Abstract
Current theories consider motor imagery, the mental representation of action, to have considerable functional overlap with the processes involved in actual movement preparation and execution. To test the neural specificity of motor imagery, we conducted a series of 3 experiments using transcranial magnetic stimulation (TMS). We compared changes in corticospinal excitability as people prepared and implemented actual or imagined movements, using a delayed response task in which a cue indicated the forthcoming response. TMS pulses, used to elicit motor-evoked responses in the first dorsal interosseous muscle of the right hand, were applied before and after an imperative signal, allowing us to probe the state of excitability during movement preparation and implementation. Similar to previous work, excitability increased in the agonist muscle during the implementation of an actual or imagined movement. Interestingly, preparing an imagined movement engaged similar inhibitory processes as that observed during actual movement, although the degree of inhibition was less selective in the imagery conditions. These changes in corticospinal excitability were specific to actual/imagined movement preparation, as no modulation was observed when preparing and generating images of cued visual objects. Taken together, inhibition is a signature of how actions are prepared, whether they are imagined or actually executed.
Keywords: action, inhibition, motor imagery, preparation, TMS
Introduction
Mental movement simulation, or motor imagery, has been the subject of considerable studies, shedding light on a number of important issues in the cognitive neuroscience of motor control (Jeannerod 1994; Wolpert and Flanagan 2001; Michel et al. 2013; Gueugneau et al. 2015). During motor imagery, we internally represent an action in the absence of movement execution. Several neurophysiological investigations have shown that actual and imagined movements activate common brain areas (for a review, see Hetu et al. (2013)) and motor representations (Munzert et al. 2009). Notably, motor imagery can facilitate decision-making through internal simulation of possible actions, as well as enhance skill acquisition through mental practice (Jeannerod 1994; Wolpert and Flanagan 2001; Gentili et al. 2010; Avanzino et al. 2015; Gentili and Papaxanthis 2015).
A fundamental question is whether motor imagery is a reactive or a proactive neural process. In other words, do we activate the motor representation of the imagined movement only after an imperative signal? Or can we prepare to imagine a movement in advance, i.e., in the same way we prepare to actually move? According to the simulation theory (Jeannerod 1994, 2001) and the emulation theory of mental actions (Grush 2004), an important aspect of motor preparation is to retrieve and activate the appropriate motor representations required for a particular action. Neuroimaging studies showed similar activations in cortical motor areas during the preparation of actual and imagined movement (Cunnington et al. 1996; Kranczioch et al. 2009; Angelini et al. 2015). This task-related preparation process could be associated to the movement-related contingent negative variation. Indeed, this physiological marker, measured at the vertex, is considered a cortical signature of a forthcoming action (Walter et al. 1964; Rohrbaugh et al. 1976; Yazawa et al. 1997). While these studies revealed brain activation during the preparation of imagined movements, less is known about the underlying excitatory and inhibitory neurophysiological mechanisms.
Transcranial magnetic stimulation (TMS) studies have revealed that corticospinal (CS) excitability progressively decreases during response preparation of actual movements (Davranche et al. 2007; Duque and Ivry 2009; Duque et al. 2010; van den Wildenberg et al. 2010; Tandonnet et al. 2011). Preparatory inhibition is observed quite broadly, evident both in muscles associated with responses that are not selected for the forthcoming trial as well as in muscles that are not associated with potential effectors (Greenhouse, Sias, et al. 2015b), effects that have been hypothesized to reflect processes associated with response selection (Duque et al. 2010, 2017; Labruna et al. 2014). Interestingly, preparatory inhibition is greatest in the agonist for the forthcoming movement (Davranche et al. 2007; Duque and Ivry 2009; Duque et al. 2010; van den Wildenberg et al. 2010). One hypothesis is that the reduction in CS excitability in the selected effector helps prevent premature movement (Duque and Ivry 2009). Alternatively, this inhibition may facilitate response preparation by reducing motor noise (Greenhouse, Sias, et al. 2015b).
We consider 3 possible outcomes concerning how CS excitability might change when participants prepare an imaginary movement. First, given the similar patterns of activity observed in neuroimaging studies, it is reasonable to suppose that the modulation of CS excitability during the preparation of imagined movements would mirror that observed during the preparation of actual movements. According to this hypothesis, we would expect a decrease of CS excitability during the preparation of imagined actions. Moreover, similar to that observed with actual movement preparation, there would be additional inhibition in the effector selected for the forthcoming action. Second, changes in CS excitability might exhibit a generic level of inhibition during the preparation of motor imagery, without exhibiting the effects for selection. This outcome would indicate that inhibitory processes targeted at the selected effector, either to prevent premature movement or enhance the signal to noise ratio, are only engaged when an actual movement is prepared. Third, it may not be possible to prepare to a specific imagined movement; rather, the process of imagery itself may constitute a form of action preparation. By this hypothesis, we would not expect to observe inhibition during the preparation of imagined movement. Rather, we would see the modulation in CS excitability deferred until the imperative to imagine the movement, with an increase in CS excitability of the selected effector (for review, see Stinear 2010; Ruffino et al. 2017). Here we would not expect any changes in the effector not involved in the forthcoming action.
