Abstract
Polymeric surfactants (a.k.a. molecular micelles, MoM) with a variety of chiral head groups and chain lengths may be the most promising chiral selectors when used for sensitive detection of chiral compounds in micellar electrokinetic chromatography-mass spectrometry (MEKC-MS). Various carbohydrate based MS compatible surfactants with phosphate and sulfate head groups have been recently synthesized in our laboratory for its application in CMEKC-MS. In this chapter, we illustrate that the synthesized glucopyranoside based MoMs are fully compatible with electrospray ionization MS and can be successfully used as a chiral selector for high throughput screening of multiple chiral compounds using MRM mode in CMEKC-MS/MS experiments. This chapter describes detail synthesis and utility of α- and β-glucopyranoside based polymeric surfactant with two different chain lengths and head groups. The presented examples optimize the effect of appropriate millimolar concentration of monomer sugar surfactants required for polymerization as it affects the separations of acidic and basic compounds. Under the optimized concentration of the monomer needed for polymerization (i.e., an equivalent monomer concentration of MoMs), the superiority of MEKC-MS over MEKC-UV is evident. Structurally similar basic drugs with the difference in hydrophobicity are first tested in MEKC-MS to find the optimum head group and optimum chain length for developing a widely applicable polymeric glucopyranoside based MoM. The partial enantioselection of several structurally similar basic compounds is significantly improved when switching from one to another head group of the glucopyranoside MoMs. Thus, complementary separations using poly-N-β-D-SUGP versus poly-N-β-DSUGS were seen. This phenomenon also exists when comparing the MoMs, which differ in an anomeric configuration such as poly-N-α-D-SUGP and poly N-β-D-SUGP.
Keywords: Capillary electrophoresis, Chiral micellar electrokinetic chromatography, Polymeric glucopyranoside surfactants, molecular micelles (MoM), poly N-α-D-SUGP, poly-N-α-D-SUGS, poly N-β-D-SUGP, poly N-β-D-SUGS
1. Introduction
Polymeric surfactants [a.k.a. molecular micelles, MoM) prepared from amino acid-based surfactants and peptide-based surfactants having anionic head groups are abundantly applied to the separation of enantiomers of racemic compounds containing positively charged secondary or tertiary amino group [1-5]. In addition, enantioseparations of neutral, negatively and positively charged atropisomers [6-7] and derivatized amino acids (8-9) can be achieved by micellar electrokinetic chromatography (MEKC). Recently, several classes of cationic [10] and anionic drugs [11] along with their metabolites are simultaneously enantioseparated using polymeric amino acid and dipeptide surfactants by MEKC-mass spectrometry (MS) by Shamsi and coworkers. Thus, the continuing development of new and improved polymeric chiral surfactants with exceptional discrimination abilities is needed for the development of both fast and sensitive MEKC-MS.
A new generation of polymeric surfactants, based on two sugar types (α-D and β-D) N-alkenyl glucopyranoside was recently developed in our laboratory (Fig.1). Several key advantages of this new generation of glucopyranoside based polymeric surfactants are noted: (a) multiple stereogenic centers favors enantioseparation of diverse group of analytes; (b) compatibility with ESI-MS and significantly lower ion-suppression compared to unpolymerizable monomers; (c) zero critical micelle concentration (CMC) allowing its use at significantly lower concentration resulting in lower operating current for CE-MS; (d) environmentally friendly and biodegradable; (e) separation of multichiral center compounds using only a single chiral selector is possible. In particular, the advantages (b) and (c) noted above enables this class of polymeric surfactants to be fully compatible with electrospray ionization (ESI)-MS.
Fig.1.
Chemical structures of sugar-based polymeric surfactants. (a) polysodium N-undecenyl-β-D-glucopyranoside-4, 6-hydrogen phosphate (poly-β-D-SUGP). (b) polysodium N-undecenyl-α-D-glucopyranoside-4, 6-hydrogen phosphate (poly-α-D-SUGP). (c) polysodium N-octenyl-β-D-glucopyranoside-4,6-hydrogen phosphate (poly-β-D-SOGP). (d) polysodium N-octenyl-α-D-glucopyranoside-4,6-hydrogen phosphate (poly-α-D-SOGP). (e) polysodium N-undecenyl-β-D-glucopyranoside-6-hydrogen sulfate (poly-β-D-SUGS), and (f) polysodium N-octenyl-β-D-glucopyranoside-6-hydrogen sulfate (poly-β-D-SOGS).
In this chapter, we describe in details the preparation and application of α-D and β-D N-alkenyl glucopyranoside with different chain lengths and head groups as chiral selectors for MEKC-MS/MS. The first type of glucose based i.e., polymeric α-D-glucopyranoside-based surfactants with different chain lengths and head groups are successfully synthesized and characterized as chiral selectors in MEKC-MS/MS [12]. First, the effect of polymerization concentration of the monomer surfactant, N-undecenyl α-D-glucopyranoside 4,6-hydrogen phosphate, sodium salt (α-SUGP), was evaluated by enantioseparation of one anionic compound [1,1′-binaphthyl-2,2′diyl-hydrogen phosphate (BNP)] and one zwitterionic compound (dansylated phenylalanine) by MEKC-UV to find the optimum molar concentration for polymerization. Next, MEKC-UV and MEKC-MS were compared for the enantioseparation of BNP. The influence of polymeric glucopyranoside-based surfactant containing phosphate and sulfate head groups as well as C8 and C11 chain lengths on chiral Rs was evaluated for two classes of cationic drugs (ephedrine alkaloids and β-blockers). Finally, enantioselective MEKC-MS of ephedrine alkaloids and β-blockers were profiled at their optimum pH 5.0 and 7.0, respectively using ammonium acetate buffer and optimum polymeric N-α-D-glucopyranoside surfactant. The LOD for most of the enantiomers ranges from 10 ng/mL-100 ng/ml with S/N of at least ≥ 3.0.
In a recent study, the second type, i.e., polymeric β−D-glucopyranoside-based surfactants with phosphate and sulfate head groups containing C8 and C11 hydrocarbon tail was compared at the optimum pH 5.0 for MEKC-MS/MS [13]. It has been shown that the selectivity of poly-β-D-SUGS and poly−β-D-SUGP are complementary to one another. Therefore, a chiral compound not separated using one polymeric glucopyranoside has a high probability of being enantio-resolved when using the other glucopyranoside. In addition, the enantioresolution of two isomeric surfactants, which differ in the anomeric orientation (containing phosphate or sulfate head group) shows that the β-form of poly-D-SUGS or poly-D-SUGP is a preferred orientation with a high success rate for enantioseparation.
