Abstract
Differences in avian eggshell pigmentation could be an honest signal of female quality that males use to inform their nestling provisioning effort. We investigated whether among-individual variation in protoporphyrin-based eggshell pigmentation in house wrens (Troglodytes aedon) reflects female fitness-associated traits and whether males use that information. Females laying lighter clutches were older and larger than females laying darker clutches. Nestlings hatching from lighter clutches had greater size-corrected mass on post-hatch day 11, a measure that strongly predicts survival and recruitment to the breeding population. To test whether male provisioning effort responds to clutch pigmentation, we used a reciprocal clutch cross-fostering design, swapping dark with light clutches and light with dark; in controls, we swapped light with light clutches and dark with dark. Shortly before hatching, clutches were returned to their original nest to avoid confounding effects of nestling quality on male provisioning. Contrary to the sexual selection hypothesis, clutch pigmentation had no effect on male provisioning. Males were probably able to observe eggshell pigmentation and thus had information about female quality, but they did not use this information to modulate their nestling provisioning. This may be because of constraints on species-specific reproductive opportunities, or because variation in eggshell protoporphyrin serves other functions.
Keywords: house wren, maculation, oxidative stress, protoporphyrin, sexual selection, Troglodytes aedon
Introduction
Avian eggshell coloration varies extensively among species, ranging from white to a broad spectrum of browns, blues or greens. In addition to these base colours, eggshells may show a wide gamut of maculation (i.e. spotting, speculation) patterns and variable amounts of ultraviolet reflection (Hauber, 2014). This diversity of pigmentation has long been an area of investigation (Gosler et al., 2005; Higham & Gosler, 2006; Kilner, 2006; Reynolds et al., 2009; Cherry & Gosler, 2010) that has focused primarily on two questions: (1) what are the proximate mechanisms by which eggshells derive their pigmentation, and (2) what is the adaptive significance, if any, of variation in eggshell pigmentation?
With regard to the first question, the majority of all eggshell coloration comes from just two pigments: biliverdin and protoporphyrin IX (hereafter protoporphyrin) (Mikšík et al., 1996; Moreno & Osorno, 2003; Gosler et al., 2005; Cassey et al., 2012). Biliverdin is responsible for blue-to-green hues, and protoporphyrin for browns, reds and pinks. Both pigments occur in the blood and body fluids of vertebrates because they are involved in the creation and destruction of red blood cells (London et al., 1949; Grinstein et al., 1959). Protoporphyrin plays a major role as an intermediary in haem synthesis in the production of haemoglobin (London et al., 1949; Grinstein et al., 1959) and is a strong pro-oxidant, reacting with oxygen and light to generate free radicals (Afonso et al., 1999). When levels of protoporphyrin rise, free radicals increase and the level of oxidative stress increases (Afonso et al., 1999). Excess protoporphyrin is removed by the liver for excretion via the intestines (Casini et al., 2001). Deposition of protoporphyrin to an eggshell takes place in the shell gland at the end of egg formation when epithelial cells deposit pigments on the layers of the shell (Board & Sparks, 1991; Brulez et al., 2015). How the pigments arrive at the shell gland is not well understood; they may be filtered from circulating blood and fluid or synthesized within the shell gland itself, or even some combination of both (Kennedy & Vevers, 1973; Lang & Wells, 1987; Samiullah et al., 2015; Hargitai et al., 2017a).
Empirical work addressing the adaptive significance of inter- and intraspecific differences in eggshell pigmentation has been a long-running, ongoing effort (Underwood & Sealy, 2002; Moreno & Osorno, 2003; Reynolds et al., 2009; Hanley et al., 2010; Dearborn et al., 2012). Interspecifically, it is clear that in at least some open-nesting species, eggshell pigmentation camouflages eggs against egg predators (Weidinger, 2001; Westmoreland & Kiltie, 2007; Duval et al., 2016), whereas in some brood-parasitized species it makes it possible for the host to distinguish its eggs from those of the parasite (Kilner, 2006). However, such explanations, as well as others, for interspecific differences in eggshell pigmentation are unlikely to explain the widespread occurrence of significant intraspecific variation in pigmentation.
Extensive variation among clutches produced by females of the same species and, even, by females in the same population can be dramatic (Bischoff & Murphy, 1993; Lahti & Lahti, 2002; Griffith et al., 2009), as shown by variation in protoporphyrin-based eggshell pigmentation in our study population of house wrens (Troglodytes aedon, Vieillot 1809) (Fig. 1). Several hypotheses have been proposed to explain such intraspecific variation (summarized in Holveck et al., 2019), including the possibility that protoporphyrin functions as a sexually selected, honest signal of female quality (Moreno & Osorno, 2003). The sexually selected hypothesis proposes that eggshell pigmentation (both biliverdin and protoporphyrin) provides an honest signal of female quality, and hence fitness, with the amount of pigment providing an indication of the level of oxidative stress, i.e. excessive production of reactive oxygen species (Sies, 1997), to which a laying female is subject (Moreno & Osorno, 2003). As oxidative stress is damaging to a wide array of physiological functions (Finkel & Holbrook, 2000), selection for the ability to withstand this stress should be strong (Dowling & Simmons, 2009), and the quantity of protoporphyrin in eggshells may reflect the physiological condition and oxidative balance of females (Moreno & Osorno, 2003; De Coster et al., 2013). If differences in eggshell pigmentation can be used to discriminate between females and their resultant offspring with high and low resistance to oxidative stress, then male birds could use this information to modify their parental investment to maximize their own reproductive success (Trivers, 1972; Moreno & Osorno, 2003; but see Reynolds et al., 2009).
Figure 1.
House wren (Troglodytes aedon) eggs from different nests produced on the study area in 2017.
Regardless of the direction of any relationship between eggshell pigmentation and female health, the sexual selection hypothesis proposes that the birds themselves could be paying attention to eggshell appearance (Moreno & Osorno, 2003; Moreno et al., 2006b). Male birds, in particular those that make a major investment in provisioning their offspring, may be selected to evaluate female traits, including eggshell pigmentation, and adjust their subsequent reproductive effort to maximize their own fitness (Burley, 1986; Jones et al., 2001; Edwards & Chapman, 2011). The evidence that males do use such information is, at best, mixed.
Most studies investigating eggshell coloration, maternal condition and paternal investment have been carried out on species with blue–green eggshells (e.g. Moreno et al., 2006b; Hanley et al., 2008; English & Montgomerie, 2011; Johnsen et al., 2011; Fronstin et al., 2016). Among the exceptions that have considered protoporphyrin-based eggshell coloration is a reciprocal cross-fostering experiment in which heavier (and probably in better condition) female great tits (Parus major) laid paler, less-speckled eggs but their mates did not alter their provisioning in relation to the extent of egg speculation (Stoddard et al., 2012). Likewise, in northern lapwings (Vanellus vanellus), there was no connection between clutch maculation and male investment in the form of male incubation (Bulla et al., 2012). However, Poláček et al. (2017) found that although protoporphyrin content was not related to measures of female health or condition, it was positively correlated with egg volume and nestling mass and male provisioning in tree sparrows (Passer montanus). In house wrens, female provisioning rate was positively related to foster-egg brightness, but male provisioning rate was unrelated to foster-egg colour, although the design of this experiment did not account for the possibly confounding influence of nestling quality (Walters & Getty, 2010). In contrast to this result, in a later experiment, male house wrens increased their provisioning of nestlings in nests to which there earlier had been added an artificially light egg compared with those receiving a dark egg (Walters et al., 2014). The results of studies on the relationship between eggshell protoporphyrin pigmentation and female condition and male provisioning are limited and conflicting, and therefore there is clearly a need for further investigation.
In this study we aimed to investigate the potential for differences in protoporphyrin-based pigmentation in the eggshells of house wrens to serve as an honest signal of female quality to which males respond. We examined whether the extensive among-individual variation in egg pigmentation in the study population (Fig. 1) reflects differences among females in traits related to fitness, a critical assumption of the sexually selected hypothesis. We used a reciprocal clutch cross-fostering design to test the hypothesis that males adjust their parental investment, namely nestling provisioning effort, based on variation in egg pigmentation. A reciprocal, cross-fostering design was used because we were concerned that a simple cross-fostering experimental design would not identify the cause of any observed differences in provisioning. If we did a single swap and detected an effect on male provisioning behaviour, we would not be able to determine whether this was based on a pre-hatch assessment of eggshell pigmentation or a post-hatch assessment of nestling quality or behaviour, or both (Riehl, 2011; Stoddard et al., 2012). Furthermore, nestlings may advertise aspects of their fitness in various forms, such as begging (Kilner, 2002; Sacchi et al., 2002), and parents may use such advertisements to influence their investment (Leonard & Horn, 2001; MacGregor & Cockburn, 2002; Bowers et al., 2019), although this is not always the case (Barnett et al., 2011). To decouple ‘expected’ nestling quality (based on eggshell pigmentation) from ‘actual’ nestling quality (possibly conveyed through begging or another nestling feature), we included a second swap (returning eggs and, hence, eventually nestlings to their biological parents) in our cross-fostering design.