In the current study, we used TMS to assess the dynamics of CS excitability during the preparation and the implementation phases of actual and imagined movements. We delivered single-pulse TMS to the cortical representation of the first dorsal interosseous (FDI) muscle of the right hand, comparing conditions in which this muscle was either involved or not involved in the movement. We also included a condition that involved mental but not motor imagery, allowing us to assess if changes in CS excitability were specific to motor imagery or resulted from more general processes associated with mental engagement.
Materials and Methods
Participants
Eighteen right-handed participants (11 males, mean age 23 ± 2 years) were recruited from the Université de Bourgogne Franche-Comté (France). The experimental protocol was approved by the University’s institutional review board. All the participants were tested in Experiment 1. Fourteen of the participants (3 women, mean age 24 ± 2 years), who took part in Experiment 1, were tested in Experiment 2.
General Experimental Procedures
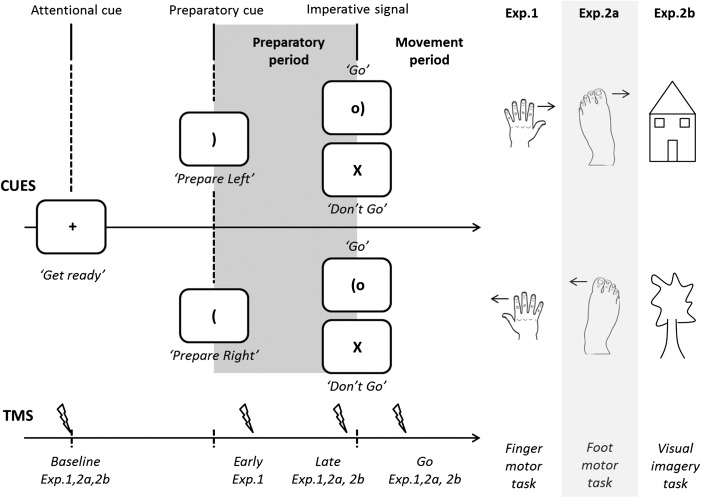
The tasks for the 2 experiments are described in Figure 1. The participant sat in front of a computer screen with both hands resting on the lap, palms down. In delayed response tasks, a preparatory cue, indicating the forthcoming response, was followed, after a fixed 900 ms delay, by an imperative signal in which that response was to be initiated as fast as possible. Catch trials, in which the imperative signal was replaced by a NoGo stimulus, were included to ensure that the participants did not prematurely initiate the prepared response. In Experiment 1, the response was either an actual or imagined movement of the left or right index finger. In Experiment 2a, finger movements were replaced by foot movements, in order to test if the pattern of inhibition observed in Experiment 1 would be reproduced when the right target index was not part of the response set. In Experiment 2b, the motor imagery task was replaced by an object imagery task (visual imagery).
Figure 1.
Experimental procedure. Each trial began with the brief presentation (100 ms) of a fixation marker at the center of the screen (Fig. 1). After a blank screen of 600 ms, a preparatory cue appeared at the center position. The cue was a bracket, described as a “soccer goal”, and the orientation indicated the effector required for the forthcoming action (Exp. 1: finger; Exp 2a: foot). After a 900 ms delay, a circle was added to the display. This circle, described as the “ball”, was present for 300 ms and served as the imperative signal. Participants were instructed to move/imagine moving as quickly as possible after the onset of the imperative and to maintain the actual or imagined contraction until the signal disappeared. In the control imagery task (Exp. 2b), participants were to imagine a house or a tree, in response to the left and right bracket, respectively. On some trials, the cue was replaced by an “X” at the center of the screen (catch trials). Participants were instructed to not respond to the “X” stimulus. In Experiment 1, the right FDI was considered as a potential effector of the action; and in Experiments 2a and 2b, the right FDI was not involved in the task since it was never included in the response set. To access CS excitability, single-pulse TMS was applied over left M1 at the onset of the fixation marker (baseline), during the preparatory period (early probe: 250 ms after the cue, E1 only; late probe: 50 ms prior to the imperative), or 300 ms after the imperative (Go or X).
Motor imagery, visual imagery and actual trials were tested in separate blocks, with the order counterbalanced across individuals. Participants were always informed about the upcoming condition before each block. Within each condition, the cues were selected in a random order, with the constraint that each occurred equally often. Participants completed 3 short practice blocks prior to the experimental session: 20 trials with actual movement (Exp. 1 and 2a, only), 20 imagined trials, and 20 imagined trials with TMS.
