Abstract
Objectives:
The mean platelet volume (MPV) and the MPV-to-platelet (PLT) count ratio have long been reported as inflammation markers. In this study, we aimed to investigate the predictive value of the MPV and the MPV-to-PLT ratio on surgical wound healing in patients who underwent abdominal hysterectomy and experienced infections at the surgical site following surgery, despite adequate antimicrobial treatment.
Methods:
A total of 100 patients who encountered surgical wound infection (SWI) after abdominal hysterectomy were enrolled retrospectively. Samples for complete blood count were drawn the day before the operation. All patients received preoperative and postoperative antibiotic prophylaxis and proper antimicrobial treatment following the SWI development. Patients’ condition resolved after standard care and antimicrobial agents were classified as the standard care group. Others, in whom an improvement despite the standard care was not observed, underwent delayed primary closure and were classified as the delayed primary closure group.
Results:
The PLT count was decreased (319.5±66 103/µL vs. 392±121 103/µL; p<0.05), MPV(9.2±1.3 fL vs. 8.2±1.5 fL; p<0.05), and the MPV-to-PLT ratio (0.030±0.006 vs. 0.024±0.014; p<0.05) was increased in the delayed primary closure group compared to the standard care group. A receiver operating characteristic curve analysis was performed to determine the predictive value of these parameters on the response to standard care measures providing 8.28fL as a cut-off value for MPV (AUC=0.647, 72% sensitivity and 52% specificity) and 0.025 as a cut-off value for the MPV-to-PLT ratio (AUC=0.750, 75% sensitivity and 67% specificity) for predicting nonresponsiveness.
Conclusion:
An increased preoperative MPV and the MPV-to-PLT ratio may predict poor wound healing following total abdominal hysterectomy.
Keywords: Delayed primary closure, mean platelet volume, platelet count, surgical wound infection, total abdominal hysterectomy
Total abdominal hysterectomy (TAH) is one of the most common surgical procedures carried out by gynecologists across the world, and a huge variety of complications may accompany the procedure. Postoperative infections, particularly surgical wound infections (SWI) following TAH, constitute an origin for further complications associated with an increased hospital stay, increased morbidity, repeated surgery, and excessive medical costs.[1] Preoperative prophylactic use of antibiotics and strict following of antiseptic measures have been reported to reduce the rate of postoperative SWIs.[2]
Platelets (PLT) are enucleated cells, which are traditionally thought to be solely involved in primary hemostasis and coagulation as a response to various kinds of physiologic triggers. However, emerging evidence supports their additional role in other physio-pathological processes including inflammation, tissue regeneration, and wound healing.[3, 4] Their ability in releasing cytokines and corroborating leukocyte response, in addition to their interaction with bacteria and endothelium, clarify the protective role of these cells in localized infection.[5] However, there are limited data regarding the potential impact of platelet dysfunction and platelet activity on SWIs. In this study, we aimed to assess the predictive value of the mean platelet volume (MPV) and the MPV-to-PLT ratio on surgical wound healing in patients who underwent abdominal hysterectomy and experienced surgical site infections following surgery, despite adequate antimicrobial treatment.
Methods
Subject Selection
This study was approved by the Institutional Ethical Committee, and it was performed in accordance with the most recent version of the Helsinki Declaration (October-2013). We retrospectively evaluated abdominal hysterectomy patients, and 100 patients who encountered wound infection after abdominal hysterectomy were enrolled in the study. All patients received preoperative and postoperative antibiotic prophylaxis, and SWI was diagnosed according to clinical and laboratory results. Patients were given medical therapy including proper antibiotics and anti-inflammatory agents. Some of the patients fully recovered, and wound infection were resolved. We labeled this group as the standard care group (67 patients). Some patients needed a delayed primary closure (surgical toilet, leave open and close later). We labeled this group the delayed primary closure group (33 patients). We compared the demographic data, preoperative PLT count, mean PLT volume, and MPV-to-PLT between the groups.
