Abstract
Poor adherence to cardiovascular disease medications carries significant psychological, physical, and economic costs, including failure to achieve therapeutic goals, high rates of hospitalization and health care costs, and incidence of death. Despite much effort to design and evaluate adherence interventions, rates of adherence to cardiovascular-related medications have remained relatively stagnant. We identify two major reasons for this: First, interventions have not addressed the time-varying reasons for nonadherence, and 2nd, interventions have not explicitly targeted the self-regulatory processes involved in adherence behavior. Inclusion of basic and applied psychological science in intervention development may improve the efficacy and effectiveness of behavioral interventions to improve adherence. In this article, we use a taxonomy of time-based phases of adherence—including initiation, implementation, and discontinuation—as context within which to review illustrative studies of barriers to adherence, interventions to improve adherence, and self-regulatory processes involved in adherence. Finally, we suggest a framework to translate basic psychological science regarding self-regulation into multicomponent interventions that can address multiple and time-varying barriers to nonadherence across the three adherence phases. The field of psychology is essential to improving medication adherence and associated health outcomes, and concrete steps need to be taken to implement this knowledge in future interventions.
Keywords: cardiovascular disease, medication adherence, self-regulation
Editor’s note.
This article is part of a special issue, “Cardiovascular Disease: Psychological, Social, and Behavioral Influences,” published in the November 2018 issue of American Psychologist. Catherine M. Stoney, Peter G. Kaufmann, and Susan M. Czajowski were the scholarly leads. Timothy W. Smith and Anne E. Kazak served as editors of the special issue.
The importance of adherence to medications (i.e., the behavior of taking medication as prescribed) is acutely observed among people with cardiovascular disease (CVD). CVD is the leading cause of mortality in the United States (Mozaffarian et al., 2015). Although Western countries have seen a 50% reduction in mortality from coronary artery disease over the past 20 years, half of these mortalities are due to CVD (Task Force Members et al., 2013), and this reduction in mortality is largely a result of the development of effective CVD medications. Mortality risk can be reduced by up to 75% when people are adherent to all four medication classes (statins, aspirin, beta-blockers, and angiotensin-converting enzyme inhibitors; Hippisley-Cox & Coupland, 2006). Conversely, lack of adherence to such medications is linked with higher mortality rates (Rasmussen, Chong, & Alter, 2007).
Most individuals with CVD have hypertension, which requires at least one antihypertensive medication to achieve effective blood pressure control (Chobanian et al., 2003). Patients with CVD may have additional comorbidities, such as hypercholesterolemia or diabetes, which necessitate the use of additional medications (Balkrishnan et al., 2003; Elliott, Maddy, Toto, & Bakris, 2000). Some comorbidities associated with CVD, such as heart failure, are symptomatic, whereas others, such as elevated blood pressure and cholesterol, are asymptomatic. Regardless, patients with CVD and associated comorbidities typically must take lifelong medications to reduce the risk for cardiovascular events and death. Patients with these CVD-related comorbidities might be prescribed antiplatelets, antihypertensives, or antihyperglycemics, as well as antilipemics. Common side effects for these medications might include, depending on the medication class, dry cough, cold hands, fatigue, dizziness, weakness, increased urination, nausea, increased bleeding, muscle pain, hypoglycemia, and weight gain.
Perhaps due to the symptomatic versus asymptomatic nature of cardiovascular comorbidities, as well as the varying side effects associated with the different medications, adherence rates vary by type of medication. For example, in one study, adherence was 71% for aspirin, 46% for beta-blockers (hypertension medication), and 44% for statins for hyperlipidemia (Newby et al., 2006). In another study, self-reported adherence rates to common cardiovascular medications were 83% for aspirin, 63% for lipid-lowering agents, 61% for beta-blockers, and 39% for aspirin + beta-blocker + lipid-lowering agent postmyocardial infarction (Ho et al., 2006). In a meta-analysis of 376,162 patients prescribed CVD medications as primary or secondary preventive therapy across 20 studies, approximately one third of patients with a history of myocardial infarction and half of patients without a history had an inadequate supply of medications, as indicated by prescription refill data (Naderi, Bestwick, & Wald, 2012).
It is unsurprising that efforts to understand the determinants and facilitators of, and challenges to, adherence have proliferated in medicine and the behavioral sciences. Yet, interventions remain relatively ineffective. In the context of a time-based taxonomy of medication adherence (Vrijens et al., 2012), we review previous medication adherence interventions and discuss two limitations that reduce their efficacy and effectiveness: lack of attention to the time-varying reasons for nonadherence and the lack of focus on self-regulatory processes involved in adherence. We conclude with recommendations for how psychological science can be utilized to benefit the understanding of, and intervention on, medication nonadherence.
ABC Taxonomy as a Time-Varying Framework for Medication Adherence
The ABC taxonomy proposes three time-based phases of medication adherence: initiation, implementation, and discontinuation (Urquhart, 2001; Vrijens et al., 2012). The first phase, initiation, refers to the time from prescription until the patient takes the first dose of a prescribed medication and is measured as a time-to-event variable. The second phase, implementation, refers to the period from initiation until the last dose is taken and is measured as a continuous measure of the difference between prescribed and taken medication. The third phase, discontinuation, refers to the end of therapy, when the next dose to be taken is omitted and no more doses are taken thereafter. Persistence, a commonly used term, is a time-to-event variable representing the duration of time a patient is in the implementation phase. A failure to identify barriers to adherence, adapt an intervention, and identify the self-regulatory processes involved in these phrases is likely to result in poor long-term adherence.
Most studies on medication adherence have focused on the implementation phase, as evidenced by the fact that intervention studies focus on patients who have a current (rather than new) prescription for medication(s) and the fact that the outcome typically is proportion of doses taken, usually measured at several time points (rather than time until taking the first dose). Significantly less attention has been devoted to initiation or the reinitiation that would occur when a patient discontinues a medication or a provider prescribes a new medication instead of, or in addition to, an existing regimen. Depending on which phase of this trajectory is of interest, different interventions might be better suited than others (Iglay et al., 2015). In the next sections, we review challenges of a general and a theoretical examination of medication adherence interventions, followed by a clearer picture of medication adherence interventions and self-regulatory processes in the context of the three phases of medication adherence.
