Abstract
Purpose:
To identify distinct diet trajectories after breast cancer (BC) diagnosis, and to examine characteristics associated with diet trajectories.
Methods:
We analyzed 2,865 Pathways Study participants who completed ≥2 food frequency questionnaires at the time of BC diagnosis (baseline), and 6 and 24 months after baseline. Trajectory groups of fruit and vegetable (F/V) intake, % calories from dietary fat, and alcohol intake over 24 months were identified using group-based trajectory modeling. Associations between diet trajectories and sociodemographic, psychosocial, and clinical factors were analyzed using multinomial logistic regression.
Results:
Analyses identified 3 F/V trajectory groups, 4 dietary fat groups, and 3 alcohol groups. All 3 F/V trajectory groups reported slightly increased F/V intake post-diagnosis (mean increase=0.2–0.5 serving/day), while 2 groups (48% of participants) persistently consumed <4 servings/day of F/V. Dietary fat intake did not change post-diagnosis, with 45% of survivors maintaining a high-fat diet (>40% of calories from fat). While most survivors consumed <1 drink/day of alcohol at all times, 21% of survivors had 1.4–3.0 drinks/day at baseline and temporarily decreased to 0.1–0.5 drinks/day at 6 months. In multivariable analysis, diet trajectory groups were significantly associated with education (ORs: 1.93–2.49), income (ORs: 1.32–2.57), optimism (ORs: 1.93–2.49), social support (OR=1.82), and changes in physical well-being (ORs: 0.58–0.61) and neuropathy symptoms after diagnosis (ORs: 1.29–1.66).
Conclusions:
Pathways Study participants reported slightly increasing F/V and decreasing alcohol intake after BC diagnosis. Nearly half of survivors consumed insufficient F/V and excessive dietary fat. It is important to prioritize nutrition counseling and education in BC survivors.
INTRODUCTION
A healthy diet may prevent and alleviate common late effects of breast cancer and its treatments, including cardiovascular disease [1], diabetes and other metabolic disorders [2], and osteoporosis [3]. The American Cancer Society and the American Institute of Cancer Research have developed dietary recommendations for cancer survivors based on lifestyle guidelines for cancer prevention. Specifically, the recommendations advise that cancer survivors consume at least 5 servings of fruit and vegetables (F/V) per day, eat whole grains, avoid high-fat and processed foods, and that female cancer survivors limit alcohol intake to less than 1 drink per day [4,5]. However, recent population-based studies in the United States show that only 8–18% of breast cancer survivors meet the recommended F/V intake, and only 7.5% reported <20% of total energy from calories attributed to solid fat, alcohol, and added sugar (also known as “empty calories”), which is the limit set forth by the Healthy Eating Index (HEI) 2010 [6–8]. These data suggest a large gap between the latest dietary recommendations for cancer survivors and breast cancer survivors’ actual dietary habits. Understanding how women change their diet following a breast cancer diagnosis is an important step in developing dietary interventions to close this gap.
Several prospective studies have examined changes in F/V [9–15], dietary fat [9,16,17,10,12–14], and alcohol intake [18,19,9,12,14] after a breast cancer diagnosis. There is consistent evidence that some women reduce alcohol intake after a breast cancer diagnosis, whereas studies of F/V and dietary fat often report small to no change. One limitation of these studies is the analytical approach used to understand dietary change, which relies on testing the overall mean change in diet between two time points. This type of analysis does not consider the variation in intrapersonal change trajectories and provides little insight into the type of dietary change. Analysis of mean change also inherently assumes that dietary change occurs linearly over time, while changing diet is a dynamic process influenced by multiple factors in the physical environment, social environment, and medical care [20]. Group-based trajectory modeling (GBTM) is a type of analysis that aims at identifying clusters of participants, also known as trajectory groups, who followed similar developmental trajectories of a single outcome over a period of time [21]. A trajectory group is a collection of individuals who have approximately the same shape and level of developmental trajectory. GBTM allows researchers to categorize individual developmental trajectories into subgroups and examine predictors of each trajectory group. This analytical method allows for the modeling of non-linear trajectories, which is particularly suitable for capturing non-persistent changes in diet during the period of active cancer treatment to early survivorship. Another limitation of prior studies is the lack of examination of the clinical and psychosocial predictors of dietary change among breast cancer survivors, which limits our understanding of the target population for whom dietary interventions are most needed.
This analysis aims to address these limitations by using GBTM to identify distinct trajectories of F/V, dietary fat, and alcohol intake among breast cancer survivors over the first 24 months following breast cancer diagnosis. The analysis used data from the Pathways Study, a population-based prospective cohort of 4,505 women with newly diagnosed invasive breast cancer within Kaiser Permanente Northern California (KPNC) [22]. We hypothesized that the distinct diet trajectories following a breast cancer diagnosis would include the following: 1) maintenance of baseline diet level, 2) a stable increase 3) a stable decrease, 4) a temporary increase, and 5) a temporary decrease. We examined potential predictors of diet trajectories, including socioeconomic status (SES), psychosocial factors related to stress coping, and side effects of cancer treatment.
MATERIALS AND METHODS
Study participants
This analysis used data from the Pathways Study, a population-based prospective cohort of women with newly diagnosed invasive breast cancer at KPNC. Details of the Pathways Study are described elsewhere [22]. Briefly, eligible participants were women who were at least 21 years of age at diagnosis, a current KP member, had a recent diagnosis of invasive breast cancer, had no previous history of malignant cancer, spoke English, Spanish, Cantonese, or Mandarin, and lived within a 65-mile radius of a field interviewer. Women were recruited from January 2006 to April 2013 approximately two months post-diagnosis (mean time = 1.8 months, range = 0.3–7.2 months). Participants completed an in-person baseline interview to collect demographic, lifestyle behaviors, psychosocial, and anthropometric information. Follow-up data were collected via mailed questionnaires with interviewer assistance at 6 and 24 months after study enrollment. A total of 2,874 (63.8%) and 2,666 (59.2%) participants completed the 6- and 24-month follow-ups, respectively. This analysis was restricted to 2,865 participants (64%) who completed at least 2 of the 3 dietary questionnaires administered at baseline, 6 months, and 24 months follow up. The study was approved by the institutional review boards of all collaborating institutions, and all participants provided written informed consent.
Measurement of diet
Dietary history was collected using a 139-item modified version of the Block 2005 food frequency questionnaire (FFQ) (NutritionQuest, Berkeley, CA) [23]. The FFQ included food items selected by identifying the top population contributors of nutrients among Whites, African Americans and Hispanics in the United States. For this analysis, the primary dietary variables included daily intakes of fruits and vegetables (F/V), dietary fat and alcohol. Vegetable groups included dark-green vegetables, deep-yellow vegetables, tomatoes, white potatoes, fried potatoes, legumes, other starchy vegetables, avocado and similar, and other vegetables (measured in cups). Fruit groups included citrus fruit and other fruit (measured in cups). Dietary fat included total fat, saturated fat, monounsaturated fatty acids, polyunsaturated fatty acids, and trans-fats (measured in grams). Dietary fat was analyzed as nutrient density, which was calculated as 100% × (Dietary fat intake (grams) × 9)/ Total energy intake (kcal) [24]. Daily alcohol intake was measured by the FFQ and converted into daily ethanol intake (in grams).