MATERIAL AND METHODS
Study site and species
The experiment took place on the Mackinaw study area in McLean County, Illinois (40.665°N, 88.89°W), USA, in 2017. This 130-ha site has 700 nestboxes spaced 30 m apart in north–south rows 60 m apart, 5.4 boxes/ha. Each box is mounted on a 1.5-m metal pole above a 43.8-cm aluminium disc that serves as a predator deterrent. Details regarding nestbox design and construction materials are given in Lambrechts et al. (2010).
House wrens are small (10–12 g), insectivorous songbirds that readily accept nestboxes in lieu of natural cavities for nesting. House wrens in the central Illinois study population are migratory, arriving on the study area in late April to early May and leaving in September. They typically produce two broods in each breeding season, the first in May with clutches of 6–8 eggs and the second in late June to early July with 5–7 eggs. Females lay one egg per day until the clutch is completed; they then incubate the eggs, which hatch after ~12 days, and brood young nestlings through the first two-thirds of the nestling period. Both males and females provision the nestlings after hatching. Asymptotic nestling body condition (size-adjusted mass) is positively related to longevity (Bowers et al., 2014a), and male provisioning effort on brood-day 4 or 5 (4–5 days after the first nestling hatched on brood-day 0) is positively correlated with the growth, survival and recruitment of nestlings to subsequent breeding populations (Bowers et al., 2014b). Nestlings fledge 15–18 days after hatching. Johnson (2014) provides additional information on house wren biology.
Field procedures
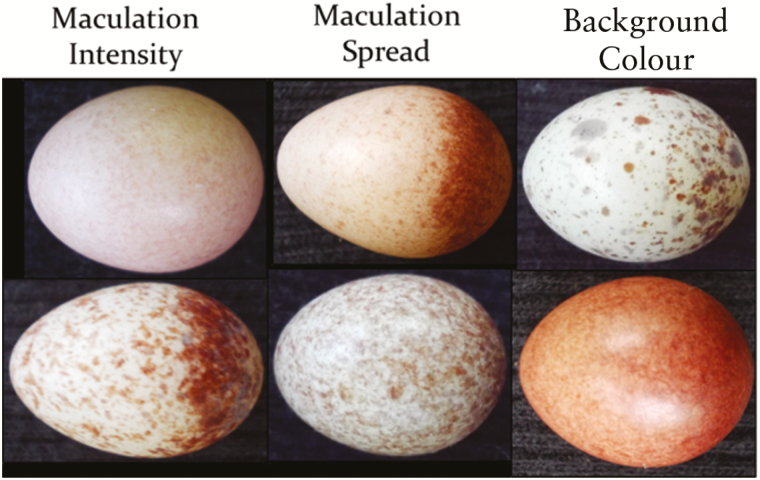
We used nests from the early-season broods (May–early June) and began monitoring nestboxes for signs of occupation in early May. Once egg-laying began, we visited the boxes daily to check for the onset of incubation and to sort clutches visually into ‘light’ and ‘dark’ categories using Gosler indices (Gosler et al., 2000, 2005). A single observer (K.E.H.) assigned a score to each egg of a clutch based on three characteristics: the darkness of the maculations, the distribution of maculations and the darkness of the eggshell background. Each characteristic was scored on a scale of 0–5 in 0.5 unit increments, with higher scores indicating increased darkness or increased spread of maculation (Fig. 2). The combined score of each characteristic formed the scores for each individual egg, which were averaged for the entire clutch. A midway point of 9.5 was chosen as the cut-off point, with clutch scores <9.5 considered light and scores ≥9.5 considered dark.
Figure 2.
Each egg in each clutch was digitally photographed after being visually scored based on three parameters: maculation intensity (darkness), maculation spread and background pigmentation darkness. Scores for each parameter ranged from 0, representing the lower extreme (very pale maculations, very concentrated maculation spread, very pale eggshell background), to 5, representing the upper extreme (very dark maculations, dispersed maculations, very dark eggshell background). The scores for each category were added to create a total score for each egg, which was then averaged over the clutch. Higher scores denote more-pigmented or darker clutches; lower scores indicate less-pigmented or lighter clutches.
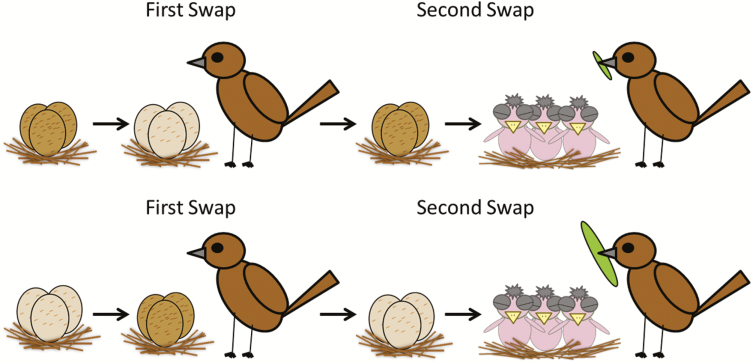
To assess the effects of clutch pigmentation on paternal provisioning, we used a reciprocal cross-fostering design (Fig. 3). Once assigned to a category, all the eggs in a clutch were swapped 2 days after incubation began with all the eggs in a second clutch in the same or opposite category. Eggs from the second nest were taken to the first nest to replace the artificial eggs left there earlier to keep females from abandoning an empty nest; females readily accepted and incubated these plastic eggs, which fall within the range of size and mass of natural house wren eggs. Cross-fostered clutches were left in their new nests for 10 days to give males adequate exposure to them, after which they were returned to their original nests 2 days before their predicted hatching for their biological parents to rear (Fig. 3). For transport on foot between nests, eggs were placed in a padded cardboard egg carton. Clutches to be swapped were chosen based on similar laying date (no more than 1-day difference in clutch-initiation date), clutch size (no more than one egg difference) and distance (shorter travel times to minimize cooling during transport). This design created two experimental groups, light–dark clutch swaps (females producing light eggs incubating dark eggs) and dark–light swaps (females producing dark eggs incubating light eggs) and two control groups, dark–dark and light–light clutch swaps.
Figure 3.
Schematic of clutch cross-fostering design used to test whether male provisioning responds to clutch pigmentation. In experimental replicates, dark clutches were swapped with light clutches 2 days into incubation, whereas in control replicates, light clutches were swapped with light or dark clutches with dark. Foster eggs remained in the nest for 10 days to allow the male ample opportunity to observe and evaluate them. At an estimated 2 days before hatching, clutches were returned to their original nest to avoid the confounding effect of nestling quality on male provisioning effort.
To evaluate male and female provisioning effort, we placed a dummy camera in a mobile-phone holster mounted on a 1.5-m pole ~1 m from the entrance of each nestbox ~24 h before recording began. On brood-day 4 or 5, the dummy camera was replaced with a real camera (Kodak Zx1 or Zx5 digital camera, Eastman Kodak Company, Rochester, NY, USA) and ~1.5-h recordings made between 06:00 and 09:00 h Central Daylight Time, which ensured that we obtained 60 min of undisturbed provisioning behaviour that began after the parents resumed normal activity (typically <5 min). Studies of three different species have demonstrated that 60 min provides an accurate estimate of provisioning rates (Pagani-Núñez & Senar, 2013; Lendvai et al., 2015; Murphy et al., 2015). Furthermore, the amount of food delivered by parents at this age based on this 1-h sampling interval is positively predictive of nestling growth, fledging success and the recruitment of offspring to the breeding population (Bowers et al., 2014a, 2015). Provisioning behaviour was scored blind to the treatment by one person (C.F.T.), who recorded the number of provisioning trips by both males and females. Not all nests survived to the end of the experiment, so if a swap partner was depredated before eggs could be returned to their natal nest, they were left with their foster parents. A total of 53 swaps yielded offspring that survived to fledging (13 light–light, 14 light–dark, nine dark–dark, 17 dark–light), of which provisioning was recorded at 37 nests (eight light–light, 12 light–dark, six dark–dark, 11 dark–light).