Examining Medication Adherence Barriers
To date, most interventions have been “one size fits all,” meaning that all participants receive the same intervention, which typically addresses a single barrier. This presumes that patients miss doses of their medications for the same reason and the same reasons are always salient. Several evidence reviews have indicated that such interventions have modest effects in promoting both overall and condition-specific medication adherence (Buntin, Burke, Hoaglin, & Blumenthal, 2011; Cebul, Love, Jain, & Hebert, 2011; Kripalani, Yao, & Haynes, 2007; Nieuwlaat et al., 2014; Viswanathan et al., 2012; Zullig, Peterson, & Bosworth, 2013). In a recent meta-analysis of 182 randomized clinical trials of interventions to improve adherence to prescribed medications, effects were inconsistent, and only a minority of randomized clinical trials improved both adherence and clinical outcomes (Nieuwlaat et al., 2014). In one review, among 37 eligible trials (including 12 informational, 10 behavioral, and 15 combined informational, behavioral, and/or social investigations), 20 studies reported a significant improvement in at least one adherence measure (Nieuwlaat et al., 2014). Adherence increased most consistently with behavioral interventions that reduced dosing demands (three of three studies, all with large effect sizes) and involved monitoring and feedback (three of four studies, with small to medium effect sizes). Adherence also improved in eight combined interventions (with medium to large effect sizes). Eleven studies (four informational, three behavioral, and four combined) demonstrated improvement in at least one clinical outcome, but effects sizes were variable and not consistently related to changes in adherence (Kripalani, Yao, & Haynes, 2007). In the most recent Cochrane review, only five of 109 studies demonstrated improvements in adherence and a clinical outcome (Nieuwlaat et al., 2014). Further, only 17 of the 109 trials identified were low in risk of bias, which limits the conclusions one can make. In general, multifactorial, tailored approaches were more effective than one-size-fits-all approaches that focus on only one component.
Poor adherence rates to CVD medications are likely attributable to several factors. A significant body of literature has focused on medication adherence as one construct, often dichotomized as yes-no at 80%; however, this conceptualization often fails to consider the specific behavior being targeted and does not address the time-varying nature of adherence (Gellad, Thorpe, Steiner, & Voils, 2017). Thus, to improve medication adherence, one must know not only whether an individual is adherent but the reason(s) why an individual does not adhere. Table 1 presents a list of common reasons for nonadherence. These reasons can be present during any phase of adherence and can vary across time, populations, diseases, drug classes. For example, medication adherence may vary by the perceived acute phase of CVD (e.g., postdischarge for myocardial infarction) versus long-term self-management such as taking daily aspirin or antihypertensives. Similarly, adherence may vary according to whether the disease is symptomatic; thus, individuals with CVD often need to consider tradeoffs with respect to short-term side effects for long-term gain for asymptomatic diseases, which affects adherence rates. Variation in adherence rates across medications is likely due to many factors that catalyze, inhibit, or otherwise influence medication adherence.
Table 1.
Barriers Associated With Medication Adherence
| Patient-related barriers |
|
| Medication-related barriers |
|
| Clinician-related barriers |
|
| Health system barriers |
|
The tendency to focus on one medication adherence barrier is exemplified in a recent trial of 53,000 individuals with suboptimal adherence. The trial focused on forgetfulness, an important barrier to medication adherence, but one of many. Patients were randomized to receive in the mail a pill bottle strip with toggles, digital timer cap, or standard pillbox. The control group received usual care, which involved neither notification nor a device. There was no statistically significant difference in the odds of optimal adherence between the control and any of the devices (including standard pillbox, digital timer cap, and pill bottle strip with toggles; Choudhry et al., 2017). In another study, however, eliminating medication copayments and providing medication for free improved adherence rates modestly (~5%) for patients for whom cost was a primary reason for poor adherence (Choudhry, Avorn, et al., 2011). Eliminating medication copayments will likely not improve adherence by people who miss medications due to, for example, side effects or negative beliefs about medications (Voils et al., 2012). The focus on one or a small number of barriers to adherence partially explains the small effects typically observed in prior studies.
Another limitation of medication adherence interventions, particularly for CVD, is that interventions are typically focused on short-term time intervals despite the likelihood that individuals may use these medications for decades. Medication adherence interventions are generally more successful for short-term treatments than for long-term, chronic illness management (Haynes, Ackloo, Sahota, McDonald, & Yao, 2008). This tendency to detect shorter term effects may be due to reasons for poor medication adherence that become more or less salient over time as circumstances change. This decrease in salience is a challenge for the long-term treatment of CVD. Those few interventions that have demonstrated effectiveness for long-term care have generally been complex interventions including number of delivery formats (e.g., telephone, digital delivery), behavior change techniques (e.g., self-monitoring, reinforcement), and therapeutic approach (e.g., individual counseling, family therapy; Nieuwlaat et al., 2014).
In sum, the multifaceted and long-term nature of medication adherence can be difficult to assess and harder to intervene upon without an organizing framework. In Table 2, the first two columns illustrate the typical process of one intervention targeting primarily one barrier to medication adherence, as described earlier. However, as seen in the third column and further described later, a time-varying taxonomy is helpful to organize interventions, including their strengths and weaknesses.
Table 2.
Barriers and Actions to Improve Medication Adherence as Related to Organizing Principles of Phase and Psychological Underpinning
| Example barriers | Common behavior-change strategy used in interventions | ABC taxonomy phase | Psychological science underpinning |
|---|---|---|---|
| Forgetting | Encourage patients to use medication reminders (e.g., promote pill boxes, alarms, vibrating watches, and smartphone applications). | Initiation Implementation |
Goal formation Self-regulation Habit formation |
| Inadequate instructions | Provide all prescription instructions clearly in writing and verbally.
|
Initiation | Goal formation Self-regulation Habit formation |
| Health literacy | Ensure patients understand their risks if they do not take medications as directed. Ask patients about these risks, and have patients restate the positive benefits of taking their medications. | Initiation Implementation Discontinuation |
Goal formation Self-regulation |
| Side effects | Discuss with patients potential side effects of any medications when initially prescribed and at every office visit thereafter. Provide information on what to expect with potential side effects and what to do if issues are experienced. | Initiation Implementation |
Goal formation Self-regulation |
| Financial challenges | Provide rewards for medication adherence.
|
Initiation Implementation Discontinuation |
Goal formation Self-regulation |
| Inadequate health coverage | Prescribe medications included in the patient’s insurance coverage formulary when possible. | Initiation | Goal formation Self-regulation |
| No. of pills | Simplify regimen: Prescribe once-daily regimens or fixed-dose combination pills. | Initiation Implementation |
Goal formation Self-regulation Habit formation Automatic priming |
| Chaotic lifestyle | Implement frequent follow-ups (e.g., e-mail, phone calls, text messages) to ensure patients adhere to their medication regimen.
|
Initiation Implementation Discontinuation |
Goal formation Self-regulation Habit formation Automatic priming |
Note. Barriers and behavior change strategies listed are examples only and not exhaustive.
