Abstract
Fifty-six piglets (6.26 ± 0.64 kg BW) were weaned at 21 d and randomly assigned to one of the eight dietary treatments with seven replicate pens for a 14-d experimental period. The eight experimental diets were prepared via a 2 × 4 factorial arrangement with citric acid (CA; 0% and 0.3%) and dietary electrolyte balance (dEB, Na + K − Cl mEq/kg of the diet; −50, 100, 250, and 400 mEq/kg). Varying dEB values were obtained by altering the contents of calcium chloride and sodium bicarbonate. An interaction (P < 0.05) between dEB and CA in diarrhea score and the number of goblet cell in jejunum were observed. Ileum pH significantly decreased in weaned piglets fed 250 mEq/kg dEB diet compared with those fed −50 and 400 mEq/kg dEB diets (P < 0.05). Supplementation of 0.3% CA decreased the number of goblet cell in the ileal crypt (P < 0.05) and the relative mRNA expression of cystic fibrosis transmembrane conductance regulator, tumor necrosis factor-α, interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-10 (IL-10), zona occludens-1, and Claudin-1 (P < 0.05). Increasing dEB values increased the number of goblet cells in the jejunal crypt (P < 0.05). A 250-mEq/kg dEB diet decreased the relative mRNA expression of IFN-γ, IL-1β, and IL-10 (P < 0.05) than 100-mEq/kg dEB diet. The interaction between dEB and CA on the relative abundances of Cyanobacteria and Saccharibacteria was observed (P < 0.05). Supplementation of 0.3% CA increased relative abundances of and Streptococcus hyointestinalis. Piglets fed 250-mEq/kg diet increased relative abundances of Firmicutes and Lactobacillus rennini, and decreased the relative abundance of Proteobacteria, Veillonella, Actinobacillus minor, and Escherichia–Shigella.In conclusion, supplementation of 0.3% CA resulted in differential expression of inflammatory cytokines, ion transporters, and tight junction proteins, and changes in the microbial community composition. A 250-mEq/kg dEB diet reduced gastrointestinal pH and promoted the enrichment of beneficial microbes in the gut microbiota, thereby suppressing inflammation and harmful bacteria. However, the addition of CA to diets with different dEB values did not promote intestinal function in weaned piglets.
Keywords: cytokines, dietary electrolyte balance, gastrointestinal pH, goblet cells, ion transporters, microbiota
Introduction
During weaning, young pigs are exposed to various stress factors which may be psychological, dietary, and/or environmental, with a consequent incidence of intestinal dysfunction with diarrhea and reduction of growth performance (Pluske et al., 1997; Heo et al., 2013). Thus, it is necessary to identify possible nutritional mitigation strategies to improve intestinal health and function in weaned piglets to control the negative effects of post-weaning stress. Sodium (Na), potassium (K), and chlorine (Cl), as the main cations and anions in the body, play an important role in maintaining normal body fluid, osmotic pressure, acid–base balance, and water–salt metabolism, participating in nutrient metabolism, and neuroregulation. However, changing the relative proportion of these ions may alter the dietary electrolyte balance (dEB, Na + K – Cl, reported in mEq/kg of diet; Mongin, 1981) inducing an electrical imbalance which is the predominant cause of acid–base disorders (Adeva-Andany et al., 2014), which may lead to diarrhea in animals (Okada et al., 2018).
In addition, the colon has the capacity to store water and electrolytes, which is about three times normal, but, if it is exceeded or damaged, it will lead to a net water loss and ultimately lead to diarrhea (Rao, 2019). Therefore, NRC (2012) recommended that the optimal dEB for pigs is approximately 250 mEq/kg. Although a number of studies have found that dEB changed blood, urine, and gastric pH and influenced the acid–base buffer system, nutrient digestibility, and growth performance in pigs (Patience et al., 1987; Haydon et al., 1990; Dersjant-Li et al., 2002; DeRouchey et al., 2003; Cheng et al., 2015; Guzmán-Pino et al., 2015), few studies were observed that suggested dEB affected intestinal health and its mechanism is still unclear.
Since antibiotics have been banned as growth promoters in animal production, organic acids have been proposed to replace them to promote growth and reduce post-weaning diarrhea. Organic acids improve intestinal health by reducing the pH value of digestive tract, increasing the number of lactic acid bacteria and Bifidobacteria, reducing Escherichia coli, and promoting the development of intestinal morphology, thus enhancing growth performance and nutrient digestibility of pigs (Zhai et al., 2017; Long et al., 2018). In addition, some organic acids participate in energy metabolism and enzyme catalysis in animals, thus promoting the proliferation and differentiation of intestinal cells such as lactic acid which is a final product of glycolysis and can release energy through gluconeogenesis (Hui et al., 2017). Citric acid (CA) participates in the tricarboxylic acid cycle as an energy sources, avoiding the tissue degradation caused by gluconeogenesis and fat decomposition, thus further increasing the digestibility of nutrients (Bodner, 1986). More importantly, CA increases the number of goblet cells in the gut (Khosravinia et al., 2015).
Intestinal microbiota plays a key role in maintaining intestinal health, including gut barrier function and immune system maturation (Min and Rhee, 2015; Thaiss et al., 2016). Alterations in gut microbiota cause the decrease in intestinal digestion and absorption capacity, the proliferation of pathogenic bacteria, and ultimately diarrhea (Huang et al., 2019). CA showed a high bacteriostatic effect on pathogenic bacteria such as coliform bacteria and Staphylococcus aureus (Aydin et al., 2010; Olaimat et al., 2017). However, it is still unknown whether there are interactions between dEB and CA. The present study was conducted to test the hypothesis that the effects of dEB may modulate the intestinal microbial structure and there may be interactions between dEB and CA.
Materials and Methods
The care and handling of the weaned piglets used in this study complied with the standards adopted by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China.
Animals and experimental design
Fifty-six weaned piglets (Duroc × Landrace × Yorkshire, barrows), 21 d of age and initially weighing 6.26 ± 0.64 kg, were randomly assigned to one of the eight dietary treatments with seven replicate pens (one piglet per replicate pen) for a 14-d period. Eight experimental diets were prepared using a 2 × 4 factorial arrangement of dEB (−50, 100, 250, and 400 meq/kg) and CA (0% and 0.3%) with similar nutritional levels. The value of dEB was calculated using this formula: dEB (mEq/kg) = [(Na × 434.98) + (K × 255.74) − (Cl × 282.06)] (Mongin, 1981). The dEB values were obtained by varying calcium chloride and sodium bicarbonate concentrations. The compositions of the basal diets are shown in Table 1. All diets were formulated to meet or exceed all nutrient concentrations recommended by the NRC (2012). Before starting the experiment, the diets were analyzed for Na, K, and Cl contents. The contents of Na and K (method 985.01) were determined according to AOAC (2007) procedures and Cl (method 6495-1) was determined according to ISO (2005) procedures. Weaned piglets had ad libitum access to water and the experimental diet throughout the experiment. Room temperature was maintained at 29 ± 1 °C during week 1 and then reduced by 1.5 °C each week. The health status was observed and recorded daily throughout the experimental period.
Table 1.
