Abstract
Artificial nerve guidance conduits (NGCs) are being investigated as an alternative to autografts, since autografts are limited in supply. A polycaprolactone (PCL)-based spiral NGC with crosslinked laminin on aligned nanofibers was evaluated in vivo post a successful in vitro assessment. PC-12 cell assays confirmed that the aligned nanofibers functionalized with laminin were able to guide and enhance neurite outgrowth. In the rodent model, the data demonstrated that axons were able to regenerate across the critical nerve gap, when laminin was present. Without laminin, the spiral NGC with aligned nanofibers group resulted in a random cluster of extracellular matrix tissue following injuries. The reversed autograft group performed best, showing the most substantial improvement based on nerve histological assessment and gastrocnemius muscle measurement. Nevertheless, the recovery time was too short to obtain meaningful data for the motor functional assessments. A full motor recovery may take up to years. An interesting observation was noted in the crosslinked laminin group. Numerous new blood capillary-like structures were found around the regenerated nerve. Owing to recent studies, we hypothesized that new blood vessel formation could be one of the key factors to increase the successful rate of nerve regeneration in the current study. Overall, these findings indicated that the incorporation of laminin into polymeric nerve conduits is a promising strategy for enhancing peripheral nerve regeneration. However, the best combination of contact-guidance cues, haptotactic cues, and chemotactic cues have yet to be realized. The natural sequence of nerve regeneration should be studied more in-depth before modulating any strategies.
Keywords: Tissue engineering, Biomaterial, Peripheral nerve regeneration, Nerve conduit, Aligned nanofiber, Laminin
1. Introduction
Peripheral nerve injury affects thousands of Americans every year. On average, there are over 250,000 procedures require a nerve guidance conduit (NGC) or an autograft annually. The predominantly preferred treatment is still an autograft [1]. A tissue engineered NGC is a viable clinical alternative for clinicians to treat peripheral nerve injury [2–5]. This construct has the potential to replace the use of autografts, thus eliminating some of the limitations, such as limited supply, diminished Schwann cell viability after harvest, size mismatch, and donor site morbidity. [6]. Although commercial options are available in terms of entubulation (tubular nerve guide), none of them have contact-guidance, haptotactic, or chemotactic cues that are presented in their devices. In an effort to improve functional recovery across large gaps (beyond the critical gap, i.e. 15 mm in rodents), the creation of a more conductive microenvironment is crucial. Failure in regeneration can be due to a variety of factors: (1) inadequate ECM formation; (2) insufficient neurotrophic support; or (3) inadequate Schwann cells numbers, etc. [7].
To date, numerous tissue engineering approaches have been utilized to mimic biologic tissues. For instance, contact-guidance cues, haptotactic cues, and chemotactic cues have been identified to facilitate nerve regeneration. Contact-guidance cues play a role in response to physically guide injured tissue toward the desired destination, owing to its unique geographic design. Haptotactic cues are the incorporation of contact-mediated cues such as insoluble ECM proteins to guide axonal elongation to the target organ [8]. ECM proteins such as collagen, fibronectin, and laminin have considerable positive effects on nerve regeneration [9–14]. For example, laminin is one of the series of structural proteins that activates the β1-integrin receptor and is a critical player of the peripheral nerve basal lamina, which is required to promote Schwann cell migration and axonal elongation [15–19]. However, improper concentrated laminin may hinder nerve regeneration [18, 20]. The optimal concentration range for promoting nerve regeneration is still unknown. Therefore, further investigation is needed.
In our previous study (10 mm rat sciatic nerve defect) [21], we demonstrated the polycaprolactone (PCL)-based spiral NGC with aligned nanofibers met the mechanical and nutrient transport requirements for nerve regeneration. A highly porous spiral channel augmented surface areas for cell invasion and proliferation, and aided nerve tissue regrowth. In addition, electrospun nanofibers coated on spiral channel provided a guidance cue to ensure that regenerated axons follow the shortest distance across the gap toward the distal nerve stump. Finally, the outer nanofibrous tube can prevent scar tissue infiltration and provided a durable mechanical strength through out the entire nerve regeneration.
The current objective was to introduce haptotactic cue (i.e. laminin) onto our novel spiral NGC. Due to the absence of functional groups in PCL, amine-containing polyethylene glycol (PEG-diamine) was incorporated onto PCL aligned nanofibers. The PCL/ PEG-diamine aligned nanofibers were then covalently crosslinked with laminin, providing the contact guidance cue and contact mediated cue simultaneously to facilitate axonal regeneration. Prior to in vivo, the crosslinking kinetic of laminin was determined in vitro. The in vitro evaluation would ensure crosslinked laminin was sufficiently present on the PCL/ PEG-diamine aligned nanofibers and promoted cellular activities. Based on the benefits of a novel spiral structure, aligned nanofibers, and an additive, we hypothesize that the novel spiral NGC with crosslinked laminin on aligned nanofibers would provide the suitable macro-environment, transport features, and ideal mechanics for enhancing the rate of nerve regeneration across a critical size (15 mm) nerve defect within 12 weeks. The efficacy of the proposed study was investigated based on the results of walking track analysis, electrophysiology, gastrocnemius muscle measurement, and histological assessment relative to the autograft.
2. Materials and Methods
2.1. Materials
Polycaprolactone (PCL, MW 70-90 kDa) and polyethylene glycol-diamine (PEG-diamine, average MW 3,000) were purchased from Sigma Aldrich (St. Louis, MO). Sodium Chloride (NaCl) was procured from Fisher Scientific (Pittsburgh, PA). 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) was obtained from Oakwood Products, while dichloromethane (DCM) came from (Pharmco-AAPER, Brookfield, CT). Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY), RPMI medium, PC-12 (PC-12 (ATCC CRL-1721, Manassas, VA), laminin (L4544, Sigma Aldrich), Laminin Antibody conjugated Alexa Fluor 488 (NB300-144AF488, Novusbio), laminin ELISA kit (abl 19599; Abcam, Cambridge, MA), Anti-Neurofilament 200 antibody (N4142, Sigma Aldrich), goat anti-rabbit IgG (H+L) with fluorescein isothiocyanate 1 (FITC) (111-095-045, Jackson Immunoresearch Laboratories, Philadelphia, PA) were used in this study.
2.2. PCL sheet fabrication
8% (w/v) PCL in 4 ml of DCM was fabricated into a PCL sheet using a solvent evaporation technique and salt-leaching approach [22]. The PCL solution was poured onto a glass petri dish to air dry in the hood for an hour. Afterwards, the PCL sheet was cut into a rectangular dimension 17 mm x 13.5 mm. Two smaller rectangles were then cut off along the longitudinal end to create chambers at the two ends of the conduit.
2.3. Electrospun PCL/PEG-diamine nanofiber for laminin crosslinking
PCL/PEG-diamine nanofiber were electrospun on PCL sheets, using a conductible rotating disk. Briefly, 50 mg of PEG-(diamine) was dissolved in 4 ml of 16 % (w/v) PCL/ HFIP solution for electrospinning. The solution was electrospun at 11 kV, 0.25 ml/hr with a distance of 5.5 cm. Aligned nanofibers were coated directly on PCL sheets, which were attached to the edge of a rotating disk. For the control, there was no PEG-diamine blended into 4 mL of 16% PCL (w/v) solution.