Transcranial Magnetic Stimulation
CS excitability was assessed by recording motor-evoked potentials (MEPs) from the right FDI muscle in response to single-pulse TMS applied over the hand area of the left primary motor cortex. We chose to stimulate the left primary cortex since previous studies have indicated stronger involvement of the left hemisphere during motor imagery (Sabate et al. 2004; Stinear and Byblow 2004). A Magstim 200 stimulator was used to activate a figure-of-eight coil (diameter of wings 70 mm). The coil was placed tangentially on the scalp with the handle oriented toward the back of the head and laterally at a 45° angle. This orientation is assumed to be approximately perpendicular to the central sulcus. At the start of each session, the experimenter identified the optimal spot for eliciting MEPs in the right FDI and marked this position on the participant’s scalp. The resting motor threshold (rMT) was then determined, defined as the minimal TMS intensity required to evoke MEP peak-to-peak amplitudes of ~50 μV in the targeted muscle on 5 out of 10 consecutive trials. EMG activity was recorded by surface electrodes placed over the right and left FDI (all experiments), as well as over the right and left Tibialis Anterior (TA) muscle (Experiment 2a, only).
The TMS intensity was set to 120% of the participant’s rMT. The TMS pulse was triggered at one of 4 times (Fig. 1): 1) During the inter-trial interval (baseline); 2) During the early probe of the preparatory period (250 ms after the preparatory cue); 3) During the late probe of preparatory period (50 ms prior to the imperative signal); 4) 300 ms after the imperative signal. We opted to use a 300 ms post-imperative probe to ensure that there was sufficient time for the responses, either actual or imagined, to be initiated.
MEPs were measured as the peak-to-peak amplitude of the response elicited after the TMS stimulation. Changes in CS excitability were expressed as a percentage of baseline: (Condition − Baseline)/Baseline × 100, with values greater than zero indicating an increase in CS excitability and values less than zero indicating a decrease in CS excitability.
EMG data were collected for 2.1 s on each trial, starting 100 ms prior to TMS. The EMG signals were amplified and bandpass filtered on-line (10–5000 Hz, Biopac Systems Inc.) and digitized at 2000 Hz for off-line analysis. Trials with EMGRMS above 10 μV, during the preparation of actual and imagined trials and during motor imagery, were discarded (1.66% of all trials).
Experiment 1
Participants completed 8 blocks of 31 trials each, 4 blocks with actual movements and 4 blocks with imagined movements following the imperative signals. The preparatory cue indicated if the forthcoming movement should be performed with the right or left index finger (see Fig. 1 for illustration). Although the cue was always valid; on 32% of the trials, the imperative signal was replaced by an “X”, indicating to the participants that they should not initiate the cued response (catch trial).
For motor imagery trials, participants were provided with specific instructions (in French): “When the cue appears, prepare to imagine the correct movement. When you see the “go” signal, imagine making the movement. You should try to feel the movement, imagining the muscle contraction and tension that you would expect to experience in actual action. Be sure to not contract any muscles during the task and keep your eyes open. When you see the “X” signal, do not imagine the movement.”
A single TMS pulse was applied on 216 of the 248 trials. Within each task (actual movement and motor imagery), TMS was applied at baseline on 12 trials, and at one of these conditions (early preparation, late preparation, post-imperative, post-catch) on 24 trials (12 for the right and 12 for the left cue). The mean TMS intensity for the 18 participants was 50.3 ± 13.6% of the maximum stimulator output.
Experiment 2
There were 2 phases in Experiment 2, with all 14 participants completing Experiment 2a prior to completing Experiment 2b. In Experiment 2a, we asked whether changes in CS excitability could be observed in muscles that were not part of the response set. To this end, MEP measurements were again limited to right FDI, but now participants had to prepare and implement actual or imagined movements of the left or the right foot.
Participants were tested on 4 blocks of 42 trials each, 2 with actual movement and 2 with imagined movement. The cue was evenly distributed between left and right cues (for foot movements) and 16% of the trials were catch trials. Within each block, TMS pulses were applied on 32 of the 42 trials, either at baseline (6 trials), at the late preparatory time (12 trials), or after the imperative (12 go, 2 catch). We did not include the early preparatory pulses given the null results observed at this time point in Experiment 1. The mean TMS intensity for the 14 participants was 57.3 ± 13.6% of the maximum stimulator output.
