Etienne-Manneville previews work from Grimsley-Myers et al., which examines the regulation of VE-cadherin endocytosis by p120catenin to modulate collective cell migration during angiogenesis.
Abstract
How cells can stick together while moving through a complex environment is not fully understood. In this issue, Grimsley-Myers et al. (2020. J. Cell Biol. https://doi.org/10.1083/jcb.201909081) demonstrate that, in the case of blood vessel formation, the balance between the maintenance of endothelial integrity and the dynamics of cell-cell contacts required for collective migration relies on VE-cadherin endocytosis.
Collective cell behaviors are essential during morphogenesis, tissue homeostasis, and specific tissue functions. One such collective cell behavior is collective migration, which requires streams, groups, chains, or sheets of cells to polarize in a common direction and to migrate at a similar speed. Collective migration has been observed during development, when it plays key roles in border cell migration or dorsal closure in Drosophila, as well as in gastrulation, neural tube closure, brain development, and blood vessel formation in mammals. It is also crucial in the adult, where collective migration is involved in epithelial tissue renewal, wound healing, and in tumor angiogenesis and cancer cell spreading.
One typical example of collective migration is angiogenesis, when new blood vessels form by sprouting from existing vessels. In this case, an elongated group of endothelial cells migrate away from, while continuously maintaining contacts with, the existing vessel wall. Migrating endothelial cells polarize in a direction dictated by environmental factors such as a VEGF gradient (Gerhardt et al., 2003). The migrating collective is led by a couple of leader cells forming the tip of the sprouting structure. For the vessel to form in continuity with the blood vessel network, migrating cells—from the preexisting blood vessel to the leaders—must maintain stable intercellular contacts. These cell–cell contacts, including VE-cadherin (VE-cad)-mediated adherens junctions, stabilize the endothelial structure and participate in the coordination of cytoskeletal dynamics and cell polarization, both required for collective movement (Sauteur et al., 2014; Lagendijk and Hogan, 2015). But, at the same time, cell–cell interactions must allow highly dynamic movements to enable cells to exchange position with their neighbors and to change shape in order to navigate through a complex environment. How the delicate balance between stability and malleability of cell–cell contacts is achieved remains unclear.
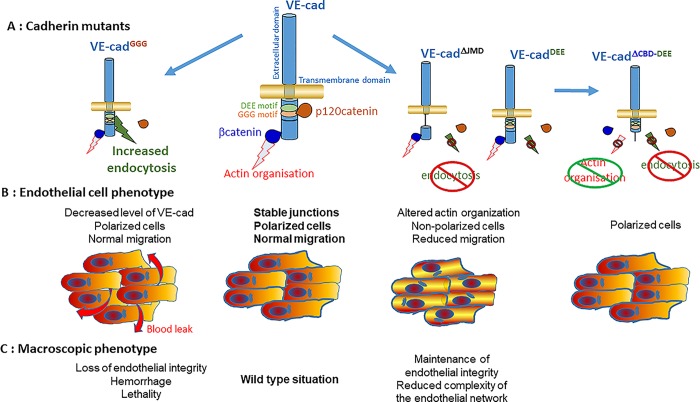
In this issue, Grimsley-Myers et al. (2020) have used a CRISPR/Cas9 approach to generate knockin mice expressing various mutants of VE-cad (Fig. 1 A) and determine whether VE-cad endocytosis affects blood vessel formation. VE-cad endocytosis is controlled by the binding of the cytosolic p120catenin to a highly conserved GGG sequence located in the juxtamembrane domain of the cadherin tail (Xiao et al., 2005). In the absence of p120catenin binding, the endocytic machinery binds to the adjacent DEE motif to promote endocytosis (Nanes et al., 2012). VE-cad mutation of the GGG motif to an AAA sequence (VE-cadGGG) abrogates p120catenin recruitment and increases VE-cad endocytosis (Fig. 1 B) as confirmed by the decreased recruitment of both proteins at endothelial cell–cell contacts. This mutation also causes a downregulation of VE-cad possibly due to the role of p120catenin binding in preventing the degradation of endocytosed VE-cad. Homozygous VE-cadGGG/GGG mice show leaky blood vessels and hemorrhages associated with a higher postnatal mortality than their wild-type littermates (Fig. 1 C). This in vivo observation suggests an important role for p120catenin-mediated VE-cad endocytosis in the stabilization of endothelial cell–cell contacts, also confirming the crucial function of p120catenin in endothelial integrity (Oas et al. 2010). Despite the destabilization of VE-cad-mediated cell–cell contacts, the overall architecture of blood vessel organization observed in the postnatal retina does not show any gross defect indicating that endothelial cell migration occurs despite the deficient adherens junctions (Fig. 1 C). The normal migratory behavior of VE-cadGGG-expressing cells was also confirmed in in vitro wound healing assays. These results suggest that VE-cadherin-mediated adherens junctions are dispensable for endothelial cell collective behavior and thus raise the question of what other cell–cell interactions are responsible for cell coordination. Whether N-cadherin can compensate for VE-cad in this situation or whether desmosomal or tight junctions can coordinate neighboring cell behavior would be worth investigating.
