Abstract
Human coronavirus-associated myocarditis is known, and a number of coronavirus disease 19 (COVID-19)–related myocarditis cases have been reported. The pathophysiology of COVID-19–related myocarditis is thought to be a combination of direct viral injury and cardiac damage due to the host’s immune response. COVID-19 myocarditis diagnosis should be guided by insights from previous coronavirus and other myocarditis experience. The clinical findings include changes in electrocardiogram and cardiac biomarkers, and impaired cardiac function. When cardiac magnetic resonance imaging is not feasible, cardiac computed tomographic angiography with delayed myocardial imaging may serve to exclude significant coronary artery disease and identify myocardial inflammatory patterns. Because many COVID-19 patients have cardiovascular comorbidities, myocardial infarction should be considered. If the diagnosis remains uncertain, an endomyocardial biopsy may help identify active cardiac infection through viral genome amplification and possibly refine the treatment risks of systemic immunosuppression. Arrhythmias are not uncommon in COVID-19 patients, but the pathophysiology is still speculative. Nevertheless, clinicians should be vigilant to provide prompt monitoring and treatment. The long-term impact of COVID-19 myocarditis, including the majority of mild cases, remains unknown.
Keywords: Arrhythmias, Coronavirus disease 2019, Endomyocardial biopsy, Fulminant myocarditis, Interleukin 6, SARS-CoV-2
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) was first described in Wuhan, China, in patients complaining of flulike symptoms.1 The virus was isolated and identified as a new strain of coronavirus, now named SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The mortality rate of COVID-19 is estimated to be <1%,2 mainly due to severe acute respiratory syndrome and multiorgan dysfunction. Cardiovascular complications of COVID-19 have received less medical attention; nevertheless, the first cases of myocarditis in COVID-19 patients have been reported,3, 4, 5, 6 and myocarditis has been recognized as the cause of death in some COVID-19 patients.7 Pathology usually is focal within the myocardium, but there is a risk of arrhythmia as well as progression to fulminant heart failure and cardiogenic shock. This article outlines the possible pathophysiology of COVID-19–related myocarditis, its clinical presentation, and the associated arrhythmias. Criteria for screening and management of myocarditis relating to COVID-19 are proposed.
Pathophysiology of COVID-19–related myocarditis
General knowledge about viral myocarditis
Myocarditis is an inflammatory disease of the heart characterized by inflammatory infiltrates and myocardial injury without an ischemic cause.8 The most commonly identifiable cause of myocarditis in the United States and other developed countries is viral.9 , 10 Esfandiarei and McManus8 proposed that the pathophysiology of viral myocarditis is a combination of direct cell injury and T-lymphocyte–mediated cytotoxicity, which can be augmented by the cytokine storm syndrome. Interleukin 6 (IL-6) seems to be the central mediator of cytokine storm, in which it orchestrates the proinflammatory responses from immune cells, including the T lymphocytes.11 This process causes T-lymphocyte activation and a further release of inflammatory cytokines, which stimulate more T lymphocytes, leading to a positive feedback loop of immune activation and myocardial damage. Cardiotropism of the T lymphocytes is thought to arise from interaction between heart-produced hepatocyte growth factor (HGF) and c-Met, an HGF receptor on naïve T lymphocytes.12
Known human coronaviruses as the etiologic agents of myocarditis