We measured body size (tarsus length measured to the nearest 0.1 mm with dial callipers) and mass [weighed to the nearest 0.1 g on a Pocket Pro PP201 or PP250B balance (Acculab, Edgewood, NY, USA), or an AC-100 balance (American Weigh Scales, Cumming, GA, USA)] of all adults and nestlings. Males and females were caught during incubation and fitted with a numbered, aluminium US Geological Survey ring; males were given additional three-coloured Darvic rings (Biodiversity Research Institute, Portland, ME, USA) (two rings per leg) to facilitate identification during provisioning. Nestlings were ringed on brood-day 11, weighed and measured. A small (~40 µL) sample of blood, taken from the brachial vein of adults and nestlings, was stored on dry ice in the field and upon return to the laboratory was transferred to a −80 °C freezer and stored for a different study. Nests were then monitored daily for fledging success. We ringed and collected a blood sample from 94 females and 315 nestlings.
Because visual scoring of egg pigmentation is not always repeatable (Stoddard et al., 2012; Brulez et al., 2014; Wegmann et al., 2015), we also took digital photographs of each egg before swapping. We photographed 653 eggs from 97 nests in the field on a standard black background from ~15 cm away (lighting conditions varied, but efforts were made to take all photos in moderate shade). These photos were later used to verify the visual classifications (see next section).
Photo processing and machine learning
To address the issue of poor repeatability of visual scoring of pigmentation, we sought to verify the scoring system using computational methods. Using the software program ImageMagick (v.7.0.7-22, http://www.imagemagick.org), digital photographs of eggs were centred, and a 10% crop was applied resulting in a central strip running pole to pole to represent the entirety of the egg. These strips were further subdivided into five equal regions for evaluation of variation across each egg from pole to pole. A common threshold was applied to each image to differentiate maculation from background and to calculate percentage maculation. Numerical data were individually extracted from the strips and strip subregions, and from the maculation and background regions of each strip, giving a total of 17 parameters (see Table 1). Darkness parameters were extracted from grayscale-transformed red–green–blue (RGB) images to represent achromatic measures, and the images were split into separate RGB channels to extract colour parameters. The repeatability of a subset of these characters within clutches was calculated following Lessells & Boag (1987), which revealed that different dimensions of eggshell pigmentation are significantly repeatable within clutches (Supporting Information, Table S1).
Table 1.
The 17 variables extracted from digital photographs of eggs
| Parameter | Description |
|---|---|
| Mean Darkness | Mean pixel darkness value from the whole egg slice |
| Minimum Darkness | Minimum pixel darkness value from the whole egg slice |
| Maximum Darkness | Maximum pixel darkness value from the whole egg slice |
| Standard Deviation | Standard deviation of pixel darkness from the whole egg slice |
| Red | Mean red channel pixel value of whole egg slice |
| Green | Mean green channel pixel value of whole egg slice |
| Blue | Mean blue channel pixel value of whole egg slice |
| Coefficient of Variation | Coefficient of variation among regions of egg slice |
| % Pigmented | Per cent of whole egg slice covered by darker maculations |
| Unpigmented Mean Darkness | Mean pixel darkness value from the lighter background area of the whole egg slice |
| Unpigmented Red | Mean red channel value from the lighter background area of the whole egg slice |
| Unpigmented Green | Mean green channel value from the lighter background area of the whole egg slice |
| Unpigmented Blue | Mean blue channel value from the lighter background area of the whole egg slice |
| Pigmented Mean Darkness | Mean pixel darkness value from the maculations of the whole egg slice |
| Pigmented Red | Mean red channel value from the maculations of the whole egg slice |
| Pigmented Green | Mean green channel value from the maculations of the whole egg slice |
| Pigmented Blue | Mean blue channel value from the maculations of the whole egg slice |
To classify each egg as dark or light, we used an unsupervised machine learning approach to cluster our samples employing all of the image data as input features. We determined the hierarchical relationships among the samples using a bottom-up agglomerative clustering approach (AGNES; implemented in the ‘cluster’ package in R; Maechler et al., 2019). Clusters were calculated using Ward’s minimum variance method to create a dendrogram (‘factoextra’ package in R; Kassambara & Mundt, 2017). To determine the number of clusters to use in our new classification, we used a silhouette plot, which revealed that three clusters (representing light-, intermediate- and dark-pigmentation categories) were better supported than our original, visually based two. Thus, we re-classified the clutches into one of three categories, going from the darkest eggs in cluster 1 to the lightest eggs in cluster 3.
To independently validate and aid in interpretation of the clusters identified by machine learning, we performed a principal components analysis (PCA) of four egg parameters that we had identified a priori as probably best capturing the different dimensions of Gosler scores: percentage maculation (percentage of the slice covered by pigment), mean darkness (an achromatic measure of the average darkness of all pixels across the slice), red value and coefficient of variation in pigmentation darkness across the five regions. The PCA only returned one PC with an Eigenvalue greater than 1 and which explained 65% of all the variation in the four original variables (Supporting Information, Table S2). Based on the loadings of the original variables on PC1, PC1 scores can be interpreted as follows: high PC1 scores indicate lighter eggs, with less pigment coverage, higher red values and reduced variation across the slice, whereas low PC1 scores indicate darker eggs, with greater pigment coverage, browner eggs and increased variation across the slice. We then used a mixed-model ANOVA with PC1 score as the dependent variable, AGNES cluster classification as the main effect and nest as the random effect to evaluate how closely AGNES cluster predicts pigmentation darkness as assessed using PCA. AGNES cluster explained a significant proportion of the variation in PC1 scores (F2,554 = 261.1, P < 0.0001), and least-squares mean PC1 scores (±SE) aligned closely with our machine-learning cluster classifications [cluster 1 (dark): −0.93 ± 0.09; cluster 2 (intermediate): 0.45 ± 0.12; cluster 3 (light): 1.44 ± 0.10].
We performed all statistical analyses using the median AGNES cluster score of a clutch as a categorical variable representing the degree of whole-clutch pigmentation, except in one analysis where we used the median PC1 score as a dependent variable to evaluate the effect of female age and size on natal-clutch pigmentation.
Additional statistical analyses
We used SAS statistical software (v.9.4; SAS Institute, Cary, NC, USA) and all tests were two-tailed (α = 0.05). We used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. Where appropriate, we included nest as a random effect to account for the statistical non-independence of observations within the same nest.
We used a generalized linear model with a negative binomial response and log link function (PROC GLIMMIX) to assess the effect of both natal- and foster-clutch pigmentation on subsequent provisioning by males on brood-day 4, with brood size and female provisioning rate (number of female feeds per hour) included as covariates. Two replicates were excluded from this analysis because of missing values for either natal- or foster-clutch pigmentation.
We similarly used generalized linear models to evaluate the effects of natal-clutch pigmentation on various components of the within-season reproductive success of females, including clutch size, hatching success and the number of fledglings produced. We initially included hatching date (i.e. time of season) as a covariate in these analyses, but after finding it had no influence, omitted it from the final models. To determine the effect of natal-clutch pigmentation on the propensity of females to produce a second brood later in the breeding season, we used a generalized linear mixed model (PROC GLIMMIX) with a binary response (produced a second brood or did not), with pigmentation category as the main effect, nest as a random effect and hatching date as a covariate.
To assess the effects of female size and age on egg pigmentation and maculation, we used the median PC1 score derived for each clutch as the dependent variable, female age as the main effect (1 or 2 years old) and tarsus length as a covariate.
To examine the effect of natal-clutch pigmentation on the size-adjusted mass of nestlings, we used a linear mixed model with natal-clutch pigmentation and foster-clutch pigmentation as main effects, tarsus length as a covariate and nest as a random effect. We included foster-clutch pigmentation based on our prediction that this would influence parental provisioning, and that this post-natal effect could, in turn, affect the condition of the offspring.
To determine the effect of natal-clutch pigmentation on female return rates the following breeding season, we used a generalized linear mixed model (PROC GLIMMIX) with a binary response (returned or did not), with pigmentation as the main effect and nest as a random effect. Similarly, to assess the effect of natal-clutch pigmentation on recruitment of offspring to the breeding population, we used a generalized linear mixed model with a binary response (recruited or did not), with nest as a random effect.
