Abstract
Introduction
On March 11, 2020, the novel coronavirus was declared a global pandemic. The disease was named COVID-19 standing for coronavirus disease 2019. The objectives were to determine the epidemiological, clinical, laboratory, and radiological characteristics of COVID-19 patients.
Methods
In this prospective descriptive study, 15 confirmed hospitalized cases of COVID-19 between 18th March and April 7, 2020 were followed-up till discharge.
Results
There were 15 reported patients infected by 3 imported index cases from Europe. The mean age of the patients was 28.06 (SD: 16.42 years). The patients’ age stratification was as follows: 0–5 (2, 13.3%); 6–18 (2, 13.3); 19–50 (10, 66.7%), and 51–64 years (1, 6.7%). The patients were male (9, 60.0%) and female (6, 40.0%). Most of the patients had mild disease severity (13, 86.7%), followed by mild-moderate (1, 6.7%) and moderate-severe (1, 6.7%). The study revealed that 6 patients were asymptomatic, and 9 patients were symptomatic. The most common symptoms were: fever (n = 8; 53.3%), cough (n = 7; 46.7%), shortness of breath (n = 3; 20.0%), fatigue (n = 3; 20.0%), and taste and smell disorders (n = 4; 26.7%). All patients were recovered and discharged over a median of 8 between 8 and 21 days. The mean and Std. deviation values of the hematological were: WBC: 6.57 (1.86); neutrophil count: 3.75 (1.26); lymphocyte count: 1.87 (0.41); Hb: 13.89 (1.26); platelet count: 207.67 (52.21).
Conclusion
All COVID-19 cases were linked to foreign visits with few local transmissions to close contacts without community transmission. The majority of cases were mild illnesses with full recovery.
Keywords: Infection, Respiratory disorders, Clinical manifestations
1. Introduction
On March 11, 2020, the novel coronavirus was declared a global pandemic by the World Health Organization (WHO). The disease was later named COVID-19 standing for coronavirus disease 2019 [1]. Most countries were affected by the disease and Iraqi Kurdistan was not an exempt. The rising cases of COVID-19 in European countries and a neighboring country, Iran had been alarming. Therefore, an action was carried out, especially after the first case was reported in Iran with an increasing trend after February 2020 [2]. The Kurdistan Regional Government (KRG) dispatched health measures for travelers and raised awareness in the general population. Hence, public and private schools and universities were announced to be closed from February 26 until May 2, 2020. Curfew was imposed on March 13 until April 23, 2020. Any traveler arriving from an epidemic area of COVID-19 is quarantined for at least 14 days and tested by molecular assay for the novel virus [3].
The disease is caused by a variant virus with an incompletely understood clinical course [4]. The frequent clinical manifestations are fever, dry cough, shortness of breath (SOB), and pneumonia; whereas less frequent features are headache, diarrhea, productive cough, runny nose, and hemoptysis [5]. Overall, the clinical features of the disease range from mild to severe illnesses. The severe form occurs in patients with risk factors such as old age (≥65 years), smokers, and comorbid diseases e.g. diabetes mellitus, hypertension … etc. [6]. The median incubation period of the disease is 5–6 days; however, it can take up to 24 days [7]. Although the transmission may occur early in the course of the disease, the period of infectivity is uncertain [8]. The spread of the virus has great epidemiological concerns; hence preventing the transmission is the primary goal toward controlling the disease. The COVID-19 patients may show varying degrees of laboratory abnormalities e.g. leukopenia, leukocytosis, lymphopenia … etc [9]. The chest x-ray (CXR) and CT scan findings are usually normal in early stages of the disease; however, it may show bilateral infiltrates and ground glass opacity in late stages and more severe forms [9,10].
There are several studies about COVID-19 from countries over all continents characterizing the nature of the disease; however, no studies were reported from Iraq. Hence, we present the first report from Duhok, Iraqi Kurdistan to determine the epidemiological, clinical, laboratory, and radiological characteristics of COVID-19 patients.
