Abstract
We conducted a blinded, case‐control, retrospective study in pediatric patients hospitalized at The Children’s Hospital, Denver, Colorado, to determine whether human coronavirus (HCoV)–NL63 infection is associated with Kawasaki syndrome (KS). Over the course of a 7‐month period, nasopharyngeal‐wash samples from 2 (7.7%) of 26 consecutive children with KS and 4 (7.7%) of 52 matched control subjects tested positive for HCoV‐NL63 by reverse transcription–polymerase chain reaction. These data suggest that, although HCoV‐NL63 was circulating in children in our community during the time of the study, the prevalence of infection with HCoV‐NL63 was not greater in patients with KS than in control subjects.
It has been almost 40 years since the initial description of a series of patients with Kawasaki syndrome (KS) [1]. Although the etiology of KS remains unknown, accumulated evidence suggests an infectious cause. Focal epidemics with a wavelike spread of illness have been reported in many countries. Outbreaks of KS usually occur during the winter and spring. The clinical features are characteristic of a severe, acute childhood infection. The peak incidence is in the toddler age group, with only rare cases in adults, which suggests widespread immunity to a common infectious agent. Similarly, the rarity of cases in infants <3 months old suggests the possibility of protection by passively acquired maternal antibodies [2, 3].
Since the initial description, >30 infectious agents have been implicated as potential etiological agents of KS [2, 3]. To date, no pathogen has been confirmed as the cause of KS. Recently, an association was reported between KS and the New Haven (NH) strain of a newly identified human coronavirus (HCoV), HCoV‐NL63 [4]. HCoV‐NL63 was initially discovered by researchers in The Netherlands who used a novel method to look for unidentified viral pathogens in children with lower‐respiratory‐tract disease [5]. Other researchers found the same virus by searching, using broad‐reacting polymerase chain reaction (PCR) primer sets specific for the Coronaviridae family, for previously unknown HCoVs in respiratory samples from children [6]. The NH strain of HCoV‐NL63 was discovered by screening respiratory samples with primers that targeted conserved regions of the CoV replicase 1a gene [7]. Using primers that were specific for the spike and replicase 1a genes of the NH strain of HCoV‐NL63, Esper et al. [4] detected viral sequences in respiratory specimens from 8 (72.7%) of 11 patients with acute KS but in only 1 (4.5%) of 22 concurrently collected specimens from age‐matched control subjects.
Other researchers have attempted to duplicate the results of Esper et al. but have failed to confirm their findings. Ebihara et al. [8] found no HCoV‐NL63–positive respiratory samples from 19 children with KS, and, in a separate report, Belay et al. [9] did not find HCoV‐NL63 RNA in 10 respiratory samples from children with KS. Shimizu et al. [10] identified 48 patients with acute KS from geographically and ethnically diverse populations. Using 15 different primer sets, they detected HCoV‐NL63 in only 1 (2%) of 48 patients with KS. Additionally, Chang et al. [11] did not find HCoV‐NL63 RNA by PCR testing of nasopharyngeal aspirates, throat swabs, rectal swabs, and peripheral blood mononuclear cells from 53 patients with KS in Taiwan.
However, since the initial report of the association of HCoV‐NL63 with KS, no case‐control studies have been conducted. Denver has a relatively high incidence of KS, and the majority of patients with KS admitted to our institution have a nasopharyngeal wash collected as part of their diagnostic workup. We therefore used an inclusive, consecutive set of respiratory samples from patients with KS to conduct a blinded, retrospective, case‐control study to assess the relationship between HCoV‐NL63 and KS in children in Denver.