RESULTS
Effects on within-season reproductive success
There were no differences in clutch size in relation to the pigmentation category of the natal clutch (F2,47 = 0.59, P = 0.56), although structurally larger females (as reflected by tarsus length) produced significantly larger clutches (parameter estimate ± SE 0.38 ± 0.18; F2,47 = 2.09, P = 0.042). The mean clutch size of all females was 6.88 ± 0.1 SE eggs. There were no differences in hatching success in relation to the pigmentation category of the natal clutch (F2,47 = 0.60, P = 0.55), and hatching success exceeded 86% in all three groups. There were also no differences among pigmentation categories in the total number of fledglings produced (F2,48 = 0.22, P = 0.80). The likelihood of a female producing a second brood later in the breeding season was unrelated to the pigmentation of her first clutch (F2,46.51 = 0.18, P = 0.84), but females producing their first brood earlier in the season showed a higher tendency to produce a second brood (parameter estimate ± SE −0.096 ± 0.049; F1,47 = 3.79, P = 0.0574).
Egg pigmentation and maculation in relation to female size and age
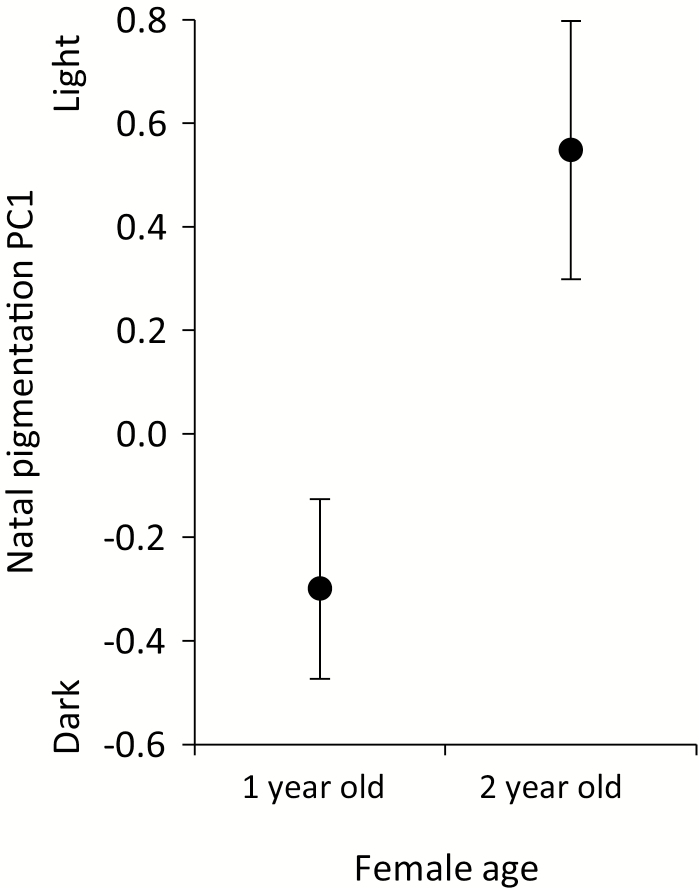
There was a significant effect of female age (i.e. year of ringing) on natal-clutch pigmentation (Fig. 4; F1,48 = 7.37, P = 0.0092). Older females laid significantly lighter, less-maculated eggs than younger females, which produced darker, more-maculated eggs; the effect size, r (calculated after Nakagawa & Cuthill, 2007), was 0.36 (0.10–0.58) (± 95% confidence interval), considered a medium effect. The effect of structural body size (i.e. tarsus length) was nearly significant (F1,48 = 3.35, P = 0. 0733). The results (not shown) were qualitatively the same when we included female body mass in the model (i.e. age remained statistically significant and tarsus length, nearly so), but we opted for the simpler model because of missing values for body mass for three females.
Figure 4.
Natal eggshell pigmentation in relation to female age.
Effects on offspring
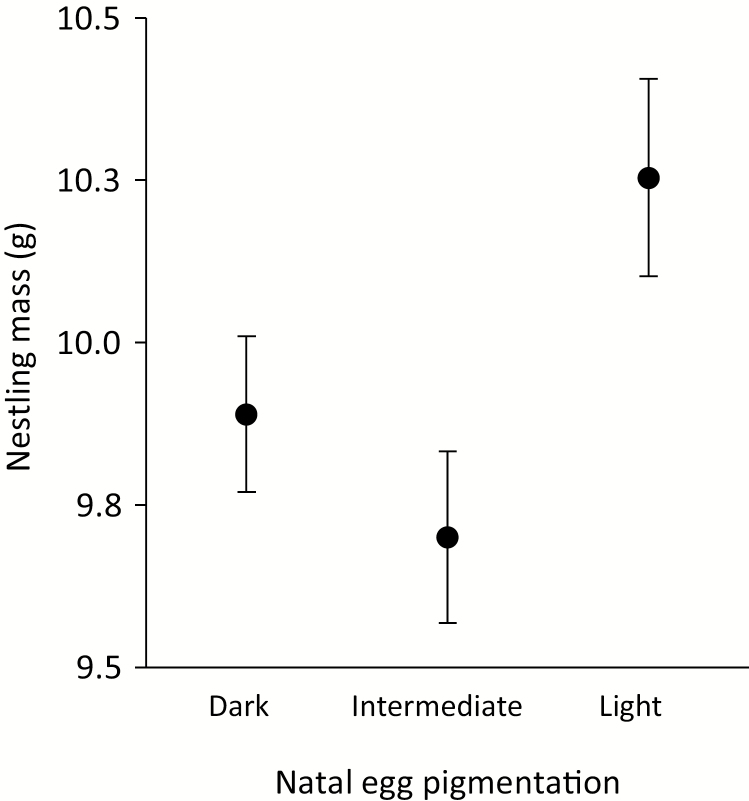
There was a significant effect of natal-clutch pigmentation on size-adjusted nestling mass on brood-day 11, but there was no influence of the pigmentation of the foster clutch (Table 3). Nestlings hatching from lighter, less-maculated eggs were heavier than those hatching from darker, more-maculated eggs (Fig. 5).
Table 3.
Effects of natal- and foster-egg maculation and pigmentation on size-adjusted nestling mass on brood-day 11
| Effect | F | d.f. | P |
|---|---|---|---|
| Natal-egg pigmentation | 3.80 | 2, 43.51 | 0.0302 |
| Foster-egg pigmentation | 1.50 | 2, 43.55 | 0.235 |
| Tarsus length | 83.45 | 1, 289.4 | <0.0001 |
Figure 5.
Least-squares mean nestling size-corrected body mass (± SE) in relation to natal-eggshell pigmentation (AGNES cluster category).
Effects on adult return rates and offspring recruitment
Of the 51 females for which we recorded natal-clutch pigmentation in 2017, 15 returned to breed the following year. There were no significant differences in adult return rates in relation to the pigmentation category of the natal clutch (F2,48 = 0.59, P = 0. 56).
Of the 303 ringed offspring produced in 2017, 14 were recruited to the breeding population (12 in 2018 and two additional offspring in 2019). There were no significant differences in offspring recruitment in relation to the pigmentation category of their natal clutch (F2,56.83 = 2.03, P = 0.14).
Effects on paternal provisioning
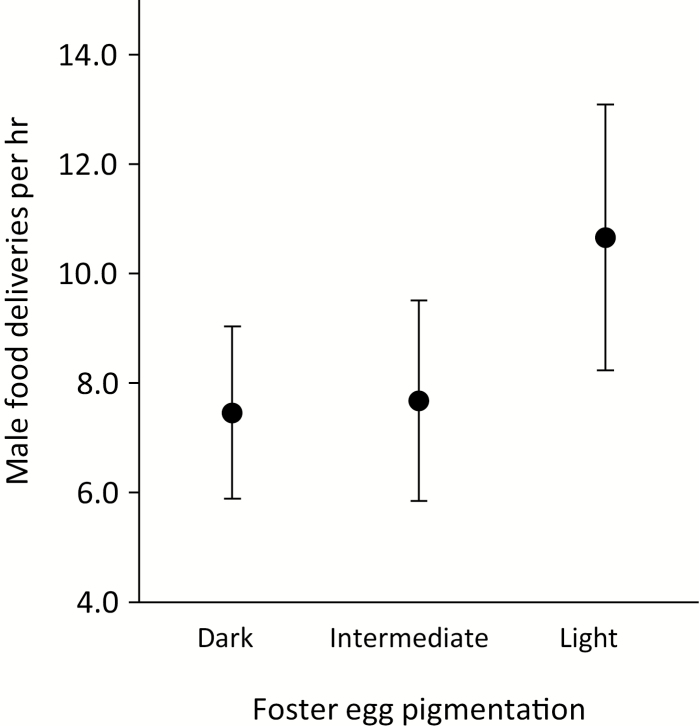
There was no effect of pigmentation of either the natal or the foster clutch on subsequent provisioning by males on brood-days 4–5, nor was the interaction between natal- and foster-clutch pigmentation significant (Fig. 6). Paternal provisioning of nestlings was inversely related to the provisioning rate of the female (parameter estimate ± SE −0.154 ± 0.045) and increased with the number of nestlings in the brood (parameter estimate ± SE 0.259 ± 0.095; Table 2).