Despite being a minor cause of all viral myocarditis cases, human coronaviruses have been linked to myocarditis in patients of all age groups.13, 14, 15 The viral RNAs of Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV, which are close relatives of SARS-CoV-2, were found in the heart tissues of infected animals, suggesting that these coronaviruses possess cardiotropism.16 , 17 Furthermore, some coronavirus proteins were shown to render them highly infectious to the human cells. Nakagawa et al18 demonstrated that MERS-CoV-Δ4a, a mutant MERS-CoV strain that lacks the 4a accessory protein, had less efficient replication in HeLa/CD26 cells compared to the wild-type MERS-CoV. The 4a accessory protein is thought to inhibit protein kinase R–mediated phosphorylation of eukaryotic initiation factor 2.18 Failure to phosphorylate the eukaryotic initiation factor 2 impairs stress granule formation. The stress granule helps sequester the host proteins important for translation and attenuates viral protein syntheses; therefore, its suppression promotes viral replication. Similarly, SARS-CoV enhances its RNA translation via the Nsp1 protein.19
Possible pathophysiology of SARS-CoV-2–related myocarditis
SARS-CoV-2 gains entry into human cells by binding its spike protein to the membrane protein angiotensin-converting enzyme 2 (ACE2).20 However, the spike protein must first be cleaved at the S1/S2 and subsequently at the S2ʹ sites to enable binding to ACE2. Cleavage at the S1/S2 site seems to be mediated by TMPRSS2, a serine protein.20 ACE2 can be found on the ciliated columnar epithelial cells of the respiratory tract, type II pneumocytes, and cardiomyocytes.21 , 22 Therefore, it is plausible that SARS-CoV-2 infects the human heart, especially in case of heart failure as ACE2 is upregulated,23 although the presence of viral receptors does not always predict tropism.24
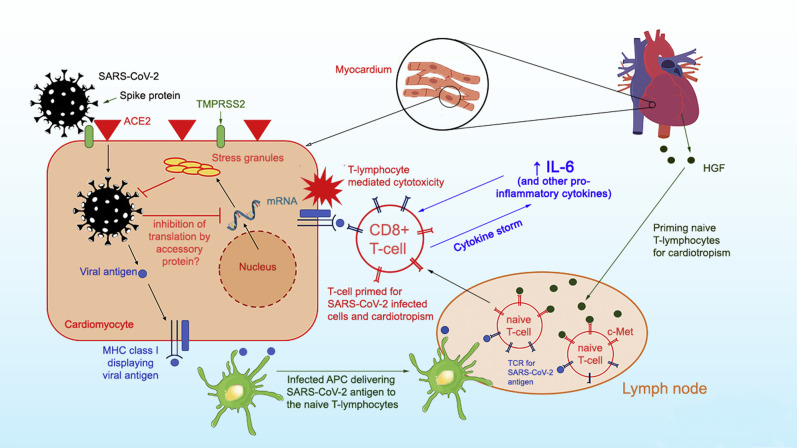
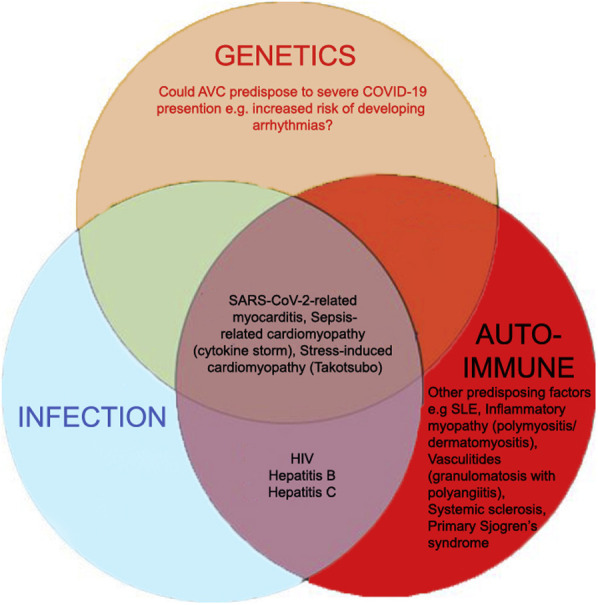
At least 6 known accessory proteins can be transcribed by the SARS-CoV-2 genome.25 Whether any of the accessory proteins provide an infectivity advantage, as in MERS-CoV and SARS-CoV, remains to be determined. Figure 1 summarizes the possible mechanism of SARS-CoV-2 myocardial infection. The speculated risk factors for developing COVID-19–related myocarditis are shown in Online Figure 1.
Figure 1.
Proposed pathophysiology of SARS-CoV-2 myocarditis. SARS-CoV-2 utilizes the spike protein (primed by TMPRSS2) to bind ACE2 to allow cell entry. Intracellular SARS-CoV-2 might impair stress granule formation via its accessory protein. Without the stress granules, the virus is allowed to replicate and damage the cell. Naïve T lymphocytes can be primed for viral antigens via antigen-presenting cells and cardiotropism by the heart-produced HGF. The HGF binds c-Met, an HGF receptor on T lymphocytes. The primed CD8+ T lymphocytes migrate to the cardiomyocytes and cause myocardial inflammation through cell-mediated cytotoxicity. In the cytokine storm syndrome, in which proinflammatory cytokines are released into the circulation, T-lymphocyte activation is augmented and releases more cytokines. This results in a positive feedback loop of immune activation and myocardial damage. ACE2 = angiotensin-converting enzyme 2; APC = antigen-presenting cell; HGF = hepatocyte growth factor; IL-6 = interleukin 6; MHC = major histocompatibility complex; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TCR = T-cell receptor.
Incidence of myocarditis in COVID-19 patients
The prevalence of myocarditis among COVID-19 patients is unclear, partly because the early reports often lacked the specific diagnostic modalities to assess myocarditis. Some argued that up to 7% of COVID-19–related deaths were attributable to myocarditis.26 However, this was assumed and not based on confirmatory diagnoses of myocarditis and thus may be an overestimate. In contrast, many laboratories are rationing tests and are first screening for known pathogens resulting in flulike symptoms. If a positive pathogen is identified, then testing for SARS-CoV-2 is not performed. This is erroneous and likely to miss the true frequency of SARS-CoV-2 infection because it assumes mutual exclusivity of SARS-CoV-2. One report identified 24.5% of COVID-19 patients as having coinfection with other viruses.27 Hence, it is possible that many cases of COVID-19–related myocarditis were missed due to a lack of SARS-CoV-2 diagnosis.
