Abstract
Aims: To evaluate the efficacy of low‐concentration chlorine dioxide (ClO2) gas against model microbes in the wet state on a glass surface.
Methods and Results: We set up a test room (39 m3) and the ClO2 gas was produced by a ClO2 gas generator that continuously releases a constant low‐concentration ClO2 gas. Influenza A virus (Flu‐A), feline calicivirus (FCV), Staphylococcus aureus and Escherichia coli were chosen as the model microbes. The low‐concentration ClO2 gas (mean 0·05 ppmv, 0·14 mg m−3) inactivated Flu‐A and E. coli (>5 log10 reductions) and FCV and S. aureus (>2 log10 reductions) in the wet state on glass dishes within 5 h.
Conclusions: The treatment of wet environments in the presence of human activity such as kitchens and bathrooms with the low‐concentration ClO2 gas would be useful for reducing the risk of infection by bacteria and viruses residing on the environmental hard surfaces without adverse effects.
Significance and Impact of the Study: This study demonstrates that the low‐concentration ClO2 gas (mean 0·05 ppmv) inactivates various kinds of microbes such as Gram‐positive and Gram‐negative bacteria, enveloped and nonenveloped viruses in the wet state.
Keywords: bacteria, chlorine dioxide, disinfectant, gas, microbe, virus
Introduction
In recent years, emergence and re‐emergence of serious infectious disease occurred frequently throughout the world. Relevant pathogens include Escherichia coli O157:H7, Mycobacterium tuberculosis, norovirus, rotavirus, swine‐origin influenza A virus (H1N1) and severe acute respiratory syndrome (SARS) coronavirus. Annually, there are 1·7 million deaths from diarrhoeal diseases and 1·5 million deaths from respiratory infections worldwide (Nada and Gregory 2006). For centuries, it was assumed that infectious diseases were spread primarily by the airborne route or through the direct patient contact, and the environmental surfaces played little or no significant role in the disease transmission (Goldmann 2000; Cozad and Jones 2003; Boone and Gerba 2007). However, there is growing evidence that contaminated fomites or surfaces play a key role in the spread of viral infections (Barker et al. 2001; Hota 2004). Influenza A virus was detected on over 50% of the fomites from bathrooms, kitchens, and play areas in homes and day care centres during the influenza season (Boone and Gerba 2005). Norovirus was detected on swabs taken from kitchen and bathroom surfaces in five outbreaks (2009a, 2009b). Furthermore, the home environment, such as the kitchen and bathroom, serves as a reservoir of large numbers of microbes, particularly Enterobacteriaceae, and the infectious disease transmission has been demonstrated to occur in 6–60% of households in which one member is ill (Kagan et al. 2002). From these reports, we focused on the reduction in microbes on environmental surfaces, particularly, under moist conditions. In this report, to evaluate the disinfecting activity of a low‐concentration chlorine dioxide (ClO2, CAS no. 10049‐04‐4) gas, we set up a test room (39 m3) and used four types of model microbes such as influenza A virus (Flu‐A, enveloped virus), feline calicivirus (FCV, a norovirus surrogate, nonenveloped virus), Staphylococcus aureus (S. aureus, Gram‐positive bacterium) and Escherichia coli (E. coli, Gram‐negative bacterium).