2.4. Integrated spiral NGC with outer nanofibrous tube
PCL sheets with aligned nanofibers were rolled up into spiral structures. Finally, random nanofibers were electrospun onto the outer layer of spiral NGCs to form an outer nanofibrous tube. The outer nanofibrous tube secured the spiral channel and enhanced the mechanical strength. A light microscope and a scanning electron microscope (SEM) were utilized to characterize the NGCs. Specimens were gold coated and examined for pore shape and interconnection of the pores, morphology, and structure of matrices
2.5. Cross-linking laminin on PCL/ PEG-diamine aligned nanofibers
Various concentrations of laminin at 0.5, 2, and 4 mg/ml were prepared by dissolving laminin in 2 ml PBS. The PEG-diamine bearing functionalities were crosslinked using a standard well established carbodiimide chemistry according to a published procedure [23, 24]. Spiral NGC with PCL/PEG-diamine aligned nanofibers were incubated with aforementioned laminin concentration in a buffer containing 48 mM 1-EDC and 6 mM N-Hydroxysuccinimide (NHS) (w/v) for 2 hours at 37 °C, following by 3 times of DI water washes to remove excess cross-linking agents. In addition to compare the efficiency, 2 mg/ml laminin was also applied to the spiral NGC with PCL aligned nanofibers (without PEG-diamine) to serve as control, following the same crosslinking procedure.
2.6. Analysis of the cross-linked laminin
The amount of laminin crosslinked on PCL/PEG-diamine aligned nanofibers was determined using a known concentration to subtract the amount of remaining non-crosslinked laminin in the tube. The amount of non-crosslinked laminin was measured with a human laminin ELISA kit.
To visualize the laminin crosslinked on the PCL/PEG-diamine aligned nanofibers, a Laminin Antibody conjugated Alexa Fluor 488 was prepared by dilute the antibody solution into 1:100. 200 μl of the above solution was spread on the laminin crosslinked PCL/PEG-diamine aligned nanofibers evenly in a dark room for an hour. The nanofibers were then washed with PBS and imaged with a Nikon Eclipse 1000U microscope.
2.7. PC-12 cell differentiation assay
To determine the optimal concentration of laminin on PCL/PEG-diamine aligned nanofibers for stimulating best axonal regeneration, various concentrations of laminin (0, 0.5, 2, and 4 mg/ml) were evaluated by observing neurite outgrowth from PC-12 cells. Prior to the PC-12 cell study, all scaffolds were sterilized with 70% isopropyl alcohol for 10 minutes, followed by 3 times of PBS washes. PC-12 cells were then seeded at a density of 100 cell clusters/conduit in a 12-well plate in 2 ml of RPMI 1640 medium supplemented with 10% horse serum, 5% fetal bovine serum, and 1% penicillin streptomycin. Cells were maintained in a humid, 5% CO2 incubator, at 37 °C. The medium was replaced with low serum medium (5% horse serum) next day and changed every other day. At least three samples were used per time point, per sample type. NGF supplemented medium (50 ng/mL) was added into each group up to 7 days.
2.8. Neurite outgrowth
PC-12 cells were stained for neurofilaments to evaluate neurite outgrowth. Cells were fixed in 4% paraformaldehyde for 25 minutes and followed by a wash of PBS. Monoclonal anti-neurofilament 100 was diluted 1:150 and applied to cells. All samples were incubated overnight at 4 °C. The second conjugated antibody (anti-mouse IgG; Sigma) was then applied for 25 minutes after a wash with PBS. Following incubation, the NGCs were viewed using confocal laser scanning microscopy. At least five frames per well were imaged. Neurite length and the percentage of PC-12 cells with neurite outgrowth were quantitatively determined. By definition, neurite-bearing cells are the ones with processes (neurites) greater than or equal to the cell body diameter.
2.9. Animal study
The efficacy of the NGC was investigated in vivo on 15 mm rat sciatic nerve gap. Two types of tissue-engineered NGCs and a reversed Autograft were evaluated (n = 9), and the specific categories are shown in Table 1. Specifically, the groups include: (1) Reversed autograft; (2) Spiral NGC with aligned nanofibers wrapped with outer nanofibrous tube (SAT); (3) Spiral NGC with aligned nanofibers wrapped with outer nanofibrous tube and crosslinked with 2 mg/ml of laminin (SAT+Laminin). The reversed autograft group was the positive control and the SAT group was the negative control, to assess the efficacy of laminin on SAT. The degree of nerve regeneration was assessed after 12 weeks post-implantation as described in the Table 1 below. All animals were carefully maintained according to methods approved by the Institutional Animal Care and Use Committee at the Stevens Institute of Technology and the National Institutes of Health regulations and standards for animal usage were followed.
Table 1.
NGCs Study Groups
| Type of Implant | In vivo | Duration of Implantation | Characterizations |
|---|---|---|---|
| Reversed autograft | N = 9 | 12 weeks | Walking track analysis, electrophysiology, gastrocnemius muscle measurement, and histological assessment. |
| SAT | N = 9 | 12 weeks | |
| SAT+Laminin | N = 9 | 12 weeks |
2.10. Surgical procedures
200-250 grams Male Sprague-Dawley (SD) rats were utilized for the experiment. Prior to implantation, rats were anesthetized, shaved and prepped. A 20-mm incision was made along the femoral axis. After separating the thigh muscles, the sciatic nerve was dissected free and a 5 mm piece of nerve, ending 5 mm proximal to the sciatic trifurcation, was explanted. The proximal and distal stump was secured 15 mm apart and 17 mm long spiral NGCs would be connected using a 10-0 Nylon monofilament suture. Harvested autografts were trimmed to 15 mm length and reversed for implantation. The musculature around the defect was closed with a single continuous resorbable suture, and the incision was closed with surgical staples. Pain was managed with injections of Meloxicam every 24 hours as needed for 48 hours following surgery. NIH guidelines for using experimental animals was followed both pre- and post-operatively. At the endpoint (12 weeks), animals were euthanized via intra-cardiac perfusion fixation. Briefly, animals were anesthetized with ketamine/xylazine (via intraperitoneal injection). Whole-body perfusion commenced using heparinized saline, followed by 4% paraformaldehyde and 0.25% gluteraldehyde.
2.11. Walking track analysis
Walking track analysis is a method to test the functional muscle reinnervation [25, 26]. Pre-operatively, the right hind paws of the rats were painted with trypan blue dye, and the right hind paw prints were recorded by training the animal to walk in a box. The parameters from paw prints taken before and after surgery was considered to be normal and experimental, respectively. The SFI was calculated every 4 weeks post-surgery until the end point of the study. The paw prints from the experimental and control hind limbs were compared.
As the Sciatic Functional Index (SFI) value approaches zero, the corresponding functional recovery was better. Three parameters were derived from the paw prints: print length (PL), toe spread (TS; distance from toe 1 to toe 5), and intermediate toe spread (IT; distance from toe 2 to toe 4). The sciatic functional index was calculated by using the following formula:
2.12. Electrophysiological testing
Electrophysiological studies were performed using an AD instruments Power Lab 4/25 stimulator and Bio AMP amplifier to test the restoration of functionality of the regenerated nerve at 12 weeks post-implantation [27, 28]. Each rat was re-anesthetized during the course of the data collection. Recording needle electrodes were placed in the gastrocnemius muscle and stimulation electrodes were placed directly posterior to the tibia; the sciatic nerve was stimulated with two stainless wire electrodes connected to an electrical stimulator. A ground electrode was placed in the surrounding muscle tissues to remove conduction of stimulation through muscle tissues. The amplitude of evoked compound muscle action potentials (CMAPs) and nerve conduction velocity (NCV) were calculated.
