Abstract
Peroxisome proliferator-activated receptors (PPARs) are a family of transcription factors with a key role in glucose and lipid metabolism. PPARs are expressed in many cell types including pancreatic beta cells and immune cells, where they regulate insulin secretion and T cell differentiation, respectively. Moreover, various PPAR agonists prevent diabetes in the non-obese diabetic (NOD) mouse model of type 1 diabetes. PPARs are thus of interest in type 1 diabetes (T1D) as they represent a novel approach targeting both the pancreas and the immune system. In this review, we examine the role of PPARs in immune responses and beta cell biology and their potential as targets for treatment of T1D.
1. Introduction
T1D is an autoimmune disease caused by the pancreatic beta cells being dysfunctional or killed by autoreactive T cells resulting in reduced insulin production and hyperglycemia [1, 2]. The incidence of T1D is increasing, and estimates from the International Diabetes Federation suggests that the number of patients (age < 20 years) has doubled from 2015 to 2017 [3, 4]. However, the incidence varies geographically with high rates in Finland (>60 cases/100.000/year) and Sardinia (~40 cases/100.000/year), while China has less than one case/100.000/year [5]. The strongest genetic susceptibility is the HLA haplotypes DR3-DQ2 and DR4-DQ8 with 90% of diagnosed children having one or both haplotypes in Scandinavia [6, 7]. Over 50 genetic loci contribute to the genetic disease predisposition, although the molecular mechanisms often remain unknown [8]. Less than 10% of genetically susceptible individuals develop T1D, demonstrating that environmental factors such as diet and microorganisms play a pivotal role in T1D pathology [9, 10]. It was previously believed that patients had an almost complete loss of beta cells at onset of disease. However, several recent studies have shown that new-onset T1D patients retain up to 40% of insulin-positive islets [11–13]. Furthermore, islets isolated from T1D patients can regain their ability to secrete insulin when cultured in a nondiabetogenic environment in vitro [14]. Thus, beta cell dysfunction is likely to play an important role in T1D pathology. Current therapeutic approaches have, with limited clinical efficacy, focused on suppressing the ongoing immune attack or stimulating beta cell regeneration [15, 16]. Therefore, strategies that both dampen the immune response and promote beta cell function are in high need. The PPAR family is an ideal target for such a strategy, as PPARs have both anti-inflammatory properties, regulate beta cell biology, and modulate the pancreatic lipidome.
2. PPARs
PPARs were identified in the 1990s as mediators of peroxisome proliferation [17]. They belong to the nuclear receptor class II superfamily of transcription factors and regulate a range of biological processes by modulating gene expression. In mammals, three isoforms have been identified: PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3), which predominately control genes involved in lipid metabolism including transport, storage, lipogenesis, and fatty acid oxidation (FAO) [17]. PPARs are important targets for metabolic disorders and multiple drugs targeting PPARα (fibrates, e.g., fenofibrate, bezafibrate, and clofibrate) and PPARγ (thiazolidinediones, e.g., troglitazone, rosiglitazone, pioglitazone, and ciglitazone) which have been used to treat hyperlipidemia and type 2 diabetes. PPARs are dynamic as they shuttle between the nucleus and cytoplasm, though they are mainly and constitutively present in the nucleus [18, 19]. The nuclear-cytoplasmic shuttling of PPARs is regulated by binding of PPAR ligands to the C-terminal domain (Figure 1) [19]. Binding of ligands induces a conformational change leading to heterodimerization with members of the retinoid X receptor (RXR) family [20, 21]. This complex binds to specific DNA sequences, termed peroxisome proliferator response elements (PPRE) through the highly conserved zinc finger DNA-binding domain in the N terminus [22]. Binding of ligands also results in dissociation of corepressors and recruitment of coactivator proteins, resulting in enhancement of target gene transcription [23]. In the absence of ligands, PPARs instead recruit corepressors that repress transcription of target genes [24]. PPARs are involved in a mechanism termed “transrepression,” which is a ligand-dependent but PPRE-independent mechanism of gene repressions through interactions with other proteins such as NFκB, AP1, and STAT [25–27]. This generates and stabilizes corepressing complexes, which typically bind to and repress proinflammatory genes [21].
Figure 1.
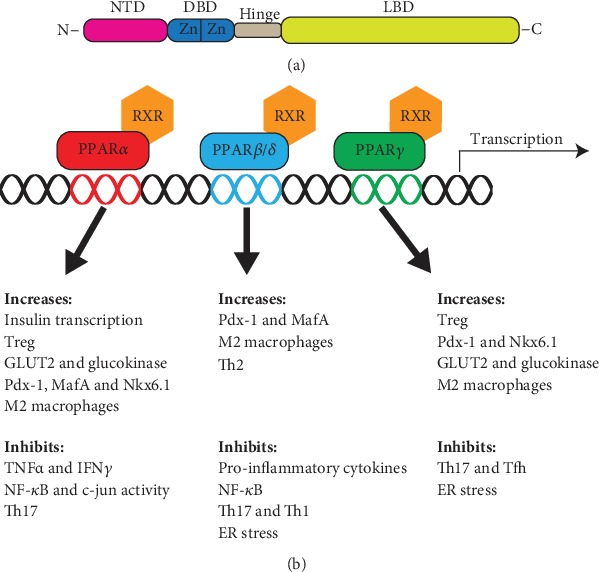
Structure and function of PPARs. (a) The peroxisome proliferator-activated receptor (PPAR) isoforms have a large degree of structural overlap, consisting of an N-terminal ligand-independent transactivation domain (NTD). The DNA-binding domain (DBD) contains two zinc finger (Zn) domains, which bind to peroxisome proliferator response element (PPRE) sequences. The DBD is connected through a hinge domain to the C terminal ligand-binding domain (LBD). (b) Illustration of the biological role of PPARs. PPARs heterodimerize with members of the retinoid X receptor (RXR) family. The isoforms are involved in a variety of pathways; shown are pathways with relation to type 1 diabetes. c-jun: transcription factor c-Jun; GLUT2: glucose transporter 2; MafA: MAF bZIP transcription factor A; NFκB: nuclear Factor-kB; Nkx6.1: NK6 homeobox 1; Pdx-1: pancreatic and duodenal homeobox 1; Tfh: follicular helper T cells; Th1: T helper 1 cells; Th17: T helper 17 cells; Th2: T helper 2 cells; TNFα: tumor necrosis factor alpha; Treg: regulatory T cells.
The PPAR isoforms have a great degree of structural and functional overlap but their expression patterns differ. PPARα is highly expressed in metabolically active tissues including liver, kidney, and adipose tissue. PPARα is activated during fasting and is involved in controlling ketogenesis, lipoproteins, gluconeogenesis, amino acid catabolism, FAO, and inflammatory responses [28]. PPARβ/δ is nearly ubiquitously expressed and involved in FAO and activation has an anti-inflammatory effect with reduced secretion of proinflammatory cytokines [29]. PPARγ is expressed in various tissues including adipose, intestine, liver, and kidney [30, 31]. It is involved in regulating fat cell differentiation, lipid storage, and differentiation of monocytes into macrophages [32, 33]. PPARs have, due to their immune regulatory functions, been linked to several autoimmune diseases, i.e., multiple sclerosis [34], lupus erythematosus [35], autoimmune thyroiditis [36], Graves ophthalmopathy [37], rheumatoid arthritis [38], psoriasis [39], and Guillain–Barré [40]. Similarly, PPARs have also been suggested as targets to treat chronic inflammatory diseases [20, 41]. An interesting feature is that women seem to be more susceptible than men to develop autoimmune diseases [42]. This might be connected to PPAR expression as mouse studies have found that male mice have higher expression of PPARα in T cells compared to female mice, and that expression was androgen sensitive [43].