Experimental diets’ ingredients and chemical composition (as-fed basis)
| 0% CA | 0.3% CA | |||||||
|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | |||||||
| Ingredient | −50 | 100 | 250 | 400 | −50 | 100 | 250 | 400 |
| Ingredients,% | ||||||||
| Corn | 66.00 | 66.12 | 64.14 | 61.48 | 65.70 | 65.82 | 63.84 | 61.18 |
| Soybean meal | 6.33 | 6.31 | 6.72 | 7.27 | 6.33 | 6.31 | 6.72 | 7.27 |
| Soy–protein concentrates | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Whey | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Fish meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Spray-dried plasma protein | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Limestone | 0.06 | 0.88 | 1.07 | 1.06 | 0.06 | 0.88 | 1.07 | 1.06 |
| Dicalcium phosphate | 0.40 | 0.40 | 0.41 | 0.42 | 0.40 | 0.40 | 0.41 | 0.42 |
| CA | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 | 0.30 | 0.30 | 0.30 |
| Chromic oxide | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| l-Lys•HCl (98%) | 0.53 | 0.53 | 0.52 | 0.51 | 0.53 | 0.53 | 0.52 | 0.51 |
| dl-Met | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| l-Thr | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| l-Trp | 0.05 | 0.05 | 0.05 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 |
| Soybean oil | 1.30 | 1.27 | 1.90 | 2.75 | 1.30 | 1.27 | 1.90 | 2.75 |
| Calcium chloride | 1.08 | 0.21 | 0.00 | 0.00 | 1.08 | 0.21 | 0.00 | 0.00 |
| Sodium bicarbonate | 0.00 | 0.00 | 0.96 | 2.23 | 0.00 | 0.00 | 0.96 | 2.23 |
| Choline chloride (50%) | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Antioxidant | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin and mineral premix1 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Calculated composition | ||||||||
| CP, % | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| ME3, kcal/kg | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 | 3,350 |
| Calcium, % | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Phosphorus, % | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| Lys2, % | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 |
| MET2,% | 0.39 | 0.39 | 0.39 | 0.40 | 0.39 | 0.39 | 0.39 | 0.40 |
| Met+Cys2,% | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| Thr2,% | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Trp2,% | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Analyzed composition | ||||||||
| Na,% | 0.17 | 0.18 | 0.44 | 0.78 | 0.16 | 0.18 | 0.41 | 0.82 |
| K,% | 0.65 | 0.65 | 0.65 | 0.65 | 0.57 | 0.67 | 0.70 | 0.66 |
| Cl,% | 1.05 | 0.52 | 0.39 | 0.38 | 0.95 | 0.55 | 0.40 | 0.43 |
| dEB3, mEq/kg | -55.99 | 97.86 | 247.62 | 398.33 | -52.59 | 94.51 | 244.54 | 404.19 |
1Vitamin-mineral premix supplied per kilogram of feed: 10,000 IU of vitamin A, 100 IU of vitamin D3, 80 IU of vitamin E, 2.0 mg of vitamin K3, 0.03 mg of vitamin B12, 12 mg of riboflavin, 40 mg of niacin, 25 mg of d-pantothenic acid, 0.25 mg of biotin, 1.6 mg of folic acid, 3.0 mg of thiamine, 2.25 mg of pyridoxine, 300 mg of choline chloride, 150 mg of Fe (FeSO4), 100 mg of Zn (ZnSO4), 30 mg of Mn (MnSO4), 25 mg of Cu (CuSO4), 0.5 mg of I (KIO3), 0.3 mg of Co (CoSO4), 0.3 mg of Se (Na2SeO3), and 0.4 mg of ethoxyquin.
2Standardized ileal-digestible.
3dEB was calculated using the following equation: dEB (mEq/kg) = [(Na × 434.98) + (K × 255.74) − (Cl × 282.06)].
Sample treatment and collection
All piglets were fasted overnight, weighed in the morning, and then fed to ensure the sampling of intestinal contents approximately 4 h before slaughter. Then, the weaned piglets in each treatment group were euthanized with Zoletil (15 mg tiletamine/kg body weight, 15 mg zolazepam/kg body weight, intramuscular injection). Their abdominal cavities were opened, and the small intestines were separated from the mesentery. The boundaries between the stomach, duodenum, jejunum, ileum, cecum, and colon were ligatured to prevent chyme flow into other parts of the intestine. The pH values of digesta were measured with a Testo 205 pH meter (Testo AG, Germany), and then the ileal digesta samples were collected with 5 mL sterile and enzymatic eppendorf tube and immediately frozen in liquid nitrogen and stored at −80 °C until required for microbiota analysis. Intestinal tissues from the middle part of the jejunum and ileum were collected (approximately 20 cm of each tissue) after being washed with phosphate-buffered saline (pH = 7.2 to 7.4), and then the glass slide was used for the collection of mucosa. The mucosal tissue samples were immediately frozen in liquid nitrogen and stored at –80 °C for use in the mRNA analysis.
Diarrhea score
We used diarrhea incidence as described by Li et al. (2018). Briefly, the clinical signs of diarrhea were visually assessed daily by observers blinded to the treatments. Fecal scoring is as follows: 1 = hard; 2 = slightly soft; 3 = soft, partially formed; 4 = loose, semi-liquid; and 5 = watery, mucous-like. The diarrhea score was calculated by the following formula: diarrhea score = sum of the morning and evening diarrhea total scores for each weaned piglet/repetition/experiment days.
RNA extraction and real-time quantitative PCR
Real-time quantitative PCR analysis was conducted according to a previous study (Chen et al., 2019). Briefly, total RNA was isolated from jejunum samples, frozen in liquid nitrogen using TRIZOL reagent (TaKaRa, Dalian, China), and treated with DNase I (TaKaRa, Dalian, China) to remove trace DNA. RNA was reverse transcribed to cDNA using the high-capacity cDNA Reverse Transcription Kit (TaKaRa, Dalian, China). Primers against nutrient transporters were designed using Primer-BLAST (National Center for Biotechnology Information, Bethesda, MD) (Table 2). The expression of all target genes was normalized to that of β-actin. Relative transcript abundance was determined by the comparative ∆∆CT method. Real-time PCR was performed according to previous publications by our laboratory.
Table 2.
Primers used for real-time PCR analysis
| Gene1 | Sequence(5′-3′)2 | Product size, bp | Accession no. |
|---|---|---|---|
| AQP3 | F: TGACCTTCGCTATGTGCTTCC | 212 | NM_001110172.1 |
| R: GTCCAAGTGTCCAGAGGGGTAG | |||
| AQP8 | F: GGTGCCATCAACAAGAAGACG | 227 | NM_001112683.1 |
| R:CCGATAAAGAACCTGATGAGCC | |||
| NKCC1 | F: CCAATGCTGTTGCAGTTGCT | 264 | XM_005661615.2 |
| R: TGGGCTTCTTGCTCTCCAAG | |||
| NHE3 | F: AGCTGGAGATCATAGACCAGGTG | 147 | AF_123280 |
| R: CGGTGAAGAAGATGACGATGAG | |||
| CFTR | F: ACTATGGACCCTTCGAGCCT | 123 | NM_001104950.1 |
| R: CGCATTTGGAACCAGCGTAG | |||
| TNF-α | F: ACAGGCCAGCTCCCTCTTAT | 102 | NM_214022.1 |
| R: CCTCGCCCTCCTGAATAAAT | |||
| IFN-γ | F: CCATTCAAAGGAGCATGGAT | 146 | NM_213948.1 |
| R: GAGTTCACTGATGGCTTTGC | |||
| IL-1β | F: CCTGGACCTTGGTTCTCT | 123 | XM_021085847.1 |
| R: GGATTCTTCATCGGCTTCT | |||
| IL-10 | F: GGGCTATTTGTCCTGACTGC | 105 | NM_214041.1 |
| R: GGGCTCCCTAGTTTCTCTTCC | |||
| Occludin | F: GAGTGATTCGGATTCTGTCT | 181 | XM_005672525.3 |
| R: TAGCCATAACCATAGCCATAG | |||
| ZO-1 | F: TTGATAGTGGCGTTGACA | 126 | XM_021098896.1 |
| R: CCTCATCTTCATCATCTTCTAC | |||
| Claudin-1 | F: CTAGTGATGAGGCAGATGAA | 250 | XM_005670262.3 |
| R: AGATAGGTCCGAAGCAGAT | |||
| β- actin | F:AGTTGAAGGTGGTCTCGTGG | 216 | XM_003357928.4 |
| R: TGCGGGACATCAAGGAGAAG |
1AQP3, aquaporin 3; AQP8, aquaporin 8; NHE3, Na+/H+ exchanger 3.
2F, forward primer; R, reverse primer.
Alcian blue–periodic acid–Schiff staining
Tissue samples fixed in formalin were dehydrated in a graded series of ethanol solutions and embedded in paraffin wax. Sections of 5-μm-thick tissue samples were cut using a microtome (RM2235; Leica; Germany) and then mounted for staining with Alcian blue–periodic acid–Schiff staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Briefly, the slices were cleaned twice in xylene for 10 min each. The slices were dewaxed with xylene twice for 10 min each time and rehydrated with 95%, 70%, and 30% ethanol and with distilled water for 2 min each. All slices were treated with Alcian blue dye for 15 min, washed with distilled water for 1 min, and incubated at room temperature for 10 min. After washing with distilled water for 3 min, the sections were incubated with Schiff’s staining for 5 min. All the slices were washed with tap water for 5 min, following which they were dehydrated through xylene and covered with a coverslip. The stained slides were examined under a light microscope (Leica DM3000; Leica Microsystems, Wetzlar, Germany). The number of goblet cells in both villus and crypt was counted manually in 30 complete villus or crypts.
Immunoblotting
Samples of jejunal mucosa were powdered in liquid nitrogen and then lysed in ice-cold radioimmunoprecipitation assay buffer supplemented with protease inhibitor phenylmethanesulfonyl fluoride (Beyotime Biotechnology, Shanghai, China). The lysates were centrifuged at 12,000 × g for 10 min at 4 °C. The protein concentration was quantified using Bicinchoninic Acid assay (Beyotime Biotechnology, China), and then all the samples were adjusted to 2 μg/μL.