2. Patients and methods
2.1. Setting
The Burn and Plastic Surgery hospital was allocated for COVID-19 in early March 2020, hence named Corona hospital. It has 50 beds, some equipped with ventilators. The hospital is admitting all confirmed cases of COVID-19 regardless of the disease’s severity. The hospital is not intended for suspected cases.
2.2. Study design and patients
In this prospective descriptive study, a total of 15 cases diagnosed with COVID-19 in Duhok were reported. The patients were Kurdish and diagnosed between 18th March and April 7, 2020. The patients were diagnosed based on the WHO interim guidance for COVID-19 [11].
2.3. Classification of the disease severity
The criteria for severity of COVID-19 were defined according to the diagnosis and treatment protocol for novel coronavirus pneumonia (Version 7) as mild, moderate, severe, and critical [12].
Mild cases: The clinical symptoms were mild with no sign of pneumonia on imaging.
Moderate cases: The patient shows fever and respiratory symptoms with radiological findings of pneumonia.
Severe cases:
Adult cases have any of the following criteria:
-
(1)
Respiratory distress (≧30 breaths/min);
-
(2)
Oxygen saturation≤93% at rest;
-
(3)
Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2)≦300 mmHg (l mmHg = 0.133 kPa).
In high-altitude areas (at an altitude of over 1000 m above the sea level), PaO2/FiO2 shall be corrected by the following formula:
| PaO2/ FiO2 x[Atmospheric pressure (mmHg)/760] |
Cases with chest imaging with obvious lesion progression within 24–48 h > 50% are managed as severe cases.
Child cases with any of the following criteria:
-
(1)
Tachypnea (RR ≥ 60 breaths/min for infants aged < 2 months; RR ≥ 50 BPM for infants aged 2–12 months; RR ≥ 40 BPM for children aged 1–5 years, and RR ≥ 30 BPM for children above 5 years old) independent of fever and crying;
-
(2)
Oxygen saturation ≤ 92% on finger pulse oximeter taken at rest;
-
(3)
Labored breathing (moaning, nasal fluttering, and infrasternal, supraclavicular and intercostal retraction), cyanosis, and intermittent apnea;
-
(4)
Lethargy and convulsion;
-
(5)
Difficulty feeding and signs of dehydration.
Critical cases
Cases with any of the following criteria:
Respiratory failure and requiring mechanical ventilation.
Shock.
With other organ failure that requires ICU care.
2.4. Management
The included patients had different stages of illnesses. The patients in mild stage had normal CXR at admission (n = 13; 86.67%). One patient had mild to moderate disease (6.7%), which showed bilateral pulmonary infiltrate in lower zones of the lungs, particularly the right side. Whereas another had moderate to severe pneumonia that had bilateral pulmonary infiltrates on CXR, and the CT scan showed diffused bilateral multi-lobar peripheral ground glass and consolidative opacity lesions in both lungs.
The treatment of the cases was performed according to the medical regulation issued by the Ministry of Health of KRG numbered 4504 on February 30, 2020. The treatment regimens and their indications are shown below:
Two drugs regimen (COVID-19 patient without pneumonia in the ward): Hydroxycholoquine 400 mg po bid on the first day, then 200 mg po bid for 5 days + Azithromycin 500 mg po for the first day, then 250 mg po qd for 5 days.
Three drugs regimen (COVID-19 patient with pneumonia in the ward): Hydroxycholoquine 400 mg po bid on the first day, then 200 mg po bid for 14 days + Azithromycin 500 mg po on the first day, then 250 mg po qd for 14 days + Oseltamivir 75 mg po bid for 5 days.
Four drugs regimen (COVID-19 patient with pneumonia in the ICU): Hydroxycholoquine 400 mg po bid on the first day, then 200 mg po bid for 14 days + Azithromycin 500 mg po on the first day, then 250 mg po qd for 14 days + Oseltamivir 75 mg po bid for 5 days + Kaletra (Lopinavir/Ritonavir [200/50 mg]) po 2 tablets bid for 5 days. Antibiotics can be added accordingly.