Figure 6.
Least-squares mean number of male feeding trips (± SE) on brood-day 4 in relation to foster-eggshell pigmentation (AGNES cluster category).
Table 2.
Effects of foster-egg maculation and pigmentation on paternal provisioning
| Effect | F | d.f. | P |
|---|---|---|---|
| Natal-egg pigmentation | 2.39 | 2, 24 | 0.11 |
| Foster-egg pigmentation | 0.78 | 2, 24 | 0.47 |
| Natal- ×foster-egg pigmentation | 1.77 | 4, 24 | 0.17 |
| Number of female feeds | 11.81 | 1, 24 | 0.0022 |
| Number of young on brood-day 4 | 7.49 | 1, 24 | 0.0115 |
Discussion
Eggshell pigmentation and female quality
We found an association between clutch pigmentation and both female and offspring quality, so our findings join a growing number of studies that suggest that eggshell pigmentation can be used to infer female quality. As in our study, some of these have found that decreased eggshell pigmentation is associated with traits indicative of higher female quality (Martínez-de la Puente et al., 2007; Stoddard et al., 2012; Duval et al., 2013b, 2016; Badás et al., 2017) . However, there are still numerous studies with results that conflict with this pattern, finding that increased brown or blue–green pigmentation is reflective of higher female quality (Krist & Grim, 2007; Sanz & García-Navas, 2009; Martínez-Padilla et al., 2010; Holveck et al., 2012, 2019) or higher egg or nestling quality (Holveck et al., 2012; Poláček et al., 2017). Only a few studies have reported no connection between the degree of protoporphyrin-based pigmentation and female condition (Duval et al., 2013a; Giordano et al., 2015).
We found that older females laid significantly lighter eggs and tended to have a larger body size. The level of pigmentation attributable to brown and blue–green pigments in a female’s clutch has been shown to change because of environmental influences, including food availability (Moreno et al., 2006a; Morales et al., 2011; Duval et al., 2016; Hargitai et al., 2017b) and climate (Avilés et al., 2007). However, there is also evidence of heritable genetic variation in certain aspects of eggshell pigmentation (Gosler et al., 2000; Morales et al., 2010) and, thus, eggshell pigmentation may remain fairly consistent over a female’s lifetime. As lifespan is probably associated with an individual’s condition and is predictive of lifetime reproductive success in the study population (Bowers et al., 2017), as well as in other small passerines (McCleery & Perrins, 1989; Grant & Grant, 2000; Merilä & Sheldon, 2000), older females might be considered to be of higher quality. Given that older females laid significantly lighter, less-maculated eggs, it follows that the pigmentation of the eggs that a female lays has the potential to convey reliable information about her age/quality, and that increased clutch pigmentation may be a sign of poorer quality.
Offspring that hatched from lighter, less-maculated clutches of eggs were also of higher quality with respect to their size-adjusted mass, which is frequently taken as a measure of individual body condition (Barnett et al., 2015), and is positively correlated with nestling survival and predictive of recruitment to the breeding population at our study site (Bowers et al., 2014b). Although we did not find any evidence that the nestlings from lighter clutches were more likely to be recruited to subsequent breeding populations, the number of recruits was probably too low to detect a difference (only 14 nestlings were recruited to the breeding population). Note that the greater size-adjusted mass of nestlings hatching from lighter eggs cannot be attributed to increased parental provisioning. Thus, the effect on nestlings was probably not environmental, but related instead to maternal condition, although how the mother confers this benefit to her offspring, genetically, nutritionally or hormonally via the egg, is unknown. Regardless of the underlying mechanism, this finding also supports the notion that eggshell pigmentation has the potential to be informative and that, at least in our house wren population, increased pigmentation is reflective of lower-quality individuals.
Although the consensus seems to be that eggshell pigmentation is informative, the direction of any relationship between eggshell pigmentation and female health (or oxidative status) is difficult to ascertain with any certainty. It is likely that the connection between protoporphyrin-based pigmentation and female quality varies from species to species and even among populations of the same species, and future research should attempt to investigate what connects those species and populations that fall on one side of the divide and those that fall on the other.
Eggshell pigmentation and male provisioning
The results of this study do not support the honest signalling hypothesis that male provisioning is informed by eggshell pigmentation. Male provisioning was instead influenced by the number of nestlings and the degree of female provisioning.
Our study is one of only a handful that has investigated whether protoporphyrin-based pigmentation affects the level of male parental investment. Both Stoddard et al. (2012) and Bulla et al. (2012) found, as we did, no effect of pigmentation on paternal effort, although it is worth noting that the latter was an observational study focused on male incubation behaviour and not nestling provisioning. Sanz & García-Navas (2009) found a higher rate of male provisioning to nestlings hatching from eggs with greater pigment ‘spread’, but their study design did not account for the effect of nestling quality. In an observational study, male tree sparrows increased their provisioning to nestlings hatching from more heavily pigmented eggs than those hatching from less-pigmented eggs (Poláček et al., 2017). In another mid-latitude population of house wrens, males provisioned more to nests in which an artificial white egg had been added than to nests in which an artificial brown egg had been added before hatching (Walters et al., 2014). However, this experiment also did not consider the potential effect of the confounding factors of female or nestling quality. With this limited amount of evidence, it is difficult to assert with any certainty whether male birds attend to the protoporphyrin-based eggshell pigmentation of their mates when subsequently provisioning their nestlings. Our study suggests they do not, despite evidence from us and other investigators that eggshell pigmentation can reveal important information about the quality of females. This raises the question: why do males not use this information to inform their investment in nestling provisioning?
One possibility is that males responded to the pigmentation of their mate’s eggs only during the brief time they were in the nest at the beginning and end of the incubation period, something we cannot completely rule out. However, we consider this to be unlikely given that the 10 days the eggs were present was over 80% of the incubation period, and that the only data available on male visitations to the nest during incubation suggest that the males enter nests less often late in incubation than during mid-incubation (Johnson et al., 2008). Another possible explanation for the lack of an effect of eggshell pigmentation on male provisioning is that males cannot detect differences in pigmentation. This could be caused by poor lighting in cavity nests, which would limit a male’s ability to observe clutch pigmentation (Cassey, 2009; Reynolds et al., 2009). However, the results of modelling studies by Holveck et al. (2010) and Avilés et al. (2011) indicate that this may not be an issue, although both concluded that more research is needed. Another hinderance to a male’s observance of eggshell pigmentation may be opportunity. In species with female-only incubation, perhaps males simply do not have access to eggs to make an evaluation based on pigmentation (Reynolds et al., 2009; Stoddard et al., 2012). This claim has been disputed in the case of blue tits (Cyanistes caeruleus; Holveck et al., 2010) and pied flycatchers (Ficedula hypoleuca; Moreno et al., 2005). As is the case in both of these cavity nesters, male house wrens both on our study site (K.E.H., C.F.T., unpubl. obs.) and in a Wyoming population (Johnson et al., 2008) frequently enter or partially enter the nestbox during the egg-laying and incubation periods when the female is absent. Thus, although they cannot be ruled out, a lack of ability or of opportunity to observe eggshell pigmentation are unlikely explanations for the absence of an effect of differences in eggshell pigmentation on male provisioning.
