ABSTRACT
Menstruation is one of the basic but poorly understood life processes in primates during which females shed inner uterine lining every month only to be completely regenerated back within a week. The definitive evidence for the existence and/or identity of stem cells responsible for this process has remained elusive for more than six decades now. Recently, we reported Axin2, a classical Wnt reporter gene, as a marker for endometrial stem cells that also serve as the cells of origin for endometrial cancer.
KEYWORDS: Endometrial stem cells, cancer, regeneration, Axin2, Wnt signaling
Endometrial regeneration following menses and parturition occurs without scarring and is a remarkable example of controlled tissue remodeling unparalleled in other organs.1 The average woman undergoes >400 cycles of endometrial degeneration and regeneration in her reproductive life span.2 This immense regenerative potential of the endometrium and its importance in female fertility and diseases such as endometriosis and endometrial cancer has sparked a significant interest in the endometrial stem cells. However, till date, the true identity of such cells has remained elusive.
Over the past six decades, although our understanding of uterine biology has improved, the ability to precisely mark and isolate the cell responsible for endometrial regeneration and cancer has been evasive. In fact, independent studies have suggested numerous different cell types as endometrial stem cells, including bone marrow-derived cells, hematopoietic stem cells, pericytes, and a rare population of epithelial and/or stromal cells.2 In addition, the role of mesenchymal–epithelial transition3,4 and self-duplication of mature epithelial cells5 has also been suggested in the endometrial regeneration and homeostasis. These findings remain ambiguous and inconclusive partly due to the fact that most of these suggestions are based on in vitro and transplantation assays, rather than direct lineage tracing analysis. The precise identification and isolation of endometrial stem cells could markedly accelerate our investigations into the origins of diseases such as endometrial cancer and endometriosis. Given the existing ambiguity in the actual identity of endometrial stem cells, the pressing question is whether a single multipotent stem cell gives rise to different mesenchymal and epithelial lineages of the uterus, or if separate uni-potent/bi-potent stem cells exist.
Recently, we used a suite of transgenic mouse models for in vivo lineage tracing and inducing ablation and oncogenic transformation of specific cells in vivo to identify endometrial stem cells.6 We first assessed the flux of genetically labeled cells between epithelial and mesenchymal lineages, to rule out the mesenchymal–epithelial transition and any contribution of mesenchyme-derived cells in endometrial regeneration and homeostasis as has been suggested in previous studies. Our data suggested that neither bone marrow-derived cells nor stromal cells contribute toward endometrial epithelial regeneration, and showed that endometrial epithelium self-renews during development, normal growth, and regeneration throughout life. Naturally, the next step was to focus on endometrial epithelium itself and distinguish between the possibilities of its self-renewal occurring via self-duplication of mature epithelial cells or being fueled by a sub-population of specialized progenitors. We used a well-established serial thymidine analogue labeling technique to detect multiple rounds of cell division and also traced the fate of epithelial cells labeled at clonal density and showed that a proliferative compartment of progenitors exist that fuels the endometrial epithelial self-renewal.
The importance of Wnt signaling in endometrial gland maintenance and our initial observations that endometrial epithelium displays a Wnt activity gradient from gland bases to gland mouths and the adjoining luminal epithelium similar to that found in the intestinal crypt-villus axis lead us to believe that the endometrial progenitors are Wnt-responsive cells residing in gland bases (Figure 1). Indeed, we showed that these progenitors are marked by Axin2 that give rise to both the ciliated and non-ciliated cells of the entire endometrial epithelium during homeostasis as well as regeneration. The fact that these cells contribute to luminal epithelium only in the long run suggests that Axin2 marks the long-lived bipotent progenitors of the endometrial epithelium that replace the short-lived luminal progenitors over time. These Axin2+ endometrial stem cells express some of the well-known stem cell markers and have the exclusive ability to form fully functional Wnt-responsive endometrial organoids. Furthermore, diphtheria toxin induced ablation of Axin2+ stem cells in the uterine gland bases resulted in the dramatic collapse of endometrial glands and severely compromised the regenerative capacity of the Axin2+ cell-depleted uterus and the organoid formation efficiency of Axin2+ cell-depleted endometrial epithelium. More importantly, when we conditionally activated Wnt and phosphoinositide 3-kinase (Pi3k) signaling pathways specifically in Axin2+ cells, it resulted in the development of endometrial adenocarcinoma within just 4 weeks. Moreover, such mutations also endowed these cells with the growth advantages outside their resident niche. These findings suggested the possibility of mutated Axin2+ cells also being the cancer-initiating cells of endometrial cancer.
Figure 1.