2.13. Nerve histological assessment
At the endpoint (12 weeks), rats were euthanized via intra-cardiac perfusion fixation. The surgical site was reopened to explant the NGCs for a histological analysis. An entire NGC with regenerated nerves tissue was explanted and stored in 4% paraformaldehyde. A 2 mm segment at the gap mid-point of an NGC was placed in 1% PBS-buffered osmium tetroxide at room temperature for 2 h, dehydrated with a series of ethanol washes, and embedded in resin. Same procedure was applied to a reversed autograft. Sections (900 nm) were sliced with an ultramicrotome (Leica EM UC6 ultracut, Wetzlar, Germany) then stained with 1% toluidine blue for analysis of morphometry. Normal nerves (n=9) were also taken for related examinations. The 40x magnification images were acquired under a light microscope. Specific regions of cross-sectional nerves were analyzed by ImageJ. Percent reinnervation at the gap midpoint and the total number of myelinated axons were reported.
2.14. Gastrocnemius muscle measurement
As the gastrocnemius muscle started to atrophy after sciatic nerve injury, its mass was proportional to extent of sciatic nerve innervation. Therefore, the gastrocnemius muscle mass provided indirect evidence for evaluating functional sciatic nerve regeneration. The relative gastrocnemius muscle weight (RGMW), which is defined as the ratio of the gastrocnemius muscle weight from the experimental (right) side to that of the normal (left) side, was used as the parameter that represents the “functional” consequences of sciatic nerve regeneration.
2.15. Statistical analysis
The data was expressed as mean values ± standard deviation. Statistical analysis was performed with one-way analysis of variance (ANOVA) with Tukey’s post hoc multiple comparison test. A value of p <0.05 was considered to be statistically significant.
3. Results
3.1. Morphological images
The measurement results from the light microscope (Fig. 1A – B) confirmed that the final spiral NGC prototype achieved the following dimensions: 17 mm in length, 1.8 mm in outer diameter, and a 50 μm thick spiral wall. In (Fig. 1B) a transection cross sectional view of the spiral NGC showed the number of spiral layers secured by an outer nanofibrous tube. The gap between the spiral channels was fixed at 100-150 μm. The macro-porous PCL sheets were coated with PCL aligned nanofibers (via electrospinning). The conduit’s topography as shown in Fig. 1C SEM images indicated that the enhanced porous structure with inter-connection pores and aligned nanofibers, which were coated uni-axially on the spiral wall. The size of the inner aligned fibers was 1090.3±56.1nm. The pore size was approximately 147.2±45.5 μm.
Figure 1.

The morphology of novel spiral NGC: (A) a 17 mm SAT; (B) cross-section view of SAT; (C) SEM image revealed aligned nanofibers on the conduit’s porous wall. Scale bar represents 200 μm.
3.2. Laminin crosslinked onto PCL/PEG-diamine aligned nanofibers
Table 2 indicated the amount of laminin crosslinked onto aligned nanofibers via the functional groups from PEG-diamine. The amount of crosslinked laminin was increased with increasing laminin concentration.
Table 2.
Summary of the amount of crosslinked laminin on aligned nanofibers
| 2 mg/ml Laminin (without PEG-diamine) | 0.5 mg/ml Laminin | 2 mg/ml Laminin | 4 mg/ml Laminin | |
|---|---|---|---|---|
| Laminin per NGC (mg) | 0 | 0.04±0.02 | 0.17±0.05 | 0.46±0.17 |
As seen in Fig 2, it was evident that laminin was uniformly crosslinked onto PCL/PEG-diamine aligned nanofibers, indicated by fluorescent labeled laminin on the microscope images. There was no specific binding observed in Fig. 2A; therefore, confirming the presence of laminin was absent on the PCL aligned nanofibers without PEG-diamine. In case of laminin crosslinked PCL/PEG-diamine aligned nanofibers (Figure 2B–D), uniform distribution of the laminin was observed, owing to increased concentration of laminin on the PCL/PEG-diamine aligned nanofibers.
Figure 2.
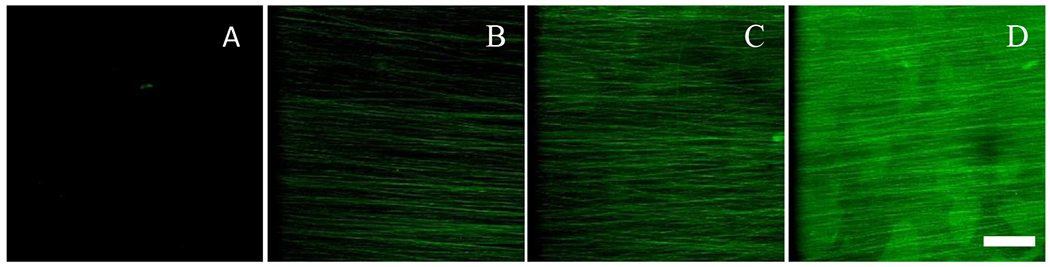
The florescence images: (A) laminin was absent on PCL aligned nanofibers without PEG-diamine. (B-D) various laminin concentrations (from left to right: 0.5 mg/ml, 2 mg/ml, and 4 mg/ml) were crosslinked uniformly on the PCL/PEG-diamine aligned nanofibers. Scale bar represents 50 μm.
3.3. PC-12 cell differentiation assay
To optimize the contact-mediated signal, PC-12 cells were seeded on the PCL/PEG-diamine nanofibers with various concentration of crosslinked laminin. As shown in Fig. 3A–D, the laminin containing groups had a significant effect on PC-12 cell differentiation. The low concentration (0.5 mg/ml) laminin group, as shown in Fig. 3B, did not display as many neurite outgrowth as medium (2 mg/ml) and high (4 mg/ml) concentration. In contrast, with 2 mg/ml laminin (Fig. 3C) and 4 mg/ml of laminin (Fig. 3D) yielded the highest neurite extension and density. The negative control (without laminin) the neurites appeared to be shorter than the rest of groups. A significantly small number of cells bearing neurites as well as rounded cell morphologies were observed.
Figure 3.
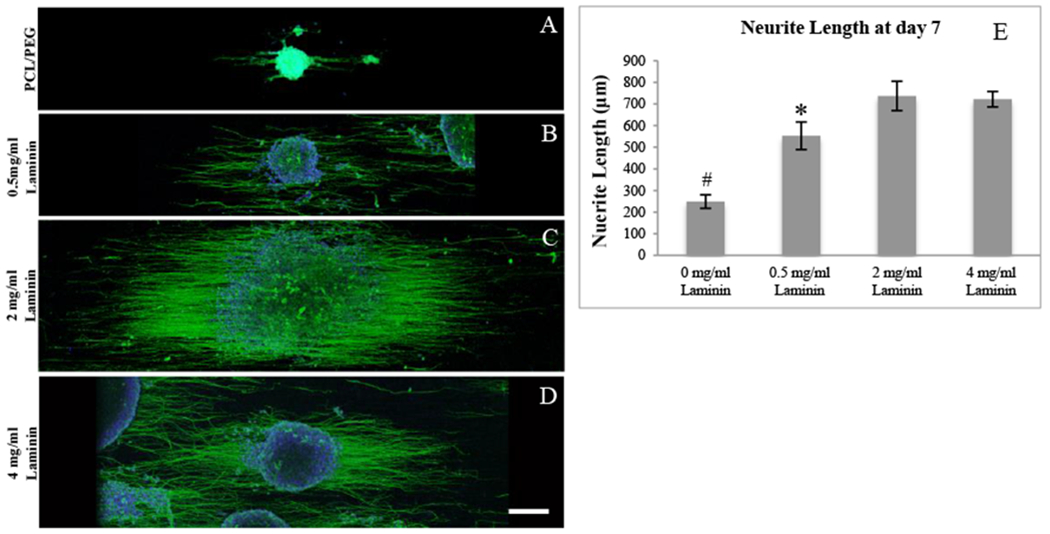
PC-12 neurite elongation. (A) PCL/PEG-diamine nanofibers; (B) PCL/PEG-diamine nanofibers with 0.5 mg/ml laminin, (C) PCL/PEG-diamine nanofibers with 2 mg/ml laminin; (D) PCL/PEG-diamine nanofibers with 4 mg/ml laminin; and (E) Quantification of PC-12 neurite length. * indicates P < 0.05. # indicates P < 0.01. Scale bar represents 200 μm.