In Experiment 2b, the participants were tested in a visual imagery condition. The goal here was to determine if changes in CS excitability were specific to the imagery conditions requiring movement preparation. Participants were required to imagine a house or tree. The targeted object was cued by the presentation of either a left (house) or right (tree) bracket. They were instructed (in French) to use the cue to select the object and to engage in active imagery at the onset of the imperative: ‘When the cue appears, prepare to mentally picture the correct image. When you see the “go” signal, imagine vividly the drawing previously presented. Be sure to stay relaxed and keep your eyes open’. Participants were tested on 2 blocks of 42 trials each, with the same structure as in Experiment 2a.
Data and Statistical Analysis
The Shapiro–Wilk test was used to evaluate whether the dependent variables were normally distributed. This test revealed normal distribution for the normalized MEPs (P > 0.05), but not for EMGRMS and RT (P < 0.05). Therefore, we used parametric tests to analyze MEP values and non-parametric tests to analyze EMGRMS and RT values.
The EMG data were used to measure reaction time (RT) and MEPs, as well as to monitor for premature responses and/or motor activation. RT for actual movement trials was defined as the interval between the onset of the imperative signal and the point at which the EMGRMS in the appropriate effector was 2.5 times above the baseline level. To ensure that participants did not move during the delay period (actual and imagined) or during motor imagery, we calculated the EMGRMS activity in the 100 ms epoch prior to the TMS pulse. Trials with EMGRMS greater than 2.5 standard deviations above baseline were discarded (0.40% of all trials).
To assess whether the TMS pulses influenced RT on the actual finger movement trials (Exp.1), we compared RT between TMS timings (baseline, early, late) with 2 Friedman ANOVAs, one for each finger. We then used 3 Wilcoxon paired tests, one for each timing, to assess the effect of selection (right or left finger) on RT. Alpha P-levels were Bonferroni-corrected to 0.025 (0.05/2) and to 0.017 (0.05/3) for the Friedman and the Wilcoxon tests, respectively. In actual foot movement trials (Exp.2a), we used 2 Wilcoxon paired tests each to test the effect of TMS timing (baseline or late) and Selection (right or left foot). Alpha P-levels were Bonferroni-corrected to 0.025 (0.05/2).
To test whether EMGRMS differed across the different TMS timings (baseline, early, late and post-imperative), we used Friedman ANOVAs, run separately for each muscle (right and left FDI) in Experiment 1. This was repeated for each movement (right and left finger movement) and each task (actual and imagined movement), with Wilcoxon tests used for post hoc comparisons. Alpha P-levels were Bonferroni-corrected to 0.012 (0.05/4) and to 0.008 (0.05/6) for the Friedman and the Wilcoxon tests, respectively. In Experiment 2, Friedman ANOVAs were conducted to test TMS timing effects (baseline, late and post-imperative) for each muscle (right and left FDI, and right and left TA in Exp.2a; right and left FDI in Exp.2b). Alpha P-levels were Bonferroni-corrected to 0.012 (0.05/4) and to 0.017 (0.05/3) for the Friedman and the Wilcoxon tests, respectively.
For the MEP analyses, the data sets for the baseline and preparatory probes were pooled across the Go and catch trials for each condition. This was justified by the fact that the Go and catch trials were identical at these time points, distinguished only with the appearance of the imperative (or catch signal). For all experiments, we performed one-sample t-tests to evaluate whether the normalized MEPs were different from baseline for each condition. For Experiment 1, we then evaluated the preparatory phase data for the actual and imagined movement conditions separately, analyzing CS excitability during the delay period with a repeated measure ANOVA involving the factors “Selection” (Selected vs. Non-Selected) and “TMS timing” (Early vs. Late). To compare the actual and MI conditions, one rmANOVA was conducted with factors “Task” (Actual vs. Imagery) and “Selection”. This analysis was restricted to the late TMS pulses because previous work showed that inhibition of CS excitability is greatest at this temporal probe (Duque et al. 2010; Lebon et al. 2016). For the post-imperative phase (actual or imagined movement), we analyzed the MEP data obtained 300 ms after the imperative. Here, we used paired t-tests to compare right and left finger movement trials, restricted to those trials in which the imperative appeared. We also calculated the Pearson correlation between normalized MEPs of actual and imagined trials at the late pulse across participants, performing separate correlations for right finger and left finger movements.
To assess the relationship between preparation and motor imagery, we performed a Pearson correlation between normalized MEPs obtained from the late timing and post-imperative pulses. We excluded actual movement trials from this analysis, because individual variability in volitional muscle contraction contaminated EMGRMS. However, we tested the correlation between normalized MEPs at late pulse and RTs for actual trials.
For Experiments 2a and 2b, rmANOVAs were conducted with the normalized MEP data from the late timing and post-imperative Go pulses. For experiment 2a, we also performed one rmANOVA with the factors Task (Actual vs. Imagery) and “Selection” (right vs. left), again, restricting this to the late pulse MEPs.