Examining the Medication Adherence Problem Through the ABC Taxonomy Framework
Initiation: Evidence and Challenges
Despite the seminal influence of this phase, and maximal possibilities for intervention as seen in Table 2, adherence to medication initiation is rarely examined at all, let alone in the context of an intervention. One reason may be that measuring initiation often requires linking nontraditional data sources such as e-prescribing information and pharmacy fill data. To date, the most thorough examination of medication nonadherence during the initiation phase came with an examination of linked e-prescription and initial pharmacy fill data among almost 200,000 prescriptions across over 75,000 patients (Fischer et al., 2010). This data illustrated that roughly 30% of patients with CVD failed to fill their initial prescription. Moreover, filling a prescription does not equate to taking the medication, and thus this examination likely still provides an underestimate.
Interventions focused on patients initially discharged from the hospital, who are often prescribed new medications, provide a common example of interventions targeting the unique barriers related to initiation of medications (Institute of Medicine, 2001). Yet these interventions rarely focus on addressing medication adherence during the initiation phase, in favor of early and sustained medication adherence during the implementation phase. This focus on early implementation instead of initiation may be the result of a high degree of overlap in the phases within the common recruitment and intervention methods currently in place. Of the example interventions presented in Table 2, only two of these would occur strictly in the initiation phase.
In a study of individuals discharged with heart failure, a nurse implemented self-management training before discharge that focused on identification of medication goals, facilitation of medication–symptom associations, and use of a symptom response plan. The comparison was an attention control group. Patients in the intervention group were several times more likely to be adherent to CVD-related medications compared with patients in the attention control group (Granger et al., 2015). One particular strength of this intervention often not present in other studies is the incorporation of patient perspectives and shared decision-making between the patient and health care provider from the outset of initiating new medication. Although this collaboration early on can help foster more patient-driven maintenance based on the psychological principles discussed in the next section, there is still no examination of patient-driven initiation. Future studies should address the impact of an intervention on the probability or lag time to an initial pharmacy fill, and more ideally to the first dose taken.
Implementation and Discontinuation: Evidence and Challenges
The bulk of interventions to improve CVD medication adherence have focused on the implementation phase, the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen, from initiation until the last dose is taken (Vrijens et al., 2012). Yet because both implementation and discontinuation involve the time period between initiation of a medication and the last dose taken, the unit of analysis focused on is often the largest distinction between the two—interventions focusing on implementation focus on mean percentage of medications taken, whereas interventions focusing on time until discontinuation (or similarly, patients’ persistence during the regimen) focus on the time-to-event, or potentially as seen in the example discussed next, mean percentage of patients who are adherent to the regimen at a fixed time point. We provide two examples to illustrate this difference, as well as the strengths of each study that are lacking in other studies.
The first example is a multifaceted, pharmacist-led, randomized controlled trial that focuses on the implementation phase (Ho et al., 2014). The 1-year intervention encompassed medication reconciliation and tailoring, patient education, collaborative care between pharmacist and patients’ physician, and voice messaging (Ho et al., 2014). There were 253 patients discharged from the hospital with acute coronary syndrome from four Veterans Affairs Medical Centers. The primary outcome was pharmacy-based medication adherence (e.g., proportion of days covered). In the intervention group, 89.3% of patients were adherent compared with a significantly lower 73.9% in the usual care group (Ho et al., 2014).
The Ho et al. (2014) trial demonstrated improvements in implementation of medication-taking behaviors over time, thanks to a complex intervention involving multiple components. Multifaceted interventions might be difficult to translate into “real world” settings and to sustain over time; there is a need for conceptual models to take an ecological perspective and include patient factors (e.g., literacy, cognitive function) including pharmacogenetics, provider factors (e.g., complex regimens), social–community factors (e.g., access to providers and pharmacy), health care factors (e.g., interaction with health care system, trust, prior authorization, fragmentation), and policy (e.g., coverage of medication; Bosworth & Voils, 2006; Gellad, Grenard, McGlynn, 2009; Osterberg & Blaschke, 2005). Focusing on a single barrier to medication nonadherence like forgetfulness rarely results in aggregate improvements in a community. For example, Volpp et al. (2017) recently reported no difference in adherence to CVD medications or clinical outcomes in a trial examining the impact of medication reminders among 1,500 individuals who recently experienced an acute myocardial infarction. Thus, effective interventions to improve appropriate medication use likely need to adopt comprehensive approaches, routinely combining numerous strategies.
The second example is a recent trial focused on reducing discontinuation of guideline-recommended cardiac medications post-ST-elevation myocardial infarction (STEMI), which is common and is associated with increased mortality. STEMI patients from one health region in Ontario, Canada, who underwent an angiogram during their admission and survived to discharge were cluster-randomized (by primary care provider) to intervention or usual care. The intervention was an automated system of personalized, educational reminders sent to patients and their family physician, urging long-term use of secondary-prevention CVD medications. The primary outcome, defined as the self-reported proportion of participants taking (persistence) all four cardiovascular medication classes (acetylsalicylic acid, angiotensin-blockers, statin, and beta-blocker) at 12 months, was roughly equivalent to around ~58% (Schwalm et al., 2015).
The strength of Schwalm et al.’s (2015) study—not found in many other studies—is twofold. First, by the very nature of examining the discontinuation of a medication regimen, this study represents an outcome and phase of adherence rarely addressed by interventions. Second, this study focused on adherence to multiple medications and diseases, acknowledging the challenges of addressing the complexity of CVD. Most interventions have focused on a single medication or disease, yet, in the context of CVD, the majority of individuals take multiple medications and rarely have one condition-disease (Choudhry, Fischer, et al., 2011). These external factors regarding interventions abound; however, it is important not to lose sight of the fact that individuals, with their own psychology, are the focus of these interventions.
Examining the Medication Adherence Problem Through Psychological Science
A newly prescribed course of treatment for cardiovascular disease requires the initiation and maintenance of new behaviors (e.g., taking medications). Irrespective of the roles of health care providers, health systems, and social supports, initiation of a new behavior requires goal formation and self-regulation on the part of the patient, defined as “the ongoing process of managing goal pursuit in the face of personal, interpersonal, and environmental forces that would derail it” (Hoyle & Gallagher, 2015, p. 189). Maintenance of the behavior during the implementation phase can be accomplished via habit formation or automatic priming of goals. A substantial body of psychological research on the mechanisms and processes involved in goal pursuit offers promise for understanding failures of medication adherence and opportunities for intervention. The last column in Table 2 provides an overview of how these psychological processes might parallel and complement the ABC taxonomy phases, contextualized within the same examples of current interventions and the primary barrier they focus on. Similarly, Figure 1 walks through these psychological processes in more detail, independent of any particular intervention or barrier to medication adherence. The top half of the General Process section presents a time-invariant process for successful self-regulation in general, which is paralleled by a more specific process for successful medication adherence (a specific self-regulatory behavior), shown in the bottom half. The Time-Varying Process section presents this self-regulatory process within the time-varying framework of the ABC taxonomy, where early self-regulatory processes are eventually replaced by more automatic cues and goals. Each of these terms, and their progression over time, is described in the next sections.