2. Materials
2.1. Instrumentation and materials
A commercial CE instrument hyphenated to a mass spectrometer. In the present study, an Agilent 7100 series CE instrument interfaced to a 6410 series triple quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA) can be used. The Agilent Mass Hunter Workstation Data Acquisition software should be used to acquire data and the Mass Hunter Qualitative Analysis software (B.07.00 version) for chromatographic data analysis. The Agilent Optimizer software to optimize MRM (multiple reaction monitoring) parameters for each analyte through flow injection analysis experiment.
A commercial HPLC pump for delivering the sheath liquid. In the present set-up an Agilent 1100 series isocratic HPLC pump equipped with a 1:100 splitter.
A Cobalt 60 panoramic irradiator for polymerization of the surfactants (Phoenix Memorial Laboratory, University of Michigan, Ann Arbor, MI, USA).
A flash chromatography fritted column (18” length, 1.8” i.d.)
A commercial ultrasonic bath for degassing mobile phase.
A commercial pH meter for mobile phase and sheath liquid pH adjustment.
Fused silica capillaries 50 μm i. d., 360 μm o. d. (e.g. from Polymicron Technologies, Phoenix, AZ, USA).
0.45 μm Nylon syringe filters for filtration of the polymeric surfactant solution.
0.2 μm filters for filtering the sheath liquid.
A 1000 MW cut-off dialysis cellulose ester membrane for polymer dialysis (e.g., 1000 MW cut-off Spectra/Por, Rancho Dominguez, CA, USA).
Silica gel for flash chromatography (pore size 60 Å)
2.2. Solutions and background electrolyte
Use HPLC grade organic solvents and ultrapure water obtained from a suitable water purifications system.
Sample solutions: Prepare stock solutions of the chiral analytes () at a concentration of 1-2 mg/mL in methanol and store at −20 °C. Prepare working solutions of the analytes at a concentration of about 0.1 mg/mL by dilution of the stock solutions with water/methanol (90:10, v/v). Prepare fresh working solutions on a daily basis.
Ammonium acetate solution (7.5 M): Dissolve 57.81 g ammonium acetate in 100.0 mL water in a volumetric flask. A commercial ready-made solution can also be used.
Background electrolyte: Dilute 7.5 M ammonium acetate solution with triply deionized water to the required concentration (usually 5-200 mM). For example, prepare a 20 mM solution by pipetting 266.7 μL of 7.5 M ammonium acetate solution and make it up to 100.0 mL with triply deionized water in a volumetric flask. Adjust the pH as needed using 14.8 M ammonium hydroxide solution for pH range 7-12 or with glacial acetic acid in the pH range of 3-7. Dissolve the molecular micelles (typically 10-100 mg) in 5-10 mL of ammonium acetate buffer to obtain the desired equivalent monomer concentration (EMC). The EMC is defined as the mM concentration of the polymeric surfactant, with the same mass concentration as the corresponding monomer (see Note 1).
TLC visualization solution: Add 2.5 mL concentrated sulfuric acid to 50 mL methanol.
2.4. Sheath liquid solution
Mix the triply deionized water and HPLC grade methanol at various volume fractions in the ratios of 20/80, 30/70, 50/50, 70/30, or 80/20 (v/v).
To each of this mixture, add 7.5 M ammonium acetate solution until the final concentration of ammonium acetate is obtained (typically in the range of 5-20 mM).
Add 1-5% (v/v) concentrated acetic acid or 1-5% (v/v) concentrated ammonium hydroxide solution to the sheath liquid to promote the ionization of cationic and anionic analytes, respectively in the gas phase.
When running MEKC-ESI-MS separation of amines at very low pH with sulfated polymeric surfactant, add 1% (v/v) valeric acid to the sheath liquid containing methanol/water in ratio of 80/20 (v/v).
3. Methods
3.1. Synthesis of polymeric surfactants
The details synthetic procedures for various derivatives of carbohydrate based chiral polymeric surfactant with d-optical configuration are provided below: The polymeric surfactant included are four α- and β-forms of phosphorylated d-glucose head groups with C8 and C11 hydrocarbon chains (Figure 1a-d) and two β-forms of sulfated d-sugars (Figure 1e-f). All steps are carried out at room temperature unless noted otherwise. All synthetic steps should be performed in a well-ventilated hood. Appropriate safety regulation for specific chemicals have to be applied.
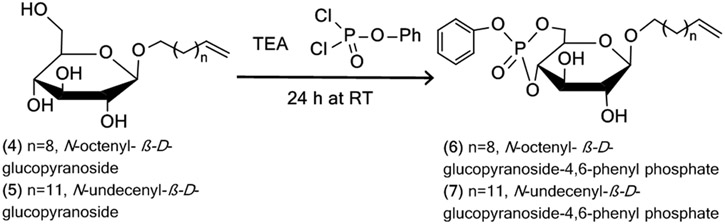
3.1.1. Synthesis of carbohydrate based surfactant containing phosphated glucose as head group and N-undecenyl and N-octenyl hydrocarbon chain with α-, and/or β-configuration
The steps of the synthesis is outlined in scheme 1 to 4. The numbers in the text refer to the structures in the schemes.
To synthesize β-configuration of phosphated sugar surfactants of eight and eleven hydrocarbon chain length, dissolve equimolar amounts (5.0 g, 0.0128 mol) of β-d-glucose pentaacetate (1) and (1.6 mL, 0.0128 mol) borontrifluoride diethyletherate in 50 mL of anhydrous dichloromethane under nitrogen in a 250 mL round-bottom flask. Add 3.9 mL (0.0192 mol) of 10-undecen-1-ol or 2.9 mL (0.0192 mole) or 7-octene-1-ol and stir overnight (~18 h) to yield N-octenyl or N-undecenyl-β-D-glucopyranoside pentaacetate (2 or 3, Scheme I, Glycosylation).
Prepare mL of a saturated solution sodium bicarbonate solution by stirring ~30 g (0.36 mol) sodium bicarbonate in 250 mL water in a 500 mL beaker. Add the saturated sodium bicarbonate solution dropwise to the product (obtained in step 1) to obtain a pH ~7 to neutralize the excess boron trifluoride (see Note 2).