Certain ethnic groups may be disproportionately affected by SARS-CoV-2. COVID-19 death rates were shown to be higher among the African American population than other ethnicities in many American states.28 Although this may partially be explained by the greater number of cardiovascular risk factors or the genetic predisposition to poorer cardiac outcomes,29 health care disparities cannot be dismissed. Bias in the health and care provisions may be the driving force behind disproportionate suffering in minorities.
Incidence and possible mechanism of arrhythmias in COVID-19–related myocarditis
Arrhythmia is recognized as one of the possible clinical manifestations of COVID-19 patients. One observational study of the clinical characteristics of COVID-19 patients in Hubei, China, reported a 7.3% incidence of heart palpitations among its 137 patients.30 Moreover, Wang et al31 reported that arrhythmia was a cause of intensive care unit transfer in 44.4% of COVID-19 patients. Caution is encouraged when interpreting these data, as the sample size tends to be small and hence prone to overestimation. The exact nature of the arrhythmias was not usually reported, so assessing whether the arrhythmias are secondary to other conditions such as electrolyte imbalance or pre-existing arrhythmias is difficult. Therefore, the actual prevalence of arrhythmias in COVID-19 patients remains unknown. Nevertheless, arrhythmias could occur in the context of myocarditis. Peretto et al32 reported in a recent study that 78.7% of myocarditis patients exhibited some form of ventricular arrhythmia. The characteristics of arrhythmias differ between active and healed myocarditis, suggesting that the pathophysiology is dependent on the stage of myocardial injury.32
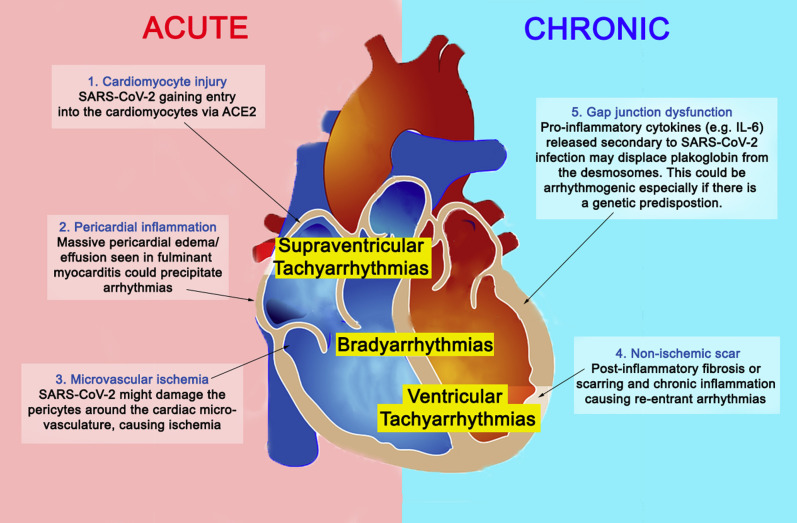
Concerning SARS-CoV-2, the possible pathophysiology of arrhythmias includes (1) direct injury to cardiomyocytes disrupting the plasma membrane and electrical conduction; (2) infection of the pericardium causing massive edema; (3) ischemia from microvascular disease due to possible infection of the pericytes33 , 34; (4) re-entrant arrhythmias due to myocardial fibrosis or scars; and (5) proinflammatory cytokines predisposing to arrhythmogenicity (Figure 2 ). Scenarios 1, 2, and 3 could occur in the acute setting, whereas scenarios 4 and 5 occur in chronic or healed myocarditis. In scenario 5, proinflammatory cytokines (eg, IL-6) might cause displacement of plakoglobin, a desmosomal protein, from the cardiomyocyte membrane.35 This could be arrhythmogenic, as inadequate cell-to-cell adherence is postulated to damage the cell membrane, leading to cardiac cell death and fibrofatty replacement.36 Moreover, reduced surface expression of desmosomal proteins is a known etiology of arrhythmogenic cardiomyopathies.36 Substantial evidence now suggests an increase in serum IL-6 in COVID-19 patients, especially in those with severe presentations.37 Therefore, it is plausible that SARS-CoV-2 infection precipitates arrhythmias in patients with a genetic predisposition. Clinicians should be vigilant for arrhythmias, especially in areas where both the COVID-19 burden and the arrhythmogenic cardiomyopathy prevalence are high, such as the North-Eastern (Veneto) region of Italy.36
Figure 2.
Arrhythmogenesis in SARS-CoV-2–related myocarditis. Possible mechanisms responsible for arrhythmias in SARS-CoV-2–related myocarditis are shown. Mechanisms 1, 2, and 3 could occur in the acute setting, whereas mechanisms 4 and 5 occur in chronic/healed myocarditis. Abbreviations as in Figure 1.