Polymorphisms in PPARβ/δ and PPARγ promoter regions contribute to the genetic predisposition to T1D and affect the severity of islet autoimmunity [44]. Additionally, PPARγ is associated with the development of insulin resistance and type 2 diabetes [45].
3. PPARs and the Immune System
The pathogenesis of T1D includes interactions between beta cells and components of both the innate and adaptive immune system [46]. Many different immune cells have been implicated including B cells and macrophages [47, 48]. However, focus has primarily been on T cells where evidence suggests that T1D develops due to a defect in regulatory T cell (Treg) function [2, 46]. Studies of postmortem pancreas samples from T1D patients revealed that CD8+ T cells are the most predominant population in the islet infiltrate followed by (in declining order) macrophages, CD4+ T cells, B cells, and plasma cells [49]. Why tolerance is lost in some individuals remains unknown.
The metabolic pathway for ATP production has an important role in regulating immune cell function. Differentiation of activated CD4+ T cells thus depends on the metabolic pathway; Th1, Th2, and Th17 cells use glycolysis while Tregs have a high level of lipid oxidation [50, 51]. In this way, T cell differentiation can be manipulated as inhibition of glycolysis blocks Th17 and promotes Treg differentiation [51]. The inflammatory M1 phenotype of macrophages uses glycolysis while the anti-inflammatory M2 phenotype utilizes lipid oxidation [52]. Hence, modulation of FAO through PPARs can induce immunological changes. PPARs are expressed in various types of immune cells including macrophages, dendritic cells, B cells, and T cells, and all three isoforms have anti-inflammatory activities [53]. Activation of all PPARs potentiates the polarization of mouse macrophages to the anti-inflammatory M2 phenotype, while M2 is diminished in PPARγ and PPARβ/δ knockouts [20, 32, 54, 55]. Deletion of PPARγ in macrophages blocks FAO and renders the macrophages incapable of making a full conversion to the M2 phenotype. Only PPARγ seems to have the same role in human macrophages [20]. This anti-inflammatory effect appears to depend on the repression of NFκB and AP-1 [20, 54, 56, 57].
The role of PPARs in T cell regulation is more complex with differences between the isoforms. Tregs from PPARα knockout mice have impaired suppressive activities towards both CD4+ and CD8+ T cells [58]. This was associated with reduced migratory abilities and diminished expression of several chemokine receptors. In support of this, PPARα knockout mice have prolonged inflammatory response to inflammatory agents such as arachidonic acid [59]. The PPARα agonist fenofibrate has been demonstrated to promote FOXP3+ regulatory T cells in mice [60, 61]. PPARα is involved in regulating effector T cells with high expression of PPARα leading to increased production of Th2 cytokines and knockout mice having increased differentiation towards a Th1 phenotype [43]. Also, fenofibrate treatment prevented the differentiation of Th17 cells in mice [62]. In addition, PPARα agonist WY14643 diminishes human T cell proliferation and induce T cell depletion by trapping the cells in the G2/S phase [63]. In hyperlipidemia patients, treatment with fenofibrate decreases TNFα and IFN-γ levels [64]. These findings were validated in PPARα knockout mice as they had increased levels of TNFα and IFN-γ [43].
PPARβ/δ activation inhibits Th1 and Th17 while enhancing Th2 [65–67]. Deletion of PPARβ/δ gives the opposite result. This is likely a consequence of PPARβ/δ increasing FAO [68], thereby blocking the proliferative burst following antigen recognition in T cells as a consequence of a shift from oxidative metabolism to glycolysis [20, 69].
PPARγ seems to have a role in regulating the balance between regulatory and effector T cells. Reduced PPARγ activity increases the amount of effector T cells as evidenced by increased antigen-specific proliferation and overproduction of IFN-γ in response to IL-12 in PPARγ knockout mice [70]. There is also evidence indicating that PPARγ inhibits expression of RORγt and thereby differentiation of Th17 cells in both mice and humans [71]. PPARγ appears to be involved in the formation of follicular helper T cells (Tfh) as mice with a knockout in CD4 cells had increased Tfh cell activation and increased formation of germinal centers [72]. PPARγ agonist troglitazone and rosiglitazone have additional in a mouse model of colitis been shown to shift the immune response from Th1 towards Th2, with a corresponding decrease in Th1-associated transcription factors, cytokine and chemokine, and an increase in Th2-associated factors [73, 74]. On the other hand, PPARγ deficiency leads to a decreased number of CD4+FOXP3+ regulatory T cells [75]. This is exemplified by the identification of a specific Treg population with a high expression of PPARγ in visceral adipose tissue [76]. PPARγ is the major orchestrator of these Tregs, and Treg-specific deletion of PPARγ prevented the formation of this cell type. Furthermore, the loss of PPARγ in Tregs leads to increased effector T cell responses while PPARγ activation increases the amount of FOXP3+ regulatory T cells [70, 75, 77]. Another study has though described how rosiglitazone had no effect on Tregs in a mouse model of allergic asthma [78], thereby suggesting the effect of PPARγ on Tregs might be tissue-specific.
4. PPARs and Pancreatic Islets
Beta cells are highly specialized cells each making millions of insulin molecules per day [79]. This puts tremendous pressure on the cells, as insulin is prone to misfolding with approximately 20% of all insulin molecules failing to reach its mature conformation [80]. Misfolded insulin can lead to ER stress, which again can lead to the formation of neoantigens and activate the immune system resulting in further beta cell death and loss of insulin production [81]. As described above, beta cell dysfunction rather than beta cell death has recently been emphasised as a major contributor to T1D. Thus, the possibility of restoring beta cell function has become an alluring research area. In this regard, the PPAR isoforms are possible targets as they are expressed in pancreatic islets [82–84] and appear to have important roles as regulators of beta cell biology.
PPARα is expressed in pancreatic islets and beta cell lines with expression depending on glucose level [85]. High glucose represses PPARα in isolated rat islets and INS-1E cells [86, 87]. The glucose-dependent upregulation of insulin expression might rely on PPARα as glucose did not increase insulin expression in islets from PPARα knockout mice [88]. PPARα knockout mice have reduced mRNA levels of insulin, Nkx6.1 (a transcription factor essential for maintaining functionally mature beta cells [89]), MafA (regulator of insulin secretion [90]), GLUT2, and glucokinase [91]. PPARα has likewise been found to upregulate Pdx-1 (transcription factor with a critical role in pancreas and beta cell development [92]) in INS-1 cells and isolated rat islets [93, 94]. On a whole-body level, PPARα knockout mice are normoglycemic in a fed state but hyperglycemic when fasted [85]. This was associated with a 55% higher plasma insulin level. The mice had improved glucose tolerance and increased insulin secretion from isolated islets.
PPARβ/δ is the most abundant PPAR isoform in beta cells [83, 95]; however, not much is known about its role in beta cell biology. PPARβ/δ appears to have an important role in pancreas development as pancreatic PPARβ/δ knockout mice had an increased number of pancreatic islets and a 2-fold increase in beta cell mass [96]. This was associated with increased plasma insulin levels, hypoglycemia, and improved glucose tolerance, while isolated islets had an increased second-phase insulin secretion. This suggests that PPARβ/δ is a negative regulator of insulin secretion in the mature pancreas, which is in contrast to a study demonstrating that PPARβ/δ promotes beta cell differentiation from stem cells by upregulating Pdx-1 [97]. GW501516, a PPARβ/δ agonist, was shown to attenuate dysfunction of palmitate-induced insulin secretion by promoting MafA [98]. Furthermore, this agonist promoted FAO and protected against palmitate-induced ER stress in a beta cell line [99]. PPARβ/δ was also demonstrated to reduce ER stress in rodent models [100, 101]. Additionally, GW501516 improved beta cell mitochondrial function in Desnutrin knockout mice and reduced lipolysis, which resulted in improved glucose tolerance and glucose-stimulated insulin secretion (GSIS) [95].