ClO2 is a strong oxidant and a yellow to reddish‐yellow gas at room temperature (Budavari 2001). Gaseous ClO2 dissolves readily in water at room temperature. The aqueous ClO2 has been used as a disinfectant, and it has been widely used for drinking water treatment in North America (Gates 1998). Recently, antibacterial and antifungal activities have been reported for gaseous ClO2 (Lee et al. 2004; Sy et al. 2005; Wilson et al. 2005). However, the concentration of ClO2 gas used in previous reports to inactivate bacteria and fungi was extremely high (250–3500 ppmv); its concentration was much higher than a lethal concentration 50% kill (LC50) value (32 ppmv, 90 mg m−3) in rats as a single exposure (Dobson 2002). Little is known whether a low‐concentration ClO2 gas has an antibacterial and antiviral activity. Previous studies in our laboratory demonstrated that a low‐concentration ClO2 gas (mean 0·08 ppmv, 0·22 mg m−3) inactivated FCV on a hard surface in the dry state within 10 h in 75–85%‘high’ relative humidity (r.h.) (>3 log10 reductions), but had little virucidal effect in 45–55%‘moderate’ r.h. (Morino et al. 2009). These results suggest that the atmospheric moisture plays an important role in the inactivation by a low‐concentration ClO2 gas of FCV on hard surfaces. The moisture may be indispensable for a low‐concentration ClO2 gas to inactivate microbes on hard surfaces. Therefore, we evaluated the efficacy of a low‐concentration ClO2 gas against model microbes in the wet state on a glass surface, which means without any drying process.
The purpose of this study was to investigate the possibility of using a low‐concentration ClO2 gas as a method for reducing the risk of infectious disease by various microbes on environmental surfaces, particularly under moist conditions such as kitchens and bathrooms.
Materials and methods
Test bacteria
Escherichia coli and S. aureus were obtained from the National Institute of Technology and Evaluation (NITE) Biological Resource Center (NBRC; 3972 and 13276) in Japan. One loop (c. 10 μl) of each bacterium was taken from the stock culture stored under refrigeration, transferred into 5 ml of Soybean Casein Digest (SCD, 393‐00185; Nihon Seiyaku, Tokyo, Japan) solution and incubated at 37°C for 18 h. Cells were collected by centrifugation at 1400 g for 15 min at 25°C, washed three times with Dulbecco’s phosphate‐buffered saline (D‐PBS) and resuspended in D‐PBS as inocula (1 × 108 cells ml−1).
Viable cell counts
The viable cell counts were determined by serial tenfold dilutions followed by an inoculation of 100 μl onto SCD agar culture plates. After incubation at 37°C for 24 h (E. coli) or 48 h (S. aureus), the number of colonies was counted and the viable cell counts on the glass dish after exposure to ClO2 gas or ordinary air were determined as colony‐forming units (CFU) per dish.
Test viruses
FCV strain F9 was obtained from the American Type Culture Collection (ATCC, VR‐782; Manassas, VA, USA), and Flu‐A subtype H1N1 strain New Caledonia/20/99 was kindly provided by Dr Yoshinobu Okuno. Crandell–Reese feline kidney (CRFK) cells for FCV infection and Madin–Darby canine kidney (MDCK) cells for Flu‐A infection were obtained from the ATCC (CCL‐94; CRFK and CCL‐34; MDCK). The CRFK cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat‐inactivated foetal bovine serum (FBS; Sigma, St. Louis, MO, USA), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. The MDCK cells were grown in Eagle’s minimum essential medium (MEM) supplemented with 10% (v/v) heat‐inactivated FBS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. The cells were inoculated with each virus and incubated at 37°C in a humidified atmosphere containing 5% CO2 until a cytopathic effect (CPE) was observed. Then, the viruses were released from the cells by freezing and thawing and centrifuged at 12 000 g for 30 min to remove the cell debris. The supernatant was filtered through a 0·45 μm membrane filter (SCHVU11RE; Millipore Co., Billerica, MA, USA) and ultra‐centrifuged at 70 000 g for 3 h (for FCV) or 2 h (for Flu‐A) at 5°C. The precipitate was suspended in D‐PBS, and the suspension was centrifuged at 13 800 g for 10 min.