Figure 1.
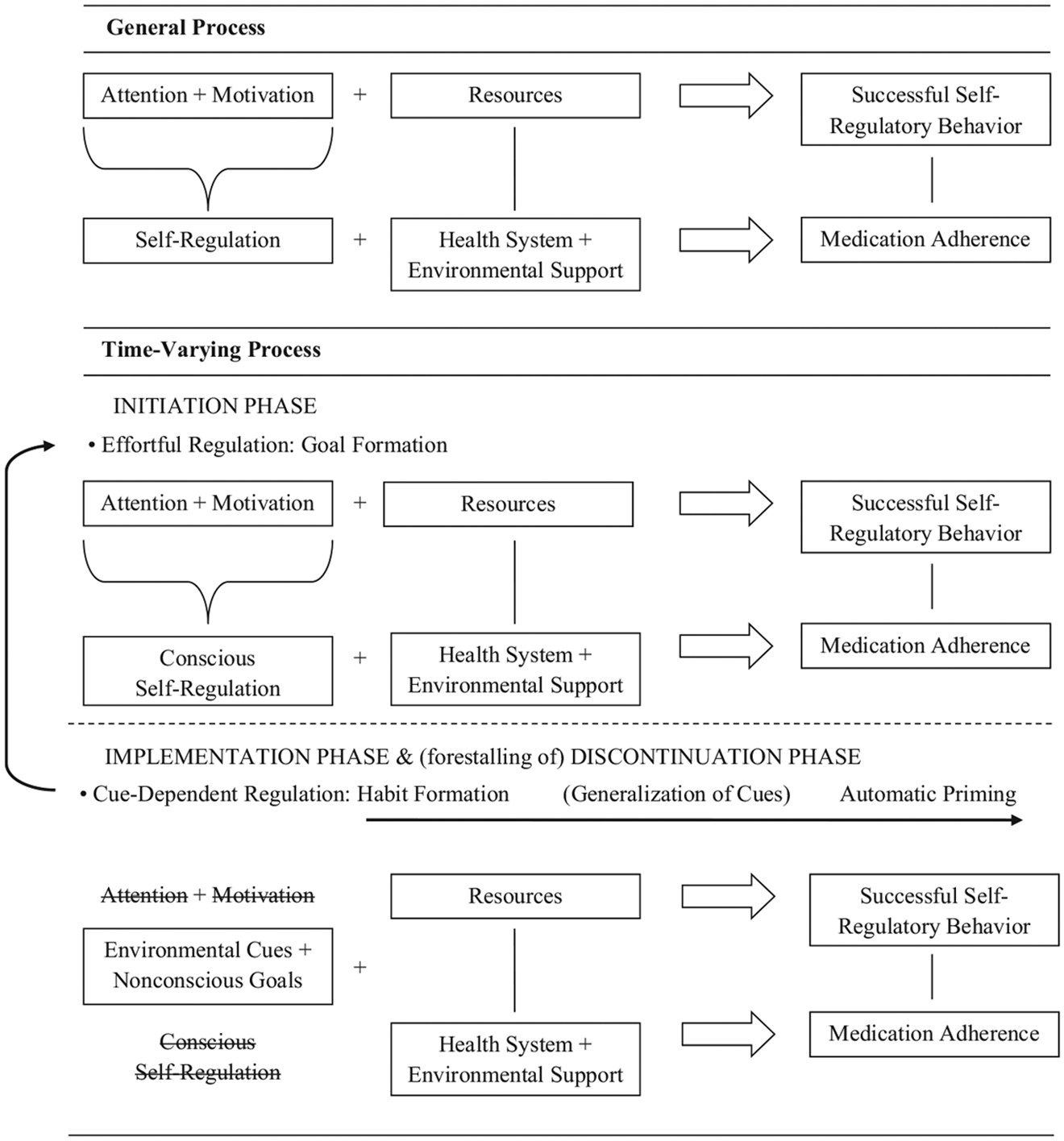
The psychological process of medication adherence during initiation and implementation. This figure illustrates the process of self-regulation generally (flowing from left to right), paralleled below by the self-regulatory process of medication adherence specifically. In the first frame, the process is general and time-invariant. The second frame illustrates the time-varying process, where early self-regulatory processes are eventually replaced by more automatic cues and goals (denoted by strike-throughs of the original self-regulatory processes). As these cues generalize, the habit formation also generalizes into automatic priming.
Initiation: Underpinnings in Psychological Science
Goal formation.
In basic terms, a goal is a mental representation of a desired future state that the individual is motivated to pursue (Elliot & Fryer, 2008; Kruglanski, Shah, Fishbach, et al., 2002). Without a goal, self-regulation is not possible. As such, the first order of business for patients facing a prescription for new medicines is to adopt concrete goals toward which progress can be monitored and rewarded (Latham & Locke, 1991).
A critical concern for the patient is that the more concrete the goal and the nearer the time horizon, the higher the number of goals required to bring about the desired changes in behavior. Imagine, for instance, that the patient is given a new prescription (perhaps more than one) and recommendations to avoid certain foods and take daily walks. The behavior-change battle must now be waged on multiple fronts, with strategies likely to support one behavior change not likely to support the others. For example, adopting the behaviors of taking pills as instructed and taking daily walks are approach goals that require self-control by initiation. Reducing or eliminating certain foods is an avoidance goal that requires self-control by inhibition (Rothman, 2000). Moreover, these goals are inserted into a potentially large and complex network of goals the individual is already pursuing (Kruglanski, Shah, Fishbach, et al., 2002), some of them consistent with and others in opposition to the new goals.
Self-regulation.
Self-regulation theories suggest that patients’ adherence is influenced by illness experiences (e.g., symptoms, medication side effects), social interactions, sources of information, and cognitive-affective processes (Kalichman, Kalichman, Cherry, & Grebler, 2016). The self-regulatory challenge associated with managing multiple new behavior change goals while continuing with ongoing goal pursuits is twofold and generally concerns the limits associated with the psychological resources that support self-regulation (Baumeister, Bratslavsky, Muraven, & Tice, 1998). A first concern is the management of attention—specifically, the challenge of maintaining attention to the new goal when other goals and temptations demand attention. A second, not unrelated, concern is the management of motivation. As attention shifts to alternative goals, motivation often follows (Inzlicht & Schmeichel, 2012). Moreover, the ability to muster motivation repeatedly or sustain it over time is limited (Baumeister et al., 1998). Thus, this latter issue of changing alternative goals and shifting motivation is likely one of the larger factors involved in the onset of the discontinuation phase of medication adherence.
