Abstract
Background
Nipah virus (NiV) and Hendra virus (HeV) are zoonotic paramyxoviruses that cause severe disease in both animals and humans. There are no approved vaccines or treatments for use in humans; however, therapeutic treatment of both NiV and HeV infection in ferrets and non-human primates with a cross-reactive, neutralizing human monoclonal antibody (mAb), m102.4, targeting the G glycoprotein has been demonstrated. In a previous study, we isolated, characterized, and humanized a cross-reactive, neutralizing anti-F mAb (h5B3.1). The mAb h5B3.1 blocks the required F conformational change needed to facilitate membrane fusion and virus infection, and the epitope recognized by h5B3.1 has been structurally defined; however, the efficacy of h5B3.1 in vivo is unknown.
Methods
The post-infection antiviral activity of h5B3.1 was evaluated in vivo by administration in ferrets after NiV and HeV virus challenge.
Results
All subjects that received h5B3.1 from 1 to several days after infection with a high-dose, oral-nasal virus challenge were protected from disease, whereas all controls died.
Conclusions
This is the first successful post-exposure antibody therapy for NiV and HeV using a humanized cross-reactive mAb targeting the F glycoprotein, and the findings suggest that a combination therapy targeting both F and G should be evaluated as a therapy for NiV/HeV infection.
Keywords: F glycoprotein, Hendra virus, membrane fusion, monoclonal antibody, Nipah virus
Nipah virus (NiV) and Hendra virus (HeV) are closely related highly pathogenic zoonotic viruses and are the type species within the paramyxovirus genus Henipavirus [1]. Both viruses can cause significant morbidity and mortality in a variety of vertebrate species including humans [2]. Pteropid bats (family Pteropodidae), commonly known as flying foxes, are the predominant natural reservoirs for both HeV and NiV [3–7], although evidence of henipavirus infection has now been reported in a wider range of both frugivorous and insectivorous bats (reviewed in [8]).
HeV spillovers, predominantly into horses, have occurred in Australia 61 times since the first occurrence in 1994, resulting in the death or euthanasia of more than 100 horses across 39 different locales in Queensland and New South Wales, including the euthanasia of 2 HeV-seropositive dogs [9]. These occurrences of HeV also resulted in 7 known human infections with 4 case fatalities all resulting from contact with sick horses, and 13 cases of significant risk of HeV infection resulting in the compassionate emergency use of a human monoclonal antibody (mAb) known as m102.4 as a postexposure therapy in people (reviewed in [10]) (E. G. Playford, unpublished data, 2019). Since the emergence of the Malaysian strain of NiV (NiV-MY) in 1998 [11] and followed by the recognition of NiV infections in Bangladesh and India by a Bangladesh strain of NiV (NiV-BD) [12, 13] and NiV-MY in the Philippines [14], NiV has repeatedly caused spillover events involving hundreds of human cases, with significant amounts of human-to-human transmission and high case fatality rates (40%–100%). Nipah virus was recently classified by the World Health Organization (WHO) as an epidemic threat needing urgent research and development action and is included in the WHO R&D Blueprint list of priority pathogens with epidemic potential [15]. In addition, NiV and HeV also have a remarkably broad host tropism spanning 6 mammalian orders and are a significant transboundary threat capable of causing an often fatal widespread systemic disease in horses, pigs, cats, dogs, ferrets, hamsters, guinea pigs, and non-human primates [2, 16, 17].
Infection of host cells by NiV and HeV requires 2 membrane-anchored envelope glycoproteins; the attachment (G) glycoprotein, which recognizes the ephrin-B2 or ephrin-B3 entry receptor, and the fusion (F) glycoprotein, which drives virus-host cell membrane merger [18–20]. Both glycoproteins are major targets of the host neutralizing antibody response [21]. In a previous study, we developed a cross-reactive, neutralizing, human mAb (m102.4) that recognizes and blocks the ephrin entry receptor binding site on the G glycoprotein [22–24]. m102.4 can potently neutralize HeV and NiV-M and NiV-BD in vitro, and when delivered as a therapeutic modality it has been shown to protect ferrets and nonhuman primates (African green monkeys [AGMs]) from lethal disease even when administered up to several days postinfection [25–28]. It has also been shown that immunization of mice with recombinant trimeric prefusion NiV or HeV F glycoprotein ectodomains could induce strong serum neutralization titers, whereas trimeric postfusion F ectodomains failed to elicit robust neutralizing responses [29]. We isolated a mouse mAb that specifically recognizes the prefusion NiV and HeV F glycoprotein trimers, designated 5B3 [29, 30], and recently we reported the cloning, sequencing, and humanization of 5B3 (h5B3.1) along with the cryo-electron microscopy (cryoEM) structure of the NiV F trimer in complex with 5B3 Fab. Structural analysis revealed the interaction of 5B3 with a prefusion-specific quaternary epitope conserved in F, and it demonstrated that it locks F in the prefusion conformation preventing its membrane fusion activity, providing a molecular mechanism of its neutralizing activity against NiV-M, NiV-B, HeV neutralizing potency [31]. In this study, we examine the protective efficacy of a humanized version of 5B3 (h5B3.1) and report that it can effectively prevent lethal NiV and HeV disease in ferrets when administered as a post-infection therapeutic modality, providing further evidence that mAb-based therapeutics may be possible for treating NiV- and HeV-infected individuals.
