Abstract
Twenty-one patients aged 4–78 years with influenza A virus–associated acute encephalopathy were studied. Influenza A virus could be detected only in a cerebrospinal fluid (CSF) specimen obtained from 1 of 18 patients, despite the use of a highly sensitive polymerase chain reaction assay. Six patients experienced influenzal encephalopathy during the course of respiratory illness. Five of these patients had hypoprothrombinemia and 4 had increased serum creatinine levels, indicating hepatic and/or renal dysfunction. Fourteen patients experienced postinfluenzal encephalopathy ⩽3 weeks after resolution of acute respiratory symptoms. In 6 patients, focal areas of high signal intensity were visible on T2-weighted magnetic resonance images of the brain. Adenovirus DNA was detected in CSF specimens obtained from 4 (36%) of 11 patients with postinfluenzal encephalopathy. Thus, influenzal encephalopathy is frequently associated with metabolic disorders, whereas postinfluenzal encephalopathy appears to have different possible etiologies.
Involvement of the CNS in influenza virus infection is very rare, but serious manifestations, such as convulsions, psychosis, stupor, and coma, have been described in individual cases [1]. For almost a century, there has been debate about a causal link between severe neurological disease and influenza virus infection. After the development of laboratory diagnostic assays, it became apparent that only very few of the patients with acute encephalopathy or encephalitis associated with an influenza-like illness were actually infected with influenza virus [2, 3]. Consequently, most of the current knowledge about influenza-associated neurological disorders was derived from reports of a small number of well-documented cases (reviewed in [4]). An outbreak of influenza-associated encephalopathy in Japan was reported recently [5], but clinical presentation in this large cohort was distinct from that observed previously in patients with influenza-associated encephalopathy.
Attempts to demonstrate the presence of the virus in the CSF or brains of patients suspected of having influenza virus encephalitis have only been successful on rare occasions in the past [6, 7]. Recently, detection rates of influenza viruses in CSF samples have increased with the application of highly sensitive, influenza virus–specific PCR assays [5, 8]. It is unclear, however, whether the use of PCR assays for routine CSF diagnostics may further increase the frequency of influenza virus detection in the CSF and how influenza virus–positive PCR results should be interpreted. This is especially important in light of frequent detection of other viral pathogens in CSF samples obtained from patients with influenza-associated encephalopathy [9, 10]. The aim of this study was to gain insight into influenza-associated acute encephalopathy by evaluating virological, clinical, laboratory, and neuroradiographic features in a well-defined cohort of patients.
Materials and Methods
Patient population. During the periods of epidemics of influenza infection in 1998–1999, 1999–2000, and 2000–2001, influenza A virus infection was diagnosed in 571 hospitalized patients by serological testing (407 patients; defined by a ⩾4-fold increase in the titer or a titer of >64 determined with use of a complement fixation [CF] test) or by virus detection (164 patients; determined by virus isolation, antigen ELISA, or both). These periods of epidemic influenza activity lasted 7–10 weeks, and the predominant circulating influenza strains were A/H1N1 and A/H3N2.
Patients with laboratory-confirmed influenza A virus infection and a discharge diagnosis of acute encephalopathy, encephalitis, meningitis, or transverse myelitis were retrospectively identified through a review of case histories. We only included patients who additionally had a febrile, influenza-like illness, defined by acute onset of fever (temperature, >37.8°C) with cough, rhinorrhea, or sore throat during periods of epidemic activity of influenza infection. Patients who had postictal unconsciousness with prompt recovery or febrile convulsions without prolonged unconsciousness were excluded from the study.
A review of medical records was performed in accordance with the recommendations of the Institutional Review Board of the University of Vienna (Austria). The information collected included demographic characteristics, clinical signs and symptoms, history of chronic illnesses, and onset and duration of respiratory and neurological symptoms. Patients were assigned to 2 groups of encephalopathy types on the basis of the timing of acute respiratory tract illness caused by influenza A virus and onset of acute neurological symptoms: influenzal encephalopathy (i.e., acute encephalopathy occurring in the course of the acute respiratory illness) and postinfluenzal encephalopathy (i.e., acute encephalopathy occurring within 3 days to 3 weeks after resolution of the influenza-related respiratory symptoms).