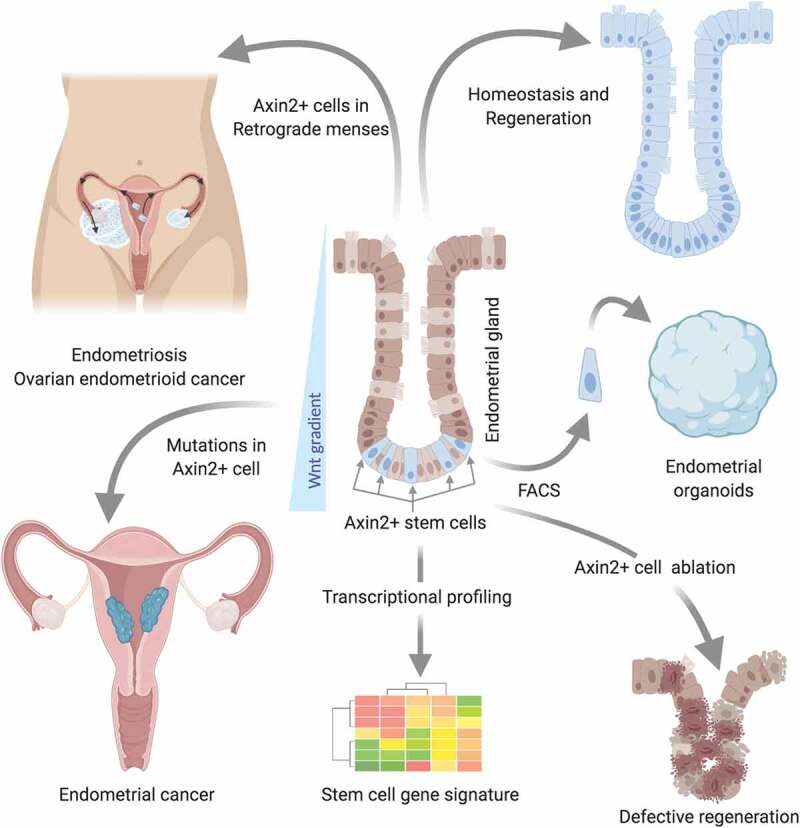
Identification of Axin2+ cells as stem cells of endometrial tissues and cancers via in vivo lineage tracing, ablation, and oncogenic transformation.
Axin2+ cells residing at gland bases are responsible for endometrial epithelial renewal during homeostasis and regeneration. Ablation of these cells compromises epithelial regeneration. These cells display a stem cell signature and have the exclusive capacity to form endometrial organoids. Oncogenic transformation of Axin2+ cells results in endometrial cancer development. When dislodged in retrograde menstruation these cells can seed in the peritoneal cavity and/or ovarian surface and possibly result in the growth of endometriotic tissue and ovarian endometrioid adenocarcinoma.
Aberrant uterine regeneration including the ectopic growth of endometrial tissue lies at the core of reproductive disorders including Asherman’s syndrome, endometriosis, endometrial cancer, adenomyosis, and ovarian endometrioid cancer. Identification of Axin2 as a marker of cells responsible for endometrial regeneration as well as the development of endometrial cancer promises a solid foundation for elucidating further the mechanisms underlying these disease processes. Furthermore, it highlights the need to revisit the existing strategies of using mesenchymal stem cells and bone marrow-derived cells to treat various uterine disorders.7–9 However, it remains to be determined whether Axin2 marks a homogeneous population of cells. Endometrial cancer precursor lesions have been demonstrated to exhibit monoclonal composition.10 This monoclonality could be the result of neutral competition between Axin2-expressing cells such that the progenies of any one stem cell dominate and repopulate an individual gland. Alternatively, there exists a distinct heterogeneity in the proliferative potential of these cells. Nevertheless, our findings suggest the possibility that aberrations in the self-renewal and/or differentiation of Axin2+ cells lie at the core of various gynecological diseases.
Acknowledgments
Work in the Tanwar’s lab was in part supported by funding from the National Health and Medical Research Council (NHMRC), Ovarian Cancer Research Foundation Australia, and The Dorothy Jean Cunningham Endometrial Cancer Research Bequest. Figure was created using Biorender.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ludwig H, Spornitz UM.. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann NY Acad Sci. 1991;622:1–3. doi: 10.1111/nyas.1991.622.issue-1. [DOI] [PubMed] [Google Scholar]
- 2.Gargett CE, Schwab KE, Deane JA.. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7(8):e44285. doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22(6):964–974. doi: 10.1089/scd.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 6.Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, Whan RM, Alterman D, Tanwar PS. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26(1):64–80 e13. doi: 10.1016/j.stem.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Cervello I, Gil-Sanchis C, Santamaria X, Cabanillas S, Diaz A, Faus A, Pellicer A, Simón C. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104(6):1552–60 e1–3. doi: 10.1016/j.fertnstert.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med. 2017;22(1):67–76. doi: 10.1111/jcmm.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovic AS, Boynton KA, Mutter GL. Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res. 1996;56(8):1917–1921. [PubMed] [Google Scholar]