Demographics, clinical characteristics, psychosocial factors, and cancer treatment side effects
Information about participants’ age, race/ethnicity, income and education were collected during baseline interview. Breast tumor and cancer treatment data were available from the KPNC Cancer Registry and the electronic health record (EHR) at approximately 6 months after diagnosis. Baseline psychosocial evaluated in this analysis included baseline depressive symptoms measured by the Center for Epidemiological Studies Depression Scale (CES-D) [25], dispositional optimism measured by the Life Orientation Test (LOT) [26], and social support measured by the Medical Outcome Study (MOS) Social Support Survey Instrument [27]. The scales and scoring of these psychosocial measures are summarized in Supplemental Table 1. The Pathways Study also measured self-reported cancer treatment-related side effects using the Functional Assessment of Cancer Therapy-Breast (FACT-B) [28,29], which quantified the symptoms of chemotherapy-induced peripheral neuropathy (CIPN) and loss of physical well-being from baseline to 6 months (Supplemental Table 1).
Statistical analysis
The analysis used semi-parametric, group-based trajectory modeling (GBTM) [21] to identify latent groups of diet trajectories and the shape parameters. The outcome variables were daily intake of F/V, % energy from dietary fat, and ethanol from baseline to 6 and 24 months. For each outcome variable, a single-group model saturated with quadratic parameters was tested initially, and then one additional group was included in each successive model. Analysis of F/V trajectory adjusted for total energy intake at each time point. Based on a priori hypothesis of the trajectory groups, we tested models composed of 1 to 6 trajectory groups to find the optimal number of trajectories. The best number of groups is determined through stepwise comparisons of the Bayesian information criterion (BIC) between the model with k groups vs. model with k+1 groups. The models with the lowest BIC and all group size ≥5% of the total sample were selected as the best models [21]. The selected models were evaluated using the average posterior probability of assignment for each group, odds of correct classification, and by comparing the actual and estimated proportion of groups [21]. Trajectory groups were labeled by the relative level of baseline diet (high, medium, or low), the direction of change (increaser, decreaser, or maintainer), and the sustainability of change (temporary or not). To evaluate the influence of loss to follow-up on trajectory group identification, a sensitivity analysis was performed by applying inverse probability weights (IPW) [30] of those remaining in follow up at 24 months to the GBTMs. Group memberships under IPW-weighted GBTM were preferred if they were found to be discordant with membership identified with unweighted GBTM based on Cohen’s kappa (kappa<0.8 as being discordant). Another sensitivity analysis examined the trajectory groups after excluding diet data with extreme total energy intake (>3 standard deviations of the mean, cutoff values were 3398.3 kcal/day at baseline, 3084.6 kcal/day at 6 months and 3175.2 kcal/day at 24 months), and evaluated their concordance with trajectory groups based on all available dietary data using Cohen’s kappa.
The average dietary intake and average change from baseline to 24 months by trajectory group were analyzed in generalized estimating equation models adjusting for total energy intake. We tested the association between SES, psychosocial factors, and cancer treatment side effects with each diet trajectory using multivariable multinomial logistic regression. The multivariable models controlled for a priori confounders, including age, race, menopausal status, baseline BMI, tumor stage, number of positive nodes, receipt of surgery, chemotherapy, hormonal therapy, and radiation therapy. Analyses were conducted in SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.4.3 (R Core Team, Vienna, Austria). GBTM was performed using the PROC TRAJ command in SAS. All statistical tests were two-sided with α=0.05.
RESULTS
Participant characteristics
A total of 2,865 participants were included in the analyses, whose characteristics are summarized in Table 1. Of these participants, 2,865 had diet data at baseline, 2,733 at 6 months, and 2,067 at 24 months; 65% participants had diet data at all 3 time points and 35% had data at 2 time points. On average, participants were diagnosed at the age of 61 years (range: 26–94 years) and were enrolled 2 months after diagnosis (range: 0–8 months). The majority of women were non-Hispanic White (69%), postmenopausal (74%), and overweight or obese (60%) at baseline. Nearly all participants were diagnosed with stage I-III breast cancer, 84% had estrogen or progesterone-receptor positive tumor, and 13% were positive for human epidermal growth factor receptor 2 (HER-2). Almost all participants received lumpectomy or mastectomy, 48% chemotherapy, 76% hormonal therapy, and 45% radiation therapy. At 6 months, 20–23% of participants reported worse CIPN and worse physical well-being.
Table 1.
Descriptive characteristics of 2,865 Pathways Study participants
| Variable | N (%) or mean (range) | |
|---|---|---|
| Age, years | <50 | 526 (18%) |
| 50–59 | 768 (27%) | |
| 60–70 | 922 (32%) | |
| 70+ | 649 (23%) | |
| Mean age, years | 61 (26–94) | |
| Mean time from diagnosis to baseline, months | 2 (0–8) | |
| Race/ethnicity | White | 1983 (69%) |
| Black | 163 (6%) | |
| Asian | 347 (12%) | |
| Hispanic | 302 (11%) | |
| Other | 70 (2%) | |
| Education | High school or less | 402 (14%) |
| Some college | 964 (34%) | |
| College or above | 1496 (52%) | |
| Household income | <$50K | 1259 (44%) |
| $50K–$89K | 1318 (46%) | |
| $90K+ | 288 (10%) | |
| Menopausal status | Premenopausal | 758 (26%) |
| Postmenopausal | 2107 (74%) | |
| Baseline BMI | Underweight (BMI <18.5 kg/m ) | 31 (1%) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 1110 (39%) | |
| Overweight (bMi 25.0–29.9 kg/m2) | 862 (30%) | |
| Obese (BMI >30.0 kg/m2) | 854 (30%) | |
| Tumor stage | I | 1600 (56%) |
| II | 966 (34%) | |
| III | 270 (9%) | |
| IV | 29 (1%) | |
| Number of positive nodes | 0 | 126 (4%) |
| 1 | 613 (21%) | |
| 2+ | 2126 (74%) | |
| HER2 positivity | Negative | 2380 (83%) |
| Positive | 372 (13%) | |
| ER/PR positivity | Negative | 446 (16%) |
| Positive | 2414 (84%) | |
| Surgery type | Lumpectomy | 1321 (46%) |
| Mastectomy | 1523 (53%) | |
| None | 21 (1%) | |
| Received chemotherapy | No | 1538 (54%) |
| Neoadjuvant | 121 (4%) | |
| Adjuvant | 1199 (42%) | |
| Received hormonal therapy | No | 690 (24%) |
| Yes | 2157 (76%) | |
| Received radiation | No | 1581 (55%) |
| Yes | 1283 (45%) | |
| Depressive symptom | Low | 2165 (76%) |
| High | 655 (23%) | |
| Dispositional optimism | Low | 1898 (66%) |
| High | 925 (32%) | |
| Perceived social support | Low | 902 (31%) |
| High | 1922 (67%) | |
| Worse physical well-being at 6 months | No | 2016 (70%) |
| Yes | 577 (20%) | |
| Worse CIPN at 6 months | No | 1276 (45%) |
| Yes | 655 (23%) | |
Note
Abbreviations: HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor; CIPN, chemotherapy-induced peripheral neuropathy
Characteristics of diet trajectory groups
The GBTM analysis identified a 3-group model for F/V, a 4-group model for dietary fat, and a 3-group model for alcohol intake. The model fitting statistics and distribution of posterior probability are shown in Supplemental Table 2. All models met the criteria for adequate model fit (Supplemental Table 3). Results based on GBTM were highly concordant with trajectory groups identified under IPW-weighted GBTM (all kappa>0.9). Therefore, trajectory groups derived by the unweighted GBTM were selected. Diet trajectory groups remained largely unchanged after excluding food records with energy intake greater than 3 standard deviations of the mean (kappa=0.98–0.99; Supplemental Table 4).