Electroencephalographic and neuroradiographic findings (by CT and MRI) were obtained and selected blood chemical values determined at the time of admission to the hospital. CSF WBC count, CSF total protein level, and albumin level were determined. To investigate whether the clinical signs of encephalopathy observed could be attributed to a metabolic disorder, the following blood chemical values were evaluated: serum alcohol, aspartate aminotransferase, alanine aminotransferase, bilirubin, prothrombin time, serum creatinine, blood urea nitrogen, serum potassium, serum sodium, and serum glucose. The ratio of serum albumin to CSF albumin was assessed to investigate the integrity of the blood-brain barrier, and a ratio of >8 was considered indicative of increased passive permeability.
Laboratory diagnosis of influenza virus infection and other viral infections. Influenza A virus–specific antibodies were detected with use of the CF test and hemagglutination inhibition test, as described elsewhere [11]. Virus isolation was performed with Madin-Darby canine kidney cells (American Type Culture Collection), and influenza A strains isolated during the study period were typed and subtyped by immunofluorescence staining with use of the monoclonal antibodies MAB8252 (H1N1) and MAB8254 (H3N2) (Chemicon International), as described elsewhere [11]. Our in-house ELISA was used as described elsewhere [12].
Antibodies specific for enteroviruses, influenza B viruses, human cytomegalovirus, measles, mumps, and rubella were detected with use of CF test, and antibodies specific for Epstein-Barr virus capsid antigen were identified by indirect immunofluorescence, as described elsewhere [11]. The detection of tickborne encephalitis virus (TBEV) infection was based on the presence of virus-specific IgM antibodies in serum and CSF specimens [13].
Detection of virus-specific nucleic acid sequences. Blood and CSF samples that were sent to the laboratory to screen for a viral cause of the encephalopathy were tested with use of an influenza A virus–specific RT-PCR assay, as described elsewhere [14]. After addition of 1 µL of RNase inhibitor (Boehringer) in a final concentration of 0.01 U/µL, viral RNA was extracted from 140 µL of the sample with use of a QIAamp Viral RNA kit (Qiagen). Subsequently, reverse transcription at 60°C and amplification of viral RNA were performed by means of a nested PCR protocol. The PCR amplicons were visualized by gel electrophoresis and ethidium bromide staining on 3% NuSieve agarose gel (FMC) with 0.5 µg of ethidium bromide per milliliter in the gel.
The following precautions were taken to avoid degradation of the viral genome in clinical specimen and false-positive results. All samples were stored at -70°C and were thawed only once for RNA extraction. Every positive PCR result was confirmed by testing a different aliquot of the original sample. Each PCR experiment included 2–5 positive controls, several negative controls, and 2–3 respiratory virus–positive specimens (respiratory syncytial virus, enteroviruses, rhinoviruses, coronavirus, parainfluenza viruses, or adenoviruses) interposed between the samples tested. For the detection of other viral nucleic acids in the CSF specimen, the following published PCR assays were used: herpes simplex virus type 1 (HSV-1) PCR [15], herpes simplex virus type 2 (HSV-2) PCR [16], varicella zoster virus (VZV) PCR [17], and adenovirus PCR [18].
Statistical analysis. Comparison of the 2 groups was performed using the Mann-Whitney U test, for quantitative parameters, and Fisher's exact test, for categorical parameters. For all statistical tests, P <.05 was considered to be statistically significant. All statistical analyses were performed with use of SPSS commercial software, version 10.0 (SPSS).
Results
Study population and clinical features. A total of 21 patients met the selection criteria of acute encephalopathy associated with an influenza A virus infection during the influenza epidemics of 1998–1999, 1999–2000, and 2000–2001. The discharge diagnoses for the 21 patients were acute encephalopathy (2 patients), encephalitis (13 patients), meningitis (5 patients), and transverse myelitis (1 patient). The virological diagnosis of an influenza A virus infection was established for 19 of these patients by serological testing (i.e., a ⩾4-fold increases in the CF titer [3 patients] or a CF titer of >64 [16 patients]), and, in 2 patients, influenza A/H3N2 virus was recovered from nasopharyngeal secretions by virus isolation. The specificity of the increased CF titer could be confirmed in all 19 patients by a hemagglutination inhibition antibody titer to influenza A virus of >1 : 80. The results of serologic screening for other viral infections (enterovirus infections, influenza B virus infections, cytomegalovirus infections, measles, mumps, rubella, Epstein-Barr virus infection, and TBEV infection) were negative for all of these patients.
