Abstract
Objectives:
This study aimed to analyze the efficacy of single-dose tranexamic acid (TA) 20 mg/kg preoperatively to reduce blood loss in patients undergoing total knee replacement (TKR).
Methods:
A total of 387 patients (82 males, 305 females) undergoing TKR between January 2014 and December 2018 were included in the study. The T + group was administrated intravenous (iv) TA 20 mg/kg 20 min before the skin incision. We determined perioperative blood loss, the amount of drainage postoperative 24 h, the amount of drainage after postoperative 24–48 h, total volume of drains, total volume of blood loss, postoperative hemoglobin and hematocrit levels, and amount of total blood transfusion.
Results:
In terms of demographic data, no statistically significant difference was observed between the groups. Perioperative blood loss and total volume of blood loss was found statistically higher in T − group compared to T + group. Postoperatively, the mean hemoglobin and hematocrit levels of T − group were statistically significantly lower than T + group.
Conclusion:
A single 20 mg/kg iv TA administration before TKR reduces bleeding during surgery and within 24 h postoperatively.
Keywords: Blood loss, deep vein thrombosis, knee replacement, tranexamic acid
Knee replacement surgery is one of the most common procedures for knee osteoarthritis. This procedure can lead to significant blood loss, and the blood transfusion rate is high. In 34% of patients who underwent total knee replacement (TKR), perioperative blood transfusions are being performed at least once.[1] Allogeneic blood transfusion is associated with a variety of risks such as transfusion reaction, volume overload of the heart, and inhibition of immune system.[2–4] Blood transfusion increases hospitalization cost.[5]
In clinical practice, different protective measures such as autologous blood transfusion have been used to reduce postoperative blood transfusion rates.[6–8] Other methods for prevention of perioperative blood loss include preoperative administration of erythropoietin, preoperative administration of iron supplements, normovolemic hemodilution, controlled hypotension, tourniquet use, and application of antifibrinolytic agents.[9–12]
Tranexamic acid (TA) is a lysine analog that blocks plasminogen-binding sites by preventing complex formation between plasminogen, fibrin, and tissue plasminogen activator.[13–15] TA is an inexpensive and easily accessible synthetic product.[16–19] TA has been shown as an effective and safe product to reduce blood transfusion and blood loss in TKR without increasing thromboembolic complications in TKR.[20, 21] Several previous clinical studies and meta-analysis reports have demonstrated the efficacy of TA administered intraoperatively in the prevention of blood loss in TKR.[22–26]
Methods
This study was designed as a retrospective clinical study and was been approved by University of Health Sciences, Şişli Hamidiye Etfal Training and Research Hospital Clinical Research Ethics Committee. A total of 387 patients (82 men, 305 women) who underwent primary cemented TKR between January 2014 and December 2018 for knee osteoarthritis were included in this study. The mean age was 67.2 years (range 60–84 years). Exclusion criteria were having chronic renal, liver, rheumatic, or hematological diseases; history of thromboembolism; cerebrovascular diseases; simultaneous bilateral knee replacement; having undergone revision surgery; prolonged use of anticoagulant medication (≥3 months, The American College of Chest Physicians (ACCP) guidelines 2012); thrombocyte level below 150.000; and INR level above 1.4.
According to whether TA was given or not, patients were divided into two groups: T – group and T + group. The T + group was administrated intravenous (iv) TA 20 mg/kg 20 min before the skin incision. TA was not administered to the T − group patients. All patients underwent spinal or combined spinal epidural anesthesia. TKR was performed with standard medial parapatellar incision. Intramedullary guides were used for femoral cuts, and extramedullary guides were used for tibial cuts. The tourniquet was used only during cementing. In all TKR operations, the same type of knee implant that protects the posterior cruciate ligaments was used. The drain remained open for 48 h from the end of the surgery. Patients were allowed partial weight bearing within the first 24 h after surgery and were mobilized with the help of crutches. The drainage was recorded at the 24th and 48th hour. Standard thromboembolism prophylaxis was applied in accordance with ACCP guidelines 2012, independent of age and weight. One dose of 0.4 ml (4000 IU) of enoxaparin was subcutaneously (sc) given 12 h prior to surgery. All patients received 0.4 ml (4000 IU) per day of enoxaparin sc for 14 days after discharge.