Solubilized proteins from jejunal mucosa were separated by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride (Millipore, Billerica, MA) membranes and blocked with 5% nonfat milk in tris-buffered saline with tween (Applygen Technologies Inc., Beijing, China) for 1 h. The primary antibodies for β-actin (SC-47778; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:3,000 dilution), anti-occludin (66378-1-Ig, Proteintech Group, Inc. Los Angeles, CA, USA; 1:1,000 dilution), and anti-claudin 1 (ab129119; Abcam, Cambridge, MA; 1:8,000 dilution) were incubated overnight at 4 °C, followed by horseradish peroxidase-linked secondary antibodies (Santa Cruz Biotechnology Inc.) incubated for 1 h at 25 °C before the development of the blots using enhanced chemiluminescence (Applygen Technologies Inc.). Target protein abundance was normalized via β-actin. AlphaImager 2200 software (Alpha Innotech Corporation, CA, USA) was used to quantify the bands of each protein per sample.
Ileum content microflora 16S rRNA sequencing
Based on the diarrhea scores of weaned piglets, four groups (−50 mEq/kg dEB, 250 mEq/kg dEB, −50 mEq/kg dEB + 0.3% CA, and 250 mEq/kg dEB + 0.3% CA) were selected for ileum microbiota analysis. Microbial DNA was extracted from approximately 0.25 g of each fecal sample using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Successful DNA isolation was performed by 2% agarose gel electrophoresis. The 16S rRNA gene V3–V4 region was amplified from genomic DNA using the universal bacterial primers 515F (5′-ACTCCTACGGGAGGCAGCAG-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The specific sequencing method was done as previously reported (Zhao et al., 2018). Briefly, paired-end sequenced on an Illumina HiSeq2500platform (Illumina, USA) at Novogene Bioinformatics Technology (Beijing, China). Raw sequencing data were assembled and filtered using QIIME (Version 1.9.1) and the UPARSE (Uparse v7.0.1001) software to obtain clean data. Clustering and species classification analysis were conducted using the operational taxonomic units (similarity level of 97%) of the clean data. Then, the alpha and beta diversity measurements were done using QIIME (Version 1.9.1) software.
Statistical analysis
SPSS (Version 17.0) analyzed all data as a 2 × 4 factorial arrangement of treatments in a randomized complete block design. Pen (N = 56) was the experimental unit. Data were analyzed by a two-way ANOVA for dEB (−50, 100, 250, 400 mEq/kg) and CA (0%, 0.3%), and their interaction (dEB × CA). When the effect of dEB or CA was significant, statistical differences between treatments were compared further using the Duncan’s multiple range test. Alpha and β diversity were analyzed using QIIME (v1.9.1). All data are expressed as the means ± SEM. Statistical significance was determined at P < 0.05 and tendencies at P < 0.10.
Results
Diarrhea score and gastrointestinal tract pH
An interaction between dEB and CA in diarrhea scores was observed (P < 0.05), whereas no such effect was found on gastrointestinal tract pH (Table 3). CA supplementation did not affect stomach, jejunum, ileum, cecum, or colon pH. Ileum pH significantly decreased in weaned piglets fed 250 mEq/kg dEB diet compared with those fed −50 mEq/kg and 400 mEq/kg dEB diets (P < 0.05). Colon pH showed decreasing trend in piglets fed 250 mEq/kg dEB diet than −50 mEq/kg dEB diet (P < 0.10). Piglets fed 100 mEq/kg and 250 mEq/kg dEB diets decreased stomach pH compared with those fed −50 mEq/kg dEB diet (P < 0.10). However, jejunum and cecum pH were not significantly affected.
Table 3.
Effect of dEB and CA on diarrhea score and gastrointestinal tract pH of weaned piglets1
| 0% CA | 0.3% CA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | P-value | ||||||||||
| Item | −50 | 100 | 250 | 400 | −50 | 100 | 250 | 400 | SEM | CA2 | dEB | CA × dEB |
| Diarrhea score | 1.80ab | 1.32bc | 1.22c | 1.50abc | 1.32bc | 1.73abc | 1.59abc | 1.87a | 0.06 | 0.160 | 0.433 | 0.026 |
| Stomach | 4.51 | 3.52 | 2.86 | 4.66 | 5.21 | 3.10 | 3.84 | 3.38 | 0.25 | 0.992 | 0.091 | 0.308 |
| Jejunum | 6.74 | 6.30 | 6.18 | 6.45 | 6.11 | 6.11 | 6.29 | 6.35 | 0.10 | 0.380 | 0.855 | 0.685 |
| Ileum | 7.00 | 6.79 | 6.35 | 6.99 | 7.26 | 6.69 | 6.63 | 6.86 | 0.08 | 0.586 | 0.020 | 0.615 |
| Cecum | 5.84 | 5.70 | 5.70 | 5.68 | 5.94 | 5.74 | 5.64 | 5.69 | 0.04 | 0.800 | 0.321 | 0.953 |
| Colon | 6.16 | 6.07 | 5.96 | 5.91 | 6.17 | 6.01 | 5.80 | 6.19 | 0.04 | 0.796 | 0.052 | 0.127 |
1Data were means of seven piglets per treatment.
2CA × dEB = the interaction between citric acid and dietary electrolyte balance.
a–cWithin a row, means with different superscripts differ (P < 0.05).
Intestinal goblet cells
There was an interactive effect of dEB and CA on the number of goblet cells in jejunal villus and crypt (P < 0.05), whereas no such effect was observed on ileum (Table 4). Supplementation of 0.3% CA decreased the number of goblet cells in the ileal crypt (P < 0.05), but no effect was found in ileal villus, jejunal villus, and crypt. Increasing dEB values increased the number of goblet cells in jejunal crypt (P < 0.05). However, the number of goblet cells in ileal villus and crypt, and jejunal villus was not significantly affected.
Table 4.
Effect of dEB and CA on the number of goblet cell in weaned piglets1
| 0% CA | 0.3% CA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | P-value | ||||||||||
| Item | −50 | 100 | 250 | 400 | −50 | 100 | 250 | 400 | SEM | CA2 | dEB | CA × dEB |
| Ileum | ||||||||||||
| Villus | 19.99 | 15.37 | 14.57 | 16.89 | 15.43 | 14.79 | 17.09 | 15.32 | 0.60 | 0.387 | 0.491 | 0.234 |
| Crypt | 27.37 | 24.22 | 23.96 | 26.47 | 22.35 | 22.26 | 22.71 | 22.76 | 0.35 | <0.001 | 0.248 | 0.241 |
| Jejunum | ||||||||||||
| Villus | 14.17ab | 18.83a | 12.66b | 15.45ab | 14.91ab | 13.23b | 16.62ab | 16.49ab | 0.54 | 0.974 | 0.639 | 0.025 |
| Crypt | 21.45ab | 20.51ab | 18.49b | 22.58a | 18.11b | 21.63ab | 22.97a | 23.70a | 0.42 | 0.322 | 0.047 | 0.020 |
1Data were means of seven piglets per treatment.
2CA × dEB = the interaction between citric acid and dietary electrolyte balance.
a,bWithin a row, means with different superscripts differ (P < 0.05).
The expression of intestinal aquaporins, ion transporters, cytokines, and tight junction proteins
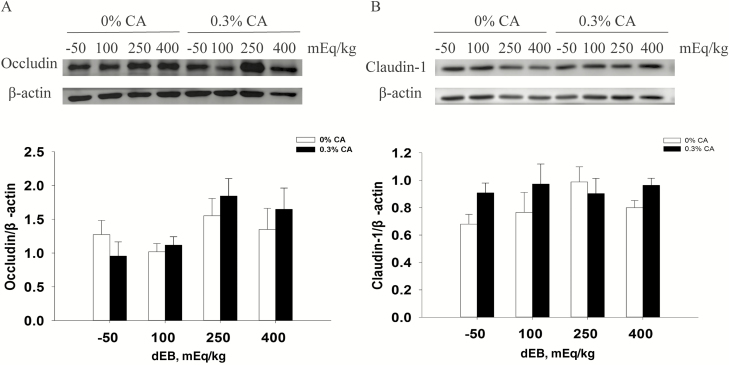
No significant interaction effects were observed on transporters mRNA expression (Table 5). Supplementation of 0.3% CA decreased the relative mRNA expression of cystic fibrosis transmembrane conductance regulator (CFTR), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β IL-1β), interleukin-10 (IL-10), zona occludens-1 (ZO-1), Claudin-1 (P <0.05). Moreover, the relative mRNA expression of Na-K-Cl cotransporter (NKCC1, P < 0.10) showed a decreased tendency in 0.3% CA diet. A 250-mEq/kg dEB diet decreased the relative mRNA expression of IFN-γ, IL-1β, IL-10 (P < 0.05), and Claudin-1 (P < 0.10) than 100 mEq/kg dEB diet. A 250-mEq/kg dEB diet decreased the relative mRNA expression of TNF-α (P < 0.10) than −50 and 100 mEq/kg dEB diets. However, no significant effects were observed on occludin and claudin-1 proteins expression in the jejunum (Figure 1).
Table 5.