MATERIALS AND METHODS
NiV and HeV
NiV number 1999011924 (GenBank NC_002728) was obtained from a patient from the 1999 outbreak in Malaysia (kindly provided by Dr. Thomas Ksiazek, University of Texas Medical Branch). HeV (GenBank NC_001906) was obtained from a patient during the 1994 outbreak in Australia and was kindly provided by Dr. Thomas Ksiazek. Each virus was propagated on Vero-E6 cells in Eagle’s minimal essential medium supplemented with 10% fetal calf serum. The NiV and HeV challenge virus stocks were assessed for the presence of endotoxin using the Endosafe-Portable Test System (PTS) (Charles River Laboratories, Wilmington, MA). Each virus preparation was diluted 1:10 in Limulus Amebocyte Lysate (LAL) Reagent Water per manufacturer’s instructions, and endotoxin levels were tested in LAL Endosafe-PTS cartridges as directed by the manufacturer. Each preparation was found to be below detectable limits, whereas positive controls showed that the tests were valid.
Ethics Approval
The animal studies were performed at the Galveston National Laboratory, University of Texas Medical Branch at Galveston (UTMB), and were approved by the UTMB Institutional Animal Care and Use Committee (IACUC). This facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Eleven female ferrets weighing 0.75–1 kg were socially housed and placed into treatment cohorts (Tables 1 and 2): no treatment (NiV-C and HeV-C), treatment on day 1 and day 3 post-NiV challenge (NiV-1–3), and treatment on day 3 and 5 postchallenge with NiV (NiV-4–6) or HeV (HeV-1–3). For virus challenge and procedures, animals were anesthetized by intramuscular injection with a ketamine-acepromazine-xylazine cocktail. Animals were inoculated intranasally (i.n.) with ~5 × 103 plaque-forming units (pfu) of NiV or HeV in 1 mL Dulbecco’s minimal essential medium (Sigma-Aldrich, St. Louis, MO) on day 0 (Figures 1A and 2A, respectively). After challenge, ferrets in the treated cohorts were given a 20 mg/kg dose of mAb h5B3.1 by intraperitoneal (i.p.) injection on day 1 and 3 post challenge for the D1/D3 cohort or day 3 and 5 post challenge for the D3/D5 cohorts; a dosage similar to prior studies in ferrets and non-human primates with an antiviral human mAb [25–28]. Animals were anesthetized for clinical examination including measuring temperature, respiration quality, and blood collection on days 0, 3, 6, 8, 11, 21, and 34 post challenge. Before and after challenge, animals’ weights, temperatures, and appearance were assessed daily, and animals were scored based on coat appearance, body weight loss, social behavior, and provoked behavior; animals scoring 9 or greater were euthanized per IACUC protocol. Subjects in the h5B3.1 treatment cohorts were euthanized at the study endpoint on day 34 postchallenge.
Table 1.
Clinical Description and Outcome of NiV-Challenged Ferrets
| Subject No. | Group | Clinical Illness | Clinical and Gross Pathology |
|---|---|---|---|
| NiV-C | Control | aFever (d5–7); facial edema (d6–8); nasal and ocular discharge (d6–8); sneezing (d6); depression (d7–8); loss of appetite (d7–8); labor breathing (d7–8); head and neck myoclonus (d8). Animal euthanized on d8 | bLymphopenia (d6, 8); thrombocytopenia (d8); hypoalbuminemia (d8); >2-fold increase in BUN (d8). Enlarge mottled spleen; diffuse reticulation of the liver; few pinpoint renal hemorrhages; lungs with few pinpointed hemorrhages; congestion of brain |
| NiV-1 | D1/D3 | None | None |
| NiV-2 | D1/D3 | Fever (d11–13) | None |
| NiV-3 | D1/D3 | None | >2-fold increase in BUN (d11, 21) |
| NiV-4 | D3/D5 | None | None |
| NiV-5 | D3/D5 | None | >2-fold increase in BUN (d6, 11, 21, 34) |
| NiV-6 | D3/D5 | Fever (d8–10); minor facial/ear twitching (d11–14) | Thrombocytopenia (d11); hypoalbuminemia (d11) |
Abbreviations: BUN, blood urea nitrogen; d/D, day; NiV, Nipah virus.
aFever is defined as a temperature more than 1.0°C over baseline or at least 1.5°C over baseline and ≥40°C.
bLymphopenia, thrombocytopenia, and hypoalbuminemia are defined by a ≥30% drop in lymphocytes, platelets, and albumin, respectively.
Table 2.
Clinical Description and Outcome of Hendra Virus-Challenged Ferrets
| Subject No. | Group | Clinical Illness | Clinical and Gross Pathology |
|---|---|---|---|
| HeV-C | Control | aFever (d4–7); nasal and ocular discharge (d4–8); sneezing (d4–6); loss of appetite (d7–8); depression (d6–8); facial edema (d7–8); labor breathing (d7–8). Animal died on d9 | bLymphopenia (d6); hypoalbuminemia (d6); enlarge mottled spleen; enlarge axillary lymph nodes; lungs with severe congestion and hemorrhage of all lobes; serosanguineous fluid in pleural cavity; congestion of brain; small focal hemorrhages on mucosal surface of urinary bladder |
| HeV-1 | D3/D5 | Mild fever (d7) | Lymphopenia (d6) |
| HeV-2 | D3/D5 | Mild fever (d7) | 2-fold increase in BUN (d11, 21) |
| HeV-3 | D3/D5 | None | 2-fold increase in BUN (d11) |
Abbreviations: BUN, blood urea nitrogen; d/D, day.
aFever is defined as a temperature more than 1.0°C over baseline or at least 1.5°C over baseline and ≥40°C.
bLymphopenia and hypoalbuminemia are defined by a ≥30% drop in lymphocytes and albumin, respectively.
Figure 1.