Diagnosing COVID-19–related myocarditis
Clinical presentation
Clinical presentation of SARS-CoV-2 myocarditis varies among cases. Some patients may present with relatively mild symptoms, such as fatigue and dyspnea,4 , 5 whereas others report chest pain or chest tightness on exertion.3 , 6 Many patients do deteriorate, showing symptoms of tachycardia and acute-onset heart failure with cardiogenic shock.3, 4, 5 In these severe cases, patients may also present with signs of right-sided heart failure, including raised jugular venous pressure, peripheral edema, and right upper quadrant pain.10 The most emergent presentation is fulminant myocarditis, defined as ventricular dysfunction and heart failure within 2–3 weeks of contracting the virus.8 The early signs of fulminant myocarditis usually resemble those of sepsis: the patient often presents febrile with low pulse pressure, cold or mottled extremities, and sinus tachycardia.10
Investigations
Results of blood tests from myocarditis patients often show elevated levels of lactate and other inflammatory markers, including C-reactive protein, erythrocyte sedimentation rate, and procalcitonin, which usually are raised in keeping with the clinical presentation of infection. It is critical to distinguish fulminant myocarditis from sepsis because fluid resuscitation, a common sepsis protocol, exacerbates fulminant myocarditis with fluid overload. We encourage testing patients for baseline cardiac enzymes (eg, troponin and N-terminal pro–B-type natriuretic peptide [NT-proBNP]) on hospital admission, as cardiac troponin I (cTnI), cardiac troponin T (cTnT), NT-proBNP, and BNP levels usually are elevated in myocarditis due to acute myocardial injury and possible ventricular dilation. Elevations of both troponin and NT-proBNP levels were observed in the COVID-19–related myocarditis cases.3, 4, 5, 6 Although a negative troponin result cannot exclude myocarditis, particularly for atypical forms such as giant cell myocarditis or for those patients in the chronic phase, negative serial high-sensitivity cardiac troponin still is helpful in the acute phase and makes diagnosis of acute myocarditis significantly less likely. In COVID-19 patients, the (NT-pro)BNP level also could increase secondary to myocardial stress, a possible knock-on effect from severe respiratory illness.38
Electrocardiogram (ECG) abnormalities commonly seen with pericarditis, such as ST elevation and PR depression, may be observed in myocarditis9; however, these findings are not sensitive in detecting the disease and their absence is not exclusionary. For example, one COVID-19–related myocarditis case showed neither ST elevation nor PR depression.3 Other ECG abnormalities, including new-onset bundle branch block, QT prolongation, pseudoinfarct pattern, premature ventricular complexes, and bradyarrhythmia with advanced atrioventricular nodal block, can be observed in myocarditis.
Differential diagnoses
Acute coronary syndrome
In the context of raised cardiac troponin levels, the acute coronary syndrome is highly suspicious, but epicardial disease can be ruled out by coronary angiography. However, many COVID-19 patients were reported to have a detectable level of cTnI, even when they had no overt cardiac symptoms38 and the finding does not generally represent type 1 myocardial infarction.39 It is possible that the raised troponin level is a result of an exacerbation of the patient’s subclinical coronary artery disease by sepsis, which increases cardiac oxygen demand. This worsens oxygen supply–demand mismatch, which could precipitate ischemia that results in type 2 myocardial infarction. Serial cardiac biomarkers can help detect myocardial injury, especially in the event of rising biomarker trend.
Sepsis-related cardiomyopathy
A case series study showed that 67% of critically ill COVID-19 patients required vasopressor and that 33% developed cardiomyopathy.40 This raises suspicion of sepsis-related cardiomyopathy, a disease characterized by reversible myocardial dysfunction. The myocardial injury is thought to arise from increased nitric oxide production, which suppresses the cardiomyocyte’s response to calcium and downregulates the heart’s β1-adrenergic receptors.41 The 3 cardinal signs of sepsis-related cardiomyopathy are (1) left ventricular dilation; (2) impaired ejection fraction; and (3) recovery in 7–10 days.41
Stress-induced cardiomyopathy (Takotsubo cardiomyopathy)
Takotsubo cardiomyopathy is a nonischemic cardiomyopathy characterized by transient weakening of the cardiomyocytes and subsequent ballooning of the apex. Its clinical manifestations mimic those of acute coronary syndrome (eg, chest pain, ECG abnormalities, and elevations of cardiac biomarkers); however, Takotsubo cardiomyopathy usually is preceded by an emotional or physical stressor.42 Of note, a case report described a COVID-19 patient who initially presented as having reverse Takotsubo cardiomyopathy, a variant form of Takotsubo cardiomyopathy.6
Diagnostic evaluation for COVID-19–related myocarditis
The American Heart Association (AHA) recommends further testing for patients having signs consistent with myocarditis with 1 or more cardiac imaging methods such as echocardiogram or cardiovascular magnetic resonance (CMR).10 The echocardiogram usually is more readily deployed because it is portable. Especially under time and resource constraints, devices such as the hand-held, point-of-care ultrasound machine can be advantageous in terms of accessibility and the relative ease of disinfecting the device for infection control. The cardinal signs of myocarditis on echocardiogram are increased wall thickness, chamber dilation, and pericardial effusion in the background of ventricular systolic dysfunction. Although CMR offers major imaging advantages over echocardiography, it is limited by out-of-hours availability, the requirement for some breathholding, slower throughput, and, given the high contagiousness of COVID-19, the requirement for deep cleaning after use. If CMR is performed, the results should be interpreted according to the revised Lake Louise consensus criteria43: (1) edema; (2) irreversible cell injury; and (3) hyperemia or capillary leak. Figures 3 A through 3F show the typical CMR findings of myocarditis. CMR myocardial edema and/or scarring were observed in all of the SARS-CoV-2–related myocarditis cases for which CMR results were reported.4, 5, 6 If CMR proves prohibitive, cardiac computed tomography (CT) scan with contrast enhancement and ECG gating is an effective alternative, especially when the patient is to undergo high-resolution CT scan of the chest for assessment of acute respiratory distress syndrome (Figure 3J). Contrast-enhanced cardiac CT will add very little scanning time and in this situation is particularly useful to perform a test rapidly with minimal requirement for breathholding.44 Without CMR or contrast-enhanced CT results, distinguishing myocarditis from other differential diagnoses is difficult.