Virus assay
The infectivity titres of viruses were determined as the 50% tissue culture infectious dose (TCID50) per 50 μl according to the Spearman–Kärber method (Hierholzer and Killington 1996). Cells were seeded into 96‐well, flat‐bottomed microtitre plates (FALCON, Franklin Lakes, NJ, USA) and allowed to grow to confluence. Regarding FCV, CRFK cells in 50 μl of DMEM with 10% FBS per well were infected by the addition of 50 μl of serial 10‐fold dilutions of the virus in DMEM supplemented with 2% FBS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin per well, with four replicates per dilution. The cells were incubated for 3 days at 37°C. Regarding Flu‐A, after MDCK cells were washed two times with D‐PBS, the cells were infected by the addition of 50 μl of serial 10‐fold dilutions of the virus in MEM supplemented with 0·2% BSA, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin per well for 2 h at 37°C, with four replicates per dilution. After 2 h, the medium in wells was discarded and 100 μl of MEM supplemented with 2·5 μg ml−1 trypsin was added to the well, and the cells were incubated for 4 days at 37°C.
Experimental set‐up
The microbe suspension was prepared as follows: bacteria (106–108 cells ml−1) and virus (108·3–109·3 TCID50 ml−1) were suspended in D‐PBS with 0, 0·1, 0·25, 0·5, 0·75 and 1% FBS. Hundred microlitres of microbe suspension was placed on glass dishes (5 cm diameter). Drying was not performed for bacteria or viruses in the wet state. These preparations were placed in the test room as described later and were exposed to ClO2 gas or ordinary air for 5 h (organic load experiments). In the time course experiments, the preparations of microbe in the wet state without FBS were placed in the test room with a low‐concentration ClO2 gas (mean 0·05 ppmv) or ordinary air for 0, 1, 2, 3, 4 and 5 h. Hundred micro litre of the medium was added to the dishes after the exposure to ClO2 gas or ordinary air, and then a sample was collected with a cell scraper (179693; Nunc, Rochester, NY, USA). To evaluate the efficacy of low‐concentration ClO2 gas against surface microbes, the glass dishes with bacteria or viruses were placed on a table in a test room, which was 4·8 m long, 3 m wide and 2·7 m high (39 m3) (Fig. 1a). The ClO2 gas in the test room was produced by a ClO2 gas generator (Cleverin generator, LISPASS®S; Taiko Pharmaceutical Co., Ltd, Osaka, Japan) that continuously releases a constant, low‐concentration ClO2 gas. The experiments were performed under fluorescent lights, and the concentration of ClO2 gas in the test room was measured by a ClO2 measuring device (0–1000 ppb, Model 4330‐SP; Interscan Corporation, Chatsworth, CA, USA). Similarly, a condition without ClO2 gas (in ordinary air) was set up as a control. The r.h. and temperature in the test room were recorded by a portable thermometer/hygrometer with the accuracy of ±3%r.h. (DL‐8829; CUSTOM, Tokyo, Japan) under ordinary air and ClO2 gas conditions. Values in this study indicate the mean of four experiments.
Figure 1.
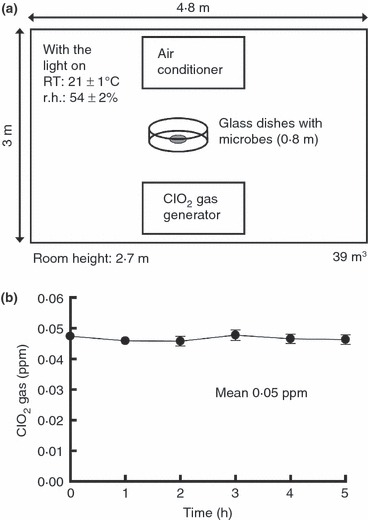
Schematic diagram of a test room and time course of changes in concentration of ClO2 gas. (a) Schematic diagram of bacteria and viruses on the glass surface in a test room. The microbes in the wet state on the glass dishes were placed at the centre of the room. The 0·8 m given in a parenthesis in the figure shows the height of glass dishes with the microbes placed above the floor. (b) Time course of changes in concentration of ClO2 gas in the test room. The graph presents the mean of eight experiments; each error bar indicates the SD. The background level of ClO2 gas in ordinary air was below 0·01 ppmv.
Reagents
All other reagents were of the highest grade commercially available.