Figure 1.
VE-cadherin mutations impact collective endothelial cell migration and vascular development. (A) Schematics of the VE-cadherin (VE-cad) mutants used in the study by Grimsley-Myers et al. (B) Major phenotypes observed in endothelial cells expressing the VE-cad mutant shown above (in A). (C) Phenotype observed in homozygous mice expressing the VE-cad mutant shown above (in A).
In theory, by preventing p120catenin binding, mutation of the GGG motif binding has a dual effect both increasing VE-cad endocytosis and degradation and releasing p120catenin in the cytoplasm, where it can control the activity of several RhoGTPases and cell migration (Grosheva et al., 2001). To distinguish between these two possibilities, the authors use a VE-cad deletion mutant (VE-cad ΔJMD) lacking the juxtamembrane domain, thus preventing both VE-cad endocytosis and p120catenin binding (Fig. 1 A). In these conditions, VE-cad-mediated adherens junctions appear intact except for p120catenin, which localizes similarly as in VE-cadGGG mutant cells. VE-cad ΔJMD/ΔJMD mice do not display hemorrhaging and postnatal mortality as observed in VE-cadGGG/GGG animals (Fig. 1 C). Similar results are obtained in mice expressing VE-cadDEE/DEE, in which the DEE motif is mutated to AAA to prevent endocytosis while also inhibiting p120catenin recruitment to adherens junctions (Fig. 1 A), clearly showing that the cytosolic accumulation of p120catenin is not responsible for the VE-cadGGG/GGG mice phenotype. To confirm this finding, Grimsley-Myers et al. (2020) showed in mosaic tissues that absence of p120catenin, which causes a dramatic decrease of VE-cad expression at cell–cell contacts, does not severely affect endothelial integrity. Again, this result leaves open the possibility that alternative adhesion molecules maintain endothelial cell interactions. Nevertheless, deletion of endothelial p120catenin in conditional knockout mice was largely embryonic lethal (Oas et al. 2010). Co-expression of VE-cadDEE/DEE in p120catenin-depleted endothelium rescued VE-cadherin localization at cell–cell contacts and mice survival rate, indicating that VE-cad destabilization is primarily responsible for the high lethality of p120catenin knockout animals. Altogether these results demonstrate that the main function of p120catenin during blood vessel formation is to control VE-cad endocytosis rather than its signaling activity.