Blood counts were evaluated at the 6th hours postoperatively. Blood transfusion indication was given when the hemoglobin level was below 8 or 9 g/dL. When the hemoglobin values decreased under 9 g/dl, the patients received one unit of allogeneic erythrocytes suspension. When the hemoglobin values decreased under 8 g/dl, the patients received two units of allogeneic erythrocytes suspension. Fresh frozen plasma was not used. Intraoperative blood loss amount was calculated from aspiration and irrigation fluids.
The basic characteristics of groups were based on the mean age, male to female ratio, body mass index (BMI), and mean preoperative hemoglobin and hematocrit values. Perioperative blood loss, amount of blood drainage in postoperative 24 h and 48 h, total volume of drains, total volume of blood loss, and amount of blood transfusion were reviewed and compared between the two groups.
Statistical Analysis
The SPSS 15.0 for Windows 7 program was used for statistical analysis. Descriptive statistics were described as number and percentage for categorical variables, and mean and standard deviation for numerical variables. When numerical variables provided normal distribution condition, independent two-group comparisons were made by Student’s t test; and when normal distribution condition was not provided, Mann–Whitney U test was used. The ratios in groups were compared with the chi-square test. Statistical significance level of alpha was accepted as p<0.05.
Results
No statistically significant difference was observed between groups with respect to the mean age, male to female ratio, BMI, American Society of Anesthesiologists grade ratios, and mean preoperative hemoglobin and hematocrit values (Table 1). There was no significant difference in preoperative comorbidity rates (Table 2).
Table 1.
Preoperative demographics
| T - group | T + group | p | |
|---|---|---|---|
| Age (year) (mean±SD) | 67.4±3.9 | 67.0±4.5 | 0.079 |
| Gender | 0.601 | ||
| Female, n (%) | 218 (78.1) | 87 (80.6) | |
| Male, n (%) | 61 (21.9) | 21 (19.4) | |
| Body mass index (kg/m2) | 31.5±2.9 | 31.4±2.6 | 0.767 |
| (mean±SD) | |||
| American Society of | 0.739 | ||
| Anesthesiologists grade | |||
| Grade I, n (%) | 27 (9.7) | 10 (9.3) | |
| Grade II, n (%) | 45 (16.1) | 21 (19.4) | |
| Grade III, n (%) | 207 (74.2) | 77 (71.3) | |
| Preoperative hemoglobin (g/dl) (mean±SD) | 12.1±1.2 | 12.2±1.2 | 0.537 |
| Preoperative hematocrit (mean±SD) | 36.2±3.6 | 36.5±3.6 | 0.333 |
The values are expressed as mean±SD or numbers of patients (n (%)). There are no significant differences between groups. P<0.05 was considered to indicate statistical significance.
Table 2.
Preoperative comorbidities
| T – group n (%) | T + group n (%) | p | |
|---|---|---|---|
| Asthma | 4 (1.4) | 2 (1.9) | 0.673 |
| CAD | 9 (3.2) | 3 (3.1) | 0.714 |
| DM | 89 (31.9) | 29 (26.9) | 0.333 |
| Dyslipidemia | 17 (6.1) | 8 (7.4) | 0.637 |
| HT | 123 (44.1) | 53 (49.1) | 0.377 |
| Thyroid diseases | 14 (5.0) | 4 (3.7) | 0.582 |
The values are expressed as numbers of patients (n (%)).There are no significant differences between groups. CAD: Coronary artery diseases. DM: Diabetes mellitus. HT: Hypertension. P<0.05 was considered statistically significant.