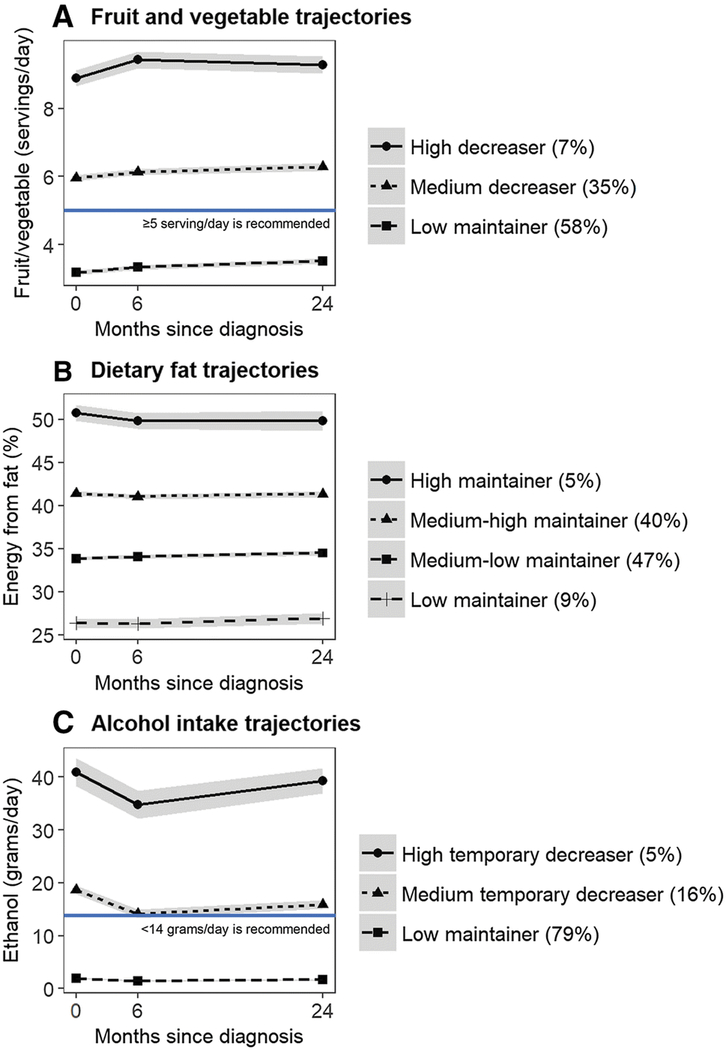
There were 3 distinct F/V trajectory groups characterized by an increasing trend in F/V, including the “high increaser” (11%), “medium increaser” (41%), and “low increaser” (48%) (Figure 1A), which on average reported 8.9, 6.0 and 3.2 servings/day of F/V at baseline, respectively. F/V intake significantly increased by 0.4–0.5 serving/day in the high increased group and by 0.2–0.3 serving/day in the medium and low increased groups (Supplemental Table 5) over the 24 months post-diagnosis. Most participants maintained stable dietary fat intake over the 24 months after diagnosis, with 5% of participants identified as “high maintainer” of dietary fat (51% calories from fat at baseline), 40% as “medium-high maintainer” (41% calories from fat), 47% as “medium-low maintainer” (34% calories from fat), and 9% as “low maintainer” (26% calories from fat) (Figure 1B). For alcohol intake, 79% of participants were “low maintainer” who consumed 1.9 grams/day of ethanol (< half drink/day) over the 24 months after diagnosis. Approximately 16% of participants consumed 18.7 grams/day of ethanol (1.4 drinks/day) and 5% consumed 40.9 grams/day of ethanol (3 drinks/day) at baseline and significantly reduced ethanol intake by 4.6–6.1 grams/day (<0.5 drinks/day) at 6 months only, and therefore were categorized as “medium temporary decreaser” and “high temporary decreaser”, respectively (Figure 1C).
Figure 1.
Mean and 95% confidence interval of dietary intakes by distinct diet trajectories during the first 24 months following a breast cancer diagnosis. The figure include trajectory groups of fruit/vegetable intake (Figure 1A), % energy from dietary fat (Figure 1B), and alcohol intake (Figure 1C). Mean and 95% confidence interval were calculated using generalized estimating equation model adjusting for total energy intake.
Factors associated with diet trajectory groups
Fruits and vegetables.
In multivariable analyses, compared with the low increasers of F/V, the high and medium increasers were more likely to be college educated or higher relative to those had a high school education or less (medium vs. low increasers: OR=2.18, 95% CI: 1.55, 3.06; high vs low increaser: OR=2.49, 95% CI: 1.49–4.17), and have household income of $50,000-$89,000 relative to <$50,000 (medium vs. low increasers: OR=1.32, 95% CI: 1.04–1.68). The high and medium F/V increasers reported higher dispositional optimism (medium vs. low increaser: OR=1.46, 95% CI: 1.15–1.84; high vs. low increaser: OR=1.75, 95% CI: 1.25–2.44), and perceived greater social support (high vs. low increasers: OR=1.82, 95% CI: 1.25–2.67) at baseline. Medium F/V increasers were also more likely to experience worse CIPN at 6-months compared to low increasers (OR=1.29, 95% CI: 1.02–1.64). Groups with higher F/V intakes were also less likely to be non-Hispanic and Asian compared to non-Hispanic white, and less likely to be overweight or obese at baseline (Table 2).
Table 2.