The role of PPARγ in insulin secretion is not fully understood. Some studies have demonstrated that PPARγ activation or overexpression suppresses insulin secretion and proinsulin biosynthesis [102–106]. For example, it was shown that overexpressing PPARγ in INS-1E cells leads to impairment of GSIS [105]. However, other studies have demonstrated that PPARγ activation or overexpression potentiates GSIS in beta cells and isolated islet [107–110]. What we do know is that PPARγ is involved in controlling several key beta cell genes. Activation of PPARγ by troglitazone (a PPARγ agonist) leads to upregulation of Pdx-1, Nkx6.1, glucokinase, and GLUT2 [111, 112]. In addition, PPARγ pancreatic knockout mice had reduced Pdx-1 protein levels in islets [113]. This is supported by findings of PPRE sequences in the promoter region of GLUT2 [114], glucokinase [115], and Pdx-1 [111, 113]. Furthermore, troglitazone was demonstrated to increase the half-life of Pdx-1 and MafA by inhibiting ubiquitination, which otherwise targets them for degradation by the proteasome [116]. The role of PPARγ in pancreas development is not completely understood as PPARγ pancreatic knockout mice are hyperglycemic despite having a normal pancreas morphology [113]. In vivo studies found that long-term rosiglitazone (a PPARγ agonist) or troglitazone treatment maintains beta cell proliferation and prevents the age-related loss of pancreatic mass in rats and mice [117–119]. Troglitazone can also prevent age-related pancreatic abnormalities and increases in fasting insulin levels [120, 121].
Other studies have shown that PPARγ agonists improve beta cell function and prevent mitochondrial alterations and diabetes in obese mice and rats [117, 118, 122]. In addition, activation of PPARγ protects against cytokine-induced apoptosis [123], lipotoxicity [124], and human islet amyloid polypeptide toxicity [125, 126]. A molecular explanation for these findings might be that activation of PPARγ is associated with a reduced amount of reactive oxygen species by inhibiting iNOS through NFκB [123]. PPARγ activation reduces islet ER stress in db/db mice and a diabetic ER stress mouse model [112, 127].
5. PPARs Regulate Sphingolipid Metabolism
We have recently described how the onset of T1D is associated with an abnormal sphingolipid metabolism in pancreatic islets. This was illustrated by newly diagnosed T1D patients having a reduced amount of the sphingolipid sulfatide and altered expression of several enzymes involved in sphingolipid metabolism in islets [44]. Sphingolipid metabolism is also altered before the onset of diabetes. Peripheral blood mononuclear cells from children progressing to T1D have altered levels of several sphingolipid species and altered expression of genes involved in sphingolipid metabolism [128]. PPARα is known to control the expression of cerebroside sulfotransferase (CST), which catalyses the last step in sulfatide biosynthesis. PPARα knockout mice had decreased CST expression associated with decreased serum sulfatide [129]. PPARα activation by fenofibrate leads to increased sulfatide concentration in the pancreas and multiple other organs [44, 130, 131]. This was associated with an increased CST expression in the corresponding tissue [130, 131]. Similarly, fatty acids have been shown to activate PPARα and increase sulfatide levels through SPTLC2 (subunit of serine palmitoyltransferase), which regulates the first step in sphingolipid synthesis [132]. Treatment with PPARα agonist WY14643 or bezafibrate leads to increased expression of SPTLC2 in various cell types [133–135]. SPTLC2 and CST both have PPARα binding sequences in their promoter region [132]. PPARα is similarly involved in regulating the composition of sulfatide species with C16 (insulin folding and secretion) and C24 (immune regulation) having different functions [136, 137]. In the pancreas, fenofibrate especially increased the amount of C24 sulfatide thereby creating an anti-inflammatory sulfatide composition [138].
Another sphingolipid with a suspected role in T1D pathology is the proapoptotic ceramide of which C16 promotes apoptosis, mitochondrial dysfunction, and insulin resistance [139–142], while C24 has beneficial roles in regulating metabolic health [141, 143]. Recently, we demonstrated that fenofibrate altered ceramide composition in the pancreas of NOD mice increasing C24 and decreasing C16, hence creating a more beneficial ceramide composition [138]. WY14643 was otherwise found to increase ceramide levels in rat hearts [134], suggesting organ-specific regulation of ceramide synthesis. PPARβ/δ and PPARγ are both known to regulate sphingolipid metabolism with PPARβ/δ agonist GW0742 and PPARγ agonist troglitazone increasing de novo synthesis in rat hearts [144].
6. PPAR Activation Prevents Diabetes in NOD Mice
NOD mice share many autoantigens and biomarkers with human patients, and much has been learned from this model concerning the identification of genetic and environmental risk factors [145]. Experiments on NOD mice are primarily performed on females owing to a diabetes incidence of approximately 80%, compared to approximately 20% in males [146]. The higher incidence in females might be connected to the gender-specific changes in the expression of PPARα and PPARγ. Female NOD mice had increased expression of PPARα, while PPARγ was decreased in macrophages and CD4+ lymphocytes compared to male NOD mice [147]. Additionally, NOD mice have altered expression of PPARα and PPARγ in CD4+ or CD8+ lymphocytes and macrophages compared to non-obese diabetic-resistant (NOR) mice [148].
We and others have demonstrated that activation of PPARα by fenofibrate or PPARγ by troglitazone and rosiglitazone results in reduced autoimmune diabetes incidence [44, 149]. Fenofibrate treatment initiated after disease onset could even reverse diabetes in 46% of female NOD mice [44]. In addition, troglitazone prevents hyperglycemia and reduces insulitis in mice following streptozotocin injections [150]. PPARs are also regulated by various naturally occurring agonists, of which several have been examined for their effect on autoimmune diabetes in NOD mice (Table 1). This includes epigallocatechin [151, 152], curcumin [153, 154], cannabidiol [155, 156], omega 3 fatty acids [157], and capsaicin [158, 159], which induce PPAR activity and protect against autoimmune diabetes in NOD mice.
Table 1.
Overview of treatments that promote PPAR expression and prevent autoimmune diabetes in NOD mice.
| Drug | Delivery | Diabetes incidence | Reference |
|---|---|---|---|
| Fenofibrate | Diet from age 3 weeks | 0% | [44] |
| Troglitazone | Oral gavage from age 3 weeks | 22% | [149] |
| Rosiglitazone | Oral gavage from age 3 weeks | 22% | [149] |
| Epigallocatechin | Water from age 5 weeks | 25% | [151] |
| Curcumin | i.p. every other day | 33 % (CYP-induced diabetes) | [153] |
| Cannabidiol | i.p. age 6-12 weeks | 30% | [155] |
| Capsaicin | Oral gavage at age 9 or 10 weeks | 20% | [158] |
| Taurine | Water to pregnant mothers | 40% (after 24 weeks) | [160] |
| Omega-3 | Diet from age 5 weeks | 33% | [157] |
| Gluten-free diet | Diet from breeding | 15% | [163] |
Taurine, which stimulates PPARα, in the diet during gestation and lactation reduces diabetes development in offspring of NOD mice [160, 161]. On a similar note, a gluten-free diet, which leads to increased expression of PPARα and PPARγ [162], was found to reduce diabetes incidence in NOD mice [163], even after exclusive exposure of the diet in utero [164, 165].