Effects of dEB and CA on aquaporins, ion transporters, cytokines, and tight junction proteins of jejunum in weaned piglets1
| 0% CA | 0.3% CA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | P-value | ||||||||||
| Item2 | −50 | 100 | 250 | 400 | −50 | 100 | 250 | 400 | SEM | CA3 | dEB | CA×dEB |
| AQP3 | 1.07 | 0.97 | 1.21 | 0.91 | 0.71 | 0.90 | 0.85 | 1.16 | 0.07 | 0.390 | 0.886 | 0.441 |
| AQP8 | 1.25 | 1.33 | 1.14 | 1.27 | 1.76 | 1.43 | 0.93 | 1.10 | 0.13 | 0.831 | 0.598 | 0.756 |
| NKCC1 | 1.11 | 0.68 | 0.66 | 0.74 | 0.53 | 0.72 | 0.43 | 0.65 | 0.06 | 0.066 | 0.400 | 0.282 |
| NHE3 | 1.02 | 1.26 | 0.76 | 1.00 | 0.76 | 0.97 | 0.84 | 0.91 | 0.05 | 0.197 | 0.202 | 0.589 |
| CFTR | 1.02 | 1.43 | 0.94 | 1.00 | 0.48 | 0.79 | 0.80 | 0.90 | 0.09 | 0.047 | 0.530 | 0.587 |
| TNF-α | 1.07 | 0.94 | 0.55 | 0.69 | 0.36 | 0.53 | 0.29 | 0.50 | 0.06 | <0.001 | 0.075 | 0.238 |
| IFN-γ | 1.06 | 1.09 | 0.71 | 1.14 | 0.35 | 0.92 | 0.19 | 0.46 | 0.08 | <0.001 | 0.044 | 0.510 |
| IL-1β | 1.02 | 1.36 | 0.66 | 1.03 | 0.39 | 1.00 | 0.43 | 0.53 | 0.09 | 0.011 | 0.047 | 0.838 |
| IL-10 | 1.04 | 1.19 | 0.59 | 0.92 | 0.39 | 0.78 | 0.32 | 0.47 | 0.07 | <0.001 | 0.023 | 0.734 |
| Occludin | 1.04 | 0.78 | 0.75 | 1.17 | 0.58 | 0.79 | 0.67 | 0.78 | 0.06 | 0.042 | 0.351 | 0.344 |
| ZO-1 | 1.02 | 0.84 | 0.65 | 0.84 | 0.51 | 0.75 | 0.60 | 0.74 | 0.04 | 0.028 | 0.396 | 0.195 |
| Claudin-1 | 1.04 | 1.94 | 1.17 | 1.62 | 0.76 | 1.59 | 0.94 | 1.32 | 0.10 | 0.136 | 0.011 | 0.997 |
1Data were means of seven piglets per treatment.
2AQP3, aquaporin 3; AQP8, aquaporin 8; NHE3, Na+/H+ exchanger 3.
3CA × dEB = the interaction between citric acid and dietary electrolyte balance.
Figure 1.
Effect of dEB (Na + K – Cl) and CA on expression of tight junction protein in the jejunum of weaned piglets. Protein expression of occluding (A) and claudin-1(B) was determined by western blotting and normalized to β-actin. The data were analyzed by two-way ANOVA for dEB (−50, 100, 250, and 400 mEq/kg), CA (0% and 0.3%), and their interaction (dEB × CA). All data are expressed as means ± SEM (n = 4), and statistical significance was indicated by P < 0.05. (A) CA (P = 0.71); dEB (P = 0.24); dEB × CA (P = 0.80). (B) CA (P = 0.14); dEB (P = 0.66); dEB × CA (P = 0.55).
Composition of the ileal microbiota
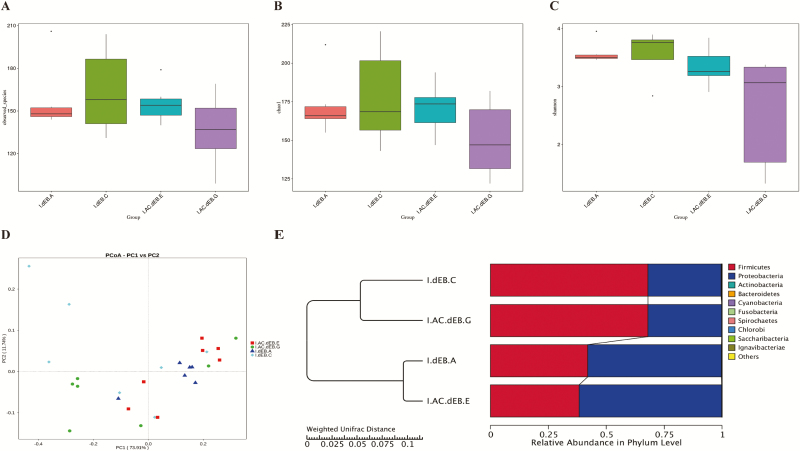
The microbiota composition and diversity of the ileal samples in 2-wk-old piglets were assessed by deep sequencing of the V3–V4 region of the 16S rRNA genes. Alpha-diversity measures (observed species and Chao1 indices) of the fecal bacteria community showed no differences between weaned piglets of dEB and CA (Table 6 and Figure 2A and B). Piglets fed the 250 mEq/kg dEB diet increased the Shannon index, but the addition of CA to the 250 mEq/kg dEB diet did not lead to a further increase, so the interaction effect (P < 0.10) between dEB and CA was observed (Table 6 and Figure 2C). Dietary supplementation with 0.3% CA decreased the Shannon index (P < 0.05). The 250 mEq/kg dEB diet decreased (P < 0.10) the Shannon index compared with the −50 mEq/kg dEB diet. Unconstrained principal co-ordinate analyses of weighted UniFrac distances were performed to investigate the patterns of separation between microbial communities revealed that the gut microbiota showed obvious segregation in weaned piglets from −50 dEB diet to 250 dEB diet (Figure 2D). Furthermore, the unweighted pair-group method with arithmetic mean analysis was applied to the weighted UniFrac distances and the phenogram showed the relationship of all the observed samples (Figure 2E).
Table 6.
Effects of dEB and CA on α-diversity indices of bacterial communities in ileal digesta in weaned piglets1
| 0% CA | 0.3% CA | |||||||
|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | P-value | ||||||
| Item | −50 | 250 | −50 | 250 | SEM | CA2 | dEB | CA×dEB |
| Observed_species | 157.50 | 164.00 | 154.86 | 136.57 | 4.52 | 0.110 | 0.521 | 0.183 |
| Shannon | 3.58a | 3.58a | 3.35a | 2.55b | 0.10 | 0.006 | 0.070 | 0.069 |
| Chao1 | 172.70 | 178.41 | 170.44 | 150.57 | 4.52 | 0.110 | 0.442 | 0.171 |
1Data were means of seven piglets per treatment.
2CA × Deb = the interaction between citric acid and dietary electrolyte balance.
a,bWithin a row, means with different superscripts differ (P < 0.05).
Figure 2.
Effect of dEB (Na + K – Cl) and CA on microbial community diversity in ileal digesta of weaned piglets. (A) Comparison of the number of observed operational taxonomic units (OTUs), (B) Chao1, and (C) Shannon index. (D) Principle co-ordinates analysis (PCoA) of microbial communities. (E) Unweighted pair-group method with arithmetic mean (UPMGA) cluster analysis of microbial communities. I.dEB.A: −50 mEq/kg dEB; I.dEB.C: 250 mEq/kg dEB; I.AC.dEB.E: −50 mEq/kg dEB with 0.3% CA; I.AC.dEB.G: 250 mEq/kg dEB with 0.3% CA.
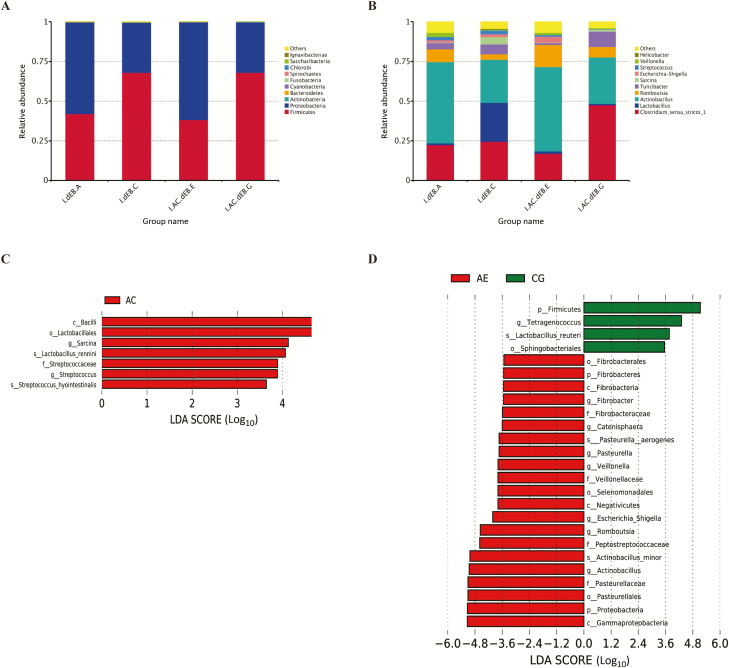
The top 10 phyla and genus in the relative abundance of the fecal microbiota present in piglets were also displayed in Table 7 and Figure 3A and B. At the phyla level, Firmicutes and Proteobacteria were the most dominated phyla in both dEB and CA piglets; these phyla accounted for more than 99% of total sequences. The interaction between dEB and CA on the relative abundance of Cyanobacteria and Saccharibacteria was observed (P < 0.05). Dietary supplementation with 0.3% CA decreased the relative abundance of Bacteroidetes and Saccharibacteria (P < 0.10). Piglets fed 250 mEq/kg dEB diet increased the relative abundance of Firmicutes and decreased the relative abundance of Proteobacteria (P < 0.05).