Statistical analysis
Statistical significance was determined using Student’s t‐test. P values less than 0·05 or 0·01 (two‐tailed) were considered to be statistically significant.
Results
The concentration of ClO2 gas in the test room
The time course of changes in concentration of ClO2 gas in the test room is shown in Fig. 1b. The mean value of ClO2 gas concentration was 0·05 ppmv, and the minimum and maximum values were 0·04 and 0·06 ppmv in the test room, respectively. The concentration of ClO2 gas was measured nearby the glass dishes containing the test microbes. The temperature and RH in the test room were 21 ± 1°C and 54 ± 2%, respectively.
Inactivation of bacteria in the wet state
We evaluated the efficacy of low‐concentration ClO2 gas against E. coli and S. aureus in the wet (without any drying process) state without FBS on a glass surface. The effect of low‐concentration ClO2 gas (mean 0·05 ppmv) against E. coli showed reductions of >2 log10 after 3 h (P < 0·05) as compared with control values (in ordinary air) and was below the detection limit (<12 CFU per dish) after 4 h (P < 0·01) (Fig. 2a). The effect of low‐concentration ClO2 gas (mean 0·05 ppmv) against S. aureus showed reductions of >2 log10 after 5 h (P < 0·05) as compared with control values (Fig. 2b).
Figure 2.
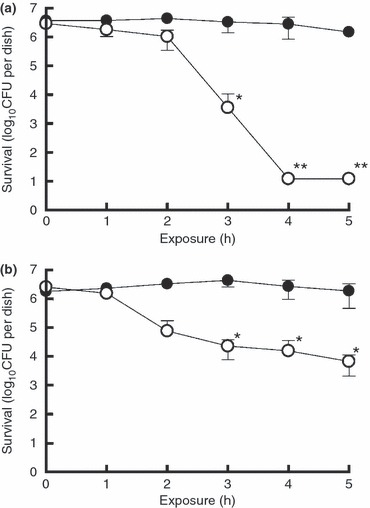
Inactivation of Escherichia coli (a) and Staphylococcus aureus (b) in the wet state by low‐concentration ClO2 gas. Drying was not performed for bacteria in the wet state (see Materials and methods). This represents samples with no organic load. The asterisks indicate reductions of >2 log10 (*) and >5 log10 (**) as compared with control values (in ordinary air). The double asterisks represent below the detection limit (<12 CFU per dish) of the assay. Data represent mean ± SD (n = 4). (•) air (control); (○) ClO2 gas (mean 0·05 ppmv).
Inactivation of viruses in the wet state
We evaluated the efficacy of low‐concentration ClO2 gas against Flu‐A and FCV in the wet state without FBS on a glass surface. The effect of low‐concentration ClO2 gas (mean 0·05 ppmv) against Flu‐A was below the detection limit (<0·5 log10[TCID50 per 50 μl]) after 3 h (P < 0·01) (Fig. 3a). The effect of low‐concentration ClO2 gas (mean 0·05 ppmv) against FCV showed reductions of >2 log10 after 4 h (P < 0·05) as compared with control values (Fig. 3b).
Figure 3.

Inactivation of Flu‐A (a) and FCV (b) in the wet state by low‐concentration ClO2 gas. Drying was not performed for viruses in the wet state (see Materials and methods). This represents samples with no organic load. The asterisks indicate titre reductions of >2 log10 (*) and >5 log10 (**) as compared with control values (in ordinary air). The double asterisks represent below the detection limit (<0·5 log10[TCID50 per 50 μl]) of the assay. Data represent mean ± SD (n = 4). (•) air (control); (○) ClO2 gas (mean 0·05 ppmv).
Effect of organic load on inactivation of bacteria and viruses in the wet state by low‐concentration ClO2 gas
It is known that ClO2 in solution loses its bactericidal activity in the presence of organic substances. Therefore, we investigated the effect of organic substance load (0–1% FBS) on the inactivation of bacteria and viruses in the wet state by a low‐concentration ClO2 gas. The low‐concentration ClO2 gas inactivated S. aureus and FCV with 0·5% FBS (1·8 log10 reductions) and E. coli and Flu‐A with 1% FBS (>2 log10 reductions) (Table 1).
