Gout is an inflammatory arthropathy with increasing global incidence and association with cardiovascular disease and mortality(1). Smoking is a well-established risk factor for coronary artery calcium(CAC) and adverse cardiovascular outcomes, yet smoking has been associated with reduced serum uric acid and decreased gout incidence, albeit in mostly young men(1). The impact of gout on CAC and cardiovascular outcomes in an older population with more significant smoking history is unknown.
This is a single center, retrospective analysis of 1033 U.S. Veterans who underwent USPSTF guideline-recommended(30pack-year smokers, aged 55–80years, active or quit <15years) lung cancer screening CT(LCSCT) between October 1, 2013 and May 31, 2014(2). Patients were excluded if they had lung cancer or prior percutaneous intervention with stent, which affects CAC quantification.
LCSCTs were not ECG-gated and were performed using a 128-slice CT scanner(Siemens), using 128×0.6mm collimation, 0.5second rotation, 0.84pitch, 380mm field of view, and matrix of 512×512pixels. Tube voltage and tube current were 120kV and 40mA. Image reconstruction thickness was 1.0–1.25mm. Minimum area required to identify calcium was 0.55mm2. Agatston CAC score(CACS) was quantified using a semi-automated imaging workstation with readers blinded to patient data(3). The kappa for interobserver agreement was 0.92(0.89–0.95).
The VA electronic medical record(EMR) was searched for patient demographics, cardiovascular risk factors, serum uric acid, and diagnosis of gout. Coronary artery disease(CAD) was defined as history of myocardial infarction(MI), coronary artery bypass graft surgery, or abnormalities in cardiac testing(ETT, echocardiography, myocardial perfusion, cardiac CT, or coronary angiography). Gout diagnosis was defined as ICD-9/10 codes of 274.XX/M10.XX, or the use of gout-related medications.
The primary outcome was CACS. The secondary outcome was MACE, defined as the composite endpoint of EMR-documented cardiovascular death, nonfatal MI, and nonfatal cerebral vascular accident(CVA)(4). Cardiovascular death includes sudden cardiac death or death from MI, heart failure, CVA, cardiovascular procedure or other cardiovascular causes. MI was defined as STEMI or NSTEMI. CVA included ischemic stroke and transient ischemic attack. Adjudicators of clinical outcomes were blinded to CACS.
Comparisons included normally distributed continuous variables(Student’s t-test), continuous variables not normally distributed(Mann-Whitney U), and categorical variables(chi-square). CACS between gout and gout-free patients were compared by linear regression adjusted for covariates with P-values less than 0.2 in the univariate model. MACE between gout and gout-free patients were compared by Cox regression, adjusted for age and CAD or for CACS.
The median age of this mostly Caucasian(94%), male(96%) population was 65(IQI:61,68) years and 9.3% had gout. The median ASCVD score was 18(IQI:12,28)%. 59% of patients had hypertension, 73% had dyslipidemia, 28% had diabetes, 19% had stage 3 or higher CKD (GFR<60 mL/min), and 17% had CAD. The overall median CACS was 452(IQI:90,1345) Agatston units(AU). During the 48 months after LCSCT, MACE occurred in 9.7% of the patients.
Patients with gout had higher median CACS than those without; 768(IQI:253,1966)AU vs. 437(IQI:87,1261)AU(P=0.001). Gout was associated with higher CACS(β-coefficient[95%CI],736[446,1027];P<0.001), and this association remained significant after adjustment for age, sex, BMI, DM, hypertension, hyperlipidemia, CKD, statin use, and CAD(β-coefficient[95%CI],437[173,701];P=0.001). Gout was also associated with higher serum uric acid; 7.0(IQI:5.7,8.6)mg/dL vs. 5.8(IQI:4.9,6.9)mg/dL(P<0.001).
MACE occurred in 18(19%) gout patients and 83(9%) gout-free patients(Fig.1A). When patients were stratified by CACS, gout was associated with increased annualized MACE at high CACS, though median CACS was significantly increased for gout in that category(Fig.1B). By Cox regression, gout was associated with MACE(HR[95%CI],2.23[1.35,3.74];P=0.002), and this association remained significant after adjustment for age and CAD(HR[95%CI],1.79[1.07,2.99];P=0.026). After adjustment for CACS, the association between gout and MACE did not remain significant(HR[95%CI],1.50[0.86,2.62];P=0.152), supporting CAC as a possible mechanism for the increased MACE.
Figure1. Gout is associated with increased MACE and higher CACS.