Table 1 summarizes clinical presentation data, laboratory and neuroradiographic findings, and outcome for patients with influenza-associated encephalopathy. The mean age (±SD) of the patients was 40.9 ± 25.7 years (range, 4–78 years), and none of the patients had received an influenza vaccination or had been treated with antiviral drugs effective against influenza viruses. In the 6 patients with influenzal encephalopathy, neurological complaints began a median of 3 days (range, 1–10 days) after the onset of influenza-related symptoms, and, in the 14 patients with postinfluenzal encephalopathy, neurological complaints began a median of 9.5 days (range, 7–23 days) after the onset of influenza.
Table 1.
Clinical and laboratory features of 21 patients with influenza-associated encephalopathy.
One patient contracted an influenza A virus infection while convalescing from HSV-1 encephalitis. HSV-1 encephalitis was confirmed by the detection of HSV-1 DNA in a CSF specimen on the fifth day of neurological disease. The first influenza-related respiratory symptoms were observed 15 days after the onset of encephalitis. No deterioration of residual neurological symptoms was observed during the course of influenza.
Patients with influenzal encephalopathy improved rapidly after admission to the hospital. They were hospitalized for a significantly shorter period of time (median, 9.5 days vs. 29 days; P =.035, determined by Mann-Whitney U test) and less frequently had neurological sequelae (0 of 6 patients vs. 9 of 14 patients; P =.015, determined by Fisher's exact test) compared with patients who had postinfluenzal encephalopathy.
Virological findings. The 18 CSF samples available for virological investigation were obtained during the symptomatic period of encephalopathy within a median of 2 days (interquartile range, 1–3 days) after the onset of neurological illness. Influenza A virus RNA could be detected in only 1 of 18 CSF samples. This sample was obtained from the patient with preceding HSV-1 encephalitis 3 days after the onset of the respiratory illness and 18 days after the onset of HSV-1 encephalitis.
To evaluate the presence of viral pathogens other than influenza A virus in the CSF, all CSF samples available were also tested with use of virus-specific PCR assays for the presence of HSV-1, HSV-2, VZV, and, because of their possible neuropathogenic potential, adenoviruses. Adenovirus DNA was detected in 4 of 17 specimens, and VZV DNA was detected in 1 of 18 specimens; all of these specimens were obtained from patients with postinfluenzal encephalopathy (table 1).
To investigate whether detection of viral genome in CSF specimens was associated with concurrent viremia, serum samples that were obtained at the same time as the influenza A virus– or adenovirus-positive CSF samples were also tested for the presence of virus by PCR. Influenza A virus was also detected in the serum sample obtained from the patient with the influenza A virus–positive CSF. Three serum samples were available from the 4 patients whose CSF specimens tested positive for adenovirus, and these samples were also found to be adenovirus DNA positive. The albumin level could be assessed in 2 of these viremic patients, including the influenza A virus–positive patient and one adenovirus-positive patient; it was assessed to evaluate the possibility of passive diffusion of viral genome from the periphery into the CSF. An increased albumin level was detected in both patients (table 1), indicative of a possible association between viremia and viral genome present in CSF as a result of an increased permeability of the blood-brain barrier.
To evaluate whether adenovirus may also be detected in the CSF of patients with acute virus-associated encephalopathy due to other pathogens, CSF samples obtained from 17 control patients were tested by adenovirus-specific PCR. These control patients had laboratory-confirmed TBEV infection and were matched by age with the 17 patients who had influenza-associated encephalopathy revealed by PCR. None of these control samples tested positive for adenovirus DNA.
