMs leads to loss of hallmarks of maturity102. Second, immature CMs developed toward an adult-like state after being transplanted into maturing myocardium103. These studies provide the logical basis to search for CM maturation cues by dissecting the physicochemical properties of maturing myocardium.
3.a.i. Biophysical Cues (Fig. 3A)
Figure 3. Representative environmental cues that regulate CM maturation.
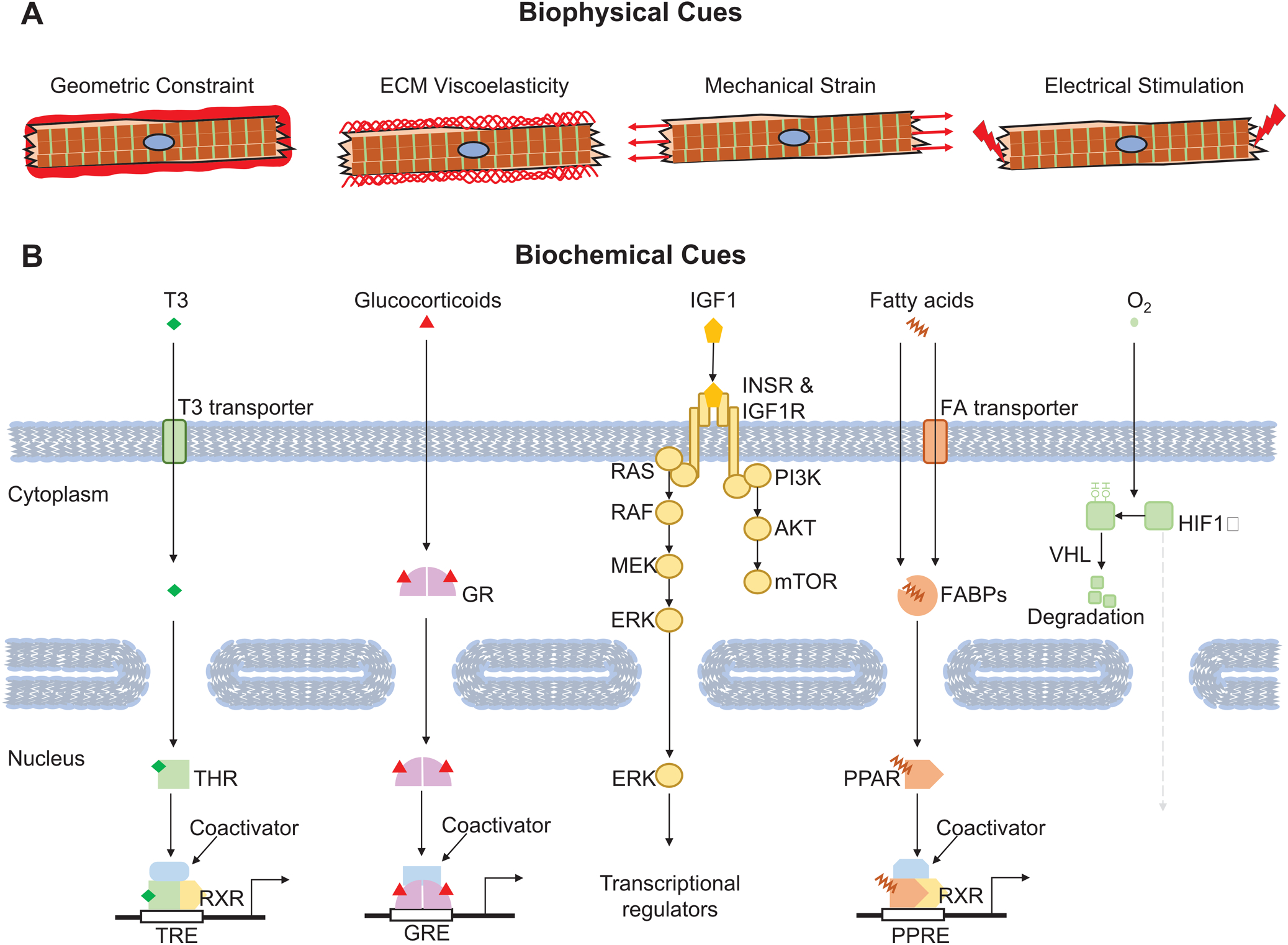
(A) Key biophysical factors that affect CM maturation. (B) Critical biochemical cues that regulate CM maturation. Representative signal receptors, messengers and transcriptional regulators are also depicted.
Adult CMs exhibit a rod shape with an average length-to-width ratio of 7:1104. This unique shape cannot be solely explained by the cell-autonomous effect of sarcomere elongation, as CMs with sarcomere ablation due to Myh6 knockout retained an elongated morphology in a genetic mosaic model in mice, although the cell width was drastically decreased21. Both neonatal and adult CMs are elongated in vivo but cannot maintain this shape after cell culture. PSC-CMs on regular cell culture dishes are round- or triangular- shaped and require physical cues to adopt a rod shape. Therefore, the microenvironment of myocardium establishes geometric cues that induce uniaxial CM elongation (Fig. 3A).
Patterning CMs to adopt a rod-shaped morphology promotes CM maturation. For example, PSC-CM growth on rectangular micropatterns105 or uniaxially aligned ridges and grooves106, 107 were sufficient to improve sarcomere organization and contractile and electrophysiological function of CMs in a two dimensional (2D) system. CM maturation was further improved by assembling CMs into three dimensional (3D) tissue with anisotropically directed strain, such as engineered heart tissue (EHT)108, 109 or cardiac microtissue (CMT)108, 109.
The viscoelastic properties of ECM also modulate CM maturation (Fig. 3A). The elastic modulus of ECM progressively increases from neonatal (<10 kPa) to adult (~25 kPa) heart110. Culturing CMs on matrix with tunable elastic moduli showed that physiological matrix stiffness is optimal for CM maturation parameters such as sarcomere organization, Ca2+ handling, and contractility111–113.
Maturing CMs experience escalating mechanical force during development114. Cyclic mechanical stress during systole and passive stretch during diastole both induced CM maturation in cell culture115–117 (Fig. 3A). Mechanical force not only improved structural maturation but also induced gene expression changes115–117. A recent study showed that cardiac contractile force regulated the distribution of vinculin and activated slingshot protein phosphatase 1 and the actin depolymerizing factor cofilin to promote myofilament maturation118. How mechanotransduction pathways convert mechanical force into transcriptional changes remains to be clarified.
Electrical pacing also enhances the ultrastructure and gene expression of cultured CMs (Fig. 3A), as well as their contractile, electrophysiological, and metabolic activity119–121. A recent study reported the production of adult-like CMs after 3D engineered heart tissue was paced at supraphysiological rates from an early point in their differentiation4, 27. The striking degree of maturation achieved in this study requires further validation and replication by other groups. The mechanisms by which electrical stimulation enhances CM maturation remain poorly explored. A key unanswered question is whether electrical pacing directly impacts CM maturation or acts indirectly through induction of mechanical stress.
3.a.ii. Biochemical Cues (Fig. 3B)
Among the best characterized biochemical cues that promote CM maturation is the thyroid hormone T3 (triiodothyronine). The serum level of T3 rises dramatically in the perinatal period. T3 exerted a broad impact on CM maturation, including isoform switching of myosin heavy chain and titin122, 123, induction of SERCA expression, hypertrophy and cell polyploidization12, 13. T3 treatment was sufficient to enhance CM contractility, Ca2+ handling, and mitochondrial respiration in vitro124, 125. One study linked a proliferative burst of mouse cardiomyocyte proliferation on postnatal day 15 to a transient surge of thyroid hormone126; however, others groups have not replicated the proposed surge of proliferating cardiomyocytes127, 128. The major thyroid hormone receptors in the heart are nuclear receptors (NRs) that are encoded by Thra and Thrb (Fig. 3B). Inactivation of Thra cell-autonomously suppressed CM maturation25.
Similar to T3, glucocorticoids also modulate CM maturation129. Glucocorticoids are ligands for the glucocorticoid receptor, another NR encoded by Nr3c1. Mutation of Nr3c1 impaired myocyte alignment, disruption of sarcomere organization and the expression of genes regulating sarcomere assembly and Ca2+ handling130.
Insulin-like growth factors (IGFs) regulate CM maturation through the insulin-like growth factor 1 receptor (IGF1R) and the insulin receptor (INSR), which are receptor tyrosine kinases that signal through the PI3K-AKT and RAF-MEK-ERK pathways. IGF1 is predominantly produced in the liver, and also locally produced in the heart131. Circulating IGF1 quickly increases after birth in response to growth hormone132, 133; changes to local production of cardiac IGF1 were not well-described. Overexpression of IGF1R in CMs caused physiological hypertrophy134. Double knockout of INSR and IGF1R in murine CMs resulted in early-onset dilated cardiomyopathy within a month after birth, with disrupted sarcomere and mitochondrial morphology and reduced heart function135. However, deletion of either INSR or IGF1R alone did not cause phenotypic abnormalities, consistent with functional redundancy.