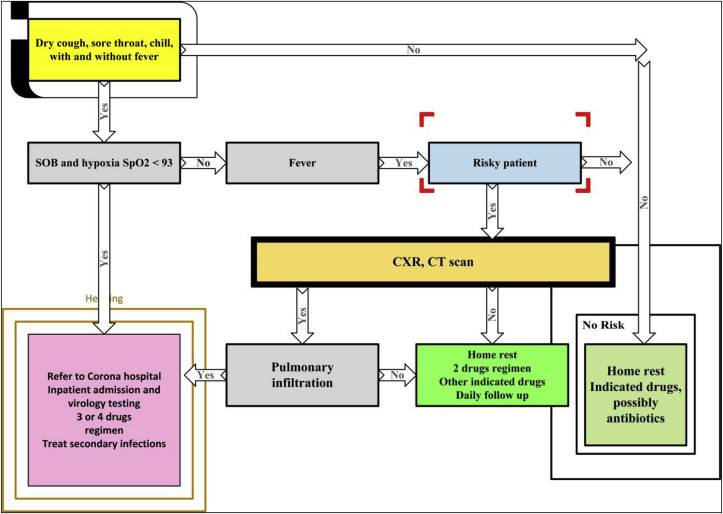
There is a local guide called “Management Guidelines of COVID-19 Patients: Outpatient and Inpatient” prepared by Dr. Muayad A. Merza, an infectious disease specialist (Fig. 1 ). If the number of patients exceeds the capacity of Duhok’s health facilities, the local guide will be in use.
Fig. 1.
Schematic guide of managing COVID-19 patients [designed by Dr. Muayad A. Merza].
The diagnostic tests were performed for each patient several times. The RT-PCR diagnostic tests were performed based on their mean recovering time. The patients were discharged from the hospital when there were clinical improvements and evidence of viral clearance over RT-PCR (two negative results at least 24 h apart). The patients were trained for the COVID-19 symptoms for a possible recurrence. They were informed to call the hotline number in case of any recurrent symptoms. We did not have any cases with recurrent symptoms by April 19, 2020. Out of 15 patients, 12 were re-tested by RT-PCR after 14 days of discharge and their results were negative. The other 3 patients will be re-tested after 14 days post-discharge.
2.5. Role of the funding source
The study was not funded by any organization. In addition, no agent had a role in the design, data curation, and designing the study draft. The corresponding author has full access to the data.
2.6. Statistical methods
The raw information of the patients was entered into statistical package for social sciences version 25 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp) for statistical calculations. Over time, the trace of COVID-19 patients was presented in a graph by Microsoft Excel 2013. The general and clinical characteristics of the patients were presented in mean (Std. deviation) or number (percentage). The rate of clinical symptoms, outcomes, and complications was determined in number and percentage. The time of disease diagnosis to discharge was determined in the median and interquartile range. The comparison of leukocyte, lymphocyte, and CRP in patients with different disease stages was presented in dot plots.
2.7. Ethical view
The ethical approval of the present protocol was obtained from the local Directorate of Health. The confidentiality of the personal information of patients was protected throughout the study steps.
3. Results
The first infected case had a travel history to Germany. This patient did not strictly follow quarantine measures when returning to Duhok. The first case infected three other people in the region through close contacts (patients 2–4). The cases were identified 4 (patient 2) and 13 days (patient 3–4) following the first case. The fifth infected case had a travel history to the United Kingdom (UK). He was diagnosed positive on March 26, 2020. His family members were diagnosed with Covid-19 the following day. Three relatives of this family were infected through family contact between March 28 and 31, 2020. The patients 13–15 also had a travel history to UK. The identified cases were confirmed by RT-PCR (Table 1 , Fig. 1).
Table 1.