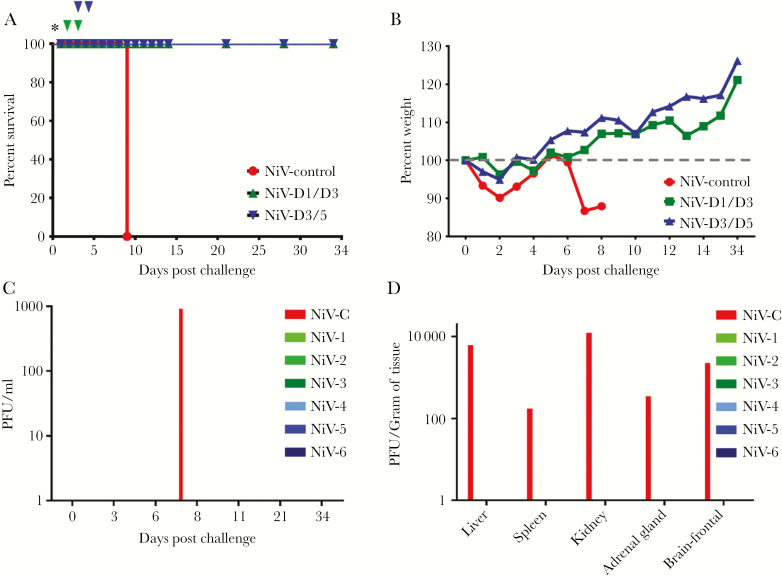
(A) Kaplan-Meier survival curve for Nipah virus (NiV)-challenged ferrets treated with h5B3.1. Red NiV control (n = 1), green D1/D3 cohort (n = 3), and blue D3/D5 cohort (n = 3). Asterisk = NiV challenge day; green nabla, 5B3.1 treatment day for D1/D3 cohort; and blue nabla, treatment day for D3/D5 cohort. (B) Percentage weight from day 0 post-NiV challenge. Red NiV control (n = 1), green D1/D3 cohort (n = 3), and blue D3/D5 cohort (n = 3). (C) Plaque-forming units (PFU) per mL isolated from whole blood on indicated days after NiV challenge. (D) The PFU per gram of tissue at the study end point for each NiV-challenged ferret in the study. NiV-C = control; NiV-1–3 green, D1/D3 cohort; and NiV-4–6 blue, D3/5 cohort.
Figure 2.
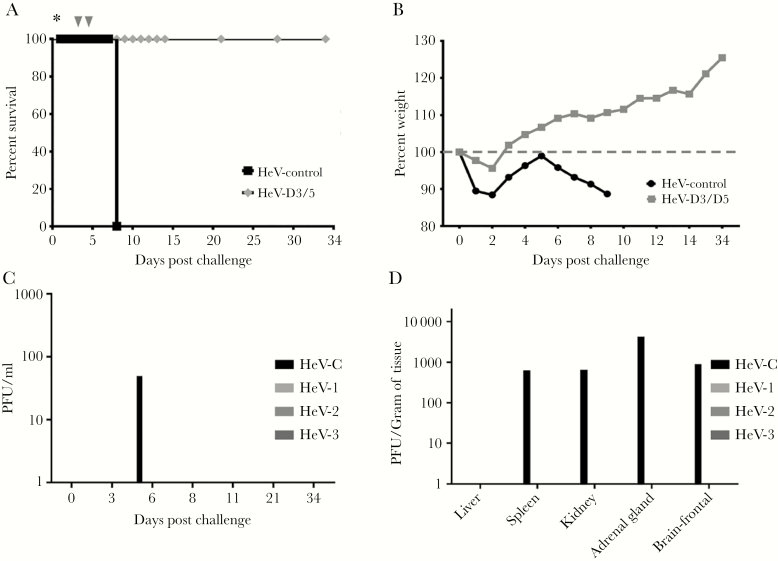
(A) Kaplan-Meier survival curve for Hendra virus (HeV)-challenged ferrets treated with h5B3.1. Black HeV control (n = 1) and gray D3/D5 cohort (n = 3). Asterisk = HeV challenge day; gray nabla, 5B3.1 treatment day for D3/D5 cohort. (B) Percentage weight from day 0 post-HeV challenge. Black HeV control (n = 1), gray D3/D5 cohort (n = 3). (C) Plaque-forming units (PFU) per mL isolated from whole blood on indicated days after HeV challenge. (D) The PFU per gram of tissue at the study end point for each HeV-challenged ferret in the study. HeV-C = control; and HeV-1–3 gray, D3/5 cohort.
Statistics
Due to the constraints of high-containment work, animal studies in biosafety level 4 restrict the number of animal subjects and the volume of biological samples, which affect the ability to repeat assays independently and thus limit statistical analysis. Data are presented as the mean calculated from replicate samples, not from replicate assays, and error bars represent the standard deviation (s.d.) between replicates.
Specimen Collection and Processing in NiV- and HeV-Infected Ferrets
On sampling days, blood was collected and placed in MiniCollect EDTA tubes (Greiner Bio-One, Monroe, NC) for virus load and hematology analysis or MiniCollect serum tubes (Greiner Bio-One) for clinical chemistry and antibody analysis. Necropsy was performed on all ferrets and tissues sampled included lungs, liver, spleen, kidney, adrenal gland, pancreas, and brain (frontal cortex). Ten percent tissue homogenates of liver, spleen, kidney, adrenal gland, and brain were used for virus load analysis.
Measurement of Virus Load
Virus titration was performed by plaque assay with Vero cells from all whole blood samples taken and tissue homogenates (10% w/v). In brief, increasing 10-fold dilutions of the samples were adsorbed to Vero cell monolayers in duplicate wells (200 µL); the limit of detection was 25 pfu/mL for whole blood and 250 pfu/gram for tissue.