Figure 3.
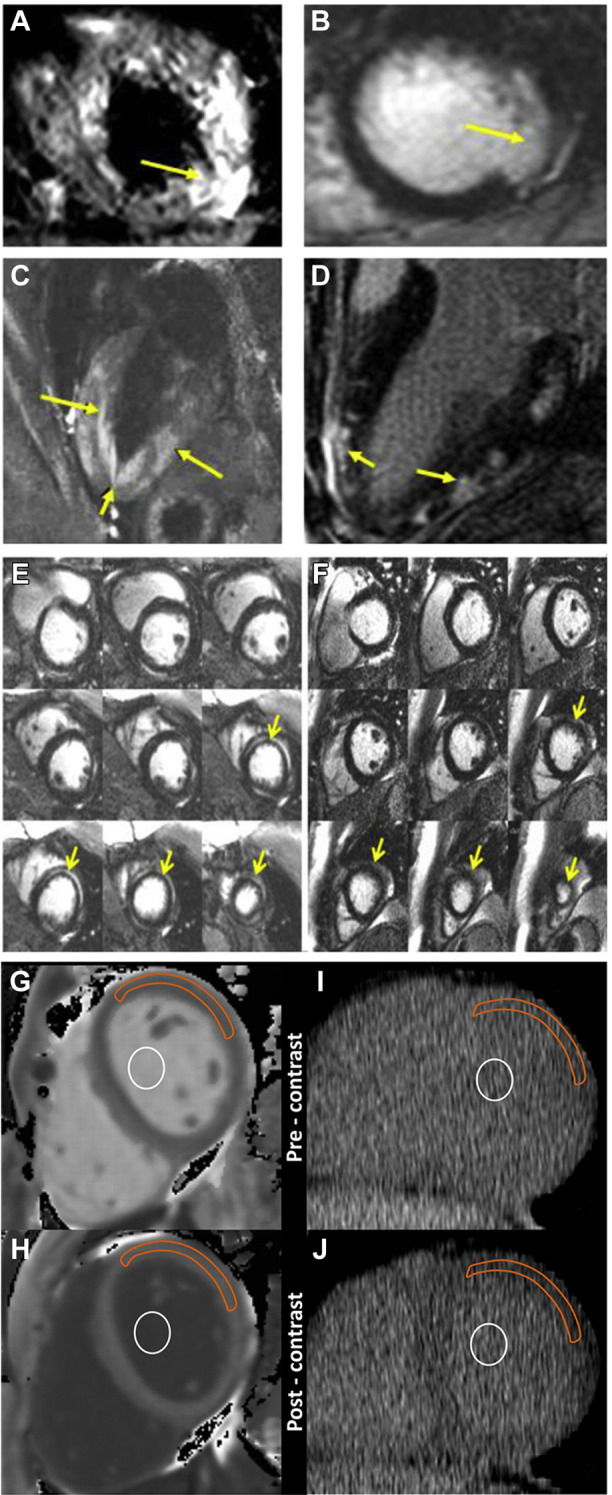
Typical cardiovascular magnetic resonance (CMR) and computed tomography (CT) findings of myocarditis. A, C: Cardiac edema (yellow arrows) in T2-weighted mode. B, D: LGE of the subepicardial region (yellow arrows) of the ventricles, a sign of myocardial fibrosis or scarring. E: Midmyocardial LGE (yellow arrows) is often present in the acute setting and resolved at follow-up. F: LGE resolved in the chronic case. These areas may initially represent acute myocardial edema (yellow arrows) and resolve over time. G, H: CMR imaging of region-of-interest measurements obtained before (G) and after (H) gadolinium chelate administration. I, J: Reformatted cardiac CT of region-of-interest measurements obtained before (I) and after (J) administration of an iodinated contrast agent. For cardiac CT, the anterolateral myocardium was most reliably identified before administration of an iodinated contrast agent. In that area, a region of interest from the anterolateral myocardium was used for attenuation measurements. A focal myocardial scar was identified on delayed CMR images and was not included in the region of interest. Orange outline indicates myocardium; white circle indicates blood pool. LGE = late gadolinium enhancement. A-F courtesy of Dr Raymond Y. Kwong (Brigham and Women's Hospital), G-J reprinted from Nacif et al44, Radiology, 2012, vol 264, p.876-883, with permission from RSNA.