General information, source of infection, and diagnosis date of COVID-19 confirmed cases.
| Case Summaries |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient (n = 15) | Age | Gender | Chronic Illness |
Smoker | Alcohol | Exposure/Setting | Diagnosis date | Stage | Hospital stay (days) |
| Patient 1 | 50 | M | No | No | Yes | Germany (Index 1) | 18 Mar | Moderate-Severe | 21 |
| Patient 2 | 20 | F | No | No | No | Close contact with index 1 | 23 Mar | Mild | 12 |
| Patient 3 | 42 | M | No | Yes | Yes | Close contact with index 1 | 31 Mar | Mild | 8 |
| Patient 4 | 28 | M | No | Yes | No | Close contact with index 1 | 31 Mar | Mild | 8 |
| Patient 5 | 45 | M | Yes | Yes | No | Index 2 (UK) | 26 Mar | Mild | 9 |
| Patient 6 | 40 | F | No | No | No | Family member of Index 2 | 27 Mar | Mild | 8 |
| Patient 7 | 15 | M | Bronchitis asthma | No | No | Family member of Index 2 | 27 Mar | Mild | 8 |
| Patient 8 | 13 | F | No | No | No | Family member of Index 2 | 27 Mar | Mild | 8 |
| Patient 9 | 5 | M | Bronchitis asthma | No | No | Family member of Index 2 | 27 Mar | Mild | 8 |
| Patient 10 | 21 | M | Normal | No | No | Close contact with index 2 | 28 Mar | Mild | 8 |
| Patient 11 | 20 | M | Normal | No | No | Close contact with index 2 | 28 Mar | Mild | 8 |
| Patient 12 | 56 | F | Normal | No | No | Close contact with index 2 | 31 Mar | Mild | 8 |
| Patient 13 | 33 | M | Normal | No | No | Index 3 (UK) | 07 Apr | Mild | 11 |
| Patient 14 | 32 | F | Normal | No | No | Index 3 (UK) | 07 Apr | Mild-moderate | 13 |
| Patient 15 | 10 | F | Normal | No | No | Index 3 (UK) | 07 Apr | Mild | 13 |
Comment: All patients have received BCG vaccine.
∗10 months.
The mean age of the patients was 28.06 (SD: 16.42 years) ranged between 10 months and 56 years. The patients’ age stratification was as follows: 0–5 (2, 13.3%); 6–18 (2, 13.3); 19–50 (10, 66.7%), and 51–64 years (1, 6.7%). The patients were male (9, 60.0%) and female (6, 40.0%). Most of the patients had mild disease severity (13, 86.7%), followed by mild-moderate (1, 6.7%) and moderate-severe (1, 6.7%). The study revealed that 6 patients were asymptomatic. The remaining nine patients had the following symptoms; fever (n = 8), cough (n = 7), shortness of breath (n = 3), fatigue (n = 3), sore throat (n = 1), chill (n = 1), and rhinorrhea (n = 2). Four patients had other symptoms, of whom 2 had both taste and smell disorder, and the other 2 with taste disorder only (Table 2 , Fig. 2 ).
Table 2.
General and clinical characteristics of COVID-19 patients.
| Patients’ characteristics (n = 15) | Statistics |
|
|---|---|---|
| Mean | Std. Deviation | |