NiV and HeV Serum Neutralization Assays
Plaque reduction neutralization titers (PRNTs) were determined using a conventional serum neutralization assay. In brief, sera were serially diluted 2-fold and incubated with ~100 pfu of NiV or HeV for 1 hour at 37°C. These virus and sera mixtures were then added to individual wells of 6-well plates of confluent Vero cell monolayers. Plates were stained with neutral red 2 days after infection, and plaques were counted 24 hours after staining. The 50% neutralization titer (PRNT50) was determined as the serum dilution at which there was a 50% reduction in plaque counts versus control wells with non-specific serum.
Measurement of Serum NiV and HeV G-Specific Antibodies
Ferret serum collected at indicated time points was tested for immunoglobulin G (IgG) antibodies against NiV or HeV G using a previously developed multiplexed microsphere assay [25]. Filter plates, 96-well, were primed with phosphate-buffered saline (PBS). Test sera were diluted in PBS at 1:10 for day 0 post challenge and 1:10000 for time points after virus challenge. Biotinylated goat antiferret IgG and streptavidin-phycoerythrin (strep-PE) (Pierce, Thermo Scientific, Rockford, IL) were also diluted in PBS. Coupled microspheres (sG-HeV, sG-NiV) were prepared by sonication for 1 minute followed by vortex mixing for 1 minute each and then diluted in PBS. Priming liquid was removed from plates using a Bio-Plex Pro II Wash Station (Bio-Rad, Hercules, CA), and 100 µL containing 1500 of each coupled microsphere was added to each well. The liquid was removed from the microsphere mixture by vacuum, and 100 µL of diluted test sera was added to appropriate wells and incubated at room temperature for 30 minutes while shaking in the dark. Diluted test samples were removed by vacuum, and 100 µL of diluted biotinylated goat anti-ferret (1:500) was added to each well and incubated as previously described above. Liquid was removed by vacuum, and 100 µL of strep-PE (1:1000) (QIAGEN, Valencia, CA) was added to each well and again incubated for 30 minutes. All liquid was removed from plates with a vacuum manifold and washed twice with 300 µL PBS, removing liquid between wash steps. Finally, 125 µL PBS was added to each well and incubated for 2 minutes as described above. Samples were assayed for mean fluorescence intensity (MFI) across at least a 100-bead region performed on the BioPlex-200 machine and analyzed using Bio-Plex Manager Software (version 6.1; Bio-Rad). The MFI and the s.d. of fluorescence intensity across 100 beads were determined for each sample and plotted.
Hematology and Serum Biochemistry
Blood and sera were collected via the anterior vena cava from all 11 ferrets on days 0, 3, 6, 8, 11, 21, and 34 post challenge. Complete blood counts of total white blood cell counts, white blood cell differentials, red blood cell counts, platelet counts, hematocrit values, total hemoglobin concentrations, mean cell volumes, mean corpuscular volumes, and mean corpuscular hemoglobin concentrations were analyzed from blood collected in MiniCollect EDTA tubes (Greiner Bio-One) using a Hemavet HV950FS instrument per manufacturer’s instructions (Drew Scientific, Oxford, CT). Blood chemistry analysis of serum was performed using a VetScan classic analyzer and comprehensive diagnostic profile rotors measuring of albumin, alanine aminotransferase, alkaline phosphatase, amylase, blood urea nitrogen (BUN), calcium, creatinine, glucose, phosphorus, sodium, total bilirubin, and total protein (Abaxis, Union City, CA). All blood and serum samples were processed and analyzed directly after collection.
RESULTS
Monoclonal Antibody h5B3.1 Efficacy Against NiV Disease in Ferrets
We previously demonstrated in vivo protective efficacy of an anti-G cross-reactive, human mAb (m102.4) [24] against NiV-mediated disease in ferrets [25] and African green monkeys [26, 27]. However, a similar approach has not been examined with any cross-reactive anti-F neutralizing mAb. Earlier, we isolated and characterized a number of mouse mAbs specific to F [29], and 1 mAb (5B3) possessed strong NiV/HeV neutralizing activity and specifically recognized the prefusion conformation of the F glycoprotein trimer [29, 30]. We recently reported the cloning, sequencing, humanization of 5B3 (h5B3.1), and the cryoEM structure of 5B3 Fab in complex with NiV F, revealing its prefusion-specific quaternary epitope and defining its neutralization mechanism of locking F in the prefusion conformation and inhibiting membrane fusion [31]. To assess the efficacy of mAb h5B3.1 against NiV-mediated disease, we treated a cohort of ferrets (NiV-D1/D3) with the antibody via the i.p. route on day 1 (day = 24 hours) after a lethal i.n. inoculation of NiV with a subsequent dose of antibody on day 3 post challenge (Figure 1A, green arrows). Based on our previous m102.4 data in AGMs [27], we were interested in examining an extended treatment window in the ferret model with h5B3.1, and an additional cohort of ferrets (NiV-D3/D5) was treated starting on day 3 post challenge with an additional treatment on day 5 (Figure 1A, blue arrows). Both groups treated with h5B3.1 survived to the end of the study (Figure 1A), gained weight over the course of the study (Figure 1B), and had minor clinical signs of NiV-mediated disease with no observable gross pathology upon necropsy at study end point (Table 1). This is in stark contrast with the untreated control ferret (NiV-C) that succumbed to NiV disease on day 8 post challenge (Figure 1A) and had over 10% weight loss from day 5–8 post challenge (Figure 1B). The untreated control subject, NiV-C, had clinical signs of NiV disease such as facial edema, nasal and ocular discharge, sneezing, loss of appetite, depression, labored breathing, and head and neck myoclonus (Table 1). Clinical pathology analysis revealed lymphopenia, thrombocytopenia, hypoalbuminemia, and increased BUN levels for NiV-C. Gross pathology at necropsy for NiV-C revealed lungs with pinpoint hemorrhage, an enlarged mottled spleen, diffuse reticulation of the liver, pinpoint hemorrhage of the kidneys, and congestion in the brain (Table 1).