By its nature, self-regulation involves effortful cognitive activity that is not sustainable for long periods at a time (Kruglanski, Shah, Pierro, & Mannetti, 2002). As such, other mechanisms and strategies are required to support self-regulation, especially when it is not at full capacity. As an example, perceived self-efficacy, especially positive beliefs concerning one’s capability of initiating new behaviors such as initiating the use of a new CVD medication (Schwarzer & Renner, 2000), in addition to contributing to self-regulation when the cognitive resources it requires are at full capacity, may serve to sustain motivation even when those resources are taxed.
Returning to the concern about the goals on which self-regulation are focused, self-efficacy beliefs about initiating new medication adherence behaviors is likely to be higher for more concrete and proximal goals (e.g., go a week without failing to take a pill as directed) than for more abstract and distal goals (e.g., be symptom-free in the future). Pursuing health goals with support from, or together with, close others (e.g., family member, pharmacist) can provide additional motivational resources and help with attention (Fitzsimons & Finkel, 2011; vanDellen, Shah, Leander, Delose, & Bornstein, 2015). Developing strategies for conserving (Muraven, Shmueli, & Burkley, 2006) and replenishing (Tice, Baumeister, Shmueli, & Muraven, 2007) cognitive and motivational resources required for effective self-regulation can decrease the likelihood of self-regulatory failures, especially during periods when multiple goals are demanding attention and motivation. Developing strategies is particularly important in the context of CVD medication adherence, given that individuals are typically on multiple medications.
Despite the challenges associated with self-regulation with reference to multiple new goals that compete for time, attention, and motivation with extant goals, self-regulation is essential during the period when new behaviors are initiated (as illustrated in the Initiation Phase section of Figure 1). Through strategic use of specific strategies such as planning, self-monitoring, and rewarding goal-consistent behavior, initiation can be accomplished through self-regulation. Planning might include such specific concerns as when to begin (e.g., when to take the first dose of a newly prescribed medication; Dai, Milkman, & Riis, 2014) and how to respond when opportunities and challenges arise (e.g., traveling or otherwise being out of routine; Gollwitzer, Oettingen, 2011). Self-monitoring, whether by traditional paper-and-pencil methods or smartphone apps, is critical for moving beyond initiation to regular engagement in the desired behaviors (e.g., Wharton, Johnston, Cunningham, & Sterner, 2014). And some strategy for evaluating progress toward the goal and acknowledging success is vital if the behavior is to gain sufficient traction to potentially become routinized with time (Gollwitzer, 1990; Voils, Gierisch, et al., 2014). Interventions that foster and support the development of strategies such as these and target more basic processes such as attention and motivation have the potential to increase the uptake of treatment and risk-reduction regimens by patients with CVD.
Implementation and Discontinuation: Underpinnings in Psychological Science
The attention and motivation required for self-regulation in pursuit of goals cannot be sustained indefinitely. The inevitable reduction in motivation that occurs as progress toward short-term behavioral goals is realized (Carver, 2003) might stem from declining attention and support from close others, an internal sense that the short-term goals have been or soon will be attained, a decrease in symptoms, or an increase in medication side effects. Thus, alternative strategies and mechanisms are required to move the behaviors from self-regulated to enacted without attention or explicit motivation (as seen in the shift in time-varying phases in Figure 1).
Habit Formation
A promising strategy for maintaining medication adherence after the use of CVD medications has been successfully initiated is habit formation. Although habitual behavior may begin as self-regulated goal pursuit, it is fundamentally different from goal-based behavior in that it requires neither motivation nor attention (Ouellette & Wood, 1998; Wood & Neal, 2007). Behaviors may become habitual when they are repeated frequently in the same context (Wood & Neal, 2007). The relevant context may be a physical location, a particular time of day, the presence of a specific person, or the enactment of another habitual behavior. In a previous study, we found that taking medications in the same context was highly associated with self-reported antihypertensive medication adherence (Voils et al., 2012). Although it is not clear how many times a behavior must be repeated in a given context before it becomes a habit, it is clear that, with repeated behavior--context pairing, habits form, and once they have formed, they persist (Neal, Wood, Wu, & Kurlander, 2011). It is important to note that when the cognitive resources that support effortful self-regulation are drained, as often they are for individuals tasked with continuing new health behaviors, habitual behavior is more likely (Neal, Wood, & Drolet, 2013).
A critical concern is how patients who successfully initiate adherence transition from self-regulation to habit as a basis for those behaviors. One strategy is to incorporate habit formation into the goal once the new behavior has been successfully initiated (Memmi, 2014). For example, the goal might shift from taking one’s medicine every day next week to taking one’s medicine after brushing one’s teeth in the morning. The potential drawback to this strategy is that, unlike goals, for which multiple means might be used to attain the goal, habits are rigid in their reliance on a single means reflected in the specific context to which the behavior is linked. People often miss medications when out of their routine. Thus, it is critical that the context that supports habitual adherence and other health behaviors be one that is highly reliable and unlikely to change, such as taking medication after brushing one’s teeth in the morning (Wood, Quinn, & Kashy, 2002).
Automatic Priming
Rather than rendering self-regulation unnecessary because the desired behaviors have become habitual, an alternative, more flexible strategy to address medication adherence involves moving self-regulation from a conscious process to an automatic one (Sheeran, Gollwitzer, & Bargh, 2013). This strategy capitalizes on the flexibility of activating goals from a range of environmental cues (“priming”), rather than activating behavioral reminders from more restricted cues in habit formation (Aarts & Dijksterhuis, 2000). Although priming can be explicit and conscious, it may also be nonconscious. As such, the attention component of explicit self-regulation is not required. Moreover, when goals are primed nonconsciously, they give rise to motivation and goal striving without conscious intent (Bargh & Barndollar, 1996). Thus, patients so primed need not muster the attention and motivation required to consciously focus on and pursue an adherence goal. The flexibility in this strategy stems from the fact that the goal may be nonconsciously primed by multiple stimuli, such as the visible presence of a pill bottle or thoughts of a friend or family member who encourages adherence (Shah, 2003) or through association with another primed mental representation such as a toothbrush in the preceding example (Ratcliff & Mckoon, 1981).
Improving the Medication Adherence Problem: Translating Basic Science to Interventions
As noted earlier, complex interventions show the most promise for improving medication adherence, and these interventions will likely work best when guided by theory and informed by relevant psychological science. The ORBIT framework for intervention design, refinement, and evaluation represents a useful method for translating the findings from psychological science that identifies and describes adherence-related psychological processes, such as self-regulation, habit formation, and automatic priming, into interventions to improve adherence (Czajkowski et al., 2015). This framework for intervention design and testing, which emphasizes a systematic, progressive, yet flexible, approach to the development and evaluation of adherence interventions, can accelerate the pathway through which novel ideas based on basic psychological and other social science research are developed into potentially more effective adherence interventions. This framework proposes that, across two preefficacy phases, the intervention would be (1) designed based on theory and findings from basic psychological science and optimized, possibly using factorial or fractional factorial designs or adaptive study designs, and(2) tested in preliminary studies using small-n, single-case designs and in larger feasibility and pilot studies. These phases are iterative and bidirectional, leading to refinement and potentially improved efficacy for the interventions being developed, which are then evaluated for efficacy and effectiveness (most often in randomized controlled trials) and ultimately implemented within clinical and community contexts.