Both the AHA and European Society of Cardiology (ESC) recommend endomyocardial biopsy (EMB) as the definitive diagnostic tool for myocarditis, but both societies recognize the limitations of EMB in terms of expertise required, contagious spread risk, and false-negative rate.9 , 10 If obtained, EMB samples should be immunohistochemically tested for inflammatory infiltrates and RNA/DNA extraction performed to test for the presence of viral genomes.45 Regarding the contagious spread risk, should coronary angiography be deemed necessary, it is reasonable to perform the right heart procedures and/or EMB concomitantly as these will add minimal risk while adding 15 minutes to the procedure time. EMB also serves as an opportunity for accurate diagnosis and provides tissues for the development of specific biomarkers, which could be useful for developing a diagnostic test for SARS-CoV-2 myocarditis. In the event of patient death, we suggest that an autopsy, including the heart, be performed because it will allow observation of the gross and microscopic pathology of the heart, including multiple left and right ventricular segments. This may illuminate the new biological pathways for COVID-19 treatments—knowledge that is critically needed and will bring immense societal benefit in this trying time.
Chronic complications of COVID-19–related myocarditis
While the acute inflammation and injury to the heart are the current focus receiving attention, the long-term effects of healed myocarditis are completely unknown. Most infected patients experience mild, self-limiting symptoms; are managed in the community; and are not undergoing clinical testing such as ECG or cardiac imaging. Because the emphasis is evaluating and admitting patients with severe lower respiratory tract symptoms, many patients with possible myocarditis will never be evaluated. Some of these patients may survive the acute event but may be at risk for subsequent arrhythmias. In a study of patients with active and healed myocarditis, monomorphic ventricular tachycardia and regular ventricular arrhythmias were more frequent in those with healed than acute myocarditis.32 However, the presence of viral genomes on EMB was not associated with the occurrence of malignant arrhythmias.32
Management of COVID-19–related myocarditis and arrhythmias
Managing myocarditis
The AHA recently published a scientific statement on the recognition and initial management of fulminant myocarditis.10 It recommends implementing the initial management protocol for cardiogenic shock in patients with fulminant myocarditis. This includes administration of inotropes and/or vasopressors and mechanical ventilation. Longer-term management involves mechanical circulatory support such as extracorporeal membrane oxygenation, ventricular assist device, or intra-aortic balloon pump. The ESC did not endorse the use of intravenous immunoglobulin due to a lack of supporting evidence,9 and it discouraged the use of corticosteroids in active-infection myocarditis, citing the ineffectiveness of corticosteroids in a randomized controlled trial.46
Case reports of coronavirus-related myocarditis provide a glimpse into the efficacy of some of the aforementioned treatments. Online Table 1 summarizes 9 case reports of patients with coronavirus-related myocarditis. Mechanical circulatory support was deployed in 2 of 7 cases (for which treatment modalities were reported).3 , 47 Presumably, in a more hemodynamically stable case, medical treatment consisting of inotropes or vasopressors was sufficient to mitigate the ventricular systolic dysfunction.4 , 13 , 48 In some cases, immunomodulatory treatments were given. Zeng et al3 and Hu et al48 reported the use of corticosteroid and intravenous immunoglobulin. A recent meta-analysis on corticosteroid and intravenous immunoglobulin use in pediatric myocarditis concluded that intravenous immunoglobulin may improve ventricular systolic function but failed to find support for corticosteroid use.49 However, immunosuppression might pose a risk for more severe clinical disease, especially in the presence of active viral replication. Therefore, it is reasonable to withhold or minimize the use of immunosuppression in SARS-CoV-2 patients, especially in the setting of a positive viral genome on EMB. Tocilizumab, an anti–IL-6 receptor monoclonal antibody, is now being tested in a multicenter randomized controlled trial that recruits COVID-19 patients with raised IL-6 levels.50 This antibody might be beneficial in the setting of cytokine storm syndrome and help reduce myocardial inflammation.