To analyze the effect that h5B3.1 treatment had on circulating infectious virus load and on infectious virus tissue load, we performed plaque assays on blood samples taken during the study and at study end point for tissue load each ferret. We were able to isolate circulating infectious NiV from NiV-C on day 8 post challenge (Figure 1C) and from the liver, spleen, kidney, adrenal gland, and brain (Figure 1D). Infectious virus could not be isolated from any subject in the cohorts treated with h5B3.1, revealing that there was no infectious virus being harbored at study endpoint (Figure 1C and D), which is similar to previous findings using a NiV/HeV anti-G glycoprotein human mAb (m102.4), where recoverable virus was undetected in any treated ferret or nonhuman primate subjects [25–28].
Monoclonal Antibody h5B3.1 Efficacy Against HeV Disease in Ferrets
Monoclonal antibodies 5B3 and h5B3.1 block membrane fusion and neutralize HeV infection in vitro [29, 31]. Based on the efficacy data of m102.4 against HeV in AGMs [28], we tested whether h5B3.1 would also have efficacy against HeV-mediated disease in the ferret model. In light of the success of h5B3.1 in preventing lethal NiV disease in ferrets in the D3/D5 cohort, we assessed the efficacy of h5B3.1 against HeV-mediated disease at only the extended treatment window, with the mAb administered via the i.p. route on day 3 after a lethal i.n. inoculation of HeV with a second dose of mAb on day 5 post challenge (Figure 2A, arrows). The HeV-D3/D5 cohort survived to the end of the study (Figure 2A), gained weight over the course of the study (Figure 2B), and had minor clinical signs of HeV-mediated disease with no observable gross pathology upon necropsy at study end point (Table 2). However, the untreated control ferret (HeV-C) that succumbed to HeV disease on day 9 post challenge (Figure 2A) had over 10% weight loss from day 4 to 9 post challenge (Figure 2B). In addition, HeV-C had clinical signs of HeV disease such as nasal and ocular discharge, sneezing, facial edema, depression, loss of appetite, and labored breathing (Table 2). Clinical pathology analysis revealed lymphopenia and hypoalbuminemia for HeV-C. Gross pathology at necropsy for HeV-C consisted of an enlarged mottled spleen, enlarged axillary lymph nodes, small focal hemorrhages of mucosal surface of the urinary bladder, serosanguinous fluid in the pleural cavity, heavily congested and hemorrhaged lung lobes, and congestion in the brain (Table 2). The disease course in the control animals infected with NiV and HeV was consistent with previous reports of henipavirus disease in ferrets [25, 32–36].
As previously stated, we analyzed the effect of h5B3.1 treatment on circulating infectious HeV load and on infectious HeV tissue load, and plaque assays on blood samples taken during the study and at study end point were carried out to determine tissue load in each subject. Circulating infectious HeV from the control subject (HeV-C) could be isolated on day 6 post challenge (Figure 2C) and also from the spleen, kidney, adrenal gland, and brain (Figure 1D). Similar to the NiV-challenged subjects, no infectious HeV was isolated from any subject in the HeV-D3/D5 cohort treated with h5B3.1, again revealing that there was no infectious virus being harbored at study endpoint (Figure 2C and D).
Neutralizing Antibody Titers and Seroconversion of h5B3.1-Treated Ferrets
All cohorts treated with h5B3.1 were protected against NiV or HeV disease, and, because this antibody has been shown to neutralize NiV and HeV in vitro [31], we assessed the circulating neutralizing antibody titers in the ferrets after treatment with h5B3.1. We analyzed sera from the ferrets with a PRNT50 assay and observed neutralizing antibody titers for each ferret from the treated cohorts on the sampling day past the first i.p. delivery where the NiV-D1/D3 cohort had neutralizing antibody on day 3 (Table 3) and the NiV-D3/D5 and HeV-D3/D5 cohorts had neutralizing antibody on day 6 (Tables 3 and 4, respectively) with neither control ferret having detectable circulating neutralizing antibody against NiV (Table 3) or HeV (Table 4).
Table 3.
Neutralization Titers for NiV-Infected Ferrets
| Treatment Regimen | Subject No. | Day 0 | Day 3 | Day 6 | Day 11 | Day 21 | Day 34 |
|---|---|---|---|---|---|---|---|
| Days 1 and 3 | NiV-1 | <20 | 80 | 40 | 80 | 1280 | 2560 |
| NiV-2 | <20 | 40 | 40 | 20 | 1280 | 5120 | |
| NiV-3 | <20 | 80 | 160 | 20 | 160 | 320 | |
| Days 3 and 5 | NiV-4 | <20 | NT | 320 | 320 | 1280 | 2560 |
| NiV-5 | <20 | NT | 160 | 160 | 160 | 320 | |
| NiV-6 | <20 | NT | 320 | 1280 | 5120 | 10 240 | |
| None | NiV-C | <20 | NT | <20 | NT | NT | NT |
Abbreviations: NiV, Nipah virus; NT, not tested.
Table 4.
Neutralization Titers for HeV-Infected Ferrets
| Treatment Regimen | Subject No. | Day 0 | Day 3 | Day 6 | Day 11 | Day 21 | Day 34 |
|---|---|---|---|---|---|---|---|
| Days 3 and 5 | HeV-1 | <20 | NT | 20 | 40 | 160 | 640 |
| HeV-2 | <20 | NT | 20 | 80 | 1280 | 1280 | |
| HeV-3 | <20 | NT | 20 | 80 | 640 | 640 | |
| None | HeV-C | <20 | NT | <20 | NT | NT | NT |
Abbreviations: HeV, Hendra virus; NT, not tested.
