Key Points
Lack of Tfh cells renders the mice susceptible to C. rodentium infection.
Tfh cell–dependent protective Abs are essential to control C. rodentium.
Tfh cells regulate IgG1 response to C. rodentium infection.
Abstract
Citrobacter rodentium colonizes at the colon and causes mucosal inflammation in mice. Previous studies have revealed the importance of the innate and adaptive immune response for controlling C. rodentium infection. In the present study, we examined the role of T follicular helper (Tfh) cells in intestinal C. rodentium infection using mice with Bcl6 deficiency in T cells. Tfh cells were absolutely required at the late, but not the early, phase to control infection. Compared with control mice, we observed systemic pathogen dissemination and more severe colitis in Tfh-deficient mice. Furthermore, the susceptibility of Tfh-deficient mice correlated with an impaired serum IgG1 response to infection, and serum Abs from infected wild-type mice protected Tfh-deficient mice from infection. The transfer of wild-type Tfh cells also restored the levels of IgG1 and led to effective clearance of the pathogens in Tfh-deficient mice. Moreover, during C. rodentium infection, IL-21– and IL-4–producing Tfh cells were increased obviously in wild-type mice, correlating with IgG1 as the major isotype in germinal center B cells. Taken together, our work highlights the requirement and the function of Tfh cells in regulating humoral response for the host protection against C. rodentium infection.
Introduction
Intestinal inflammation caused by pathogenic bacteria is a common and important health problem (1). The mouse model of Citrobacter rodentium infection provides a powerful tool for understanding the causes of pathogenesis and host responses to intestinal pathogens. It provides a mimicry for human bacterial colitis caused by enterohemorrhagic or enteropathogenic Escherichia coli (2, 3). Both species of bacteria can induce attaching and effacing lesions and cause severe diarrhea and even kidney failure (2–4).
Various types of immune cells together confer the host defense against C. rodentium, including, but not limited to, type 3 innate lymphoid cells (ILCs) (5) and adaptive immune cells comprising T and B lymphocytes (6–9). CD4+ T cell–dependent humoral immunity is critical for controlling the systemic spread of C. rodentium infection (7). Notably, pathogen-specific IgG Abs—but not IgM or IgA—are required for pathogen clearance and host survival (9). However, better understanding of which subsets of CD4+ T cells and Ab subclasses control C. rodentium and protect the host is needed.
T follicular helper (Tfh) cells, as a crucial subset of CD4+ T cells, specialize in helping B cells regulate Ab responses (10, 11). Tfh cells are required for germinal center (GC) reactions, which result in the production of high-affinity Abs. In host defense, Tfh cells play vital roles in controlling viral infection (12–14) and autoimmunity (15, 16). However, whether Tfh cells are involved in immune responses against intestinal infection is not studied.
In this study, we used mice with conditional deletion of Bcl6 in T cells to investigate the role of Tfh cells during the course of C. rodentium infection. Our results demonstrate that Tfh cells are required for pathogen-specific Ab response that protects mice from C. rodentium infection in the late phase. Interestingly, C. rodentium infection results in induction of IL-21– and IL-4–producing Tfh cells, possibly underscoring IgG1 production in GC B cells.
Materials and Methods
Mice
All experiments were performed according to protocols approved by the Tsinghua Institutional Animal Care and Use Committee. The Bcl6fl/fl mice, which had been reported previously (17), were backcrossed with C57BL/6 mice for at least eight generations and crossed with CD4cre mice.
C. rodentium infection
We grew C. rodentium strain DBS 100 on MacConkey agar and cultured it in Luria broth overnight. According to a different experiment, 3- to 5-wk-old mice were used. They were fasted 8 h prior to oral gavage with a low dose (5 × 108 CFU) or a high dose (2 × 109 CFU) per mouse. We calculated the bacterial titers in the blood or homogenous liquids from livers and spleens after culturing them on MacConkey agar.
Flow cytometry and Abs
Unless indicated, all Abs were obtained from BD Biosciences. Single-cell suspensions were prepared with a 70-μm cell strainer. Before surface staining, cells were stained with a viability dye and incubated with CD16/CD32 Ab to block unspecific staining. For cytokine staining, the cells were cultured with PMA (50 ng/ml), ionomycin (500 ng/ml), and Golgistop for 4 h. After surface staining, cells were fixed, permeabilized, and incubated with intracellular staining Abs. The following Abs were used: anti-CD3e (Thermo Fisher Scientific), anti-CD4, anti-CD44 (BioLegend), anti-CXCR5-biotin, anti-B220, anti–PD-1, anti-CD95 (Thermo Fisher Scientific), anti-GL7 (Thermo Fisher Scientific), anti-IgG1, anti-IgG2a (BioLegend), anti-IgG2b (BioLegend), anti-IgA (Thermo Fisher Scientific), anti–IFN-γ, anti–IL-4, anti-CD45 (Thermo Fisher Scientific), antilineage mixture (Thermo Fisher Scientific), anti-CD90 (Thermo Fisher Scientific), anti-RORγt, anti-Nkp46 (BioLegend), anti-KLRG1, BV421-Streptavidin (BioLegend), and anti-human-IgG (BioLegend). For IL-21 staining, cells were incubated with mouse IL-21R human Fc (R&D Systems) for 1 h in room temperature. After washed twice with buffer, cells were then stained with PE-labeled anti-human IgG Fc.
C. rodentium Ig ELISA
We detected the titers of C. rodentium–specific Ig. We coated 96-well plates with C. rodentium protein extract at 10 mg/ml in PBS (18) and left them overnight at 4°C. After washing, we blocked the plates with 2% BSA in PBS for 1 h then added serially diluted sera or feces supernatants and then incubated overnight at 4°C. The plates were washed then incubated with HRP conjugate Abs for 1 h at room temperature. They were detected by adding TMB substrate and measuring the absorbance at 450 nm.