Statistical analysis showed that perioperative blood loss, drainage blood loss 0–24 h, total volume of drains, and total volume of blood loss were statistically higher than T + group in T − group. But no statistically significant difference was observed between the two groups in the aspect of drainage blood loss between 24 h and 48 h (Table 3) (Fig. 1). When the mean hemoglobin and hematocrit values measured at the 6th hour postoperatively were examined, it was found that the T – group results was statistically lower than the T + group’s (Table 4). Allogeneic blood transfusion amount in the T − group was significantly higher than in the T + group (Fig. 2). But no statistically significant difference was observed between the two groups in terms of postoperative hospital stay (days) (Table 4).
Table 3.
Blood loss data
| T – group (mean±SD) | T + group (mean±SD) | p | |
|---|---|---|---|
| Perioperative blood loss (ml) | 493.5±42.4 | 257.9±35.3 | <0.001 |
| Drainage blood loss 0-24 h (ml) | 637.6±57.7 | 306.5±35.3 | <0.001 |
| Drainage blood loss 24-48 h (ml) | 93.3±17.7 | 92.4±16.2 | 0.932 |
| Total volume of drains (ml) | 730.9±60.5 | 398.8±41.9 | <0.001 |
| Total volume of blood loss (ml) | 1224.5±71.9 | 656.7±57.4 | <0.001 |
The values are expressed as mean±SD. P<0.05 was considered statistically significant.
Figure 1.
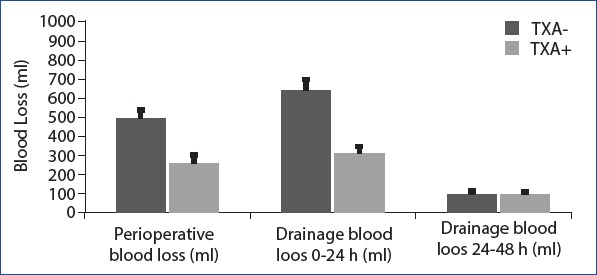
Perioperative and postoperative 48 hours blood loss(ml): Perioperative blood loss and drainage blood loss 0-24 hours were statistically higher than TXA + group in TXA - group. But there was no statistically significant difference between the two groups in the results of drainage blood loss between 24-28 hours.
Table 4.
Comparison outcomes between the groups
| T – group (mean±SD) | T + group (mean±SD) | p | |
|---|---|---|---|
| Postoperative hemoglobin | 9.0±1.2 | 10.1±1.2 | <0.001 |
| 6 h (g/dL) | |||
| Postoperative hematocrit 6 h | 26.9±3.7 | 30.4±3.6 | <0.001 |
| Postoperative hospital stay (days) | 4.3±0.6 | 4.1±0.4 | 0.337 |
| Total (perioperative and | 1.7±0.5 | 1.0±0.0 | <0.001 |
| postoperative 48 h) | |||
| transfusion (unit) |
The values are expressed as mean±SD. P<0.05 was considered statistically significant.
Figure 2.
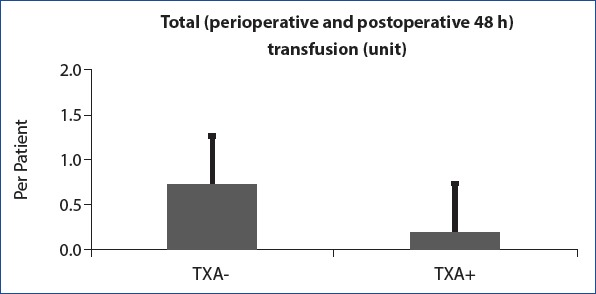
Total allogeneic blood transfusion units: Allogeneic blood transfusion level in the TXA - group was significantly higher than in the TXA + group.