Managing arrhythmias
Managing arrhythmias is crucial in mitigating a patient’s adverse health outcomes. Cardiac monitoring is advised to enable appropriate therapy for brady- and tachyarrhythmias, including atrioventricular block, and ventricular tachycardia or fibrillation. Bradyarrhythmias may require temporary cardiac pacing, and tachyarrhythmias may respond to antiarrhythmic drugs (eg, lidocaine and mexiletine) and overdrive pacing. Some centers have commenced prescription of antimalarials and macrolides, which are known to prolong the QTc interval. Caution must be taken with concomitant use of antiarrhythmic drugs.51
Cardiovascular considerations regarding COVID-19 therapeutics
Several potential pharmacologic candidates being repurposed for use in COVID-19 patients are under investigation. Currently, chloroquine is under a phase IIb clinical trial to assess its efficacy in treating COVID-19 patients with severe respiratory syndrome.52 Both chloroquine and its derivative, hydroxychloroquine, may cause QTc interval prolongation; however, their effects seem to be modest.51 Nevertheless, (hydroxy)chloroquine requires metabolism by the CYP3A4 enzyme,53 whose inhibition might raise the drug’s plasma concentration, accentuating the long QT risk. Many pharmacologic agents used empirically to treat COVID-19, including ritonavir/lopinavir and azithromycin, are known CYP3A4 inhibitors; hence, their combination therapy with (hydroxy)chloroquine should be accompanied by QTc interval monitoring.51
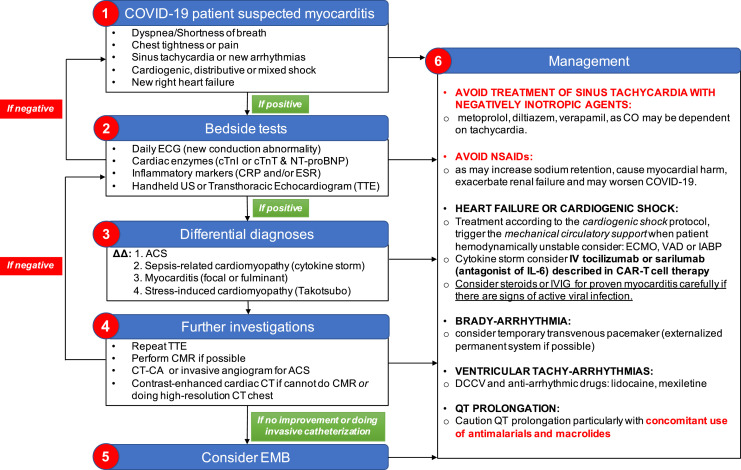
Given that SARS-CoV-2 binds to ACE2 to gain host cell entry, there is ongoing debate on whether renin-angiotensin-aldosterone system (RAAS) antagonists should be used in COVID-19 patients. Some argued that the blockade might offer clinical benefit,54 whereas others queried the possible upregulation of ACE2 as a consequence of such blockades. However, according to the current clinical evidence, the Heart Failure Society of America, the American College of Cardiology, and AHA advise continuing the RAAS antagonist regimen if prescribed for their approved indications, even if the patient contracts COVID-19 later.55 Both the AHA10 and ESC9 advised against the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in myocarditis patients because they are the known cause of renal impairment and sodium retention, which could exacerbate acute ventricular dysfunction. Our recommendation for the diagnosis and management of SARS-CoV-2–related myocarditis is summarized in Figure 4 .
Figure 4.
Suggested diagnostic and management protocol for SARS-CoV-2–related myocarditis. ΔΔ = differential diagnoses; ACS = acute coronary syndrome; CAR = chimeric antigen receptor; CMR = cardiovascular magnetic resonance; CO = cardiac output; COVID-19 = coronavirus 19; CRP = C-reactive protein; CT = computed tomography; CT-CA = computed tomography–coronary angiogram; cTnI = cardiac troponin I; cTnT = cardiac troponin T; DCCV = direct current cardioversion; ECG = electrocardiogram; ECMO = extracorporeal membrane oxygenation; EMB = endomyocardial biopsy; ESR = erythrocyte sedimentation rate; IABP = intra-aortic balloon pump; IL-6 = interleukin 6; IV = intravenous; IVIG = intravenous immunoglobulin; NSAID = nonsteroidal anti-inflammatory drug; NT-proBNP = N-terminal pro–B-type natriuretic peptide; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TTE = transthoracic echocardiogram; US = ultrasound; VAD = ventricular assist device.
Conclusion
Several cases of coronavirus-related myocarditis have been reported. Its pathophysiology likely is a combination of the direct viral insult to cardiomyocytes and the human’s immune response to virally infected myocardium. Simple bedside tests such as serial ECG and cardiac biomarkers can raise suspicion of acute-onset cardiac symptoms. Particular attention should be given to biomarkers changes or trends and not just readings obtained in isolation. Cardiac imaging techniques such as echocardiography and CMR can be used to aid diagnosis; however, distinguishing between differential diagnoses of stress-induced cardiomyopathy, sepsis-related cardiomyopathy, and acute coronary syndrome can be difficult. An invasive coronary angiogram will often be warranted, especially in older patients. The definitive diagnosis of myocarditis is obtained via EMB, and if an invasive catheterization is to be performed, concomitant EMB would add little time and no further risk of infection spread vs catheterization alone. For patients who cannot undergo CMR, contrast-enhanced cardiac CT is a swift, reproducible, precise, and reliable alternative that can be added to the sequences for high-resolution CT of the lungs to evaluate the acute respiratory distress syndrome. Initial treatment of fulminant myocarditis should follow the cardiogenic shock protocol, which includes the use of inotropes or vasopressors and mechanical ventilation. Arrhythmias can be managed by temporary cardiac pacing or antiarrhythmic medications. Then, depending on severity, the patient may require mechanical circulatory support. Cautions must be taken for the use of the NSAIDs and QTc-prolonging drugs in COVID-19 patients because these medications might exacerbate cardiac symptoms.