Serum and IgG transfer
Five-week-old C57BL/6 donor mice were infected with C. rodentium 3 wk prior to inoculating the Bcl6fl/fl/CD4cre recipient mice. On days 3, 4, and 6 after the inoculation of the Bcl6fl/fl/CD4cre mice, whole blood was collected from the donors. Their sera were sterilized through a 0.22-μm filter before immediate use. The Bcl6fl/fl/CD4cre recipients received 250 μl of serum or PBS via tail vein injection. For IgG purification and transfer experiment, IgG was purified with Protein G 4FF Chromatography Column (Yeasen Biotech) and sterilized with a 0.22-μm filter again. Protein concentration was measured with Nanodrop at 280 nm. Three-week-old Bcl6fl/fl/CD4cre mice were inoculated with C. rodentium, and received 400 μg of IgG or PBS with i.v. injection on days 3 and 4 postinfection.
Tfh cell sorting and transfer
To obtain Tfh cells, 5-wk-old C57BL/6 donor mice were inoculated with C. rodentium, and cells were isolated from spleens and mesenteric lymph nodes (MLNs) on day 8 postinfection. CD4+ T cells were first enriched with CD4 microbeads (Miltenyi Biotec) following the manufacturer instruction. Afterwards, B220−CD11c−CD3+CD4+CD44+CXCR5+PD1+ Tfh cells were further sorted using FACSAria sorter, with purity >98%. A total of 0.4 million cells were injected i.v. into Bcl6fl/fl/CD4cre mice 24 h prior to C. rodentium inoculation. Bcl6fl/fl/CD4cre control mice received PBS instead of Tfh cells.
Histological analysis
Distal sections of colon were fixed in 4% paraformaldehyde, and remaining feces were removed. Tissue sections were stained with H&E. Slides were examined with a Nikon microscope, and images were collected with NIS-Elements software. Samples were scored (0–5) based on the inflammatory cellular infiltration, mucosal erosion, crypt ulceration, loss of crypts, and goblet cells.
Lamina propria cell isolation
Colons were cut open longitudinally and feces were removed. They were subsequently incubated with constant shaking in RPMI 1640 with addition of 1 mM DTT and 2 mM EDTA at 37°C for 30 min to further remove epithelial cells. The colon pieces were then washed twice with harvesting media and digested with 0.5 mg/ml collagenase D, 1 mg/ml Dispase, and DNase I in RPMI 1640 at 37°C for 30 min. The digested colon was filtered with a 100-μm cell strainer, dissolved in 40% Percoll, and layered over 80% Percoll in a 15-ml tube. After centrifugation at 2500 rpm and 25°C for 30 min, lamina propria lymphocytes were collected from the interface between 40 and 80% Percoll.
Real-time RT-PCR
Total RNA was isolated from mouse colon and reverse transcribed to cDNA. We conducted RT-PCR with Yeasen quantitative PCR SYBR Green Master Mix. The expression levels were obtained on a Bio-Rad CFX 96 Thermal Cycler. For analysis, samples were normalized against β-actin and reported according to the 2−∆∆CT method.
Statistical analysis
Data were analyzed and graphed by GraphPad Prism 8, GraphPad Software. Mantel–Cox test was used for survival analysis. Data were analyzed by Mann–Whitney U test for comparisons of groups with nonnormal distributed variables. For normal distribution data, Student t test was performed. A p value ≤ 0.05 was considered significant.
Results
Tfh cells are essential for protection against C. rodentium infection at the late phase
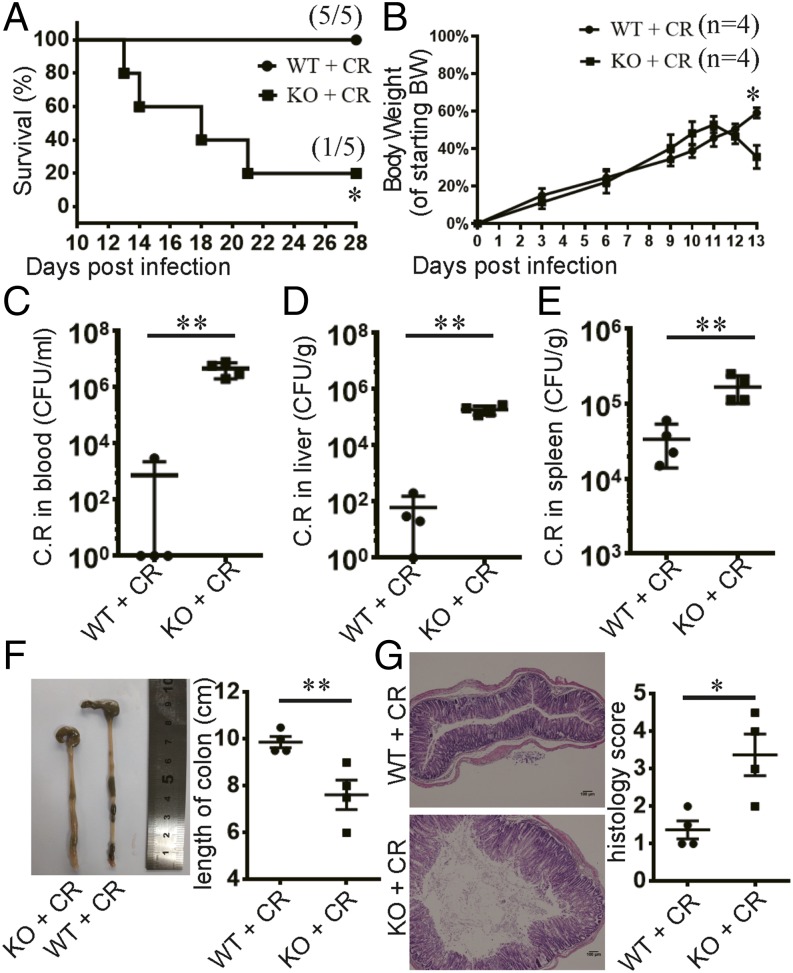
Previous research has demonstrated that both αβ T cells and B cells are important for protecting the host against C. rodentium infection (6–9), but the mechanism by which they function is unclear. In this study, we specifically assessed the importance of Tfh cells in the course of C. rodentium infection. Following C. rodentium infection, CXCR5+PD-1+ Tfh cells developed clearly in the MLNs and spleens (Supplemental Fig. 1A, 1B). These results suggest that Tfh cells may be important in host defense to the infection. We next sought to determine the role of Tfh cells in regulating C. rodentium infection using Bcl6fl/fl/CD4cre mice whose Tfh cells were selectively deficient (17). These mice showed deficiency of GC B cells and a reduction in the numbers of both IgG1+ and IgA+ B cells in the MLNs in the resting state (Supplemental Fig. 2A–C). Following oral infection with a high dose of C. rodentium, four out of five Tfh-deficient mice succumbed to death after 13–21 d, whereas there was no mortality in the littermate controls (Fig. 1A). From day 12 postinfection, Tfh-deficient mice began to show obvious body weight loss compared with control mice, although there was no weight loss in either group during the first week of infection (Fig. 1B). Consistent with the markedly higher mortality and increased weight loss observed in the Tfh-deficient mice, we detected the systemic dissemination of C. rodentium in these mice, as evidenced by elevated C. rodentium titers in the blood, liver, and spleen on day 14 postinfection (Fig. 1C–E). In contrast, we did not find any difference between these two groups on day 4 postinfection (Supplemental Fig. 3A–C). These results together indicate a defect in Bcl6fl/fl/CD4cre mice in protection against C. rodentium infection at the late but not early phase of infection.