Discussion
Primer cemented TKR can result in significant amounts of blood loss. The mean total blood loss according to various studies ranged from 400 to 2000 ml.[27] Surgical trauma increases perioperative blood loss through fibrinolysis activation.[28] TA is the inhibitor of fibrinolysis that reduces postoperative blood loss. Hippala et al.[29] first published the use of TA as an antifibrinolytic agent to reduce blood loss after TKR. TA is widely used in various surgical applications, but recently it has begun to be used in TKR. TA is administered in the form of different methods. Following iv application, TA is rapidly dispersed into synovial fluid.[12]
Many publications show that TA administration causes significant reduction in the blood loss.[29–32] Our study showed that the average amount of blood loss in the TA-receiving group was 656.7±57.4 ml and the blood loss in the non-TA group was 1224.5±71.9 ml (p<0.001) consistent with the literature. The blood loss in the T + group is close to the upper limit stated in the literature, because the tourniquet is not used during the operation. The volume of postoperative blood loss was 398.8±41.9 ml.
There is no consensus about administration time, methods, or usage volume of TA.[19, 32–35] Single-dose TA usage is claimed to be more unsuccessful than multiple dose or topical usage combined with iv administration.[34, 35] However, Hourlier et al.[33] showed that single doses of TA were as effective as the multiple-dose method. Previous studies have indicated that single-dose therapy did not show efficacy as they usually used low doses.[33] The biological half-life for TA is 3 h; 90% of the given TAs are excreted via the kidneys within 24 h.[36] TA becomes effective 15 min after administration, and remains effective for about 8 h in the serum and up to 18 h in the tissue. The fact that the majority of blood loss occurred within the first 5 h after surgery suggests that preoperative high single-dose intravenous TA treatment has a rapid enough effect on blood loss.[37] In our study, blood loss in the first 24 h after surgery was 306.5±35.3 ml in the T + group and 637.6±57.7 ml in the T − group (p<0.001). After 24 h, no significant difference was observed in blood loss between the two groups (p=0.932). Treatment with a high-dose of TA 20 min prior to skin incision in patients planning TKR results in a statistically significant reduction in blood loss volume during and after surgery. The amount of perioperative blood loss with preoperative TA treatment was significantly reduced compared to T − group (p<0.001). A meta-analysis also confirms a statistically significant decrease in blood loss.[19]
Studies have shown that patients in whom tourniquet was used suffer from blood loss of bolus despite treatment with TA.[35] In the literature, drainage is recommended after 1 h to avoid bolus blood loss.[37] The usage of tourniquet only during cementation helped to prevent bolus blood loss in our study. The application of TA before surgery was effective, and there was no bolus blood loss in our study. The usage of TA to control blood loss in a range of publications does not pose a risk for thromboembolism, and TA treatment is considered safe.[19, 33] Recent meta-analyses confirm that administration of TA reduces perioperative blood loss in TKR without increasing the incidence of thromboembolic events or infectious complications.[36] However, in a meta-analysis study, it is stated that five of the patients were diagnosed as pulmonary embolism (PE) and two of them were in TA patients.[32] In our study, 387 patients treated with anticoagulant therapy were diagnosed with deep venous thromboembolism DVT in 4 and PE in 3 were patients. Of the four patients diagnosed with DVT, one was in the TA-receiving group and three of the TA-receiving group and two in the PE-diagnosed patients were in the TA-receiving group. No significant difference was observed between groups in terms of thromboembolism (p=0.468). However, patients with renal insufficiency, cardiovascular disorders, neurovascular disorders, or history of DVT have been associated with an increased risk of ongoing thromboembolic events with the administration of TA. About comorbid conditions such as myocardial infarction history, cerebrovascular events, or hormonal replacement therapy there is still concern.[12]
The most important limitation of our study is that four different surgeons performed the surgeries. Although all surgeons have extensive experience in the field of knee replacement surgery, individual differences could affect perioperative blood loss. Another limitation is the retrospective nature of the study. On the other hand, it was the advantage of studying the inclusion of 387 patients and the use of the same type of knee implants.