Transfer the resulting solution to a 1000 mL separatory funnel and remove excess boron trifluoride diethyl etherate by shaking the funnel vigorously with 200 mL of water. Collect the lower organic layer in a clean 1000 mL beaker. Dry the organic layer using a small amount of anhydrous sodium sulfate while slowly swirling the beaker (see Note 3). Filter the organic solution into a clean 250 mL round bottom flask and remove solvent at 60 °C in a rotary evaporator to obtain the product as yellow viscous liquid.
Dissolve the entire glycosylated product N-undecenyl-β-D-glucopyranoside acetate (2) or N-octenyl-β-D-glucopyranoside acetate (3) in a 250 mL round bottom flask in 20 mL of anhydrous methanol. Add a catalytic amount (~1mL) of sodium methoxide (25% solution in methanol) to the reaction mixture using a disposable syringe and stir the mixture for 3 h on the ice bath (0°C) to yield the crude deacetylated product (Scheme II, Deacetylation).
Add about 5 g of Amberlyst 15 hydrogen form cation exchanger to the round bottom flask containing the deacetylated product until a pH ~7 is obtained in order to remove the excess sodium methoxide. Filter the resulting mixture using Whatman #42 filter paper (or a similar filter paper) to remove the cation exchange resin. Transfer the filtrate to 1000 mL round bottom flask and add ~25 g silica gel before removing the solvent in a rotary evaporator at 60°C.
Load a flash chromatography column up to 12 inches with silica gel and then load the dried deacetylated product mixed with silica gel from step 5 on to the top of the silica packed column (see Note 4).
Add about 1000 mL of the binary solvent (methanol/ethyl acetate, 0.5:10, v/v) to elute pure deacetylated sugar product. First, pass the binary solvent through packed silica column containing dry loaded deacetylated product and collect 30 fractions of 15 mL eluate each 15 mL clean falcon tubes.
Spot each fraction on a TLC plate and develop using … as mobile phase. After development dry plate at room temperature and visualize the spots by dipping the TLC plate into the visualization solution or by spraying the plate with the visualization solution and heat TLC plate on a hot plate. The spots appear as ….
Combine fractions containing pure deacetylated product as indicated by the TLC in a round bottom flask and evaporate solvents in a rotary evaporator at 60°C to yield N-octenyl-β-D-glucopyranoside (4) or N-undecenyl-β-D-glucopyranoside (5) as colorless viscous liquids. The dried product can be stored in a round bottom flask capped with rubber septum at room temperature until further use. Identify the product by NMR spectroscopy (see Note X).
Dissolve the 0.8 g (0.0027 mol) N-octenyl-β-D-glucopyranoside (4) or 1.18 g (0.0036 mol) N-undecenyl-β-D-glucopyranoside (5) in 50 mL of anhydrous dichloromethane in a 250 mL round bottom flask under nitrogen. Add 648.5μL (0.0046mol) of triethylamine using a syringe and stir for 10 min while keeping the flask on ice bath. Add 636.5μL (0.0043mol) of phenyl dichlorophosphate and stir the reaction mixture at …°C for 3 h. Check the reaction by TLC 3 h to confirm formation of phosphorylated product (Scheme III, Phosphorylation).
After stirring the reaction mixture for 24 h at … °C , add about 25 g silica gel and evaporate the solvent at 60 °C using a rotary evaporator. Load the silica gel containing phosphorylated product on a flash chromatography column and carry out purification as described in steps 6. Use a total of 1000 mL of ethyl acetate/n-hexane (2:1, v/v) for elution. Collect 15 mL fractions into falcon tubes. Spot each fraction on a TLC plate and develop using … as mobile phase. Develop plate and visualize spots as describe in step 8.
Combine fractions containing the pure product of either (6) or (7) (about 5 fractions) in a 100mL round bottom flask and evaporate solvents using a rotary evaporator at 60°C to yield a sticky white solid product (octenyl- or undecenyl-β-D-glucopyranoside 4, 6-phenyl phosphate, 6, 7).
Dissolve the entire product of either octenyl- or undecenyl-β-D-glucopyranoside phenyl phosphate (6, 7) in 20 mL of 1, 4-dioxane in a 250 mL round bottom flask. Add 110 μL of sodium hydroxide (50% in water) and stir the reaction mixture for 20 h. After the first 3 h, check the reaction mixture by TLC for multiple spots on TLC plate indicating the product formation. Use ethyl acetate/n-hexane (2:1, v/v) as mobile phase (Scheme IV, Hydrolysis).
After stirring for 20 h, evaporate solvent THF in a rotary evaporator to obtain the product as a powder. Neutralize the resulting product by dissolving it in 25 mL of triply deionized water for 30 min and add 1 M hydrochloric acid until a pH of about 7 is reached (pH paper should turn yellow).
After neutralization, transfer the solution of octenyl- or undecenyl phosphated surfactants (8, 9) to a separatory funnel and extract with 200 mL of ethyl acetate to remove organic impurities. Swirl the separatory funnels and allow the two layers to separate and clear. Collect the bottom aqueous layer in a beaker and stir overnight to remove residual ethyl acetate. Lyophilize the aqueous layer to yield solid salt form of the monomeric surfactant (i.e., either β-D-SOGP or β-D-SUGP).
Scheme I:
Reaction scheme for glycosylation of β-D-glucose pentaacetate (1) to yield N-alkylenyl-β-D-glucopyranoside pentaacetate (2, 3).
Scheme II:
Reaction scheme for deacetylation of N-alkylenyl-β-D- glucopyranoside pentaacetate (2, 3) to N-alkylenyl -β-D-glucopyranoside(4, 5).
Scheme III:
Reaction scheme for phosphorylation of N-alkylenyl-β-D-glucopyranoside (4, 5) to yield N-alkylenyl-β-D-glucopyranoside-4, 6-phenyl phosphate (6, 7).
Scheme IV:
Reaction scheme for hydrolysis of phenyl group of N-alkylenyl-β-D-glucopyranoside-4, 6-phenyl phosphate (6, 7) to yield sodium-N-alkylenyl-β-D-glucopyranoside4, 6-hydrogen phosphate (8, 9).
