Abstract
Porcine epidemic diarrhea virus (PEDV) and porcine deltacoronavirus (PDCoV) are both members of the family Coronaviridae which induce clinical signs of diarrhea, dehydration, and in some circumstances, mortality. Most research has been focused on isolation, genome sequencing, pathogenicity, and virulence of these viruses, but there is little information on long-term growth performance and tissue accretion of pigs inoculated with PEDV or PDCoV. Therefore, our objective was to determine the effect of PEDV or PDCoV infection on growth performance and tissue accretion over 42 d following inoculation. A total of 75 Choice Genetics Large White Pureline barrows and gilts (BW = 10.81 ± 0.81 kg) at approximately 2 wk post-wean and naïve for PEDV and PDCoV were selected. Pigs were allotted based on BW and sex, stratified across 3 treatments with 8 pens per treatment. Treatments were: 1) Control (n = 8); 2) PEDV inoculated (n = 8); and 3) PDCoV inoculated (n = 8). On day post inoculation (dpi) 2, 5, 7, and 14 pigs were euthanized for tissue collection and analyses from these tissues are discussed elsewhere. Pen feed intake and BW were recorded on dpi 2, 5, 7, and weekly thereafter until dpi 42. On 1 designated pig per pen, initial and final body composition was determined using dual-energy X-ray absorptiometry (DXA) and tissue accretion rates were calculated over 6 wk test period. Peak PEDV infection was noted at 3 dpi compared with 4 dpi for PDCoV pigs as determined by fecal swab quantitative real-time PCR (RT-PCR). Control pigs remained negative for PEDV and PDCoV throughout the experiment. Overall, Control and PDCoV pigs did not differ in ADG, ADFI or G:F (P > 0.05). Compared to Control and PDCoV pigs, the overall 42 d ADFI was reduced in the challenged PEDV pigs (P < 0.05) by 19 and 27%, respectively. PEDV did not significantly reduce the overall ADG or G:F compared with Control and PDCoV pigs; however, the biggest reduction in ADG and ADFI for PEDV pigs was within 14 dpi compared to the Control pigs (P < 0.05). Whole body tissue accretion was altered due to PED, with fat, lean, protein, and bone mineral accretion reductions by 24, 20, 21, and 42%, respectively (P < 0.05) compared with Control pigs. Overall, nursery pig performance was greatly impacted by PEDV challenge. Surprisingly, the PDCoV challenge did not negatively influence nursery pig performance. This study provides further insight into the longitudinal impact swine enteric coronaviruses have on growing pigs.
Keywords: Growth performance, PEDV, PDCoV, Pig, Tissue accretion
INTRODUCTION
In every stage of production pigs can encounter pathogens that may cause detrimental effects on growth performance and health. In the U.S., 2 newly-emerged enteric pathogens which compromise health and production are porcine epidemic diarrhea virus (PEDV) and porcine deltacoronavirus (PDCoV). Both of these RNA viruses are of the Coronaviridae family (Woo et al., 2012) that infect enterocytes of the gastrointestinal tract. In April 2013, PEDV was first identified in the U.S. (Stevenson et al., 2013) with clinical signs including diarrhea, dehydration, anorexia, and mortality. Severe clinical signs and mortality have been primarily observed in suckling pigs (Stevenson et al., 2013); however, PEDV clinical signs are also observed in weaned pigs with varying levels of morbidity (Madson et al., 2014) in experimental conditions. In February 2014, PDCoV was first detected in the U.S. and was shown to be related to 2 Chinese strains with clinical signs of diarrhea in sows and piglets and mortality in piglets (Wang et al., 2014). The majority of research on PDCoV has focused on etiology, propagation in cell culture, and genome sequencing (Chen et al., 2015; Hu et al., 2015; Jung et al., 2015c; Ma et al., 2015; Homwong et al., 2016); however, little research is available on the effect of PDCoV on growth performance.
There is a perception that PEDV occurrences are declining and is mainly an issue for neonatal pigs, but there are still new cases of PEDV and PDCoV occurring every week (USDA, 2016). Interestingly, the long-term effects of PEDV and PDCoV on pig performance and lean tissue accretion have not been reported. Such research can provide critical information as to how long it takes infected pigs to recover from either virus infection, and how the infection affects overall lean accretion. Therefore, our objective was to compare and determine how these 2 coronaviruses modulate nursery pig growth performance and tissue accretion rates over a 6 wk period.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee at Iowa State University (Ames, IA) approved all experimental protocols (IACUC# 11–14–7903-S).
Animals, Housing, Experimental Design, and Diets
A total of 75 maternal line Choice Genetics Large White Pureline Line 3 barrows and gilts (BW = 10.81 ± 0.81 kg) at approximately 2 wk post-wean and naïve for PEDV and PDCoV were selected, weighed, and allotted to treatments.
Pigs were allotted based on BW and sex across 3 treatments with 8 pens per treatment for a 42 d test period. Each pen had 2 to 5 pigs per pen. Treatments were: 1) Control (n = 8); 2) PEDV inoculated (n = 8); and 3) PDCoV inoculated (n = 8). Inoculum of PEDV was administered as previously described (Madson et al., 2014). Pen feeder weights and BW were recorded on day post inoculation (dpi) 0, 2, 5, 7, and weekly thereafter until dpi 42. Over the first 2 wk, pigs were sequentially removed from each pen on dpi 2, 5, 7, and 14 and euthanized for tissue collection to study the pathogenesis of these viruses. This analyses will be discussed elsewhere. From dpi 14 to 42, there was 1 pig per pen.