Circulating fatty acids also increase at birth, and this could serve as a biochemical signal for CM maturation. Culture of engineered cardiac tissues with palmitate, the most abundant long-chain free fatty acid in the neonatal circulation136, matured multiple parameters, including gene expression, contractile force, action potential, Ca2+ transient and oxidative respiration29. In another study, treatment of PSC-CMs with palmitate-albumin complexes along with carnitine, which facilitate mitochondrial fatty acid transport, promoted structural and functional maturation, suggesting that in vitro promotion of oxidative phosphorylation stimulates overall CM maturation137. However, perturbation of metabolic maturation did not impair structural maturation in a cell-autonomous manner in vivo, since neonatal, mosaic ablation of genes essential for mitochondrial function (Tfam) or dynamics (Mfn1/2) did not impair structural maturation of the mutant CMs21, 89.
Oxygen tension is another environmental cue that modulates CM maturation. Increased oxygen tension inhibits HIF1α (hypoxia-inducible factor 1α) activity and promotes the metabolic switch to oxidative phosphorylation during murine heart development138, whereas hypoxia impaired PSC-CMs differentiation and maturation in vitro139. Inhibition of HIF1α and its downstream target lactate dehydrogenase A promoted hiPSC-CM maturation, enhancing not only metabolism but also gene expression, sarcomere organization and contractility140.
Biochemical signals function synergistically to promote CM maturation. For example, T3 and dexamethasone, a synthetic glucocorticoid, in combination with culture on “matrigel mattresses” cooperatively triggered CM maturation by inducing T-tubule formation141. A cocktail of T3, dexamethasone, and IGF1 induced several adult features in iPSC-CMs cultured in 3D cardiac tissues3. Cross-talk between T3 and AKT-PI3K, a downstream branch of IGF1 signaling, stimulated TTN isoform switching in cultured, late gestation rat CMs122. Thus, a sophisticated signaling network is present that integrates diverse extracellular signals into a robust and coordinated program of CM maturation.
3.a.iii. Non-CMs
Although CMs occupy ~70–85% of myocardial volume, they constitute only ~20–30% of the total cell number80, 142, 143. Numerically, non-CMs, including endothelial cells (64%), cardiac fibroblasts (27%), and leukocytes (9%), are the major cell types in the heart143. In the fetal heart, CMs constitute a higher fraction of cells, with the proportion declining during maturation due to the greater proliferation of non-CMs.
Non-CMs regulate CM maturation, as co-culture of CMs with non-CMs promotes CM maturation in vitro144–146. The impact of non-CMs on CM maturation could occur through direct physical adhesion, and through paracrine molecules that are secreted from non-CMs and act on CMs146. In addition, non-CMs build the microenvironment that delivers biophysical and biochemical cues to CMs. For example, cardiac fibroblasts create the appropriate ECM to support CM maturation, and endothelial cells construct coronary vasculature that transport circulating signals to instruct CM maturation.
3.b. Intracellular regulation
3.b.i. Transcriptional regulation of gene expression
The coordination of diverse phenotypic changes during CM maturation and the association of those changes with altered gene expression suggest an overarching transcriptional program that orchestrates CM maturation.
Several transcriptional regulators of CM maturation have been identified. One of these is serum response factor (SRF)21. In murine CMs undergoing maturation, SRF depletion resulted in a wide spectrum of transcriptional dysregulation, including defective sarcomere isoform switching, global downregulation of the transcriptional programs of lipid metabolism, mitochondria biogenesis and oxidative respiration, and the reversal of maturational changes of key electrophysiological and Ca2+ handling genes, such as upregulation of Hcn4 and downregulation of Kcnj2, Serca2a and Ryr221. Structurally, SRF depletion impaired sarcomere expansion, T-tubule formation, and mitochondrial organization.
The broad impact of SRF on nearly every aspect of CM maturation is partly due to its key role in regulating sarcomere genes. Sarcomere disassembly by mosaic inactivation of the major Z-line protein ACTN2 not only recapitulated structural CM maturation defects, but also the transcriptomic signature of mosaic SRF depletion22. This relationship demonstrates that sarcomere-based signaling impacts gene transcription and highlights a hierarchical organization of the subprograms of CM maturation: Sarcomere maturation is upstream of most other aspects of CM maturation21, whereas metabolic maturation was dispensable for structural maturation in vivo21, 89.
Three myocardin-family transcriptional regulators, MYOCD, MRTFA and MRTFB, are major coactivators of SRF in CMs147. MRTFA and MRTFB are functionally redundant. Mrtfa−/−; Mrtfbfl/fl; Myh6Cre mice caused lethality of most mutants within a month after birth148. Myocdfl/fl; Myh6Cre mice developed later onset, lethal cardiomyopathy, with a median survival of about 10 months149. Although Mrtfa/b double knockout mice exhibit a more severe cardiac phenotype than Myocd mutant mice, both mice exhibit cardiac phenotypes that are less severe than Srf knockout mice, suggesting a synergistic role of all three factors in SRF activation and CM maturation. The MRTF-SRF axis could convert mechanical stress into transcriptional changes150, thus MRTF-SRF signaling potentially mediates regulation of CM maturation in response to biomechanical cues, including mechanical stretch and ECM matrix stiffness.
A recent transcriptomic analysis revealed another SRF-binding transcription cofactor, HOPX, as a novel activator of CM maturation, especially in the process of myofibrillar isoform switching and CM hypertrophy151. In vivo, overexpression of HOPX in CMs resulted in progressive concentric cardiac hypertrophy with preserved systolic function152, whereas Hopx knockout caused partial embryonic lethality153, 154, with postnatal survivors exhibiting normal cardiac contractility and cardiomyocyte hyperplasia due to delayed cell cycle exit154. Paradoxically, HOPX was classically thought to be a transcriptional corepressor that reduces SRF-DNA binding153, 154. Further studies are necessary to determine how SRF-HOPX interaction impacts CM maturation.
SRF functions in synergy with other transcription factors. For instance, SRF ChIP-Seq in maturing hearts revealed co-enrichment of GATA and MEF2 motifs21. GATA4 and GATA6 are the major GATA family transcription factors expressed in CMs, and these factors are redundantly essential for neonatal CM maturation25, 155. Four MEF2 family transcription factors, MEF2A~D, are expressed in hearts156 and their functions can be factor-specific, overlapping, or, in some cases, antagonistic157, 158. A systematic comparison has yet to be performed to determine the overlapping and unique roles of MEF2 factors in CM maturation.
In addition to SRF-related factors, NRs are another major group of transcription regulators that control CM maturation. Among these factors, thyroid hormone receptors and glucocorticoid receptors mediate the role of T3 and glucocorticoids in CM maturation as described in the previous section. Additional NRs play key roles in metabolic maturation. One family of such factors are peroxisome proliferator-activated receptors (PPARs), which form heterodimers with retinoid X NRs to activate and balance the transcription of genes involved in fatty acid and carbohydrate metabolism159, 160. The ligands of PPARs are fatty acid metabolites161, thus PPARs probably mediate the impact of circulating fatty acids on CM maturation. The estrogen-related receptors (ERRα, β, and γ) are another group of NRs essential for the maturational switch to oxidative respiration, by activating genes involved in fatty acid oxidation, citric acid cycle, electron transport chain, ATP synthase and mitochondrial dynamics162, 163. These factors belong to the orphan NR family and do not bind to estrogen. Interestingly, myofibril and Ca2+ handling genes are also direct downstream targets of ERRs162, 163. Both PPARs and ERRs directly interact with PGC1α/β, encoded by Ppargc1a and Ppargc1b, which are master regulators of both oxidative respiration and its associated mitochondrial biogenesis73. Interestingly, a recent study showed additional functions of PGC1/PPARα in the maturation of calcium handling and hypertrophy, implicating broader roles of these factors beyond metabolism164.
Epigenetic mechanisms, such as DNA methylation and covalent histone modifications, exert a profound impact on transcriptional regulation. DNA hypermethylation is associated with gene silencing in CM maturation, while DNA demethylation results in gene activation75, 165, 166. Activating histone modifications H3K27ac, H3K4me1, H3K4me3 and H3K9ac are associated with actively expressed genes in maturation165, 167, while repressive histone marks H3K27me3 and H3K9me2 are maintained or acquired by inactivated genes165, 167–170. Treatment of cultured human cardiac progenitor cells with polyinosinic-polycytidylic acid yielded PSC-CMs with enhanced maturity, which was attributed to “epigenetic priming” that enhanced Notch signaling and expression of cardiac myofilament genes171. Recently, a clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-based forward genetic screen in vivo identified RNF20/40 as a novel epigenetic regulator of CM maturation. This enzyme deposits histone H2B lysine 120 monoubiquitination marks at genes that are active in CM maturation25. Mutations that disrupt this pathway cause congenital heart disease24, suggesting that the same mutations that cause congenital heart disease could also impact CM maturation and late cardiac outcomes.