FIGURE 1.
Tfh cells are required for protection against C. rodentium infection. (A–G) Weaning-age Bcl6(fl/fl)/CD4cre mice and their control littermates were orally inoculated with a high dose of C. rodentium. Survival time (A) and body weight change (B) are shown. (C–E) C. rodentium titers were measured in blood, liver, and spleen homogenate cultures at day 14 postinfection. (F) Images of colons at day 14 postinfection. Colon lengths were measured. (G) The histological changes in the colon were analyzed on day 14 postinfection. Tissue sections were stained with H&E. Scale bars, 100 μm. (B–G) Four mice in each group were used. Data are shown as mean ± SEM and are from three independent experiments. *p ≤ 0.05, **p ≤ 0.01, (A) Mantel–Cox test, (B–G) Mann–Whitney U test.
At day 14 postinfection, Tfh-deficient mice exhibited more severe diarrhea, hemafecia, and worsened colon shortening than control mice (Fig. 1F). Moreover, C. rodentium infection induced severe colitis in Tfh-deficient mice, characterized by leukocyte infiltration in the lamina propria and submucosa, mucosal erosion, and loss of crypts, which was mild in control mice (Fig. 1G). Therefore, these results indicate that Tfh-deficient mice were sensitive to C. rodentium infection with more severe colitis at the late phase of infection.
Mice lacking Tfh cells exhibit impaired serum IgG1 response against C. rodentium
Because Tfh-deficient mice shared similar pathogen burdens and body weights as the control mice on day 4 postinfection, we reasoned that they may have no defect in the innate immunity to C. rodentium infection. To address this, we sacrificed mice on day 4 following infection for analysis. Between these two groups of mice, we observed comparable expression of various cytokines, including IL-22, TNF-α, IL-β, IL-6, IFN-γ, IL-17A, IL-17F, and IL-10, in the colon (Supplemental Fig. 3D–K) as well as similar cell numbers for ILC1, ILC2, and ILC3 (data not shown). These results indicate that Tfh cells are not required for the innate immunity to C. rodentium infection.
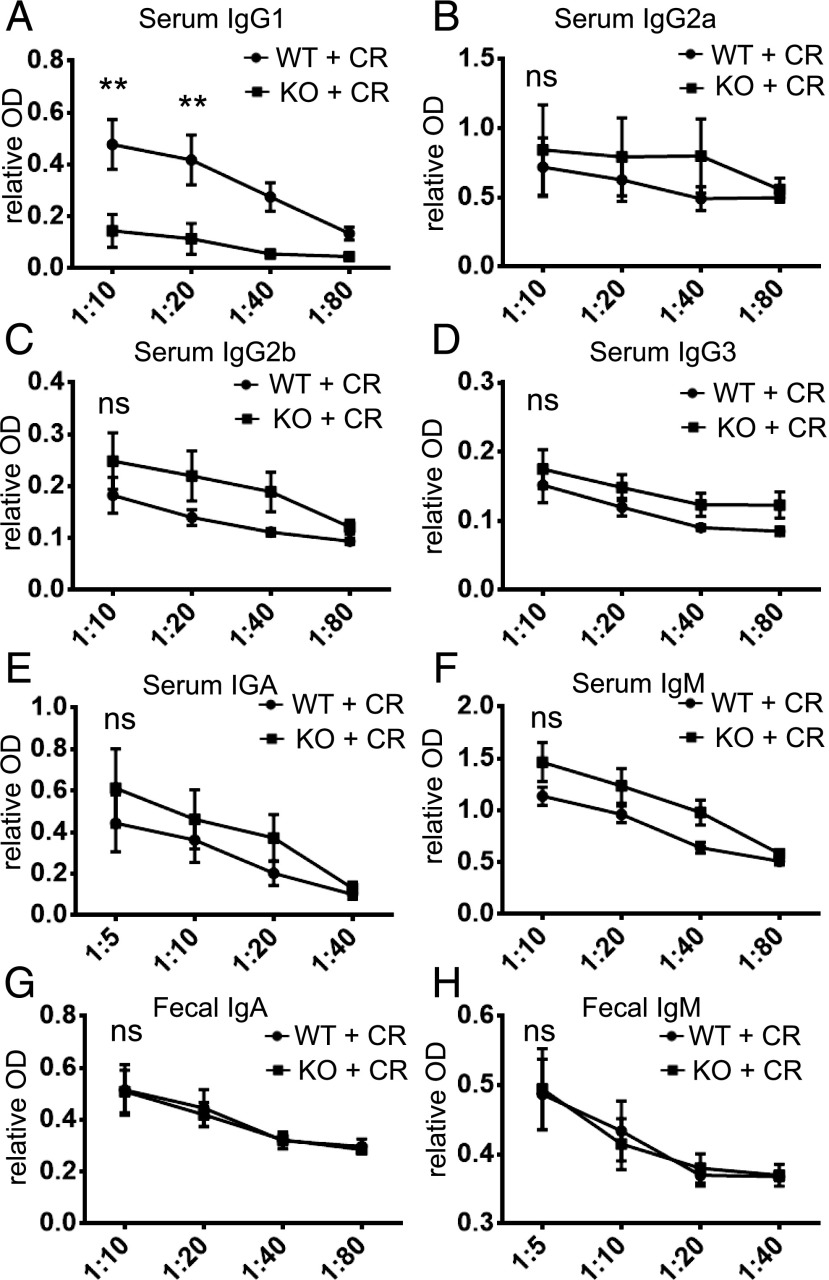
The Ab response to infection varies depending on the types of infectious pathogens (12, 19, 20). Although B cells and IgG Abs are critical for protection against C. rodentium (9), it is not clear which IgG subclasses are involved. To assess whether Tfh cells facilitate the production of class-switched Abs (21) to control C. rodentium infection, we collected sera on day 14 postinfection and examined C. rodentium–specific Ab responses. Bcl6-sufficient mice produced substantial amounts of IgG, IgA, and IgM against C. rodentium, whereas in mice lacking Bcl6 in T cells, IgG1 was significantly reduced; however, IgG2a, IgG2b, IgG3, IgA, and IgM levels were comparable in both groups of mice (Fig. 2A–F). Furthermore, there was no difference in secreted IgA or IgM levels in the feces between two groups (Fig. 2G, 2H). This suggests that Tfh cell–dependent serum IgG1 is important for protection against C. rodentium infection.
