In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in the Wuhan region of China.1 SARS-CoV-2, known to cause coronavirus disease 2019 (COVID-19), is a beta coronavirus with structural similarities to severe acute respiratory syndrome coronavirus (SARS-CoV).1 , 2 Because of the rapid spread of the disease, the World Health Organization declared a pandemic on March 11, 2020.1 , 2 The spectrum of disease severity ranges from asymptomatic to life-threatening with complications such as end-organ damage and acute respiratory distress syndrome (ARDS).3 , 4 According to the Centers for Disease Control and Prevention, SARS-CoV-2 is more likely to cause severe disease in people older than 65 years old, those with comorbid conditions (including chronic respiratory disease), and those who are immunocompromised, among others.4 Here, we describe the first clinical observation of COVID-19 in a patient with common variable immunodeficiency (CVID) treated with intravenous immunoglobulin replacement (IVIG) who fully recovered from the severe disease despite increased risk.
A 53-year-old woman presented to the emergency department with 7 days of intermittent fever of up to 38°C, chills, myalgia, generalized fatigue, headache, nonproductive cough, pleuritic chest pain, and mild shortness of breath. Her partner had recently received positive results for SARS-CoV-2 and was hospitalized for pneumonia. A review of the patient’s past medical history revealed she had CVID with stable bronchiectasis and normal absolute lymphocyte count. She was on monthly IVIG with her last infusion being 4 days before presenting to the emergency department. She also had breast cancer in remission on daily tamoxifen, hypothyroidism, and Sjogren’s syndrome on a dose of 200 mg hydroxychloroquine twice daily.
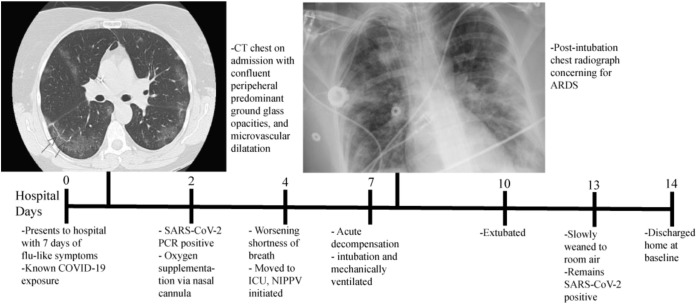
On admission, physical examination revealed the patient to be afebrile with a blood pressure of 100/58 mmHg, a heart rate of 70 beats per minute, a respiratory rate of 17 breaths per minute, and an oxygen saturation of 94% on room air. She was alert and oriented with pertinent physical examination findings of coarse crackles in bilateral lung fields without wheezing, rhonchi, or increased work of breathing. A chest computed tomography performed in the emergency department revealed multifocal opacities, confluent peripheral-predominant ground-glass opacities, and evidence of microvascular dilatation, as shown in Figure 1 . The laboratory examination on admission indicated leukopenia with a white blood cell count of 2.8 x 109 cells/L with a decreased absolute lymphocyte count of 0.77 x 109/L that was initially normal at 1.42 x 109/L 11 days before. Routine blood tests, electrolytes, renal function, liver function, and serum procalcitonin were within the normal range. Her C-reactive protein was higher than the normal at 16.66 mg/dL. The total serum immunoglobulin levels included an immunoglobulin G of 1710 mg/dL (within normal range), immunoglobulin M of 33 mg/dL (low), and immunoglobulin A of <7 mg/dL (undetectable). The antigen test results for influenza A and B and respiratory syncytial virus were negative. The patient was admitted with suspected SARS-CoV-2, with pending results for nasopharyngeal swab test for SARS-CoV-2 by polymerase chain reaction assay, and was administered ceftriaxone and doxycycline because of concern of possible superimposed bacterial pneumonia.
Figure 1.
The timeline of considerable events during a patient’s hospitalization. ACRS, acute respiratory distress syndrome; CT, computed tomography; COVID-19, coronavirus disease 2019; ICU, intensive care unit; NIPPV, nasal intermittent positive pressure ventilation; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
On hospital day 2, the patient required oxygen supplementation through a nasal cannula. Her swab results revealed positive for SARS-CoV-2 and supplemental IVIG of 500 mg/kg was given. The patient’s home hydroxychloroquine dose was increased from 200 mg twice daily to 200 mg thrice daily. Consideration was given to start azithromycin but was deferred given her history of an adverse drug reaction.