For the synthesis of α-d-configured phosphated sugar surfactants with octenyl- or undecenyl carbon chains (i.e. compounds 12 and 13), use proceed as described above for the respective β-d-configured derivatives (steps 1 to 15) except for using a different molar ratio of the reactants in step 1. Specifically, in step 1, dissolve 5 g (0.0128 moles) β-D-glucose pentaacetate (1) and 1.6 mL (0.0129 mol) boron trifluoride diethyl etherate in 50 mL of anhydrous dichloromethane under nitrogen in a 250 mL round-bottom flask. Add 2.5 g (0.0192 mol) 7-octen-1-ol or 3.3 g (0.0192 moles) 10-undecen-1-ol and stir for 72 h (Scheme V). Follow the subsequent steps 2 to 15 as described with no modifications.
Scheme V:
Reaction scheme for synthesis of N-alkylenyl-α-D-glucopyranoside pentaacetate (10, 11) and sodium-N-alkylenyl-β-D-glucopyranoside phenyl-4, 6-phosphate (12, 13). (II, III, and IV refers to reaction schemes)
3.1.2. Synthesis of carbohydrate based surfactant containing sulfated glucose as head group and N-undecenyl and octenyl hydrocarbon chain with α- and β configuration
1.Prepare N-octenyl or N-undecylenyl α- or β-D-glucopyranoside pentaacetate (2, 3, 10, and 11) as described above in steps 1 to 3 in the synthesis of α- or β-D- SUGP, and α- or β-D-SOGP (sub heading 3.1.1).
2.Dissolve all of the intermediates of N-octenyl- α- or β-D-glucopyranoside (e.g., 1.45 g or 0.005 moles) or N-undecenyl- α or β-D-glucopyranoside (e.g., 1.65 g 0.005 moles) in 80 mL of anhydrous pyridine under nitrogen blanket. Add equimolar amount (0.8 g, 0.005mol) of sulfur trioxide pyridine complex and stir for 10 min while keeping RBF on ice bath to reduce heat generated by addition of sulfur trioxide pyridine complex. Continue the reaction under nitrogen blanket for total 24 hours (Scheme VI, Sulfonation). Check the reaction with TLC after 3 hours to confirm formation of the product. Develop the TLC plate using 10:2:1 for ethyl acetate/ methanol/ water to check for product formation (See Note 5).
Scheme VI:
Reaction scheme for synthesis of N-alkylenyl-α-D and β-D-glucopyranoside-6-hydrogen sulfate (18, 19, 14, and 15)
3.After 24 hours of stirring, transfer the resulting product (s) in 1000 mL RBF using minimal amount of pyridine. Add ~25 g silica gel to the RBF and rotavaporate at 70°C to yield intermediate N-undecylenyl or N-octenyl-α-D-glucopyrano-side-6-hydrogen sulfate (14, 15) and N-undecenyl or N-octylenyl-β-D-glucopyranoside −6-hydrogen sulfate (18, 19).
4.Purify the intermediate as described in step 6, and 7 (sub section 3.1.1) using 10:2:1 ethyl acetate/ methanol/ water solvent mixture. The eluting solvent is prepared by mixing 1000 mL of HPLC grade ethyl acetate to 200 mL of methanol and 100 ml of water.
5. Collect eluate from the falcon tubes containing pure product in to 250 mL RBF rotavaporate at 60°C to yield pure intermediate (s).
6. Dissolve the pure intermediate in 50 ml of water and add 1 ml of sodium hydroxide (10%) solution and stir the mixture (Scheme VII, formation of sodium salt).
Scheme VII:
Reaction scheme for synthesis of sodium salts of N-alkylenyl-α and β-D-glucopyranoside-6-hydrogen sulfate (22, 23, 21, and 22).
7. Lyophilize the resulting solution at −50°C collector temperature and 0.05 mbar pressure for two days to yield final products (20, 21, 22, and 23) sodium N-undecylenyl or octenyl-β-D-glucopyranoside 6-hydrogen sulfate (β-D-SUGS and β-D-SOGS), and sodium N-undecenyl or octylenyl-α-D-glucopyranoside-6-hydrogen sulfate (α-D-SUGS and α-D-SOGS) monosodium salts .
3.2. Polymerization of surfactant monomers
1. Dissolve various milimolar amounts of α-D-SUGP, α-D-SOGP, β- D-SUGP, β-D-SOGP and the same chain length of α-and β- sulfated surfactants equivalent to 5 X, 12.5 X, 18.75 X and 20X the CMC in triply deionized water (18.8 mΩ) [14] in four 50 mL clear borosilicate glass bottles and sonicate to obtain a clear solution.
2.Polymerize all surfactant solutions in each 20 mL size glass vial at the same time and temperature using a cobalt-60 gamma radiation source at a total dose of 20 MRad by Phoenix laboratory, University of Michigan, Ann Arbor, MI). The polymerized bottles attained an amber color with similar intensity, suggesting the polymerization occurred homogenously.
3. Transfer the polymerized solution from each amber bottle to a cutoff dialysis membrane (See Note 6) and stir in a beaker of water for 24 hrs to remove unreacted monomers. The water solutions in the beaker should be changed every 8 hrs to promote efficient dialysis and to remove molecular impurities of less than 2000 Daltons.
4.Filter the dialyzed solutions and lyophilize at −50 °C collector temperature and 0.05 mbar until a dry powder of polymer is obtained (See Note 7). Polymerize all remaining α- and β-vinyl surfactant sugar monomers (i.e., octenyl and undecylenyl phosphated and sulfated sugar surfactants) in a similar fashion except they are polymerized at 100 mM equivalent monomer concentrations.
3.2.1. Optimization of surfactant concentrations for polymerization
1.An example of conditions for optimization of surfactant concentration for polymerization using two model test analytes: 1, 1’-binaphthyl-2, 2, dihydrogen phosphate (BNP) and dansylated phenylalanine (DNS-PA) is shown in Fig.2.
Fig.2.