When comparing the clinical features of adenovirus-positive and -negative patients, it became apparent that adenovirus-positive patients experienced convulsions significantly more frequently (4 of 4 patients vs. 2 of 13 patients; P =.006, determined by Fisher's exact text; table 1). No other significant differences between these 2 patient groups could be identified when other clinical, laboratory, and neuroradiographic parameters were evaluated.
Other laboratory findings. Blood parameters determined at the time of admission to the hospital were assessed. Table 2 compares patients who had influenzal encephalopathy with patients who had postinfluenzal encephalopathy with respect to abnormal blood parameters. A significant proportion of patients with influenzal encephalopathy had abnormal prothrombin times (range, 24%–50%), creatinine levels (range, 1.6–5.0 mg/dL), and blood urea nitrogen levels (range, 43–119 mg/dL) at the time of hospital admission, indicating that the majority of these patients had hepatic as well as renal dysfunction at the time of neurological illness. Aspartate aminotransferase, alanine aminotransferase, and bilirubin values were elevated in only 1 patient each with influenzal encephalopathy, an abnormal prothrombin time, and an abnormal creatinine level (1 of 20 patients, 1 of 19 patients, and 1 of 15 patients, respectively). Evidence of hypoglycemia or abnormal potassium and/or sodium levels as another possible cause of the acute encephalopathy was not found in the patients investigated (0 of 18 patients and 0 of 19 patients, respectively).
Table 2.
Comparison of occurrence of risk factors surveyed by the risk assessment questionnaire given to 5382 students with or without latent tuberculosis (TB) infection (tuberculin skin test [TST] induration, ⩾10 mm).
MRI. Neuroradiographic imaging studies were performed for 15 of 20 patients with influenzal or postinfluenzal encephalopathy. Evidence of acute cerebral lesions was not found in any of the patients with influenzal encephalopathy, but it was found in 6 (46%) of 13 patients with postinfectious encephalopathy. Four of these 6 patients received therapy with corticosteroids, and their conditions significantly improved.
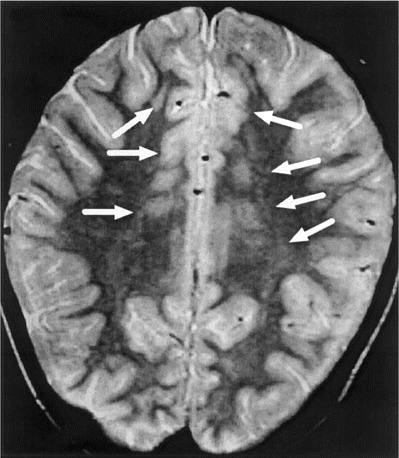
In 2 patients, a single, large area of increased signal intensity in the temporal and parietal regions of the brain associated with significant cerebral edema was observed on T2-weighted MRIs (figure 1). In the other 4 patients, MRI revealed multiple foci of high–signal intensity lesions on T2-weighted images, which were distributed asymmetrically throughout the brain, including varying areas of parietal, occipital, or temporal regions of the brain, thalamus, pons, or corpus callosum (figure 2) characteristic of postinfectious demyelinating encephalitis. Moreover, in an 11-year-old patient with postinfluenzal transverse myelitis, a single high–signal intensity lesion on T2-weighted MRIs was found in the region of the conus medullaris. Of the 4 adenovirus-positive patients with acute postinfluenzal encephalopathy, only 1 had evidence of acute lesions of the brain (table 1 and figure 1).
Figure 1.