On hospital day 4, the patient experienced progressive shortness of breath with an increasing requirement for oxygen supplementation. She was transferred to the intensive care unit and placed on noninvasive positive pressure ventilation with continuous positive airway pressure. Her oxygen requirements progressively decreased until day 7 when she acutely decompensated and required mechanical ventilation. Subsequent chest radiography findings revealed evidence of ARDS, as shown in Figure 1. The patient remained intubated for 3 days before she was successfully extubated. During the next 4 days, she improved clinically and oxygen supplementation was weaned to room air. The SARS-CoV-2 nasopharyngeal polymerase chain reaction results remained positive on hospital day 13. She was discharged home the next day.
Several potential treatments have emerged including the use of chloroquine antimalarial therapy. Hydroxychloroquine, an analog of chloroquine, has been found to have anti–SARs-CoV activity in vitro.5 Our patient’s home dose was increased from 200 mg hydroxychloroquine twice daily to 200 mg thrice daily during her hospitalization; it is unknown whether the dosing regimen affected the course of her disease. The patient received IVIG before admission and was further supplemented during hospitalization. These infusions were not derived from plasma with SARS-CoV-2 antibodies and limited evidence of its efficacy is currently available.6
Recently, COVID-19 pneumonia developed in 2 patients in Italy with x-linked agammaglobulinemia that did not require intensive care or mechanical ventilation.6 Similar to our patient, they were on long-term IVIG with normal immunoglobulin G levels at the time of diagnosis and received additional IVIG during hospitalization. In contrast, ARDS developed in our patient and required mechanical ventilation. It can be speculated that the Bruton’s tyrosine kinase mutation may have further protected those patients from experiencing the severe inflammatory disease.6 As we learn more about SARS-CoV-2 and the course of disease in patients with different primary immunodeficiencies, we will likely gain valuable knowledge of the immune system’s response to COVID-19.
Immunosuppression may be beneficial in preventing mortality, increasing syndromes of hyperinflammation and cytokine storm brought about by COVID-19 activating host immune responses, including interleukin (IL)-2, IL-6, IL-7, ferritin, and tumor necrosis factor-alpha among others.7 It is unknown whether a cytokine storm developed in our patient because markers were not evaluated; however, she developed ARDS, signifying pulmonary hyperinflammation. The patient fully recovered despite her immunodeficiency and underlying lung disease and did not require extended ventilatory support. It is undetermined whether our patient’s inability to mount a full immune response, immunoregulation with long-term hydroxychloroquine for her autoimmune disease and IVIG for her CVID, and tamoxifen therapy altogether affected the pathogenesis of SARS-CoV-2 with resultant favorable outcome in this patient. It is unknown whether the addition of tocilizumab, an IL-6 inhibitor, would have offered further protective benefit. We describe the first clinical observation of COVID-19 infection in a patient with CVID, bronchiectasis, and autoimmune disease on chronic hydroxychloroquine and her recovery.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: The authors have no funding sources to report.
Dr Fill, Dr Hadney, and Dr Graven contributed equally to this work.
References
- 1.Harapan H., Itoh N., Yufika A. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaker MS, Oppenheimer J, Grayson M, et. al. COVID-19: pandemic contingency planning for the allergy and immunology clinic [e-pub ahead of print]. J Allergy Clin Immunol Pract. 2020;S2213-2198(20)30253-1. https://doi.org/10.1016/j.jaip.2020.03.012, accessed April 20, 2020. [DOI] [PMC free article] [PubMed]
- 3.Sohrabi C., Alsafi Z., O’Neill N. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html Available at: Accessed April 16, 2020.
- 5.Perinel S, Launay M, Botelho-Nevers E, et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients [e-pub ahead of print]. Clin Infect Dis. 10.1093/cid/ciaa394, accessed April 15, 2020. [DOI] [PMC free article] [PubMed]
- 6.Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover [e-pub ahead of print]. Pediatr Allergy Immunol. 10.1111/pai.13263, accessed April 28, 2020. [DOI] [PMC free article] [PubMed]
- 7.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]