All pigs were fed the same corn-soybean meal diet that was formulated to meet or exceed the NRC (2012) requirements for nutrients and energy (Table 1). Pigs were allowed free access to feed and water. The diet was analyzed (Table 1) for DM by oven drying at 135°C for 2 h (method 930.15; AOAC Int., 2007), concentration of CP was calculated by analyzing N content via a TruMac N (LECO Corporation, St. Joseph, MO) and gross energy using bomb calorimetry (Oxygen Bomb Calorimeter 6200, Parr Instrument Company, Moline, IL). Benzoic acid was used as the standard for bomb calorimeter calibration.
Table 1.
Diet composition, as-fed basis
| Ingredient | %, as-fed |
|---|---|
| Corn | 60.93 |
| Soybean meal, 48% CP | 30.00 |
| Corn DDGS1 | 5.00 |
| Soybean oil | 1.00 |
| Limestone | 0.94 |
| l-lysine HCl | 0.50 |
| Sodium chloride | 0.35 |
| Vitamin-mineral pre-mix2 | 0.30 |
| Monocalcium phosphorous, 21% | 0.55 |
| Heat stable Optiphos 20003 | 0.02 |
| l-Threonine | 0.22 |
| dl-Methionine | 0.19 |
| Calculated composition | |
| CP, % | 21.13 |
| ME, kcal/kg | 3388 |
| NE, kcal/kg | 2433 |
| Lys, SID4% | 1.33 |
| Lys, total % | 1.48 |
| Analysis | |
| DM, % | 95.21 |
| CP, % | 21.38 |
| GE, kcal/kg | 4030 |
1DDGS = distiller's dried grains with solubles.
2Premix supplied (per kg of diet): 8820 IU vitamin A, 1653 IU vitamin D3, 33.1 IU vitamin E, 4.4 mg vitamin K, 6.6 mg riboflavin, 38.9 mg niacin, 22.1 mg pantothenic acid, 0.04 mg vitamin B12, 1.1 mg I as potassium iodide, 0.30 mg Se as sodium selenite, 60.6 mg Zn as zinc oxide, 36.4 mg Fe as ferrous sulfate, 12.1 mg Mn as manganous oxide, and 3.6 mg Cu as copper sulfate.
3Huvepharma Inc., Peachtree City, GA.
4SID = standardized ileal digestibility.
Inoculation and Sample Collection
On dpi 0, PEDV pigs were inoculated with 5 mL of 103 tissue culture infectious dose (TCID)50/ml PEDV isolate (USA/Iowa/18984/2013) via intragastric gavage as previously described (Hoang et al., 2013; Madson et al., 2014). For PDCoV pigs, plaque-cloned PDCoV isolate (US/Iowa/25573/2014) from a naturally infected sow showing clinical signs of diarrhea was used. The pigs received 5 mL of the virus at a rate of 103 TCID50/ml via intragastric gavage. Individual pig fecal swabs were taken daily for the first wk to determine virus shedding in the feces (Table 2). Fecal swabs were subjected to normal diagnostic process via quantitative real-time PCR (RT-PCR) testing for PEDV and PDCoV.
Table 2.
Fecal virus shedding of nursery pigs inoculated with porcine epidemic diarrhea virus (PEDV) or porcine deltacoronavirus (PDCoV)1
| dpi2 | |||||
|---|---|---|---|---|---|
| Item | 1 | 2 | 3 | 4 | 5 |
| Cycle threshold3 | |||||
| Control | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| PEDV | 34.73 | 31.98 | 30.12 | 27.49 | 27.86 |
| PDCoV | 40.00 | 39.07 | 32.98 | 38.43 | 39.91 |
| Positive pigs/total pigs | |||||
| Control | 0/25 | 0/25 | 0/21 | 0/21 | 0/21 |
| PEDV | 8/25 | 12/25 | 16/20 | 14/20 | 13/20 |
| PDCoV | 0/25 | 2/25 | 14/21 | 5/21 | 0/21 |
1Average cycle threshold of all pigs on test pigs. Positive pigs/total pigs represent pigs with Ct ≤ 37 that were positive for PEDV or PDCoV out of the total number of pigs sampled.
2dpi = day post inoculation.
3Cycle threshold (Ct) for positive = ≤ 37, suspect = 38 to 40, negative ≥ 40.
In brief, fecal swabs were processed on the day of collection. In brief, fecal swabs were added to 2 mL of PBS (pH 7.4) without calcium and magnesium (GIBCO/Fischer Scientific, Pittsburg, PA). Samples were vortexed, centrifuged, and the supernatant was collected and stored at -80°C. Viral RNA was extracted using the MagMAX Viral RNA Isolation Kit (Life Technologies/Fisher Scientific, Pittsburg, PA) using the manufacturer's instructions for high-volume extraction. Reverse transcription quantitative PCR was performed as previously described (Madson et al., 2014) with viral standards of known infectivity titers for quantification. A cycle threshold (Ct) less than or equal to 37 was considered positive for virus, 38 to 39 was considered suspect, and greater than or equal to 40 was considered negative.