Patients, Materials, and Methods
Patient with KS were identified from hospital discharge records from 1 December 2004 to 30 June 2005. All patients included were seen by a pediatric infectious disease specialist and had a diagnosis of classic or incomplete KS, according to standard clinical criteria [12]. As part of an ongoing epidemiological investigation of respiratory viruses at The Children’s Hospital in Denver, from November 2004 to November 2005, we archived at −70°C all respiratory specimens that tested negative by direct immunofluorescence (DFA) for respiratory syncytial virus, influenza viruses A and B, parainfluenza viruses 1–3, and adenovirus. For each patient with KS who had a banked respiratory specimen, we identified at least 2 matched control subjects from our archived specimens. Control subjects were matched by age (within 6 months if <5 years old and within 1 year if >5 years old) and by date of specimen collection (within 1 week of the case patient). All respiratory specimens used in the study were nasopharyngeal washes. All control and KS samples came from the same pool of samples that were negative for the 7 respiratory viruses tested by DFA. Use of banked specimens and clinical data was approved by the Colorado Multiple Institutional Review Board. All samples (from case patients and control subjects) were identified and coded at The Children’s Hospital microbiology/virology laboratory and then transferred to the University of Colorado Health Science Center laboratory (Aurora, CO) for blinded analysis for HCoV‐NL63 RNA by reverse‐transcription (RT)–PCR.
RNA was extracted using the QIAmp Viral RNA Mini Kit (Qiagen) in accordance with the manufacturer’s instructions. Specimens were screened for the presence of HCoV‐NL63 by RT‐PCR using Moloney murine leukemia virus reverse transcriptase (Invitrogen) with random primers, followed by PCR and/or nested PCR. Amplicons were analyzed in ethidium bromide–stained agarose gels. On the basis of results published elsewhere, 8 different primer sets were used [4, 5, 13]. Multiple primer sets were used purposely because of the potential variations in specificity and sensitivity caused by variations in the nucleotide sequences of different HCoV‐NL63 strains. Primer sets had binding sites located within the HCoV‐NL63 (NH strain) spike gene (primer set A, with nested primers B) [4], HCoV‐NL63 (NH strain) replicase 1a gene (primer set C) [4], HCoV‐NL63 nucleocapsid protein gene (primer set D) [13], and HCoV‐NL63 replicase 1b gene (primer set E with nested primer set F) [5]. Additionally, 2 sets of degenerate coronavirus primers (G and H) [4] were designed using the conserved region of the replicase 1b gene. HotStarTaq (Qiagen) was used in all PCRs, and PCR amplification programs were run as described elsewhere for each primer set. Primer set H was designed in our laboratory on the basis of a conserved region shared by HCoV‐NL63, severe acute respiratory syndrome–CoV, HCoV‐229E, and HCoV‐OC43. Primer set H consisted of forward primer 5′‐GCGCAAAATAATGAATTAATGCC‐3′ and reverse primer 5′‐CAATACAWACAAAYAGACGCWCCACC‐3′ (W, A + T; Y, C + T). The PCR amplification program was as follows: 15 min at 95°C to activate the HotStar polymerase; 10 cycles of 30 s at 94°C, 60 s at 45°C, and 45 s at 72°C; followed by 30 cycles of 30 s at 94°C, 60 s at 50°C, and 45 s at 72°C; and a final extension of 10 min at 72°C.
Positive control templates for HCoV‐NL63 consisted of either clinical specimens positive for RNA of the NH strain of HCoV‐NL63 (gift from Jeffrey S. Kahn, Yale University, New Haven, CT) or RNA extracted in the Holmes laboratory from infectious HCoV‐NL63 virus (gift from Lia van der Hoek, Academic Medical Center, University of Amsterdam, The Netherlands). To minimize the possibility of contamination, all RT reactions and PCRs were prepared in an enclosed acrylic nucleic‐acid workstation equipped with a timer‐controlled UV light (Clone Zone; USA Scientific) in a room separate from the main laboratory, and the master mix was irradiated with UV light before the addition of cDNA templates. All samples were extracted in duplicate, and all PCRs were performed on each extracted sample in duplicate. All positive results were confirmed to be HCoV‐NL63 by sequence analysis of amplicons. After all samples had been analyzed and all positive results confirmed, samples were decoded, and medical chart reviews were performed.
Statistical computations were conducted using SAS software (version 9.1.3; SAS). Significance was determined using the Wilcoxon signed‐rank test or the χ2 test.