For post hoc analyses, we used HSD Tukey’s correction for paired comparisons. We performed the statistical analysis with the STATISTICA software (Statsoft, version 6.1, Statistica, Tulsa, OK, USA).
Results
Experiment 1
Reaction Time and EMG Activity
As assessed by the Friedman ANOVAs, RTs were not affected by the timing of the TMS pulse (χ2 = 1.44, P = 0.48 and χ2 = 2.11, P = 0.35, for right and left finger responses, respectively). When the TMS pulse was applied at baseline, RTs were similar for left (265 ± 31 ms) and right hand (259 ± 33 ms) responses (Z = 0.85, P = 0.39). However, when the TMS pulse was applied during the delay period, a right hand advantage was found for the early probe (left: 277 ± 32 ms, right: 260 ± 36 ms; Z = 2.42, P = 0.01) and approached significance for the late probe (left: 274 ± 45 ms, right: 254 ± 35 ms, Z = 2.02, P = 0.04, with P-value corrected to 0.017).
Inspection of the EMG data indicated that the participants did not produce subtle movements during the preparatory period of actual and imagined trials (Table 1 presents raw EMGRMS). Similarly, there was no evidence of EMG activity when the participants imagined the movements. EMGRMS activity in the window 100 ms prior to the TMS pulse during imagined trials did not vary (right FDI, during right finger movement: χ2 = 3.40, P = 0.33 and during left finger movement: χ2 = 2.33, P = 0.51; left FDI, during left finger movement: χ2 = 0.52, P = 0.91, and during right finger movement: χ2 = 2.30, P = 0.51). As expected, a pronounced increase in EMG activity in right FDI was observed during actual right finger movement (Friedman ANOVA: χ2 = 33.7, P < 0.001; post hoc tests in comparison to EMGRMS at baseline and during preparation: for both Z > 3.72, P < 0.001), and in left FDI during actual left finger movement (χ2 = 32.6, P < 0.001; for both Z > 3.72, P < 0.001).
Table 1.
Root mean square electromyographic activity in μV (mean ± SD). EMGrms only increased after the Go signal in right FDI during actual right finger movement and in left FDI during actual left finger movement; ***P < 0.001
| Right FDI | Left FDI | ||
|---|---|---|---|
| Actual trials | Rest | 2.96 ± 1.07 | 3.13 ± 2.36 |
| Early Right | 2.85 ± 1.03 | 3.03 ± 2.14 | |
| Early Left | 3.05 ± 1.04 | 3.24 ± 2.40 | |
| Late Right | 2.94 ± 1.06 | 3.26 ± 2.51 | |
| Late Left | 2.95 ± 1.12 | 3.14 ± 2.12 | |
| Go Right | 39.9 ± 37.3*** | 3.39 ± 2.98 | |
| Go Left | 3.33 ± 1.42 | 51.9 ± 63.4*** | |
| Imagined trials | Rest | 2.70 ± 1.04 | 2.99 ± 2.27 |
| Early Right | 2.69 ± 1.06 | 2.93 ± 2.30 | |
| Early Left | 2.69 ± 1.03 | 2.96 ± 2.28 | |
| Late Right | 2.73 ± 1.01 | 2.93 ± 2.26 | |
| Late Left | 2.71 ± 1.00 | 2.94 ± 2.30 | |
| Go Right | 2.79 ± 1.00 | 2.92 ± 2.29 | |
| Go Left | 2.68 ± 1.00 | 3.28 ± 2.27 |
Corticospinal Excitability During Preparation of Actual or Imagined Movements
To assess changes in CS excitability, we focused on the changes in MEPs, relative to baseline, 250 ms after the preparatory cue (Early probe), 850 ms after the preparatory cue (Late probe, 50 ms before imperative), or 300 ms after the imperative Go signal. We did not analyze the data for trials in which the TMS pulse was applied after the Catch signal to focus on preparatory inhibition.
Relative to baseline, MEPs were inhibited during the delay period. However, this effect, while consistent across all conditions, was not significant at the early probe, regardless of whether a right or a left finger movement was prepared (Actual Movement: t = −1.75, P = 0.10 and t = −1.21, P = 0.24 for right and left actual finger movement, respectively, Figs 2A and 4A; Imagined Movement: t = −1.08, P = 0.29 and t = 0.92, P = 0.37 for right and left imagined finger movement, respectively, Figs 2B and 4B). By the late probe, the MEPs were significantly inhibited relative to baseline in all conditions (Actual Movement: t = −5.71, P < 0.001 and t = −2.50, P = 0.02 for right and left actual finger movement, respectively; Imagined Movement: t = −4.22, P < 0.001 and t = 3.50, P = 0.002 for right and left imagined finger movement, respectively).