Effect of polymerization concentrations (mM) of sodium N-undecylenyl α-D-glucopyranoside 4, 6-hydrogen phosphate (α-D-SUGP) surfactant monomers on chiral Rs of one zwitterionic compound dansylated phenyl alanine (DNS-PA, left) and one anionic compound (1,1′-binaphthyl-2,2′diylhydrogen-phosphate) (BNP, right) in MEKC. Panel (A): 56 cm effective length (375 μm O.D., 50 μm I.D.) fused silica capillary, sample concentration: 1.0 mg/mL in MeOH/-H2O (50/50). Buffer: 12.5 mM NaH2PO4 +12.5 mM Na2HPO4, pH 7.0, poly-α-D-SUGP concentration: 45mM, applied voltage: +20 kV, injection: 5 mbar, 10 s. Panel (B) Conditions for applied voltage, injection and capillary dimensions are the same as panel A. Sample concentration: 0.1 mg/mL in MeOH/H2O (50/50), buffer: 20 mM NH4OAc, pH 10.8, 15 mM poly-α-D-SUGP.
1. Polymerize α-D-SUGP at various milimolar concentrations of monomer (i.e., at equivalent monomer concentrations (EMC), total dose of 20 M Rad). Using optimized CE-UV conditions carry out chiral separations and determine the chiral resolution.
2. In the present example (Figure 2A-B), α-D-SUGP was polymerized at various milimolar concentrations from 20 mM to 100 mM EMCs. Several interesting trends are noted in the electropherograms when comparing the resolution and retention of BNP and DNS-PA enantiomeric pairs. First, it can be noted that due to the high chiral recognition capability, α-D-SUGP monomers when polymerized at 20 mM (only 5x the CMC), provided significant retention and resolution of BNP. Second, micelles formed using higher analytical concentration of α−D-SUGP always resulted in longer run times. This increase retention is primarily due to increase in aggregation number and hence hydrophobicity of the micelle. The effect of increasing migration time is less pronounced for BNP (right panel, Fig.2B) compared to DNS-PA (left panel, Fig.2A) probably because DNS-PA mainly interacts with the hydrophobic (non-enantioselective sites) of the polymeric surfactant resulting in substantially lower chiral resolution values for DNS-PA.
3.2.2. Optimization of MEKC-MS Procedures and Conditions
1.First, optimize the pH and buffer concentration as both are critical for chiral separation. Volatile buffer are the only suitable choice for MS compatibility. Buffer such as ammonium acetate, ammonium carbonate, and triethylammonium acetate over a pH range of 5 to 10 whereas ammonium formate over a pH range of 2 to 4.5 are commonly used in CMEKC-MS/MS experiments.
2.Organic modifier such as methanol and acetonitrile up to 50 and 60% respectively can be used to improve chiral separation.
3.Optimize CE parameters such as separation voltage, capillary temperature, and injection size for each chiral test analyte.
4.Optimize MS parameters such as drying gas flow rate, drying gas temperature, flow rate, capillary voltage, fragmentor voltage, and collision energy for MRM transitions. This is usually done using LC-MS by flow injection study or direct infusion.
5.Optimize the sheath liquid parameters such as sheath liquid composition, pH, and flow rate by direct infusion and online MEKC-MS experiments.
3.3. Procedures and conditions for CMEKC-UV and CMEKC-MS/MS experiments
Transfer about 200 μL of analyte solution to the cone shaped 250 μL size vials.
Dissolve surfactant in the run buffer by vortexing, filter using 0.45 μm nylon syringe filter, and sonicate for 5 min.
Transfer running buffer to the buffer vials (about 350 μL) for CMEKC-MS runs.
Use fused silica capillary with total length of 64.5 cm and effective length of 56 cm for CMEKC-UV experiments (see Note 8).
When higher resolution is required or tandem mode (CMEKC-UV-MS/MS) is used, capillary with total length of 120 cm can be used. Detection window is created at 60 cm from injection end by removing 3 mm section of polyimide coating.
When performing CMEKC-UV experiments, install fused silica capillary in CE-UV cassette (see Note 9).
For CMEKC-MS/MS experiments only, install fused silica capillary in the CE-MS cassette. Insert capillary inlet to the injector side of the CE-MS cassette and the outlet in to the nebulizer. To install capillary in to the nebulizer, first slide the capillary in to the nebulizer from top until it comes out from spray tip. Align the capillary end flat with the spray tip with a finger nail and secure the fitting screw to hold the capillary in position (see Note 10). Connect the sheath flow splitter and the nebulizing gas tubing to the nebulizer. Fill up the sheath liquid bottle with sheath liquid and prime the HPLC pump to remove any air bubbles from the tubing. An example of sheath liquid used in CMEKC-ESI-MS is as follows: 80/20 MeOH/ H2O with 5 mM NH4OAc (pH 6.8) at a flow rate of 5 μL/min at high pH and HOAc or valeric acid (1%, v/v) at low pH.
Before each CMEKC-MS/MS experiment, rinse prepunctures and electrodes with 2-propanol. For maximum sensitivity, clean the spray shield with 2-propanol using special cloth (see Note 11).
Flush the new fused silica capillary for 30 min with 1 M NH4OH followed by 20 min with triply deionized water. When performing CMEKC-UV, 1 M NH4OH can be replaced with 1 M NaOH.
Pre-condition the capillary by flushing with running buffer containing molecular micelle of various EMC concentrations (5-50 mM) for 2-5 min before each runs. Post-condition the capillary by flushing sequentially with water for 2 min, 1 M NH4OH for 4 min, and water for 2 min.
Set up the capillary temperature and the voltage polarity according to the nature of analyte.
Keep analyte solutions at 10-15 °C in the vial handler using circulator water bath. Perform hydrodynamic injection at pressure of 5-10 mbar for varying times in seconds.
Depending on analysis, scan modes such as selected ion monitoring (SIM) and multiple reaction monitoring (MRM) can be used. MS signal can be optimized by determining the fragmentor voltage and collision energy from flow injection HPLC.
Typical ESI-MS spray chamber parameters for CMEKC-ESI-MS/MS experiments are as follows: nebulizer pressure: 3-4 psi; drying gas flow rate: 5 L/min; drying gas temperature: 200 °C; capillary voltage: 3500 V; gain setting: 3. Optimization of these parameters is recommended for best S/N.