Figure 4.

Post-implantation after 12 Weeks: (A) Reversed autograft, (B) SAT, and (C) SAT+Laminin.
To evaluate the bioactivity of the laminin, the mean length of PC-12 neurite extension, as shown in Fig. 3E, was quantified using ImageJ. The crosslinked laminin groups significantly increased neurite lengths over 7 days. The 2 mg/ml laminin group had 745±68 μm mean neurite lengths and the 4 mg/ml Laminin group had 725±39 μm. Both conditions provided the most favorable surface for PC-12 cells. Their neurite lengths were significantly higher than 0.5 mg/ml laminin and PCL/PEG-diamine groups. There was no statistical difference between 2 mg/ml and 4 mg/ml of laminin. Since 2 mg/ml laminin group provided sufficient stimulation for neurite outgrowth, this concentration should be used for in vivo study.
3.4. Post-implantation
The effectiveness of a spiral NGC with laminin was evaluated, using a 15 mm long rat sciatic nerve defect. There were no obvious signs of systemic or regional inflammation and surgical complications after implantation.
3.5. Functional recovery evaluation
The SFI data of reversed autograft, SAT, and SAT+Laminin groups were shown in Fig 5. The initial SFI values from each group plunged to −90 after surgery and were then improved with each concurrent time point (Fig. 5E). As the SFI value approached zero, the corresponding functional recovery was improved. The final SFI values at the 12-week endpoint for the reversed autograft group was −71.4±4.6, followed by the SAT (−79±4.7) and SAT+Laminin (−74±7.7) groups. A significant difference (p<0.05) was observed between the reversed autograft group and the SAT group at 8-week timepoint. Each group had significant improvement (p<0.05) when comparing 12-week SFI to 4-week SFI.
Figure 5.
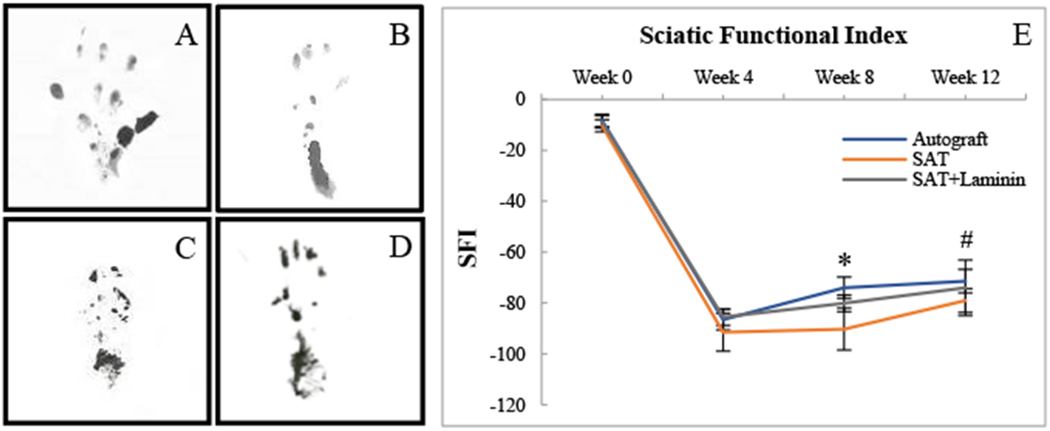
The footprints from the groups studied for SFI: (A) Normal footprint pre-surgery; (B) Reversed autograft; (C) SAT; (D) SAT+Laminin; and (E) The quantification of SFI during 12 Weeks post-implantation. * indicates the reversed autograft group has significant difference (p < 0.05) as compared to the SAT group. # indicates that the 12-week SFI from each group showed significant improvement (p<0.05) when comparing to its own 4-week SFI.
3.6. Electrophysiological assessment
Electrophysiologic assessment was performed to evaluate functional recovery after 12 weeks post-surgery. CMAPs were evoked by stimulation at the surgical limbs and recorded from gastrocnemius muscle followed by measurements of amplitude and NC V. Data was recorded and analyzed in Fig. 6E and F. As shown in Fig. 6B–D, the reversed autograft group presented strongest single with faster latency, indicating a greater presence of myelinated axons with thicker myelin sheathes.
Figure 6 -.
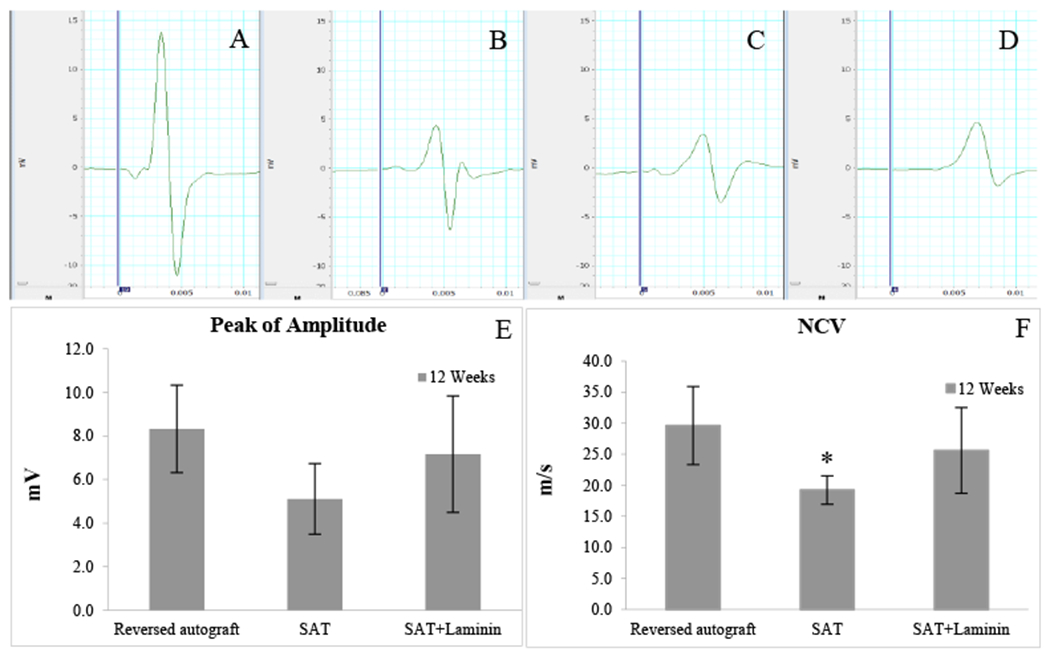
The results of the electrophysiology assessment: (A) Normal Nerve; (B) Reversed autograft; (C) SAT; (D) SAT+Laminin; and data comparison of (F) evoked CMAPs and (F) NCV. * indicates the SAT group is significant lower (p < 0.05) than the reversed autograft group.