Chromatin organization changes are also correlated with transcriptional changes in CM maturation. ATAC-Seq revealed decreased chromatin accessibility of silenced genes such as cell cycle genes between neonatal and adult hearts, while metabolic and muscle contraction genes acquired a more open chromatin state in mature hearts172. Histone remodeling factor BRG1 modulates myosin heavy chain isoform switching173. Mutation of CTCF, a crucial regulator of chromatin-architecture, was recently reported to cause premature activation of the CM maturation program in embryonic cardiomyocytes174.
3.b.ii. Post-transcriptional regulation of gene expression
RNA splicing is a critical regulatory component of CM maturation, as isoform switching often occurs through alternative splicing. One representative splicing regulator is RBM20, mutation of which causes dilated cardiomyopathy175–177. RBM20 is essential for proper splicing of Ttn (Titin) transcripts and other maturationally regulated genes175, 178.
Additional splicing regulators could potentially impact CM maturation: CELF proteins are down regulated in heart development while MBNL proteins are upregulated. The antagonistic regulation of these two splicing regulators179 has been proposed to trigger a large fraction of developmental splicing changes and to be essential for T-tubule organization and Ca2+ handling180, 181. Serine/arginine-rich family of splicing factors, including SRSF1182, SRSF2183 and SRSF10184, were each shown to regulate postnatal heart development by modulating Ca2+ handling genes. CM-specific Hnrnpu knockout resulted in splicing defects in Ttn and Ca2+ handling genes and triggered perinatal dilated cardiomyopathy185. The RNA splicing regulator RBFOX1 markedly increases in expression during CM maturation180, 186 and is another potential activator of CM maturation187.
MicroRNA (miRNA)-based mRNA silencing is another mechanism that modulates gene expression in CM maturation. For example, miR-1, a miRNA enriched in mature CMs, facilitated electrophysiological maturation in stem-cell derived CMs in vitro188. Let-7 family miRNAs were highly enriched in CMs matured for 1 year in vitro, and they were necessary and sufficient to promote hypertrophy, sarcomere organization, contractile force, and respiratory capacity of cultured PSC-CMs189. Co-culture of CMs with endothelial cells promoted CM maturation in association with upregulation of multiple miRNAs145. Overexpression of four such miRNAs (miR-125b-5p, miR-199a-5p, miR-221, and miR-222) in PSC-CMs resulted in improvement of several maturation hallmarks such as Myh6/7 switching, sarcomere alignment, mitochondrial cristae formation, and improved Ca2+ handling145. Recently, a new miRNA maturation cocktail that overexpressed Let-7i and miR-452 and repressed miR-122 and miR-200a was shown to promote transcriptomic maturation as well as contractility, cell size, and fatty acid oxidation without sharing predicted target genes with previous microRNA cocktails190.
Cardiac protein synthesis is very active at fetal and neonatal stages, but regulation of protein translation, modification, and stability in CM maturation has been poorly studied. Recent advances in proteomics have started to characterize protein changes in CM maturation191–193. Integration of these data with RNA-Seq and Ribo-Seq analyses will provide an improved understanding of regulation at the protein level.
3.b.iii. Ultrastructural regulation
Major ultrastructural maturation hallmarks -- myofibrils, mitochondria, and T-tubules -- are not independent of each other. As the major cytoskeletal structures of CMs, myofibrils are essential for the organization of other intracellular structures. Mutagenesis of key myofibril genes, such as Myh6 and Actn2, impaired mitochondrial enlargement as well as the organization of T-tubules21, 22. By contrast, perturbation of T-tubule (by mutagenesis of Jph257) or mitochondria (by mutagenesis of Mfn1/221 or Tfam89, or by overexpression of Drp121) did not impair myofibril organization. Thus, proper sarcomere organization and expansion is central to overall structural maturation.
4. Model systems to study CM maturation
Innovations in the model systems and techniques used to study CM maturation will fuel future discoveries. Here we review some of the recent advances in model systems used to study CM maturation.
4.a. Mouse genetic mosaic and Cas9-mediated somatic knockout models
Genetically modified mice have been gold standards to understand mammalian heart development. This approach is particularly important in CM maturation research because so far no in vitro system can induce, or even maintain, full maturity of CMs. However, traditional genetic manipulation of the murine heart has several caveats. First, it is slow and expensive to generate or obtain alleles to knockout each gene of interest. Achieving spatiotemporal control of the knockout in perinatal CMs requires further complexity. Second, organ-wide mutagenesis of a gene essential for CM maturation often triggers lethality or secondary effects that can confound identification of the direct functions of the gene. This is particularly problematic in CM maturation research as the secondary effects of heart dysfunction, such as fetal gene reactivation and mitochondria/T-tubule remodeling, are similar to CM maturation defects57, 194.
These problems can be circumvented using adeno-associated virus (AAV), which efficiently and stably manipulates genes in CMs following subcutaneous or intraperitoneal injection to newborn mice. Gain-of-function via AAV-directed overexpression is straightforward. Loss-of-function can be achieved by using AAV to delivery CRISPR/Cas9 components (CRISPR/Cas9 and AAV-mediated somatic mutagenesis, CASAAV, Fig. 4A)57, 195. The CRISPR/Cas9 system further reduces the need to obtain conditional alleles. This technology allows mutagenesis of many genes at once21, 57 and even high-throughput genetic screening in vivo25.
Figure 4. Model systems to study CM maturation.
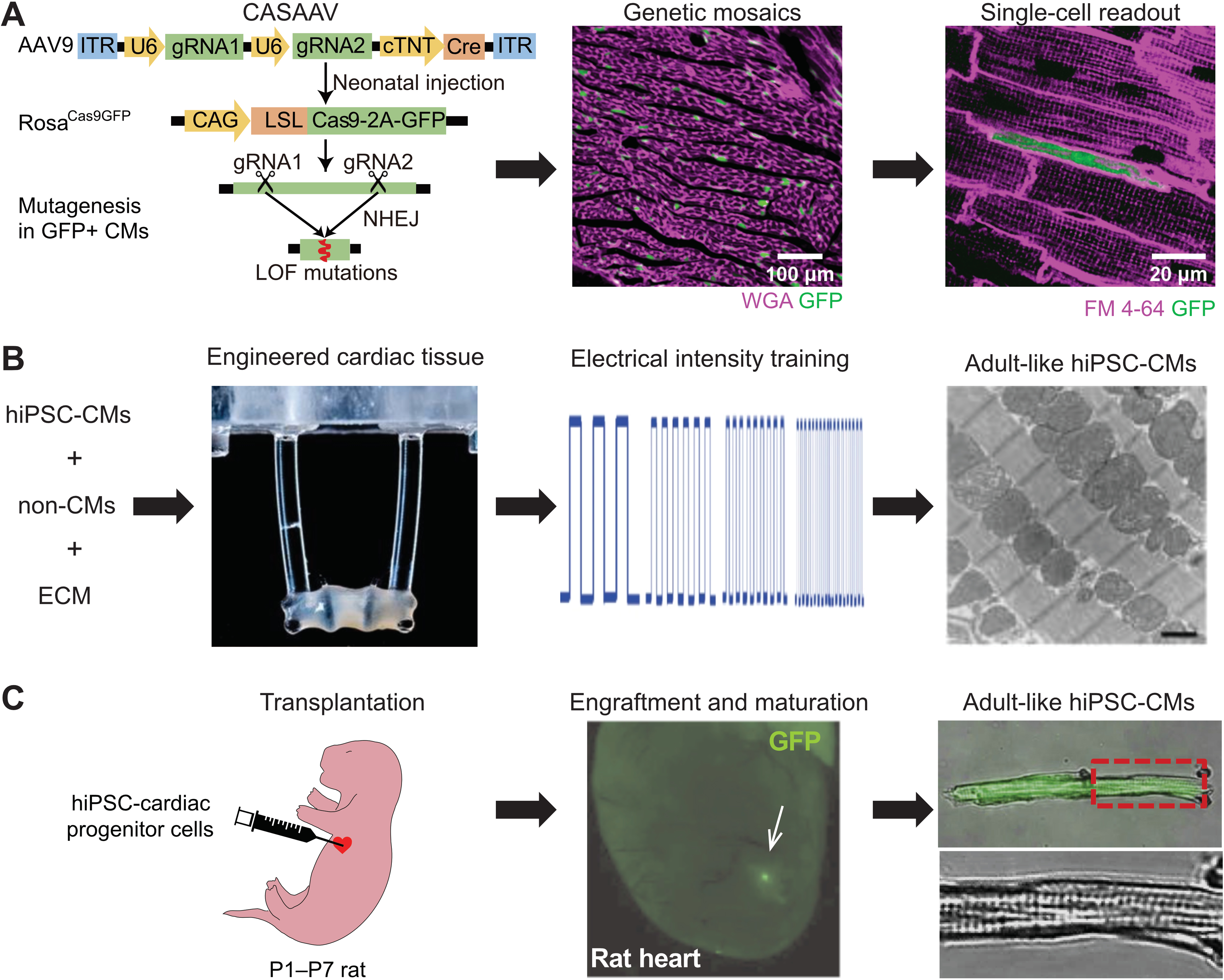
(A) CASAAV-based genetic mosaic analysis of murine CM maturation in vivo57. Expression of genome-encoded Cas9-P2A-GFP was activated by AAV-delivery of single or dual gRNAs and Cre, expressed from the cardiomyocyte-specific cTNT promoter (left). When the AAV is given at a low dose, mosaic transduction and Cas9-mediated somatic mutagenesis at genes targeted by gRNA(s) occurs (GFP+ cells, middle). The phenotype of single GFP+ cells is then analyzed (right, illustrating T-tubule and maturational growth defects caused by Srf depletion21). WGA, wheat germ agglutinin. FM 4–64, membrane dye. Left panel reprinted ref. 21. with permission. (B) In vitro maturation of PSC-CMs by tissue engineering and electrical pacing. 3D cultured engineered heart tissue was assembled from PSC-CMs (left). Elastomeric posts apply anisotropic stress on muscle bundle. Rapid electrical pacing protocol was applied from early in the PSC-CM differentiation process (middle), resulting in well organized, mature PSC-CMs, as evaluated by transmission electron microscopy (right). Reprinted from ref. 4 with permission. (C) In vivo maturation of PSC-CMs. Human PSC-CMs expressing GFP were injected into the hearts of immunodeficient neonatal rats (left). After several weeks, engrafted PSC-CMs (GFP+, middle) have mature morphology (right). Reprinted from ref.103 with permission.