fruit/vegetable intake trajectory following a breast cancer diagnosis
| Predictors | Low increaser1 (n=1,365) | Medium increaser (n=1,180) | High increaser (n=320) | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Education (ref=high school or less) | |||||
| Some college | Ref | 1.26 (0.89, 1.78) | 0.19 | 0.96 (0.55, 1.66) | 0.88 |
| College and above | Ref | 2.18 (1.55, 3.06) | <0.01 | 2.49 (1.49, 4.17) | <0.01 |
| Income (ref=<$50K) | |||||
| $50K–$89K | Ref | 1.32 (1.04, 1.68) | 0.02 | 1.03 (0.72, 1.47) | 0.89 |
| $90K+ | Ref | 0.79 (0.53, 1.17) | 0.24 | 1.25 (0.73, 2.12) | 0.42 |
| Depressive symptoms (high vs. low) | Ref | 0.84 (0.64, 1.09) | 0.19 | 0.77 (0.5, 1.17) | 0.22 |
| Optimism (high vs. low) | Ref | 1.46 (1.15, 1.84) | <0.01 | 1.75 (1.25, 2.44) | <0.01 |
| Social support (high vs. low) | Ref | 1.08 (0.86, 1.36) | 0.52 | 1.82 (1.25, 2.67) | <0.01 |
| Worse PWB at 6 months (yes vs. no) | Ref | 0.85 (0.66, 1.11) | 0.24 | 1.07 (0.72, 1.6) | 0.73 |
| Worse CIPN at 6 months (yes vs. no) | Ref | 1.29 (1.02, 1.64) | 0.03 | 0.86 (0.59, 1.25) | 0.42 |
| Age at diagnosis (ref=age<50) | |||||
| 50–59 | Ref | 1.3 (0.87, 1.94) | 0.20 | 2.76 (1.48, 5.15) | <0. 01 |
| 60–70 | Ref | 1.95 (1.2, 3.17) | 0.01 | 3.94 (1.86, 8.32) | <0.01 |
| 70+ | Ref | 1.61 (0.96, 2.72) | 0.07 | 2.02 (0.9, 4.54) | 0.09 |
| Race/ethnicity (ref=non-Hispanic White) | |||||
| non-Hispanic Black | Ref | 1.16 (0.74, 1.83) | 0.52 | 1.09 (0.53, 2.25) | 0.82 |
| non-Hispanic Asian | Ref | 0.58 (0.42, 0.81) | <0.01 | 0.7 (0.42, 1.17) | 0.17 |
| Hispanic | Ref | 0.51 (0.35, 0.75) | <0.01 | 0.92 (0.54, 1.58) | 0.78 |
| Other | Ref | 0.64 (0.33, 1.24) | 0.19 | 0.61 (0.2, 1.84) | 0.38 |
| Postmenopausal (yes vs. no) | Ref | 0.82 (0.55, 1.21) | 0.32 | 0.72 (0.41, 1.27) | 0.26 |
| Baseline BMI (ref=BMI <25 kg/m2) | |||||
| Overweight (BMI 25–29.9 kg/m2) | Ref | 0.73 (0.57, 0.94) | 0.01 | 0.6 (0.41, 0.87) | 0.01 |
| Obese (BMI 30+ kg/m2) | Ref | 0.54 (0.42, 0.7) | <0.01 | 0.46 (0.31, 0.68) | <0.01 |
| Stage (ref=Stage I) | |||||
| Stage II | Ref | 1.06 (0.81, 1.37) | 0.69 | 0.98 (0.65, 1.46) | 0.91 |
| Stage III | Ref | 0.82 (0.53, 1.28) | 0.38 | 1.14 (0.59, 2.21) | 0.70 |
| Stage IV | Ref | 1.82 (0.49, 6.76) | 0.37 | 3.75 (0.66, 21.2) | 0.13 |
| Number of lymph node removed (ref=0) | |||||
| 1 | Ref | 1.44 (0.76, 2.73) | 0.27 | 2.36 (0.71, 7.83) | 0.16 |
| 2+ | Ref | 1.31 (0.71, 2.42) | 0.38 | 2.54 (0.8, 8.11) | 0.11 0.22 |
| ER/PR status (Positive vs. negative) | Ref | 0.87 (0.55, 1.37) | 0.54 | 1.48 (0.8, 2.74) | |
| Surgery (ref=lumpectomy) | |||||
| Mastectomy | Ref | 0.99 (0.77, 1.26) | 0.90 | 0.79 (0.54, 1.14) | 0.20 |
| None | Ref | 4.52 (0.75, 27.34) | 0.10 | 1.27 (0.08, 20.79) | 0.87 |
| Chemotherapy (ref=none) | |||||
| Neoadjuvant | Ref | 1.12 (0.6, 2.09) | 0.73 | 0.68 (0.24, 1.94) | 0.47 |
| Adjuvant | Ref | 0.87 (0.65, 1.16) | 0.33 | 0.73 (0.47, 1.13) | 0.16 |
| Hormonal therapy (yes vs. no) | Ref | 1.12 (0.77, 1.64) | 0.56 | 0.56 (0.34, 0.91) | 0.02 |
| Radiation (yes vs. no) | Ref | 1.22 (0.95, 1.57) | 0.13 | 0.96 (0.65, 1.4) | 0.82 |
Note
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; CIPN, chemot herapy-induced peripheral neuropathy; PWB, physical well-being; NE, not estimable.
Trajectory groups were labeled by the relative level of baseline behavior (high, medium, or low), direction of change (increased, unchanged, or decreased), and the persistence of change (temporary or stable).
Dietary fat.
Compared to the low maintainers of dietary fat, the medium-low and medium-high maintainers were less likely to report worse physical well-being (medium-low vs. low maintainer: OR= 0.58, 95% CI:0.38, 0.89; medium-high vs. low maintainer: OR= 0.61, 95% CI: 0.40,0.94; Table 3), but more likely to experience worse CIPN from baseline to 6 months (medium-high vs. low maintainer: OR= 1.66, 95% CI: 1.08, 2.56). Other factors associated with higher dietary fat intake included Asian ethnicity, being overweight or obese at baseline, and diagnosis of ER- and/or PR-negative tumor (Table 3).
Table 3.