Table 1.
Effect of organic load on inactivation of bacteria and viruses in the wet state by low‐concentration ClO2 gas
| Exposure time (h) | FBS concentration in microbes suspension (%) | Bacteria survival (log10 CFU per dish) | Viruses infectivity (log10[TCID50 per 50 μl]) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Flu‐A | FCV | ||||||
| Air | ClO2 | Air | ClO2 | Air | ClO2 | Air | ClO2 | ||
| 5 | 0 | 4·9 | 1·1 (3·9)* | 6·0 | 2·1 (3·9)* | 5·6 | <0·5 (5·1)* | 5·0 | 2·3 (2·7)* |
| 0·1 | 4·9 | 1·1 (3·8)* | 6·1 | 3·3 (2·8)* | 6·3 | <0·5 (5·8)* | 5·1 | 2·6 (2·5) * | |
| 0·25 | 4·8 | 2·2 (2·7)* | 6·1 | 4·4 (1·7) | 6·4 | 0·6 (5·8)* | 5·4 | 3·1 (2·3)* | |
| 0·5 | 4·7 | 1·3 (3·5)* | 6·2 | 4·4 (1·8) | 6·3 | <0·5 (5·8)* | 5·3 | 3·6 (1·8) | |
| 0·75 | 4·7 | 2·3 (2·4)* | 6·3 | 5·0 (1·3) | 6·2 | <0·5 (5·7)* | 5·2 | 4·8 (0·4) | |
| 1 | 4·9 | 2·0 (2·8)* | 6·1 | 5·1 (1·0) | 6·3 | 0·6 (5·7)* | 5·3 | 5·3 (0·0) | |
CFU, colony‐forming units; TCID, tissue culture infectious dose.
Values are the mean of four experiments.
The values given in parentheses show log10 reduction.
*Values indicate reductions of >2 log10 as compared with control values (in ordinary air).
Discussion
Microbial decontamination is one of the practical issues involved in health care and food industry, etc., and many hospitals have used fumigation of formaldehyde, peracetic acid and chlorhexidine for the disinfection purpose. However, these procedures have a number of disadvantages, such as acute toxicity induced by inhalation of the disinfectant vapours and skin inflammation by contact with these disinfectants. In this study, we demonstrated that a low‐concentration ClO2 gas (mean 0·05 ppmv) inactivated E. coli, Flu‐A (>5 log10 reductions) and S. aureus, FCV (>2 log10 reductions) in the wet state within 5 h. These results suggest that the low‐concentration ClO2 may inactivate various kinds of microbes such as Gram‐positive bacteria, Gram‐negative bacteria, enveloped viruses, and nonenveloped viruses in the wet state. According to the International Chemical Safety Card, the threshold limit values for ClO2 gas are 0·1 ppmv as an 8 h time‐weighted average and 0·3 ppmv as a 15‐min short‐term exposure limit (Dobson 2002); the concentration of ClO2 gas (0·05 ppmv) used to inactivate microbes in this study was lower than these concentrations (0·1 and 0·3 ppmv). Previous studies suggest that the environmental surfaces, particularly under moist conditions, play a key role in the spread of viral infections and serve as a reservoir of various microbes (Barker et al. 2001; Boone and Gerba 2005; Boxman et al. 2009a,b; Hota 2004; Kagan et al. 2002). The gaseous agents show features of excellent diffusibility and penetrability, making it possible to access sites that are difficult to disinfect with conventional liquid agents. In other words, these features make it possible for the gas to disinfect widely dispersed sources of infection. The treatment of wet environments in the presence of human activity, such as kitchens and bathrooms, with the low‐concentration ClO2 gas would be useful for reducing the risk of infection by bacteria and viruses on the environment surfaces without adverse effects.
