Dear Editor,
An increasing number of Coronavirus Disease 2019 (COVID-19) patients shows neurological symptoms, but currently few studies have systematically analyzed the impact of SARS-CoV-2 on the Central Nervous System (CNS). Symptoms are usually mild like headache, anosmia and dysgeusia, although disturbance of consciousness, paralysis, paraesthesia and seizures have also been described (Mao et al., 2020). Interestingly, severe neurological complications could appear delayed to respiratory symptoms (Bertran Recasens et al., 2020).
In our Department we observed 3 different clinical pictures in SARS-CoV-2 infection with neurological impairment: cerebral thrombosis (CTh) with hemorrhagic infarction, demyelinating lesions (Zanin et al., 2020) and encephalopathy. We defined this condition as Neuro-COVID for the overwhelming CNS involvement in COVID-19.
Currently the diagnosis of SARS-CoV-2 infection is based on real-time reverse-transcription polymerase chain reaction (rRT-PCR) performed on nasopharyngeal swab. However, the sensitivity of this method is about 60% (Wang et al., 2020). Therefore, a negative rRT-PCR does not exclude a SARS-CoV-2 infection. The diagnostic work-up for Neuro-COVID is even more challenging. Neuroimaging cannot always provide a diagnostic certainty. A lumbar puncture could add relevant data to the diagnostic process but this test is rarely positive (Ye et al., 2020). This could be explained by the mechanism of CNS invasion by SARS-CoV-2 and the resulting pathophysiologic events.
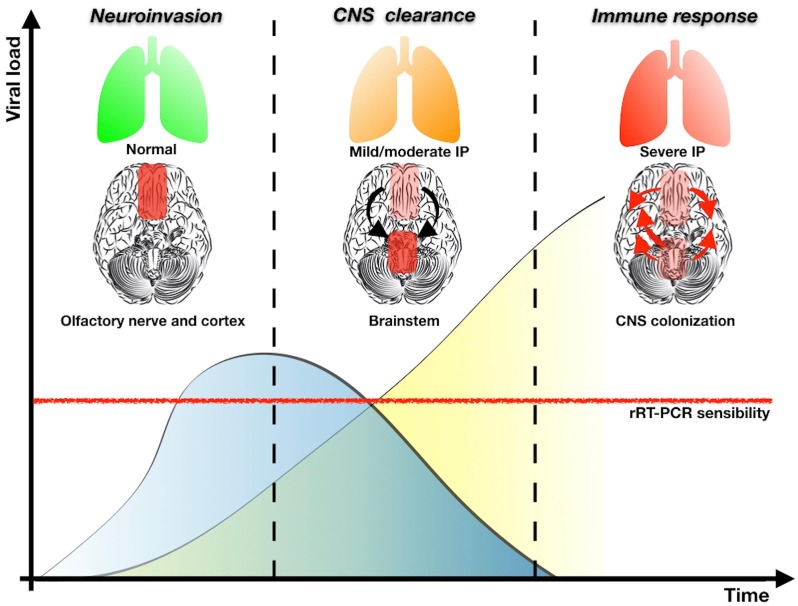
SARS-CoV-2 may lead to Neuro-COVID through three phases (Fig. 1 ): neuroinvasion, CNS clearance and immune response.
-
1)
The SARS-CoV-2 reaches the brain via the bloodstream and/or trans-cribriform route along the olfactory nerve. The viral load in the cerebrospinal fluid (CSF) should progressively increase till the second phase.
-
2)
The interaction between the spike protein S1 and the host ACE-2 receptor allows the penetration of SARS-CoV-2 into the neuronal cells (Mao et al., 2020). This mechanism determines a direct neuronal damage and the onset of symptoms such as anosmia and dysgeusia even in the early phase of infection (Mao et al., 2020). Nevertheless, a severe respiratory impairment could be observed in case of nucleus of the solitary tract involvement (Bertran Recasens et al., 2020). During the CNS clearance phase the viral load starts to decrease till the occurrence of the indirect SARS-CoV-2 effects on the CNS.
-
3)
The viral infection can lead to an immuno-mediated central nervous system damage. Moreover, SARS-CoV-2 could trigger the production of antibodies against glial cells, as a para-infective or post-infective phenomenon, similarly to Epstein Barr Virus. In this phase the respiratory system could be severely affected leading to neurotoxic hypoxia with subsequent brain damage.
Fig. 1.
According to pre-clinical model based on SARS-CoV, in the first phase of neuroinvasion the virus follows a trans-synaptic pathway along the olfactory nerve reaching the entorhinal cortex. Minimal or no respiratory symptoms are detected and CoV replication increases the viral load in CSF (blue curve). In the “CNS clearance” phase, CoV could be located in the brainstem (e.g. nucleus of the solitary tract) affecting the respiratory drive. The viral load in CSF progressively decreases but, on the contrary, SARS-CoV-2 could be detected in respiratory secretions by nasopharyngeal swab (yellow hyperbole). In the last phase, the respiratory system is severely affected leading to potential hypoxia with subsequent brain damage. IP, Interstitial pneumonia. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The Neuro-COVID clinical pictures generally occur in the last phase with different phenotypes (CTh, demyelinating lesions and encephalopathy). Nevertheless, the SARS-CoV-2 load could be significantly reduced and not detectable in the CSF. Negative results could also be explained by the lack of apoptosis and/or necrosis as observed in pre-clinical models (Netland et al., 2008), the low-sensitivity of the method and the CSF clearance of SARS-CoV-2 that lead to a low viral below the sensitivity of currently available instrumentation.
Neuro-COVID could be a severe, occasionally fatal, delayed complication of SARS-CoV-2. Prompt invasive treatment could be required to hypoxia with possible neurological impairment. Diagnosis is mainly based on clinical pictures and neuroimaging; on the other hand, CSF analysis generally fails to reveal SARS-CoV-2 and should not be considered mandatory for the treatment.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- Bertran Recasens B., Martinez-Llorens J.M., Rodriguez-Sevilla J.J., Rubio M.A. Lack of dyspnea in Covid-19 patients; another neurological conundrum? Eur. J. Neurol. 2020 doi: 10.1111/ene.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Li Y., Jin H., Hu B. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. Lancet Neurol. 2020 doi: 10.1101/2020.02.22.20026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in DIFFERENT TYPES OF CLINICAL SPECImens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani PP., Renisi G., Signorini L., Migliorati K., Fontanella MM. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochirurgica. 2020 doi: 10.1007/s00701-020-04374-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]