Whole Body Composition and Tissue Accretion
One pig per pen was designated for longitudinal assessment of body composition at dpi -3 and at the end of the study (dpi 42). These 24 pigs were selected based on the average BW. This initial and final whole body composition was assessed using nondestructive dual-energy X-ray absorptiometry (DXA; Hologic Discovery A, Bedford, MA) as previously described (Suster et al., 2003). Briefly, all pigs were fasted overnight prior to scanning and the designated 24 body composition pigs were then removed from their pens, anesthetized via intramuscular injection with telazol:ketamine:xylozene (2:1:2; 4.4 mg/kg BW and 2.2 mg/kg BW, respectively) at a dosage rate of 1 mL per 45.5 kg BW. Anesthetized pigs were placed on the DXA scan table in sternal recumbancy with fore and hind legs extended. Once scanned, pigs were allowed to recover and then returned to their pens. The DXA output provided information on whole body bone, fat, and lean tissue mass. Scan data was corrected for gut fill and blood volume using internally built calibration curves for pigs using the following regressions: Live weight, y = 1.0822x-1.826, R2 = 0.9970; Fat, y = 0.9515x-1.06, R2 = 0.9308; Bone mineral ash, y = 2.1473x-0.1411, R2 = 0.9219; Lean, y = 1.0668x-0.1411, R2 = 0.9909; Protein, y = 0.2206x-0.6611, R2 = 0.9758; where x = DXA results and y = chemical proximate on an empty whole body (i.e., no luminal, urine or gall bladder contents). These calibration curves where built as described by Suster et al. (2003).
Calculations and Statistical Analysis
Weekly ADG, ADFI, and G:F were calculated using weekly pen feed intake and BW. The accretion rates (g/d) for whole body lean, protein, fat, BW, bone mineral density (BMD) and bone mineral content (BMC) were calculated by the following formula:
 |
Data were analyzed using the MIXED procedure in SAS (SAS Inst. Inc., Cary, NC). Pen was the experimental unit for all growth performance parameters. The model included treatment as fixed effect and start BW was used as a covariate. Pig was used as the experimental unit for body composition and tissue accretion analysis. The model included treatment as fixed effect. Least square (LS) means of treatments were determined using the LS means statement and differences in LS means were produced using the pdiff option. Data are reported as LS means estimates with a pooled SEM. A P-value of 0.05 or lower was used to assess significance and a P-value less than 0.10 was considered a tendency.
Change in BW was plotted as a scatter plot using SGPLOT procedure, with regression of the change in BW over time (dpi) with 95% confidence limit around the mean BW per treatment. Predicted BW change per treatment group was determined using the MIXED procedure. The model included treatment and dpi as fixed effects. Pig nested within treatment was used in the repeated statement using spatial power covariance structure. Predicted means were plotted using the SGPLOT procedure regressing the treatment predicted means over time (dpi).
RESULTS
A common diet was fed to all treatments and was formulated to contain 21.13% CP, 3388 ME (kcal/kg), and 1.33% SID Lys on an as-fed basis. The analyzed diet was 95.21% DM, 21.38% CP, and 4030 gross energy (kcal/kg).
Control pigs were negative for PEDV and PDCoV for the entire duration of the study and performed similarly to the predicted performance for similar BW and high health pigs (NRC, 2012). The NRC predicts ADG and ADFI to be 0.59 kg and 0.95 kg, respectively for pigs weighing 11 to 25 kg, which is similar to the overall Control pig performance. Pigs inoculated with PEDV were confirmed positive via RT-PCR on dpi 1 and continued shedding virus though dpi 5, but were negative for PEDV from dpi 6 through the end of the study (Table 2). Pigs inoculated with PDCoV became suspect for virus via RT-PCR on dpi 2, were confirmed positive on dpi 3, and all pigs were negative on dpi 6. From this study, PEDV and PDCoV pigs had the highest rate of infection on dpi 3 as indicated by the greater amount of positive pigs out of total pigs sampled.
There was a tendency (P = 0.091) for start BW to be different among treatments; therefore, start BW was used as a covariate for all growth performance data (Table 3). Pigs inoculated with PEDV weighed 15% and 18% less (P = 0.012) at the end of 42 d compared with Control pigs and PDCoV pigs, respectively, which were not different from each other. From dpi 0 to 7, PEDV pigs had significantly reduced (P < 0.05) ADG and G:F (78 and 58%, respectively) compared with Control pigs and had a 32% reduction (P = 0.013) in ADFI compared to PDCoV pigs. However, pigs inoculated with PDCoV were able to maintain similar (P > 0.05) ADG, ADFI, and G:F to Control pigs from dpi 0 to 7.
Table 3.
Overall and weekly growth performance in Controls and pigs inoculated with porcine epidemic diarrhea virus (PEDV) or porcine deltacoronavirus (PDCoV) over a 42 d period
| Treatment | P-value | |||||
|---|---|---|---|---|---|---|
| Item | Control | PEDV | PDCoV | SEM | TRT | |
| Start BW, kg | 11.06 | 9.71 | 11.65 | 0.605 | 0.091 | |
| End BW, kg | 41.89a | 35.39b | 43.39a | 1.804 | 0.012 | |
| dpi 0– 71 | ||||||
| ADG | 0.49a | 0.11b | 0.44a | 0.038 | < 0.001 | |
| ADFI | 0.62ab | 0.48b | 0.71a | 0.047 | 0.013 | |
| G:F | 0.71a | 0.13b | 0.68a | 0.075 | < 0.001 | |
| dpi 8– 14 | ||||||
| ADG | 0.60 | 0.55 | 0.64 | 0.054 | 0.565 | |
| ADFI | 0.69b | 0.66b | 0.94a | 0.058 | 0.019 | |
| G:F | 0.88 | 0.86 | 0.68 | 0.089 | 0.308 | |
| dpi 15– 21 | ||||||
| ADG | 0.79 | 0.69 | 0.58 | 0.076 | 0.144 | |
| ADFI | 1.19 | 1.06 | 1.34 | 0.104 | 0.211 | |
| G:F | 0.67a | 0.67a | 0.42b | 0.045 | 0.002 | |
| dpi 22– 28 | ||||||
| ADG | 0.80 | 0.74 | 1.01 | 0.108 | 0.197 | |
| ADFI | 1.29 | 1.15 | 1.49 | 0.122 | 0.173 | |
| G:F | 0.62 | 0.68 | 0.70 | 0.085 | 0.807 | |
| dpi 29– 42 | ||||||
| ADG | 0.86 | 0.80 | 0.87 | 0.043 | 0.493 | |
| ADFI | 1.77 | 1.72 | 1.82 | 0.138 | 0.873 | |
| G:F | 0.50 | 0.48 | 0.48 | 0.040 | 0.781 | |
| Overall (dpi 0– 42) | ||||||
| ADG | 0.73 | 0.64 | 0.72 | 0.056 | 0.489 | |
| ADFI | 1.00a | 0.81b | 1.11a | 0.059 | 0.011 | |
| G:F | 0.74 | 0.76 | 0.66 | 0.055 | 0.486 | |
a,bMeans with differing superscripts indicate a significant (P < 0.05) difference.