3.4.1. Comparison of MEKC-UV and MEKC-MS/MS
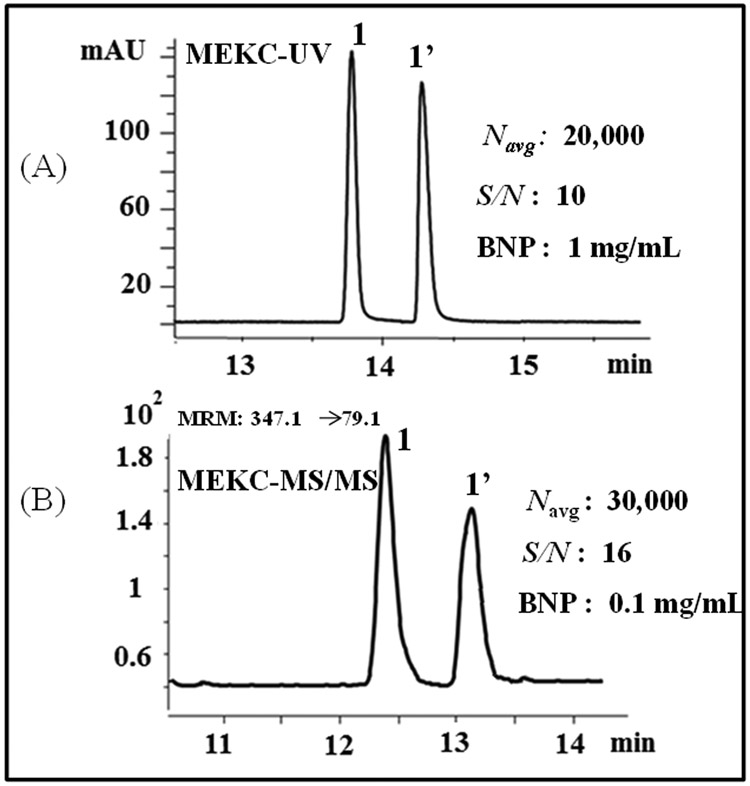
CMEKC-MS/MS is the most sensitive method for chiral separations using molecular micelle compared to CMEKC-UV. However, CMEKC-MS/MS is least sensitive method when using monomeric surfactant because of ion suppression caused by monomer fragmentation. Polymeric surfactants of high molecular weight provides higher S/N and efficiency with CMEKC-MS/MS compared to CMEKC-UV. For e.g., poly-α-D-SUGP provided higher S/N and efficiency with baseline resolution by MEKC-MS/MS when analyte with 10 times lower concentration was injected as compared to CMEKC-UV (Fig.3).
Fig.3.
Comparison of MEKC-UV (A), and MEKC-MS/MS (B) for chiral separation of BNP. Conditions: (A) 56 cm effective length (375 μm O.D., 50 μm I.D.) fused silica capillary. Buffer: 20 mM NH4OAc, pH 10.8, 15 mM poly-α-D-SUGP. Applied voltage: +20 kV, injection: 5 mbar, 10 s. The capillary dimension in (B) are the same as (A) except for 60 cm effective length. Spray chamber parameters: nebulizer pressure: 3 psi; drying gas temp: 250 °C, drying gas flow rate: 6 L/min; capillary voltage: −3000 V; fragmentor voltage: 200 V, collision energy: 41 eV, MRM transition: 347.1 −> 79.1. Sheath liquid: MeOH/H2O (80/20, v/v), 5 mM NH4OAc, pH 6.8 with a flow rate of 5 μL/min. Peak identification: 1 = R-BNP, 1’ = S’-BNP.
3.4.2. Comparison of chain length and head group of glucopyrano-side surfactants
Four polymeric surfactant with two different head groups (phosphate and sulfate), and two different chain lengths (C8 and C11) were tested to determine the optimum head group and chain length. A total of four ephedrine alkaloids and four β-blockers were tested using four polymeric surfactants with α-D configuaration at the same MEKC-MS conditions. The comparison results are illustrated in bar plots (Fig. 4 A-B). Amongst the four surfactants, poly-α-D-SUGP gave the highest chiral resolution values for all four ephedrine alkaloids whereas poly-α-D-SUGS gave baseline chiral resolution for norephedrine only. Partial chiral resolution was obtained for methylephedrine and norephedrine using poly-α-D-SUGS and poly-α-D-SOGS, respectively. However no chiral resolution was observed with poly-α-D-SOGP. Again, the highest chiral resolution was obtained for four β-blockers when poly-α-D-SUGP was used as a chiral selector. Using the other three surfactants, only atenolol was separated with partial chiral resolution irrespective of type of head group and the chain length of the polymeric surfactants. Through overall comparison, it is clear that sugar surfactants with longer chain length and phosphate head group are desirable for higher chiral selectivity.
Fig.4.
Bar plots illustrating the effect of chain length and head groups of sugar surfactants on chiral Rs of ephedrine alkaloids (top, A) at pH 5.0 and β-blockers (bottom, B) at pH 7.0 in MEKC-MS/MS. The * indicates Rs = 0. Conditions: 75 cm long (375 μm O.D., 50 μm I.D.) fused silica capillary. Buffer: 25 mM NH4OAc, pH 5.0, 30 mM poly-α-D-SUGP, poly-α-D-SUGS, poly-α-D-SOGP and poly-α-D-SOGS. Applied voltage, +20 kV, injection, 5 mbar, 10 s. Spray chamber and sheath liquid conditions are the same as Fig. 3. Sample concentration: 10 μg/mL in MeOH/H2O (10/90, v/v). MEKC-MS conditions are the same as Fig. 3 except the optimum fragmentor voltage, collision energy and MRM of ephedrines and β-blockers are: fragmentor voltage 88, 64, 98, 137, 107, 83, and 98; collision energy 25, 17, 21, 25, 17, 17, and 13; MRM transition 166.1 → 115.1, 152.2 → 117, 180.2 → 147.2, 267.2 → 145.2, 268.2 → 116.2, 293.2 → 237.2, and 364.3 → 308.3 for pseudoephedrine/ephedrine, norephedrine, methylephedrine, atenolol, metoprolol, carteolol, and talinolol, respectively.
3.4.3. Selected Application of Poly-α-D-SUGP
Analysis of ephedrine alkaloids is of serious concern in health products because of its amphetamine like effects [15]. Naturally occurring ephedrine alkaloids are reported to have often adulterated with the least expensive synthetic alkaloids which are in racemic form [15]. As different enantiomers of ephedrine alkaloids have significantly different pharmacological activities. Hence, chiral resolution is of much importance. Enantiomers of three ephedrine alkaloids were baseline separated and one of the alkaloid showed partial separation using optimized 25 mM of poly-α-D-SUGP at pH 5.0 with CMEKC-MS/MS. The enantioresolution and enantioselectivity of four ephedrine alkaloids was in following decreasing order: methyl ephedrine > ephedrine > norephedrine > pseudo-ephedrine (Fig. 5). Pseudoephedrine and ephedrine enantiomer pair has same MRM transition (Precursor-> Product) and cannot be distinguished by either accurate mass or MS/MS. However, simultaneous separation based on CMEKC retention is easily possible without cross talk of product ions.