7. Conclusions
Numerous studies have examined PPARs in relation to their role as regulators of lipid metabolism. However, the isoforms are also potent regulators of inflammation and beta cell biology (Figure 1). The effects of PPAR activation on T cell survival, activation, and differentiation are likely beneficial in a T1D setting but remain unstudied to a large extent. The same is true for studies of pancreas biology with most studies being conducted in relation to type 2 diabetes. Thus, we need further studies to determine the precise role of PPARs in T1D pathology. The beneficial effect on NOD mice by PPAR agonists is promising, and we believe that modulation of PPARs represents a novel treatment strategy targeting both the immune system and the pancreas.
Acknowledgments
This article was supported by Kirsten og Freddy Johansens Fond.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
LJH wrote the manuscript with input from MØM, MHJ, and KB. All authors have read and approved the final manuscript.
References
- 1.Frumento D., Ben Nasr M., El Essawy B., D'Addio F., Zuccotti G. V., Fiorina P. Immunotherapy for type 1 diabetes. Journal of Endocrinological Investigation. 2017;40(8):803–814. doi: 10.1007/s40618-017-0641-y. [DOI] [PubMed] [Google Scholar]
- 2.van Belle T. L., Coppieters K. T., von Herrath M. G. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiological Reviews. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 3.Federation I. D. IDF Diabetes Atlas. 7th 2015.
- 4.Federation I. D. IDF Diabetes Atlas. 8th. 2017. [PubMed]
- 5.Atkinson M. A., Eisenbarth G. S., Michels A. W. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham J., Hagopian W. A., Kockum I., et al. Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes. 2002;51(5):1346–1355. doi: 10.2337/diabetes.51.5.1346. [DOI] [PubMed] [Google Scholar]
- 7.Sanjeevi C. B., Lybrand T. P., DeWeese C., et al. Polymorphic amino acid variations in HLA-DQ are associated with systematic physical property changes and occurrence of IDDM. Members of the Swedish Childhood Diabetes Study. Diabetes. 1995;44(1):125–131. doi: 10.2337/diab.44.1.125. [DOI] [PubMed] [Google Scholar]
- 8.Groop L., Pociot F. Genetics of diabetes - Are we missing the genes or the disease? Molecular and Cellular Endocrinology. 2014;382(1):726–739. doi: 10.1016/j.mce.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Pociot F., Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 10.Rewers M., Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387(10035):2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leete P., Willcox A., Krogvold L., et al. Differential insulitic profiles determine the extent of β-Cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 12.Krogvold L., Wiberg A., Edwin B., et al. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia. 2016;59(3):492–501. doi: 10.1007/s00125-015-3820-4. [DOI] [PubMed] [Google Scholar]
- 13.Coppieters K. T., Dotta F., Amirian N., et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. The Journal of Experimental Medicine. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogvold L., Skog O., Sundstrom G., et al. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment In Vitro. Diabetes. 2015;64(7):2506–2512. doi: 10.2337/db14-1911. [DOI] [PubMed] [Google Scholar]
- 15.Cobo-Vuilleumier N., Lorenzo P. I., Rodriguez N. G., et al. LRH-1 agonism favours an immune-islet dialogue which protects against diabetes mellitus. Nature Communications. 2018;9(1):p. 1488. doi: 10.1038/s41467-018-03943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson M. A., Roep B. O., Posgai A., Wheeler D. C. S., Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. The Lancet Diabetes & Endocrinology. 2019;7(1):52–64. doi: 10.1016/S2213-8587(18)30112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menendez-Gutierrez M. P., Roszer T., Ricote M. Biology and therapeutic applications of peroxisome proliferator- activated receptors. Current Topics in Medicinal Chemistry. 2012;12(6):548–584. doi: 10.2174/156802612799436669. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi S., Gupta P., Saini A. S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. Journal of Advanced Pharmaceutical Technology and Research. 2011;2(4):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umemoto T., Fujiki Y. Ligand-dependent nucleo-cytoplasmic shuttling of peroxisome proliferator-activated receptors, PPARα and PPARγ. Genes to Cells. 2012;17(7):576–596. doi: 10.1111/j.1365-2443.2012.01607.x. [DOI] [PubMed] [Google Scholar]
- 20.Le Menn G., Neels J. G. Regulation of immune cell function by PPARs and the connection with metabolic and neurodegenerative diseases. International Journal of Molecular Sciences. 2018;19(6):p. 1575. doi: 10.3390/ijms19061575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capelli D., Cerchia C., Montanari R., et al. Structural basis for PPAR partial or full activation revealed by a novel ligand binding mode. Scientific Reports. 2016;6(1, article 34792) doi: 10.1038/srep34792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulsen L., Siersbaek M., Mandrup S. PPARs: fatty acid sensors controlling metabolism. Seminars in Cell and Developmental Biology. 2012;23(6):631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Zoete V., Grosdidier A., Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochimica et Biophysica Acta. 2007;1771(8):915–925. doi: 10.1016/j.bbalip.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Nettles K. W., Greene G. L. Ligand control of coregulator recruitment to nuclear receptors. Annual Review of Physiology. 2005;67(1):309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- 25.Ricote M., Glass C. K. PPARs and molecular mechanisms of transrepression. Biochimica et Biophysica Acta. 2007;1771(8):926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torchia J., Glass C., Rosenfeld M. G. Co-activators and co-repressors in the integration of transcriptional responses. Current Opinion in Cell Biology. 1998;10(3):373–383. doi: 10.1016/S0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 27.Yessoufou A., Wahli W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Medical Weekly. 2010;140, article w13071 doi: 10.4414/smw.2010.13071. [DOI] [PubMed] [Google Scholar]
- 28.Mandard S., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cellular and Molecular Life Sciences. 2004;61(4):393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zingarelli B., Piraino G., Hake P. W., et al. Peroxisome Proliferator-Activated Receptor δ Regulates Inflammation via NF-κB Signaling in Polymicrobial Sepsis. American Journal of Pathology. 2010;177(4):1834–1847. doi: 10.2353/ajpath.2010.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fajas L., Auboeuf D., Raspe E., et al. The organization, promoter analysis, and expression of the human PPARgamma gene. Journal of Biological Chemistry. 1997;272(30):18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 31.Ricote M., Huang J. T., Welch J. S., Glass C. K. The peroxisome proliferator-activated receptor(PPARgamma) as a regulator of monocyte/macrophage function. Journal of Leukocyte Biology. 1999;66(5):733–739. doi: 10.1002/jlb.66.5.733. [DOI] [PubMed] [Google Scholar]
- 32.Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willson T. M., Brown P. J., Sternbach D. D., Henke B. R. The PPARs: from orphan receptors to drug discovery. Journal of Medicinal Chemistry. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 34.Racke M. K., Gocke A. R., Muir M., Diab A., Drew P. D., Lovett-Racke A. E. Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis. Journal of Nutrition. 2006;136(3):700–703. doi: 10.1093/jn/136.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aprahamian T. R., Bonegio R. G., Weitzner Z., Gharakhanian R., Rifkin I. R. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology. 2014;142(3):363–373. doi: 10.1111/imm.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallahi P., Ferrari S. M., Elia G., et al. Novel therapies for thyroid autoimmune diseases. Expert Review of Clinical Pharmacology. 2016;9(6):853–861. doi: 10.1586/17512433.2016.1157468. [DOI] [PubMed] [Google Scholar]