Our results make it likely that one of the several other proposed adaptive explanations for intraspecific variation in egg pigmentation (Holveck et al., 2019), such as protoporphyrin playing a structural role when calcium is limited (Underwood & Sealy, 2002; Gosler et al., 2005; Brulez et al., 2015), applies to our study population. If, however, males are aware but do not modify their paternal effort based on eggshell pigmentation, perhaps the most parsimonious explanation is that they are constrained by the species’ mating system, limited lifetime opportunities to reproduce and reproductive costs. House wrens are primarily socially monogamous (Johnson, 2014), which means that in a population with a roughly equal sex ratio, many individuals will be paired with partners that are of lower-than-average quality. Thus, although a male may ascertain that his mate is of low quality after the clutch is completed, his opportunities to ‘trade up’ in a timely manner may be severely curtailed. Exacerbating the demand/supply mismatch with respect to the availability of high-quality mates, house wrens in our study population are short-lived and are present for only an average of 1.5 breeding seasons (Bowers et al., 2014a). We have also recently shown that male provisioning rate is negatively correlated with the likelihood of their return in the next breeding season (J. B. Jenkins et al., unpublished data). Thus, males may be selected to invest a specific level of parental effort irrespective of the quality of their mate because the alternative is possibly not to reproduce at all. Sexual selection on eggshell pigmentation is, therefore, more likely to be found in species that are longer-lived and have persistent pair-bonds (Cherry & Gosler, 2010).
In conclusion, we found that female house wrens exhibit extensive variation in eggshell pigmentation, and that various estimates of this pigmentation are significantly repeatable within clutches. Moreover, eggshell pigmentation appears to reveal reliable information about the quality of females and their offspring. Specifically, darker, more heavily maculated eggs seem to be indicative of lower-quality individuals. Notwithstanding these obvious cues, male house wrens do not appear to adjust their nestling provisioning in relation to the pigmentation of their mate’s clutch. Thus, the results are not consistent with the sexually selected hypothesis, making it likely that intraspecific variation in eggshell pigmentation, at least in the study population, has some other functional significance. In the case of a potential signalling function, the conflicting results of what eggshell pigmentation does, or does not, signal to males across different species of birds can probably best be resolved through consideration of variation in mating system and life history. However, such a comparative approach will require additional studies in which the effects of eggshell pigmentation are experimentally decoupled from the quality of nestlings in eliciting parental solicitude.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
Table S1. Repeatability of selected egg pigmentation parameters within clutches.
Table S2. Principal components analysis of selected egg pigmentation parameters.
Acknowledgments
We thank the 2017 Wren Crew members, in particular Rachael DiSciullo, Dylan Poorboy and Beth Weber, for their contributions to data collection, and Steve Juliano, Ben Sadd, Mark Hauber, Doug Mock and an anonymous reviewer for helpful comments on the manuscript. We also thank the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church and the Sears and Butler families for the use of their properties. This research was supported by grants from the National Institutes of Health (R15HD076308 to S.K.S. and C.F.T; R03AG063314 to N.T.M.; R15AR070505 to A.D.V.-M.), the American Ornithological Society (to K.E.H.), Sigma Xi Scientific Research Honor Society (to K.E.H.), the Beta Lambda Chapter of the Phi Sigma Honor Society (to K.E.H.) and a Summer Faculty Fellowship from Illinois State University (to S.K.S.). This research was carried out under US Geological Survey banding permit 09211, US Fish and Wildlife collecting permit MB692148-0, Illinois collecting permit NH15.0004a and Illinois State University Institutional Animal Care and Use Committee protocol 865938.
References
- Afonso S, Vanore G, Batlle A. 1999. Protoporphyrin IX and oxidative stress. Free Radical Research 31: 161–170. [DOI] [PubMed] [Google Scholar]
- Avilés JM, Soler JJ, Hart NS. 2011. Sexual selection based on egg colour: physiological models and egg discrimination experiments in a cavity-nesting bird. Behavioral Ecology and Sociobiology 65: 1721–1730. 10.1007/s00265-011-1180-8 [DOI] [Google Scholar]
- Avilés JM, Stokke BG, Moksnes A, Røskaft E, Møller AP. 2007. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behavioral Ecology and Sociobiology 61: 475–485. 10.1007/s00265-006-0275-0 [DOI] [Google Scholar]
- Badás EP, Martínez J, Rivero-de Aguilar J, Stevens M, van der Velde M, Komdeur J, Merino S. 2017. Eggshell pigmentation in the blue tit: male quality matters. Behavioral Ecology and Sociobiology 71: 57 10.1007/s00265-017-2286-4 [DOI] [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. 2011. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Animal Behaviour 81: 113–120. 10.1016/j.anbehav.2010.09.019 [DOI] [Google Scholar]
- Barnett CA, Suzuki TN, Sakaluk SK, Thompson CF. 2015. Mass-based condition measures and their relationship with fitness: in what condition is condition? Journal of Zoology 296: 1–5. 10.1111/jzo.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff CM, Murphy MT. 1993. The detection of and responses to experimental intraspecific brood parasitism in eastern kingbirds. Animal Behaviour 45: 631–638. 10.1006/anbe.1993.1079 [DOI] [Google Scholar]
- Board RG, Sparks NHC. 1991. Shell structure and formation in avian eggs. In: Deeming DC, ed. Egg incubation: its effects on embryonic development in birds and reptiles . Cambridge: Cambridge University Press, 71–86. [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. 2015. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist 185: 769− 783. 10.1086/681017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. 2014a. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95: 3027–3034. 10.1890/14-0418.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Jenkins JB, Mueller AJ, Miller KD, Thompson CF, Sakaluk SK. 2019. Condition-dependent begging elicits increased parental investment in a wild bird population. American Naturalist 193: 725–737. 10.1086/702848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. 2014b. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behavioral Ecology 25: 1485–1493. 10.1093/beheco/aru153 [DOI] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. 2017. Interactive effects of parental age on offspring fitness and age-assortative mating in a wild bird. Journal of Experimental Zoology 327: 302− 310. 10.1002/jez.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulez K, Choudhary PK, Maurer G, Portugal SJ, Boulton RL, Webber SL, Cassey P. 2014. Visual scoring of eggshell patterns has poor repeatability. Journal of Ornithology 155: 701–705. 10.1007/s10336-014-1053-9 [DOI] [Google Scholar]
- Brulez K, Pike TW, Reynolds SJ. 2015. Egg signalling: the use of visual, auditory, and chemical stimuli. In: Deeming DC, Reynolds SJ, eds. Nests, eggs, and incubation. Oxford: Oxford University Press, 127–141. [Google Scholar]
- Bulla M, Šálek M, Gosler AG. 2012. Eggshell spotting does not predict male incubation but marks thinner areas of a shorebird’s shells. Auk 129: 26–35. 10.1525/auk.2012.11090 [DOI] [Google Scholar]
- Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. American Naturalist 127: 415–445. 10.1086/284493 [DOI] [Google Scholar]
- Casini S, Fossi MC, Gavilan JF, Barra R, Parra O, Leonzio C, Focardi S. 2001. Porphyrin levels in excreta of sea birds of the Chilean coasts as non-destructive biomarker of exposure to environmental pollutants. Archives of Environmental Contamination and Toxicology 41: 65–72. 10.1007/s002440010221 [DOI] [PubMed] [Google Scholar]