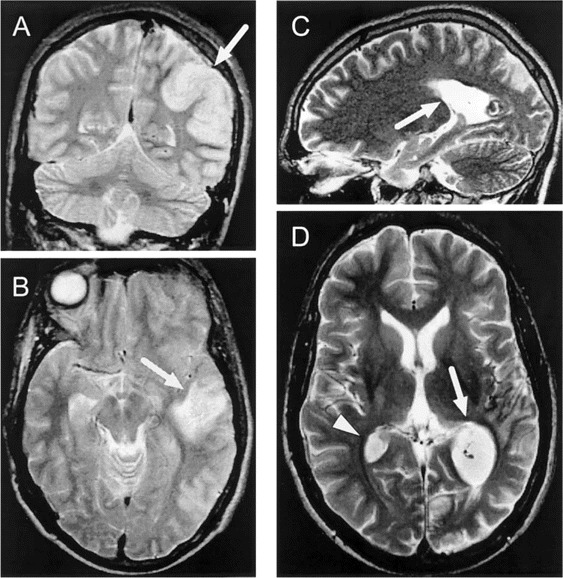
A and B, Single, large, tumorlike lesion of the brain (arrows) associated with cerebral edema in a 23-year-old patient with postinfluenzal encephalopathy noted on T2-weighted MRIs. Adenovirus DNA was detected in a CSF sample obtained from this patient 1 day earlier. C and D, MRIs for this patient 2 months later that reveal regression of this single lesion (arrow) but also an additional lesion (arrowhead).
Figure 2.
Comparison of selected laboratory parameters for patients with influenzal or postinfluenzal encephalopathy.
Discussion
The results of this study demonstrate that detection of influenza A virus RNA is only rarely achieved when testing CSF samples obtained from patients with influenza-associated acute encephalopathy. Influenza A virus RNA was detected only in the CSF sample of 1 patient, despite testing the CSF samples with a highly sensitive influenza A virus–specific PCR assay [14]. Accordingly, influenza virus could rarely be detected in CSF samples obtained from patients who had influenza-associated encephalopathy or encephalitis in the past [4, 5, 19]. It cannot be entirely discounted that, in the present cases, lumbar puncture was performed too late for the detection of influenza A virus in the CSF. However, this seems unlikely, because all CSF samples were obtained during the course of encephalopathy, and 75% of the samples were obtained ⩽3 days after the onset of neurological symptoms.
In addition, the clinical significance of the presence of influenza virus genome in the CSF is questionable. We found an increased permeability of the blood-brain barrier in the only influenza virus–positive patient, indicating passive diffusion of viral genome from the periphery into the CSF. Although the integrity of the blood-brain barrier was not evaluated in previous studies [5, 8], it has been suggested that direct invasion by influenza A virus and inflammation is unlikely to be the cause of encephalopathy [5]. Moreover, histological abnormalities of the brain are often absent in patients who die with clinical signs of influenza-associated encephalopathy [20], and despite the occurrence of massive brain edema, no influenza antigen has been detected in the brains of patients with influenza-associated encephalopathy [5]. All of these observations indicate that the integrity of the blood-brain barrier may be an important factor for the detectability of influenza A virus RNA in CSF specimens.
Adenovirus DNA could be detected in 4 (36%) of 11 CSF samples obtained from patients with postinfluenzal encephalopathy. Adenovirus infection has been reported only sporadically in association with acute CNS disease [21, 22], and, in a previous study that used the same PCR assay, we found that detection of adenovirus in CSF specimens from patients with encephalitis is unusual [15]. It is noteworthy that adenovirus DNA was detected more frequently in these patients than in control specimens, but it is unclear whether the presence of adenovirus in the CSF in these patients indicates adenovirus infection of the brain or is entirely unrelated to the patients' symptoms. In addition, the role of other pathogens in the etiology of postinfluenzal encephalopathy remains to be elucidated.
One-third of the patients had acute influenza-associated encephalopathy in the course of the acute respiratory illness, and 67% of these patients showed evidence of hepatic and renal function abnormalities. Additional observations further point to a metabolic cause of the influenzal encephalopathies observed: the most prominent neurological disorders were disorientation and altered consciousness; the duration of hospitalization was short for these patients, compared with patients who had postinfluenzal encephalopathy; and patients did not experience neurological sequelae. These findings are in concordance with previous studies of influenza-associated encephalopathy that found liver function abnormalities [5, 8, 20, 23]. Thus, metabolic encephalopathies may be more common in patients with influenza A virus infection than has previously been acknowledged as a result of their wide variety of clinical manifestations, ranging from deep coma to subtle abnormalities detectable only by psychometric testing [24]. Therefore, annual influenza vaccination and early recognition of influenza virus infection by rapid and sensitive assays seems all the more important, especially in patients who have preexisting chronic liver or kidney disease.