dietary fat intake trajectory following a breast cancer diagnosis
| Predictors | Low maintainer1(n=256) | Medium-low maintainer (n=1,336) | Medium-high maintainer (n=1,142) | High maintainer(n=131) | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Education (ref=high school or less) | |||||||
| Some college | Ref | 1.09 (0.6 1, 1.92) | 0.78 | 0.91 (0.51, 1.61) | 0.74 | 0.91 (0.38, 2.16) | 0.83 |
| College and above | Ref | 1.22 (0.70, 2.14) | 0.48 | 0.89 (0.51, 1.57) | 0.70 | 0.79 (0.33, 1.86) | 0.59 |
| Income (ref=<$50K) | |||||||
| $50K-$89K | Ref | 1.06 (0.72, 1.58) | 0.76 | 1.11 (0.75, 1.66) | 0.60 | 1.46 (0.79, 2.71) | 0.22 |
| $90K+ | Ref | 1.67 (0.86, 3.26) | 0.13 | 1.03 (0.51, 2.05) | 0.94 | t 1.02 (0.33, 3.21) | 0.97 |
| Depressive symptoms (high vs. low) | Ref | 1.12 (0.70, 1.78) | 0.63 | 0.99 (0.62, 1.59) | 0.98 | 1.13 (0.55, 2.31) | 0.74 |
| Optimism (high vs. low) | Ref | 0.91 (0.63, 1.33) | 0.63 | 0.78 (0.53, 1.15) | 0.52 | 1.43 (0.79, 2.57) | 0.24 |
| Social support (high vs. low) | Ref | 0.85 (0.57, 1.27) | 0.42 | 0.88 (0.58, 1.31) | 0.02 | 0.86 (0.46, 1.61) | 0.63 |
| Worse PWB at 6 months (yes vs. no) | Ref | 0.58 (0.38, 0.89) | 0.01 | 0.61 (0.40, 0.94) | 0.80 (0.42, 1.56) | 0.52 | |
| Worse CIPN at 6 months (yes vs. no) | Ref | 1.53 (1.00, 2.33) | 0.05 | 1.66 (1.08, 2.56) | 0.02 | 1.53 (0.80, 2.94) | 0.20 |
| Age at diagnosis (ref=age<50) | |||||||
| 50–59 | Ref | 1.04 (0.51, 2.11) | 0.91 | 0.74 (0.36, 1.52) | 0.42 | 1.88 (0.56, 6.35) | 0.31 |
| 60–70 | Ref | 0.80 (0.34, 1.87) | 0.60 | 0.78 (0.33, 1.84) | 0.57 | 1.47 (0.37, 5.94) | 0.59 |
| 70+ | Ref | 0.67 (0.27, 1.64) | 0.38 | 0.62 (0.25, 1.55) | 0.31 | 0.77 (0.17, 3.47) | 0.73 |
| Race/ethnicity (ref=non-Hispanic White) | |||||||
| non-Hispanic Black | Ref | 1.42 (0.58, 3.49) | 0.44 | 1.37 (0.55, 3.40) | 0.49 | 2.24 (0.70, 7.13) | 0.17 |
| non-Hispanic Asian | Ref | 1.76 (0.88, 3.49) | 0.11 | 2.37 (1.19, 4.73) | 0.01 | 3.37 (1.31, 8.65) | 0.01 |
| Hispanic | Ref | 1.06 (0.58, 1.96) | 0.85 | 1.06 (0.57, 1.97) | 0.86 | 0.83 (0.29, 2.35) | 0.73 |
| Other | Ref | 1.46 (0.41, 5.15) | 0.56 | 1.90 (0.54, 6.62) | 0.32 | 0.77 (0.08, 7.85) | 0.82 |
| Postmenopausal (yes vs. no) | Ref | 0.99 (0.50, 1.95) | 0.98 | 1.00 (0.50, 1.99) | 0.99 | 1.55 (0.54, 4.40) | 0.41 |
| Baseline BMI (ref=BMI <25 kg/m2) | |||||||
| Overweight (BMI 25–29.9 kg/m2) | Ref | 1.33 (0.89, 2.00) | 0.17 | 1.60 (1.06, 2.43) | 0.03 | 1.21 (0.59, 2.47) | 0.60 |
| Obese (BMI 30+ kg/m2) | Ref | 1.47 (0.93, 2.33) | 0.10 | 2.53 (1.60, 4.01) | <0.01 | 3.04 (1.54, 6.02) | <0.01 |
| Stage (ref=Stage I) | |||||||
| Stage II | Ref | 1.02 (0.65, 1.61) | 0.92 | 1.38 (0.88, 2.17) | 0.16 | 1.08 (0.52, 2.22) | 0.84 |
| Stage III | Ref | 0.90 (0.43, 1.89) | 0.78 | 0.99 (0.47, 2.08) | 0.97 | 1.86 (0.62, 5.58) | 0.27 |
| Stage IV | Ref | 2.23 (0.33, 14.9) | 0.41 | 0.58 (0.08, 4.32) | 0.59 | NE | NE |
| Number of lymph node removed (ref=0) | |||||||
| 1 | Ref | 1.60 (0.5 8, 4.41) | 0.36 | 0.85 (0.32, 2.28) | 0.75 | 0.75 (0.15, 3.76) | 0.73 |
| 2+ | Ref | 1.57 (0.60, 4.11) | 0.36 | 1.02 (0.40, 2.60) | 0.96 | 0.78 (0.17, 3.63) | 0.76 |
| ER/PR status (Positive vs. negative) | Ref | 0.41 (0.19, 0.90) | 0.03 | 0.51 (0.23, 1.14) | 0.10 | 0.34 (0.11, 1.10) | 0.07 |
| Surgery (ref=lumpectomy) | |||||||
| Mastectomy | Ref | 0.90 (0.60, 1.36) | 0.62 | 1.09 (0.72, 1.66) | 0.67 | 1.27 (0.67, 2.41) | 0.47 |
| None | Ref | 0.16 (0.02, 1.56) | 0.11 | 0.69 (0.09, 5.33) | 0.72 | NE | NE |
| Chemotherapy (ref=none) | |||||||
| Neoadjuvant | Ref | 0.64 (0.21, 1.90) | 0.42 | 0.78 (0.27, 2.30) | 0.66 | 0.29 (0.05, 1.70) | 0.170.20 |
| Adjuvant | Ref | 0.90 (0.56, 1.45) | 0.66 | 0.86 (0.53, 1.40) | 0.55 | 0.60 (0.28, 1.31) | 0.20 |
| Hormonal therapy (yes vs. no) | Ref | 1.10 (0.63, 1.94) | 0.73 | 1.08 (0.61, 1.93) | 0.78 | 0.91 (0.36, 2.30) | 0.84 |
| Radiation (yes vs. no) | Ref | 0.89 (0.59, 1.35) | 0.58 | 0.83 (0.54, 1.26) | 0.38 | 1.01 (0.52, 1.94) | 0.98 |
Note
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; CIPN, chemotherapy-induced peripheral neuropathy; PWB, physical well-being; NE, not estimable
Trajectory groups were labeled by the relative level of baseline behavior (high, medium, or low), direction of change (increased, unchanged, or decreased), and the persistence of change (temporary or stable).
Alcohol.
Temporary decreasers of alcohol intake after diagnosis were more likely to have a college education relative to those with high school or less (medium temporary decreaser vs. low maintainer: OR=1.93, 95% CI: 1.18, 3.16), and a household income of $50,000-$89,000 relative to <$50,000 (medium temporary decreaser vs. low maintainer: OR=1.62, 95% CI: 1.20, 2.20; high temporary decreaser vs. low maintainer: OR=2.57, 95% CI: 1.50, 4.41). Compared with low maintainers, temporary decreasers were less likely to be Asian or Hispanic, obese at baseline, and received mastectomy vs. lumpectomy, and were more likely to be postmenopausal (Table 4).
Table 4.