- Cassey P. 2009. Biological optics: seeing colours in the dark. Current Biology 19: 1083–1084. 10.1016/j.cub.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Cassey P, Mikšík I, Portugal SJ, Maurer G, Ewen JG, Zarate E, Sewell MA, Karadas F, Grim T, Hauber ME. 2012. Avian eggshell pigments are not consistently correlated with colour measurements or egg constituents in two Turdus thrushes. Journal of Avian Biology 43: 503–512. 10.1111/j.1600-048X.2012.05576.x [DOI] [Google Scholar]
- Cherry MI, Gosler AG. 2010. Avian eggshell coloration: new perspectives on adaptive explanations. Biological Journal of the Linnean Society 100: 753–762. [Google Scholar]
- De Coster G, De Neve L, Lens L. 2013. Intra-clutch variation in avian eggshell pigmentation covaries with female quality. Journal of Ornithology 154: 1057–1065. 10.1007/s10336-013-0974-z [DOI] [Google Scholar]
- Dearborn DC, Hanley D, Ballantine K, Cullum J, Reeder DM. 2012. Eggshell colour is more strongly affected by maternal identity than by dietary antioxidants in a captive poultry system. Functional Ecology 26: 912–920. 10.1111/j.1365-2435.2012.02001.x [DOI] [Google Scholar]
- Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proceedings of the Royal Society B: Biological Sciences 276: 1737–1745. 10.1098/rspb.2008.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Cassey P, Lovell PG, Mikšík I, Reynolds SJ, Spencer KA. 2013a. Eggshell appearance does not signal maternal corticosterone exposure in Japanese quail: an experimental study with brown-spotted eggs. PLoS ONE 8: e80485 10.1371/journal.pone.0080485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Cassey P, Mikšík I, Reynolds SJ, Spencer KA. 2013b. Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica). Journal of Experimental Biology 216: 700–708. 10.1242/jeb.077370 [DOI] [PubMed] [Google Scholar]
- Duval C, Cassey P, Lovell PG, Mikšík I, Reynolds SJ, Spencer KA. 2016. Maternal influence on eggshell maculation: implications for cryptic camouflaged eggs. Journal of Ornithology 157: 303–310. 10.1007/s10336-015-1278-2 [DOI] [Google Scholar]
- Edwards DA, Chapman T. 2011. The evolution and significance of male mate choice. Trends in Ecology and Evolution 26: 647–654. 10.1016/j.tree.2011.07.012 [DOI] [PubMed] [Google Scholar]
- English PA, Montgomerie R. 2011. Robin’s egg blue: does egg color influence male parental care? Behavioral Ecology and Sociobiology 65: 1029–1036. https://dio.org/10.1007/s00265-010-1107-9 [Google Scholar]
- Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247. https://dio.org/10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fronstin RB, Doucet SM, Christians JK. 2016. Haematocrit, eggshell colouration and sexual signaling in the European starling (Sturnus vulgaris). BMC Ecology 16: 31 https://dio.org/10.1186/s12898-016-0084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Constantini D, Pick JL, Tschirren B. 2015. Female oxidative status, egg antioxidant protection and eggshell pigmentation: a supplemental feeding experiment in great tits. Behavioral Ecology and Sociobiology 69: 777–785. 10.1007/s00265-015-1893-1 [DOI] [Google Scholar]
- Gosler AG, Barnett PR, Reynolds SJ. 2000. Inheritance and variation in eggshell patterning in the great tit Parus major. Proceedings of the Royal Society of London B: Biological Sciences 267: 2469–2473. 10.1098/rspb.2000.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosler AG, Higham JP, Reynolds SJ. 2005. Why are birds’ eggs speckled? Ecology Letters 8: 1105–1113. 10.1111/j.1461-0248.2005.00816.x [DOI] [Google Scholar]
- Grant PR, Grant BR. 2000. Non-random fitness variation in two populations of Darwin’s finches. Proceedings of the Royal Society of London B: Biological Sciences 267: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith SC, Barr I, Sheldon BC, Rowe LV, Burke T. 2009. Egg patterning is not a reliable indicator of intraspecific brood parasitism in the blue tit Cyanistes caeruleus. Journal of Avian Biology 40: 337–341. 10.1111/j.1600-048X.2009.04671.x [DOI] [Google Scholar]
- Grinstein M, Bannerman RM, Moore CV. 1959. The utilization of protoporphyrin 9 in heme synthesis. Blood 14: 476–485. [PubMed] [Google Scholar]
- Hanley D, Doucet SM, Dearborn DC. 2010. A blackmail hypothesis for the evolution of conspicuous egg coloration in birds. Auk 127: 453–459. 10.1525/auk.2009.09090 [DOI] [Google Scholar]
- Hanley D, Heibe G, Dearborn DC. 2008. Testing an assumption of the sexual-signaling hypothesis: does blue–green egg color reflect maternal antioxidant capacity? Condor 110: 767–771. 10.1525/cond.2008.8634 [DOI] [Google Scholar]
- Hargitai R, Boross N, Hámori S, Neuberger E, Nyiri Z. 2017a. Eggshell biliverdin and protoporphyrin pigments in a songbird : are they derived from erythrocytes, blood plasma, or the shell gland? Physiological and Biochemical Zoology 90: 613–626. 10.1086/694297 [DOI] [PubMed] [Google Scholar]
- Hargitai R, Boross N, Nyiri Z, Eke Zs. 2017b. Effects of food limitation on the intensity of blue-green and brown eggshell coloration: an experimental study with the canary. Journal of Avian Biology 49: e01486 10.1111/jav.01486 [DOI] [Google Scholar]
- Hauber ME. 2014. The book of eggs: a life-size guide to the eggs of six hundred of the world’s bird species. Chicago: University of Chicago Press. [Google Scholar]
- Higham JP, Gosler AG. 2006. Speckled eggs: water-loss and incubation behaviour in the great tit. Oecologia 149: 561–570. https://dio.org/10.1007/s00442-006-0484-2 [DOI] [PubMed] [Google Scholar]
- Holveck M, Guerreiro R, Perret P, Doutrelant C, Grégoire A. 2019. Eggshell coloration indicates female condition during egg-laying: a field experiment in blue tits. Biological Journal of the Linnean Society 128: 181– 200. 10.1093/biolinnean/blz082 [DOI] [Google Scholar]
- Holveck MJ, Doutrelant C, Guerreiro R, Perret P, Gomez D, Grégoire A. 2010. Can eggs in a cavity be a female secondary sexual signal? Male nest visits and modeling of egg visual discrimination in blue tits. Biology Letters 6: 453–457. 10.1098/rsbl.2009.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holveck MJ, Grégoire A, Staszewski V, Guerreiro R, Perret P, Boulinier T, Doutrelant C. 2012. Eggshell spottiness reflects maternally transferred antibodies in blue tits. PLoS ONE 7:e50389 10.1371/journal.pone.0050389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen A, Vesterkjær K, Slagsvold T. 2011. Do male pied flycatchers (Ficedula hypoleuca) adjust their feeding effort according to egg colour? Ethology 117: 309–317. 10.1111/j.1439-0310.2011.01876.x [DOI] [Google Scholar]
- Johnson LS. 2014. House wren (Troglodytes aedon). In: Poole A, ed. The Birds of North America Online, version 2.0. New York: Cornell Lab of Ornithology and American Ornithologists’ Union; https://birdsna-org.bnaproxy.birds.cornell.edu/Species-Account/bna/species/380/articles/introduction [Google Scholar]
- Johnson LS, Brubaker JL, Johnson BGP. 2008. How males in the house wren, a cavity-nesting songbird, discover that eggs have hatched and transition to provisioning nestlings. Behaviour 145: 1781–1796. [Google Scholar]
- Jones KM, Monaghan P, Nager RG. 2001. Male mate choice and female fecundity in zebra finches. Animal Behaviour 62: 1021–1026. 10.1006/anbe.2001.1843 [DOI] [Google Scholar]
- Kassambara A, Mundt F. 2017. Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.5. Available at: https://CRAN.R-project.org/package=factoextra [Google Scholar]
- Keneddy GY, Vevers HG. 1973. Eggshell pigments of the Araucano fowl. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 44: 11–25. 10.1016/0305-0491(73)90336-2 [DOI] [PubMed] [Google Scholar]
- Kilner RM. 2002. Sex differences in canary (Serinus canaria) provisioning rules. Behavioral Ecology and Sociobiology 52: 400–407. 10.1007/s00265-002-0533-8 [DOI] [Google Scholar]
- Kilner RM. 2006. The evolution of egg colour and patterning in birds. Biological Reviews of the Cambridge Philosophical Society 81: 383–406. [DOI] [PubMed] [Google Scholar]
- Krist M, Grim T. 2007. Are blue eggs a sexually selected signal of female collared flycatchers? A cross-fostering experiment. Behavioral Ecology and Sociobiology 61: 863–876. https://dio.org/10.1007/s00265-066-0315-9 [Google Scholar]