To pinpoint the direct, cell-autonomous effects of gene manipulation, the dose of AAV is titrated so that a minority (e.g. <15%) of CMs are transduced, leaving most CMs, and the overall cardiac function, unaffected. Single-cell readouts on the transduced cells are used to deduce cell-autonomous gene function21, 22, 25, 57, 89. In genetic mosaics, mutant and control CMs are mixed in the same heart, thus analysis is limited to single cell readouts, or readouts compatible with a cell purification method such as flow cytometry. These analyses rely heavily on the ability to distinguish individual mutant and control cells, usually through immunostaining of the targeted proteins or introduction of fluorescent proteins as surrogate markers. Genetic mosaic approaches are most well suited to cell autonomous phenotypes and would difficult to apply to genes that produces secreted products.
4.b. Engineered tissue model
CM maturation demonstrates substantial interspecies differences. For instance, adult zebrafish CMs lack T-tubules196 and exhibit much lower mitochondrial content14 than mammalian CMs. Mouse and human CMs also exhibit several distinct maturation features, such as Myh6/7 isoform switching, contraction rates, and action potential profiles. Therefore, a human model is necessary to validate knowledge that was learnt in other model organisms.
In addition, a major practical goal of studying CM maturation is to improve the maturation of hPSC-CMs in vitro for translational medicine. The current consensus is that 3D engineered cardiac tissues that are assembled by hPSC-CMs, non-myocytes and ECMs provide the necessary platforms to best mature CMs in vitro. Additional biochemical (T3, Dex, IGF1, palmitate) and biophysical treatments (electrical pacing; mechanical stress) on these engineered tissues are essential to produce adult-like CMs (Fig. 4B, Table 2)3, 4. These technologies are useful to validate knowledge that is generated in animal models and to allow de novo discovery of CM maturation regulators. In vivo validation is still necessary to determine the physiological relevance of novel CM maturation factors that are identified in these tissue models. Importantly, factors that drive CM maturation in vitro may incompletely overlap with those that promote maturation in vivo during normal heart development.
Disease modeling is another application of these hPSC-CMs and engineered tissues. The immaturity of these cells is an important hurdle to disease modeling. Nevertheless these model systems have yielded important insights into disease mechanisms and led to new potential therapeutic strategies197. The properties of the model system, such as its electrical or metabolic maturity, should be considered with respect to the disease being studied. Key findings may require validation in alternative model systems that exhibit greater physiological maturity.
4.c. Neonatal xenotransplantation model
Human PSC-CMs could be matured toward a near-adult state by transplantation into rat myocardium (Fig. 4C)103, 198, which is a promising solution to the partial maturation defects observed in in vitro engineered tissue models. However, human PSC-CMs matured by this method exhibit more binucleation than normal human adult CMs103, raising the question of whether the transplanted human PSC-CMs become rat-like CMs or remain human-like. Although some comparisons between donor and host CMs were documented198, a more comprehensive analysis is necessary to determine if xenotransplants are viable models to study human-specific features of CM maturation.
5. Concluding Remarks
Here we reviewed major hallmarks of CM maturation and known regulators of this process. Although differences between immature and mature CMs have been well documented, the molecular mechanisms that mediate the change from immature to mature states remain incompletely understood. Accumulated evidence demonstrates interdependence between individual maturation events. Thus, research in this area should not only study individual hallmarks, but also how the maturation events are coordinated. With technical advances in model systems and increased collaboration between basic scientists with tissue engineers, a more comprehensive picture of CM maturation is warranted in the near future. This effort is critical to design better strategies to mature PSC-CM, stimulate CM regeneration, and treat diseases that involve CM maturation defects.
Acknowledgements
We thank Blake Jardin for assistance in figure preparation. We thank Nathan VanDusen, Maksymilian Prondzynski and Justin King for constructive comments on the manuscript.
Sources of Funding
This work was supported by NIH (R01HL146634 to W.T.P.) and the American Heart Association (postdoctoral fellowship 18POST33960037 to Y.G.), and charitable donations to the Boston Children’s Hospital Department of Cardiology.
Non-standard Abbreviations and Acronyms:
- CM
cardiomyocyte
- PSC
pluripotent stem cell
- hiPSC-CM
human induced PSC-derived CM
- TEM
transmission electron microscopy
- CICR
calcium induced calcium release
- SR
sarcoplasmic reticulum
- T-tubule
transverse tubule
- ECM
extracellular matrix
- ICD
intercalated disc
- EHT
engineered heart tissue
- CMT
cardiac microtissue
- CDK
cyclin-dependent kinase
- T3
triiodothyronine
- NR
nuclear receptor
- IGF
Insulin-like growth factor
- PPAR
peroxisome proliferator-activated receptor
- ERR
estrogen-related receptor
- CRISPR
clustered regularly interspaced short palindromic repeats
- miRNA
microRNA
- AAV
adeno-associated virus
- CASAAV
CRISPR/Cas9/AAV9-mediated somatic mutagenesis
Footnotes
Disclosures
None.