Laboratory Findings
Blood samples were drawn from a forearm vein the day before the operation, collected into tubes containing ethylene diamine tetra acetic acid and stored at room temperature until measurement, which was in all cases performed within 1 h after venipuncture. Blood measurements were analyzed by an automated hematology analysis system (CELL-DYN Sapphire) that provided the PLT count (103/µL), and the MPV in the femtoliter (fL) mean corpuscular volume (fL) and red blood cell (RBC) count (106/µL).
Statistical Analysis
Statistical analysis was performed using the SPSS for Windows, version 17 (Chicago, IL, USA). All data were distributed normally. Comparisons among the groups with respect to demographic data, the MCV, RBC count, PLT count, mean PLT volume, and MLT volume/PLT count were evaluated using Student’s t-test, Pearson’s chi-squared test, and Fisher’s exact test. A two-sided p≤0.05 was interpreted as statistically significant. The MLT-to-PLT ratio and MPV values were found to be statistically different between groups, and they were analyzed for their diagnostic value in predicting delayed primary closure using a receiver operating characteristic (ROC) analysis. The cut-off values were determined.
Results
Demographic variables including age, weight, height, the PLT count, red blood cell, MLV, mean corpuscular volume, and MLT/PLT count are shown in Table 1. The age, weight, height, RBC, and MCV were not significantly different between the groups (p>0.05 for all comparisons). Proportion of smokers and diabetics were also not significant in the two groups. Time to SWI development was 0.8 weeks in the standard care group and 0.6 weeks in the delayed primary closure group (p>0.05). Time to wound healing was significantly longer in the delayed primary closure group compared to the standard care group (9.3 weeks vs. 4.1 weeks, p<0.001). The PLT count was statistically decreased in the delayed primary closure group (319.5±66 103/µL vs. 392±121 103/µL; p<0.05), MPV was statistically increased (9.2±1.3 fL vs. 8.2±1.5 fL; p<0.05) in the delayed primary closure group, and the MPV-to-PLT ratio (0.030±0.006 vs. 0.024±0.014; p<0.05) was statistically increased in the delayed primary closure group. A correlation analysis revealed that the MPV (r=0.256, p=0.024) and MPV-to-PLT (r=0.232, p=0.031) were significantly correlated with poor wound healing. To determine whether the MPV and MPV-to-PLT value was appropriate in predicting delayed primary closure, ROC curves were drawn, and the specificity and sensitivity values were calculated. The highest cut-off points for the sensitivity and specificity were determined (Figs. 1, 2). The value of 8.28 fL as a cut off value for MPV had an AUC of 0.647, and 72% sensitivity and 52% specificity. The value of 0.025 was a cut-off value for the MPV-to-PLT ratio and had an AUC of 0.750, and 75% sensitivity and 67% specificity.
Table 1.
Demographic characteristics and blood parameter results
| Standard care group (n=67) | Delayed primary closure group (n=33) | p | |
|---|---|---|---|
| Age mean (years) | 49.7±9.3 | 49.1±5.8 | NS |
| Weight (kg) | 79.7±7.7 | 78.6 ±7.9 | NS |
| Height (cm) | 170.1±8.1 | 169.1±9.1 | NS |
| Smoking (n, %) | 8 (12) | 7 (21) | 0.081 |
| Diabetes (n, %) | 6 (9) | 8 (24) | 0.063 |
| MCV (fL) | 81.1±7.9 | 92.6±9.3 | NS |
| RBC (106/µL) | 4.1±1.3 | 3.8±0.7 | NS |
| PLT (103/µL) | 392±121 | 319±66 | 0.002 |
| MPV (fL) | 8.2±1.5 | 9.2±1.3 | 0.004 |
| MPV/PLT | 0.024±0.014 | 0.030±0.006 | 0.039 |
| Time to SWI (weeks) | 0.8 | 0.6 | NS |
| Time to wound | 4.1 | 9.3 | <0.001 |
| healing (weeks) |
n: number of patients; NS: not significant; kg: kilogram; cm: centimeter; fL: femtoliter; MCV: mean corpuscular volume; RBC: red blood cell; PLT: platelet; MPV: mean platelet volume; SWI: surgical wound infection.