Figure 2.
Experiment 1. Percentage of CS Excitability during the preparation of actual and imagined finger movements: (A) Actual finger movement trials. The CS excitability in the right FDI decreased between the early and the late probe. Additional inhibition was present when the right finger was the effector of the forthcoming movement. (B) Imagined finger movement trials. The CS excitability in the right FDI decreased between the early and the late probe. (C and D) Correlation between normalized right FDI MEPs during the late phase of preparatory period for actual and imagined right (C) and left finger trials (D). A positive correlation was found between CS excitabilities; *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
Experiment 1. Typical MEP recording of the right FDI muscle during actual (A) and imagined (B) finger movement trials. The time window is between 100 ms before and 300 ms after the TMS pulse. The black line corresponds to the mean and the gray area to the standard deviation around the mean. To note that the responses at rest was similar for left and right movements.
The ANOVA confirmed the decrease in CS excitability during the delay period. In the analysis of the actual movement conditions, there was a main effect of “TMS Timing” (F(1,17) = 11.60, P = 0.003). The main effect of “Selection” was also significant (F(1,17) = 4.91, P = 0.04). Inhibition in the right FDI was greater when preparing right finger movements. While this effect is most apparent at the late probe, the interaction was not significant (F(1,17) = 2.80, P = 0.11).
Interestingly, in the imagined movement conditions, we also found a main effect of “TMS Timing” (F(1,17) = 14.84, P = 0.001). However, neither the effect of Selection (F(1,17) = 0.01, P = 0.90) nor the interaction were significant (F(1,17) = 0.11, P = 0.74). Thus, while CS excitability was inhibited when people prepared to engage in motor imagery, the degree of inhibition did not differ as a function of whether the probed muscle was related to the selected or non-selected response. To directly assess this apparent difference between the preparation of actual and imagined movement, we compared conditions at the late probe. The Task by Selection interaction was significant (F(1,17) = 5.69, P = 0.03), indicating a difference between right and left finger movement trials only for actual movements.
The preceding analyses indicate that preparing actual and imagined movements entails the recruitment of inhibitory processes. To examine the relationship between these 2 manifestations of preparatory inhibition, we correlated the degree of CS inhibition observed in the right FDI at the late preparatory pulse between the 2 conditions. This correlation was positive when preparing a right finger movement (r = 0.56, P < 0.05, Fig. 2C) or left finger movement (r = 0.74, P < 0.05, Fig. 2D). We did not find any correlation at the early stage of preparation (right finger movement: r = −0.11, P > 0.05; left finger movement: r = 0.35, P > 0.05), although as noted above, inhibition at this time point was not significantly different than baseline.
Corticospinal Excitability During Movement Execution or Motor Imagery
On trials in which the participants implemented an actual right index finger response after the imperative, MEPs elicited from the right FDI were much larger than baseline (Figs 3A and 4A, t = 7.29, P < 0.001). A similar pattern was also observed when the participants imagined making a right index finger movement (Figs 3B and 4B, t = 1.98, P = 0.032). Note that this effect was much less than that observed for actual movements (and thus the different scales for the y-axis between Fig. 3A and B). The lower MEP increase during motor imagery is due to lower activation at the cortical and/or spinal levels (Porro et al. 1996; Grospretre et al. 2016). When participants produced a left index finger movement, no inhibition was evident in right FDI following the imperative (t = −0.65, P = 0.52). However, when imagining left hand movements, right FDI continued to show inhibition at the post-imperative probe (t = −2.62, P = 0.017).
Figure 3.
Experiment 1. Percentage of CS excitability during the implementation phase of finger movements. (A) Actual finger movement trials. The CS excitability in the right FDI only increased when the right finger was activated. (B) Imagined finger movement trials. The CS excitability in the right FDI increased when the participants imagined a right finger movement and remained inhibited when the participants imagined a left finger movement; (C) Correlation between normalized right FDI MEPs during the preparatory and the implementation phases for right finger imagined movements. The greater the inhibition during preparation of right finger imagined movements, the greater the increase while imagining right finger movements; *P < 0.05, ***P < 0.001.
Dynamics Between the Preparation and Implementation Phases
Interestingly, for the imagined condition, the degree of inhibition during the late preparatory phase (late pulse) was inversely correlated to the degree of facilitation observed during the imagined movement (r = −0.58, P = 0.015; Fig. 3C). Thus, participants who exhibited the strongest inhibition when preparing an imagined movement also showed the largest facilitation during the implementation phase. This correlation was not performed for the actual condition due to the large and variable increase in the EMG induced by volitional muscle contraction. However, the MEP decrease observed at the late phase of the delay period was not correlated with RT for either right or left actual finger movements (all, P > 0.05). As it has been noted elsewhere (Greenhouse, Sias, et al. 2015b), the magnitude of preparatory inhibition is not predictive of RT.