- Lahti DC, Lahti AR. 2002. How precise is egg discrimination in weaverbirds? Animal Behaviour 63: 1135–1142. 10.1006/anbe.2002.3009 [DOI] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, Bouvier J-C, Camprodon J, Cooper CB, Dawson RD, Eens M, Eeva T, Faivre B, Garamszegi LZ, Goodenough AE, Gosler AG, Grégoire A, Griffith SC, Gustafsson L, Johnson LS, Kania W, Keiš O, Llambias PE, Mainwaring MC, Mänd R, Massa B, Mazgajski TD, Møller AP, Moreno Naef-Daenzer B, Nilsson J-Ǻ, Norte AC, Orell M, Otter KA, Park CR, Perrins CM, Pinowski J, Porkert J, Potti J, Remes V, Richner H, Rytkönen S, Shiao M-T, Silverin B, Slagsvold T, Smith HG, Sorace A, Stenning MJ, Steward I, Thompson CF , Tryjanowski P, Török J, van Noordwijk AJ, Winkler DW, Ziane N.. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica 45: 1–26. 10.3161/000164510X516047 [DOI] [Google Scholar]
- Lang MR, Wells JW. 1987. A review of eggshell pigmentation. World’s Poultry Science Journal 43: 238–246. 10.1079/WPS19870016 [DOI] [Google Scholar]
- Lendvai ÁZ, Akçay Ҫ, Ouyang JQ, Dakin R, Domalik AD, St John PS, Stanback M, Moore IT, Bonier F. 2015. Analysis of the optimal duration of behavioral observations based on an automated continuous monitoring system in tree swallows (Tachycineta bicolor): is one hour good enough? PLoS ONE 10: e0141194 https://doi:10.1371/journal.pone.0141194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard ML, Horn AG. 2001. Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor). Behavioral Ecology and Sociobiology 49: 170–175. 10.1007/s002650000290 [DOI] [Google Scholar]
- Lessells C, Boag P. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104: 116–121. https://doi:10.2307/4087240 [Google Scholar]
- London IM, Shemin D. 1949. Heme synthesis and red blood cell dynamics in normal humans and in subjects with polycythemia vera, sickle-cell anemia, and pernicious anemia. The Journal of Biological Chemistry 179: 463–484. [PubMed] [Google Scholar]
- MacGregor NA, Cockburn A. 2002. Sex differences in parental response to begging nestlings in superb fairy-wrens. Animal Behaviour 63: 923–932. 10.1006/anbe.2001.1991 [DOI] [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. 2019. Cluster: cluster analysis basics and extensions. R package version 2.1.0. [Google Scholar]
- Martínez-Padilla J, Dixon H, Vergara P, Pérez-Rodríguez L, Fargallo JA. 2010. Does egg colouration reflect male condition in birds? Naturwissenschaften 97: 469–477. 10.1007/s00114-010-0660-4 [DOI] [PubMed] [Google Scholar]
- Martínez-de la Puente J, Merino S, Moreno J, Tomás G, Morales J, Lobato E, García-Fraile S, Martínez J. 2007. Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? Journal of Avian Biology 38: 377–384. 10.1111/j.2007.0908-8857.03877.x [DOI] [Google Scholar]
- McCleery RH, Perrins CM. 1989. Great tit. In: Newton I, ed. Lifetime reproduction in birds. Oxford: Oxford University Press, 35–53. [Google Scholar]
- Merilä J, Sheldon BC. 2000. Lifetime reproductive success and heritability in nature. The American Naturalist 155: 301–310. 10.1086/303330 [DOI] [PubMed] [Google Scholar]
- Mikšík I, Holáň V, Deyl Z. 1996. Avian eggshell pigments and their variability. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 113: 607–612. 10.1016/0305-0491(95)02073-X [DOI] [Google Scholar]
- Morales J, Kim SY, Lobato E, Lobato E, Merino S, Tomás G, Martínez-De La Puente J, Moreno J 2010. On the heritability of blue–green eggshell coloration. Journal of Evolutionary Biology 23: 1783–1791. [DOI] [PubMed] [Google Scholar]
- Morales J, Velando A, Torres R. 2011. Biliverdin-based egg coloration is enhanced by carotenoid supplementation. Behavioral Ecology and Sociobiology 65: 197–203. 10.1007/s00265-010-1025-x [DOI] [Google Scholar]
- Moreno J, Morales J, Lobato E, Merino S, Tomás G. 2005. Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behavioral Ecology 16: 931–937. 10.1093/beheco/ari072 [DOI] [Google Scholar]
- Moreno J, Lobato E, Morales J, Merino S, Tomás G, Martínez-de la Puente J, Sanz JJ, Mateo R, Soler JJ. 2006a. Experimental evidence that egg color indicates female condition at laying in a songbird. Behavioral Ecology 17: 651–655. 10.1093/beheco/ark014 [DOI] [Google Scholar]
- Moreno J, Morales J, Lobato E, Merino S, Tomás G, Martínez-De La Puente J. 2006b. More colourful eggs induce a higher relative paternal investment in the pied flycatcher Ficedula hypoleuca: a cross-fostering experiment. Journal of Avian Biology 37: 555–560. 10.1111/j.2006.0908-8857.03915.x [DOI] [Google Scholar]
- Moreno J, Osorno JL. 2003. Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters 6: 803–806. 10.1046/j.1461-0248.2003.00505.x [DOI] [Google Scholar]
- Murphy MT, Chutter CM, Redmond LJ. 2015. Quantification of avian parental behavior: what are the minimum necessary sample times? Journal of Field Ornithology 86: 41–50. 10.1111/jofo.12087 [DOI] [Google Scholar]
- Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society 82: 591–605. [DOI] [PubMed] [Google Scholar]
- Pagani-Núñez E, Senar JC. 2013. One hour of sampling is enough: great tit Parus major parents feed their nestlings consistently across time. Acta Ornithologica 48: 194–200. [Google Scholar]
- Poláček M, Griggio M, Mikšík I, Bartíková M, Eckenfellner M, Hoi H. 2017. Eggshell coloration and its importance in postmating sexual selection. Ecology and Evolution 7: 941–949. 10.1002/ece3.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SJ, Martin GR, Cassey P. 2009. Is sexual selection blurring the functional significance of eggshell coloration hypotheses? Animal Behaviour 78: 209–215. 10.1016/j.anbehav.2009.03.003 [DOI] [Google Scholar]
- Riehl C. 2011. Paternal investment and the “sexually selected hypothesis” for the evolution of eggshell coloration: revisiting the assumptions. Auk 128: 175–179. 10.1525/auk.2011.10171 [DOI] [Google Scholar]
- Sacch R, Saino N, Galeotti P. 2002. Features of begging calls reveal general condition and need of food of barn swallow (Hirundo rustica) nestlings. Behavioral Ecology 13: 268–273. 10.1093/beheco/13.2.268 [DOI] [Google Scholar]
- Samiullah S, Roberts JR, Chousalkar K. 2015. Eggshell color in brown-egg laying hens – a review. Poultry Science 94: 2566–2575. 10.3382/ps/pev202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JJ, García-Navas V. 2009. Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. The Journal of Animal Ecology 78: 31–41. [DOI] [PubMed] [Google Scholar]
- Sies H. 1997. Oxidative stress: oxidants and antioxidants. Experimental Physiology 82: 291–295. 10.1113/expphysiol.1997.sp004024 [DOI] [PubMed] [Google Scholar]
- Stoddard MC, Fayet AL, Kilner RM, Hinde CA. 2012. Egg speckling patterns do not advertise offspring quality or influence male provisioning in great tits. PLoS ONE 7 10.1371/journal.pone.0040211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R. 1972. Parental investment and sexual selection. In: Campbell B, ed. Sexual selection and the descent of man 1871–1971. Chicago: Aldine Press, 136–179. [Google Scholar]
- Underwood TJ, Sealy SG. 2002. Adaptive significance of egg coloration. In: Deeming DC, ed. Avian incubation. Oxford: Oxford University Press, 280–298. [Google Scholar]
- Walters LA, Getty T. 2010. Are brighter eggs better? Egg color and parental investment by house wrens. Journal of Field Ornithology 81: 155–166. 10.1111/j.1557-9263.2010.00273.x [DOI] [Google Scholar]
- Walters LA, Olszewski N, Sobol K. 2014. Male house wrens provide more parental provisioning to nests with a brighter artificial egg. Wilson Journal of Ornithology 126: 508–515. 10.1676/13-221.1 [DOI] [Google Scholar]
- Wegmann M, Vallat-Michel A, Richner H. 2015. An evaluation of different methods for assessing eggshell pigmentation and pigment concentration using great tit eggs. Journal of Avian Biology 46: 597–607. 10.1111/jav.00495 [DOI] [Google Scholar]
- Weidinger K. 2001. Does egg colour affect predation rate on open passerine nests? Behavioral Ecology and Sociobiology 49: 456–464. 10.1007/s002650100324 [DOI] [Google Scholar]
- Westmoreland D, Kilti RA. 2007. Egg coloration and selection for crypsis in open-nesting blackbirds. Journal of Avian Biology 38: 682–689. 10.1111/j.2007.0908-8857.04066.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.