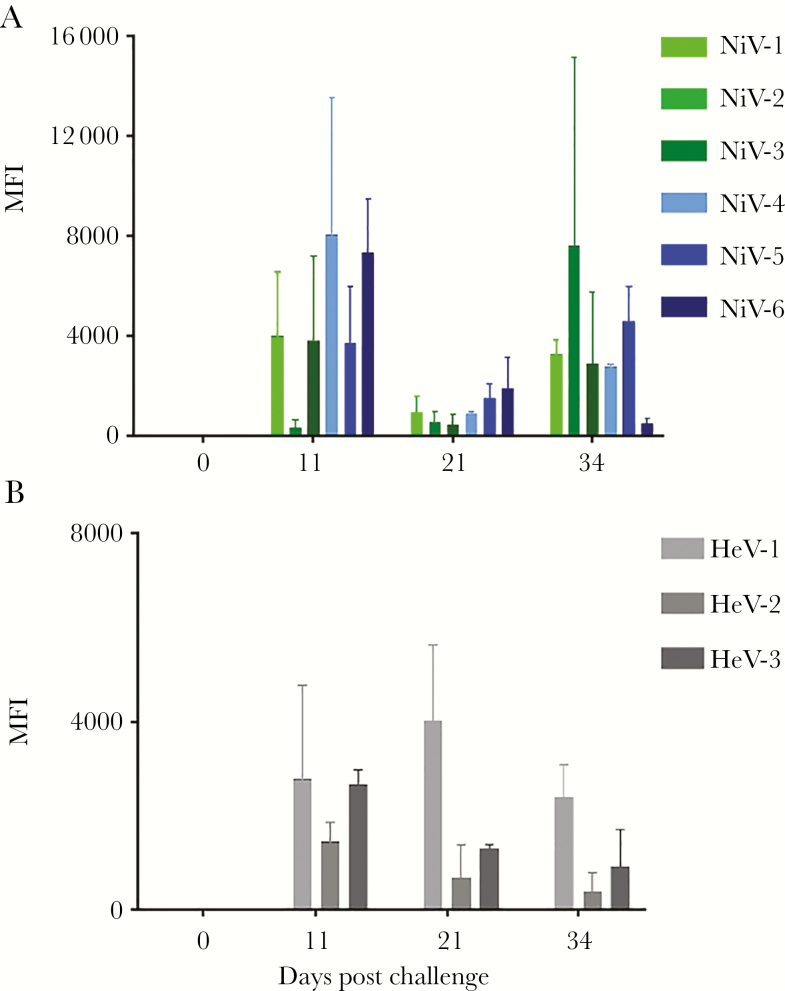
Regardless of the cohort, all treated ferrets developed an increased neutralization antibody titer by the end of the study. To assess the role of the host immune response in providing protection after treatment with h5B3.1, we measured circulating levels of IgG antibodies directed against the NiV G glycoprotein (Figure 3A) or the HeV G glycoprotein (Figure 3B), and, as expected, we detected anti-NiV and anti-HeV G antibodies in all treated ferrets by day 11 post challenge when an IgG response was expected to be present. These observations were similar to previous studies using a therapeutic human mAb to the NiV/HeV G glycoprotein where after a therapeutic treatment and survival of AGMs, a rise in specific antibody to the NiV and HeV F glycoprotein was measured [27, 28].
Figure 3.
Detection of specific anti-NiV G (A) and anti-HeV G (B) immunoglobulin G (IgG) antibodies circulating in ferrets at indicated days post virus challenge. NiV-C = control; NiV-1–3 green, D1/D3 cohort; and NiV-4–6 blue, D3/5 cohort. HeV-C = control; and HeV-1–3 gray, D3/5 cohort. Mean fluorescence intensities (MFI) are shown on the y-axis and represent binding of specific IgG. Error bars represent the standard deviation of fluorescence intensity across 100 beads for each sample.
DISCUSSION
For the paramyxoviruses, the attachment and fusion glycoproteins are the main targets of the virus-neutralizing humoral immune response, and neutralizing antibodies are the key vaccine-induced protective mechanism for many, including the parainfluenza viruses, mumps virus, and measles virus (reviewed in [37–39]). However, in the absence of an active vaccination strategy, a passive immunization approach to prevent or treat viral infections can often be an effective measure [40]. Although there are presently no licensed therapeutics available to treat infection caused by HeV or NiV for use in humans, it has been demonstrated that human, a human mAb targeting the NiV/HeV glycoprotein (m102.4) has been shown to provide therapeutic protection in ferrets and non-human primates when administered up to several days postinfection [25–28]. Furthermore, anti-F mouse polyclonal antibodies and mAbs were shown to protect hamsters from NiV and HeV challenge [41, 42], and in this study we examined a well characterized, cross-reactive (NiV-M, NiV-B and HeV), neutralizing humanized mAb (h5B3.1), with the potential for human use, that targets the F glycoprotein as a postinfection therapeutic.
The paramyxovirus F glycoprotein, and its pre-fusion to post-fusion conformational transition, is the driver of virion and host membrane merger initiating virus infection (reviewed in [43]). The 5B3 mAb and its humanized derivative, h5B3.1, target a conserved epitope in the pre-fusion conformation of the NiV/HeV F glycoprotein [29–31]. The mechanism underlying 5B3 and h5B3.1 NiV/HeV neutralization is its ability to lock F in the pre-fusion conformation, preventing its membrane fusion activity. In this study, we explored whether h5B3.1 could also be effective in vivo in preventing lethal NiV and HeV disease in a well characterized animal model.