Collectively, the results for Experiment 1 reveal a decrease in CS excitability during a preparatory delay period when participants prepare an imagined movement, a pattern that is similar to that observed during actual movement preparation. However, we also observed one marked difference between the preparation of actual and imagined movement: In the former case, inhibition is larger when the effector has been cued for the forthcoming movement. In contrast, the degree of CS inhibition in the right FDI was equivalent when preparing an imagined movement of either the left or the right index finger.
Experiment 2a
To further examine the specificity of the preparatory inhibition during imagined movement, we conducted a second experiment in which we measured CS excitability in hand muscles while the participants prepared and performed actual or imagined movements with the foot (Fig. 1). Here, we assessed CS dynamics by comparing MEPs elicited 50 ms prior to the imperative (late preparation) or 300 ms after the imperative (implementation phase), normalized to baseline.
Reaction Time and EMG Activity
When the TMS pulses were applied at baseline, RTs were similar for left and right foot responses (respectively, 260 ± 43 ms and 264 ± 41 ms; Z = 0.34, P = 0.72). Relative to baseline, RTs were delayed when the TMS pulses were applied during the preparatory period, for left (332 ± 77 ms; Z = 3.10, P = 0.001) and right foot responses (340 ± 83 ms; Z = 2.98, P = 0.002).
As in Experiment 1, background EMG was similar to baseline during the preparatory period or after the imperative in the imagined movement conditions (Table 2 presents the raw EMGRMS). EMGRMS activity of all muscles (right and left FDI, and right and left TA) during imagined foot movements and of right and left FDI during actual foot movements did not vary (for all Friedman ANOVAs, P > 0.05). As expected, there was in increase in EMG activity in the right TA during actual right foot movement (Friedman ANOVA: χ2 = 18.6, P < 0.001; post hoc tests in comparison to EMGRMS at baseline and during preparation: for both Z > 3.06, P < 0.01), and in left TA during actual left foot movement (χ2 = 15.2, P < 0.001; for both Z > 2.74, P < 0.006).
Table 2.
Root mean square electromyographic activity in μV (mean ± SD). EMGrms only increased after the Go signal in right TA during actual right foot movement and in left TA during actual left foot movement; **P < 0.01
| Right FDI | Left FDI | Right TA | Left TA | ||
|---|---|---|---|---|---|
| Experiment 2a | |||||
| Actual trials | Rest | 2.31 ± 0.82 | 2.39 ± 0.93 | 1.61 ± 0.58 | 1.67 ± 0.36 |
| Late Right | 2.21 ± 0.83 | 2.40 ± 0.95 | 1.60 ± 0.61 | 1.78 ± 0.69 | |
| Late Left | 2.27 ± 0.84 | 2.38 ± 0.90 | 1.67 ± 0.59 | 1.99 ± 1.19 | |
| Go Right | 2.28 ± 0.84 | 2.37 ± 0.96 | 26.2 ± 21.7** | 1.65 ± 0.36 | |
| Go Left | 2.28 ± 0.82 | 2.44 ± 0.98 | 1.88 ± 0.96 | 19.4 ± 17.8** | |
| Imagined trials | Rest | 2.35 ± 0.77 | 2.13 ± 1.03 | 1.56 ± 0.38 | 1.77 ± 0.46 |
| Late Right | 2.41 ± 0.73 | 2.20 ± 1.04 | 1.55 ± 0.37 | 1.70 ± 0.44 | |
| Late Left | 2.39 ± 0.70 | 2.27 ± 0.97 | 1.54 ± 0.39 | 1.91 ± 0.56 | |
| Go Right | 2.41 ± 0.79 | 2.17 ± 1.05 | 1.75 ± 0.61 | 1.71 ± 0.43 | |
| Go Left | 2.38 ± 0.72 | 2.21 ± 1.07 | 1.55 ± 0.39 | 1.95 ± 0.69 | |
| Experiment 2b | |||||
| Visual imagery trials | Rest | 2.37 ± 0.72 | 2.36 ± 1.12 | ||
| Late House | 2.37 ± 0.72 | 2.35 ± 1.00 | |||
| Late Tree | 2.34 ± 0.74 | 2.32 ± 1.12 | |||
| Go House | 2.36 ± 0.75 | 2.43 ± 1.03 | |||
| Go Tree | 2.44 ± 0.76 | 2.36 ± 1.11 | |||
Corticospinal Excitability During Preparation of Actual or Imagined Movements
MEPs elicited from right FDI were significantly suppressed 50 ms prior to an imperative cue, signaling that the participant produce or imagine producing a foot movement (Actual Movement: t = −4.42, P < 0.001 and t = −3.51, P = 0.004 for left and right foot movement, respectively, Figs 5A left side and 6A upper chart; Imagined Movement: t = −4.53, P < 0.001 and t = 4.25, P = 0.001, respectively, Figs 5B left side and 6B lower chart). The amount of inhibition in the finger muscle was similar between actual and imagined foot movement preparation (no main effect of Task, F(1,11) = 0.005, P = 0.94 or Selection, F(1,11) = 1.60, P = 0.23; nor did these factors interact, F(1,11) = 1.67, P = 0.22).