Table 7.
Effects of dEB and CA on the composition of the ileal microbiota in weaned piglets1
| 0% CA | 0.3% CA | |||||||
|---|---|---|---|---|---|---|---|---|
| dEB, mEq/kg | dEB, mEq/kg | P-value | ||||||
| Item | −50 | 250 | −50 | 250 | SEM | CA2 | dEB | CA×dEB |
| Phylum, % | ||||||||
| Firmicutes | 42.067 | 67.994 | 38.263 | 68.067 | 5.20 | 0.859 | 0.013 | 0.854 |
| Proteobacteria | 57.706 | 31.559 | 61.555 | 31.774 | 5.20 | 0.847 | 0.013 | 0.863 |
| Actinobacteria | 0.073 | 0.201 | 0.055 | 0.088 | 0.03 | 0.214 | 0.132 | 0.368 |
| Bacteroidetes | 0.127 | 0.167 | 0.077 | 0.045 | 0.02 | 0.094 | 0.938 | 0.476 |
| Cyanobacteria | 0.006b | 0.053a | 0.014b | 0.006b | 0.01 | 0.106 | 0.102 | 0.028 |
| Fusobacteria | 0.005 | 0.013 | 0.019 | 0.013 | 0.00 | 0.463 | 0.924 | 0.435 |
| Spirochaetes | 0.004 | 0.007 | 0.005 | 0.004 | 0.00 | 0.606 | 0.632 | 0.259 |
| Chlorobi | 0.000 | 0.000 | 0.003 | 0.000 | 0.00 | 0.273 | 0.273 | 0.273 |
| Saccharibacteria | 0.008a | 0.001b | 0.001b | 0.003b | 0.00 | 0.068 | 0.096 | 0.007 |
| Ignavibacteriae | 0.001 | 0.000 | 0.002 | 0.000 | 0.00 | 0.516 | 0.241 | 0.516 |
| Others | 0.004 | 0.005 | 0.005 | 0.000 | 0.00 | 0.358 | 0.295 | 0.078 |
| Genus, % | ||||||||
| Clostridium_sensu_stricto_1 | 22.350 | 24.500 | 16.900 | 47.586 | 4.850 | 0.341 | 0.083 | 0.129 |
| Lactobacillus | 1.050b | 24.443a | 1.529b | 0.857b | 3.722 | 0.097 | 0.103 | 0.085 |
| Actinobacillus | 51.350 | 27.386 | 53.029 | 29.386 | 5.199 | 0.854 | 0.024 | 0.987 |
| Romboutsia | 8.083 | 3.214 | 14.029 | 6.414 | 1.934 | 0.237 | 0.111 | 0.719 |
| Turicibacter | 3.850 | 6.429 | 1.329 | 9.786 | 1.423 | 0.880 | 0.055 | 0.292 |
| Sarcina | 0.200 | 4.514 | 0.057 | 0.914 | 0.712 | 0.167 | 0.061 | 0.200 |
| Escherichia-Shigella | 1.583 | 1.743 | 3.929 | 0.214 | 0.654 | 0.751 | 0.176 | 0.142 |
| Streptococcus | 2.083 | 2.271 | 1.014 | 0.257 | 0.357 | 0.033 | 0.679 | 0.494 |
| Veillonella | 2.550 | 0.400 | 1.271 | 0.514 | 0.327 | 0.346 | 0.025 | 0.262 |
| Helicobacter | 0.003 | 0.754 | 0.045 | 0.003 | 0.185 | 0.347 | 0.347 | 0.295 |
| Others | 6.883 | 4.371 | 6.857 | 4.014 | 0.474 | 0.821 | 0.004 | 0.845 |
1Data were means of seven piglets per treatment.
2CA × dEB = the interaction between citric acid and dietary electrolyte balance.
a,bWithin a row, means with different superscripts differ (P < 0.05).
Figure 3.
Effect of dEB (Na + K – Cl) and CA on microbial compositions in ileal digests of weaned piglets. (A) The abundance of top 10 bacteria at phylum level. (B) The abundance of top 10 bacteria at genus level. I.dEB.A: −50 mEq/kg dEB; I.dEB.C: 250 mEq/kg dEB; I.AC.dEB.E: −50 mEq/kg dEB with 0.3% CA; I.AC.dEB.G: 250 mEq/kg dEB with 0.3% CA. (C and D) Linear discriminant analysis coupled with effect size (LEfSe) analysis of the ileal bacterial community. LDA, linear discriminant analysis. AC: 0.3% CA; AE: −50 mEq/kg dEB; CG: 250 mEq/kg dEB.
At the genus level, Clostridium_sensu_stricto_1, Lactobacillus, and Actinobacillus are the most dominant in both dEB and CA piglets; these genera accounted for more than 77% of total sequences. In 0% CA diets, increasing dEB levels from 100 to 250 mEq/kg increased the relative abundance of Lactobacillus, whereas in 0.3% CA diets, no effect of dEB level was observed (dEB × CA, P < 0.10). Dietary supplementation with 0.3% CA decreased the relative abundance of Streptococcus (P < 0.05) and Lactobacillus (P < 0.10). Piglets fed 250 mEq/kg dEB diet increased the relative abundance of Clostridium_sensu_stricto_1, Turicibacter, and Sarcina (P < 0.10) and decreased the relative abundance of Actinobacillus and Veillonella (P < 0.05).
Microbial compositions between CA and dEB piglets were further analyzed using the linear discriminant analysis coupled with effect size (Figure 3C and D). The results showed that class Bacilli, order Lactobacillales, family Streptococcaceae, genus Sarcina and Streptococcus, and species Lactobacillus_rennini were significantly enriched in 0.3% CA. Phylum Firmicutes, order Sphingobacteriales, genus Tetragenococcus, and species Lactobacillus_rennini were significantly enriched in 250 mEq/kg dEB. However, phyla Fibrobacteres and Proteobacteria; genus Fibrobacter, Catenisphaera, Pasteurella, Veillonella, Escherichia_Shigella, Romboutsia, and Actinobacillus; class Fibrobacteria, Negativicutes, and Gammaproteobacteria; order Fibrobacterales, Selenomonadales, and Pasteurellales; familiae Fibrobacteraceae, Veillonellaceae, Peptostreptococcaceae, and Pasteurellaceae; and species Pasteurella__aerogenes and Actinobacillus_minor in −50 mEq/kg dEB were enriched.
Discussion
It has been reported that diarrhea results when there is loss of the dynamic and finely balanced absorption and secretion of water and electrolytes within the gut (Kelly et al., 2018). In the present study, the occurrence rate of diarrhea among weaned piglets was not affected by increase in dEB levels. Although nonsignificant result was obtained for dEB addition, yet minimum diarrhea rate was gained by the weaned piglets fed 250 mEq/kg dEB diet likely due to the decrease of ileum and colon pH levels. A reduction in intestinal pH inhibits the growth of pathogenic bacteria and promotes the proliferation of probiotics (Kim et al., 2005).
Previous studies reported that the increase of the pH in the small intestine of weaned piglets would cause a decrease of pepsin activation and the proliferation of pathogenic bacteria which resulted in diarrhea among weaned piglets (Kim et al., 2005; Cheng et al., 2006; Jia et al., 2010). Therefore, many literature suggested that pigs fed 166 to 250 mEq/kg dEB diet improved growth performance (NRC 2012; Xin et al., 2017; Jones et al., 2019). The diarrhea rate among weaned piglets was affected by the inclusion of 0.3% CA to a dEB diet in this study. This showed that the effects of adding CA may be further influenced by a different dEB, which changed intestinal health.