Figure 1.
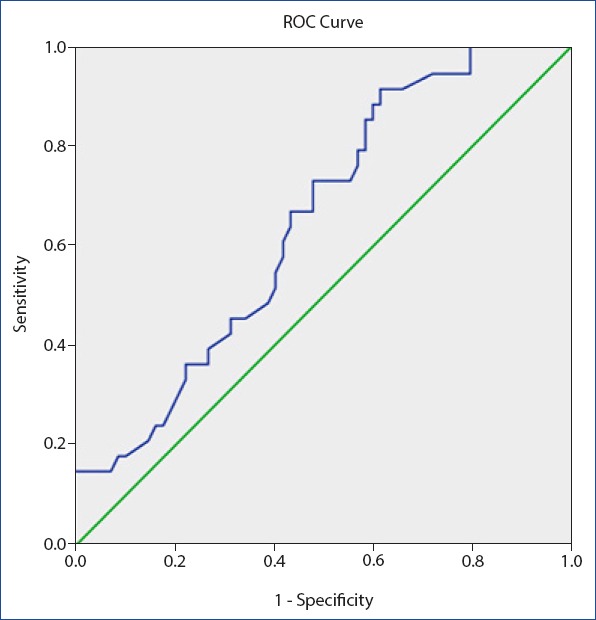
Receiver operating characteristic curve for mean platelet volume value for delayed primary closure. Diagonal segments are produced by ties. Area under the curve 0.64, 8.28 fL as a cut off value for MPV, sensitivity 72% and specificity 52%.
Figure 2.
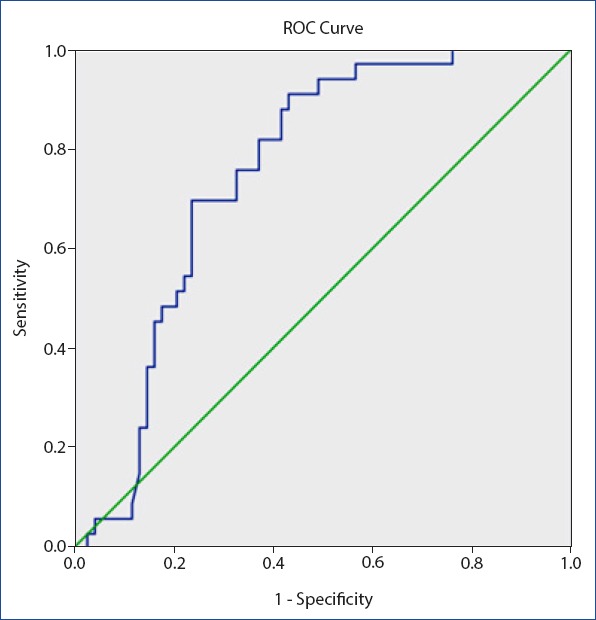
Receiver operating characteristic curve for mean platelet volume/Platelet for delayed primary closure. Diagonal segments are produced by ties. Area under the curve 0.750, 0.025 as a cut of value for MPV/PLT sensitivity 75% and specificity 67%.
The MPV and MPV-to-PLT measured 4 weeks following the wound healing were similar to preoperative measurements in the delayed primary closure group (9.2±1.3 vs. 9.0±1.6, p>0.05 for MPV and 0.030±0.006 vs. 0.028±0.005, p>0.05 for MPV-to-PLT), although they were lower compared to preoperative measurements in the standard care group (8.2±1.5 vs. 7.4±1.1, p=0.034 for MPV and 0.024±0.014 vs. 0.019±0.006, p=0.044 for MPV-to-PLT).
Discussion
In this study, we found that a lower PLT count, higher MPV value, and higher MPV-to-PLT ratio predict the need for a delayed primary closure in patients experiencing SWI following TAH. This study is, to the best of our knowledge, the first to demonstrate the predictive value of the MPV and the MPV-to-PLT ratio in SWI.