1dpi = days post inoculation.
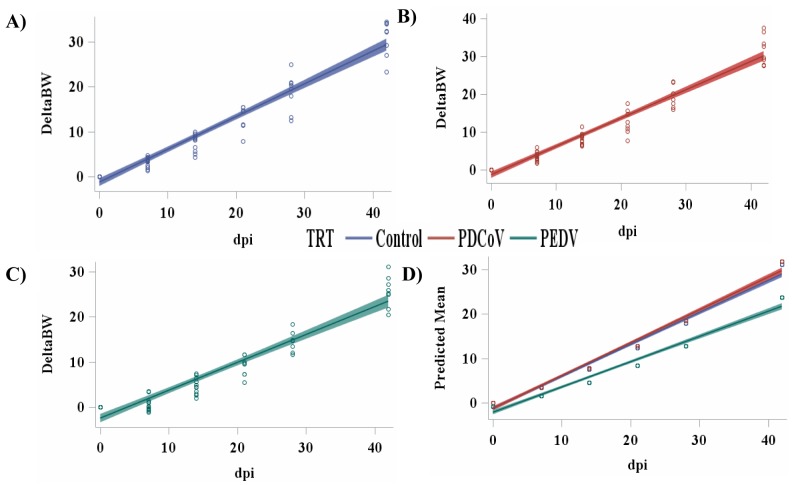
From dpi 8 to 14, PEDV pigs had a 30% reduction (P = 0.019) in ADFI compared with PDCoV pigs, but were similar to Control pigs. In contrast, pigs inoculated with PDCoV consumed 27% more feed per d compared with Control pigs. However, all treatments had similar (P > 0.05) G:F from dpi 8 to 14. From dpi 15 to 21, pigs inoculated with PDCoV had a 37% reduction in G:F compared with Control and PEDV pigs, which were not statistically different from each other. Weekly performance from 22 to 42 dpi, showed no difference (P > 0.05) in ADG, ADFI, and G:F among treatment groups. From 0 to 42 dpi, pigs inoculated with PEDV had a 19 and 27% reduction (P = 0.011) in ADFI compared with Control and PDCoV pigs, respectively. The overall reduction in ADFI is primarily driven by the reductions in ADFI and feed efficiency during dpi 0 to 7 which agrees with the peak impact of PEDV being within dpi 0 to 7 as indicated by number of positive pigs for fecal viral shedding. The rate of BW change was plotted over the 42 d period (Fig. 1). The rate was similar for Control and PDCoV pigs; however, the rate of BW change was visibly decreased for PEDV pigs.
Figure 1.
Scatter plot of the cumulative change in BW over 42 d of A) Controls and pigs inoculated with B) porcine deltacoronavirus (PDCoV) or C) porcine epidemic diarrhea virus (PEDV) with a 95% confidence limit. D) Predicted mean cumulative change in BW over 42 d for Controls (y = 0.72x – 1.24) and pigs inoculated with PDCoV (y = 0.74x – 1.28) or PEDV (y = 0.56x – 2.13).
There was no difference (P > 0.05) in initial body composition among pigs including: fat, lean, lean to fat ratio, BW, BMC, and BMD (Table 4). Pigs inoculated with PEDV had a 22 and 27% reduction (P = 0.018) in final whole body fat mass compared with Control and PDCoV pigs, respectively, which were not different from each other. Similarly, PEDV pigs had a 15 and 17% reduction (P = 0.012) in final whole body lean mass compared with Control and PDCoV pigs, respectively, which were not different form each other. There was a tendency (P = 0.056) for PEDV pigs to have greater lean to fat ratio compared with PDCoV pigs, but were not different from Control pigs. The DXA predicted BW reported a 16 and 18% reduction (P = 0.012) in final BW for PEDV pigs compared with Control and PDCoV pigs, respectively. Similar trends were observed for final whole body protein mass, final BMC, and final BMD where PEDV pigs had less final whole body protein mass (P = 0.013), final BMC (P = 0.034), and final BMD (P = 0.029) compared with Control and PDCoV pigs.
Table 4.