References
- 1.Tu C, Chao BS and Wu JC. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2018;123:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan S and Kwon C. Regulation of cardiomyocyte maturation during critical perinatal window. J Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CY, Maia-Joca RPM, Ong CS, Wilson I, DiSilvestre D, Tomaselli GF and Reich DH. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. Journal of Molecular and Cellular Cardiology. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M and Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X and Murry CE. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 2020: 10.1038/s41569-019-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scuderi GJ and Butcher J. Naturally Engineered Maturation of Cardiomyocytes. Front Cell Dev Biol. 2017;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H-P, Laflamme MA and Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jopling C, Sleep E, Raya M, Martí M, Raya A and Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DYR and Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP and Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN and Sadek HA. Transient Regenerative Potential of the Neonatal Mouse Heart. Science. 2011;331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL and Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012;26:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, Smith M, Gillett E, Muroy SE, Schmid T, Wilson E, Field KA, Reeder DM, Maden M, Yartsev MM, Wolfgang MJ, Grützner F, Scanlan TS, Szweda LI, Buffenstein R, Hu G, Flamant F, Olgin JE and Huang GN. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI and Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP and Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja P, Perriard E, Perriard J-C and Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci. 2004;117:3295–3306. [DOI] [PubMed] [Google Scholar]
- 17.O’Meara CC, Wamstad JA, Gladstone RA, Fomovsky GM, Butty VL, Shrikumar A, Gannon JB, Boyer LA and Lee RT. Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circ Res. 2015;116:804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monroe TO, Hill MC, Morikawa Y, Leach JP, Heallen T, Cao S, Krijger PHL, de Laat W, Wehrens XHT, Rodney GG and Martin JF. YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo. Dev Cell. 2019;48:765–779.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, Ali H, Braga L, Gorgodze N, Bernini F, Burchielli S, Collesi C, Zandonà L, Sinagra G, Piacenti M, Zacchigna S, Bussani R, Recchia FA and Giacca M. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yotti R, Seidman CE and Seidman JG. Advances in the Genetic Basis and Pathogenesis of Sarcomere Cardiomyopathies. Annu Rev Genomics Hum Genet. 2019;20:129–153. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Jardin BD, Zhou P, Sethi I, Akerberg BN, Toepfer CN, Ai Y, Li Y, Ma Q, Guatimosim S, Hu Y, Varuzhanyan G, VanDusen NJ, Zhang D, Chan DC, Yuan G-C, Seidman CE, Seidman JG and Pu WT. Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor. Nat Commun. 2018;9:3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Jardin BD, Sethi I, Ma Q, Moghadaszadeh B, Troiano EC, Trembley MA, Small EM, Yuan G-C, Beggs AH and Pu WT. Sarcomeres regulate cardiomyocyte maturation through MRTF-SRF signaling. bioRxiv. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gifford CA, Ranade SS, Samarakoon R, Salunga HT, de Soysa TY, Huang Y, Zhou P, Elfenbein A, Wyman SK, Bui YK, Cordes Metzler KR, Ursell P, Ivey KN and Srivastava D. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science. 2019;364:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robson A, Makova SZ, Barish S, Zaidi S, Mehta S, Drozd J, Jin SC, Gelb BD, Seidman CE, Chung WK, Lifton RP, Khokha MK and Brueckner M. Histone H2B monoubiquitination regulates heart development via epigenetic control of cilia motility. Proc Natl Acad Sci U S A. 2019;116:14049–14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDusen NJ, Lee JY, Gu W, Sethi I, Zheng Y, King JS, Zhou P-Z, Suo S, Guo Y, Ma Q, Yuan G-C and Pu WT. In vivo CRISPR screening identifies RNF20/40 as epigenetic regulators of cardiomyocyte maturation. bioRxiv. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CY, Peres Moreno Maia-Joca R, Ong CS, Wilson I, DiSilvestre D, Tomaselli GF and Reich DH. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J Mol Cell Cardiol. 2020;138:1–11. [DOI] [PubMed] [Google Scholar]
- 27.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M and Vunjak-Novakovic G. Author Correction: Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2019;572:E16–E17. [DOI] [PubMed] [Google Scholar]
- 28.Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME and Bursac N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun. 2017;8:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang Q-D, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER and Hudson JE. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 2017;114:E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M and Murry CE. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation. 2016;134:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A and Eschenhagen T. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014;74:151–61. [DOI] [PubMed] [Google Scholar]
- 32.Mannhardt I, Breckwoldt K, Letuffe-Breniere D, Schaaf S, Schulz H, Neuber C, Benzin A, Werner T, Eder A, Schulze T, Klampe B, Christ T, Hirt MN, Huebner N, Moretti A, Eschenhagen T and Hansen A. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Reports. 2016;7:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemoine MD, Mannhardt I, Breckwoldt K, Prondzynski M, Flenner F, Ulmer B, Hirt MN, Neuber C, Horvath A, Kloth B, Reichenspurner H, Willems S, Hansen A, Eschenhagen T and Christ T. Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci Rep. 2017;7:5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G and Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautel M and Djinovic-Carugo K. The sarcomeric cytoskeleton: from molecules to motion. J Exp Biol. 2016;219:135–45. [DOI] [PubMed] [Google Scholar]
- 36.Henderson CA, Gomez CG, Novak SM, Mi-Mi L and Gregorio CC. Overview of the Muscle Cytoskeleton. Compr Physiol. 2017;7:891–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarkova I and Perriard J-C. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. [DOI] [PubMed] [Google Scholar]
- 38.Reiser PJ, Portman MA, Ning XH and Schomisch Moravec C. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;280:H1814–20. [DOI] [PubMed] [Google Scholar]
- 39.Cui Y, Zheng Y, Liu X, Yan L, Fan X, Yong J, Hu Y, Dong J, Li Q, Wu X, Gao S, Li J, Wen L, Qiao J and Tang F. Single-Cell Transcriptome Analysis Maps the Developmental Track of the Human Heart. Cell Rep. 2019;26:1934–1950.e5. [DOI] [PubMed] [Google Scholar]
- 40.Selewa A, Dohn R, Eckart H, Lozano S, Xie B, Gauchat E, Elorbany R, Rhodes K, Burnett J, Gilad Y, Pott S and Basu A. Systematic Comparison of High-throughput Single-Cell and Single-Nucleus Transcriptomes during Cardiomyocyte Differentiation. bioRxiv. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E and Chien KR. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J Biol Chem. 1994;269:16961–70. [PubMed] [Google Scholar]
- 42.O’Brien TX, Lee KJ and Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci U S A. 1993;90:5157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedada FB, Chan SSK, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M and Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahmers S, Wu Y, Call DR, Labeit S and Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–513. [DOI] [PubMed] [Google Scholar]
- 45.Weeland CJ, van den Hoogenhof MM, Beqqali A and Creemers EE. Insights into alternative splicing of sarcomeric genes in the heart. J Mol Cell Cardiol. 2015;81:107–113. [DOI] [PubMed] [Google Scholar]
- 46.Liu A, Tang M, Xi J, Gao L, Zheng Y, Luo H, Hu X, Zhao F, Reppel M, Hescheler J and Liang H. Functional characterization of inward rectifier potassium ion channel in murine fetal ventricular cardiomyocytes. Cell Physiol Biochem. 2010;26:413–420. [DOI] [PubMed] [Google Scholar]
- 47.Goversen B, van der Heyden MAG, van Veen TAB and de Boer TP. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacol Ther. 2018;183:127–136. [DOI] [PubMed] [Google Scholar]
- 48.Yu L, Gao S, Nie L, Tang M, Huang W, Luo H, Hu X, Xi J, Zhu M, Zheng Y, Gao L, Zhang L, Song Y, Hescheler J and Liang H. Molecular and functional changes in voltage-gated Na⁺ channels in cardiomyocytes during mouse embryogenesis. Circ J. 2011;75:2071–2079. [DOI] [PubMed] [Google Scholar]
- 49.Haufe V, Camacho JA, Dumaine R, Günther B, Bollensdorff C, von Banchet GS, Benndorf K and Zimmer T. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu Y and Boutjdir M. Gene expression of SERCA2a and L- and T-type Ca channels during human heart development. Pediatr Res. 2001;50:569–574. [DOI] [PubMed] [Google Scholar]