Footnotes
This contemporary review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr Nazarian has been a consultant for CardioSolv and Circle Software; and has received grants from SIEMENS, Imricor, Biosense Webster, and the National Institutes of Health (NIH). All other authors have reported that they have no conflicts relevant to the contents of this paper to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2020.05.001.
Appendix. Supplementary data
Supplementary Figure 1.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. Covid-19: death rate is 0.66% and increases with age, study estimates. BMJ. 2020;369:m1327. doi: 10.1136/bmj.m1327. [DOI] [PubMed] [Google Scholar]
- 3.Zeng J.-H., Liu Y.-X., Yuan J. First case of COVID-19 infection with fulminant myocarditis complication: case report and insights. Published online April 10. Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim I.-C., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esfandiarei M., McManus B.M. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 9.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 10.Kociol R.D., Cooper L.T., Fang J.C. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komarowska I., Coe D., Wang G. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes t cell migration to the heart via autocrine chemokine release. Immunity. 2015;42:1087–1099. doi: 10.1016/j.immuni.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantreuil J., Favrais G., Soule N. Tachycardie atriale chaotique au cours d’une infection respiratoire à coronavirus NL63. Arch Pediatr. 2013;20:278–281. doi: 10.1016/j.arcped.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riski H., Hovi T., Frick M.H. Carditis associated with coronavirus infection. Lancet. 1980;2:100–101. doi: 10.1016/S0140-6736(80)92989-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrawal A.S., Garron T., Tao X. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaecher S.R., Stabenow J., Oberle C. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology. 2008;380:312–321. doi: 10.1016/j.virol.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa K., Narayanan K., Wada M., Makino S. Inhibition of stress granule formation by Middle East respiratory syndrome coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J Virol. 2018;92:e00902–e00918. doi: 10.1128/JVI.00902-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanan K., Huang C., Lokugamage K. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol. 2008;82:4471–4479. doi: 10.1128/JVI.02472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Z., Travanty E.A., Oko L. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J., Wei X., Li Q. Single-cell RNA analysis on ACE2 expression provides insight into SARS-CoV-2 blood entry and heart injury. Preprint. Posted online April. 2020;4 doi: 10.1101/2020.03.31.20047621. medRxiv 2020.03.31.20047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S.R. Forty years with coronaviruses. J Exp Med. 2020;217 doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah N. Higher co-infection rates in COVID19. March 18. 2020. https://medium.com/@nigam/higher-co-infection-rates-in-covid19-b24965088333
- 28.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 29.Myers V.D., Gerhard G.S., McNamara D.M. Association of variants in BAG3 with cardiomyopathy outcomes in African American individuals. JAMA Cardiol. 2018;3:929–938. doi: 10.1001/jamacardio.2018.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peretto G., Sala S., Rizzo S. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Peretto G., Sala S., Rizzo S. Arrhythmias in myocarditis: state of the art. Heart Rhythm. 2019;16:793–801. doi: 10.1016/j.hrthm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asimaki A., Tandri H., Duffy E.R. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:743–752. doi: 10.1161/CIRCEP.111.964890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gemayel C., Pelliccia A., Thompson P.D. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2001;38:1773–1781. doi: 10.1016/s0735-1097(01)01654-0. [DOI] [PubMed] [Google Scholar]
- 37.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Preprint. Posted online April. 2020;3 doi: 10.1101/2020.03.30.20048058. medRxiv 2020.03.30.20048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Januzzi J.L. Troponin and BNP use in COVID-19. Cardiology Magazine. March. 2020;18 https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19 [Google Scholar]
- 39.Clerkin K.J., Fried J.A., Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 40.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato R., Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48. doi: 10.1186/s40560-015-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelliccia F., Kaski J.C., Crea F., Camici P.G. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nacif M.S., Kawel N., Lee J.J. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264:876–883. doi: 10.1148/radiol.12112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leone O., Veinot J.P., Angelini A. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Mason J.W., O’Connell J.B., Herskowitz A. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 47.Rao S., Sasser W., Diaz F., Sharma N., Alten J. Coronavirus associated fulminant myocarditis successfully treated with intravenous immunoglobulin and extracorporeal membrane oxygenation. Chest. 2014;146:336A. [Google Scholar]
- 48.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Yu Y., Chen S., Liao Y., Du J. Corticosteroids and intravenous immunoglobulin in pediatric myocarditis: a meta-analysis. Front Pediatr. 2019;7:342. doi: 10.3389/fped.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease. 2019. https://clinicaltrials.gov/ct2/show/NCT04310228
- 51.Wu C.I., Postema P.G., Arbelo E. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA network open NLM (Medline) 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K.A., Park J.Y., Lee J.S., Lim S. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res. 2003;26:631–637. doi: 10.1007/BF02976712. [DOI] [PubMed] [Google Scholar]
- 54.Sun M.L., Yang J.M., Sun Y.P., Su G.H. [Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:219–222. doi: 10.3760/cma.j.issn.1001-0939.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 55.HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. ACC New Story. March 16, 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.