Results
From December 2004 to June 2005, we found 30 patients with a diagnosis of KS. From these 30 patients, 29 frozen nasopharyngeal‐wash samples from 26 patients (87%) were available for analysis. Frozen nasopharyngeal‐wash samples from 52 matched control subjects were identified and analyzed. All control patients had nasopharyngeal‐wash samples sent as part of a diagnostic workup for a respiratory illness. Seventy‐five percent of these patients were hospitalized inpatients.
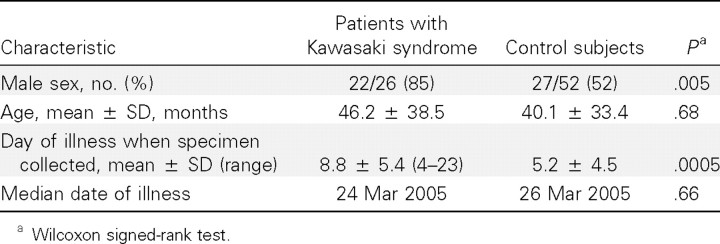
Table 1 shows the demographic characteristics of patients with KS and control subjects. Mean ages and dates of illness were not significantly different between groups. As is commonly seen in KS, the majority of our patients with KS were male. The majority of patients with KS (21 [81%]) had specimens obtained within 10 days of the onset of fever.
Table 1. .
Demographics of patients.
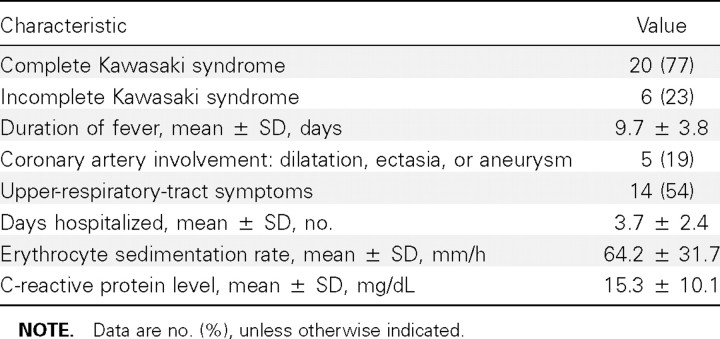
The clinical characteristics of KS patients are shown in table 2. A total of 20 (77%) of 26 patients fulfilled clinical criteria for KS, and 6 (23%) of 26 patients had incomplete KS. All patients had fever for at least 5 days, and the mean ± SD duration of fever was 9.7 ± 3.8 days. We found that 5 (19%) of 26 patients had coronary artery involvement, either dilatation, aneurysm, or ectasia. Four of 26 patients received treatment with 2 doses of intravenous immunoglobulin (IVIG), and 2 of these patients received a third treatment modality—infliximab infusion—before symptoms resolved. In addition to typical symptoms of KS, 54% of the patients had upper‐respiratory‐tract symptoms at presentation, and 9 patients (35%) reported a recent or concurrent household member with a respiratory‐tract infection.
Table 2. .
Clinical characteristics of patients with Kawasaki syndrome (n=26).
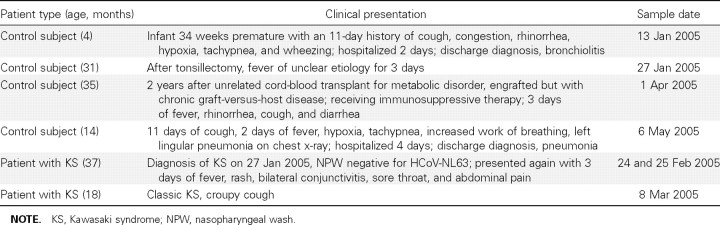
Seven (8.6%) of 81 total samples were positive for HCoV‐NL63 RNA by RT‐PCR (table 3). Two of the positive samples were positive for all primer sets tested (HCoV‐NL63 NH strain replicase and spike and HCoV‐NL63 replicase and nucleocapsid genes). Four of the positive samples were positive for HCoV‐NL63 replicase and nucleocapsid genes. The remaining sample was positive only for the HCoV‐NL63 NH strain spike gene. Two of the positive samples were from the same patient. Thus, of a total of 78 patients, 6 (7.7%) were positive for HCoV‐NL63 RNA.