| Age (8 months-56 years) | 28.06 | 16.42 |
| Number | Percentage | |
| Age categories n (%) | ||
| 0–5 years | 2 | 13.3 |
| 6–18 years | 2 | 13.3 |
| 19–50 years | 10 | 66.7 |
| 51–64 years | 1 | 6.7 |
| Gender | ||
| Male | 9 | 60.0 |
| Female | 6 | 40.0 |
| Disease Stage | ||
| Mild | 13 | 86.7 |
| Mild-Moderate | 1 | 6.7 |
| Moderate-Severe | 1 | 6.7 |
| Clinical Symptoms n (%) | ||
| Fever | 8 | 53.3 |
| Cough | 7 | 46.7 |
| Shortness of breath | 3 | 20.0 |
| Fatigue | 3 | 20.0 |
| Sore throat | 1 | 6.7 |
| Chill | 1 | 6.7 |
| Rhinorrhea | 2 | 13.3 |
| Other symptoms (Taste and smell disorders) | 4 | 26.7 |
| Type of infection, n (%) | ||
| Symptomatic | 9 | 60.0 |
| Asymptomatic | 6 | 40.0 |
| Clinical outcome, n (%) | ||
| Recovered and discharged | 15 | 100 |
| Died in hospital | 0 | 0.0 |
| Admitted to ICU | 0 | 0.0 |
| Required ICU care | 0 | 0.0 |
| Required assisted ventilation | 0 | 0 |
| Complications related to Covid-19 | ||
| Acute lung injury/ARDS | 0 | 0.0 |
| Acute kidney injury | 0 | 0.0 |
| Liver dysfunction | 0 | 0.0 |
| Rhabdomyolysis | 0 | 0.0 |
| Pneumothorax | 0 | 0.0 |
| Arrhythmias | 0 | 0.0 |
| Sepsis | 0 | 0.0 |
| Seizures | 0 | 0.0 |
| Time from illness diagnosis to discharge (Range: 8–21 days) | Median: 8.0 | Interquartile range: 3.25 |
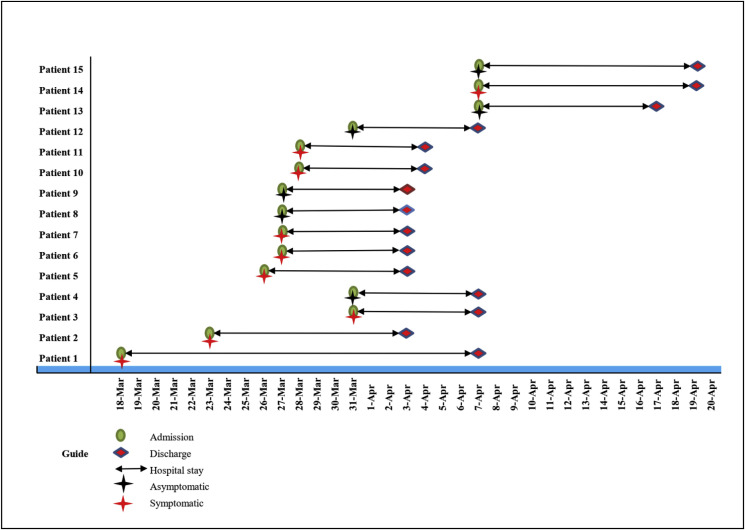
Fig. 2.
Timeline of patients with COVID-19 from admission to discharged a
) Comparison of WBC count in patients with different disease stages. b
) Comparison of lymphocyte count in patients with different disease stages. c
) Comparison of CRP in patients with different disease stages.
All patients were recovered and discharged over a median of 8, between 8 and 21 days. Only one patient (first patient) was discharged after 21 days due to moderate to severe illness and delayed viral clearance. No patient died in the hospital and no complication related to COVID-19 was found in the admitted patients. In addition, no patient was admitted to the ICU. We did not observe any of the following complications by the discharge date: Acute Respiratory Distress Syndrome (ARDS), acute kidney injury, liver dysfunction, rhabdomyolysis, pneumothorax, arrhythmias, Sepsis, and seizures (Table 2, Fig. 2).
The findings of medical laboratory of the patients were presented in Table 3 . The mean and Std. deviation values of the hematological were: WBC: 6.57 (1.86), neutrophil count: 3.75 (1.26), lymphocyte count: 1.87 (0.41), Hb: 13.89 (1.26), and platelet count: 207.67 (52.21). Serum procalcitonin was measured for the moderate-severe case only (first case), which was negative.
Table 3.
Laboratory findings of COVID-19 patients.