Goblet cells are specialized secretory cells found throughout mucosal epithelia; they play an important role in maintaining tissue homeostasis by secreting a variety of factors, including proteins, trefoil factors, and mucins, all of which contribute to the mucus layer protecting mucosal epithelium (Mccauley and Guasch, 2015). ZO-1, occludin, and claudins are the main components of the tight junctions, which involved in maintaining the function of cell polarity and tight junction barrier. Our study observed that a CA-free diet increased the number of goblet cells in ileal crypt and the mRNA expression of cytokines such as TNF‑α, IFN-γ, IL-1β, IL-10, and tight junction proteins such as occluding and ZO-1. These results indicated that inflammation may damage the integrity of intestinal epithelium, leading to increased permeability which may exacerbate immune system activation. In contrast, Morel et al. (2019) reported that the supplementation of benzoic acid in the diet had no effect on the number of villus and crypt goblet cells in grower-finisher pigs. Giannenas et al. (2016) indicated that dietary inclusion of 5 g/kg benzoic acid was not affected in jejunal goblet cell numbers in fattening pigs. The result of these conflicts may be that the diet without CA significantly increases the number of goblet cells in response to allergens or inflammation. An increase in the number of goblet cells or the production of mucus is a normal physiological response of mucosal epithelium to harmful stimuli (Deplancke and Gaskins, 2001; Asselin and Gendron, 2014). IFN-γ, the pro-inflammatory cytokine, has been reported to induce intestinal epithelial cells expression of IL-10 receptor and signaling the maintenance and restitution of epithelial barrier (Lu et al., 1998; Jarry et al., 2008; Suenaert et al., 2010; Kominsky et al., 2014). IL-36, a member of the IL-1 family, plays a role in promoting repair of the intestinal epithelial barrier by recruiting neutrophils and releasing IL-22 (Medina-Contreras et al., 2016; Scheibe et al., 2017), indicating a dynamic interaction between inflammatory cytokines and barrier function. Therefore, cytokines and tight junction proteins are upregulated at the same time, which paradoxically promotes the repair and strengthening of intestinal barrier. However, the occluding and claudin-1 protein expression was not consistent with the mRNA expression in this study, which may be due to the regulation of occludin and claudin-1 expression at the transcriptional level.
In the present study, increasing dEB values increased the number of goblet cells in the jejunal crypt. This may have a protective effect on the intestinal mucosa. In addition, piglets fed 250 mEq/kg dEB diet downregulated cytokines and tight junction protein expression may be related to the numerical reduction of diarrhea rate. Furthermore, 250 mEq/kg dEB reduces the pH of the gastrointestinal tract, which also inhibits the growth of harmful bacteria and promotes intestinal health. The interactions between CA and dEB in goblet cells of jejunum were observed, which indicated that optimal dEB for weaned piglets were affected by the addition of CA in the diet. Piglets fed the 250 mEq/kg dEB diet without CA showed reduced goblet cells of jejunum, whereas piglets fed the 400 mEq/kg dEB diet with 0.3% CA showed a increase. This interaction may be related to diarrhea among piglets. Diarrhea causes the destruction of the intestinal mucosal barrier, while a transient increase in goblet cell numbers may be a crucial response to inflammation.
In the pathophysiology of diarrheal disease, alterations in fluid and electrolyte transport are of prime importance. Diarrheal disorders are almost always associated with changes in fluid and electrolyte movement, especially accompanied with a decrease in absorption and/or an increase in secretion (Dennehy and Penelope, 2005). CFTR is a cAMP-dependent chloride channel and is considered to be the major channel for Cl– secretion in the intestine (Keely and Barrett, 2000). CFTR was also essential for HCO3 secretion, especially in maintaining pH and mucus production in the small intestine (Venkatasubramanian et al., 2010). The basolateral NKCC1 is a key determinant of transepithelial chloride secretion, and dysregulation of chloride secretion is a common feature of many diseases including secretory diarrhea (Tang et al., 2010). The present study observed that mRNA expression of NKCC1 and CFTR in a CA-free diet was upregulated. Inflammation has a profound impact on intestinal hydroelectrolytic transport. The activation of CFTR is thought to be one of the main causes of cholera-induced diarrhea (Rao, 2004). Increase in intracellular cAMP induced by toxins or another pathologic condition may activate NKCC1 and CFTR (Lee et al., 2019). Zhu et al. (2017) reported that enterotoxigenic Escherichia coli (ETEC) K88 treatment increased the expression of NKCC1 in the jejunum, ileum, and colon of piglets. In this study, we observed a simultaneous increase in cytokines and ion transports. Interestingly, however, increased Cl– secretion did not lead to an increase in diarrhea. This may be due to the CA-free diet increasing the number of goblet cells and mRNA expression of tight junction proteins.
Intestinal microbiota plays a key role in the maturation of the immune system and efficient absorption/utilization of nutrients (Min and Rhee, 2015; Thaiss et al., 2016). A study reported that after 2 d post-weaning of piglets, intestinal Lactobacillus decreased sharply, while the number of coliforms increased (Mathew et al., 1996). Lactobacillus is an important probiotic, which may regulate intestinal flora, enhances immunity, improves intestinal function, and prevents diarrhea (Bauer et al., 2006; Lebeer et al., 2008; Li et al., 2015). The anti-inflammatory effect of Sarcina in the intestine has been reported by Getachew et al. (2018). Moreover, a potentially novel protease-insensitive antimicrobial produced by Streptococcus hyointestinalis was observed (O’Shea et al., 2009). Hence, the improvement of these intestinal microorganisms by a CA-free diet may contribute to the maintenance of intestinal mucosal integrity.
As shown in our study, the microbiota in 250 mEq/kg dEB group increased the proportion of Firmicutes and decreased the population of Proteobacteria and Actinobacillus compared with −50 mEq/kg dEB group. Firmicutes are the dominant beneficial bacteria, whereas Proteobacteria and Actinobacillus are usually considered as pathogens. These results demonstrated that 250 mEq/kg dEB regulates the abundance of Firmicutes, Proteobacteria, and Actinobacillus, thus improving intestinal microecology. Enterotoxigenic and Shiga toxin-producing E. coli are one of the most important pathogenic bacteria that cause diarrhea among newborn and weaned piglets (Cheng et al., 2006). Huang et al. (2019) found that the abundance of Escherichia–Shigella and Veillonella increased in fecal microbiota of piglets due to dysbiosis induced by porcine epidemic diarrhea virus infection. In the present study, piglets fed 250 mEq/kg dEB diet significantly decreased the abundance of Escherichia–Shigella and Veillonella and increased Lactobacillus reuteri in the ileum. The changes in these bacteria reflect that the proliferation of probiotics may inhibit the growth of pathogenic microorganisms in the intestine, thereby maintaining intestinal health. However, the abundances of Actinobacillus minor, Pasteurella aerogenes, and Gammaproteobacteria were increased in the −50 mEq/kg dEB group.
Previous studies have shown that Actinobacillus minor and Pasteurella aerogenes were opportunistic pathogens in humans or animals (Kuhnert et al., 2000; Arya and Niven, 2011; Xu et al., 2019). Gammaproteobacteria and Proteobacteria were increased in inflammation in the distal gut (Winter and Bäumler, 2014). The tilt of the balance of healthy flora to pro-inflammatory microbial species may lead to intestinal inflammation (Tamboli et al., 2004; Kaur et al., 2011). Here also, as noted earlier, −50 mEq/kg dEB diet may cause intestinal microbial dysbiosis and increase the risk of intestinal inflammation among piglets. Interestingly, the abundance of Fibrobacter significantly increased in the −50 mEq/kg dEB group. Fibrobacter is one of fiber-degrading bacteria, which consumes cellulose in the intestine to maintain the growth characteristics of the host animals. Furthermore, fiber improves the thickness of intestinal mucus layer, which serves as a primary defense against enteric pathogens (Mahesh et al., 2016). Therefore, enrichment of Fibrobacter in −50 mEq/kg dEB may be a unique response to anti-inflammation. Increasing dEB values from −50 to 250 mEq/kg without CA increased Cyanobacteria and Lactobacillus, and decreased Saccharibacteria, but no such effect was observed on the diets with 0.3% CA. This suggested that the addition of CA to the dEB diets affected the intestinal microbial composition of the weaned piglets.
Conclusion
Supplementation of 0.3% CA resulted in differential expression of inflammatory cytokines, ion transporters, and tight junction proteins, and changes in microbial community composition. A 250-mEq/kg dEB diet reduced gastrointestinal pH and promoted the enrichment of beneficial microbes in the gut microbiota, thereby suppressing inflammation and harmful bacteria. The interaction between dEB and CA affected the diarrhea score, intestinal goblet cells, and microbial composition in weaned piglets. However, the addition of CA to diets with different dEB values did not promote intestinal function in weaned piglets.
Acknowledgments
This work was supported by Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSWSMC008), National Key R&D Program (2016YFD0501201), and Natural Science Foundation of Hunan Province (2017JJ1020).