FIGURE 2.
Impaired serum IgG1 response to C. rodentium infection in Tfh-deficient mice. We orally inoculated mice with a low dose of C. rodentium. The levels of C. rodentium–specific IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM (A–F) in the sera, IgA, and IgM in feces (G and H) were detected by ELISA, using serial dilutions. Four mice in each group were used. Data are presented as mean ± SEM and derived from three independent experiments. **p ≤ 0.01, Mann–Whitney U test.
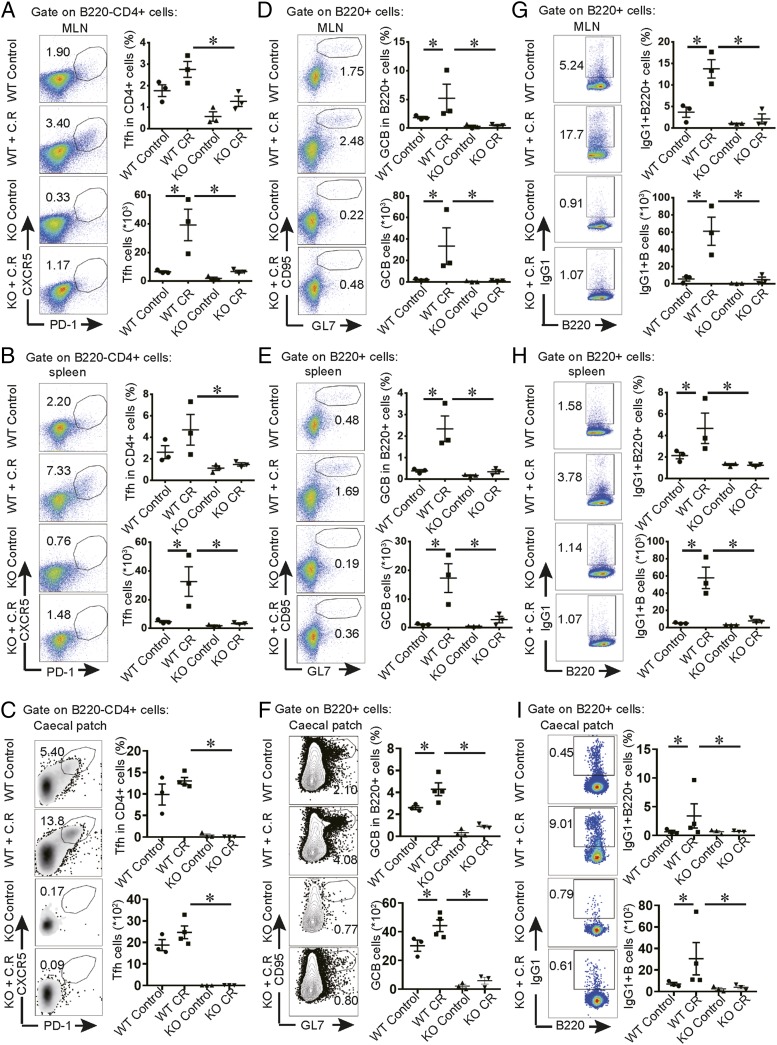
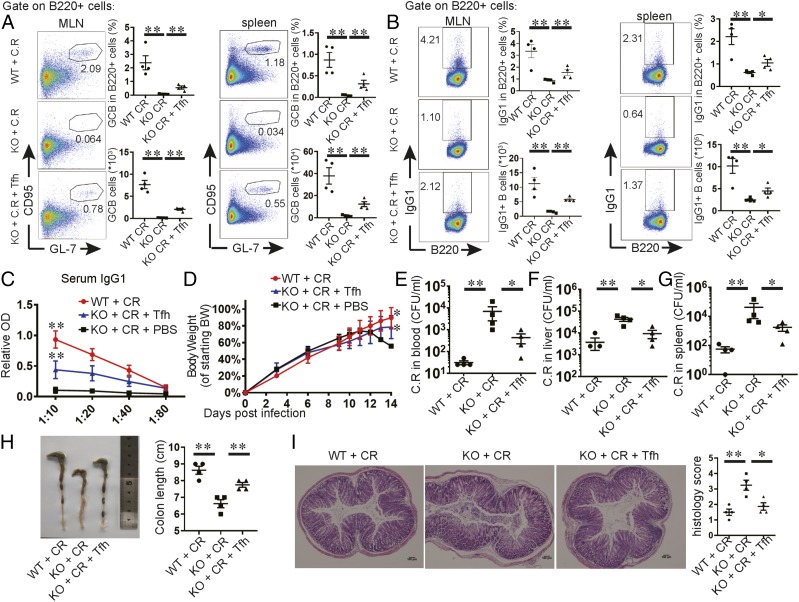
To further understand the regulation of serum IgG1 production in C. rodentium infection, we sacrificed mice to examine the responses of both T cells and B cells in caecal patches, MLNs, and spleens. In contrast to infected control mice, activated CD4+CD44+ T cells were obviously reduced in the infected knockout mice (data not shown). Tfh cells accounted for this difference: Bcl6-deficient mice were impaired dramatically in their Tfh cell response to infection (Fig. 3A, 3B). In addition, CD95+GL7+ GC B cells and IgG1+ B cells expanded in response to infection in the control mice but were almost completely absent in mice with Bcl6-deficient T cells (Fig. 3C–F). Therefore, our results demonstrate that C. rodentium–induced IgG1 response is dependent on Tfh cells, which might be important for protection against infection at the late phase.