Fig.5.
Electropherograms for enantioseparation of ephedrine alkaloids using sugar surfactant with optimum head group and chain length at optimum pH 5.0 in MEKC-MS/MS. The MEKC conditions and spray chamber parameters are the same as Fig.4. Peak identifications: 1 = (1R,2S)-(−) norephedrine, 1’ =(1S,2R)-(+)norephedrine, 2 = (1R, 2R ) (−) pseudoephedrine, 2’ = (1S,2S)(+) pseudoephedrine; 3 = (1R,2S)-(−)ephedrine, 3’ = (1S,2R)-(+)ephedrine; 4 = (1R,2S)-(−)N-methylephedrine, 4’ = (1S,2R)-(+)N-methylephedrine.
Using optimized 25 mM poly-α-D-SUGP at pH 5, chiral separation of four β-blocker drugs was achieved as showed in Fig.6. Interestingly, the poly-α-D-SUGP was able to separate four β-blockers atenolol, metoprolol, cartelol, and talinolol based on their differences in hydrophobicity (log P). In contrary, resolution and retention factor did not follow the hydrophobicity trend but rather chiral resolution was higher for the early eluting compounds.
Fig.6.
Electropherograms for enantioseparation of β-blockers using sugar surfactant with optimum head group and chain length at optimum pH 7.0 in MEKC-MS/MS. The MEKC conditions and spray chamber parameters are the same as Fig.4. Peak identifications: 1,1’ = atenolol, 2,2’ = carteolol, 3,3’ = metoprolol, 4,4’ = talinolol. For each β-blocker the R-enantiomer always eluted earlier than S-enantiomer.
3.4.4. Comparison of poly-β-D-SUGS and poly-β-D-SUGP
Polymeric glucopyranoside surfactants with sulfate and phosphate head groups are shown to have complementary effects on chiral resolution. This means a partial enantio-resolution obtained with one glucopyranoide surfactant increases the possibility of obtaining baseline resolution with the other glucopyranoside. One example shown in Fig.7 compares the chiral resolution of two very structurally similar tropane alkaloids (atropine and homatropine). It can be seen that chiral Rs of atropine was dramatically improved using poly-β-D-SUGS, whereas entirely opposite results were seen for homatropine, which provide much higher resolution value (Rs =5.0) using poly-β-D-SUGP. In addition, note that poly-β-D-SUGS showed longer retention for the two drugs with strong dependence of retention on chiral resolution. In contrary, poly-β-D-SUGP did resolve enantiomers of homatropine but not atropine. The retention value for the first eluting enantiomer of the two drugs is very similar.
Fig.7.
Electropherograms comparing the effect of head group of polymeric sugar surfactants on chiral separation of atropine and homatropine in MEKC-MS/MS. Conditions: 25 mM NH4OAc, pH 5.0, using 15 mM poly-β-D-SUGS or poly-β-D-SUGP. Sample concentration: 100 μg/mL in MeOH/H2O (10/90, v/v).
3.4.5. Comparison of poly-α-D-SUGP and poly-β-D--SUGP
The structural orientation of the carbon chain at the anomeric carbon (e.g. α, β) of glucose surfactants plays an important role in chiral selectivity. Example shown in Fig. 8 shows the complementary effect of α- and β- configurations of poly-D-SUGP surfactants on chiral separations of pseudoephedrine and β-blockers. However, a detail screening of 43 drugs using α- or β− form of poly-D-SUGP clearly indicates that the β-anomer resolved enantiomers with 65% success rate Thus, the β-anomer is significantly a superior chiral selector compared to the α-anomer which has only 35 % success rate Similar trends were also seen for chiral screening of α-and β-form of poly-D-SUGS. Other research group have also shown that pyranosiide unpolymerized micelles formed with β-configuration has higher chiral selectivity [16]. Molecular modeling studies done by this group suggested that β-micelle are less compact and the amount of solvent accessible chiral head groups (i.e., chiral face) is more compared for β- compared to α-micelle. Thus, the β-micelle results in better chiral recognition of enantiomers of DNS-AAs.
Fig.8.
Electropherograms comparing the effect of anomeric configuration of polymeric phosphated sugar on the enantioseparation of (A-B) pseudoephedrine and (C-D) metoprolol in MEKC-MS/MS. Buffer: 25 mM NH4OAc, pH 5.0 and 7.0 for pseudoephedrine and metoprolol respectively. Sample concentration: 100 μg/mL in MeOH/H2O (10/90, v/v). Other conditions are the same as Fig.7. The bar plots in (E) showing the chiral resolution values from the chiral screening using sugar surfactant polymers with different anomeric configurations. The Rs values are classified as poor (Rs = 0), fair-to-good (0 < Rs < 1.5), and excellent (Rs > 1.5).
5. Acknowledgements
This work was supported by NIH grant (5-R21MH107985-02)
Footnotes
pH of the MEKC buffer is always adjusted before the addition of polymeric surfactant.
pH is checked by placing few drops of the product on a pH paper and comparing the color of pH paper with color indicator chart.
Organic solvent is dried by adding small amount of anhydrous sodium sulfate and shaking the beaker. When sodium sulfate is added to the organic solvent, it absorbs water and clumps up. If no water is left in the organic solvent to absorb, additional sodium sulfate will no longer clump and usually floats freely indicating complete removal of residual water.
Chromatography column is packed by pouring dry silica using plastic funnel while applying slight vacuum at the bottom end. Tap the column gently while packing to allow silica to settle down and pack tightly. Load the silica containing dry product over silica bed in the column tap the column gently to make even layer of product. Solvent is poured slowly on the top of column bed without disturbing product layer. Product can be covered with layer of silica before adding solvent to prevent uneven spreading of product.
Reaction progress is monitored by spotting reaction mixture along with starting material on TLC plate and developing with suitable solvent system. Multiple spots and spot other than starting material on developed TLC plate suggests formation of product.