Table 3. .
Characteristics of human coronavirus (HCoV)–NL63–positive patients.
The sensitivity of the PCRs varied for the different primer pairs. When infectious HCoV‐NL63 propagated in tissue culture with a TCID50 of 105 cells/mL was used, viral RNA was detectable by different primer sets at a lower limit of detection in a range of 0.01–1 TCID50.
Samples from 2 (7.7%) of 26 patients with KS (1 had 2 positive specimens) were positive by RT‐PCR for HCoV‐NL63, and 4 (7.7%) of 52 control subjects were positive. The clinical summaries of the HCoV‐NL63–positive patients are shown in table 3. The first patient with KS was an 18‐month‐old boy who presented with 6 days of fever, diffuse rash, bilateral conjunctivitis, red and cracked lips, strawberry tongue, and swelling and erythema of his hands and feet. His nasopharyngeal‐wash sample, collected on day 6 of his illness, was positive for HCoV‐NL63. Laboratory studies were notable for aseptic meningitis, sterile pyuria, thrombocytosis, increased alanine aminotransferase levels, and hypoalbuminemia. In addition to classic symptoms of KS, he also had a very pronounced croupy cough, tachypnea, and several bouts of emesis. He clinically responded to 1 infusion of IVIG but was found to have bilateral saccular coronary aneurysms on a 2‐week follow‐up echocardiogram.
The second patient with KS was a 3‐year‐old boy who presented with 14 days of rash, 6 days of fever, bilateral conjunctivitis, red lips, strawberry tongue, and swollen hands. He received 1 dose of IVIG, was hospitalized for 4 days, and was discharged after he became afebrile and his inflammatory markers had decreased. A nasopharyngeal‐wash sample obtained at day 6 of fever (day 14 of rash) during this hospitalization was negative for HCoV‐NL63. He presented again to the emergency room for evaluation for possible recurrent KS, 26 days after discharge, with a 3‐day history of fever, rash, red eyes, sore throat, and abdominal pain. Repeat inflammatory markers measured at that time were all normal. The patient was evaluated by the infectious disease consultation service, which found no evidence for recurrent KS, and he was discharged from the emergency department with a diagnosis of viral syndrome. Two nasopharyngeal‐wash samples obtained on days 2 and 3 of this second illness (25 and 26 days after discharge for the KS admission) were both positive for HCoV‐NL63.
Discussion
The identification of an infectious etiology of KS has proved to be very difficult. Our data, to our knowledge the only case‐control study to date to use an inclusive series of consecutively identified patients with KS, failed to demonstrate an association between HCoV‐NL63 and KS. Eighty‐seven percent of patients with KS seen during the study period had nasopharyngeal‐wash specimens available for analysis. Nasopharyngeal‐wash specimens are routinely collected from patients with KS, regardless of the presence or absence of upper‐respiratory‐tract symptoms, to search for viral respiratory pathogens that may be producing rash and fever or coinfecting patients with clinical KS. The prevalence (7.7%) of HCoV‐NL63 was identical in patients with KS and control subjects.
Our results failed to confirm those of Esper et al. [4]. That article reported that 53 children with KS were identified during the study period. However, respiratory specimens were available from only 11 children (7/11 had symptoms consistent with an upper‐respiratory‐tract infection). This suggests the possibility of selection bias in determining which KS samples were tested for CoV RNA.
The exact prevalence and disease associations of HCoV‐NL63 in children are now being studied at a number of institutions. Several recent studies that have examined respiratory specimens from children with respiratory illnesses of unknown etiology found prevalences of 2.1%–9.3% [5–7 , 13–17 ]. Our data, which show a similar prevalence of 7.7%, further support the conclusions that HCoV‐NL63 was circulating in our community, was detectable by our methods, and was not more prevalent in patients with KS than in control subjects with upper‐respiratory‐tract infections that were not caused by 7 common respiratory viruses.