alcohol intake trajectory following a breast cancer diagnosis
| Predictors | Low maintainer1 (n=2,269) | Medium temporary decreaser(n=459) | High temporary decreaser(n=137) | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Education (ref=high school or less) | |||||
| Some college | Ref | 1.50 (0.90, 2.49) | 0.12 | 0.62 (0.30, 1.31) | 0.21 |
| College and above | Ref | 1.93 (1.18, 3.16) | 0.01 | 0.74 (0.36, 1.49) | 0.40 |
| Income (ref=<$50K) | |||||
| $50K-$89K | Ref | 1.62 (1.20, 2.20) | <0.01 | 2.57 (1.50, 4.41) | <0.01 |
| $90K+ | Ref | 0.84 (0.49, 1.43) | 0.51 | 0.18 (0.02, 1.38) | 0.10 |
| Depressive symptoms (high vs. low) | Ref | 0.94 (0.66, 1.34) | 0.74 | 1.34 (0.76, 2.35) | 0.31 |
| Optimism (high vs. low) | Ref | 0.99 (0.74, 1.31) | 0.92 | 1.14 (0.70, 1.87) | 0.59 |
| Social support (high vs. low) | Ref | 1.27 (0.94, 1.73) | 0.13 | 1.07 (0.64, 1.80) | 0.80 |
| Worse PWB at 6 months (yes vs. no) | Ref | 0.89 (0.63, 1.27) | 0.53 | 1.03 (0.57, 1.85) | 0.93 |
| Worse CIPN at 6 months (yes vs. no) | Ref | 0.92 (0.68, 1.26) | 0.61 | 1.12 (0.67, 1.87) | 0.68 |
| Age at diagnosis (ref=age<50) | |||||
| 50–59 | Ref | 0.84 (0.51, 1.40) | 0.50 | 0.94 (0.37, 2.40) | 0.90 |
| 60–70 | Ref | 0.77 (0.41, 1.42) | 0.40 | 0.52 (0.18, 1.48) | 0.22 |
| 70+ | Ref | 0.90 (0.46, 1.73) | 0.74 | 0.40 (0.12, 1.28) | 0.12 |
| Race/ethnicity (ref=non-Hispanic White) | |||||
| non-Hispanic Black | Ref | 0.70 (0.37, 1.33) | 0.28 | NE | NE |
| non-Hispanic Asian | Ref | 0.17 (0.09, 0.31) | <0.01 | 0.09 (0.02, 0.39) | <0.01 |
| Hispanic | Ref | 0.45 (0.26, 0.78) | <0.01 | 0.14 (0.03, 0.59) | 0.01 |
| Other | Ref | 0.87 (0.39, 1.96) | 0.73 | 0.35 (0.05, 2.63) | 0.31 |
| Postmenopausal (yes vs. no) | Ref | 1.12 (0.68, 1.85) | 0.65 | 2.89 (1.24, 6.76) | 0.01 |
| Baseline BMI (ref=BMI <25 kg/m2) | |||||
| Overweight (BMI 25–29.9 kg/m2) | Ref | 0.91 (0.68, 1.22) | 0.53 | 1.20 (0.71, 2.04) | 0.49 |
| Obese (BMI 30+ kg/m2) | Ref | 0.40 (0.28, 0.58) | <0.01 | 0.66 (0.37, 1.19) | 0.17 |
| Stage (ref=Stage I) | |||||
| Stage II | Ref | 0.89 (0.64, 1.25) | 0.51 | 0.69 (0.38, 1.27) | 0.24 |
| Stage III | Ref | 0.96 (0.54, 1.71) | 0.90 | 1.08 (0.42, 2.78) | 0.87 |
| Stage IV | Ref | 1.30 (0.36, 4.74) | 0.69 | NE | NE |
| Number of lymph node removed (ref=0) | |||||
| 1 | Ref | 0.79 (0.36, 1.71) | 0.55 | NE | NE |
| 2+ | Ref | 0.83 (0.40, 1.73) | 0.62 | NE | NE |
| ER/PR status (Positive vs. negative) | Ref | 1.35 (0.76, 2.39) | 0.30 | 0.74 (0.25, 2.14) | 0.57 |
| Surgery (ref=lumpectomy) | |||||
| Mastectomy | Ref | 0.97 (0.71, 1.32) | 0.85 | 0.54 (0.32, 0.92) | 0.02 |
| None | Ref | 0.42 (0.04, 4.06) | 0.46 | NE | NE |
| Chemotherapy (ref=none) | |||||
| Neoadjuvant | Ref | 0.88 (0.38, 2.02) | 0.76 | 0.39 (0.05, 3.33) | 0.39 |
| Adjuvant | Ref | 0.83 (0.58, 1.20) | 0.33 | 0.71 (0.37, 1.34) | 0.29 |
| Hormonal therapy (yes vs. no) | Ref | 1.05 (0.67, 1.63) | 0.83 | 1.69 (0.68, 4.15) | 0.26 |
| Radiation (yes vs. no) | Ref | 1.29 (0.94, 1.76) | 0.12 | 0.74 (0.42, 1.28) | 0.28 |
Note
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; CIPN, chemotherapy -induced peripheral neuropathy; PWB, physical well-being; NE, not estimable.
Trajectory groups were labeled by the relative level of baseline behavior (high, medium, or low), direction of change (increased, unchanged, or decreased), and the persistence of change (temporary or stable).
DISCUSSION
Our results show that women generally reported slight increases in F/V intake and temporary decreased alcohol consumption after a breast cancer diagnosis, while there was a lack of change in dietary fat intake. Even with the beneficial changes in F/V and alcohol intake, 48% of survivors reported <5 servings/day of F/V intake and 21% had >1 drinks/day of alcohol throughout this period, indicating non-adherence to the current nutrition recommendations for cancer survivors. This analysis also identified factors associated with diet trajectories. Specifically, higher education and income, higher dispositional optimism, and higher perceived social support at baseline were associated with groups with higher F/V intake. Higher education and income were also associated with groups with higher alcohol intakes. Survivors in groups with higher F/V and dietary fat intake experienced more neuropathy symptoms but fewer declines in physical well-being. Other factors associated with diet trajectories included race/ethnicity, menopausal status, baseline BMI, hormone receptor positivity, and receipt of surgery. Of note, this information is readily available in clinical records and in the future could be used to identify patients for whom nutrition counseling is warranted.
A number of studies have described the spontaneous changes in diet after a breast cancer diagnosis. There is a consensus that women may reduce alcohol intake after a breast cancer diagnosis [18,19,9,12,14], while studies of F/V and dietary fat often report mixed results [9–17]. For instance, a recent analysis based on 246 breast cancer survivors in the French NutriNet-Santé cohort reported that women decreased alcohol and F/V intake and increased dietary fat intake during an average of 48 months postdiagnosis [14]. However, other studies in European breast cancer survivors reported that women increased F/V intake by 0.4–2.0 serving/day at 1 year after diagnosis
[11,12,15]. Some studies have also observed small decreases in dietary fat intake postdiagnosis. For example, the Health, Eating, Activity, and Lifestyle study (HEAL) study (n=260) and the DietCompLyf study (n=1,560) reported that breast cancer survivors on average reduced 8 grams/day in total fat intake at 1 year after diagnosis [12] and 3.6 grams/day at 2 years post-diagnosis [13], respectively. The mixed results in previous analyses could be due to difference in dietary assessment method (24-hour recalls vs. FFQ) and timing of diet data collection (from diagnosis to 1, 2, and 4 years postdiagnosis).
To our knowledge, this is the first analysis to identify the longitudinal trajectories of diet in breast cancer survivors. Our findings contribute to the literature by showing the heterogeneity in dietary change in relation to pre-diagnosis diet, i.e., patients with high F/V intake at diagnosis increased more F/V after diagnosis compared to patients with insufficient F/V intake at diagnosis. In addition, the post-diagnosis change in F/V intake was generally sustainable, while the reduction in alcohol consumption tended to be short-term. These results confirm the working model that cancer diagnosis is a “teachable moment” for healthy lifestyle changes [31], as we observed favorable dietary change following breast cancer diagnosis. However, the spontaneous changes alone were insufficient to meet national guidelines, because survivors who were not adherent to the nutrition guidelines at diagnosis (i.e., >5 servings/day of F/V, <1 drink/day of alcohol) were still unable to meet these recommendations post-diagnosis. Therefore, nutrition assessment and counseling in routine oncology care may be necessary to promote effective and sustainable dietary change.