To begin, the researcher would identify relevant basic psychological science findings—or conduct such studies if they are lacking—related to adherence behavior. As mentioned previously, people may miss medications due to a variety of factors, and these factors may change over time. Repeated-measures studies such as daily diaries or event sampling might be conducted to identify how frequently nonadherence episodes occur and factors contributing to those episodes, including whether those factors tend to be stable or vary between and within individuals (Voils, King, et al., 2014). For example, prescribing a different medication may help a patient avoid side effects, but if the new medication is costlier, then cost-related nonadherence may result. This notion of changes over time becomes easier to grasp when one examines the behavior of medication adherence as a process that ideally begins with goal-setting and effortful self-regulatory actions during the initiation phase, transitions into conscious but less effortful habit, and eventually becomes effortless, automatic behavior during the implementation phase. Findings from such studies could efficiently inform intervention content and dose and increase the chances of constructing an effective intervention by knowing why medication adherence is improved. As mentioned previously, Table 2 represents an initial attempt to integrate current interventions into multiple theories across medical and psychological science.
In addition to a focus on early phase translation and intervention development, consideration for later phase translation (i.e., implementation of efficacious programs) is necessary. The transfer of research findings into clinical practice is often a slow and haphazard process (Agency for Healthcare Research and Quality, 2001; Graham et al., 2006). This minimal translation of many interventions into practice may be attributed to lack of planning for future scalability. Scaling-up a successful program and broadly implementing it (or translating knowledge into action), perhaps in the context of a health care system, could maximize potential impacts on individual and population health. Regardless of whether an organization is developing or identifying and/or adapting an existing program, the sustainability of the program must be established. Sustainability can be thought of in two dimensions: program sustainability over time or maintaining the improvement in outcome. Because interventions occur in complex societal systems, sustaining an intervention may require action at many levels, ranging from knowledge to individual change, community engagement, and institutional change (Graham et al., 2006; Swerissen & Crisp, 2004). Several elements, including planning, gathering relevant evidence, seeking commitment and support, developing partnerships, identifying program champions, building capacity, embedding into core policy, evaluating effectiveness and outcomes, evolving and adapting, and securing funding, may predict program sustainability (Whelan et al., 2014). There is an increased emphasis on bringing such elements into the early design phases of intervention development to ensure successful implementation at later phases (Whelan et al., 2014).
Conclusion
Factors affecting medication adherence occur at multiple levels: patient; provider; the health care system; and broader sociocultural, community, and societal levels. Research on the phenomenon of medication adherence and interventions to improve it has been informative and shown some benefits, but perhaps in an inefficient manner. To further improve the understanding of and intervention upon medication adherence, broader frameworks are necessary to organize that understanding. Acknowledging and capturing the time-varying nature of medication adherence is a first crucial step to improve adherence, not only in the short-term but to create flexible points of intervention that yield lasting behavioral changes.
New research in the behavioral sciences is also elucidating the basic psychological, cognitive, social, and behavioral processes underlying behavior and behavior change related to medication adherence. These processes are promising targets for the development of new, more effective behavioral interventions. Findings in this area could be developed into new strategies targeting adherence behaviors, but a mechanism for developing and testing novel ideas is needed. Bringing together collaborative, interdisciplinary teams of basic behavioral scientists and clinically oriented behavioral researchers could spur development and testing of innovative new approaches to medication adherence-related behavioral problems.
Acknowledgments
This work was supported by the Center of Innovation for Health Services Research in Primary Care (CIN 13-410) at the Durham Veterans Affairs Medical Center. Hayden B. Bosworth and Corrine I. Voils are partially funded by Career Scientist Awards RCS 08-027 and RCS 14-443, respectively, from the Health Services Research & Development service of the Department of Veterans Affairs. Rick H. Hoyle received partial funding from National Institute on Drug Abuse Grant P30 DA023026 during the writing of this article. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Biographies
Hayden B. Bosworth

Dan V. Blalock

Rick H. Hoyle

Susan M. Czajkowski

Corrine I. Voils

Contributor Information
Hayden B. Bosworth, Durham Veterans Affairs Medical Center, Durham, North Carolina, and Duke University Medical Center
Dan V. Blalock, Durham Veterans Affairs Medical Center, Durham, North Carolina, and Duke University School of Medicine
Rick H. Hoyle, Duke University
Susan M. Czajkowski, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Corrine I. Voils, William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, and University of Wisconsin School of Medicine and Public Health
References
- Aarts H, & Dijksterhuis A (2000). Habits as knowledge structures:Automaticity in goal-directed behavior. Journal of Personality and Social Psychology, 78, 53–63. 10.1037/0022-3514.78.1.53 [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality. (2001). Translating research into practice (TRIP)-II. Retrieved from https://archive.ahrq.gov/research/findings/factsheets/translating/tripfac/trip2fac.pdf
- Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, & Anderson RT (2003). Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: A longitudinal cohort study. Clinical Therapeutics, 25, 2958–2971. 10.1016/S0149-2918(03)80347-8 [DOI] [PubMed] [Google Scholar]
- Bargh JA, & Barndollar K (1996). Automaticity in action: The unconscious as repository of chronic goals and motives In Gollwitzer PM & Bargh JA (Ed.), The psychology of action: Linking cognition and motivation to behavior (pp. 457–471). New York, NY: Guilford Press. [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, & Tice DM (1998). Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology, 74, 1252–1265. 10.1037/0022-3514.74.5.1252 [DOI] [PubMed] [Google Scholar]
- Bosworth H, & Voils C (2006). Theoretical models to understand adherence In Bosworth H, Oddone E, & Weinberger M (Eds.), Patient treatment adherence: Concepts, interventions, and measurement (pp. 13–46). Mahwah, NJ: Erlbaum. [Google Scholar]