The disinfectants such as alcohol, triclosan and chlorhexidine are effective against enveloped viruses, but are limited against nonenveloped viruses, suggesting that nonenveloped viruses have a higher resistance to disinfectants (Rotter 1997). In fact, we found that a ClO2 solution was more effective against enveloped viruses than nonenveloped viruses (Sanekata et al. 2010). Similarly, antiviral activity of the low‐concentration ClO2 gas was higher against enveloped viruses than nonenveloped viruses (Fig. 3). In the case of bacteria, a ClO2 solution was more effective against E. coli (Gram‐negative) than S. aureus (Gram‐positive) (Toda et al. 2006). Similarly, antibacterial activity of the low‐concentration ClO2 gas was higher against E. coli than S. aureus (Fig. 2). It is known that a ClO2 solution loses its bactericidal activity in the presence of organic substances. In this report, the low‐concentration ClO2 gas also decreased its antimicrobial activity in the presence of organic substances (up to 1% FBS), except for Flu‐A (Table 1). Taken together, the findings from research on antimicrobial activity of a ClO2 solution may be utilized to simulate the activity of low‐concentration ClO2 gas against various kinds of microbes. Additional studies will be needed to clarify the relationship between the antimicrobial activity of a ClO2 solution and that of low‐concentration ClO2 gas. Regarding the mechanism of inactivation of microbes by ClO2, Ogata (2007) discussed that the inactivation of microbes by ClO2 was caused by the oxidative modification of their tryptophan and tyrosine residues. The difference in the efficacy of ClO2 against microbes described earlier may be due to the difference in the reactivity of ClO2 against tryptophan and tyrosine residues on key proteins that are indispensable to the growth of bacteria and to the infectivity of viruses.
Staphylococcus aureus is the most important pathogen among staphylococci. Traditionally, methicillin‐resistant S. aureus (MRSA) infections have occurred predominantly in hospitals. However, there has been an increasing incidence of community‐acquired MRSA (CA‐MRSA) infections for recent years (Otto 2010). Recently, Scott et al. reported that MRSA was found on a variety of household surfaces, including the kitchen and bathroom sinks as wet environments (2008, 2009). Although the efficacy of low‐concentration ClO2 gas against MRSA was not determined in this report, the efficacy of low‐concentration ClO2 gas against methicillin‐sensitive S. aureus (MSSA) showed reductions of >2 log10 after 5 h as compared with control values (Fig. 2b). Furthermore, inactivation of a ClO2 solution against MRSA was nearly equal to that against MSSA (S. Yamasaki and A. Hinenoya 2011, personal communication). These results suggest that the low‐concentration ClO2 gas may have an inactivation activity against MRSA and CA‐MRSA. Influenza viruses cause significant morbidity and mortality among human populations worldwide. In April 2009, the swine‐origin Flu‐A (H1N1) first appeared in Mexico and spread worldwide, presumably as a result of frequent, intercontinental airplane travels. In June 2009, the World Health Organization raised its pandemic level to the highest level of phase 6. The pandemic caused many hospitalizations and deaths world‐wide (Al Hajjar and McIntosh 2010). In this study, the efficacy of low‐concentration ClO2 gas against the swine‐origin Flu‐A (H1N1) was not determined. However, the efficacy of low‐concentration ClO2 gas against identical subtype Flu‐A (H1N1) strain New Caledonia/20/99 resulted in the reduction in viral activity below the detection limit after 3 h (Fig. 3a). In addition, the inactivation of a ClO2 solution against swine‐origin Flu‐A (H1N1) was nearly equal to that against Flu‐A (H1N1) strain New Caledonia/20/99 (Fukuda et al. 2011). These results suggest that the low‐concentration ClO2 gas may inactive the swine‐origin Flu‐A (H1N1). Boone and Gerba (2005) reported that Flu‐A was detected from over 50% of the fomites collected from wet environments such as bathrooms and kitchens in homes and day care centres during the influenza season. From these data, we propose that the low‐concentration ClO2 gas would be useful for reducing the risk of MRSA and swine‐origin Flu‐A infections in wet environments such as kitchens and bathrooms, without necessitating the evacuation of residents.