Initial and final body composition, and accretion of fat, lean, protein, BW, bone mineral content (BMC), and bone mineral density (BMD) in Controls and pigs inoculated with porcine epidemic diarrhea virus (PEDV) or porcine deltacoronavirus (PDCoV) from dpi -3 to 421
| Treatment | P-value | ||||
|---|---|---|---|---|---|
| Item | Control | PEDV | PDCoV | SEM | TRT |
| Initial composition | |||||
| Fat, kg | 0.88 | 0.80 | 0.84 | 0.08 | 0.736 |
| Lean, kg | 8.93 | 9.01 | 8.59 | 0.68 | 0.899 |
| Protein, kg | 1.23 | 1.24 | 1.17 | 0.11 | 0.899 |
| Lean:Fat | 10.43 | 11.62 | 10.55 | 0.68 | 0.421 |
| BW, kg | 10.02 | 9.97 | 9.65 | 0.76 | 0.931 |
| BMC2, kg | 0.23 | 0.25 | 0.25 | 0.02 | 0.589 |
| BMD3, g/cm3 | 0.40 | 0.40 | 0.40 | 0.01 | 0.984 |
| Final composition | |||||
| Fat, kg | 5.55a | 4.35b | 5.96a | 0.34 | 0.018 |
| Lean, kg | 37.12a | 31.69b | 38.05a | 1.47 | 0.012 |
| Protein, kg | 6.14a | 5.13b | 6.31a | 0.27 | 0.013 |
| Lean:Fat | 6.83ab | 7.34a | 6.49b | 0.24 | 0.059 |
| BW, kg | 44.15a | 37.25b | 45.64a | 1.91 | 0.012 |
| BMC2, kg | 0.69a | 0.52b | 0.73a | 0.06 | 0.034 |
| BMD3, g/cm3 | 0.68ab | 0.63b | 0.71a | 0.02 | 0.029 |
| Whole body accretion | |||||
| Total body, g/d | 726.1a | 580.4b | 734.6a | 38.65 | 0.016 |
| Fat, g/d | 99.3a | 75.8b | 104.6a | 7.69 | 0.034 |
| Lean, g/d | 599.8a | 482.6b | 601.2a | 30.06 | 0.015 |
| Protein, g/d | 104.5a | 82.8b | 104.9a | 5.54 | 0.014 |
| BMC2, g/d | 9.8a | 5.7b | 9.9a | 1.04 | 0.014 |
a,bMeans with differing superscripts indicate a significant (P < 0.05) difference.
1Initial composition was determined by dual x-ray absorptiometry (DXA) scan 3 d prior to inoculation. Final composition was determined by DXA scan at 42 dpi. Whole body accretion was calculated by (corrected final composition– corrected initial composition)/d on test.
2BMC = bone mineral content.
3BMD = bone mineral density.
Whole body protein accretion rates (Table 4) of Control pigs (104.5 g/d) was similar to that estimated for this size pig by the NRC (2012) modeling at 105 g/d. Pigs inoculated with PEDV had a 24 and 28% reduction (P = 0.034) in whole body fat accretion compared with Control and PDCoV pigs, respectively, which were not different from each other. In PEDV pigs, whole body lean and protein accretion was reduced (P = 0.015) by 20 and 21% compared with Control and PDCoV pigs, respectively, which did not differ from each other. The DXA predicted whole body accretion rates were similar to scale based overall ADG as reported in Table 3 (Controls 0.73 vs. 0.73 kg/d; PEDV 0.58 vs. 0.64 kg/d; and PDCoV 0.73 vs. 0.73 kg/d, respectively). For PEDV pigs, the whole body accretion rate were 20 and 21% less than Control and PDCoV pigs, respectively which was similar to the scale-based reduction in overall ADG of 16 and 18%, respectively.
DISCUSSION
Pigs that have poor health status or are raised in conditions of high pathogen loads have antagonized growth performance. This is evident in work by Williams et al. (1997a, b, c), in which grow-finish pigs raised in unsanitary conditions have reduced ADG, G:F, and predicted lean accretion. Further, Escobar et al. (2004) showed that nursery pigs challenged with porcine reproductive and respiratory syndrome virus (PRRSV) had profoundly impacted whole body protein accretion. This was particularly evident in the first week of PRRSV infection where whole protein accretion is impeded up to 60% from negative pigs and up to 33% in wk 2 (Escobar et al., 2004). In weaned pigs, PRRSV infection has also been shown to decrease ADFI and G:F ratios within the first 14 dpi compared with negative pigs (Rochell et al., 2015). Interestingly, there is limited data showing the extent to which enteric pathogens alter pig feed efficiency, body composition and tissue accretion rates.
Enteric viruses infect the gastrointestinal tract typically causing clinical signs of diarrhea and dehydration with varying degrees of morbidity, and mortality. These clinical signs may be due to altered digestive function and gut integrity. Nursery pigs infected with PEDV have been shown to have decreased brush border digestive enzymes (Jung et al., 2006) and redistribution of tight junction proteins zona-occludin 1 and E-cadherin (Jung et al., 2015b). In addition, growing pigs inoculated with PEDV showed increased permeability and decreased intestinal integrity in the jejunum as indicated by increased FITC-dextran permeability and decreased transepithelial electrical resistance, respectively, compared with negative pigs (Schweer et al., 2015). These changes at the gastrointestinal tract level are evident during periods of high pathogen load; however, the long-term impacts on growth performance and lean accretion have not been reported.
The viral shedding pattern of PEDV observed in this study was as expected in this age pig and is consistent with previous literature that reports viral shedding after dpi 1 (Madson et al., 2014; Jung et al., 2015a; Kim et al., 2015). Kim et al. (2015) observed viral shedding through dpi 7 compared with Madson et al. (2014) who observed viral shedding until dpi 24; however, pigs were different sizes, 2 kg and 6.5 kg, respectively and inoculum dose and strain differed between studies. As with PEDV, PDCoV onset of viral shedding is relatively quick (within dpi 0 to 2). The viral shedding period in PDCoV pigs was 3 d and fairly consistent among pigs. In suckling pigs (∼2 kg BW), viral shedding was variable where some pigs began to shed virus at dpi 2 and others at dpi 5 (Chen et al., 2015). Ma et al. (2015) orally inoculated four 10 d-old piglets with 106 plaque-forming units (PFU) of PDCoV and observed clinical signs of diarrhea at dpi 1, peak viral shedding at dpi 7, and viral persistence in feces through dpi 21. Pigs in this study had an average start BW of 10.81 kg and may have had a more developed immune system (Annamalai et al., 2015) compared with younger pigs used in aforementioned studies and variability in age, genetics, health status, diet, inoculum strain virulence, inoculum dose, and housing conditions, etc., may influence viral shedding among studies.