Conclusion
In conclusion, the use of single and high doses of TA in patients with TKRs significantly reduces blood loss during surgery and in the first 24 h postoperatively. There is no significant reduction in blood loss after 24 h. Preoperative iv TA administration significantly reduced the rate of allogenic erythrocyte suspensions. The above results showed that administration of intravenous high-dose TA prior to TKR did not increase complications, contributed significantly to the vital stability of the patient by reducing blood loss, and thus should be routinely performed in appropriate patients.
Disclosures
Ethics Committee Approval: This study was designed as a retrospective clinical study and was been approved by University of Health Sciences, Şişli Hamidiye Etfal Training and Research Hospital Clinical Research Ethics Committee.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – M.A.T.; Design – M.A.T.; Supervision – M.A.G.; Data collection &/or processing – M.A.G.; Analysis and/or interpretation – M.S.S.; Literature search – H.M.Ö., S.E.B.; Writing – M.A.T.; Critical review – H.M.Ö.
References
- 1.Camarasa MA, Ollé G, Serra-Prat M, Martín A, Sánchez M, Ricós P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement:a randomized clinical trial. Br J Anaesth. 2006;96:576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 2.Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32:517–24. doi: 10.1046/j.1537-2995.1992.32692367194.x. [DOI] [PubMed] [Google Scholar]
- 3.Lannan KL, Sahler J, Spinelli SL, Phipps RP, Blumberg N. Transfusion immunomodulation--the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61–8. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumberg N, Heal JM. Immunomodulation by blood transfusion:an evolving scientific and clinical challenge. Am J Med. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 5.Aydın BK, Durgut F, Erkoçak ÖF, Acar MA. Other benefits of intra-articular injection of tranexamic acid in primarytotal knee arthroplasty apart from reducing blood transfusion rates. Eklem Hastalik Cerrahisi. 2017;28:25–9. doi: 10.5606/ehc.2017.52725. [DOI] [PubMed] [Google Scholar]
- 6.Woolson ST, Marsh JS, Tanner JB. Transfusion of previously deposited autologous blood for patients undergoing hip-replacement surgery. J Bone Joint Surg Am. 1987;69:325–8. [PubMed] [Google Scholar]
- 7.Mawatari M, Higo T, Tsutsumi Y, Shigematsu M, Hotokebuchi T. Effectiveness of autologous fibrin tissue adhesive in reducing postoperative blood loss during total hip arthroplasty:a prospective randomised study of 100 cases. J Orthop Surg (Hong Kong) 2006;14:117–21. doi: 10.1177/230949900601400202. [DOI] [PubMed] [Google Scholar]
- 8.Benoni G, Lethagen S, Nilsson P, Fredin H. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthop Scand. 2000;71:250–4. doi: 10.1080/000164700317411834. [DOI] [PubMed] [Google Scholar]
- 9.Conteduca F, Massai F, Iorio R, Zanzotto E, Luzon D, Ferretti A. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. Int Orthop. 2009;33:1609–13. doi: 10.1007/s00264-008-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F. Which Route of Tranexamic Acid Administration is More Effective to Reduce Blood Loss Following Total Knee Arthroplasty?Arch Bone Jt Surg. 2016;4:65–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18:132–8. [PubMed] [Google Scholar]
- 12.Lacko M, Cellar R, Schreierova D, Vasko G. Comparison of intravenous and intra-articular tranexamic acid in reducing blood loss in primary total knee replacement. Eklem Hastalik Cerrahisi. 2017;28:64–71. doi: 10.5606/ehc.2017.54914. [DOI] [PubMed] [Google Scholar]
- 13.Santos AT, Splettstosser JC, Warpechowski P, Gaidzinski MM. Antifibrinolytics and cardiac surgery with cardiopulmonary bypass [Article in Portuguese] Rev Bras Anestesiol. 2007;57:549–64. doi: 10.1590/s0034-70942007000500011. [DOI] [PubMed] [Google Scholar]