We have discussed that the moisture may be indispensable for a low‐concentration ClO2 gas to inactivate microbes on hard surfaces. ClO2 gas had little effect even when the FCV in the dry state was exposed to high‐concentration ClO2 gas (8 ppmv) for 24 h (Morino et al. 2009). In the efficacy experiment of ClO2 gas for S. aureus and FCV, the effect of ClO2 gas exposed for 3–5 h (Fig. 2b) and 4–5 h (Fig. 3b) did not significantly differ from the natural reduction in these microorganisms over time during these late‐phase period of time courses. This finding may be due to that, in this experiment, the residual amounts of water were 100 μl (initial), 59 ± 5 μl (after 1 h), 26 ± 8 μl (after 2 h), 9 ± 8 μl (after 3 h), 3 ± 3 μl (after 4 h) and <2 μl (after 5 h). Therefore, the remaining amount of water beyond 3 h was less than 10% of the initial amount. It is likely that the effect of ClO2 gas may be influenced by various factors such as inoculum volume, inoculum surface area, humidity, temperature and airflow. However, we were unable to investigate the effect of these factors in detail, because this study focused mainly on the simulation of realistic circumstances, employing a practical assessment system (test room, 39 m3). In the future, with respect to the effect of ClO2 gas and the factors that affect such effect, a further study that enables the detailed investigation will be needed, using, for example, a small size chamber in which the temperature and the humidity can be strictly regulated.
Acknowledgements
We are grateful to Ms Tomoko Koizumi for technical assistance and to Dr Yoshinobu Okuno for the preparation of New Caledonia strain influenza A virus.
References
- Al Hajjar, S. and McIntosh, K. (2010) The first influenza pandemic of the 21st century. Ann Saudi Med 30, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, J. , Stevens, D. and Bloomfield, S.F. (2001) Spread and prevention of some common viral infections in community facilities and domestic homes. J Appl Microbiol 91, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone, S.A. and Gerba, C.P. (2005) The occurrence of influenza A virus on household and day care center fomites. J Infect 51, 103–109. [DOI] [PubMed] [Google Scholar]
- Boone, S.A. and Gerba, C.P. (2007) Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 73, 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman, I.L. , Dijkman, R. , te Loeke, N.A. , Hägele, G. , Tilburg, J.J. , Vennema, H. and Koopmans, M. (2009a) Environmental swabs as a tool in Norovirus outbreak investigation, including outbreaks on cruise ships. J Food Prot 72, 111–119. [DOI] [PubMed] [Google Scholar]
- Boxman, I. , Dijkman, R. , Verhoef, L. , Maat, A. , van Dijk, G. , Vennema, H. and Koopmans, M. (2009b) Norovirus on swabs taken from hands illustrate route of transmission. J Food Prot 72, 1753–1755. [DOI] [PubMed] [Google Scholar]
- Budavari, S. (2001) Monographs In The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th edn ed. O’Neil M.J., Smith A., Heckelman P.E. and Budavari S. pp. 362. Whitehouse Station, NJ, USA: Merck & Co., Inc. [Google Scholar]
- Cozad, A. and Jones, R.D. (2003) Disinfection and the prevention of infectious disease. Am J Infect Control 31, 243–254. [DOI] [PubMed] [Google Scholar]
- Dobson, S. (2002) Effects on laboratory mammals and in vitro test systems. Previous evaluations by international bodies In Concise International Chemical Assessment Document 37—Chlorine Dioxide (Gas) ed. Dobson S. and Cary R. pp. 1–26. Geneva: World Health Organization. [Google Scholar]
- Fukuda, T. , Miura, T. , Morino, H. and Kase, T. (2011) The 85th annual meeting of the Japanese association for infectious diseases, abstract.