Using the ferret model, we challenged subjects with NiV or HeV, and then we administered h5B3.1 mAb up to several days after virus administration. All subjects that received h5B3.1 survived NiV and HeV infection; however, in contrast, our untreated, infected controls in the 100% lethal models succumbed. The survival of all subjects in both the NiV and HeV post challenge groups in the present study represents an additional and major step forward towards a viable passive immunotherapeutic approach to counter NiV and HeV infection. Furthermore, the protective efficacy of h5B3.1 in preventing lethal NiV and HeV disease shown here, when administered as a post-infection therapeutic modality, provides further evidence that mAb-based therapeutics for the prevention and treatment of NiV- and HeV-infected individuals may be possible, and based on prior studies in both ferrets and non-human primates using another anti-NiV/HeV human mAb, therapeutic treatment will likely also reduce the possibility of infectious virus shedding [25–28].
CONCLUSIONS
However, the isolation of mAb neutralization escape mutants of HeV and NiV is also possible and has been demonstrated by passaging in presence of m102.4 [22] and h5B3.1 [31], but virus escape has never been observed during m102.4 or h5B3.1 in vivo efficacy testing presumably due to the very high doses of mAb used in conjunction with an effective adaptive immune responses in virus-challenged subjects. Nevertheless, the use of antibody cocktails has been either proposed and/or used in vivo for Ebola virus [44–46] or severe acute respiratory syndrome coronavirus [47] to prevent and/or limit the possible emergence of such mutants as well as enhance neutralization potency. Taken together, our findings here imply a similar strategy, and the combination of h5B3.1 and m102.4, or other anti-NiV/HeV mAbs, targeting antigenic sites on G and F, should be tested in future studies in the African green monkey model to investigate proper dosage to achieve therapeutic benefit. Indeed, such an antiviral mAb cocktail approach could be implemented for treating NiV and HeV infections in people, providing for both a viable prophylaxis against disease and possibly an effective therapeutic strategy.
Notes
Acknowledgments. We thank the University of Texas Medical Branch Animal Resource Center for husbandry support of laboratory animals and Natalie Dobias for expert histology and immunohistochemistry support.
Disclaimer. The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense or the University of Texas Medical Branch at Galveston.
Authors’ contributions. C. C. B., C. E. M., and T. W. G. conceived and designed the experiments. Y.-P. C. and C. C. B. developed the h5B3.1 antibody used in the studies. H. V. D., D. V., Y.-P. C., and C. C. B. provided the structural and mechanism data on the h5B3.1 antibody. C. E. M., V. B., and R. W. C. performed the Nipah and Hendra challenge experiments at the Galveston National Laboratory. K. N. A. performed the clinical pathology assays. C. E. M. performed necropsies for gross pathology. K. A. F. performed gross pathologic analysis of the data. V. B. performed the HeV and NiV infectivity and neutralization assays. Y.-P. C. and L. Y. developed and optimized the anti-F antibody assays and K. N. A. performed the anti-F antibody assays. C. E. M., T. W. G., Y.-P. C., K. N. A., R. W. C., V. B., K. A. F., and C. C. B. analyzed the data. C. C. B. and C. E. M. wrote the paper. C. C. B., C. E. M., T. W. G., R. W. C., Y.-P. C., and D. V. edited the manuscript. C. C. B. prepared the final versions of manuscript. All authors approved the final version of the manuscript.
Financial support. This study was funded by the National Institutes of Health (Grants AI054715 and AI077995 [to C. C. B.] and AI182121 [to T. W. G.]); D.V. is supported by National Institutes of Health Grant HHSN272201700059C, an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund, and a Pew Biomedical Scholars Award.
Potential conflicts of interest. C. C. B. is a US federal employee, and Y.-P. C. and C. C. B. are coinventors on U.S. Patent 9,982,038: “Antibodies against F glycoprotein of Hendra and Nipah viruses.” (assignees are The United States of America as represented by the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc. [Bethesda, MD]). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wang LF, Mackenzie JS, Broder CC. Henipaviruses. In: Knipe DM, Howley PM, eds. Fields Virology. Vol. 1 Philadelphia: Lippincott Williams & Wilkins, 2013: pp 1070–85. [Google Scholar]
- 2. Geisbert TW, Feldmann H, Broder CC. Animal challenge models of henipavirus infection and pathogenesis. Curr Top Microbiol Immunol 2012; 359:153–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halpin K, Hyatt AD, Fogarty R, et al. ; Henipavirus Ecology Research Group Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am J Trop Med Hyg 2011; 85:946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Field HE. Hendra virus ecology and transmission. Curr Opin Virol 2016; 16:120–5. [DOI] [PubMed] [Google Scholar]
- 5. Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 2000; 81:1927–32. [DOI] [PubMed] [Google Scholar]
- 6. Chua KB, Koh CL, Hooi PS, et al. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect 2002; 4:145–51. [DOI] [PubMed] [Google Scholar]
- 7. Anderson DE, Islam A, Crameri G, et al. Isolation and full-genome characterization of Nipah viruses from bats, Bangladesh. Emerg Infect Dis 2019; 25:166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henipavirus gap analysis workshop report. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service, 2018. Available at: http://go.usa.gov/xnHgR. Accessed 23 August 2019. [Google Scholar]
- 9. Summary of Hendra virus incidents in horses. 2019. Available at: https://www.business.qld.gov.au/industries/service-industries-professionals/service-industries/veterinary-surgeons/guidelines-hendra/incident-summary. Accessed 23 August 2019. [Google Scholar]
- 10. Broder CC, Weir DL, Reid PA. Hendra virus and Nipah virus animal vaccines. Vaccine 2016; 34:3525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chua KB, Bellini WJ, Rota PA, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science 2000; 288:1432–5. [DOI] [PubMed] [Google Scholar]