As the technical improvement limited the complications of major surgery, SWIs still constitute a major problem following TAH. Surgical site infections have the third highest incidence of the infections that necessitate hospitalization, comprising 14%–16% of total infections.[6] Poorly controlled diabetes, tobacco use, prolonged steroid use, high body mass index, malnutrition, low socioeconomic status, prolonged hospital stay, and coincidental infections are risk factors for SWI.[7] In addition, inadequate skin preparation, not to pay required attention to antisepsis measures, poor surgical technique and suboptimal use of prophylactic antimicrobial agents may give rise to the development of SWI. Total abdominal hysterectomy carries a higher risk for SWI compared to minimal invasive hysterectomy and laparoscopic hysterectomy.[8] Cautious patient selection and correction of retrievable risk factors for preceding surgery may be helpful in minimizing postoperative complications, including SWI. Moreover, antibiotic therapy should be employed preoperatively and postoperatively in accordance with the suggestions by the American College of Obstetricians and Gynecologists in patients who are potential candidates for the development of SWI.[9] However, despite the administration of antibiotics and appropriate skin preparation, postoperative infections may still occur, and a minority of wounds might be obliged to left delayed primary closure, which brings out many additional risks for further complications and morbidities. Although not preferred frequently by the surgeons, and used as a bail-out strategy, delayed primary closure remains critical when early fascial closure is not feasible or prudent, as in patients with a high intra-abdominal pressure, accompanying uncontrolled intra-abdominal infections, or postoperative wound infections, which could also be found in some of our study population. Despite these benefits provided by a delayed late closure technique, given the increased mortality recorded after these procedures, early wound closure and healing is one of the main targets in patients who underwent TAH.[10]
Platelets function as first responders during the wounding process and hemostasis. Following surgical incision, platelets actively transmigrate across the leaky or inflamed vessel wall to aid in wound sterilization and tissue regeneration.[11] Platelets initiate inflammation by releasing various kinds of cytokines and adhesion molecules, directly activating responses for monocytes, neutrophils, and T-lymphocytes.[12] They also activate the complement system that augments the inflammatory responses. In patients with severe sepsis, lower platelet counts have been reported in a substantial number of studies.[13]
The mean PLT volume is a measurement of the average size of platelets found in the blood stream, which is universally available with routine blood counts. A higher MPV indicates an increased activity of platelets independent of the technique used to measure MPV.[14] Previously, the role of platelets and MPV as a surrogate marker of platelet activity has been shown in several studies. An elevation in the MPV level has been shown in many clinical conditions that are explicit with chronic inflammation.[15–17] MPV levels are elevated in chronic inflammatory diseases, including ankylosing spondylitis, rheumatoid arthritis, chronic cardiovascular diseases, and hypertension and dyslipidemia, and all usually represent a chronic inflammatory state.[18–22] The MPV is increased in low-grade inflammation, whereas lower MPV values have been reported in high-grade inflammation. MPV is also increased in a wide variety of clinical conditions, including smoking, hypertension, dyslipidemia, and diabetes, which might complicate tissue healing in the perioperative period.[23] The MPV-to-PLT ratio, as an emerging marker of inflammation, has also been studied in the diagnosis of patients with sepsis and SIRS.[14] Previous studies investigating the role of platelets in miscellaneous types of major infections and sepsis have shown that MPV values were increased and PLT counts were decreased in those clinical conditions.[24] The MPV also proved to be a good predictor of the outcome in patient with sepsis. Guclu et al.[25] have found that a level of MPV >8 fl was associated with moderate sensitivity (53.47%) and excellent diagnostic specificity for the diagnosis and prognosis of sepsis. With these substantial data, it’s evident that a higher MPV value and a lower MPV-to-PLT ratio are indicative of an increased PLT activity and thus more intense inflammation. Although the literature contains extensive data regarding the role of MPV and MPV-to-PLT ratio in chronic inflammatory diseases, data addressing the role of these markers in SWI remain limited.