FIGURE 3.
Tfh-deficient mice exhibit impaired GC and IgG1 response in MLN, spleen, and caecal patch to pathogens. We orally inoculated 3- to 4-wk-old mice with a low dose of C. rodentium and sacrificed the mice at day 7 postinfection to analyze immune response in caecal patches. For analysis on MLNs and spleens, mice were sacrificed at day 9 postinfection. Flow cytometric analysis of B220−CD4+CXCR5+PD1+ Tfh cells (A–C), B220+CD95+GL7+ GC B cells (D–F), and B220+IgG1+ cells (G–I) in MLNs, spleens, and caecal patches. We calculated the cell numbers. Each point represents an individual mouse. Data are shown as mean ± SEM and derived from two independent experiments. *p ≤ 0.05, Mann–Whitney U test.
Serum Abs from infected normal mice protect Tfh-deficient mice from C. rodentium infection
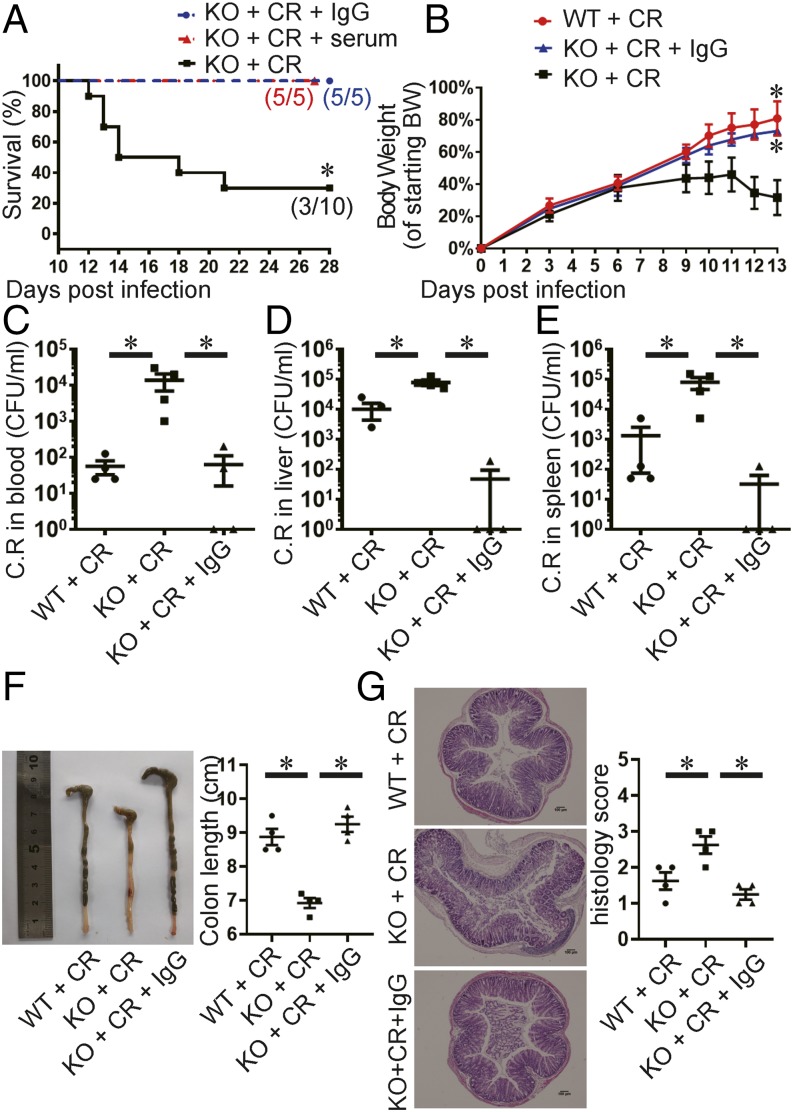
Our above observations indicate that the susceptibility of Tfh-deficient mice correlated with impaired serum IgG1 response to infection. Therefore, we further determined whether serum Abs can protect Bcl6fl/fl/CD4cre mice during infection. We adoptively transferred the sera or purified IgG from infected wild-type mice to infected Bcl6fl/fl/CD4cre mice. All Tfh-deficient mice receiving serum treatment survived. Furthermore, purified IgG from infected wild-type mice also protected all the recipient knockout mice, whereas 7 out of 10 mice receiving PBS died (Fig. 4A).
FIGURE 4.
Serum Ab from infected wild-type mice protect Tfh-deficient mice from C. rodentium infection. The weaning-age Bcl6(fl/fl)/CD4cre mice were orally inoculated with a high dose of C. rodentium and received sera or purified IgG from acutely infected wild-type mice i.v. for treatment. As parallel, mice received PBS i.v. instead as controls. Survival time (A) and body weight change (B) are shown. (C–E) C. rodentium titers were measured in blood, liver, and spleen homogenate cultures at day 14 postinfection. (F) Images of colons at day 14 postinfection. Colon lengths were measured. (G) The histological changes in the colon were analyzed on day 14 postinfection. Tissue sections were stained with H&E. Scale bars, 100 μm. (B–G) Four mice in each group were used. Data are shown as mean ± SEM and are from two independent experiments. *p ≤ 0.05, (A) Mantel–Cox test, (B–G) Mann–Whitney U test.
Next, we sought to determine the effect of Ab treatment on bacterial burdens in the target organs. We sacrificed mice at day 14 posttransfer. Compared with Bcl6fl/fl/CD4cre mice that received no IgG treatment, less-severe weight loss and improved bacterial clearance in the target organs were found in Bcl6fl/fl/CD4cre mice that received IgG treatment (Fig. 4B–E). In addition, colon length shortening and histology scores were also significantly improved to the similar levels as in infected wild-type mice (Fig. 4F, 4G). Collectively, these results confirm that impaired humoral immune response resulted from Tfh deficiency in Bcl6fl/fl/CD4cre mice renders their susceptibility to C. rodentium infection.