The dialysis membrane must be soaked for at least 3 hours in a beaker filled to remove traces of sodium and unpolymerized monomers. Rinse the membrane several times. Construct a dialysis bag from the membrane using gentle sealing ridges universal closure (clips) to fill the polymerized solution for dialysis.
Almost all molecular micelle are hygroscopic and should be stored in a desiccator for better run time reproducibility and longer shelf life.
For CMEKC-MS/MS experiments, 50 cm or longer fused silica capillary should be used because of instrument constraints.
Install capillary in the alignment interface by first pushing alignment interface against the capillary insertion tool and sliding the capillary. Once UV detection window is aligned with the alignment window, release the interface and place it in the interface holder of an empty cassette. Wind the capillary around the reel of the cassette if the capillary is too long. Avoid any contact between the capillary windings to prevent any heating due to high voltage. Finally, close the cassette cover and make sure that both the capillary ends are of same length as the cassette guiding pins. Install the cassette in the CE instrument carefully while guiding capillary ends to the electrodes.
Distance between capillary tip and the spray tip can be adjusted if necessary by turning the adjustment screw on the nebulizer. For e.g. turning adjustment screw clockwise will retreat the capillary inside the nebulizer and turning anti-clockwise will stick the capillary tip outside the nebulizer. The position of the capillary tip relative to the spray tip needs to be optimized for CMEKC-MS/MS analysis. Several experiments may be needed to be optimize the position of the capillary tip relative to spray tip for stable current without compromising the sensitivity.
MS spray chamber should be cleaned with lint-free cloth (Agilent part # 05980-60051). If spray shield is too dirty, an abrasive paper (8,000 grit, Agilent part # 8660-0852) can be used to remove stains.
References:
- 1.Wang J, Warner IM (1994) Chiral Separations Using Micellar Electrokinetic Capillary Chromatography and a Polymerized Chiral Micelle. Anal Chem 66: 3773–3776 [Google Scholar]
- 2.Billiot EJ, Thibodeaux SJ, Shamsi SA, Warner IM (2000) Evaluating Chiral Separation Interactions by Use of Diastereomeric Polymeric Dipeptide Surfactants. Anal Chem 71: 4044–4049. [DOI] [PubMed] [Google Scholar]
- 3.Rizvi SA, Zheng J, Apkarian RP, Shamsi SA (2006) Polymeric Sulfated Amino Acid Surfactants: A Class of Versatile Chiral Selectors for Micellar Electrokinetic Chromatography (MEKC) and MEKC-MS. Anal Chem 79: 879–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, He J, Shamsi SA (2011) A high throughput multivariate optimization for the simultaneous enantioseparation and detection of barbiturates in micellar electrokinetic chromatography-mass spectrometry. J. Chromatographic Science. 48:572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Shamsi SA (2013) Chiral separations, methods and protocols (second edition), In Scriba GKE (ed) CMEKC-MS with polymeric surfactants, Chapter 21. Humana Press, New York: pp.319–348 [Google Scholar]
- 6.He J, Shamsi SA (2009) Multivariate approach for the enantioselective analysis in MEEKC-MS: II. Optimization of 1, 1’-binapthyl-2, 2’-diamine in positive ion mode. J.Sep Sci 32: 1916–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billiot EJ, Warner IM (2000) Examination of Structural Changes of Polymeric Amino Acid-Based Surfactants on Enantioselectivity: Effect of Amino Acid Order, Steric Factors, and Number and Position of Chiral Centers Anal Chem. 72: 1740–1748. [DOI] [PubMed] [Google Scholar]
- 8.Valle BC, Morris KF, Fletcher KA, Fernand V, Sword DM, Eldridge S, Larive CK, Warner IM (2006) Understanding chiral micellar separations using steady state fluorescence anisotropy, capillary electrophoresis and NMR. Langmuir 23: 425–435 [DOI] [PubMed] [Google Scholar]
- 9.Agnew-Heard KA.; Shamsi SA, Warner IM (2000) Optimizing enantioseparation of phenylthiohydantoin amino acids with polymerized sodium undecanoyl-L-valinate in chiral electrokinetic chromatography. J. Liq. Chromatogr Relat Technol 239: 1301–1317. [Google Scholar]
- 10.Liu Y, Shamsi SA (2015) Development of novel micellar electrokinetic chromatography mass spectrometry for simultaneous enantioseparation of venlafaxine and dimethyl-venlafaxine: Application to analysis of drug-drug interactions.” J. Chromatogr A 1420: 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Hou J, Shamsi SA (2013) Development of a novel chiral micellar electrokinetic chromatography-tandem mass spectrometry assay for simultaneous analysis of warfarin and hydroxywarfarin metabolites: Application to the analysis of serum samples of patients undergoing warfarin therapy. J. Chromatogr A, 1271: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Lin B, Wang P, Shamsi SA (2016) “Synthesis, characterization and application of polymeric α-D-glucopyranoside based surfactant: Application for enantioseparation of chiral pharmaceuticals in micellar electrokinetic chromatography-tandem mass spectrometry.” Electrophoresis 37: 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y (2016) Chiral capillary electrophoresis-mass spectrometry: Developments and applications of novel glucopyranoside molecular micelles. Ph.D dissertation, Georgia State University, Atlanta, GA: 30303 [Google Scholar]
- 14.Liu Y, Wu B, Wang P, & Shamsi SA (2016). Synthesis, characterization and application of polysodium N-alkylenyl α-D-glucopyranoside surfactants for micellar electrokinetic chromatography-tandem mass spectrometry. Electrophoresis, 37(7-8), 913–923. 10.1002/elps.201500434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Marriott PJ, Chan W, Lee AW, & Huie CW (2006). Enantiomeric separation and quantification of ephedrine-type alkaloids in herbal materials by comprehensive two-dimensional gas chromatography. Journal of Chromatography A, 1112(1-2), 361–368. doi: 10.1016/j.chroma.2005.12.043 [DOI] [PubMed] [Google Scholar]
- 16.Tickle D, George A, Jennings K, Camilleri P, & Kirby AJ (1998). A study of the structure and chiral selectivity of micelles of two isomeric D-glucopyranoside-based surfactants. Journal of the Chemical Society, Perkin Transactions 2,(3), 467–474. doi: 10.1039/a708418h [DOI] [Google Scholar]