Pathogens can be recognized by macrophages which respond by secreting cytokines to initiate an immune response and cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-ɑ have been shown to decrease appetite (Johnson, 1998). This information, along with published performance data of PEDV and PDCoV infected pigs (Kim and Lee, 2014; Chen et al., 2015; Ma et al., 2015; Schweer et al., 2015), led us to expect a reduction in ADG, ADFI, and G:F in pigs inoculated with either PEDV or PDCoV. Pigs inoculated with PEDV have been reported to have severe reductions in ADG (-18 g/d) compared to their negative counterparts (27 g/d) after 7 dpi (Kim and Lee, 2014). In pigs that were similar in size to our current study and inoculated with the same PEDV strain, there was a 29% decrease in ADFI for PEDV pigs compared with negative pigs (Schweer et al., 2015) which was similar to the 23% reduction during dpi 0 to 7 which was not statistically significant in the current study. However, this paper reported a 47 and 28% reductions in ADG and G:F, respectively, of pigs inoculated with PEDV which was less than our observed reduction of 78 and 58%, for ADG and G:F, respectively, during dpi 0 to 7. Although we observed a decrease in feed efficiency from dpi 0 to 7, we did not observe an overall effect of PEDV on 42 d feed efficiency.
To our knowledge, feed intake and feed efficiency of pigs inoculated with PDCoV has not been published. However, there have been reports of ADG and BW changes due to PDCoV challenges. Chen et al. (2015) inoculated 5-d old pigs with 10 mL of 3 × 103 TCID50/mL of a cell culture isolate of PDCoV (USA/IL/2014), and reported that after dpi 4 and 7, PDCoV pigs did not differ in BW or ADG compared with negative pigs. However, in 10 d-old pigs, Ma et al. (2015) observed a 10 to 15% reduction in BW of pigs inoculated with 106 PFU of PDCoV, MI strain compared with negative pigs after dpi 10. We did not observe a difference in ADG or G:F of PDCoV pigs compared with Control pigs. As with PEDV, these differences may be due to strain of PDCoV used, virulence of inoculum, age, genetics, housing conditions, or health status. For both PEDV and PDCoV, we did not observe any differences in growth performance after dpi 21 compared with Control pigs. This indicates that pigs infected with either of these viruses at this BW can recover and begin to perform similar to their negative counterparts. However, the initial impact of PEDV on ADFI and ADG will increase the number of d to reach a desirable final market weight compared to negative pigs.
The data herein is the first report on whole body tissue accretion rates of pigs inoculated with PEDV and PDCoV that we are aware of. In our study, we determined that PEDV decreased lean, protein, fat, and BMC accretion compared with Control pigs over a 6 wk period, but PDCoV did not differ from the Control pigs. There are few published studies on the effect of an immune challenge on whole body composition and tissue accretion of pigs (Escobar et al., 2002; Escobar et al., 2004; Gabler et al., 2013). Escobar et al. (2002 and 2004) used the serial slaughter technique to determine whole body composition and calculated tissue accretion rates. After 7 d of PRRSV infection in 4 wk-old pigs, there was a 41 and 63% reduction in protein and lipid accretion, respectively, compared with negative pigs (Escobar et al., 2004). After 80 d of PRRSV infection in pigs (start BW = 33 kg), there was a 14% reduction in lean and protein accretion and an 18% reduction in fat accretion compared with negative pigs (Gabler et al., 2013). These studies show that PRRSV not only impacts short-term tissue accretion, but also reduces tissue accretion long-term. Peak viremia of PRRSV is usually within 7 d after inoculation (Johnson et al., 2004; Islam et al., 2013) and reduced feed intake is observed within dpi 0 to 14 (Schweer et al., 2015) which may explain a bigger reduction in tissue accretion during a shorter time period after onset of infection. During an immune challenge, there is a demand for energy and nutrients at a time that is coupled with reduced feed intake. This situation may cause mobilization of AA and FA from muscle and adipose, respectively, thus altering body composition. Pigs inoculated with PEDV had severe reductions in ADFI within and began to perform similar to Control pigs after dpi 21, but we still observed reductions in tissue accretion over 42 d for PEDV pigs.
The observed decrease in protein accretion for PEDV compared with Control pigs may be due to overall decreased feed intake and thus, energy and AA are repartitioned in different tissues in the body. In 26-d old pigs, immune stimulation, as initiated by lipopolysaccharide (LPS) injection, increased circulating total AA concentrations during the fasted state (Orellana et al., 2012). In rotavirus-infected pigs, total circulating AA concentrations were also increased during the fed state (Rhoads et al., 2007). Increasing circulating AA concentration during an immune stimulation indicates that the body is repartitioning its AA pool to tissues in need of AA and one of the major AA pools in the body is muscle. In fasted 7-d and 26-d old piglets, LPS injection was used to determine severity of muscle degradation during systemic inflammation (Orellana et al., 2012). Despite the high protein turnover rate in 7-d old piglets compared with 26-d old piglets (Davis and Fiorotto, 2009), both age groups had increased abundance of proteins involved with skeletal muscle degradation including: NF-ϗB, caspase 3, AMPK, E3 ligases (MuRF1 and atrogin1), and cleaved ɑ-actin, a result of muscle proteolysis.