One potential weakness of our data is that samples from patients with KS were collected later during their illness than were samples from control subjects. All studies of KS are limited by the time of patient presentation. It is possible that this could have falsely decreased the recovery of an etiological infectious agent. However, 80% of our samples were collected on or before day 10 of illness, and restricting our analysis to these samples did not significantly change our results. In a recent report that examined infants with lower‐respiratory‐tract infections, Kaiser et al. [18] were able to recover HCoV‐NL63 RNA from 3 (50%) of 6 patients in follow‐up samples obtained 3 weeks after their acute illness, which suggests that prolonged viral shedding can occur in the respiratory tract.
One‐third of our patients with KS reported exposure to a family member with a respiratory‐tract illness, and more than one‐half had concurrent respiratory symptoms. Other researchers have made similar observations, and a recent report by Benseler et al. [19] found that one‐third of patients presenting with KS had a concurrent confirmed viral or bacterial infection. In addition, Rowley et al. [20] discovered that an antigen detected by immunohistochemical analysis using synthetic KS antibodies in ciliated bronchial epithelium from patients with acute KS is found in cytoplasmic inclusion bodies that are consistent with aggregates of viral proteins. This suggests that infection with an as‐yet‐unidentified infectious agent may be important in the pathogenesis of KS.
There are several possibilities regarding the etiology of KS. The disease may be caused by a single pathogen or by interactions between multiple pathogens. An initial infectious agent(s) may predispose to a secondary infection that leads to KS. Alternatively, numerous pathogens may cause a cascade of signs that result in a common clinical presentation diagnosed as KS.
In conclusion, the results of the present case‐control study do not support an etiological association between HCoV‐NL63 and KS. Further studies are needed to determine the real etiological agent(s) of KS.
Acknowledgments
We thank the staff of the Microbiology and Virology Laboratory, Children’s Hospital, Denver, CO, for their assistance; James K. Todd, for statistical assistance; and M. K. Smith, Sonia Tusell, and Scott Jeffers, for intellectual support.
Footnotes
(See the editorial commentary by Rowley, on pages 1635–7.)
Financial support: Roche Laboratories (Pediatric Infectious Disease Society Fellowship Award to S.R.D.); National Institutes of Health (grant AI‐059576 to K.V.H.).
Potential conflicts of interest: none reported.
References
- 1.Kawaski T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children.] Arergui. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9:185–94. doi: 10.1016/j.ijid.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 4.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–8. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebihara T, Endo R, Ma X, Ighifuor N, Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192:351–2. doi: 10.1086/430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belay ED, Erdman DD, Anderson L, et al. Kawasaki disease and human coronavirus. J Infect Dis. 2005;192:352–3. doi: 10.1086/431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis. 2005;192:1767–71. doi: 10.1086/497170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L‐Y, Chiang B‐L, Kai C‐L, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV‐NH, and Kawasaki disease in Taiwan. J Infect Dis. 2006;193:283–6. doi: 10.1086/498875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis, and Kawasaki disease. Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 13.Bastien N, Robinson JL, Tse A, et al. Human coronavirus NL‐63 infections in children: a 1 year study. J Clin Microbiol. 2005;43:4567–73. doi: 10.1128/JCM.43.9.4567-4573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vabret A, Mourez T, Dina J, et al. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225–9. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–9. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis. J Med Virol. 2005;75:463–5. doi: 10.1002/jmv.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arden KE, Nissen MD, Sloots TP, Mackay IM. New human coronavirus NL63 associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–62. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser L, Regamey N, Roiha H, Deffenez C, Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life. Pediatr Infect Dis J. 2005;24:1015–7. doi: 10.1097/01.inf.0000183773.80217.12. [DOI] [PubMed] [Google Scholar]
- 19.Benseler SM, McCrindle BW, Silverman ED, Tyrrell PN, Wong J, Yeung RS. Infections and Kawasaki disease: implications for coronary artery outcome. Pediatrics. 2005;116:e760–6. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 20.Rowley AH, Baker SC, Shulman ST, et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192:1757–66. doi: 10.1086/497171. [DOI] [PMC free article] [PubMed] [Google Scholar]