Transfer of Tfh cells restores IgG1 levels and offers protection in C. rodentium–infected Tfh-deficient mice
Because Tfh deficiency correlated with impaired IgG1 production in Bcl6fl/fl/CD4cre mice, we sought to determine whether transfer of Tfh cell is sufficient to restore IgG1 levels and re-establish resistance in these mice to infection. CXCR5+PD-1+ Tfh cells were sorted from the spleens and MLNs of infected wild-type mice and adoptively transferred into Bcl6fl/fl/CD4cre recipient mice, which were subsequently challenged with C. rodentium 24 h later. At day 14 postinfection, mice receiving Tfh cell transfer had restored GC B cells and IgG1-producing B cells in MLNs and spleens (Fig. 5A, 5B). The serum IgG1 levels were also recovered (Fig. 5C). In addition, Tfh cell transfer resulted in less worsened weight loss (Fig. 5D) and improved bacterial clearance in target organs (Fig. 5E–G) compared with the mice without Tfh cell transfer. Consistently, the reduced pathogen burden in mice following Tfh cell transfer was accompanied by improved colon length shortening and reduced pathology scores in the colon compared with the control mice without cell transfer. (Fig. 5H, 5I). As a control, transfer of total CD4+ T cells from naive wild-type mice was ineffective in restoring the IgG1 levels and failed in improving protection against C. rodentium infection (data not shown). Therefore, these results establish a central role of Tfh cells in regulating protective IgG1 response following C. rodentium infection.
FIGURE 5.
Transfer of Tfh cells restores IgG1 levels and protects Tfh-deficient mice from C. rodentium infection. The weaning-age Bcl6(fl/fl)/CD4cre mice received i.v. treatment with CXCR5+PD1+ Tfh cells in MLNs and spleens from infected wild-type mice and were orally inoculated with a high dose of C. rodentium 24 h later. In parallel, mice received PBS i.v. instead as controls. (A) Flow cytometric analysis of B220+CD95+GL7+ GC B cells in MLNs and spleens. (B) Flow cytometric analysis of IgG1+B220+ cells in MLNs and spleens. We calculated the cell numbers. Each point represents an individual mouse. (C) The levels of C. rodentium–specific IgG1 in the sera were detected by ELISA, using serial dilutions. (D) Body weight changes are shown. (E–G) C. rodentium titers were measured in blood, liver, and spleen homogenate cultures at day 14 postinfection. (H) Images of colons at day 14 postinfection. Colon lengths were measured. (I) The histological changes in the colon were analyzed on day 14 postinfection. Tissue sections were stained with H&E. Four mice in each group were used. Data are shown as mean ± SEM and are from two independent experiments. *p ≤ 0.05, **p ≤ 0.01, (A–I) Mann–Whitney U test.
IL-21 and IL-4 are produced in Tfh cells during infection
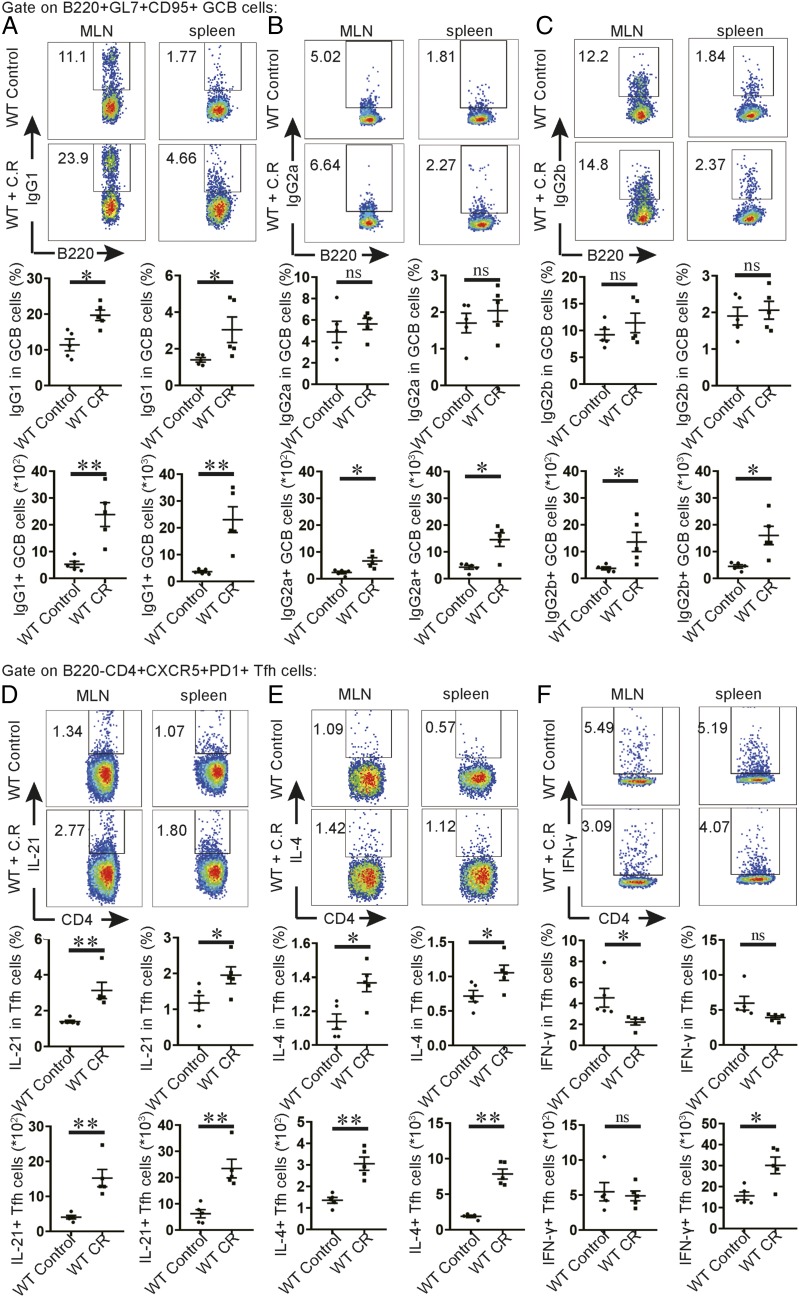
To understand the functional mechanisms of Tfh cells in C. rodentium infection, we measured Ab isotype class switching in GC B cells. We found that IgG1+ GC B cells were increased by ∼4-fold in MLNs and spleens following infection, whereas IgG2a and IgG2b production in GC B cells was only modestly increased (Fig. 6A–C). This observation indicates that IgG1 may be a major isotype in GC B cells following C. rodentium infection. In addition, we measured IgG2a and IgG2b responses in MLNs and spleens in mice lacking Bcl6 in T cells following infection. In contrast to almost absent levels of IgG1, the production of IgG2a and IgG2b in B cells from MLNs and spleens was normal in these mice (Supplemental Fig. 4A, 4B). This finding is consistent with our results in Fig. 2 showing comparable levels of IgG2 in sera between mice with or without Tfh cells. Thus, IgG2a and IgG2b can be induced in mice without Tfh response following C. rodentium infection. These results support the notion that Tfh-dependent IgG1 production may be a major response of GC B cells following C. rodentium infection.