- 12. Chadha MS, Comer JA, Lowe L, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis 2006; 12:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu VP, Hossain MJ, Parashar UD, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis 2004; 10:2082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ching PK, de los Reyes VC, Sucaldito MN, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis 2015; 21:328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweileh WM. Global research trends of World Health Organization’s top eight emerging pathogens. Global Health 2017; 13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong KT, Ong KC. Pathology of acute henipavirus infection in humans and animals. Patholog Res Int 2011; 2011:567248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weingartl HM, Berhane Y, Czub M. Animal models of henipavirus infection: a review. Vet J 2009; 181:211–20. [DOI] [PubMed] [Google Scholar]
- 18. Bossart KN, Fusco DL, Broder CC. Paramyxovirus entry. Adv Exp Med Biol 2013; 790:95–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonaparte MI, Dimitrov AS, Bossart KN, et al. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A 2005; 102:10652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Negrete OA, Levroney EL, Aguilar HC, et al. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 2005; 436:401–5. [DOI] [PubMed] [Google Scholar]
- 21. Broder CC, Geisbert TW, Xu K, et al. Immunization strategies against henipaviruses. Curr Top Microbiol Immunol 2012; 359:197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu K, Rockx B, Xie Y, et al. Crystal structure of the Hendra virus attachment G glycoprotein bound to a potent cross-reactive neutralizing human monoclonal antibody. PLoS Pathog 2013; 9:e1003684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Dimitrov AS, Bossart KN, et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol 2006; 80:891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu Z, Bossart KN, Bishop KA, et al. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J Infect Dis 2008; 197:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bossart KN, Zhu Z, Middleton D, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog 2009; 5:e1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mire CE, Satterfield BA, Geisbert JB, et al. Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: implications for antibody Therapy. Sci Rep 2016; 6:30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geisbert TW, Mire CE, Geisbert JB, et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci Transl Med 2014; 6:242ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bossart KN, Geisbert TW, Feldmann H, et al. A neutralizing human monoclonal antibody protects African green monkeys from Hendra virus challenge. Sci Transl Med 2011; 3:105ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan YP, Lu M, Dutta S, et al. Biochemical, conformational, and immunogenic analysis of soluble trimeric forms of henipavirus fusion glycoproteins. J Virol 2012; 86:11457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong JJ, Paterson RG, Lamb RA, Jardetzky TS. Structure and stabilization of the Hendra virus F glycoprotein in its prefusion form. Proc Natl Acad Sci U S A 2016; 113:1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang HV, Chan YP, Park YJ, et al. A potent cross-neutralizing antibody targeting the fusion glycoprotein inhibits Nipah virus and Hendra virus infection. Nat Struct Mol Biol 2019; 26:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clayton BA, Middleton D, Arkinstall R, Frazer L, Wang LF, Marsh GA. The nature of exposure drives transmission of Nipah viruses from Malaysia and Bangladesh in ferrets. PLoS Negl Trop Dis 2016; 10:e0004775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leon AJ, Borisevich V, Boroumand N, et al. Host gene expression profiles in ferrets infected with genetically distinct henipavirus strains. PLoS Negl Trop Dis 2018; 12:e0006343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pallister J, Middleton D, Wang LF, et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine 2011; 29:5623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallister JA, Klein R, Arkinstall R, et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol J 2013; 10:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Satterfield BA, Cross RW, Fenton KA, et al. The immunomodulating V and W proteins of Nipah virus determine disease course. Nat Commun 2015; 6:7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karron RA, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, eds. Fields Virology. 6 ed Vol. 1 Philadelphia: Lippincott Williams & Wilkins, 2013: pp 996–1023. [Google Scholar]
- 38. Rubin SA, Sauder CJ, Carbone KM. Mumps virus. In: Knipe DM, Howley PM, eds. Fields Virology. 6 ed Vol. 1 Philadelphia: Lippincott Williams & Wilkins, 2013: pp 1024–41. [Google Scholar]
- 39. Griffin DE. Measles virus. In: Knipe DM, Howley PM, eds. Fields Virology. 6 ed Vol. 1 Philadelphia: Lippincott Williams & Wilkins, 2013: pp 1042–69. [Google Scholar]
- 40. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695–703. [DOI] [PubMed] [Google Scholar]
- 41. Guillaume V, Wong KT, Looi RY, et al. Acute Hendra virus infection: analysis of the pathogenesis and passive antibody protection in the hamster model. Virology 2009; 387:459–65. [DOI] [PubMed] [Google Scholar]
- 42. Guillaume V, Contamin H, Loth P, et al. Antibody prophylaxis and therapy against Nipah virus infection in hamsters. J Virol 2006; 80:1972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jardetzky TS, Lamb RA. Activation of paramyxovirus membrane fusion and virus entry. Curr Opin Virol 2014; 5:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wec AZ, Bornholdt ZA, He S, et al. Development of a human antibody cocktail that deploys multiple functions to confer pan-ebolavirus protection. Cell Host Microbe 2019; 25:39–48.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bornholdt ZA, Herbert AS, Mire CE, et al. A two-antibody pan-ebolavirus cocktail confers broad therapeutic protection in ferrets and nonhuman primates. Cell Host Microbe 2019; 25:49–58.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rockx B, Donaldson E, Frieman M, et al. Escape from human monoclonal antibody neutralization affects in vitro and in vivo fitness of severe acute respiratory syndrome coronavirus. J Infect Dis 2010; 201:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]