Same samples in some groups failed to record electrophysiological signals. The size of samples was varied: reversed autograft (n=8); SAT (n=3); and SAT+Laminin (n=6). Each group’s amplitude was recorded as follows: Normal nerve (25.2±2.3mV); Reversed autograft (8.3±2 mV); SAT (5.1±1.6 mV); and SAT+Laminin (7.2±2.7 mV), respectively. All groups were statistically insignificant to each other. The results of NCV was recorded as follow: Normal nerve (32.7±1.6 m/s); Reversed autograft (29.6±6.3 m/s); SAT (19.2±2.3 m/s); and SAT+Laminin (24.8±4.5 m/s), respectively. The mean NCV value of the reversed autograft group was statistically higher (p < 0.05) than the SAT group.
3.7. Percent reinnervation at the gap midpoint
Table 3 indicates that myelinated axons success regeneration across the nerve defect at the midpoint. Previous experiments performed in Yannas’s lab had demonstrated that using the gap mid-point with short-term study would yield meaningful data based on percent reinnervation [2, 29]. In our current study, the reversed autograft group showed the highest successful rate with 89.9 % of rats showing reinnervation. An unexpected high failure rate was observed in the SAT group. The SAT group showed promising outcomes while bridging a 10 mm nerve gap within 6 weeks in our previous study [21]. The absence of an additive resulted in a lower successful rate of the SAT group of 33.3%. However, the SAT+Laminin group increased the rate up to 77.8%.
Table 3.
Summary of nerve regeneration in control and experimental groups
| Groups | No. of rats with regeneration | % Reinnervation |
|---|---|---|
| Reversed autograft | 8/9 | 89.9% |
| SAT | 3/9 | 33.3% |
| SAT+ Laminin | 7/9 | 77.8% |
3.8. Nerve histological assessment
The histological assessment is a critical assessment to evaluate nerve regeneration as it provides information of how effective a method of treatment is, based on the number of axons that travel from the proximal end to the distal end. High magnifications of micrographs (Fig. 7A–D) provided detailed views of the myelinated axons at gap mid-point. Only the reversed autograft group showed the regenerated axons with large fiber diameters and was encased in myelin sheaths of uniform thickness. The SAT group had lowest myelinated axons with various unmyelinated axons. The SAT+Laminin group had small myelinated axons with many capillary vessels around.
Figure 7.
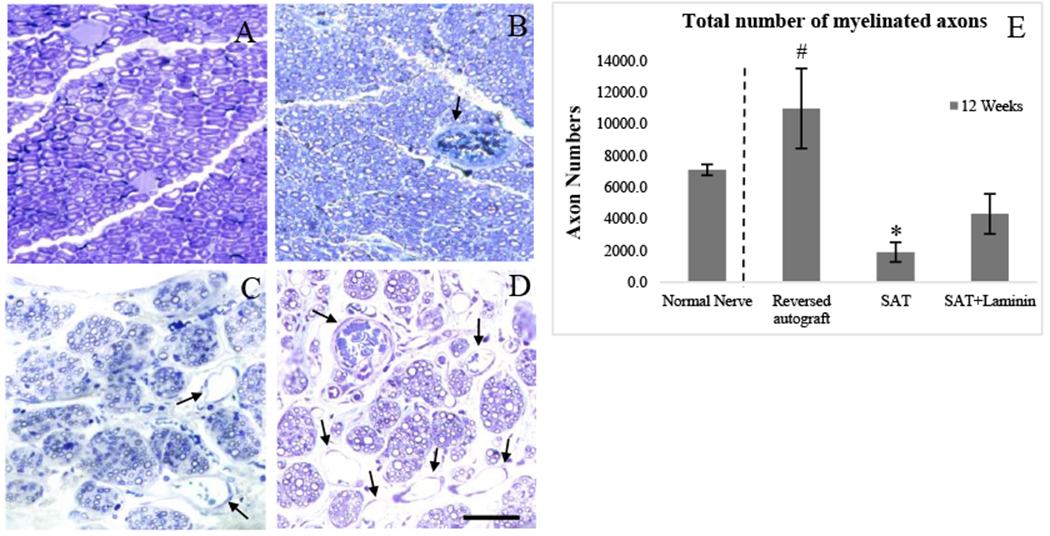
The nerve histological image showing myelinated axons at the mid-point after 12 Week post-implantation: (A) Normal nerve; (B) Reversed Autograft; (C) SAT; (D) SAT+Laminin; and (E) Total number of myelinated axons at gap midpoint. * indicates a significantly difference (p < 0.05). # indicates a highly significantly difference (p < 0.01). Black line arrows indicate blood vessel formation. Scale bar represents 25 μm.
Quantitative analysis of total myelinated axons (Fig. 7E) was measured. The size of samples was varied. Only those cases which were successfully regenerated were recorded: Normal nerve (n=9); Reversed autograft (n=8); SAT (n=3); and SAT+Laminin (n=7). Rat normal sciatic nerve contains −7,100 myelinated nerve fibers. The reversed autograft contained the highest myelinated axon counts (10,974±2,525.2) and showed a significant difference to the other two groups (p<0.01). Similarly, the experimental group (SAT+Laminin: 4,310±1,264) was statistically greater than the negative control (SAT: 1,896.7±617.3) (p<0.05).
3.9. Relative gastrocnemius muscle weight
Relative gastrocnemius muscle weight (RGMW) provides indirect evidence for evaluating functional sciatic nerve regeneration since it undergoes atrophy after sciatic nerve injury; its mass is proportional to extent of sciatic nerve innervation. The reversed autograft group was statistically different relative to the rest of the groups; however, it can be seen that the additive (i.e. laminin) improved the reinnervation of the gastrocnemius muscle evident by the higher mean values. Independently, the laminin improved the efficacy of the SAT to restore gastrocnemius muscle weight reinnervation. Each group’s value was recorded as follow: Autograft (57.5±8.6%), SAT (26.1±4.3%), and SAT+Laminin (35.2±3.6%). Muscle weight ratio of the reversed autograft group was highly statistically greater as compared to the other two groups (p<0.01). However, there was no significant difference between the SAT and SAT+Laminin groups (p>0.05).
4. Discussion
It is important to understand cell-biomaterial interaction, when using synthetic biomaterials to repair tissues. Their surface characteristics may not be always satisfactory for inducing the required effects. This leads to reduce their capability for tissue regeneration. Surface modification with bioactive molecules is beneficial as this can create a biomimetic environment to further promote cell adhesion, proliferation and differentiation [9, 30–34]. Laminin was chosen in the present study because laminin is one of important ECM components in nerves. Laminin can be found in the peripheral nerve basal lamina that is effective in promoting Schwann cell migrations and neurite outgrowth [32–34]. It interacts with Schwann cell integrins, which is essential for successful nerve regeneration [9–14]. Armstrong et al. [9] demonstrated that Schwann cells proliferation was influenced more by laminin than collagen and fibronectin. Therefore, incorporation of laminin on nanofibers could promote Schwann cell number and enhance the nerve regeneration due to its natural biochemical properties [10].