Enteric infections have been shown to modulate protein synthesis machinery in a tissue dependent manner (Rhoads et al., 2007). One of the main pathways involved in protein synthesis is the mammalian target of rapamycin (mTOR) pathway. This pathway is regulated by many inputs including energy and amino acid status, stress, and growth factors. Two proteins downstream of mTOR that are thought to be involved in the initiation of protein synthesis are p70s6k and its downstream target ribosomal protein S6. In rotavirus-challenged 4-d old pigs, protein abundance of total and activated (phosphorylated) p70S6K were increased during peak infection in the jejunum compared to negative pigs (Rhoads et al., 2007). In that study, another group of pigs was rotavirus-infected and malnourished by diluting the complete formula by 50% with an electrolyte solution. Regardless of having reduced energy intake, the malnourished-infected pigs also had increased protein abundance of total and activated (phosphorylated) p70S6K in the jejunum. However, in the muscle of both infected groups, there was a reduction of total S6 protein during peak infection compared to healthy controls. A reduction in protein synthesis in the muscle agrees with the current study that there was a decrease in protein accretion in PEDV pigs. The degradation occurring in the muscle most likely is due to the increased demand of AA in the intestine to aid in recovery of the epithelium. This indicates that the effects of enteric challenges are not driven by reduced feed intake alone, but there are post-absorptive changes during an enteric challenge that can lead to changes in body composition due to metabolic shifts within tissues.
In conclusion, reductions in tissue accretion and feed intake appear to be pathogen specific. We determined that overall 6 wk reductions in tissue accretion and ADFI were only observed in PEDV, but not in PDCoV inoculated pigs. We could not determine from this study if the reduction in tissue accretion is primarily driven by the reduction in feed intake, changes in digestibility, or other modifications to post-absorptive metabolism resulting from the PEDV challenge itself. Regardless, the energy and AA requirements of health-challenged pigs may be different from their healthy counterparts during infection and warrants further investigation.
Footnotes
Funding for this research was provided by the Agriculture and Food Research Initiative Competitive Grant no. 2016–67015–24574 and Animal Health Hatch funding from the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Annamalai T., Saif L. J., Lu Z., Jung K. 2015. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 168:193–202. doi: 10.1016/j.vetimm.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC Int 2007. 18th ed. Rev. 2Hortwitz W., Latimer G. W., Jr editors. AOAC Int., Gaithersburg, MD. [Google Scholar]
- Chen Q., Gauger P., Stafne M., Thomas J., Arruda P., Burrough E., Madson D., Brodie J., Magstadt D., Derscheid R., Welch M., Zhang J. 2015. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology 482:51–59. doi: 10.1016/j.virol.2015.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. A., Fiorotto M. L. 2009. Regulation of muscle growth in neonates. Curr. Opin. Clin. Nutr. Metab. Care 12:78–85. doi: 10.1097/MCO.0b013e32831cef9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J., Van Alstine W. G., Baker D. H., Johnson R. W. 2002. Growth performance and whole-body composition of pigs experimentally infected with Mycoplasma hyopneumoniae. J. Anim. Sci. 80:384–391. doi: 10.2527/2002.802384x [DOI] [PubMed] [Google Scholar]
- Escobar J., Van Alstine W. G., Baker D. H., Johnson R. W. 2004. Decreased protein accretion in pigs with viral and bacterial pneumonia is associated with increased myostatin expression in muscle. J. Nutr. 134:3047–3053. [DOI] [PubMed] [Google Scholar]
- Gabler N. K., Schweer W., Patience J. F., Karriker L., Sparks J. C., Gourley G., FitzSimmons M., Schwartz K., Burkey T. E. 2013. The impact of PRRSV on feed efficiency, digestibility and tissue accretion in grow-finisher pigs. Allen D. Leman Swine Conference. No. 40. p. 135–136. Veterinary Continuing Education, St. Paul, Mn, USA. [Google Scholar]
- Hoang H., Killian M. L., Madson D. M., Arruda P. H., Sun D., Schwartz K. J., Yoon K. J. 2013. Full-Length Genome Sequence of a Plaque-Cloned Virulent Porcine Epidemic Diarrhea Virus Isolate (USA/Iowa/18984/2013) from a Midwestern U.S. Swine Herd. Genome Announc. 1(6): e01049–13. doi: 10.1128/genomeA.01049-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homwong N., Jarvis M. C., Lam H. C., Diaz A., Rovira A., Nelson M., Marthaler D. 2016. Characterization and evolution of porcine deltacoronavirus in the United States. Prev. Vet. Med. 123:168–174. doi: 10.1016/j.prevetmed.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Jung K., Vlasova A. N., Chepngeno J., Lu Z., Wang Q., Saif L. J. 2015. Isolation and Characterization of Porcine Deltacoronavirus from Pigs with Diarrhea in the United States. J. Clin. Microbiol. 53:1537–1548. doi: 10.1128/JCM.00031-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z. U., Bishop S. C., Savill N. J., Rowland R. R., Lunney J. K., Trible B., Doeschl-Wilson A. B. 2013. Quantitative analysis of porcine reproductive and respiratory syndrome (PRRS) viremia profiles from experimental infection: A statistical modelling approach. PLoS One 8:e83567. doi: 10.1371/journal.pone.0083567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. W. 1998. Immune and endocrine regulation of food intake in sick animals. Domest. Anim. Endocrinol. 15:309–319. doi: 10.1016/S0739-7240(98)00031-9 [DOI] [PubMed] [Google Scholar]