Despite the absence of macroscopic phenotype, the authors observe that in VE-cadDEE/DEE mice endothelial sprouting is altered, reducing the endothelial network complexity and 3D endothelial sprouting (Fig. 1 C). To further explore the impact of VE-cadDEE/DEE on endothelial collective migration, Grimsley-Myers et al. (2020) compare primary endothelial cells from the dermis of WT and VE-cadDEE/DEE mice in an in vitro wound healing assay. Stabilization of VE-cad at cell–cell contacts (using VE-cadDEE/DEE) decreases wound closure, which may explain the reduced endothelial sprouting observed in vivo. How does a decrease in VE-cadherin endocytosis impact wound closure? The authors propose several, possibly related, mechanisms. They first show that VE-cadDEE/DEE expression delays centrosome and Golgi reorientation (Fig. 1 B). This may affect the direction of movement or delay the initiation of migration at least during the first 8 hr of migration, after which the mutant cells correctly polarize. Whether the effect of VE-cadDEE on centrosome and Golgi orientation may result from the absence at cell–cell contacts of p120catenin, which is known to interact with the microtubule network, was not investigated here. The authors instead show that the stabilization of adherens junctions is associated with a reorganization of the actin cytoskeleton (Fig. 1 B). VE-cadDEE/DEE cells displayed more longitudinal cortical bundles along cell–cell junctions and less actin transverse arcs. These observations, in agreement with the known function of adherens junctions in the regulation of the actin cytoskeleton (Leckband and de Rooij, 2014), show that VE-cad endocytosis plays a crucial role in modulating actin dynamics during collective endothelial migration. Transient alteration of the actin cytoskeleton by cytochalasin D to uncouple actin cables from cell–cell junctions rescues Golgi orientation. Even more convincing is the finding that expression of a VE-cadherin mutant bearing the DEE mutation together with a deletion of the β−catenin-binding domain to uncouple adherens junctions from the actin cytoskeleton (Fig. 1 A) also rescues Golgi orientation, indicating that the defect in actin organization caused by the stabilization of junctions is responsible for delayed cell polarization (Fig. 1 B). Such a delay, possibly associated with an altered directionality of migration, may be responsible for the slower wound closure. However, it also likely that the altered actin organization directly impacts cell migration speed. A precise tracking of individual migrating cells should answer this question.
Overall, Grimsley-Myers et al. (2020) point to VE-cadherin endocytosis as a critical event balancing the stability of endothelial cell contacts and the remodeling of adherens junctions necessary for collective migration during blood vessel formation. The beautiful combination of in vivo studies with the careful analysis of cytoskeletal organization in in vitro assays strongly suggests that p120catenin binding is the major regulator of the dynamics of VE-cad at cell–cell contacts. These results certainly constitute a significant progress in our understanding of how both stability and dynamics of cell–cell contacts are simultaneously achieved during angiogenesis. Of course, they also lead to more questions: How is p120catenin binding regulated in time and space in the migrating group? Is its role similar in leaders and in followers? Cell biological approaches have previously revealed that phosphorylation of p120catenin influences its interaction with N-cadherin and that p120catenin phosphorylation is higher at the rear than at the front of the cells to promote the polarized recycling of cadherin and the continuous renewal of adherens junctions (Peglion et al., 2014). VE-cad has also been shown to contribute to the transmission of polarity between leaders and followers in endothelial migrating collectives (Hayer et al., 2016), suggesting that its stability at the front and rear of migrating cells may have a different significance at the lateral edges and at the front and rear sides of migrating cells. The precise dynamic regulation of VE-cadherin in particular, and more generally of cell–cell contacts during angiogenesis, certainly needs to be explored in more details to help us better control this process in pathological situations and to better elucidate the fundamental mechanisms underlying collective cell behaviors.
Acknowledgments
The author declares no competing financial interests.
References
- Gerhardt H., et al. J. Cell Biol. 2003 doi: 10.1083/jcb.200302047. [DOI] [Google Scholar]
- Grimsley-Myers C.M., et al. J. Cell Biol. 2020 doi: 10.1083/jcb.201909081. [DOI] [Google Scholar]
- Grosheva I., et al. 2001. J. Cell Sci. 114:695–707. [DOI] [PubMed] [Google Scholar]
- Hayer A., et al. Nat. Cell Biol. 2016 doi: 10.1038/ncb3438. [DOI] [Google Scholar]
- Lagendijk A.K., and Hogan B.M. Curr. Top. Dev. Biol. 2015 doi: 10.1016/bs.ctdb.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Leckband D.E., and de Rooij J. Annu. Rev. Cell Dev. Biol. 2014 doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- Nanes B.A., et al. J. Cell Biol. 2012 doi: 10.1083/jcb.201205029. [DOI] [Google Scholar]
- Oas R.G., et al. Circ. Res. 2010 doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peglion F., et al. Nat. Cell Biol. 2014 doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- Sauteur L., et al. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Xiao K., et al. Mol. Biol. Cell. 2005 doi: 10.1091/mbc.e05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]