Although laminin was successfully crosslinked onto PCL/PEG-diamine aligned nanofibers via covalent crosslinking strategy, the loading efficiency was trivial as compared to direct blending strategy. This indicates a higher concentration of laminin is required. Based on the ELISA data (Table 2), even the highest concentration (4 mg/ml) had only 12% of Laminin crosslinking onto nanofibers. However, the crosslinking method can avoid potentially denaturing interactions between laminin and an organic solvent during electrospinning process. Previous study showed biological activity of biomolecules significantly decreased when exposed to an organic solvent [35]. Moreover, unlikely blending method, crosslinked laminin can fully expose itself on the surface of nanofibers, providing the direct contact mediate signal to cells. The evidence can be visualized in Fig 2. The fluorescent-labeled laminin was successfully crosslinked on the nanofibers. With increasing concentration of laminin solution, the intensity of fluorescent-labeled laminin also increased. Furthermore, as seen in Fig. 2A, it confirmed that laminin cannot be crosslinked onto PCL aligned nanofibers without any functional group.
The PC-12 based bioassay was used primarily as a quantitative and qualitative tool to examine the bioactivity of laminin at various concentration over 7days of culturing. The results not only demonstrated all laminin crosslinked groups had neurite promotion, also the aligned nanofibers oriented PC-12 cell neurites along the longitudinal axis of the conduits. Laminin crosslinked PCL/PEG-diamine aligned nanofibers showed more favorable for cellular attachment, and yielded longer neurite elongation (Fig. 3B, C and D). The 2mg/ml and 4 mg/ml laminin groups significantly stimulated the cells bearing longer neurites over those non-treated or low concentration groups (Fig. 3 A and B). It is important to note that there is no significant difference between the 2 mg/ml and 4 mg/ml of laminin. Since 2 mg/ml laminin has provided sufficient stimulation for neurite outgrowth, suggesting 2 mg/ml laminin should be carried for in vivo study.
The reversed autograft group remained a gold standard based on the results of nerve histological assessment (Fig. 7) and gastrocnemius muscle measurement (Fig. 8), showing statistical difference compared to the SAT and the SAT+Laminin groups. This is due to the fact that autografts provide ideal microstructures (e.g. endoneurial tubes) and contain growth factors, proteins, and cells for optimal recovery [36]. On the other hand, the SAT group did not receive many successful cases in the current study. Although nerve regeneration decreased with increasing gap length, it was unexpected to yield a high failure rate. Since the SAT was designed to optimize contact guidance cue, of which the efficacy was confirmed in a 10 mm nerve gap [21]. One of the possibilities could be discontinuous fibrin cables and Schwann cell tubes were formed in a manner, leading to inappropriate regenerated axons. Rrecent studies have shown that blood vessel formation and immune cells (e.g. macrophage) could also play crucial roles in promoting nerve regeneration [37–45]. Cattin et al. [37] showed Schwann cells appear unable to migrate within the 3D matrix but situation improved efficiently when physical surface of blood vessels were present. Without sufficient biomolecule and cellular support, it can lead to reduce functional regeneration [46, 47]. Therefore, SAT limited the degree of nerve regeneration resulted in random clusters of extracellular matrix tissue in most cases (supplement Figure 1). Likewise, Mackinnon’s findings [36] also explained lack of endoneurial microstructure tubes disabled NGC to support regeneration equivalent to that of an isograft.
Figure 8.
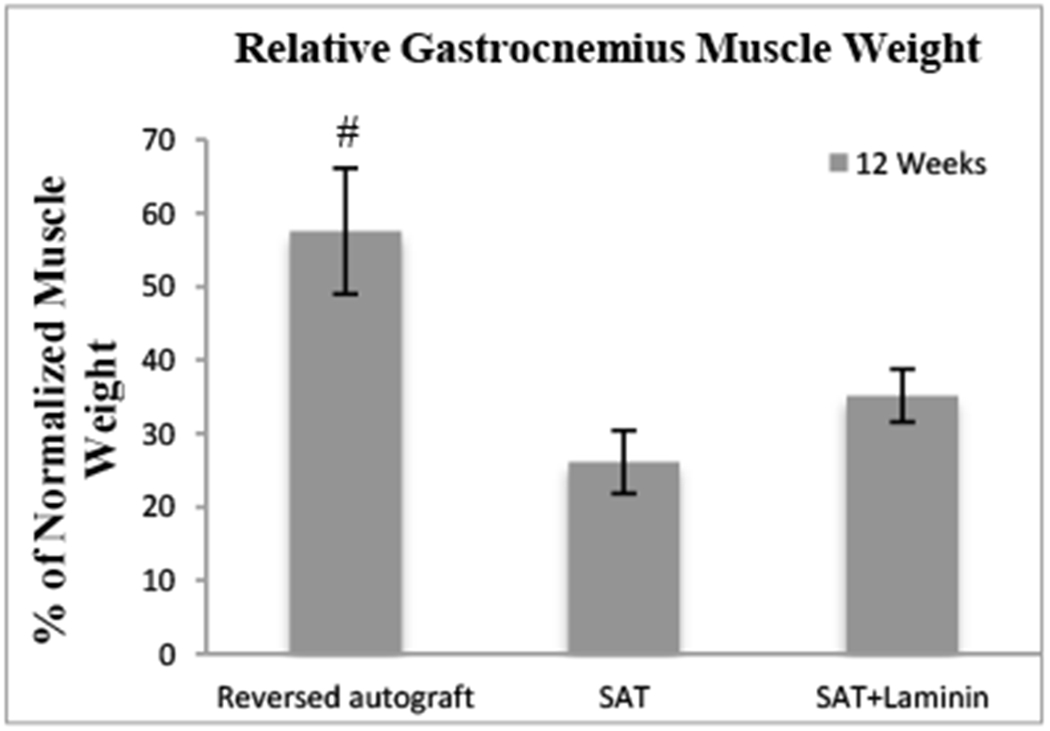
The RGMW after 12 Weeks post-implantation. # indicates a highly significant difference (p < 0.01) was found as compared to the SAT and SAT+Laminin groups.
In contrast, crosslinked laminin successfully functionalized the SAT and provided robust regeneration. The degree of sensory and motor functions was enhanced by crosslinked laminin, which significantly improved the percent of reinnervation and promoted axon regeneration. This supports our earlier hypothesis that a SAT with crosslinked laminin could provide a more favorable environment for nerve regeneration. Although laminin is an effective biological molecule for enabling nerve regeneration, it was expected that sensory and motor recovery would be lower than the reversed autograft group.
In terms of the functional assays, SAT+Laminin group did not show statistically differences at all time points as compared to the SAT group or the reversed autograft group. Most of data sit in between these two groups. But, early healing was still revealed from each functional assay. The reversed autograft group had faster healing rate (higher mean values), followed by SAT+Laminin group then SAT group. These functional assays correlating with the number of functional axons and the degree of myelination indicate the quality of nerve regeneration affected by different treatment strategies. It was suggested that a long-term study should take place in order to obtain meaningful data. Yet, a full reinnervation may take up to years [5]. Following nerve healing, the gastrocnemius muscle undergoes reinnervation. All the groups with nerve injuries had reduced muscle weight ratio. When nerve injury occurs, the gastrocnemius muscle degenerates; and as the nerve heals, the gastrocnemius muscle undergoes reinnervation. The reversed autograft group revealed the earliest reinnervation to prevent the gastrocnemius muscle atrophy, thus it stored the muscle mass quicker than the other two groups. Laminin also improved the reinnervation of the gastrocnemius muscle, as it can be seen with the higher mean values than the SAT group. Independently, laminin improved the efficacy of the SAT. The aforementioned data was consistent with the nerve histological assessment. The degree of myelinated axons for the reversed autograft and the SAT+Laminin groups were statistically greater than for the SAT group. Especially for the reversed autograft group, it showed better quality of regenerated axons in terms of higher axon density and thicker myelin sheaths. These findings provided valuable insights to determine whether regenerated axons have yielded reinnervation. A study with longer follow-up of regeneration may increase measurement values for reversed autograft and SAT+Laminin group. However, qualitatively, it still remains a challenge to guide the regenerated axons to their original target organs.