- 51.Link S, Meissner M, Held B, Beck A, Weissgerber P, Freichel M and Flockerzi V. Diversity and Developmental Expression of L-type Calcium Channel β2 Proteins and Their Influence on Calcium Current in Murine Heart. Journal of Biological Chemistry. 2009;284:30129–30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parton RG, Way M, Zorzi N and Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol. 1997;136:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant SM, Kong CHT, Watson JJ, Gadeberg HC, Roth DM, Patel HH, Cannell MB, James AF and Orchard CH. Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular ICa density in mouse ventricular myocytes. American Journal of Physiology-Heart and Circulatory Physiology. 2018;315:H1101–H1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong T, Yang H, Zhang S-S, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marbán E, Jan LY and Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De La Mata A, Tajada S, O’Dwyer S, Matsumoto C, Dixon RE, Hariharan N, Moreno CM and Santana LF. BIN1 Induces the Formation of T-Tubules and Adult-Like Ca Release Units in Developing Cardiomyocytes. Stem Cells. 2019;37:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeshima H, Komazaki S, Nishi M, Iino M and Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y, VanDusen NJ, Zhang L, Gu W, Sethi I, Guatimosim S, Ma Q, Jardin BD, Ai Y, Zhang D, Chen B, Guo A, Yuan G-C, Song L-S and Pu WT. Analysis of Cardiac Myocyte Maturation Using CASAAV, a Platform for Rapid Dissection of Cardiac Myocyte Gene Function In Vivo. Circ Res. 2017;120:1874–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C, Spinozzi S, Chen J-Y, Fang X, Feng W, Perkins G, Cattaneo P, Guimarães-Camboa N, Dalton ND, Peterson KL, Wu T, Ouyang K, Fu X-D, Evans SM and Chen J. Nexilin Is a New Component of Junctional Membrane Complexes Required for Cardiac T-Tubule Formation. Circulation. 2019;140:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JJ, Yang L, Lin B, Zhu X, Sun B, Kaplan AD, Bett GCL, Rasmusson RL, London B and Salama G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J Mol Cell Cardiol. 2015;81:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neubauer S The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 61.Lopaschuk GD and Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–140. [DOI] [PubMed] [Google Scholar]
- 62.Schaper J, Meiser E and Stämmler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Liu Y and Dorn GW, 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC and Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song M, Franco A, Fleischer JA, Zhang L and Dorn GW 2nd. Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab. 2017;26:872–883.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, Tonkonogi M, Sahlin K, Kay L, Appaix F, Braun U, Eimre M and Saks VA. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;1504:379–395. [DOI] [PubMed] [Google Scholar]
- 67.Dai D-F, Danoviz ME, Wiczer B, Laflamme MA and Tian R. Mitochondrial Maturation in Human Pluripotent Stem Cell Derived Cardiomyocytes. Stem Cells Int. 2017;2017:5153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B and Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. [DOI] [PubMed] [Google Scholar]
- 69.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L and De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. [DOI] [PubMed] [Google Scholar]
- 70.Jans DC, Wurm CA, Riedel D, Wenzel D, Stagge F, Deckers M, Rehling P and Jakobs S. STED super-resolution microscopy reveals an array of MINOS clusters along human mitochondria. Proc Natl Acad Sci U S A. 2013;110:8936–8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikon N and Ryan RO. Cardiolipin and mitochondrial cristae organization. Biochim Biophys Acta Biomembr. 2017;1859:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiko C, Davies KM, Shinzawa-Itoh K, Tani K, Maeda S, Mills DJ, Tsukihara T, Fujiyoshi Y, Kühlbrandt W and Gerle C. Bovine F1Fo ATP synthase monomers bend the lipid bilayer in 2D membrane crystals. Elife. 2015;4:e06119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dorn GW 2nd, Vega RB and Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uosaki H, Cahan P, Lee DI, Wang S, Miyamoto M, Fernandez L, Kass DA and Kwon C. Transcriptional Landscape of Cardiomyocyte Maturation. Cell Rep. 2015;13:1705–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sim CB, Ziemann M, Kaspi A, Harikrishnan KN, Ooi J, Khurana I, Chang L, Hudson JE, El-Osta A and Porrello ER. Dynamic changes in the cardiac methylome during postnatal development. FASEB J. 2015;29:1329–1343. [DOI] [PubMed] [Google Scholar]
- 76.Fritz HL, Smoak IW and Branch S. Hexokinase I expression and activity in embryonic mouse heart during early and late organogenesis. Histochem Cell Biol. 1999;112:359–365. [DOI] [PubMed] [Google Scholar]
- 77.Calmettes G, John SA, Weiss JN and Ribalet B. Hexokinase-mitochondrial interactions regulate glucose metabolism differentially in adult and neonatal cardiac myocytes. J Gen Physiol. 2013;142:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, Kathiriya IS, Hinson JT, Homsy J, Gray J, Pu W, Bruneau BG, Seidman JG and Seidman CE. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev Cell. 2016;39:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, Vergnes L, Fu K, Morselli M, Dunham C, Ding X, Stieg AZ, Gimzewski JK, Pellegrini M, Clark PM, Reue K, Lusis AJ, Ribalet B, Kurdistani SK, Christofk H, Nakatsuji N and Nakano A. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H and Frisén J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–1575. [DOI] [PubMed] [Google Scholar]
- 81.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S and Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohamed TMA, Ang Y-S, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S and Srivastava D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018;173:104–116.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bersell K, Arab S, Haring B and Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. [DOI] [PubMed] [Google Scholar]
- 84.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL and Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morikawa Y, Heallen T, Leach J, Xiao Y and Martin JF. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. 2017;547:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JTT and Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, VanDusen N, Guo Y, Zhang J, Stevens SM, Liang F, Quan Q, van Gorp PR, Li A, Dos Remedios C, He A, Bezzerides VJ and Pu WT. Acetylation of VGLL4 Regulates Hippo-YAP Signaling and Postnatal Cardiac Growth. Dev Cell. 2016;39:466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang D, Li Y, Heims-Waldron DA, Bezzerides VJ, Guatimosim S, Guo Y, Gu F, Zhou P, Lin Z, Ma Q, Liu J, Wang D-Z and Pu WT. Mitochondrial Cardiomyopathy Caused by Elevated Reactive Oxygen Species and Impaired Cardiomyocyte Proliferation. Circ Res. 2017;122:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soonpaa MH, Kim KK, Pajak L, Franklin M and Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–9. [DOI] [PubMed] [Google Scholar]
- 91.Li F, Wang X, Capasso JM and Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–1746. [DOI] [PubMed] [Google Scholar]
- 92.Brodsky VY, Arefyeva AM, Gvasava IG, Sarkisov DS and Panova NW. Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Arch. 1994;424:429–435. [DOI] [PubMed] [Google Scholar]
- 93.Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR and Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J Mol Cell Cardiol. 1996;28:1463–1477. [DOI] [PubMed] [Google Scholar]
- 94.Patterson M, Barske L, Van Handel B, Rau C, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien C-L, Makita T, Lusis AJ, Kumar SR and Sucov HM. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49:1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H, Zhang C-H, Ammanamanchi N, Suresh S, Lewarchik C, Rao K, Uys GM, Han L, Abrial M, Yimlamai D, Ganapathy B, Guillermier C, Chen N, Khaladkar M, Spaethling J, Eberwine JH, Kim J, Walsh S, Choudhury S, Little K, Francis K, Sharma M, Viegas M, Bais A, Kostka D, Ding J, Bar-Joseph Z, Wu Y, Yechoor V, Moulik M, Johnson J, Weinberg J, Reyes-Múgica M, Steinhauser ML and Kühn B. Control of cytokinesis by β-adrenergic receptors indicates an approach for regulating cardiomyocyte endowment. Sci Transl Med. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.González-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE and Geoffrey Burns C. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Developmental Cell. 2018;44:433–446.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frawley LE and Orr-Weaver TL. Polyploidy. Curr Biol. 2015;25:R353–8. [DOI] [PubMed] [Google Scholar]
- 98.Vermij SH, Abriel H and van Veen TAB. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res. 2017;113:259–275. [DOI] [PubMed] [Google Scholar]
- 99.Epifantseva I and Shaw RM. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim Biophys Acta Biomembr. 2018;1860:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peter AK, Cheng H, Ross RS, Knowlton KU and Chen J. The costamere bridges sarcomeres to the sarcolemma in striated muscle. Prog Pediatr Cardiol. 2011;31:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chopra A, Kutys ML, Zhang K, Polacheck WJ, Sheng CC, Luu RJ, Eyckmans J, Hinson JT, Seidman JG, Seidman CE and Chen CS. Force Generation via β-Cardiac Myosin, Titin, and α-Actinin Drives Cardiac Sarcomere Assembly from Cell-Matrix Adhesions. Dev Cell. 2018;44:87–96.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA and Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol. 1993;265:H747–54. [DOI] [PubMed] [Google Scholar]
- 103.Cho G-S, Lee DI, Tampakakis E, Murphy S, Andersen P, Uosaki H, Chelko S, Chakir K, Hong I, Seo K, Chen H-SV, Chen X, Basso C, Houser SR, Tomaselli GF, O’Rourke B, Judge DP, Kass DA and Kwon C. Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy. Cell Rep. 2017;18:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP and Schocken DD. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. [DOI] [PubMed] [Google Scholar]
- 105.Heidi Au HT, Cui B, Chu ZE, Veres T and Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9:564–575. [DOI] [PubMed] [Google Scholar]
- 106.Kim D-H, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh K-Y, Tung L and Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang P-Y, Yu J, Lin J-H and Tsai W-B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7:3285–3293. [DOI] [PubMed] [Google Scholar]
- 108.Zimmermann WH, H. Zimmermann W, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL and Eschenhagen T. Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circulation Research. 2002;90:223–230. [DOI] [PubMed] [Google Scholar]
- 109.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB and Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhana B, Iyer RK, Chen WLK, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA and Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. [DOI] [PubMed] [Google Scholar]