In our study, we found a higher MPV, lower PLT count, and higher MPV-to-PLT ratio in patients who developed surgical site incisional infections and did not respond to standard care and antimicrobial agents. We suppose that a higher MPV and higher MPV-to-platelet ratio measured preoperatively might indicate a preexisting chronic inflammatory state in addition to the presence of unfavorable risk factors for postoperative wound healing such as smoking, hypertension, dyslipidemia, and diabetes. Also, when the primary responsive role of platelets in wound healing was kept in mind, lower levels of platelets in the delayed primary closure group is consistent with previous data. It is also remarkable that in the delayed primary closure group, the high MPV and MPV-to-PLT ratio measured preoperatively continued to be high even 4 weeks following wound healing. With this background in mind, we suggest that a high MPV and MPV/PLT ratio are the indicators of proinflammatory state, may predict poor wound healing following TAH, and may be a warning for postponing the operation until the modifiable risk factors are corrected.
Limitations
This study has several limitations. The present study is a retrospective, single-center study, and it does not have a follow-up period for further complications. MPV values were not recorded serially in the preoperative period, and a single MPV and PLT count were used for statistical analysis. However, given its pivotal role in SWI, this study might constitute a core for future studies in this area.
Conclusion
Increased preoperative MPV values and MPV-to-PLT ratios may predict poor wound healing following TAH. Strict compliance with the guidelines and utilization of less-invasive surgical techniques might be required in these patients. Modifiable unfavorable risk factors influencing wound healing should also be corrected in these patients before scheduling surgery.
Disclosures
Ethics Committee Approval: The study was approved by the Kanuni Sultan Suleyman Ethics Committee (KAEK/2018.10.33).
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept –A.A., G.Y., N.K.; Design –A.A., G.Y., N.K.; Supervision – A.A., G.Y., N.K.; Materials –A.A., G.Y.; Data collection &/or processing –A.A., N.K.; Analysis and/or interpretation – A.A.; Literature search – A.A., G.Y.; Writing – A.A., G.Y.; Critical review – A.A., G.Y.
References
- 1.Olsen MA, Higham-Kessler J, Yokoe DS, Butler AM, Vostok J, Stevenson KB, et al. Prevention Epicenter Program, Centers for Disease Control and Prevention. Infect Control Hosp Epidemiol. 2009;30:1077–83. doi: 10.1086/606166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. American Society of Health-System Pharmacists (ASHP);Infectious Diseases Society of America (IDSA);Surgical Infection Society (SIS);Society for Healthcare Epidemiology of America (SHEA). Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 3.Garraud O, Hamzeh-Cognasse H, Pozzetto B, Cavaillon JM, Cognasse F. Bench-to-bedside review:Platelets and active immune functions - new clues for immunopathology? Crit Care. 2013;17:236. doi: 10.1186/cc12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Moure JS, Van Eps JL, Cabrera FJ, Barbosa Z, Medrano Del Rosal G, Weiner BK, et al. Platelet-rich plasma:a biomimetic approach to enhancement of surgical wound healing. J Surg Res. 2017;207:33–44. doi: 10.1016/j.jss.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets:versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34:5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madeira MZ, Trabasso P. Surgical site infections in women and their association with clinical conditions. Rev Soc Bras Med Trop. 2014;47:457–61. doi: 10.1590/0037-8682-0125-2014. [DOI] [PubMed] [Google Scholar]
- 7.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 8.Göksever Çelik H, Çelik E, Turan G, Seçkin KD, Gedikbaşı A. Risk factors for surgical site infection after hysterectomy. J Infect Dev Ctries. 2017;11:355–60. doi: 10.3855/jidc.9053. [DOI] [PubMed] [Google Scholar]