An unanticipated outcome was identified on the nerve histological micrograph (Fig. 7D). We suspected laminin might synergistically effect on regenerated axons and new blood capillary formation during nerve regeneration. However, the weakness of the study lacks for immunohistochemistry staining to confirm our observation. Due to the use of gluteraldehyde and osmium tetroxide (OsO4) during whole body fixation and histological preparation, the harvested tissues may be damaged and antibodies were unable to bind to specific antigens. Although we could identify wall of capillaries, structure of red blood cells, myelin sheaths, and regenerated axons from nerve histological micrographs, further immuno-staining, such as alpha-smooth muscle actin, CD31, von Willebrand factor (VWR), S100b, and neurofilament, is needed to reevaluate the results. Even so, the degree of interaction between nerve and blood vessels was underestimated in the past. The blood vessels and macrophage are necessary during nerve regeneration development. Cattin et al. [37] demonstrated that following an injury, macrophages in the nerve gap secreted VEGF-A to promote the formation of new blood vessels that can guide Schwann cells out from nerve stumps and bridge the gap. Laminin is well known for promoting new blood vessel formation [48, 49] and regulating macrophage activity [50, 51]. This may explain why the percent of reinnervation in the SAT+Laminin group could increase to 77.8%. Herein, we hypothesized that the use of laminin in our model enhances macrophage cellular activity at the beginning. VEGF-A secreted from macrophages then mediates endothelial cells to form new blood vessels along with aligned nanofibers. Schwann cells, therefore, could utilize these newly formed blood capillaries as a track to bridge the gap and facilitate regenerated axons crossing the gap.
5. Conclusions
In conclusion, PC-12 cell assay suggested 2 mg/ml of laminin was the optimal concentration for neurite outgrowth. In this rat model, the reversed autograft group showed substantial improvement based on motor functional assessments. The SAT group exhibited statistically different patterns of nerve regeneration as compared to the SAT+Laminin group based on percent of reinnervation and nerve histological assessment. The data demonstrated that nerve regeneration only occurs when laminin was present. The SAT group (6 out of 9 rats) resulted in random clusters of extracellular matrix tissue. In contrast, motor functional assessments for current study were far to match the performance of normal nerve. Fully motor recovery may take up to years. It was noted an unanticipated result was found from the SAT+Laminin group that laminin may simultaneously mediate the formation of blood vessels during the nerve regeneration. Overall, these findings suggest that laminin increase the efficiency by encouraging blood vessel formation and Schwann cell proliferation into the NGC to provide a more conducive environment for peripheral nerve regeneration. Although a number of interesting strategies have been proposed to attempt to bridge nerve gaps, none have shown efficacy comparable to an autograft treatment especially at the critical size defect or beyond. The best combination of contact-guidance cues, haptotactic cues, and chemotactic cues have yet to be realized. The natural sequence of nerve regeneration should be studied more in-depth before modulating any strategies.
Supplementary Material
6. Acknowledgement
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Orthopaedic Research Program under Award No. [W81XWH-13-1-0320], the U.S. National Institutes of Health grant [NIH-R15 NS074404], the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health [R01EB020640], and the U.S. National Science Foundation through [NSF Grant 1545992].
References
- [1].Brattain K, Analysis of the peripheral nerve repair market in the United States, Magellan Medical Technology Consultants, Inc: Minneapolis, MN, 2014. [Google Scholar]
- [2].Chamberlain L, Yannas I, Hsu H, Strichartz G, Spector M, Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft, Experimental neurology 154(2) (1998) 315–329. [DOI] [PubMed] [Google Scholar]
- [3].Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S, Nerve regeneration in silicone chambers: influence of gap length and of distal stump components,Experimental Neurology 76(2) (1982) 361–375. [DOI] [PubMed] [Google Scholar]
- [4].Li S-T, Archibald SJ, Krarup C, Madison RD, Peripheral nerve repair with collagen conduits, Clinical materials 9(3–4) (1992) 195–200. [DOI] [PubMed] [Google Scholar]
- [5].Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD, A collagen-based nerve guide conduit for peripheral nerve repair: An electrophysiological study of nerve regeneration in rodents and nonhuman primates, Journal of Comparative Neurology 306(4) (1991) 685–696. [DOI] [PubMed] [Google Scholar]
- [6].Safa B, Buncke G, Autograft substitutes: conduits and processed nerve allografts, Hand clinics 32(2) (2016)127–140. [DOI] [PubMed] [Google Scholar]
- [7].Daly W, Yao L, Zeugolis D, Windebank A, Pandit A, A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery, Journal of the Royal Society Interface 9(67) (2011) 202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tessier-Lavigne M, Goodman CS, The molecular biology of axon guidance, Science 274(5290) (1996) 1123. [DOI] [PubMed] [Google Scholar]
- [9].Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ, ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth, Tissue engineering 13(12) (2007) 2863–2870. [DOI] [PubMed] [Google Scholar]
- [10].Carbonetto S, The extracellular matrix of the nervous system, Trends in Neurosciences 7(10) (1984) 382–387. [Google Scholar]
- [11].Bailey S, Eichler M, Villadiego A, Rich K, The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers, Journal of neurocytology 22(3)(1993)176–184. [DOI] [PubMed] [Google Scholar]
- [12].Chen Y-S, Hsieh C-L, Tsai C-C, Chen T-H, Cheng W-C, Hu C-L, Yao C-H, Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin, Biomaterials 21(15)(2000)1541–1547. [DOI] [PubMed] [Google Scholar]
- [13].Madison R, da Silva CF, Dikkes P, Chiu T-H, Sidman RL, Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel, Experimental neurology 88(3) (1985) 767–772. [DOI] [PubMed] [Google Scholar]
- [14].Madison RD, da Silva C, Dikkes P, Sidman RL, Chiu T-H, Peripheral nerve regeneration with entubulation repair: comparison of biodegradeable nerve guides versus polyethylene tubes and the effects of a laminin-containing gel, Experimental neurology 95(2) (1987) 378–390. [DOI] [PubMed] [Google Scholar]
- [15].Podratz JL, Rodriguez E, Windebank AJ, Role of the extracellular matrix in myelination of peripheral nerve, Glia 35(1) (2001) 35–40. [DOI] [PubMed] [Google Scholar]
- [16].Rivas RJ, Burmeister DW, Goldberg DJ, Rapid effects of laminin on the growth cone, Neuron 8(1) (1992)107–115. [DOI] [PubMed] [Google Scholar]
- [17].McLoon SC, McLoon LK, Palm SL, Furcht LT, Transient expression of laminin in the optic nerve of the developing rat, Journal of Neuroscience 8(6) (1988) 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dodla MC, Bellamkonda RV, Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps, Biomaterials 29(1) (2008) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Z-L, Yu W-M, Strickland S, Peripheral Regeneration, Annual Review of Neuroscience 30(1) (2007) 209–233. [DOI] [PubMed] [Google Scholar]