This analysis also revealed several factors associated with diet trajectory in breast cancer survivors. Higher education and higher income were associated with higher F/V in breast cancer survivors. It is possible that patients with advantageous socioeconomic status had greater knowledge and awareness of healthier diet [32,33], easier access to healthy food options [34], and greater economic resources to afford healthier foods [33]. The association between higher education and income and higher alcohol intake is consistent with findings in the general population [35]. Further, our results suggest that higher dispositional optimism and greater social support were associated with higher F/V intake, which could be due to better stress coping after cancer diagnosis [36]. Finally, our results suggest that higher F/V was associated with worse CIPN, and higher dietary fat was associated with worse CIPN but better physical well-being. These findings are provocative but consistent with a recent analysis based on the SWOG S0221 trial showing higher intake of citrus fruits was associated with higher risk of CIPN [37]. These associations could be due to reverse causation when patients increased F/V to manage neuropathy symptoms, or that patients consumed more energy-dense foods due to treatment-induced deficits in taste sensitivity [38,39]. Future studies are needed to better understand the impact of diet on cancer treatment side effects.
Our analysis has several methodological strengths. We used GBTM to identify subpopulations of women with distinct trajectories of dietary components, which revealed insights into the complex behavior change processes in breast cancer survivors that could not be observed in analysis of average change. GBTM also allows for missing intermittent data over follow-up and estimated trajectory parameters using Full Information Maximum Likelihood (FIML) estimation, which is less biased than methods that delete observations with incomplete data [40]. Second, we assessed the influence of loss to follow-up on the identification of trajectory groups to minimize the influence of selection bias. Third, energy adjustment was used in analyzing dietary change, which reduced bias due to decreased overall food intake that many breast cancer survivors experience during active treatment [41]. Finally, the racial/ethnic diversity of Pathways Study participants makes our analysis more generalizable to other breast cancer survivor populations in the US.
This analysis has some limitations. First, diet was assessed using the FFQ which has lower validity and weaker association with total energy intake compared to 24-hour recalls [42]. It has been shown that the FFQ tends to underestimate F/V intake [43] and overestimate dietary fat intake [42] compared to multi-day food records and thus may bias our estimates in similar directions. Second, self-reported diet data may be inaccurate due to social desirability bias, which refers to the tendency to over- or under-report particular behaviors in order to avoid being viewed negatively [44]. As such, our findings of the general increasing trend in F/V and decreasing alcohol intake could be due to the increased desire to present oneself as one who conforms to a healthier diet. Third, this analysis may suffer from selection bias, as more than 50% of eligible women chose not to enroll in the Pathways Study. However, our sensitivity analysis showed minimal impact of loss to follow-up on the trajectory analysis. Fourth, these trajectory groups were identified through GBTM in a data-driven approach, not by a priori cutoff values for the baseline dietary intake or magnitude of change. As such, the labeling of these trajectory groups does not reflect the clinical classifications of dietary intake level or clinically meaningful dietary change. Finally, there was no appreciable difference between the 6 months and 24 months across the 3 dietary outcomes, except for the “high temporary decreaser” group of alcohol intake, suggesting that GBTM did not reveal dietary change patterns that would not be obtained from mean change measures.
In summary, this analysis indicates that breast cancer survivors made small favorable changes in increasing F/V and decreasing alcohol intake over 24 months after diagnosis in the Pathways Study, with nearly half of survivors persistently consuming <5 servings/day of F/V and >40% of calories from dietary fat, while one in five survivors having >1 drink/day of alcohol during this period. Socioeconomic status, baseline dispositional optimism and social support, as well as changes in physical well-being and neuropathy symptoms were associated with diet trajectories. Better diet has been associated with 26–60% lower all-cause mortality [45,46] and 28–56% lower noncancer mortality [47,45] in breast cancer survivors. Given the large proportion of breast cancer survivors who do not adhere to the nutrition recommendations before and after cancer diagnosis, and the lack of significant, self-motivated change, it is important to prioritize nutrition counseling and education in breast cancer survivors who maintain an unhealthy diet post-diagnosis.
Supplementary Material
Acknowledgments
DISCLOSURES
SUPPORT/FUNDING: Supported by NCI/NIH R01CA105274 (to LHK), U01CA195565 (to LHK), and China Scholarship Council predoctoral training award No. 201208000008 (to ZS).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest
REFERENCES
- 1.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD (2011) Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13 (3):R64. doi: 10.1186/bcr2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL (2015) Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med 163 (6):437–451. doi: 10.7326/M15-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwan ML, Lo JC, Tang L, Laurent CA, Roh JM, Chandra M, Hahn TE, Hong CC, Sucheston-Campbell L, Hershman DL, Quesenberry CP Jr., Ambrosone CB, Kushi LH, Yao S (2014) Bone health history in breast cancer patients on aromatase inhibitors. PLoS One 9 (10):e111477. doi: 10.1371/journal.pone.0111477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62 (4):243–274. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research (2018) Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report. [Google Scholar]
- 6.BlancharM, CourneyS, SteiK, American Cancer Society’S, II (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26 (13):2198–2204. doi: 10.1200/JCO.2007.14.6217 [DOI] [PubMed] [Google Scholar]
- 7.Milliron BJ, Vitolins MZ, Tooze JA (2014) Usual dietary intake among female breast cancer survivors is not significantly different from women with no cancer history: results of the National Health and Nutrition Examination Survey, 2003–2006. J Acad Nutr Diet 114 (6):932–937. doi: 10.1016/j.jand.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM (2013) Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 113 (4):569–580. doi: 10.1016/j.jand.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costanzo ES, Lutgendorf SK, Roeder SL (2011) Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psychooncology 20 (1):53–61. doi: 10.1002/pon.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skeie G, Hjartaker A, Braaten T, Lund E (2009) Dietary change among breast and colorectal cancer survivors and cancer-free women in the Norwegian Women and Cancer cohort study. Cancer Causes Control 20 (10):1955–1966. doi: 10.1007/s10552-009-9390-3 [DOI] [PubMed] [Google Scholar]
- 11.Steinhilper L, Geyer S, Sperlich S (2013) Health behavior change among breast cancer patients. Int J Public Health 58 (4):603–613. doi: 10.1007/s00038-013-0444-7 [DOI] [PubMed] [Google Scholar]
- 12.Boulton R, Chin K (2010) A post-clinic questionnaire survey following patient discharge with primary mastalgia, in a District General Hospital setting. European Journal of Surgical Oncology (EJSO) 36 (11):1107–1108. doi: 10.1016/j.ejso.2010.08.005 [DOI] [Google Scholar]