- 111.Jacot JG, McCulloch AD and Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodriguez AG, Han SJ, Regnier M and Sniadecki NJ. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J. 2011;101:2455–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL and Palecek SP. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taber LA. Biomechanics of Cardiovascular Development. Annual Review of Biomedical Engineering. 2001;3:1–25. [DOI] [PubMed] [Google Scholar]
- 115.Mihic A, Li J, Miyagi Y, Gagliardi M, Li S-H, Zu J, Weisel RD, Keller G and Li R-K. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014;35:2798–2808. [DOI] [PubMed] [Google Scholar]
- 116.Abilez OJ, Tzatzalos E, Yang H, Zhao M-T, Jung G, Zöllner AM, Tiburcy M, Riegler J, Matsa E, Shukla P, Zhuge Y, Chour T, Chen VC, Burridge PW, Karakikes I, Kuhl E, Bernstein D, Couture LA, Gold JD, Zimmermann WH and Wu JC. Passive Stretch Induces Structural and Functional Maturation of Engineered Heart Muscle as Predicted by Computational Modeling. Stem Cells. 2018;36:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H and Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukuda R, Gunawan F, Ramadass R, Beisaw A, Konzer A, Mullapudi ST, Gentile A, Maischein H-M, Graumann J and Stainier DYR. Mechanical Forces Regulate Cardiomyocyte Myofilament Maturation via the VCL-SSH1-CFL Axis. Dev Cell. 2019;51:62–77.e5. [DOI] [PubMed] [Google Scholar]
- 119.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE and Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sathaye A, Bursac N, Sheehy S and Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J Mol Cell Cardiol. 2006;41:633–641. [DOI] [PubMed] [Google Scholar]
- 121.Martherus RSRM, Vanherle SJV, Timmer EDJ, Zeijlemaker VA, Broers JL, Smeets HJ, Geraedts JP and Ayoubi TAY. Electrical signals affect the cardiomyocyte transcriptome independently of contraction. Physiol Genomics. 2010;42A:283–289. [DOI] [PubMed] [Google Scholar]
- 122.Krüger M, Sachse C, Zimmermann WH, Eschenhagen T, Klede S and Linke WA. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/ AKT pathway. Circ Res. 2008;102:439–447. [DOI] [PubMed] [Google Scholar]
- 123.Haddad F, Jiang W, Bodell PW, Qin AX and Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee Y-K, Ng K-M, Chan Y-C, Lai W-H, Au K-W, Ho C-YJ, Wong L-Y, Lau C-P, Tse H-F and Siu C-W. Triiodothyronine Promotes Cardiac Differentiation and Maturation of Embryonic Stem Cells via the Classical Genomic Pathway. Molecular Endocrinology. 2010;24:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang X, Rodriguez M, Pabon L, Fischer KA, Reinecke H, Regnier M, Sniadecki NJ, Ruohola-Baker H and Murry CE. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J Mol Cell Cardiol. 2014;72:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM and Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL and Bergmann O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell. 2015;163:1026–36. [DOI] [PubMed] [Google Scholar]
- 128.Hirai M, Cattaneo P, Chen J and Evans SM. Revisiting Preadolescent Cardiomyocyte Proliferation in Mice. Circ Res. 2016;118:916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rog-Zielinska EA, Richardson RV, Denvir MA and Chapman KE. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J Mol Endocrinol. 2014;52:R125–35. [DOI] [PubMed] [Google Scholar]
- 130.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC and Chapman KE. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22:3269–3282. [DOI] [PubMed] [Google Scholar]
- 131.Winn N, Paul A, Musaró A and Rosenthal N. Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease. Cold Spring Harb Symp Quant Biol. 2002;67:507–518. [DOI] [PubMed] [Google Scholar]
- 132.Gluckman PD and Butler JH. Parturition-related changes in insulin-like growth factors-I and -II in the perinatal lamb. J Endocrinol. 1983;99:223–232. [DOI] [PubMed] [Google Scholar]
- 133.Daughaday WH, Parker KA, Borowsky S, Trivedi B and Kapadia M. Measurement of somatomedin-related peptides in fetal, neonatal, and maternal rat serum by insulin-like growth factor (IGF) I radioimmunoassay, IGF-II radioreceptor assay (RRA), and multiplication-stimulating activity RRA after acid-ethanol extraction. Endocrinology. 1982;110:575–581. [DOI] [PubMed] [Google Scholar]
- 134.McMullen JR, Shioi T, Huang W-Y, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM and Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J Biol Chem. 2004;279:4782–4793. [DOI] [PubMed] [Google Scholar]
- 135.Laustsen PG, Russell SJ, Cui L, Entingh-Pearsall A, Holzenberger M, Liao R and Kahn CR. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27:1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bougnères PF, Karl IE, Hillman LS and Bier DM. Lipid transport in the human newborn. Palmitate and glycerol turnover and the contribution of glycerol to neonatal hepatic glucose output. J Clin Invest. 1982;70:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, Ritterhoff J, Zhao L, Kolwicz SC Jr., Pabon L, Reinecke H, Sniadecki NJ, Tian R, Ruohola-Baker H, Xu H and Murry CE. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Reports. 2019;13:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Menendez-Montes I, Escobar B, Palacios B, Gómez MJ, Izquierdo-Garcia JL, Flores L, Jiménez-Borreguero LJ, Aragones J, Ruiz-Cabello J, Torres M and Martin-Puig S. Myocardial VHL-HIF Signaling Controls an Embryonic Metabolic Switch Essential for Cardiac Maturation. Dev Cell. 2016;39:724–739. [DOI] [PubMed] [Google Scholar]
- 139.Medley TL, Furtado M, Lam NT, Idrizi R, Williams D, Verma PJ, Costa M and Kaye DM. Effect of Oxygen on Cardiac Differentiation in Mouse iPS Cells: Role of Hypoxia Inducible Factor-1 and Wnt/Beta-Catenin Signaling. PLoS ONE. 2013;8:e80280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu D, Linders A, Yamak A, Correia C, Kijlstra JD, Garakani A, Xiao L, Milan DJ, van der Meer P, Serra M, Alves PM and Domian IJ. Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1α and LDHA. Circ Res. 2018;123:1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tønnessen T, Kryshtal DO, Louch WE and Knollmann BC. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2017;121:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou P and Pu WT. Recounting Cardiac Cellular Composition. Circ Res. 2016;118:368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA and Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim C, Majdi M, Xia P, Wei KA, Talantova M, Spiering S, Nelson B, Mercola M and Chen H-SV. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee DS, Chen J-H, Lundy DJ, Liu C-H, Hwang S-M, Pabon L, Shieh R-C, Chen C-C, Wu S-N, Yan Y-T, Lee S-T, Chiang P-M, Chien S, Murry CE and Hsieh PCH. Defined MicroRNAs Induce Aspects of Maturation in Mouse and Human Embryonic-Stem-Cell-Derived Cardiomyocytes. Cell Rep. 2015;12:1960–1967. [DOI] [PubMed] [Google Scholar]
- 146.Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K and Sawa Y. Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol Ther. 2018;26:2681–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Posern G and Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. [DOI] [PubMed] [Google Scholar]
- 148.Mokalled MH, Carroll KJ, Cenik BK, Chen B, Liu N, Olson EN and Bassel-Duby R. Myocardin-related transcription factors are required for cardiac development and function. Dev Biol. 2015;406:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang J, Min Lu M, Cheng L, Yuan L-J, Zhu X, Stout AL, Chen M, Li J and Parmacek MS. Myocardin is required for cardiomyocyte survival and maintenance of heart function. Proc Natl Acad Sci U S A. 2009;106:18734–18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mendez MG and Janmey PA. Transcription factor regulation by mechanical stress. The International Journal of Biochemistry & Cell Biology. 2012;44:728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Friedman CE, Nguyen Q, Lukowski SW, Helfer A, Chiu HS, Miklas J, Levy S, Suo S, Han J-DJ, Osteil P, Peng G, Jing N, Baillie GJ, Senabouth A, Christ AN, Bruxner TJ, Murry CE, Wong ES, Ding J, Wang Y, Hudson J, Ruohola-Baker H, Bar-Joseph Z, Tam PPL, Powell JE and Palpant NJ. Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation. Cell Stem Cell. 2018;23:586–598.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kook H, Lepore JJ, Gitler AD, Lu MM, Yung WW-M, Mackay J, Zhou R, Ferrari V, Gruber P and Epstein JA. Cardiac hypertrophy and histone deacetylase–dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest. 2003;112:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC and Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. [DOI] [PubMed] [Google Scholar]
- 154.Shin CH, Liu Z-P, Passier R, Zhang C-L, Wang D-Z, Harris TM, Yamagishi H, Richardson JA, Childs G and Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. [DOI] [PubMed] [Google Scholar]
- 155.Prendiville TW, Guo H, Lin Z, Zhou P, Stevens SM, He A, VanDusen N, Chen J, Zhong L, Wang D-Z, Gao G and Pu WT. Novel Roles of GATA4/6 in the Postnatal Heart Identified through Temporally Controlled, Cardiomyocyte-Specific Gene Inactivation by Adeno-Associated Virus Delivery of Cre Recombinase. PLoS One. 2015;10:e0128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iida K, Hidaka K, Takeuchi M, Nakayama M, Yutani C, Mukai T and Morisaki T. Expression of MEF2 genes during human cardiac development. Tohoku J Exp Med. 1999;187:15–23. [DOI] [PubMed] [Google Scholar]
- 157.Desjardins CA and Naya FJ. The Function of the MEF2 Family of Transcription Factors in Cardiac Development, Cardiogenomics, and Direct Reprogramming. J Cardiovasc Dev Dis. 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Desjardins CA and Naya FJ. Antagonistic regulation of cell-cycle and differentiation gene programs in neonatal cardiomyocytes by homologous MEF2 transcription factors. J Biol Chem. 2017;292:10613–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee W-S and Kim J. Peroxisome Proliferator-Activated Receptors and the Heart: Lessons from the Past and Future Directions. PPAR Res. 2015;2015:271983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc Res. 2007;73:269–277. [DOI] [PubMed] [Google Scholar]