- [20].Labrador RO, Buti M, Navarro X, Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair, Experimental neurology 149(1) (1998) 243–252. [DOI] [PubMed] [Google Scholar]
- [21].Chang W, Shah MB, Lee P, Yu X, Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration, Acta Biomaterialia 73 (2018) 302–311. [DOI] [PubMed] [Google Scholar]
- [22].Hutmacher D, Goh J, Teoh S, An introduction to biodegradable materials for tissue engineering applications, Annals of the Academy of Medicine, Singapore 30(2) (2001) 183. [PubMed] [Google Scholar]
- [23].N.A.A. Cheng Y, Valmikinathan CM, Lee P, Liang D, Yu X, and Kumbar SG, Collagen Functionalized Bioactive Nanofiber Matrices for Osteogenic Differentiation of Mesenchymal Stem Cells: Bone Tissue Engineering, J. Biomed. Nanotech (2013). [DOI] [PubMed] [Google Scholar]
- [24].Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo M-M, Li S, Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance, Nano letters 7(7) (2007) 2122–2128. [DOI] [PubMed] [Google Scholar]
- [25].Hare G, Evans P, Mackinnon S, Best T, Bain J, Szalai J, Hunter D, Walking track analysis: a long-term assessment of peripheral nerve recovery, Plastic and reconstructive surgery 89(2) (1992) 251–258. [PubMed] [Google Scholar]
- [26].Bain J, Mackinnon S, Hunter D, Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat, Plastic and reconstructive surgery 83(1) (1989) 129–138. [DOI] [PubMed] [Google Scholar]
- [27].Zeng L, Worseg A, Albrecht G, Grisold W, Hopf R, Redl H, Schlag G, A noninvasive functional evaluation following peripheral nerve repair with electromyography in a rat model, Plastic and reconstructive surgery 94(1) (1994) 146–151. [DOI] [PubMed] [Google Scholar]
- [28].Wolthers M, Moldovan M, Binderup T, Schmalbruch H, Krarup C, Comparative electrophysiological, functional, and histological studies of nerve lesions in rats, Microsurgery: Official Journal of the International Microsurgical Society and the European Federation of Societies for Microsurgery 25(6) (2005) 508–519. [DOI] [PubMed] [Google Scholar]
- [29].Spilker MH, Peripheral nerve regeneration through tubular devices: a comparison of assays of device effectiveness, Massachusetts Institute of Technology, 2000. [Google Scholar]
- [30].Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Endo K, Shimizu Y, Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves, Brain research 868(2) (2000) 315–328. [DOI] [PubMed] [Google Scholar]
- [31].Rangappa N, Romero A, Nelson KD, Eberhart RC, Smith GM, Laminin-coated poly (L-lactide) filaments induce robust neurite growth while providing directional orientation, Journal of biomedical materials research 51(4) (2000) 625–634. [DOI] [PubMed] [Google Scholar]
- [32].Itoh S, Takakuda K, Ichinose S, Kikuchi M, Schinomiya K, A study of induction of nerve regeneration using bioabsorbable tubes, Journal of reconstructive microsurgery 17(02) (2001) 115–124. [DOI] [PubMed] [Google Scholar]
- [33].Toba T, Nakamura T, Shimizu Y, Matsumoto K, Ohnishi K, Fukuda S, Yoshitani M, Ueda H, Hori Y, Endo K, Regeneration of canine peroneal nerve with the use of a polyglycolic acid-collagen tube filled with laminin-soaked collagen sponge: a comparative study of collagen sponge and collagen fibers as filling materials for nerve conduits, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 58(6) (2001) 622–630. [DOI] [PubMed] [Google Scholar]
- [34].Koh H, Yong T, Teo W, Chan C, Puhaindran M, Tan T, Lim A, Lim B, Ramakrishna S, In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration, Journal of neural engineering 7(4) (2010) 046003. [DOI] [PubMed] [Google Scholar]
- [35].Madurantakam PA, Rodriguez IA, Beckman MJ, Simpson DG, Bowlin GL, Evaluation of biological activity of bone morphogenetic proteins on exposure to commonly used electrospinning solvents, Journal of Bioactive and Compatible Polymers 26(6) (2011) 578–589. [Google Scholar]
- [36].Mackinnon SE, Nerve endoneurial microstructure facilitates uniform distribution of regenerative fibers: a post hoc comparison of midgraft nerve fiber densities, Journal of reconstructive microsurgery 27(2)(2011)83–90. [DOI] [PubMed] [Google Scholar]
- [37].Cattin A-L, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Calavia NG, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves, Cell 162(5) (2015) 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen P, Piao X, Bonaldo P, Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury, Acta neuropathologica 130(5) (2015) 605–618. [DOI] [PubMed] [Google Scholar]
- [39].Potas JR, Haque F, Maclean FL, Nisbet DR, Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo, Journal of immunological methods 420 (2015) 38–49. [DOI] [PubMed] [Google Scholar]
- [40].Mokarram N, Dymanus K, Srinivasan A, Lyon JG, Tipton J, Chu J, English AW, Bellamkonda RV, Immunoengineering nerve repair, Proceedings of the National Academy of Sciences 114(26) (2017) E5077–E5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Perry VH, Brown MC, Macrophages and nerve regeneration, Current opinion in neurobiology 2(5) (1992) 679–682. [DOI] [PubMed] [Google Scholar]
- [42].Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng X-L, The effect of thick fibers and large pores of electrospun poly (ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration, Biomaterials 35(22) (2014) 5700–5710. [DOI] [PubMed] [Google Scholar]
- [43].Hobson M.l., Green CJ, Terenghi G, VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy, The Journal of Anatomy 197(4) (2000) 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Carmeliet P, Blood vessels and nerves: common signals, pathways and diseases, Nature Reviews Genetics 4(9) (2003) 710. [DOI] [PubMed] [Google Scholar]
- [45].Best TJ, Mackinnon SE, Peripheral nerve revascularization: a current literature review, Journal of reconstructive microsurgery 10(03) (1994) 193–204. [DOI] [PubMed] [Google Scholar]
- [46].Williams LR, Longo FM, Powell HC, Lundborg G, Varon S, Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay, Journal of Comparative Neurology 218(4) (1983) 460–470. [DOI] [PubMed] [Google Scholar]
- [47].Cai J, Peng X, Nelson KD, Eberhart R, Smith GM, Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 75(2) (2005) 374–386. [DOI] [PubMed] [Google Scholar]
- [48].Grant DS, Lelkes P.l., Fukuda K, Kleinman HK, Intracellular mechanisms involved in basement membrane induced blood vessel differentiation in vitro, In Vitro Cellular & Developmental Biology-Animal 27(4) (1991) 327–336. [DOI] [PubMed] [Google Scholar]
- [49].Grant DS, Tashiro K.-l., Segui-Real B, Yamada Y, Martin GR, Kleinman HK, Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro, Cell 58(5)(1989)933–943. [DOI] [PubMed] [Google Scholar]
- [50].Wang G-Y, Hirai K-L, Shimada H, Taji S, Zhong S-Z, Behavior of axons, Schwann cells and perineurial cells in nerve regeneration within transplanted nerve grafts: effects of anti-laminin and anti-fibronectin antisera, Brain research 583(1–2) (1992) 216–226. [DOI] [PubMed] [Google Scholar]
- [51].Mercurio AM, Shaw LM, Macrophage interactions with laminin: PMA selectively induces the adherence and spreading of mouse macrophages on a laminin substratum, The Journal of cell biology 107(5) (1988) 1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