- 14.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty:results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–7. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 15.Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of Tranexamic Acid in Total Knee Arthroplasty - Prospective Randomized Trial. Open Orthop J. 2017;11:1049–1057. doi: 10.2174/1874325001711011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S, Issa K, Pivec R, McElroy MJ, Khanuja HS, Harwin SF, et al. Intraoperative pharmacotherapeutic blood management strategies in total knee arthroplasty. J Knee Surg. 2013;26:379–85. doi: 10.1055/s-0033-1353992. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35:1639–45. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement:a systematic review and meta-analysis. Knee. 2013;20:300–9. doi: 10.1016/j.knee.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty:a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742–52. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Ma XL, Ma JX. Efficiency and Safety of Intravenous Tranexamic Acid in Simultaneous Bilateral Total Knee Arthroplasty:A Systematic Review and Meta-analysis. Orthop Surg. 2016;8:285–93. doi: 10.1111/os.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jhurani A, Shetty GM, Gupta V, Saxena P, Singh N. Effect of Closed Suction Drain on Blood Loss and Transfusion Rates in Simultaneous Bilateral Total Knee Arthroplasty:A Prospective Randomized Study. Knee Surg Relat Res. 2016;28:201–6. doi: 10.5792/ksrr.2016.28.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement:a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–85. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hiparthroplasty:a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25:129–33. doi: 10.1007/s00590-014-1457-5. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen CS, Jans Ø, Ørsnes T, Foss NB, Troelsen A, Husted H. Combined Intra-Articular and Intravenous Tranexamic Acid Reduces Blood Loss in Total Knee Arthroplasty:A Randomized, Double-Blind, Placebo-Controlled Trial. J Bone Joint Surg Am. 2016;98:835–41. doi: 10.2106/JBJS.15.00810. [DOI] [PubMed] [Google Scholar]
- 26.Oremus K, Sostaric S, Trkulja V, Haspl M. Influence of tranexamic acid on postoperative autologous bloodretransfusion in primary total hip and knee arthroplasty:a randomizedcontrolled trial. Transfusion. 2014;54:31–41. doi: 10.1111/trf.12224. [DOI] [PubMed] [Google Scholar]
- 27.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86:561–5. [PubMed] [Google Scholar]
- 28.Kambayashi J, Sakon M, Yokota M, Shiba E, Kawasaki T, Mori T. Activation of coagulation and fibrinolysis during surgery, analyzed by molecular markers. Thromb Res. 1990;60:157–67. doi: 10.1016/0049-3848(90)90294-m. [DOI] [PubMed] [Google Scholar]
- 29.Hiippala S, Strid L, Wennerstrand M, Arvela V, Mäntylä S, Ylinen J, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534–7. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 30.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty:a prospective randomised controlled trial of 29 patients. Knee. 2006;13:106–10. doi: 10.1016/j.knee.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen RE, Husted H. Tranexamic acid reduces blood loss and the need of blood transfusion after knee arthroplasty [Article in Danish] Ugeskr Laeger. 2002;164:326–9. [PubMed] [Google Scholar]
- 32.Wu Q, Zhang HA, Liu SL, Meng T, Zhou X, Wang P. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty?A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25:525–41. doi: 10.1007/s00590-014-1568-z. [DOI] [PubMed] [Google Scholar]
- 33.Hourlier H, Reina N, Fennema P. Single dose intravenous tranexamic acid as effective as continuous infusion in primary total knee arthroplasty:a randomised clinical trial. Arch Orthop Trauma Surg. 2015;135:465–71. doi: 10.1007/s00402-015-2168-z. [DOI] [PubMed] [Google Scholar]
- 34.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty:a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012;470:2605–12. doi: 10.1007/s11999-012-2310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30:776–80. doi: 10.1016/j.arth.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 36.McCormack PL. Tranexamic acid:a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585–617. doi: 10.2165/11209070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Jung WH, Chun CW, Lee JH, Ha JH, Kim JH, Jeong JH. No difference in total blood loss, haemoglobin and haematocrit between continues and intermittent wound drainage after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21:2831–6. doi: 10.1007/s00167-012-2253-6. [DOI] [PubMed] [Google Scholar]