- 37.Pawlak-Adamska E., Daroszewski J., Bolanowski M., et al. PPARg2 Ala12 variant protects against Graves' orbitopathy and modulates the course of the disease. Immunogenetics. 2013;65(7):493–500. doi: 10.1007/s00251-013-0702-0. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto H., Iwamoto T., Kotake S., Momohara S., Yamanaka H., Kamatani N. Inhibition of NF-κB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clinical and Experimental Rheumatology. 2005;23(3):323–330. [PubMed] [Google Scholar]
- 39.Lima E. A., de Andrade Lima M. M. D., Marques C. D. L., Duarte A. L. B. P., da Rocha Pita I., da Rocha Pita M. G. Peroxisome proliferator-activated receptor agonists (PPARs): a promising prospect in the treatment of psoriasis and psoriatic arthritis. Anais Brasileiros de Dermatologia. 2013;88(6):1029–1035. doi: 10.1590/abd1806-4841.20132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramkalawan H., Wang Y. Z., Hurbungs A., et al. Pioglitazone, PPARγ agonist, attenuates experimental autoimmune neuritis. Inflammation. 2012;35(4):1338–1347. doi: 10.1007/s10753-012-9447-4. [DOI] [PubMed] [Google Scholar]
- 41.Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2011;1812(8):1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beeson P. B. Age and sex associations of 40 autoimmune diseases. The American journal of medicine. 1994;96(5):457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 43.Dunn S. E., Ousman S. S., Sobel R. A., et al. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. Journal of Experimental Medicine. 2007;204(2):321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holm L. J., Krogvold L., Hasselby J. P., et al. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia. 2018;61(7):1650–1661. doi: 10.1007/s00125-018-4614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janani C., Ranjitha Kumari B. D. PPAR gamma gene - A review. Diabetes and Metabolic Syndrome. 2015;9(1):46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Hull C. M., Peakman M., Tree T. I. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia. 2017;60(10):1839–1850. doi: 10.1007/s00125-017-4377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szablewski L. Role of immune system in type 1 diabetes mellitus pathogenesis. International Immunopharmacology. 2014;22(1):182–191. doi: 10.1016/j.intimp.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 48.Bloem S. J., Roep B. O. The elusive role of B lymphocytes and islet autoantibodies in (human) type 1 diabetes. Diabetologia. 2017;60(7):1185–1189. doi: 10.1007/s00125-017-4284-5. [DOI] [PubMed] [Google Scholar]
- 49.Willcox A., Richardson S. J., Bone A. J., Foulis A. K., Morgan N. G. Analysis of islet inflammation in human type 1 diabetes. Clinical and Experimental Immunology. 2009;155(2):173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L. Z., Wang R., Huang G., et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of Experimental Medicine. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalek R. D., Gerriets V. A., Jacobs S. R., et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of Immunology. 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang S. C., Everts B., Ivanova Y., et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature Immunology. 2014;15(9):846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi J. M., Bothwell A. L. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Molecules and Cells. 2012;33(3):217–222. doi: 10.1007/s10059-012-2297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penas F., Mirkin G. A., Vera M., et al. Treatment in vitro with PPAR α and PPARγ ligands drives M1-to-M2 polarization of macrophages from T. cruzi -infected mice. Biochimica et Biophysica Acta. 2015;1852(5):893–904. doi: 10.1016/j.bbadis.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 55.Odegaard J. I., Ricardo-Gonzalez R. R., Red Eagle A., et al. Alternative M2 Activation of Kupffer Cells by PPARδ Ameliorates Obesity- Induced Insulin Resistance. Cell Metabolism. 2008;7(6):496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo W., Xu Q., Wang Q., Wu H., Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Scientific Reports. 2017;7(1, article 44612) doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng X., Zhang P., Liang T., Deng S., Chen X., Zhu L. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARγ and NF-κB pathways. International Journal of Molecular Medicine. 2015;36(2):449–454. doi: 10.3892/ijmm.2015.2230. [DOI] [PubMed] [Google Scholar]
- 58.Hichami A., Yessoufou A., Ghiringhelli F., et al. Peroxisome proliferator-activated receptor alpha deficiency impairs regulatory T cell functions: Possible application in the inhibition of melanoma tumor growth in mice. Biochimie. 2016;131:1–10. doi: 10.1016/j.biochi.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Devchand P. R., Keller H., Peters J. M., Vazquez M., Gonzalez F. J., Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384(6604):39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Z., Liang Y., Gao Y., Kong W., Feng J., Wang X. Fenofibrate Enhances the In Vitro Differentiation of Regulatory T Cells in Mice. PPAR Research. 2012;2012:10. doi: 10.1155/2012/529035.529035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng H., Xi Y., Chi X., Wu Y., Liu G. Fenofibrate treatment of rats with experimental autoimmune myocarditis by alleviating Treg/Th17 disorder. Central-European Journal of Immunology. 2016;1(1):64–70. doi: 10.5114/ceji.2016.58817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Z., Sun W., Liang Y., et al. Fenofibrate Inhibited the Differentiation of T Helper 17 Cells In Vitro. PPAR Research. 2012;2012:10. doi: 10.1155/2012/145654.145654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morse E., Selim E., Cunard R. PPARα ligands cause lymphocyte depletion and cell cycle block and this is associated with augmented TRB3 and reduced Cyclin B1 expression. Molecular Immunology. 2009;46(16):3454–3461. doi: 10.1016/j.molimm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Madej A., Okopien B., Kowalski J., et al. Effects of fenofibrate on plasma cytokine concentrations in patients with atherosclerosis and hyperlipoproteinemia IIb. International Journal of Clinical Pharmacology and Therapeutics. 1998;36(6):345–349. [PubMed] [Google Scholar]
- 65.Dunn S. E., Bhat R., Straus D. S., et al. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. Journal of Experimental Medicine. 2010;207(8):1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanakasabai S., Chearwae W., Walline C. C., Iams W., Adams S. M., Bright J. J. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;130(4):572–588. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanakasabai S., Walline C. C., Chakraborty S., Bright J. J. PPARδ deficient mice develop elevated Th1/Th17 responses and prolonged experimental autoimmune encephalomyelitis. Brain Research. 2011;1376:101–112. doi: 10.1016/j.brainres.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 68.Mothe-Satney I., Murdaca J., Sibille B., et al. A role for peroxisome proliferator-activated receptor beta in T cell development. Scientific Reports. 2016;6(1, article 34317) doi: 10.1038/srep34317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frauwirth K. A., Riley J. L., Harris M. H., et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 70.Hontecillas R., Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. Journal of Immunology. 2007;178(5):2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 71.Klotz L., Burgdorf S., Dani I., et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. Journal of Experimental Medicine. 2009;206(10):2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park H. J., Kim D. H., Choi J. Y., et al. PPARγ negatively regulates T cell activation to prevent follicular helper T cells and germinal center formation. PLoS One. 2014;9(6, article e99127) doi: 10.1371/journal.pone.0099127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saubermann L. J., Nakajima A., Wada K., et al. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflammatory Bowel Diseases. 2002;8(5):330–339. doi: 10.1097/00054725-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Celinski K., Dworzanski T., Fornal R., et al. Comparison of anti-inflammatory properties of peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone in prophylactic treatment of experimental colitis. Journal Of Physiology and Pharmacology. 2013;64(5):587–595. [PubMed] [Google Scholar]