Glossary
Abbreviations
- CA
citric acid
- CFTR
cystic fibrosis transmembrane conductance regulator
- dEB
dietary electrolyte balance
- ETEC
enterotoxigenic Escherichia coli
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- IL-1β
interleukin-1β
- NKCC1
Na-K-Cl cotransporter
- TNF-α
tumor necrosis factor-α
- ZO-1
zona occludens 1
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Adeva-Andany M. M., Carneiro-Freire N., Donapetry-García C., Rañal-Muíño E., and López-Pereiro Y.. . 2014. The importance of the ionic product for water to understand the physiology of the acid-base balance in humans. Biomed. Res. Int. 2014:695281. doi: 10.1155/2014/695281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC. 2007. Official methods of analysis. 18th ed. Washington, DC: AOAC.
- Arya G., and Niven D. F.. . 2011. Acquisition of haemoglobin-bound iron by strains of the Actinobacillus minor/“porcitonsillarum” complex. Vet. Microbiol. 148:283–291. doi: 10.1016/j.vetmic.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Asselin C., and Gendron F. P.. . 2014. Shuttling of information between the mucosal and luminal environment drives intestinal homeostasis. FEBS Lett. 588:4148–4157. doi: 10.1016/j.febslet.2014.02.049 [DOI] [PubMed] [Google Scholar]
- Aydin A., Pekel A. Y., Issa G., Demirel G., and Patterson P. H.. . 2010. Effects of dietary copper, citric acid, and microbial phytase on digesta pH and ileal and carcass microbiota of broiler chickens fed a low available phosphorus diet. J. Appl. Poultry Res. 19:422–431. doi: 10.3382/japr.2009-00123 [DOI] [Google Scholar]
- Bauer E., Williams B. A., Smidt H., Verstegen M. W., and Mosenthin R.. . 2006. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 7:35–51. [PubMed] [Google Scholar]
- Bodner G. M. 1986. Metabolism part ii: the tricarboxylic acid (TCA), citric acid, or Krebs cycle. J. Chem. Educ. 63:673–677. doi: 10.1021/ed063p673 [DOI] [Google Scholar]
- Chen C. C., Wang Z. B., Li J. Z., Li Y. L., Huang P. F., Ding X. Q., Yin J., He S. P., Yang S. H., and Yin Y. L.. . 2019. Dietary vitamin E affect small intestinal histomorphology, digestive enzyme activity and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets. J. Anim. Sci. 97:1212–1221. doi: 10.1093/jas/skz023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D., Sun H., Xu J., and Gao S.. . 2006. PCR detection of virulence factor genes in Escherichia coli isolates from weaned piglets with edema disease and/or diarrhea in China. Vet. Microbiol. 115:320–328. doi: 10.1016/j.vetmic.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Wang L., Chen X. L., Shi B. M., and Shan A. S.. . 2015. Effects of dietary electrolyte balance on the performance, plasma biochemistry parameters and immunoglobulin of sows during late gestation and lactation. Anim. Feed Sci. Tech. 200:93–101.doi: 10.1016/j.anifeedsci.2014.12.011 [DOI] [Google Scholar]
- Dennehy M. D., and Penelope H.. . 2005. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect. Dis. Clin. N. Am. 19:585–602. doi: 10.1016/j.idc.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Deplancke B., and Gaskins H. R.. . 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S [DOI] [PubMed] [Google Scholar]
- DeRouchey J. M., Hancock J. D., Hines R. H., Cummings K. R., Lee D. J., Maloney C. A., Dean D. W., Park J. S., and Cao H.. . 2003. Effects of dietary electrolyte balance on the chemistry of blood and urine in lactating sows and sow litter performance. J. Anim. Sci. 81:3067–3074. doi: 10.2527/2003.81123067x [DOI] [PubMed] [Google Scholar]
- Dersjant-Li Y., Verstegen M. W., Jansman A., Schulze H., Schrama J. W., and Verreth J. A.. . 2002. Changes in oxygen content and acid-base balance in arterial and portal blood in response to the dietary electrolyte balance in pigs during a 9-h period after a meal. J. Anim. Sci. 80:1233–1239. doi: 10.2527/2002.8051233x [DOI] [PubMed] [Google Scholar]
- Getachew B., Aubee J. I., Schottenfeld R. S., Csoka A. B., Thompson K. M., and Tizabi Y.. . 2018. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 18:222. doi: 10.1186/s12866-018-1373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannenas I., Doukas D., Karamoutsios A., Tzora A., Bonos E., Skoufos I., … Florou-Paneri P.. . 2016 Effects of Enterococcus faecium, mannan oligosaccharide, benzoic acid and their mixture on growth performance, intestinal microbiota, intestinal morphology and blood lymphocyte subpopulations of fattening pigs. Anim. Feed Sci. Tech. 220: 159–167.doi: 10.1016/j.anifeedsci.2016.08.003 [DOI] [Google Scholar]
- Guzmán-Pino S. A., Solà-Oriol D., Davin R., Manzanilla E. G., and Pérez J. F.. . 2015. Influence of dietary electrolyte balance on feed preference and growth performance of postweaned piglets. J. Anim. Sci. 93:2840–2848. doi: 10.2527/jas.2014-8380 [DOI] [PubMed] [Google Scholar]
- Haydon K. D., West J. W., and McCarter M. N.. . 1990. Effect of dietary electrolyte balance on performance and blood parameters of growing-finishing swine fed in high ambient temperatures. J. Anim. Sci. 68:2400–2406. doi: 10.2527/1990.6882400x [DOI] [PubMed] [Google Scholar]
- Heo J. M., Opapeju F. O., Pluske J. R., Kim J. C., Hampson D. J., and Nyachoti C. M.. . 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl). 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Huang A., Cai R., Wang Q., Shi L., Li C., and Yan H.. . 2019. Dynamic change of gut microbiota during porcine epidemic diarrhea virus infection in suckling piglets. Front. Microbiol. 10:322. doi: 10.3389/fmicb.2019.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S., Ghergurovich J. M. Morscher R. J., Jang C., Teng X., W. Lu, L. A. Esparza, T. Reya, L. Zhan, and J. Y. Guo.. 2017. Glucose feeds the TCA cycle via circulating lactate. Nature. 000:1–16.doi: 10.1038/nature24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO. 2005. Animal feeding stuffs. Determination of water- soluble chlorides content. Part 1: titrimetric method. Geneva (Switzerland): British Standards Institution. [Google Scholar]
- Jarry A., Bossard C., Bou-Hanna C., Masson D., Espaze E., Denis M. G., and Laboisse C. L.. . 2008. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J. Clin. Invest. 118:1132–1142. doi: 10.1172/JCI32140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Yan. J. Y., Cai J. Y., and Wang K. N.. . 2010. Effects of encapsulated and non-encapsulated compound acidifiers on gastrointestinal pH and intestinal morphology and function in weaning piglets. J. Anim. Feed Sci. 19:81–92. doi: 10.4081/ijas.2010.e88 [DOI] [Google Scholar]
- Jones A. M., Wu F., Woodworth J. C., Dritz S. S., Tokach M. D., DeRouchey J. M., and Goodband R. D.. . 2019. Evaluation of dietary electrolyte balance on nursery pig performance. Transl. Anim. Sci. 3:161–167. doi: 10.1093/tas/txy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N., Chen C. C., Luther J., and Kao J. Y.. . 2011. Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes 2:211–216. doi: 10.4161/gmic.2.4.17863. [DOI] [PubMed] [Google Scholar]
- Keely S. J., and Barrett K. E.. . 2000. Chapter 7 Integrated signaling mechanisms that regulate intestinal chloride secretion. Curr. Top. Membr. 50:249–299. doi: 10.1016/s1063-5823(00)50009-x [DOI] [Google Scholar]
- Kelly L., Jenkins H., and Whyte L.. . 2018. Pathophysiology of diarrhoea. Paediatr. Child. doi: 10.1016/j.paed.2018.09.002. [DOI] [Google Scholar]
- Khosravinia H., Nourmohammadi R., and Afzali N.. . 2015. Productive performance, gut morphometry, and nutrient digestibility of broiler chicken in response to low and high dietary levels of citric acid. J. Appl. Poult. Res. 00:1–11. doi: 10.3382/japr/pfv050 [DOI] [Google Scholar]
- Kim Y. Y., Kil D.Y. Oh H. K., and Han I. K.. . 2005. Acidifier as an alternative material to antibiotics in animal feed. Asian-Australas. J. Anim. Sci. 18:1048–1060. doi: 10.5713/ajas.2005.1048 [DOI] [Google Scholar]