Unlike patients with influenzal encephalopathy, 54% of patients with postinfluenzal encephalopathy had cerebral or spinal lesions detectable by MRI. Three different patterns of CNS lesions were identified: multiple, small disseminated lesions resembling those seen in postinfectious encephalomyelitis; single, large, tumorlike lesions of the brain; and a single lesion located at the conus medullaris.
MRI revealed that all of these lesions had a demyelinating nature, and the patients' conditions improved soon after corticosteroid therapy was initiated. Nevertheless, evidence of demyelination is only suggestive because no brain biopsies were performed, and postinfectious demyelination in the CNS is not usually thought to result in large lesions like those found in 2 of the patients we describe. It was suggested previously that large, focal, tumorlike demyelinating lesions of the brain represent an entity intermediately between classic multiple sclerosis and postinfectious encephalomyelitis [25], which should also be considered for the present cases.
In conclusion, influenzal encephalopathy was frequently associated with metabolic disturbances, whereas postinfluenzal encephalopathy seemed to be a less distinct clinical entity. Divergent CSF WBC values, presence of viruses other than influenza virus in the CSF, and diverse MRI findings suggest that the clinical diagnosis of postinfluenzal encephalopathy, established by the close timing between acute respiratory tract illness and onset of acute neurological symptoms, has a number of different possible etiologies.
Acknowledgements
We thank Thomas Urbanek, Carolin Günther, Claudia Kellner, Edith Mlynar, and Karin Kraus, for their excellent technical assistance, and the many physicians at the following Austrian hospitals, for their excellent cooperation and for providing clinical data: Department of Pediatrics, Hospital Barmherzigen Brüder, Eisenstadt; Intensive Care Unit, Hanusch Hospital, Vienna; Department of Pediatrics, Hospital Oberwart, Oberwart; Department of Internal Medicine, Wilhelminenspital, Vienna; Department of Neurology, Hospital Klagenfurt, Klagenfurt; Department of Pediatrics, Hospital Amstetten, Amstetten; Department of Internal Medicine, Kaiserin Elisabethspital, Vienna; Department of Pediatrics, Donauspital–Sozialmedizinischen Zentrum Ost, Vienna; Neurological Hospital Rosenhügel, Vienna; Department of Internal Medicine; Children's Hospital Linz, Linz; Department of Internal Medicine; Sozialmedizinisches Zentrum Otto Wagner-Spital, Vienna; Department of Internal Medicine and Department of Neurology, Hospital Lainz, Vienna; Hospital Göttlicher Heiland, Vienna; Department of Pediatrics, Hospital St. Pölten, St. Pölten; Department of Neurology, Hospital Villach, Villach; Department of Neurology, Hospital Rudolfstiftung, Vienna.
References
- 1.Wright PF, Webster RG. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Field's virology. 4th ed. Philadelphia: Lippincott Raven; 2001. pp. 1533–79. [Google Scholar]
- 2.Mellman WJ. Influenza encephalitis. J Pediatr. 1958;1:292–7. doi: 10.1016/s0022-3476(58)80214-0. [DOI] [PubMed] [Google Scholar]
- 3.Flewett TH, Hoult JG. Influenza encephalopathy and postinfluenza encephalitis. Lancet. 1958;2:11–5. doi: 10.1016/s0140-6736(58)90003-5. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Webster RG, Hay AJ. Textbook of influenza. London: Blackwell Science; 1998. [Google Scholar]
- 5.Morishima T, Togashi T, Yokota S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–7. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 6.Paisley JW, Bruhn FW, Lauer BA, McIntosh K. Type A2 influenza viral infections in children. Am J Dis Child. 1978;132:34–6. doi: 10.1001/archpedi.1978.02120260036007. [DOI] [PubMed] [Google Scholar]