- 75.Guri A. J., Mohapatra S. K., Horne W. T., 2nd, Hontecillas R., Bassaganya-Riera J. The role of T cell PPAR γ in mice with experimental inflammatory bowel disease. BMC Gastroenterology. 2010;10(1):p. 60. doi: 10.1186/1471-230X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cipolletta D., Feuerer M., Li A., et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404, article BFnature11132):549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wohlfert E. A., Nichols F. C., Nevius E., Clark R. B. Peroxisome proliferator-activated receptor γ (PPARγ) and immunoregulation: enhancement of regulatory T cells through PPARγ-Dependent and -independent mechanisms. Journal of Immunology. 2007;178(7):4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 78.Maslanka T., Otrocka-Domagala I., Zuska-Prot M., Gesek M. Beneficial effects of rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, in a mouse allergic asthma model is not associated with the recruitment or generation of Foxp3-expressing CD4(+) regulatory T cells. European Journal of Pharmacology. 2019;848:30–38. doi: 10.1016/j.ejphar.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 79.Rorsman P., Ashcroft F. M. Pancreatic β-Cell electrical activity and insulin secretion: of mice and men. Physiological Reviews. 2018;98(1):117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xin Y., Dominguez Gutierrez G., Okamoto H., et al. Pseudotime ordering of single human β-Cells reveals states of insulin production and unfolded protein response. Diabetes. 2018;67(9):1783–1794. doi: 10.2337/db18-0365. [DOI] [PubMed] [Google Scholar]
- 81.Thomaidou S., Zaldumbide A., Roep B. O. Islet stress, degradation and autoimmunity. Diabetes Obesity and Metabolism. 2018;20(Supplement 2):88–94. doi: 10.1111/dom.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eizirik D. L., Sammeth M., Bouckenooghe T., et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8(3, article e1002552) doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravnskjaer K., Frigerio F., Boergesen M., Nielsen T., Maechler P., Mandrup S. PPARdelta is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction. Journal of Lipid Research. 2010;51(6):1370–1379. doi: 10.1194/jlr.M001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dillon J. S., Yaney G. C., Zhou Y., et al. Dehydroepiandrosterone sulfate and beta-cell function: enhanced glucose-induced insulin secretion and altered gene expression in rodent pancreatic beta-cells. Diabetes. 2000;49(12):2012–2020. doi: 10.2337/diabetes.49.12.2012. [DOI] [PubMed] [Google Scholar]
- 85.Gremlich S., Nolan C., Roduit R., et al. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146(1):375–382. doi: 10.1210/en.2004-0667. [DOI] [PubMed] [Google Scholar]
- 86.Roduit R., Morin J., Masse F., et al. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-alpha gene in the pancreatic beta -cell. Journal of Biological Chemistry. 2000;275(46):35799–35806. doi: 10.1074/jbc.M006001200. [DOI] [PubMed] [Google Scholar]
- 87.Ravnskjaer K., Boergesen M., Dalgaard L. T., Mandrup S. Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. Journal of Molecular Endocrinology. 2006;36(2):289–299. doi: 10.1677/jme.1.01965. [DOI] [PubMed] [Google Scholar]
- 88.Bihan H., Rouault C., Reach G., Poitout V., Staels B., Guerre-Millo M. Pancreatic islet response to hyperglycemia is dependent on peroxisome proliferator-activated receptor alpha (PPARalpha) FEBS Letters. 2005;579(11):2284–2288. doi: 10.1016/j.febslet.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 89.Taylor B. L., Liu F. F., Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Reports. 2013;4(6):1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y., Liu Q., Zhou Z., Ikeda Y. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Research and Therapy. 2017;8(1):p. 240. doi: 10.1186/s13287-017-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yessoufou A., Ategbo J. M., Attakpa E., et al. Peroxisome proliferator-activated receptor-alpha modulates insulin gene transcription factors and inflammation in adipose tissues in mice. Molecular and Cellular Biochemistry. 2009;323(1-2):101–111. doi: 10.1007/s11010-008-9968-1. [DOI] [PubMed] [Google Scholar]
- 92.Babu D. A., Deering T. G., Mirmira R. G. A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis. Molecular Genetics and Metabolism. 2007;92(1-2):43–55. doi: 10.1016/j.ymgme.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo H., Sun S., Zhang X., Zhang X. J., Gao L., Zhao J. J. AMPK enhances the expression of pancreatic duodenal homeobox-1 via PPARalpha, but not PPARgamma, in rat insulinoma cell line INS-1. Acta Pharmacologica Sinica. 2010;31(8):963–969. doi: 10.1038/aps.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Y., Zhang L., Gu H. F., et al. Peroxisome proliferator-activated receptor-alpha regulates the expression of pancreatic/duodenal homeobox-1 in rat insulinoma (INS-1) cells and ameliorates glucose-induced insulin secretion impaired by palmitate. Endocrinology. 2008;149(2):662–671. doi: 10.1210/en.2007-1275. [DOI] [PubMed] [Google Scholar]
- 95.Tang T., Abbott M. J., Ahmadian M., Lopes A. B., Wang Y., Sul H. S. Desnutrin/ATGL Activates PPARδ to Promote Mitochondrial Function for Insulin Secretion in Islet β Cells. Cell Metabolism. 2013;18(6):883–895. doi: 10.1016/j.cmet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iglesias J., Barg S., Vallois D., et al. PPARβ/δ affects pancreatic β cell mass and insulin secretion in mice. Journal of Clinical Investigation. 2012;122(11):4105–4117. doi: 10.1172/JCI42127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li L., Li T., Zhang Y., et al. Peroxisome proliferator-activated receptor β/δ activation is essential for modulating p-Foxo1/Foxo1 status in functional insulin-positive cell differentiation. Cell death and disease. 2015;6(4, article e1715) doi: 10.1038/cddis.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao M., Long Y., Tong Y., Wan J., Tong N. Activation of PPARδ up-regulates the expression of insulin gene transcription factor MafA and ameliorates glucose-induced insulin secretion impaired by palmitate. Molecular and Cellular Biochemistry. 2012;366(1-2):183–189. doi: 10.1007/s11010-012-1296-9. [DOI] [PubMed] [Google Scholar]
- 99.Cao M., Tong Y., Lv Q., et al. PPAR δ Activation Rescues Pancreatic β -Cell Line INS-1E from Palmitate-Induced Endoplasmic Reticulum Stress through Enhanced Fatty Acid Oxidation. PPAR Research. 2012;2012:8. doi: 10.1155/2012/680684.680684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silva-Veiga F. M., Rachid T. L., de Oliveira L., Graus-Nunes F., Mandarim-de-Lacerda C. A., Souza-Mello V. GW0742 (PPAR-beta agonist) attenuates hepatic endoplasmic reticulum stress by improving hepatic energy metabolism in high-fat diet fed mice. Molecular and Cellular Endocrinology. 2018;474:227–237. doi: 10.1016/j.mce.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 101.Tong Q., Wu L., Gao Q., Ou Z., Zhu D., Zhang Y. PPARβ/δ agonist provides neuroprotection by suppression of IRE1α–Caspase-12-Mediated endoplasmic reticulum stress pathway in the rotenone rat model of Parkinson's disease. Molecular Neurobiology. 2016;53(6):3822–3831. doi: 10.1007/s12035-015-9309-9. [DOI] [PubMed] [Google Scholar]