| Lab investigations (n = 15) | Statistics |
||
|---|---|---|---|
| Range | Mean | Std. Deviation | |
| WBC (10⁹ cells per L) | 3.7–9.5 | 6.57 | 1.86 |
| Neutrophil count (10⁹ cells per L) | 2.1–6.0 | 3.75 | 1.26 |
| Lymphocyte count (10⁹ cells per L) | 1.0–6.0 | 1.87 | 0.41 |
| Hb (g/L) | 11.9–15.8 | 13.89 | 1.26 |
| Platelet count (10⁹ cells per L) | 120.0–317.0 | 207.67 | 52.21 |
| ESR (mm/hr) | 3.0–68.0 | 12.50 | 8.05 |
| Blood urea (mg/dl) | 15.0–95.0 | 25.62 | 6.54 |
| Creatinine (mg/dl) | 0.3–1.1 | 0.73 | 0.21 |
| CRP (mg/dl) | 5.0–73.5 | 5.45 | 0.28 |
| ALT (U/L) | 12.0–42.0 | 28.33 | 9.22 |
| AST (U/L) | 14.0–39.0 | 24.93 | 7.73 |
| ALP (U/L.) | 77.0–345.0 | Median: 190.0 | Interquartile range: 203.0 |
| Total bilirubin (mmol/L) | 0.30–1.10 | 0.70 | 0.23 |
| Serum ferritin (ng/ml) | 43.85–397.00 | 177.88 | 191.33 |
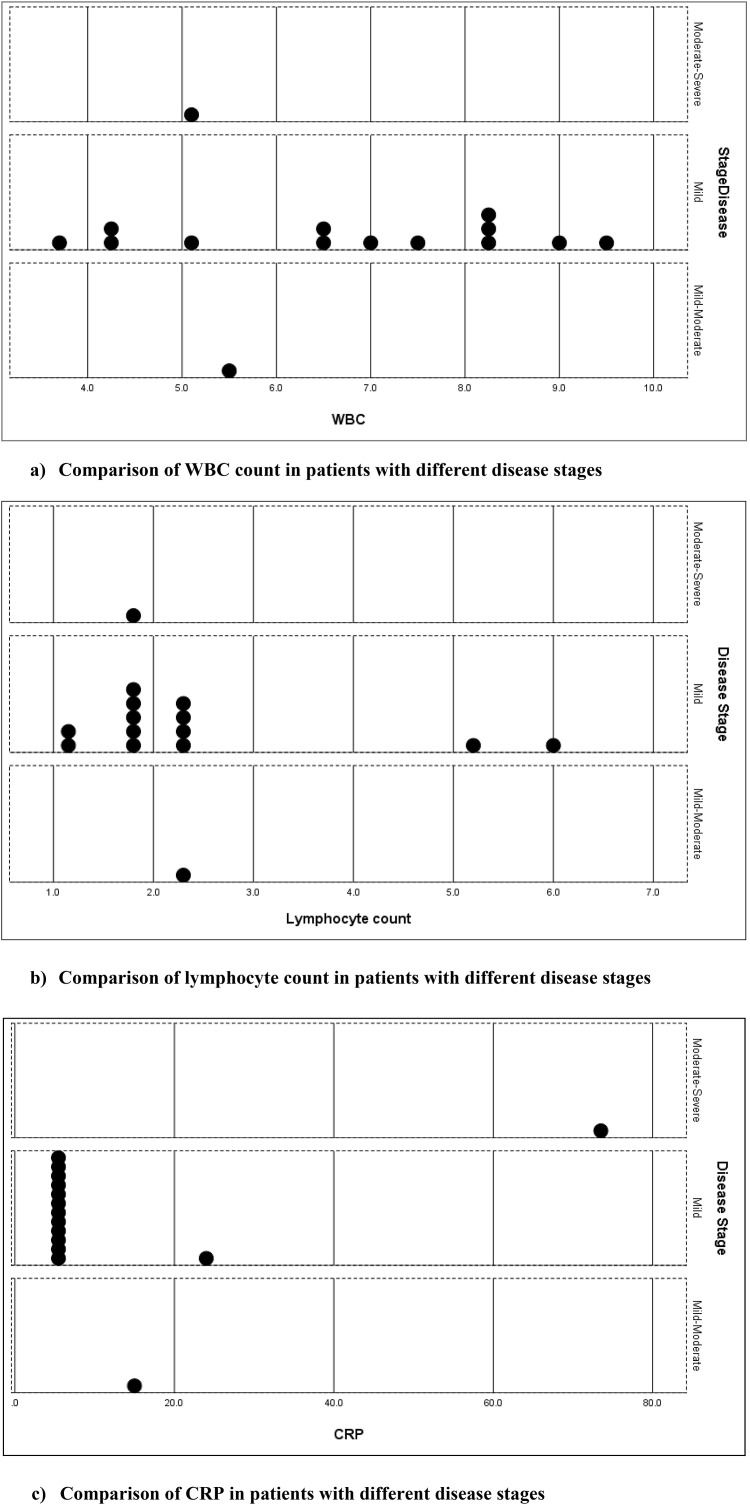
The laboratory investigations were checked for normal and abnormal values. The study revealed: two patients with high levels of lymphocyte count (13.3%), two patients with high levels of blood urea (13.3%), and three patients with high levels of CRP (20.0%). One of the mild cases revealed high CRP because of having concomitant gingival infection. The serum ferritin and ESR were high for patient no.1. The comparison of the leukocyte count, lymphocyte count, and CRP among patients with different disease stages is presented in the dot plot in Fig. 3 .