- 7.Price DA, Postlethwaite RJ, Longson M. Influenzavirus A2 infections presenting with febrile convulsions and gastrointestinal symptoms in young children. Clin Pediatr (Phila) 1976;15:361–7. doi: 10.1177/000992287601500408. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto S, Kobayashi M, Uemura O, et al. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet. 1998;352:873–5. doi: 10.1016/S0140-6736(98)12449-2. [DOI] [PubMed] [Google Scholar]
- 9.Sugaya N, Yoshikawa T, Miura M, Ishizuka T, Kawakami C, Asano Y. Influenza encephalopathy associated with infection with human herpesvirus 6 and/or human herpesvirus 7. Clin Infect Dis. 2002;34:461–6. doi: 10.1086/338468. [DOI] [PubMed] [Google Scholar]
- 10.Studahl M, Bergstrom T, Hagberg L. Acute viral encephalitis in adults—a prospective study. Scand J Infect Dis. 1998;30:215–20. doi: 10.1080/00365549850160828. [DOI] [PubMed] [Google Scholar]
- 11.Lennette EH, Schmidt NJ. Diagnostic procedures for viral, rickettsial and chlamydial infections. 5th ed. Washington, DC: American Public Health Association; 1979. [Google Scholar]
- 12.Sarkkinen HK, Halonen PE, Salmi AA. Detection of influenza A virus by radioimmunoassay and enzyme-immunoassay from nasopharyngeal specimens. J Med Virol. 1981;7:213–20. doi: 10.1002/jmv.1890070305. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann H, Frisch-Niggemeyer W, Heinz F. Rapid diagnosis of tick-borne encephalitis by means of enzyme linked immunosorbent assay. J Gen Virol. 1979;42:505–11. doi: 10.1099/0022-1317-42-3-505. [DOI] [PubMed] [Google Scholar]
- 14.Steininger C, Kundi M, Aberle SW, Aberle JH, Popow-Kraupp T. Effectiveness of reverse transcription-PCR, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza A virus infection in different age groups. J Clin Microbiol. 2002;40:2051–6. doi: 10.1128/JCM.40.6.2051-2056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puchhammer-Stöckl E, Presterl E, Croy C, et al. Screening for possible failure of herpes simplex virus PCR in cerebrospinal fluid for the diagnosis of herpes simplex encephalitis. J Med Virol. 2001;64:531–6. doi: 10.1002/jmv.1082. [DOI] [PubMed] [Google Scholar]
- 16.Aurelius E, Johansson B, Skoldenberg B, Forsgren M. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol. 1993;39:179–86. doi: 10.1002/jmv.1890390302. [DOI] [PubMed] [Google Scholar]
- 17.Puchhammer-Stöckl E, Popow-Kraupp T, Heinz FX, Mandl CW, Kunz C. Detection of varicella-zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol. 1991;29:1513–6. doi: 10.1128/jcm.29.7.1513-1516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allard A, Albinsson B, Wadell G. Detection of adenoviruses in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J Med Virol. 1992;37:149–57. doi: 10.1002/jmv.1890370214. [DOI] [PubMed] [Google Scholar]
- 19.Hoult JG, Flewett TH. Influenzal encephalopathy and post-influenzal encephalitis: histological and other observations. BMJ. 1958;1:1847–50. doi: 10.1136/bmj.1.5189.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–65. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitley RJ, Cobbs CG, Alford CA, Jr, et al. Diseases that mimic herpes simplex encephalitis: diagnosis, presentation, and outcome. NIAD Collaborative Antiviral Study Group. JAMA. 1989;262:234–9. [PubMed] [Google Scholar]
- 22.Berlin LE, Rorabaugh ML, Heldrich F, Roberts K, Doran T, Modlin JF. Aseptic meningitis in infants <2 years of age: diagnosis and etiology. J Infect Dis. 1993;168:888–92. doi: 10.1093/infdis/168.4.888. [DOI] [PubMed] [Google Scholar]
- 23.Kapila CC, Kaul S, Kapur SC, Kalayanam TS, Banerjee D. Neurological and hepatic disorders associated with influenza. BMJ. 1958:1311–4. doi: 10.1136/bmj.2.5108.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bircher J, Benhamou JP, McIntyre N, Rizzetto M, Rodés J. Oxford textbook of clinical hepatology. 2nd ed. New York: The University of Chicago Press; 1999. [Google Scholar]
- 25.Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]