- 102.Nakamichi Y., Kikuta T., Ito E., et al. PPAR-γ overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Biochemical and Biophysical Research Communications. 2003;306(4, article S0006291X03010453):832–836. doi: 10.1016/s0006-291x(03)01045-3. [DOI] [PubMed] [Google Scholar]
- 103.Bollheimer L. C., Troll S., Landauer H., Wrede C. E., Scholmerich J., Buettner R. Insulin-sparing effects of troglitazone in rat pancreatic islets. Journal of Molecular Endocrinology. 2003;31(1):61–69. doi: 10.1677/jme.0.0310061. [DOI] [PubMed] [Google Scholar]
- 104.Ito E., Ozawa S., Takahashi K., et al. PPAR-γ overexpression selectively suppresses insulin secretory capacity in isolated pancreatic islets through induction of UCP-2 protein. Biochemical and Biophysical Research Communications. 2004;324(2):810–814. doi: 10.1016/j.bbrc.2004.08.238. [DOI] [PubMed] [Google Scholar]
- 105.Ravnskjaer K., Boergesen M., Rubi B., et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology. 2005;146(8):3266–3276. doi: 10.1210/en.2004-1430. [DOI] [PubMed] [Google Scholar]
- 106.Wang X., Zhou L., Shao L., et al. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sciences. 2007;81(2):160–165. doi: 10.1016/j.lfs.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 107.Yang C., Chang T. J., Chang J. C., et al. Rosiglitazone (BRL 49653) enhances insulin secretory response via phosphatidylinositol 3-kinase pathway. Diabetes. 2001;50(11):2598–2602. doi: 10.2337/diabetes.50.11.2598. [DOI] [PubMed] [Google Scholar]
- 108.Santini E., Fallahi P., Ferrari S. M., Masoni A., Antonelli A., Ferrannini E. Effect of PPAR- Activation and inhibition on glucose-stimulated insulin release in INS-1e cells. Diabetes. 2004;53(Supplement 3):S79–S83. doi: 10.2337/diabetes.53.suppl_3.s79. [DOI] [PubMed] [Google Scholar]
- 109.Kim H. S., Noh J. H., Hong S. H., et al. Rosiglitazone stimulates the release and synthesis of insulin by enhancing GLUT-2, glucokinase and BETA2/NeuroD expression. Biochemical and Biophysical Research Communications. 2008;367(3):623–629. doi: 10.1016/j.bbrc.2007.12.192. [DOI] [PubMed] [Google Scholar]
- 110.Kim H. S., Hwang Y. C., Koo S. H., et al. PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-Cells. PLoS One. 2013;8(1, article e50128) doi: 10.1371/journal.pone.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moibi J. A., Gupta D., Jetton T. L., Peshavaria M., Desai R., Leahy J. L. Peroxisome proliferator-activated Receptor- Regulates Expression of PDX-1 and NKX6.1 in INS-1 cells. Diabetes. 2007;56(1):88–95. doi: 10.2337/db06-0948. [DOI] [PubMed] [Google Scholar]
- 112.Evans-Molina C., Robbins R. D., Kono T., et al. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Molecular and Cellular Biology. 2009;29(8):2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gupta D., Jetton T. L., Mortensen R. M., Duan S. Z., Peshavaria M., Leahy J. L. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. Journal of Biological Chemistry. 2008;283(47):32462–32470. doi: 10.1074/jbc.M801813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim H. I., Kim J. W., Kim S. H., Cha J. Y., Kim K. S., Ahn Y. H. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes. 2000;49(9):1517–1524. doi: 10.2337/diabetes.49.9.1517. [DOI] [PubMed] [Google Scholar]
- 115.Kim H. I., Cha J. Y., Kim S. Y., et al. Peroxisomal proliferator-activated Receptor- Upregulates Glucokinase Gene Expression in -Cells. Diabetes. 2002;51(3):676–685. doi: 10.2337/diabetes.51.3.676. [DOI] [PubMed] [Google Scholar]
- 116.Zhu Y., Ma A., Zhang H., Li C. PPARγ activation attenuates glycated-serum induced pancreatic beta-cell dysfunction through enhancing Pdx1 and Mafa protein stability. PLoS One. 2013;8(2, article e56386) doi: 10.1371/journal.pone.0056386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimabukuro M., Zhou Y. T., Lee Y., Unger R. H. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. Journal of Biological Chemistry. 1998;273(6):3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- 118.Higa M., Zhou Y. T., Ravazzola M., Baetens D., Orci L., Unger R. H. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proceedings of the National Academy of Sciences. 1999;96(20):11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finegood D. T., McArthur M. D., Kojwang D., et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50(5):1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 120.Jia D. M., Tabaru A., Nakamura H., Fukumitsu K. I., Akiyama T., Otsuki M. Troglitazone prevents and reverses dyslipidemia, insulin secretory defects, and histologic abnormalities in a rat model of naturally occurring obese diabetes. Metabolism. 2000;49(9):1167–1175. doi: 10.1053/meta.2000.8599. [DOI] [PubMed] [Google Scholar]
- 121.Jia D. M., Otsuki M. Troglitazone stimulates pancreatic growth in normal rats. Pancreas. 2002;24(3):303–312. doi: 10.1097/00006676-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 122.Matsui J., Terauchi Y., Kubota N., et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes. 2004;53(11):2844–2854. doi: 10.2337/diabetes.53.11.2844. [DOI] [PubMed] [Google Scholar]
- 123.Kim E. K., Kwon K. B., Koo B. S., et al. Activation of peroxisome proliferator-activated receptor-γ protects pancreatic β-cells from cytokine-induced cytotoxicity via NFκB pathway. The International Journal of Biochemistry and Cell Biology. 2007;39(6):1260–1275. doi: 10.1016/j.biocel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Shen X., Yang L., Yan S., et al. Fetuin A promotes lipotoxicity in β cells through the TLR4 signaling pathway and the role of pioglitazone in anti-lipotoxicity. Molecular and Cellular Endocrinology. 2015;412:1–11. doi: 10.1016/j.mce.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 125.Lin C. Y., Gurlo T., Haataja L., Hsueh W. A., Butler P. C. Activation of peroxisome proliferator-activated Receptor-γ by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase-dependent pathway. Journal of Endocrinology and Metabolism. 2005;90(12):6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 126.Hull R. L., Shen Z.-P., Watts M. R., et al. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54(7):2235–2244. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]
- 127.Akiyama M., Hatanaka M., Ohta Y., et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia. 2009;52(4, article 1270):653–663. doi: 10.1007/s00125-009-1270-6. [DOI] [PubMed] [Google Scholar]
- 128.Sen P., Dickens A. M., López-Bascón M. A., et al. Metabolic alterations in human peripheral blood mononuclear cells associate with progression to islet autoimmunity and type 1 diabetes. bioRxiv. 2019;(article 658500) doi: 10.1101/658500. [DOI] [Google Scholar]
- 129.Zhang X., Nakajima T., Kamijo Y., et al. Acute kidney injury induced by protein-overload nephropathy down-regulates gene expression of hepatic cerebroside sulfotransferase in mice, resulting in reduction of liver and serum sulfatides. Biochemical and Biophysical Research Communications. 2009;390(4):1382–1388. doi: 10.1016/j.bbrc.2009.10.164. [DOI] [PubMed] [Google Scholar]
- 130.Kimura T., Nakajima T., Kamijo Y., et al. Hepatic Cerebroside Sulfotransferase Is Induced by PPARα Activation in Mice. PPAR Research. 2012;2012:10. doi: 10.1155/2012/174932.174932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakajima T., Kamijo Y., Yuzhe H., et al. Peroxisome proliferator-activated receptor α mediates enhancement of gene expression of cerebroside sulfotransferase in several murine organs. Glycoconjugate Journal. 2013;30(6):553–560. doi: 10.1007/s10719-012-9454-6. [DOI] [PubMed] [Google Scholar]
- 132.Yang Y., Feng Y., Zhang X., et al. Activation of PPARα by fatty acid accumulation enhances fatty acid degradation and sulfatide synthesis. The Tohoku Journal of Experimental Medicine. 2016;240(2):113–122. doi: 10.1620/tjem.240.113. [DOI] [PubMed] [Google Scholar]