Fig. 3.
Comparison of hematological parameters in patients with different stages.
4. Discussion
This is a prospective descriptive study about COVID-19 patients admitted to Corona hospital in Duhok. It presents the first report about COVID-19 patients in Iraqi Kurdistan. There were 15 reported cases that were assumed to be infected by 3 index cases. All of the index cases were locally imported from European countries; including Germany and UK, and all of the generated cases were clustered and majorities were close contacts. It was a local transmission of the disease in Duhok with no community transmission. Despite effective control measures by KRG [3], the high transmissibility to close contacts in this study explains the high R0 of COVID-19. Moreover, quarantine and social distancing measures were not strictly followed by these patients [13]. Therefore, the preventive measures taken by the KRG to control the outbreak were promising.
In correspondence to other studies, greater number of males (60%) were observed [7]. Previous studies on other novel coronaviruses i.e. Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) also showed male predominance [14,15]. The low susceptibility to viral infection in females could be attributed to a stronger immune response [16].
In the current study, 9 patients (60.0%) were symptomatic at the time of the diagnosis. Our finding was in parallel to most reports of COVID-19 patients [5,9], whereas few reports recorded a higher rate of asymptomatic patients at the time of hospital admission [17]. The slightly high frequency (40%) of asymptomatic patients in this study can be partly justified by systematic testing of close contacts of COVID-19 index patients. The most reported symptoms were fever, cough, and SOB, which was in concordance to findings of other literatures [9,18]. Interestingly, 4 patients (26.7%) showed smell and taste disorders. This finding was in correspondence to a study from Italy on COVID-19 patients where 34% reported such disorders [19]. Although smell and taste disorder can occur in a wide range of viral infections, it might be a useful diagnostic clue in COVID-19 outbreak.
The majority of our patients (86.66%) showed mild illnesses. This deemed to be related to their young age, and no associated risk factors resulting in a better immune response. Several studies documented that COVID-19 has a more profound course in elderly patients with underlying comorbid disease due to a weak immune response [7,18,20]. Additionally, as BCG is included in universal vaccination program in Iraq for infants; it might hypothesize its role in reducing the disease’s severity. There are manuscripts, not peer reviewed, supporting the role of BCG in reducing morbidity and mortality in COVID-19 patients [21,22]. In this study, one patient had moderate to severe illness and another one had mild to moderate illness. Both patients were index cases from Europe. These findings are in line with the seventh report about rapid risk assessment of COVID-19 in Europe, which assessed the risk of severe illnesses among general population to be moderate [18]. Fortunately, we did not report any deaths, perhaps as most patients were mild cases, less than 50 years and without underlying comorbid diseases.
The median hospital stay was 8 days, as their symptoms resolved and had viral clearance on repeated testing. It has been documented that mild cases reveal early viral clearance [20,23]. Only one case with a moderate to severe form had a prolonged hospital stay (21 days), which indicates a high viral shedding due to high viral load [20,23].
In our study, the laboratory findings in most patients were normal, which can be explained by mild illnesses. This finding was in agreement with other studies [24,25]. The first patient showed a marked increase in CRP, ESR and S. ferritin while the 14th patient showed a mild elevation of CRP. These patients were classified as moderate to severe and mild to moderate, respectively. The mean lymphocyte count was 1.87 (SD: 0.41), which was close to the lower limit reference range. This finding supports the evidence that COVID-19 acts mainly on lymphocytes, which results in decreasing lymphocyte counts [25]. Hence, routine laboratory parameters might be useful predictors of the disease’s severity at hospital admission.