- Johnson W., Roof M., Vaughn E., Christopher-Hennings J., Johnson C. R., Murtaugh M. P. 2004. Pathogenic and humoral immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) are related to viral load in acute infection. Vet. Immunol. Immunopathol. 102:233–247. doi: 10.1016/j.vetimm.2004.09.010 [DOI] [PubMed] [Google Scholar]
- Jung K., Ahn K., Chae C. 2006. Decreased activity of brush border membrane-bound digestive enzymes in small intestines from pigs experimentally infected with porcine epidemic diarrhea virus. Res. Vet. Sci. 81:310–315. doi: 10.1016/j.rvsc.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Jung K., Annamalai T., Lu Z., Saif L. J. 2015a. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Vet. Microbiol. 178:31–40. doi: 10.1016/j.vetmic.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Eyerly B., Annamalai T., Lu Z., Saif L. J. 2015b. Structural alteration of tight and adherens junctions in villous and crypt epithelium of the small and large intestine of conventional nursing piglets infected with porcine epidemic diarrhea virus. Vet. Microbiol. 177:373–378. doi: 10.1016/j.vetmic.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L. J. 2015c. Pathogenicity of 2 Porcine Deltacoronavirus Strains in Gnotobiotic Pigs. Infect. Dis. 21: 650–654. doi: 10.3201/eid2104.141859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. B., Lee C. Y., Kim S. J., Han J. H., Choi K. H. 2015. Medicinal herb extracts ameliorate impaired growth performance and intestinal lesion of newborn piglets challenged with the virulent porcine epidemic diarrhea virus. J. Anim. Sci. Technol. 57: 33–39. doi: 10.1186/s40781-015-0065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lee C. 2014. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology 460–461:180–193. doi: 10.1016/j.virol.2014.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. 2015. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio 6:e00064–15. doi: 10.1128/mBio.00064-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson D. M., Magstadt D. R., Arruda P. H., Hoang H., Sun D., Bower L. P., Bhandari M., Burrough E. R., Gauger P. C., Pillatzki A. E., Stevenson G. W., Wilberts B. L., Brodie J., Harmon K. M., Wang C., Main R. G., Zhang J., Yoon K. J. 2014. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 174:60–68. doi: 10.1016/j.vetmic.2014.09.002 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of Swine. 11th ed.The National Academies Press, Washington, DC. [Google Scholar]
- Orellana R. A., Suryawan A., Wilson F. A., Gazzaneo M. C., Fiorotto M. L., Nguyen H. V., Davis T. A. 2012. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302:R682–R690. doi: 10.1152/ajpregu.00259.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads J. M., Corl B. A., Harrell R., Niu X., Gatlin L., Phillips O., Blikslager A., Moeser A., Wu G., Odle J. 2007. Intestinal ribosomal p70(S6K) signaling is increased in piglet rotavirus enteritis. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G913–G922. doi: 10.1152/ajpgi.00468.2006 [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Alexander L.S., Rocha G.C., Van Alstine W.G., Boyd R.D., Pettigrew J.E., Dilger R.N. 2015. Effects of dietary soybean meal concentration on growth and immune response of pigs infected with porcine reproductive and respiratory syndrome virus1. J. Anim. Sci. 93:2987–2997. doi: 10.2527/jas.2014-8462 [DOI] [PubMed] [Google Scholar]
- Schweer W. P., Schwartz K., Burrough E. R., Yoon K. J., Sparks J. C., Gabler N. K. 2015. The effect of porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus challenge on growing pigs I: Growth performance and digestibility1. J. Anim. Sci. 94(2):514–22. doi: 10.2527/jas.2015-9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. W., Hoang H., Schwartz K. J., Burrough E. R., Sun D., Madson D., Cooper V. L., Pillatzki A., Gauger P., Schmitt B. J., Koster L. G., Killian M. L., Yoon K. J. 2013. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 25:649–654. doi: 10.1177/1040638713501675 [DOI] [PubMed] [Google Scholar]
- Suster D., Leury B. J., Ostrowska E., Butler K. L., Kerton D. J., Wark J. D., Dunshea F. R. 2003. Accuracy of dual energy X-ray absorptiometry (DXA), weight and P2 back fat to predict whole body and carcass composition in pigs within and across experiments. Livest. Prod. Sci. 84:231–242. doi: 10.1016/S0301-6226(03)00077-0 [DOI] [Google Scholar]
- USDA 2016. Swine Enteric Coronavirus Disease (SECD) Situation Report - Nov 24, 2016, USDA-APHIS-VS. https://www.aphis.usda.gov/animal_health/animal_dis_spec/swine/downloads/secd_sit_rep_11_24_16.pdf (Accessed 29 November 2016).
- Wang L., Byrum B., Zhang Y. 2014. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 20:1227–1230. doi: 10.3201/eid2007.140296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. H., Stahly T. S., Zimmerman D. R. 1997a. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J. Anim. Sci. 75:2472–2480. doi: 10.2527/1997.7592472x [DOI] [PubMed] [Google Scholar]
- Williams N. H., Stahly T. S., Zimmerman D. R. 1997b. Effect of chronic immune system activation on the rate, efficiency, and composition of growth and lysine needs of pigs fed from 6 to 27 kg. J. Anim. Sci. 75:2463–2471. doi: 10.2527/1997.7592463x [DOI] [PubMed] [Google Scholar]
- Williams N. H., Stahly T. S., Zimmerman D. R. 1997c. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J. Anim. Sci. 75:2481–2496. doi: 10.2527/1997.7592481x [DOI] [PubMed] [Google Scholar]
- Woo P. C., Lau S. K., Lam C. S., Lau C. C., Tsang A. K., Lau J. H., Bai R., Teng J. L., Tsang C. C., Wang M., Zheng B. J., Chan K. H., Yuen K. Y. 2012. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 86:3995–4008. doi: 10.1128/JVI.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]