- 9.ACOG Committee on Practice Bulletins--Gynecology. ACOG practice bulletin No. 104:antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180–9. doi: 10.1097/AOG.0b013e3181a6d011. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Ye J, Song W, Chen J, Yuan Y, Ren J. Comparison of Outcomes between Early Fascial Closure and Delayed Abdominal Closure in Patients with Open Abdomen:A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2014;2014:784056. doi: 10.1155/2014/784056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancuso ME, Santagostino E. Platelets:much more than bricks in a breached wall. Br J Haematol. 2017;178:209–19. doi: 10.1111/bjh.14653. [DOI] [PubMed] [Google Scholar]
- 12.Iannacone M. Platelet-mediated modulation of adaptive immunity. Semin Immunol. 2016;28:555–60. doi: 10.1016/j.smim.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Orfanu AE, Popescu C, Leuştean A, Negru AR, Tilişcan C, Aramă V, et al. The Importance of Haemogram Parameters in the Diagnosis and Prognosis of Septic Patients. J Crit Care Med (Targu Mures) 2017;3:105–10. doi: 10.1515/jccm-2017-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ates S, Oksuz H, Dogu B, Bozkus F, Ucmak H, Yanıt F. Can mean platelet volume and mean platelet volume/platelet count ratio be used as a diagnostic marker for sepsis and systemic inflammatory response syndrome? Saudi Med J. 2015;36:1186–90. doi: 10.15537/smj.2015.10.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uysal P, Tuncel T, Olmez D, Babayigit A, Karaman O, Uzuner N. The role of mean platelet volume predicting acute exacerbations of cystic fibrosis in children. Ann Thorac Med. 2011;6:227–30. doi: 10.4103/1817-1737.84778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yüksel O, Helvaci K, Başar O, Köklü S, Caner S, Helvaci N, et al. An overlooked indicator of disease activity in ulcerative colitis:mean platelet volume. Platelets. 2009;20:277–81. doi: 10.1080/09537100902856781. [DOI] [PubMed] [Google Scholar]
- 17.Makay B, Türkyilmaz Z, Unsal E. Mean platelet volume in children with familial Mediterranean fever. Clin Rheumatol. 2009;28:975–8. doi: 10.1007/s10067-009-1148-5. [DOI] [PubMed] [Google Scholar]
- 18.Yazici S, Yazici M, Erer B, Erer B, Calik Y, Ozhan H, et al. The platelet indices in patients with rheumatoid arthritis:mean platelet volume reflects disease activity. Platelets. 2010;21:122–5. doi: 10.3109/09537100903474373. [DOI] [PubMed] [Google Scholar]
- 19.Choi DH, Kang SH, Song H. Mean platelet volume:a potential biomarker of the risk and prognosis of heart disease. Korean J Intern Med. 2016;31:1009–17. doi: 10.3904/kjim.2016.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sezgin M, Tecer D, Kanık A, Kekik FS, Yeşildal E, Akaslan E, et al. Serum RDW and MPV in Ankylosing Spondylitis:Can they show the disease activity? Clin Hemorheol Microcirc. 2017;65:1–10. doi: 10.3233/CH-162067. [DOI] [PubMed] [Google Scholar]
- 21.Gang L, Yanyan Z, Zhongwei Z, Juan D. Association between mean platelet volume and hypertension incidence. Hypertens Res. 2017;40:779–84. doi: 10.1038/hr.2017.30. [DOI] [PubMed] [Google Scholar]
- 22.Icli A, Aksoy F, Nar G, Kaymaz H, Alpay MF, Nar R, et al. Increased Mean Platelet Volume in Familial Hypercholesterolemia. Angiology. 2016;67:146–50. doi: 10.1177/0003319715579781. [DOI] [PubMed] [Google Scholar]
- 23.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume:a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 24.Zampieri FG, Ranzani OT, Sabatoski V, de Souza HP, Barbeiro H, da Neto LM, et al. An increase in mean platelet volume after admission is associated with higher mortality in critically ill patients. Ann Intensive Care. 2014;4:20. doi: 10.1186/s13613-014-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. Afr Health Sci. 2013;13:333–8. doi: 10.4314/ahs.v13i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]