- 13.Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R (2004) Changes in dietary intake after diagnosis of breast cancer. J Am Diet Assoc 104 (10):1561–1568. doi: 10.1016/j.jada.2004.07.028 [DOI] [PubMed] [Google Scholar]
- 14.Fassier P, Zelek L, Lecuyer L, Bachmann P, Touillaud M, Druesne-Pecollo N, Galan P, Cohen P, Hoarau H, Latino-Martel P, Kesse-Guyot E, Baudry J, Hercberg S, Deschasaux M, Touvier M (2017) Modifications in dietary and alcohol intakes between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Sante cohort. Int J Cancer 141 (3):457–470. doi: 10.1002/ijc.30704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Affret A, His M, Severi G, Mancini FR, Arveux P, Clavel-Chapelon F, Boutron-Ruault MC, Fagherazzi G (2018) Influence of a cancer diagnosis on changes in fruit and vegetable consumption according to cancer site, stage at diagnosis and socioeconomic factors: Results from the large E3N-EPIC study. Int J Cancer. doi: 10.1002/ijc.31572 [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK (2001) Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 19 (9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381 [DOI] [PubMed] [Google Scholar]
- 17.Rabin C, Pinto B (2006) Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psychooncology 15 (8):701–712. doi: 10.1002/pon.1000 [DOI] [PubMed] [Google Scholar]
- 18.Bell RJ, Lijovic M, Fradkin P, Schwarz M, Davis SR (2012) Changes in patterns of use of cigarettes and alcohol in women after a first diagnosis of invasive breast cancer: a cohort study of women from Victoria, Australia. Support Care Cancer 20 (4):783–789. doi: 10.1007/s00520-011-1150-8 [DOI] [PubMed] [Google Scholar]
- 19.Bidstrup PE, Dalton SO, Christensen J, Tjonneland A, Larsen SB, Karlsen R, Brewster A, Bondy M, Johansen C (2013) Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: a prospective study in the Danish Diet, Cancer and Health Cohort. Acta Oncol 52 (2):327–335. doi: 10.3109/0284186X.2012.746466 [DOI] [PubMed] [Google Scholar]
- 20.Tinker LF, Rosal MC, Young AF, Perri MG, Patterson RE, Van Horn L, Assaf AR, Bowen DJ, Ockene J, Hays J, Wu L (2007) Predictors of dietary change and maintenance in the Women’s Health Initiative Dietary Modification Trial. J Am Diet Assoc 107 (7):1155–1166. doi: 10.1016/j.jada.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 21.Nagin DS (1999) Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological methods 4 (2):139. [DOI] [PubMed] [Google Scholar]
- 22.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, Ashley CH, Bittner JR, Darbinian J, Stronach K, Caan BJ, Davis W, Kutner SE, Quesenberry CP, Somkin CP, Sternfeld B, Wiencke JK, Zheng S, Kushi LH (2008) The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control 19 (10):1065–1076. doi: 10.1007/s10552-008-9170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block G, Woods M, Potosky A, Clifford C (1990) Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43 (12):1327–1335 [DOI] [PubMed] [Google Scholar]
- 24.Willet W (1999) Nutritional Epidemiology. 2nd edn Oxford University Press, New York [Google Scholar]
- 25.Radloff LS (1977) The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement 1 (3):385–401 [Google Scholar]
- 26.Scheier MF, Carver CS (1992) Effects of optimism on psychological and physical well-being: Theoretical overview and empirical update. Cognitive therapy and research 16 (2):201–228 [Google Scholar]
- 27.Sherbourne CD, Stewart AL (1991) The MOS social support survey. Soc Sci Med 32 (6):705–714 [DOI] [PubMed] [Google Scholar]
- 28.Cella D, Peterman A, Hudgens S, Webster K, Socinski MA (2003) Measuring the side effects of taxane therapy in oncology: the functional assesment of cancer therapy-taxane (FACT-taxane). Cancer 98 (4):822–831. doi: 10.1002/cncr.11578 [DOI] [PubMed] [Google Scholar]
- 29.Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 1:79. doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Hernan MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168 (6):656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM (2005) Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol 23 (24):5814–5830. doi: 10.1200/JCO.2005.01.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link BG (2008) Epidemiological sociology and the social shaping of population health. J Health Soc Behav 49 (4):367–384. doi: 10.1177/002214650804900401 [DOI] [PubMed] [Google Scholar]
- 33.Cutler DM, Lleras-Muney A (2010) Understanding differences in health behaviors by education. J Health Econ 29 (1):1–28. doi: 10.1016/j.jhealeco.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson AS, Meyer KA, Howard AG, Boone-Heinonen J, Popkin BM, Evenson KR, Kiefe CI, Lewis CE, Gordon-Larsen P (2014) Neighborhood socioeconomic status and food environment: a 20-year longitudinal latent class analysis among CARDIA participants. Health Place 30:145–153. doi: 10.1016/j.healthplace.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins SE (2016) Associations Between Socioeconomic Factors and Alcohol Outcomes. Alcohol Res 38 (1):8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CL, Edmondson D, Fenster JR, Blank TO (2008) Positive and negative health behavior changes in cancer survivors: a stress and coping perspective. J Health Psychol 13 (8):1198–1206. doi: 10.1177/1359105308095978 [DOI] [PubMed] [Google Scholar]
- 37.Mongiovi JM, Zirpoli GR, Cannioto R, Sucheston-Campbell LE, Hershman DL, Unger JM, Moore HCF, Stewart JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Thomas Budd G, Albain KS, Ambrosone CB, McCann SE (2018) Associations between self-reported diet during treatment and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221). Breast Cancer Res 20 (1):146. doi: 10.1186/s13058-018-1077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berteretche MV, Dalix AM, d’Ornano AM, Bellisle F, Khayat D, Faurion A (2004) Decreased taste sensitivity in cancer patients under chemotherapy. Support Care Cancer 12 (8):571–576. doi: 10.1007/s00520-004-0589-2 [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Lara K, Sosa-Sanchez R, Green-Renner D, Rodriguez C, Laviano A, Motola-Kuba D, Arrieta O (2010) Influence of taste disorders on dietary behaviors in cancer patients under chemotherapy. Nutr J 9:15. doi: 10.1186/1475-2891-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enders C, Bandalos D (2001) The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling: A Multidisciplinary Journal 8 (3):430–457. doi: 10.1207/s15328007sem0803_5 [DOI] [Google Scholar]
- 41.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12 (5):489–495. doi: 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 42.Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, Schoeller DA, Troiano RP, Freedman LS (2003) A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol 32 (6):1054–1062 [DOI] [PubMed] [Google Scholar]
- 43.Kristal AR, Vizenor NC, Patterson RE, Neuhouser ML, Shattuck AL, McLerran D (2000) Precision and bias of food frequency-based measures of fruit and vegetable intakes. Cancer Epidemiol Biomarkers Prev 9 (9):939–944 [PubMed] [Google Scholar]
- 44.Tourangeau R, Yan T (2007) Sensitive questions in surveys. Psychol Bull 133 (5):859–883. doi: 10.1037/0033-2909.133.5.859 [DOI] [PubMed] [Google Scholar]
- 45.George SM, Ballard-Barbash R, Shikany JM, Caan BJ, Freudenheim JL, Kroenke CH, Vitolins MZ, Beresford SA, Neuhouser ML (2014) Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev 23 (4):575–583. doi: 10.1158/1055-9965.EPI-13-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George SM, Irwin ML, Smith AW, Neuhouser ML, Reedy J, McTiernan A, Alfano CM, Bernstein L, Ulrich CM, Baumgartner KB, Moore SC, Albanes D, Mayne ST, Gail MH, Ballard-Barbash R (2011) Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control 22 (4):589–598. doi: 10.1007/s10552-011-9732-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izano MA, Fung TT, Chiuve SS, Hu FB, Holmes MD (2013) Are diet quality scores after breast cancer diagnosis associated with improved breast cancer survival? Nutr Cancer 65 (6):820–826. doi: 10.1080/01635581.2013.804939 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.