- Gates, D. (1998) Introduction In The Chlorine Dioxide Handbook‐Water Disinfection Series ed. Cobban B. pp. 1–6. Denver: American Water Works Association. [Google Scholar]
- Goldmann, D.A. (2000) Transmission of viral respiratory infections in the home. Pediatr Infect Dis J 19, S97–S102. [DOI] [PubMed] [Google Scholar]
- Hierholzer, J.C. and Killington, R.A. (1996) Virus isolation and quantitation In Virology Methods Manual ed. Mahy B.W.J. and Kangro H.O. pp. 25–46. London: Harcourt Brace & Company. [Google Scholar]
- Hota, B. (2004) Contamination, disinfection, and cross‐colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 39, 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan, L.J. , Aiello, A.E. and Larson, E. (2002) The role of the home environment in the transmission of infectious diseases. J Community Health 27, 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.Y. , Costello, M. and Kang, D.H. (2004) Efficacy of chlorine dioxide gas as a sanitizer of lettuce leaves. J Food Prot 67, 1371–1376. [DOI] [PubMed] [Google Scholar]
- Morino, H. , Fukuda, T. , Miura, T. , Lee, C. , Shibata, T. and Sanekata, T. (2009) Inactivation of feline calicivirus, a Norovirus surrogate, by chlorine dioxide gas. Biocontrol Sci 14, 147–153. [DOI] [PubMed] [Google Scholar]
- Nada, O. and Gregory, H. (2006) Almost a Quarter of All Disease Caused by Environmental Exposure. Geneva, Switzerland: World Health Organization, http://www.who.int/mediacentre/news/releases/2006/pr32/en/ . [Google Scholar]
- Ogata, N. (2007) Denaturation of protein by chlorine dioxide: oxidative modification of tryptophan and tyrosine residues. Biochemistry 46, 4898–4911. [DOI] [PubMed] [Google Scholar]
- Otto, M. (2010) Looking toward basic science for potential drug discovery targets against community‐associated MRSA. Med Res Rev 30, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter, M.L. (1997) Hand washing, hand disinfection, and skin disinfection In Prevention and Control of Nosocomial Infections ed. Wenzel R.P. pp. 1052–1068. Philadelphia: Williams & Wilkins. [Google Scholar]
- Sanekata, T. , Fukuda, T. , Miura, T. , Morino, H. , Lee, C. , Maeda, K. , Araki, K. , Otake, T. et al. (2010) Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci 15, 45–49. [DOI] [PubMed] [Google Scholar]
- Scott, E. , Duty, S. and Callahan, M. (2008) A pilot study to isolate Staphylococcus aureus and methicillin‐resistant S. aureus from environmental surfaces in the home. Am J Infect Control 36, 458–460. [DOI] [PubMed] [Google Scholar]
- Scott, E. , Duty, S. and McCue, K. (2009) A critical evaluation of methicillin‐resistant Staphylococcus aureus and other bacteria of medical interest on commonly touched household surfaces in relation to household demographics. Am J Infect Control 37, 447–453. [DOI] [PubMed] [Google Scholar]
- Sy, K.V. , McWatters, K.H. and Beuchat, L.R. (2005) Efficacy of gaseous chlorine dioxide as a sanitizer for killing salmonella, yeasts, and molds on blueberries, strawberries, and raspberries. J Food Prot 68, 1165–1175. [DOI] [PubMed] [Google Scholar]
- Toda, S. , Watabe, S. , Matsuda, T. , Matsuda, Y. , Haraguchi, S. , Ikeda, K. and Okuda, K. (2006) Antimicrobial and bacteriostatic efficacy of chlorine dioxide. Kankyokansen 21, 231–235. [Google Scholar]
- Wilson, S.C. , Wu, C. , Andriychuk, L.A. , Martin, J.M. , Brasel, T.L. , Jumper, C.A. and Straus, D.C. (2005) Effect of chlorine dioxide gas on fungi and mycotoxins associated with sick building syndrome. Appl Environ Microbiol 71, 5399–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]