FIGURE 6.
IL-21 and IL-4 production by Tfh cells in C. rodentium infection. We orally inoculated 3- to 4-wk-old mice with a low dose of C. rodentium. We sacrificed the mice at day 9 postinfection to see the immune responses in MLNs and spleens. Flow cytometric analysis of IgG1+ GC B cells (A), IgG2a+ GC B cells (B), IgG2b+ GC B cells (C), IL-21+ Tfh cells (D), IL-4+ Tfh cells (E), and IFN-γ+ Tfh cells (F) in MLNs and spleens are shown. We calculated the cell numbers. Each point represents an individual mouse. Data are shown as mean ± SEM and derived from two independent experiments. *p ≤ 0.05, **p ≤ 0.01, t test.
It is known that IL-21 and IL-4 promote the production of IgG1 by B cells (22, 23), whereas IgG2 response is facilitated by IFN-γ–producing cells (24). To determine whether Tfh cells produce cytokines to facilitate the production of IgG1 Ab to control C. rodentium infection, we measured IL-21, IL-4, and IFN-γ in Tfh cells from MLNs and spleens. In CD4 T cells, IL-21 was mainly expressed by CXCR5+PD-1+ Tfh cells. Upon C. rodentium infection, we found IL-21 and IL-4 expression in Tfh cells was increased obviously in wild-type mice (Fig. 6D, 6E). In contrast, IFN-γ+ Tfh cells were only altered significantly in spleens following infection (Fig. 6F). In conclusion, our findings suggest IL-21 and IL-4 production in Tfh cells may be a major mechanism underlying Tfh cell–driven IgG1 production by GC B cells following C. rodentium infection.
Discussion
The main goal of this study is to assess the role of Tfh cells in the pathogenesis of intestinal infection using C. rodentium in mice. Although previous studies reported CD4+ T cell–dependent humoral immunity is critical for controlling the systemic spread of C. rodentium infection (6, 7), the subset of CD4+ T cells as well as the underlying functional mechanisms in control of C. rodentium infection remain to be elucidated. In this study, we provide the first evidence, to our knowledge, that Tfh cells are essential in protection against C. rodentium infection. We found that Tfh cells expanded in the MLNs and spleens following C. rodentium infection. As C. rodentium initially colonized at cecum, we examined GC response in caecal patches to infection. Different from what happened in MLNs and spleens, the size of caecal patch and the total lymphocytes within caecal patches decreased gradually from day 7 to 14 postinfection. One possible explanation might be that these cells may migrate into other sites, such as other gut-associated lymphoid tissues and MLNs. Although we did not find significant expansion of Tfh cells localized in caecal patch following infection, the increase of GC B cells in this structure also indicated the role of GC response to C. rodentium infection. Moreover, the susceptibility of Bcl6fl/fl/CD4cre mice at the late phase of infection demonstrates that protective adaptive immunity requires a systemic Tfh cell response. Additionally, the normal response at the early phase of infection in mice with conditional ablation of Bcl6 in T cells indicates that Tfh cells are not required for the innate immunity to C. rodentium infection.
Infections with virus or bacteria may result in the formation of Tfh cells, which localize in B cell follicles to provide the necessary signals for Ab production. During this process, the isotypes of the Ab responses are pathogen dependent and differ from those elicited by the Ags. IgG2 are known as effective virus-neutralizing Abs that provide protective responses (12, 19). Recent research indicates that IgG1, but not IgG2a, is required in immune protection against Salmonella typhimurium (20). It has been unclear which Ab isotype is required for the protection against C. rodentium. In the current study, we found that the susceptibility of Tfh-deficient mice to infection correlated with the defective production of IgG1, whereas the levels of IgG2, IgG3, IgM, and IgA were comparable between groups, suggesting that Tfh-dependent IgG1 response may be important in controlling C. rodentium infection. Our results support one previous study reporting that IgA, IgM, and IgG3 are not required for eradication of C. rodentium (9). In another study, sera from infected IFN-γ−/− mice can protect 100% of T cell–deficient mice to C. rodentium infection, which suggests that IFN-γ–dependent IgG2 is dispensable for protection against C. rodentium infection (6). In contrast, we analyzed the adaptive immune responses in caecal patch, MLN, and spleen to infection and found the almost absent levels of Tfh, GC B, and IgG1+ B cells in Tfh-deficient mice, which underscored the absent level of IgG1 in sera.
Furthermore, the ability of transferred immune sera or purified IgG in restoring the systemic immunity in infected Bcl6fl/fl/CD4cre mice demonstrates Tfh-dependent protective Abs are critical for the pathogen clearance. The adoptive transfer of Tfh cells from infected mice but not naive mice restored IgG1 levels in sera and protected the Bcl6fl/fl/CD4cre recipient mice from the pathogens in infecting organs. The lack of protection by total CD4+ T cells from naive wild-type mice might be because C. rodentium Ag-specific Tfh cells had not been generated. In contrast, Tfh cells in the infected wild-type mice were mostly Ag specific and could directly migrate to GC to provide the necessary signals to help the production of high-affinity C. rodentium–specific IgG1 Ab. These observations underscore the crucial role of Tfh cells in inducing IgG1 response following C. rodentium infection.