- Buntin MB, Burke MF, Hoaglin MC, & Blumenthal D (2011). The benefits of health information technology: A review of the recent literature shows predominantly positive results. Health Affairs, 30, 464–471. 10.1377/hlthaff.2011.0178 [DOI] [PubMed] [Google Scholar]
- Carver CS (2003). Pleasure as a sign you can attend to something else: Placing positive feelings within a general model of affect. Cognition and Emotion, 17, 241–261. 10.1080/02699930302294 [DOI] [PubMed] [Google Scholar]
- Cebul RD, Love TE, Jain AK, & Hebert CJ (2011). Electronic health records and quality of diabetes care. New England Journal of Medicine, 365, 825–833. 10.1056/NEJMsa1102519 [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., … the National High Blood Pressure Education Program Coordinating Committee. (2003). The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. Journal of the American Medical Association, 289, 2560–2572. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, … Shrank WH (2011). Full coverage for preventive medications after myocardial infarction. New England Journal of Medicine, 365, 2088–2097. 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, Pakes J, … Shrank WH (2011). The implications of therapeutic complexity on adherence to cardiovascular medications. Archives of Internal Medicine, 171, 814–822. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Krumme AA, Ercole PM, Girdish C, Tong AY, Khan NF, … Franklin JM (2017). Effect of reminder devices on medication adherence: The REMIND randomized clinical trial. Journal of the American Medical Association Internal Medicine, 177, 624–631. 10.1001/jamainternmed.2016.9627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson ME (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34, 971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HC, Milkman KL, & Riis J (2014). The fresh start effect: Temporal landmarks motivate aspirational behavior. Management Science, 60, 2563–2582. 10.1287/mnsc.2014.1901 [DOI] [Google Scholar]
- Elliot AJ, & Fryer JW (2008). The goal construct in psychology In Shah James Y. & Gardner Wendi L. (Ed.), Handbook of motivational science (pp. 235–250). New York, NY: Guilford Press. [Google Scholar]
- Elliott WJ, Maddy R, Toto R, & Bakris G (2000). Hypertension in patients with diabetes: Overcoming barriers to effective control. Postgraduate Medicine, 107, 29–38. 10.3810/pgm.2000.03.940 [DOI] [PubMed] [Google Scholar]
- Fitzsimons GM, & Finkel EJ (2011). Outsourcing self-regulation. Psychological Science, 22, 369–375. 10.1177/0956797610397955 [DOI] [PubMed] [Google Scholar]
- Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, & Weissman JS (2010). Primary medication non-adherence: Analysis of 195,930 electronic prescriptions. Journal of General Internal Medicine, 25, 284–290. 10.1007/s11606-010-1253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellad W, Grenard J, & McGlynn EA (2009). A review of barriers to medication adherence: A framework for driving policy options. Retrieved from https://www.rand.org/pubs/technical_reports/TR765.html
- Gellad WF, Thorpe CT, Steiner JF, & Voils CI (2017). The myths of medication adherence. Pharmacoepidemiology and Drug Safety, 26, 1437–1441. 10.1002/pds.4334 [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM (1990). Action phases and mind-sets In Higgins ET &Sorrentino RM (Eds.), Handbook of motivation and cognition: Foundations of social behavior (Vol. 2, pp. 53–92). New York, NY: Guilford Press. [Google Scholar]
- Gollwitzer PM, & Oettingen G (2011). Planning promotes goal striving In Vohs KD & Baumeister RF (Eds.), Handbook of self-regulation: Research, theory, and applications (2nd ed., pp. 162–185). New York, NY: Guilford Press. [Google Scholar]
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, & Robinson N (2006). Lost in knowledge translation: Time for a map? Journal of Continuing Education in the Health Professions, 26, 13–24. 10.1002/chp.47 [DOI] [PubMed] [Google Scholar]
- Granger BB, Ekman I, Hernandez AF, Sawyer T, Bowers MT, DeWald TA, … Bosworth HB (2015). Results of the Chronic Heart Failure Intervention to Improve MEdication Adherence study: A randomized intervention in high-risk patients. American Heart Journal, 169, 539–548. 10.1016/j.ahj.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, & Yao X (2008). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews, 2008, CD000011 10.1002/14651858.CD000011.pub3 [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, & Coupland C (2006). Effect of statins on the mortality of patients with ischaemic heart disease: Population based cohort study with nested case-control analysis. Heart, 92, 752–758. 10.1136/hrt.2005.061523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, … Del Giacco EJ (2014). Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: A randomized clinical trial. Journal of the American Medical Association Internal Medicine, 174, 186–193. 10.1001/jamainternmed.2013.12944 [DOI] [PubMed] [Google Scholar]
- Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, … Rumsfeld JS (2006). Impact of medication therapy discontinuation on mortality after myocardial infarction. Archives of Internal Medicine, 166, 1842–1847. 10.1001/archinte.166.17.1842 [DOI] [PubMed] [Google Scholar]
- Hoyle RH, & Gallagher P (2015). The interplay of personality and self-regulation In Mikulincer M, Shaver PR, Cooper ML, & Larsen RJ (Eds.), APA handbooks in psychology. APA handbook of personality and social psychology, Vol. 4. Personality processes and individual differences (pp. 189–207). Washington, DC: American Psychological Association; 10.1037/14343-009 [DOI] [Google Scholar]
- Iglay K, Cartier SE, Rosen VM, Zarotsky V, Rajpathak SN, Radican L, & Tunceli K (2015). Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Current Medical Research and Opinion, 31, 1283–1296. 10.1185/03007995.2015.1053048 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2001). Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Inzlicht M, & Schmeichel BJ (2012). What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science, 7, 450–463. 10.1177/1745691612454134 [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Kalichman MO, Cherry C, & Grebler T (2016). HIV disclosure and transmission risks to sex partners among HIV-positive men. AIDS Patient Care and STDs, 30, 221–228. 10.1089/apc.2015.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Yao X, & Haynes RB (2007). Interventions to enhance medication adherence in chronic medical conditions: A systematic review. Archives of Internal Medicine, 167, 540–550. 10.1001/archinte.167.6.540 [DOI] [PubMed] [Google Scholar]
- Kruglanski AW, Shah JY, Fishbach A, Friedman R, Chun WY, & Sleeth-Keppler D (2002). A theory of goal systems. Advances in Experimental Social Psychology, 34, 331–378. 10.1016/S0065-2601(02)80008-9 [DOI] [Google Scholar]
- Kruglanski AW, Shah JY, Pierro A, & Mannetti L (2002). When similarity breeds content: Need for closure and the allure of homogeneous and self-resembling groups. Journal of Personality and Social Psychology, 83, 648–662. 10.1037/0022-3514.83.3.648 [DOI] [PubMed] [Google Scholar]
- Latham GP, & Locke EA (1991). Self-regulation through goal-setting. Organizational Behavior and Human Decision Processes, 50, 212–247. 10.1016/0749-5978(91)90021-K [DOI] [Google Scholar]