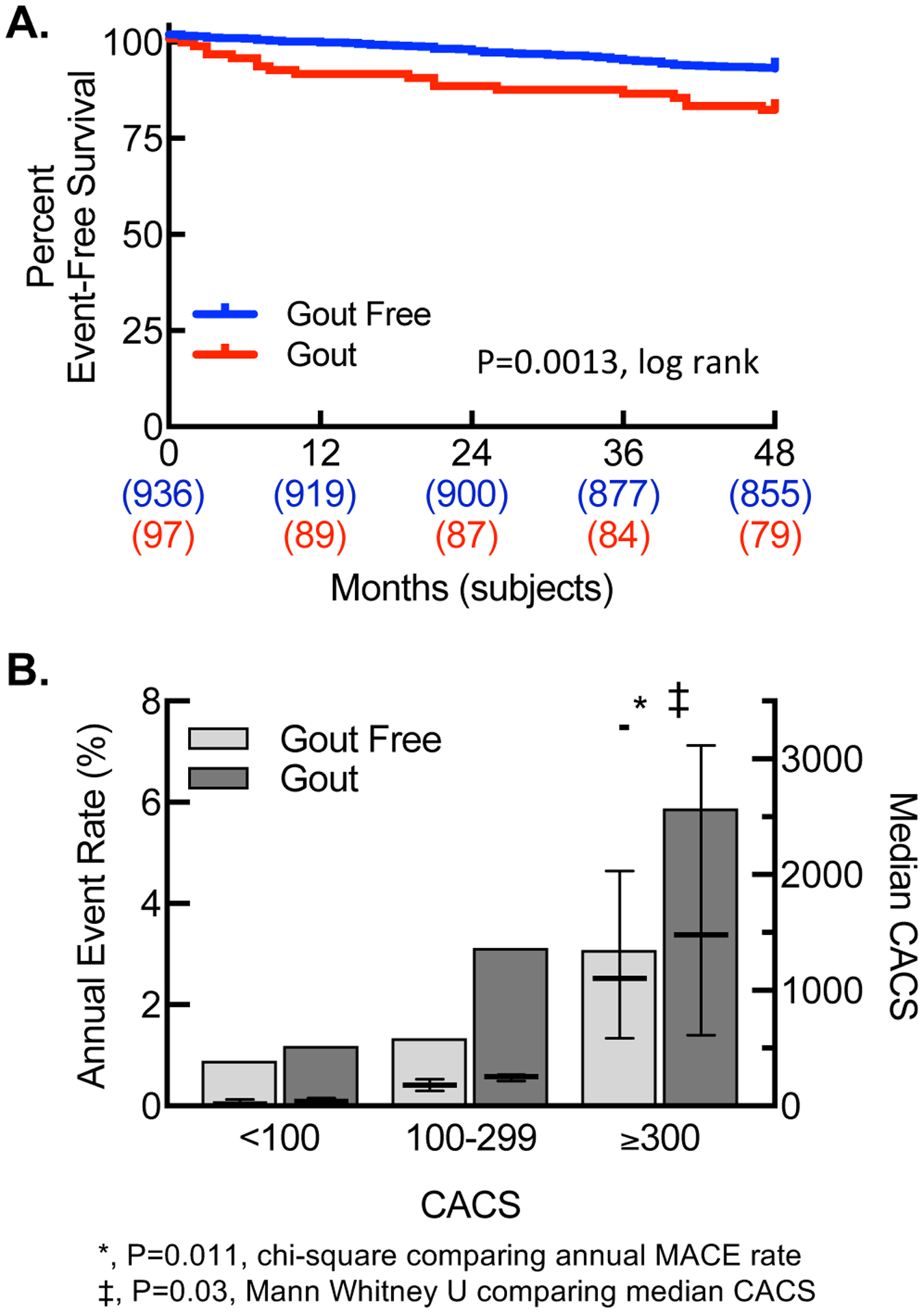
(A)Kaplan-Meier curve for MACE. (B)Annualized MACE rate(bars,left y-axis) and median CACS(dash,right y-axis) by gout status, stratified by CACS.
Our study shows, for the first time, that gout is independently associated with increased CACS and worsening cardiovascular outcomes likely due to increased CAC.
This study is limited by selection biases inherent to the study design. This particular Veteran population consisted of mostly older, white men with significant smoking histories. Our definition of gout may be less accurate than ACR-EULAR classification criteria. Diagnosis codes and events may be underestimated due to limitations inherent in extracting data or because care outside the VA was not fully captured.
Additional studies are required to assess the mechanistic relationship between uric acid, gouty inflammation and calcific atherosclerosis leading to increased events in this population, and whether this population would derive particular benefit from more aggressive gout- or inflammation-targeted clinical strategies to reduce cardiovascular events.
Research reported in this publication was supported by:
Research Project Grants NIH NHLBI R01HL139795 (A.R.M.), R01HL128661 (G.C.), and R01HL148727 (G.C.)
Institutional Development Award (IDeA) from NIH NIGMS P20GM103652 (A.R.M.) NIH NHLBI T35HL094308 (A.C.)
Career Development Award Number 7IK2BX002527 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program (A.R.M.) Veterans Affairs Merit Award I01CX001892 (G.C.) Agency for Healthcare Research and Quality Award 5K12HS022998 (N.R.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationships with Industry: None
REFERENCES
- 1.Bardin T,Richette P. Impact of comorbidities on gout and hyperuricaemia. BMCMed 2017;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer VA,Force USPST. Screening for lung cancer. AnnInternMed 2014;160:330–8. [DOI] [PubMed] [Google Scholar]
- 3.Christensen JL,et al. Impact of Slice Thickness on the Predictive Value of Lung Cancer Screening Computed Tomography in the Evaluation of Coronary Artery Calcification. JAHA 2019;8:e010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ,et al. 2013ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. JACC 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]