Previous studies have shown that cytokines play vital roles in Ab class switching. IL-21 and IL-4 are essential for the production of IgG1, whereas IFN-γ contributes to the generation of IgG2 Ab (22–24). Tfh cell is the main producer of IL-21 and IL-4 from T cells (10), indicating the potential effect on IgG1 class switching from GC B cells. Th1 cell (19) and Th1-like Tfh cell (12) could be the important mediators of IgG2 class switching to viral infection. In the current study, we detected substantial levels of IL-21 and IL-4 in Tfh cells during C. rodentium infection, correlating with the increase of IgG1 production from GC B cells. Meanwhile, IFN-γ expression in Tfh cells and IgG2 from GC B cells were moderate in response to C. rodentium infection. Our findings are in line with the previous report that the humoral response in IFN-γ−/− mice was sufficient to protect CD4+ T cell–deficient mice from infection with C. rodentium (6). Our results also suggest that IL-21 and IL-4 production may be a major function in Tfh cells, resulting in IgG1 class switching in GC B cells following C. rodentium infection.
In summary, our data demonstrate that Tfh cells are crucial at the late phase of intestinal C. rodentium infection and function to promote protective systemic humoral responses. These findings may suggest new, effective therapy for intestinal infection.
Supplementary Material
This work was supported by grants from the National Key Research and Development Program of China (2016YFC0906200 to C.D]), the National Natural Science Foundation of China (91642201 and 31821003 to C.D. and 31500743 to X.B.), Beijing Municipal Science and Technology (Z181100006318015 and Z181100001318007 to C.D.), and the China Postdoctoral Science Foundation (2016M590105 to X.B.).
The online version of this article contains supplemental material.
- GC
- germinal center
- ILC
- innate lymphoid cell
- MLN
- mesenteric lymph node
- Tfh
- T follicular helper.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Perez-Lopez A., Behnsen J., Nuccio S. P., Raffatellu M. 2016. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 16: 135–148. [DOI] [PubMed] [Google Scholar]
- 2.Ochoa T. J., Barletta F., Contreras C., Mercado E. 2008. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans. R. Soc. Trop. Med. Hyg. 102: 852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins J. W., Keeney K. M., Crepin V. F., Rathinam V. A., Fitzgerald K. A., Finlay B. B., Frankel G. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat. Rev. Microbiol. 12: 612–623. [DOI] [PubMed] [Google Scholar]
- 4.Mundy R., MacDonald T. T., Dougan G., Frankel G., Wiles S. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 7: 1697–1706. [DOI] [PubMed] [Google Scholar]
- 5.Guo X., Liang Y., Zhang Y., Lasorella A., Kee B. L., Fu Y. X. 2015. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity 42: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bry L., Brigl M., Brenner M. B. 2006. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect. Immun. 74: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bry L., Brenner M. B. 2004. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172: 433–441. [DOI] [PubMed] [Google Scholar]
- 8.Simmons C. P., Clare S., Ghaem-Maghami M., Uren T. K., Rankin J., Huett A., Goldin R., Lewis D. J., MacDonald T. T., Strugnell R. A., et al. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71: 5077–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maaser C., Housley M. P., Iimura M., Smith J. R., Vallance B. A., Finlay B. B., Schreiber J. R., Varki N. M., Kagnoff M. F., Eckmann L. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72: 3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29: 621–663. [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Nurieva R. I., Dong C. 2013. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol. Rev. 252: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang H., Tang J., Liu Z., Liu Y., Huang Y., Xu Y., Hao P., Yin Z., Zhong J., Ye L., et al. 2019. ZIKV infection induces robust Th1-like Tfh cell and long-term protective antibody responses in immunocompetent mice. Nat. Commun. 10: 3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greczmiel U., Kräutler N. J., Pedrioli A., Bartsch I., Agnellini P., Bedenikovic G., Harker J., Richter K., Oxenius A. 2017. Sustained T follicular helper cell response is essential for control of chronic viral infection. Sci. Immunol. 2: eaam8686. [DOI] [PubMed] [Google Scholar]
- 14.Sheikh A. A., Cooper L., Feng M., Souza-Fonseca-Guimaraes F., Lafouresse F., Duckworth B. C., Huntington N. D., Moon J. J., Pellegrini M., Nutt S. L., et al. 2019. Context-dependent role for T-bet in T follicular helper differentiation and germinal center function following viral infection. Cell Rep. 28: 1758–1772.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno H. 2016. T follicular helper cells in human autoimmunity. Curr. Opin. Immunol. 43: 24–31. [DOI] [PubMed] [Google Scholar]
- 16.Gensous N., Charrier M., Duluc D., Contin-Bordes C., Truchetet M. E., Lazaro E., Duffau P., Blanco P., Richez C. 2018. T follicular helper cells in autoimmune disorders. Front. Immunol. 9: 1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva R. I., Chung Y., Martinez G. J., Yang X. O., Tanaka S., Matskevitch T. D., Wang Y. H., Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koroleva E. P., Halperin S., Gubernatorova E. O., Macho-Fernandez E., Spencer C. M., Tumanov A. V. 2015. Citrobacter rodentium-induced colitis: a robust model to study mucosal immune responses in the gut. J. Immunol. Methods 421: 61–72. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi K., Sugimoto-Ishige A., Harada Y., Adachi Y., Usami Y., Kaji T., Inoue K., Hasegawa H., Watanabe T., Hijikata A., et al. 2016. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 17: 1447–1458. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Dominguez-Medina C., Cumley N. J., Heath J. N., Essex S. J., Bobat S., Schager A., Goodall M., Kracker S., Buckley C. D., et al. 2017. IgG1 is required for optimal protection after immunization with the purified porin OmpD from Salmonella typhimurium. J. Immunol. 199: 4103–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good-Jacobson K. L., Szumilas C. G., Chen L., Sharpe A. H., Tomayko M. M., Shlomchik M. J. 2010. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozaki K., Spolski R., Feng C. G., Qi C. F., Cheng J., Sher A., Morse H. C., III, Liu C., Schwartzberg P. L., Leonard W. J. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science 298: 1630–1634. [DOI] [PubMed] [Google Scholar]
- 23.Ochel M., Vohr H. W., Pfeiffer C., Gleichmann E. 1991. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J. Immunol. 146: 3006–3011. [PubMed] [Google Scholar]
- 24.Snapper C. M., Paul W. E. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.