- 133.Rivier M., Castiel I., Safonova I., Ailhaud G., Michel S. Peroxisome Proliferator-Activated Receptor-α Enhances Lipid Metabolism in a Skin Equivalent Model. Journal of Investigative Dermatology. 2000;114(4):681–687. doi: 10.1046/j.1523-1747.2000.00939.x. [DOI] [PubMed] [Google Scholar]
- 134.Baranowski M., Blachnio A., Zabielski P., Gorski J. PPARalpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. Journal of physiology and pharmacology. 2007;58(1):57–72. [PubMed] [Google Scholar]
- 135.Zabielski P., Blachnio-Zabielska A., Baranowski M., Zendzian-Piotrowska M., Gorski J. Activation of PPARα by bezafibrate negatively affects de novo synthesis of sphingolipids in regenerating rat liver. Prostaglandins and Other Lipid Mediators. 2010;93(3-4):120–125. doi: 10.1016/j.prostaglandins.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 136.Subramanian L., Blumenfeld H., Tohn R., et al. NKT cells stimulated by long fatty acyl chain sulfatides significantly reduce the incidence of type 1 diabetes in nonobese diabetic mice [corrected] PLoS One. 2012;7(5, article e37771) doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buschard K., Blomqvist M., Osterbye T., Fredman P. Involvement of sulfatide in beta cells and type 1 and type 2 diabetes. Diabetologia. 2005;48(10):1957–1962. doi: 10.1007/s00125-005-1926-9. [DOI] [PubMed] [Google Scholar]
- 138.Holm L. J., Haupt-Jorgensen M., Giacobini J. D., Hasselby J. P., Bilgin M., Buschard K. Fenofibrate increases very-long-chain sphingolipids and improves blood glucose homeostasis in NOD mice. Diabetologia. 2019;62(12):2262–2272. doi: 10.1007/s00125-019-04973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lang F., Ullrich S., Gulbins E. Ceramide formation as a target in beta-cell survival and function. Expert Opinion on Therapeutic Targets. 2011;15(9):1061–1071. doi: 10.1517/14728222.2011.588209. [DOI] [PubMed] [Google Scholar]
- 140.Grosch S., Schiffmann S., Geisslinger G. Chain length-specific properties of ceramides. Progress in Lipid Research. 2012;51(1):50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Raichur S., Wang S. T., Chan P. W., et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metabolism. 2014;20(5):p. 919. doi: 10.1016/j.cmet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 142.Zigdon H., Kogot-Levin A., Park J. W., et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. The Journal of Biological Chemistry. 2013;288(7):4947–4956. doi: 10.1074/jbc.M112.402719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park J. W., Park W. J., Kuperman Y., Boura-Halfon S., Pewzner-Jung Y., Futerman A. H. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013;57(2):525–532. doi: 10.1002/hep.26015. [DOI] [PubMed] [Google Scholar]
- 144.Baranowski M., Gorski J. Heart sphingolipids in health and disease. Advances in Experimental Medicine and Biology. 2011;721:41–56. doi: 10.1007/978-1-4614-0650-1_3. [DOI] [PubMed] [Google Scholar]
- 145.Pearson J. A., Wong F. S., Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. Journal of Autoimmunity. 2016;66:76–88. doi: 10.1016/j.jaut.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Reed J. C., Herold K. C. Thinking bedside at the bench: the NOD mouse model of T1DM. Nature Reviews Endocrinology. 2015;11(5):308–314. doi: 10.1038/nrendo.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yaacob N. S., Goh K. S., Norazmi M. N. Male and female NOD mice differentially express peroxisome proliferator- activated receptors and pathogenic cytokines. Experimental and Toxicologic Pathology. 2012;64(1-2):127–131. doi: 10.1016/j.etp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 148.Yaacob N. S., Kaderi M. A., Norazmi M. N. Differential transcriptional expression of PPARalpha, PPARgamma1, and PPARgamma2 in the peritoneal macrophages and T-cell subsets of non-obese diabetic mice. Journal of Clinical Immunology. 2009;29(5):595–602. doi: 10.1007/s10875-009-9300-1. [DOI] [PubMed] [Google Scholar]
- 149.Beales P. E., Pozzilli P. Thiazolidinediones for the prevention of diabetes in the non-obese diabetic (NOD) mouse: implications for human type 1 diabetes. Diabetes/Metabolism Research and Reviews. 2002;18(2):114–117. doi: 10.1002/dmrr.262. [DOI] [PubMed] [Google Scholar]
- 150.Ogawa J., Takahashi S., Fujiwara T., et al. Troglitazone can prevent development of type 1 diabetes induced by multiple low-dose streptozotocin in mice. Life Sciences. 1999;65(12):1287–1296. doi: 10.1016/s0024-3205(99)00364-1. [DOI] [PubMed] [Google Scholar]
- 151.Fu Z., Zhen W., Yuskavage J., Liu D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. British Journal of Nutrition. 2011;105(8):1218–1225. doi: 10.1017/S0007114510004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang S., Yang X., Luo J., et al. PPARα activation sensitizes cancer cells to epigallocatechin-3-gallate (EGCG) treatment via suppressing heme oxygenase-1. Nutrition and Cancer. 2014;66(2):315–324. doi: 10.1080/01635581.2014.868909. [DOI] [PubMed] [Google Scholar]
- 153.Castro C. N., Barcala Tabarrozzi A. E., Winnewisser J., et al. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clinical and Experimental Immunology. 2014;177(1):149–160. doi: 10.1111/cei.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mazidi M., Karimi E., Meydani M., Ghayour-Mobarhan M., Ferns G. A. Potential effects of curcumin on peroxisome proliferator-activated receptor-γin vitroandin vivo. World Journal of Methodology. 2016;6(1):112–117. doi: 10.5662/wjm.v6.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weiss L., Zeira M., Reich S., et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39(2):143–151. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- 156.O'Sullivan S. E. An update on PPAR activation by cannabinoids. British Journal of Pharmacology. 2016;173(12):1899–1910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bi X., Li F., Liu S., et al. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. Journal of Endocrinological Investigation. 2017;127(5):1757–1771. doi: 10.1172/JCI87388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nevius E., Srivastava P. K., Basu S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunology. 2012;5(1):76–86. doi: 10.1038/mi.2011.50. [DOI] [PubMed] [Google Scholar]
- 159.Kang J. H., Goto T., Han I. S., Kawada T., Kim Y. M., Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18(4):780–787. doi: 10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- 160.Arany E., Strutt B., Romanus P., Remacle C., Reusens B., Hill D. J. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia. 2004;47(10):1831–1837. doi: 10.1007/s00125-004-1535-z. [DOI] [PubMed] [Google Scholar]
- 161.Bonfleur M. L., Borck P. C., Ribeiro R. A., et al. Improvement in the expression of hepatic genes involved in fatty acid metabolism in obese rats supplemented with taurine. Life Sciences. 2015;135:15–21. doi: 10.1016/j.lfs.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 162.Soares F. L., de Oliveira M. R., Teixeira L. G., et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. The Journal of Nutritional Biochemistry. 2013;24(6):1105–1111. doi: 10.1016/j.jnutbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 163.Funda D. P., Kaas A., Bock T., Tlaskalova-Hogenova H., Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes/Metabolism Research and Reviews. 1999;15(5):323–327. doi: 10.1002/(SICI)1520-7560(199909/10)15:5<323::AID-DMRR53>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 164.Antvorskov J. C., Josefsen K., Haupt-Jorgensen M., Fundova P., Funda D. P., Buschard K. Gluten-free diet only during pregnancy efficiently prevents diabetes in NOD mouse offspring. Journal of Diabetes Research. 2016;2016:7. doi: 10.1155/2016/3047574.3047574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haupt-Jorgensen M., Larsen J., Josefsen K., et al. Gluten-free diet during pregnancy alleviates signs of diabetes and celiac disease in NOD mouse offspring. Diabetes/Metabolism Research and Reviews. 2018;34(4, article e2987) doi: 10.1002/dmrr.2987. [DOI] [PubMed] [Google Scholar]