- 161.Harmon GS, Lam MT and Glass CK. PPARs and Lipid Ligands in Inflammation and Metabolism. Chemical Reviews. 2011;111:6321–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M and Giguère V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. [DOI] [PubMed] [Google Scholar]
- 163.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, Evans RM, Patel VV and Pei L. Estrogen-related receptor α (ERRα) and ERRγ are essential coordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35:1281–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murphy MM Sean, Kervadec Anais, Kannan Suraj, Tampakakis Emmanouil, Kambhampati Sandeep, Leei Lin Brian, Paek Sam, Andersen Peter, Lee Dong-Ik, Zhu Renjun, An Steven S., Kass David A., Uosaki Hideki, Colas Alexandre R., and Kwon Chulan. PGC1/PPAR Drive Cardiomyocyte Maturation through Regulation of Yap1 and SF3B2. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gilsbach R, Preissl S, Grüning BA, Schnick T, Burger L, Benes V, Würch A, Bönisch U, Günther S, Backofen R, Fleischmann BK, Schübeler D and Hein L. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat Commun. 2014;5:5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kranzhöfer DK, Gilsbach R, Grüning BA, Backofen R, Nührenberg TG and Hein L. 5’-Hydroxymethylcytosine Precedes Loss of CpG Methylation in Enhancers and Genes Undergoing Activation in Cardiomyocyte Maturation. PLoS One. 2016;11:e0166575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ai S, Peng Y, Li C, Gu F, Yu X, Yue Y, Ma Q, Chen J, Lin Z, Zhou P, Xie H, Prendiville TW, Zheng W, Liu Y, Orkin SH, Wang D-Z, Yu J, Pu WT and He A. EED orchestration of heart maturation through interaction with HDACs is H3K27me3-independent. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thienpont B, Aronsen JM, Robinson EL, Okkenhaug H, Loche E, Ferrini A, Brien P, Alkass K, Tomasso A, Agrawal A, Bergmann O, Sjaastad I, Reik W and Roderick HL. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J Clin Invest. 2017;127:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Delgado-Olguín P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A and Bruneau BG. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat Genet. 2012;44:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou DC, Cahan P, Daley GQ, Kong SW, Orkin SH, Seidman CE, Seidman JG and Pu WT. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res. 2012;110:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Biermann M, Cai W, Lang D, Hermsen J, Profio L, Zhou Y, Czirok A, Isai DG, Napiwocki BN, Rodriguez AM, Brown ME, Woon MT, Shao A, Han T, Park D, Hacker TA, Crone WC, Burlingham WJ, Glukhov AV, Ge Y and Kamp TJ. Epigenetic Priming of Human Pluripotent Stem Cell-Derived Cardiac Progenitor Cells Accelerates Cardiomyocyte Maturation. Stem Cells. 2019;37:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quaife-Ryan GA, Sim CB, Ziemann M, Kaspi A, Rafehi H, Ramialison M, El-Osta A, Hudson JE and Porrello ER. Multicellular Transcriptional Analysis of Mammalian Heart Regeneration. Circulation. 2017;136:1123–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hang CT, Yang J, Han P, Cheng H-L, Shang C, Ashley E, Zhou B and Chang C-P. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gomez-Velazquez M, Badia-Careaga C, Lechuga-Vieco AV, Nieto-Arellano R, Tena JJ, Rollan I, Alvarez A, Torroja C, Caceres EF, Roy AR, Galjart N, Delgado-Olguin P, Sanchez-Cabo F, Enriquez JA, Gomez-Skarmeta JL and Manzanares M. CTCF counter-regulates cardiomyocyte development and maturation programs in the embryonic heart. PLoS Genet. 2017;13:e1006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Özcelik C, Saar K, Hubner N and Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 2012;18:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guo W, Zhu C, Yin Z, Wang Q, Sun M, Cao H and Greaser ML. Splicing Factor RBM20 Regulates Transcriptional Network of Titin Associated and Calcium Handling Genes in The Heart. Int J Biol Sci. 2018;14:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beraldi R, Li X, Martinez Fernandez A, Reyes S, Secreto F, Terzic A, Olson TM and Nelson TJ. Rbm20-deficient cardiogenesis reveals early disruption of RNA processing and sarcomere remodeling establishing a developmental etiology for dilated cardiomyopathy. Hum Mol Genet. 2014;23:3779–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bertero A, Fields PA, Ramani V, Bonora G, Yardimci GG, Reinecke H, Pabon L, Noble WS, Shendure J and Murry CE. Dynamics of genome reorganization during human cardiogenesis reveal an RBM20-dependent splicing factory. Nat Commun. 2019;10:1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang ET, Ward AJ, Cherone JM, Giudice J, Wang TT, Treacy DJ, Lambert NJ, Freese P, Saxena T, Cooper TA and Burge CB. Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins. Genome Res. 2015;25:858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB and Cooper TA. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci U S A. 2008;105:20333–20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Giudice J, Xia Z, Wang ET, Scavuzzo MA, Ward AJ, Kalsotra A, Wang W, Wehrens XHT, Burge CB, Li W and Cooper TA. Alternative splicing regulates vesicular trafficking genes in cardiomyocytes during postnatal heart development. Nat Commun. 2014;5:3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu X, Yang D, Ding J-H, Wang W, Chu P-H, Dalton ND, Wang H-Y, Bermingham JR Jr., Ye Z, Liu F, Rosenfeld MG, Manley JL, Ross J Jr., Chen J, Xiao R-P, Cheng H and Fu X-D. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. [DOI] [PubMed] [Google Scholar]
- 183.Ding J-H, Xu X, Yang D, Chu P-H, Dalton ND, Ye Z, Yeakley JM, Cheng H, Xiao R-P, Ross J, Chen J and Fu X-D. Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart. EMBO J. 2004;23:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feng Y, Valley MT, Lazar J, Yang AL, Bronson RT, Firestein S, Coetzee WA and Manley JL. SRp38 regulates alternative splicing and is required for Ca(2+) handling in the embryonic heart. Dev Cell. 2009;16:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ye J, Beetz N, O’Keeffe S, Tapia JC, Macpherson L, Chen WV, Bassel-Duby R, Olson EN and Maniatis T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc Natl Acad Sci U S A. 2015;112:E3020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao C, Ren S, Lee J-H, Qiu J, Chapski DJ, Rau CD, Zhou Y, Abdellatif M, Nakano A, Vondriska TM, Xiao X, Fu X-D, Chen J-N and Wang Y. RBFox1-mediated RNA splicing regulates cardiac hypertrophy and heart failure. J Clin Invest. 2016;126:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee JZ, Huang J, Rau C, Gao C, Yang Z, Wang H, Pushkarsky I, Parikh S, Di Carlo D, Knollmann B and Wang Y. Abstract 409: Regulation of Cardiomyocyte Maturation by an RNA Splicing Regulator Rbfox1. Circulation Research. 2019;125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fu J-D, Rushing SN, Lieu DK, Chan CW, Kong C-W, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ and Li RA. Distinct roles of microRNA-1 and −499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6:e27417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kuppusamy KT, Jones DC, Sperber H, Madan A, Fischer KA, Rodriguez ML, Pabon L, Zhu W-Z, Tulloch NL, Yang X, Sniadecki NJ, Laflamme MA, Ruzzo WL, Murry CE and Ruohola-Baker H. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:E2785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Miklas JW, Clark E, Levy S, Detraux D, Leonard A, Beussman K, Showalter MR, Smith AT, Hofsteen P, Yang X, Macadangdang J, Manninen T, Raftery D, Madan A, Suomalainen A, Kim D-H, Murry CE, Fiehn O, Sniadecki NJ, Wang Y and Ruohola-Baker H. TFPa/HADHA is required for fatty acid beta-oxidation and cardiolipin re-modeling in human cardiomyocytes. Nat Commun. 2019;10:4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cai W, Zhang J, De Lange WJ, Gregorich ZR, Karp H, Farrell ET, Mitchell ST, Tucholski T, Lin Z, Biermann M, McIlwain S, Ralphe JC, Kamp TJ and Ge Y. Unbiased Proteomics Method to Assess the Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Poon E, Keung W, Liang Y, Ramalingam R, Yan B, Zhang S, Chopra A, Moore J, Herren A, Lieu DK, Wong HS, Weng Z, Wong OT, Lam YW, Tomaselli GF, Chen C, Boheler KR and Li RA. Proteomic Analysis of Human Pluripotent Stem Cell-Derived, Fetal, and Adult Ventricular Cardiomyocytes Reveals Pathways Crucial for Cardiac Metabolism and Maturation. Circ Cardiovasc Genet. 2015;8:427–436. [DOI] [PubMed] [Google Scholar]
- 193.Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, Funcke S, Murphy E, Eschenhagen T and Hansen A. Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Reports. 2018;10:834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo Y and Pu WT. Genetic Mosaics for Greater Precision in Cardiovascular Research. Circ Res. 2018;123:27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.VanDusen NJ, Guo Y, Gu W and Pu WT. CASAAV: A CRISPR‐Based Platform for Rapid Dissection of Gene Function In Vivo. Curr Protoc Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brette F, Luxan G, Cros C, Dixey H, Wilson C and Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun. 2008;374:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van den Brink L, Grandela C, Mummery CL and Davis RP. Inherited cardiac diseases, pluripotent stem cells, and genome editing combined-the past, present, and future. Stem Cells. 2020;38:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kadota S, Pabon L, Reinecke H and Murry CE. In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts. Stem Cell Reports. 2017;8:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]