- Kominsky D. J., Campbell E. L., Ehrentraut S. F., Wilson K. E., Kelly C. J., Glover L. E., Collins C. B., Bayless A. J., Saeedi B., Dobrinskikh E., . et al. 2014. IFN-γ-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 192:1267–1276. doi: 10.4049/jimmunol.1301757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert P., Heyberger-Meyer B., Nicolet J., and Frey J.. . 2000. Characterization of PaxA and its operon: a cohemolytic RTX toxin determinant from pathogenic Pasteurella aerogenes. Infect. Immun. 68:6–12. doi: 10.1128/iai.68.1.6-12.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer S., Vanderleyden J., and De Keersmaecker S. C.. . 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72:728–764, doi: 10.1128/MMBR.00017-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Hong G. S., Lee S. H., Kim H., Kim A., Hwang E. M., Kim J., Lee M. G., Yang J. Y., Kweon M. N., . et al. 2019. Anoctamin 1/TMEM16A controls intestinal Cl- secretion induced by carbachol and cholera toxin. Exp. Mol. Med. 51:1–14. doi: 10.1038/s12276-019-0287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Ni K., Pang H., Wang Y., Cai Y., and Jin Q.. . 2015. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australas. J. Anim. Sci. 28:620–631. doi: 10.5713/ajas.14.0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng J., Deng K., Chen L., Zhao X. L., Jiang X., Fang Z., Che L., Xu S., Feng B., . et al. 2018. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J. Anim. Sci. 96:3302–3318. doi: 10.1093/jas/sky197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. F., Xu Y. T., Pan L., Wang Q. Q., Wang C. L., Wu J. Y., Y. Y. Wu, Y. M. Han, C. H. Yun and X. S. Piao.. 2018. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Tech. 235:23–32.doi: 10.1016/j.anifeedsci.2017.08.018 [DOI] [Google Scholar]
- Lu J., Philpott D. J., Saunders P. R., Perdue M. H., Yang P. C., and McKay D. M.. . 1998. Epithelial ion transport and barrier abnormalities evoked by superantigen-activated immune cells are inhibited by interleukin-10 but not interleukin-4. J. Pharmacol. Exp. Ther. 287:128–136. [PubMed] [Google Scholar]
- Mahesh D., Seekatz A., Koropatkin N., Kamada N., Hickey C., Wolter M., N. A. Pudlo, S. Kitamoto, N. Terrapon, and A. Muller.. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A. G., Franklin M. A., Upchurch W. G., and Chattin S. E.. . 1996. Influence of weaning age on ileal microflora and fermentation acids in young pigs. Nutr. Res. 16:817–827. doi: 10.1016/0271-5317(96)00074-7 [DOI] [Google Scholar]
- McCauley H. A., and Guasch G.. . 2015. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 21:492–503. doi: 10.1016/j.molmed.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O., Harusato A., Nishio H., Flannigan K. L., Ngo V., Leoni G., Neumann P. A., Geem D., Lili L. N., Ramadas R. A., . et al. 2016. Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J. Immunol. 196:34–38. doi: 10.4049/jimmunol.1501312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y. W., and Rhee P. L.. . 2015. The role of microbiota on the gut immunology. Clin. Ther. 37:968–975. doi: 10.1016/j.clinthera.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Mongin P. 1981. Recent advances in dietary anion-cation balance: applications in poultry. Proc. Nutr. Soc. 40:285–294. doi: 10.1079/pns19810045 [DOI] [PubMed] [Google Scholar]
- Morel P. C. H., Chidgey K. L., Jenkinson C. M. C., Lizarraga I., and Schreurs N. M.. . 2019. Effect of benzoic acid, sodium butyrate and sodium butyrate coated with benzoic acid on growth performance, digestibility, intestinal morphology and meat quality in grower-finisher pigs. Livest. Sci. 226:107–113.doi: 10.1016/j.livsci.2019.06.009 [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC):National Academy Press. [Google Scholar]
- Okada S., Muramoto M., Sunaga F., and Sato R.. . 2018. Evaluation of fecal pH as a potential diagnostic indicator of diarrhea induced metabolic acidosis in Japanese black calves. Large Anim. Rev. 24:139–142. [Google Scholar]
- O’Shea E. F., Gardiner G. E., O’Connor P. M., Mills S., Ross R. P., and Hill C.. . 2009. Characterization of enterocin- and salivaricin-producing lactic acid bacteria from the mammalian gastrointestinal tract. FEMS Microbiol. Lett. 291:24–34. doi: 10.1111/j.1574-6968.2008.01427.x [DOI] [PubMed] [Google Scholar]
- Olaimat A. N., Al-Nabulsi A. A., Osaili T. M., Al-Holy M., Ayyash M. M., Mehyar G. F., Z. W. Jaradat, and M. A. Ghoush.. 2017. Survival and inhibition of, Staphylococcus aureus, in commercial and hydrated tahini using acetic and citric acids. Food Control 77:179–186. doi: 10.1016/j.foodcont.2017.02.022 [DOI] [Google Scholar]
- Patience J. F., Austic R. E., and Boyd R. D.. . 1987. Effect of dietary supplements of sodium or potassium bicarbonate on short-term macromineral balance in swine. J. Anim. Sci. 64:1079–1085. doi: 10.2527/jas1987.6441079x [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236.doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Rao M. C. 2004 Oral rehydration therapy: new explanations for an old remedy. Annu. Rev. Physiol. 66:385–417. doi: 10.1146/annurev.physiol.66.032902.134726 [DOI] [PubMed] [Google Scholar]
- Rao M. C. 2019. Physiology of electrolyte transport in the gut: implications for disease. Compr. Physiol. 9:947–1023. doi: 10.1002/cphy.c180011 [DOI] [PubMed] [Google Scholar]
- Scheibe K., Backert I., Wirtz S., Hueber A., Schett G., Vieth M., Probst H. C., Bopp T., Neurath M. F., and Neufert C.. . 2017. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 66:823–838. doi: 10.1136/gutjnl-2015-310374 [DOI] [PubMed] [Google Scholar]
- Suenaert P., Maerten P., Van Assche G., Van Driessche W., Geboes K., Bulteel V., Simaels J., Augustijns P., Ceuppens J. L., Rutgeerts P., . et al. 2010. Effects of T cell-induced colonic inflammation on epithelial barrier function. Inflamm. Bowel Dis. 16:1322–1331. doi: 10.1002/ibd.21211 [DOI] [PubMed] [Google Scholar]
- Tamboli C. P., Neut C., Desreumaux P., and Colombel J. F.. . 2004. Dysbiosis as a prerequisite for IBD. Gut 53:1057. [PMC free article] [PubMed] [Google Scholar]
- Tang X., Tang J., Mykoniatis A., Matthews J. B., and Bouyer P.. . 2010. W1779 regulation of chloride secretion in the colonic crypt cell line T84 by inositol 1, 4, 5-trisphosphate (IP3) receptor-binding protein released with Ip3 regulates (IRBIT). Gastroenterology. 138:S–738.doi: 10.1016/s0016-5085(10)63399-9 [DOI] [Google Scholar]
- Thaiss C. A., Zmora N., Levy M., and Elinav E.. . 2016. The microbiome and innate immunity. Nature 535:65–74. doi: 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian J., Ao M., and Rao M. C.. . 2010. Ion transport in the small intestine. Curr. Opin. Gastroenterol. 26:123–128. doi: 10.1097/MOG.0b013e3283358a45 [DOI] [PubMed] [Google Scholar]
- Winter S. E., and Bäumler A. J.. . 2014. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes 5:71–73. doi: 10.4161/gmic.27129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. L., Jing Y. C., Park J. H., and Kim I. H.. . 2017. Evaluation of different dietary electrolyte balance in weanling pigs diets. Anim. Feed Sci. Tech. 226:98–102. 10.1016/j.anifeedsci.2017.02.014 [DOI] [Google Scholar]
- Xu K., Bai M. M., He W. G., Liu H. N., Duan Y. H., Li T. J., and Yin Y. L.. . 2019. The effects of dietary reduced mineral elements and coated cysteamine supplementation on bacterial diversity in the ileum of finishing pigs. Anim. Sci. J. 90:1239–1247. doi: 10.1111/asj.13267 [DOI] [PubMed] [Google Scholar]
- Zhai H., Ren W., Wang S., Wu J., Guggenbuhl P., and Kluenter A. M.. . 2017. Growth performance of nursery and grower-finisher pigs fed diets supplemented with benzoic acid. Anim. Nutr. 3:232–235. doi: 10.1016/j.aninu.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. L., Yin H. C., Lu T. F., Niu Y. J., Zhang Y. Y., Li S. Q., Wang Y. P., and Chen H. Y.. . 2018. Application of high-throughput sequencing for microbial diversity detection in feces of specific-pathogen-free ducks. Poult. Sci. 97:2278–2286. doi: 10.3382/ps/pex348 [DOI] [PubMed] [Google Scholar]
- Zhu C., Ye J. L., Yang J., Yang K. M., Chen Z., Liang R., Wu X. J., Wang L., and Jiang Z. Y.. . 2017. Differential expression of intestinal ion transporters and water channel aquaporins in young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 95:5240–5252. doi: 10.2527/jas2017.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]