- Memmi SA (2014). When the goal is a habit: Setting goals that help, not hinder, habit formation. Manuscript in preparation. [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Stroke Statistics Subcommittee. (2015). Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation, 131, e29–e322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Muraven M, Shmueli D, & Burkley E (2006). Conserving self-control strength. Journal of Personality and Social Psychology, 91, 524–537. 10.1037/0022-3514.91.3.524 [DOI] [PubMed] [Google Scholar]
- Naderi SH, Bestwick JP, & Wald DS (2012). Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. American Journal of Medicine, 125, 882–887.e1. 10.1016/j.amjmed.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Neal DT, Wood W, & Drolet A (2013). How do people adhere to goals when willpower is low? The profits (and pitfalls) of strong habits. Journal of Personality and Social Psychology, 104, 959–975. 10.1037/a0032626 [DOI] [PubMed] [Google Scholar]
- Neal DT, Wood W, Wu M, & Kurlander D (2011). The pull of the past: When do habits persist despite conflict with motives? Personality and Social Psychology Bulletin, 37, 1428–1437. 10.1177/0146167211419863 [DOI] [PubMed] [Google Scholar]
- Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, … Califf RM (2006). Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation, 113, 203–212. 10.1161/CIRCULATIONAHA.105.505636 [DOI] [PubMed] [Google Scholar]
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, … Haynes RB (2014). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews, 2014, CD000011 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg L, & Blaschke T (2005). Adherence to medication. New England Journal of Medicine, 353, 487–497. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- Ouellette JA, & Wood W (1998). Habit and intention in everyday life: The multiple processes by which past behavior predicts future behavior. Psychological Bulletin, 124, 54–74. 10.1037/0033-2909.124.1.54 [DOI] [Google Scholar]
- Rasmussen JN, Chong A, & Alter DA (2007). Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Journal of the American Medical Association, 297, 177–186. 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, & Mckoon G (1981). Automatic and strategic priming in recognition. Journal of Verbal Learning and Verbal Behavior, 20, 204–215. 10.1016/S0022-5371(81)90381-9 [DOI] [Google Scholar]
- Rothman AJ (2000). Toward a theory-based analysis of behavioral maintenance. Health Psychology, 19(1, Suppl.), 64–69. 10.1037/0278-6133.19.Suppl1.64 [DOI] [PubMed] [Google Scholar]
- Schwalm JD, Ivers NM, Natarajan MK, Taljaard M, Rao-Melacini P, Witteman HO, … Grimshaw JM (2015). Cluster randomized controlled trial of Delayed Educational Reminders for Long-term Medication Adherence in ST-Elevation Myocardial Infarction (DERLA-STEMI). American Heart Journal, 170, 903–913. 10.1016/j.ahj.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Schwarzer R, & Renner B (2000). Social-cognitive predictors of health behavior: Action self-efficacy and coping self-efficacy. Health Psychology, 19, 487–495. 10.1037/0278-6133.19.5.487 [DOI] [PubMed] [Google Scholar]
- Shah J (2003). Automatic for the people: How representations of significant others implicitly affect goal pursuit. Journal of Personality and Social Psychology, 84, 661–681. 10.1037/0022-3514.84.4.661 [DOI] [PubMed] [Google Scholar]
- Sheeran P, Gollwitzer PM, & Bargh JA (2013). Nonconscious processes and health. Health Psychology, 32, 460–473. 10.1037/a0029203 [DOI] [PubMed] [Google Scholar]
- Swerissen H, & Crisp BR (2004). The sustainability of health promotion interventions for different levels of social organization. Health Promotion International, 19, 123–130. 10.1093/heapro/dah113 [DOI] [PubMed] [Google Scholar]
- Task Force Members, Montalescot, Sechtem U, Achenbach S, Andreotti F, Arden C, … Zamorano JL (2013). 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. European Heart Journal, 34, 2949–3003. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- Tice DM, Baumeister RF, Shmueli D, & Muraven M (2007). Restoring the self: Positive affect helps improve self-regulation following ego depletion. Journal of Experimental Social Psychology, 43, 379–384. 10.1016/j.jesp.2006.05.007 [DOI] [Google Scholar]
- Urquhart J (2001). Some economic consequences of noncompliance. Current Hypertension Reports, 3, 473–480. 10.1007/s11906-001-0009-7 [DOI] [PubMed] [Google Scholar]
- vanDellen MR, Shah JY, Leander NP, Delose JE, & Bornstein JX (2015). In good company: Managing interpersonal resources that support self-regulation. Personality and Social Psychology Bulletin, 41, 869–882. 10.1177/0146167215580778 [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ,, Wines RC, … Lohr KN (2012). Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Annals of Internal Medicine, 157, 785–795. 10.7326/0003-4819-157-11-201212040-00538 [DOI] [PubMed] [Google Scholar]
- Voils CI, Gierisch JM, Yancy WS Jr., Sandelowski M, Smith R, Bolton J, & Strauss JL (2014). Differentiating behavior initiation and maintenance: Theoretical framework and proof of concept. Health Education & Behavior, 41, 325–336. 10.1177/1090198113515242 [DOI] [PubMed] [Google Scholar]
- Voils CI, King HA, Neelon B, Hoyle RH, Reeve BB, Maciejewski ML, & Yancy WS Jr. (2014). Characterizing weekly self-reported antihypertensive medication nonadherence across repeated occasions. Patient Preference and Adherence, 8, 643–650. 10.2147/PPA.S60715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voils CI, Maciejewski ML, Hoyle RH, Reeve BB, Gallagher P, Bryson CL, & Yancy WS Jr. (2012). Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Medical Care, 50, 1013–1019. 10.1097/MLR.0b013e318269e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Mehta SJ, Norton L, Zhu J, Lim R, … Asch DA (2017). Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: The Heart-Strong Randomized Clinical Trial. Journal of the American Medical Association Internal Medicine, 177, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, … Urquhart J (2012). A new taxonomy for describing and defining adherence to medications. British Journal of Clinical Pharmacology, 73, 691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton CM, Johnston CS, Cunningham BK, & Sterner D (2014). Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. Journal of Nutrition Education and Behavior, 46, 440–444. 10.1016/j.jneb.2014.04.291 [DOI] [PubMed] [Google Scholar]
- Whelan J, Love P, Pettman T, Doyle J, Booth S, Smith E, & Waters E (2014). Cochrane update: Predicting sustainability of intervention effects in public health evidence: Identifying key elements to provide guidance. Journal of Public Health, 36, 347–351. 10.1093/pubmed/fdu027 [DOI] [PubMed] [Google Scholar]
- Wood W, & Neal DT (2007). A new look at habits and the habit-goal interface. Psychological Review, 114, 843–863. 10.1037/0033-295X.114.4.843 [DOI] [PubMed] [Google Scholar]
- Wood W, Quinn JM, & Kashy DA (2002). Habits in everyday life: Thought, emotion, and action. Journal of Personality and Social Psychology, 83, 1281–1297. 10.1037/0022-3514.83.6.1281 [DOI] [PubMed] [Google Scholar]
- Zullig LL, Peterson ED, & Bosworth HB (2013). Ingredients of successful interventions to improve medication adherence. Journal of the American Medical Association, 310, 2611–2612. 10.1001/jama.2013.282818 [DOI] [PubMed] [Google Scholar]