The main limitation in this study was the small sample size where most patients had mild illnesses making it difficult to assess the risk factors among severe illnesses. Furthermore, the duration of RT-PCR negativity from the time of diagnosis in asymptomatic patients may be underestimated due to lacking presentation symptoms, making it challenging to determine when these cases were infected.
In conclusion, despite its small sample size, this study was first in the region in its nature. All COVID-19 cases were linked to foreign visits with few local transmissions to close contacts without community transmission. The majority of our cases were mild illnesses with full recovery. This is most likely due to young age and no risk factors. Universal BCG vaccination might have implicated in reduced disease severity.
Contributions
Muayad A. Merza: Concept, review, assessment, treatment, design, data collection, writing first draft, analysis, and final draft approval.
Azad A. Haleem Al Mezori: assessment, treatment, and final approval.
Hakar Mustafa Mohammed: assessment, treatment, and final approval.
Deldar Morad Abdulah: Concept, statistical extractions, first draft, and final draft approval.
Funding
The authors were only financial supporters of the study.
Declaration of competing interest
The authors do not declare any conflict of interest.
Acknowledgments
We would like to thank the staff of the corona hospital who are on the frontline for managing COVID-19 patients. We are grateful to the COVID-19 teams of directorate general of health, preventive health department and central laboratory of Duhok province.
References
- 1.World Health Organization . vol. 43. 2020. (Coronavirus disease 2019 (COVID-19): situation report). [Google Scholar]
- 2.Zandifar A., Badrfam R. Fighting COVID-19 in Iran; economic challenges ahead. Arch Iran Med. 2020;23(4):284. doi: 10.34172/aim.2020.14. [DOI] [PubMed] [Google Scholar]
- 3.Kurdistan Regional Govrnment Situation update Coronavirus (COVID-19), what the KRG is doing 2020. https://gov.krd/coronavirus-en/situation-update/
- 4.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed: Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari S.P., Meng S., Wu Y.-J. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious diseases of poverty. 2020;9(1):1–12. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for disease control prevention. Novel coronavirus. 2020. https://www.cdc.gov/coronavirus/2019-nCoV/summary.html Wuhan, China. [Google Scholar]
- 7.Kolifarhood G., Aghaali M., Saadati H.M. Epidemiological and clinical aspects of COVID-19; a narrative review. Archives of Academic Emergency Medicine. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues J., Hare S., Edey A. An update on COVID-19 for the radiologist-A British society of Thoracic Imaging statement. Clin Radiol. 2020;75(5):323. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Global surveillance for COVID-19 disease caused by human infection with the 2019 novel coronavirus. Interim guidance. 27 February 2020:2020. [Google Scholar]
- 12.Coronavirus TOCGGtDaTtN Diagnosis and treatment protocol for novel coronavirus pneumonia (version 7) https://medium.com/@balajis/the-official-chinese-government-guide-to-diagnosing-and-treating-the-novel-coronavirus-9d06868f8df4
- 13.Jones R.L., Nzekwu M.-M.U. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 14.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein S.L., Huber S. Springer; 2010. Sex differences in susceptibility to viral infection. Sex hormones and immunity to infection; pp. 93–122. [Google Scholar]
- 17.Japanese National Institute of Infectious Diseases. Field Briefing: Diamond Princess COVID-19 Cases, 20 Feb Update https://www.niid.go.jp/niid/en/2019-ncov-e/9417-covid-dp-fe-02.html. Accessed.
- 18.Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – seventh update. ECDC; Stockholm: 2020. [Google Scholar]
- 19.Giacomelli A., Pezzati L., Conti F. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2020. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study; p. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020:1–10. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 21.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. medRxiv; 2020. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. [Google Scholar]
- 22.Abdulah D.M., Hassan A.B. In: Unpublished work. Uo Duhok., editor. 2020. Effects of Bacillus Calmette–Guérin (BCG), Pneumococcal conjugate, and Measles-containing vaccine on infection and mortality rates of COVID-19 in the world; pp. 1–23. [Google Scholar]
- 23.Liu Y., Yan L.-M., Wan L. The Lancet Infectious Diseases; 2020. Viral dynamics in mild and severe cases of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y., Li T., Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]