Figure 5.
Experiments 2a and b. Percentage of CS excitability for actual (A) and imagined (B) foot movement trials, and for mental picturing of objects (C). The CS excitability in right FDI decreased during the preparation of actual and imagined foot movements, while it remained unchanged in non-motor imagery trials; ***P < 0.001.
Figure 6.
Experiment 2a. Typical MEP recording of the right FDI muscle during actual (A) and imagined (B) foot movement trials. The time window is between 100 ms before and 300 ms after the TMS pulse. The black line corresponds to the mean and the gray area to the standard deviation around the mean. To note that the responses at rest was similar for left and right movements.
Corticospinal Excitability During Movement Execution or Motor Imagery
Following the imperative, participants made an actual or imagined movement with either the right (homolateral to the recorded MEPs in the right FDI) or left foot (contralateral). When an actual right or left foot movement was executed, MEPs in the right index finger were not different than baseline at the post-imperative probe (right foot: t = 0.77, P = 0.45; left foot: t = −0.86, P = 0.40, Figs 5A right side and 6A upper chart). When the foot movement was imagined, MEPs from right FDI remained significantly inhibited in the left foot condition (t = −2.38, P = 0.03), but were not different than baseline in the right foot condition (t = −0.58, P = 0.57, Figs 5B right side and 6B upper chart). The asymmetry here may reflect some synergistic linkage of homolateral muscles (e.g., the right hand and the right foot being more coupled) or intra-hemispheric spread when executing or imagining a movement with the right foot, producing a faster return to baseline values in right FDI.
Dynamics Between the Preparation and Implementation Phases
The ANOVA confirmed the modulation in CS excitability between the preparatory and implementation epochs, with the effect of “Timing” significant for actual (F(1,12) = 6.58, P = 0.02) and imagined trials (F(1,12) = 9.44, P = 0.009). Interestingly, we observed a “Selection” by “Timing” interaction only for actual movements (F(1,12) = 6.83, P = 0.02). Post hoc tests revealed a significant difference between the preparatory and the implementation phase for right foot (P < 0.001), but not for left foot movements (P = 0.29). This pattern supports the idea that preparatory inhibition is not specific to the muscles involved in the planned response, whether it is an actual execution or a motor simulation.
As for actual trials in Experiment 1, MEP decrease at late pulse was not correlated to RTs of the right and left foot movements (all, P > 0.05).
Experiment 2b
The results of the first 2 experiments reveal marked inhibition of CS excitability when people prepare to make an imagined movement. Moreover, this inhibition was observed regardless of whether the probed effector was involved or not involved in the task. These findings raise the question of whether modulations in CS excitability are specific to motor imagery, or whether a similar pattern would be observed for any form of mental imagery. To examine this issue, the participants in Experiment 2a completed separate blocks of trials in which they performed a non-motor visual imagery task. The cue indicated whether the participants should plan to generate an image of a house or tree. These objects were selected because they have relatively weak associations with finger actions.
EMGRMS collected from the right and left FDI (Table 2) confirmed that participants remained at rest (for all ANOVAs, P > 0.03, with P-value corrected at 0.025). In addition, CS excitability was not modulated when participants prepared to imagine the visual objects (Figs 5C and 7; t = −1.00, P = 0.33 and t = −0.21, P = 0.83, for tree and house, respectively) or when imaging the objects (t = −0.53, P = 0.61 and t = 066, P = 0.52, for tree and house, respectively). These null effects in terms of comparison to baseline were also observed in an ANOVA that included the factors “Timing” (F(1,12) = 0.92, P = 0.35) and “Image” (F(1,12) = 1.81, P = 0.20).
Figure 7.
Experiment 2b. Typical MEP recording of the right FDI muscle during mental picturing of objects. The time window is between 100 ms before and 300 ms after the TMS pulse. The black line corresponds to the mean and the gray area to the standard deviation around the mean. To note that the responses at rest was similar for left and right movements.
Taken together, the results of Experiment 2 reveal the operation of inhibitory mechanisms during the preparatory of imagined movements, even when the probed muscle is not involved in the task. Experiment 2a showed that CS excitability in a finger muscle was inhibited when participants prepared to make actual or imagined foot movement. Experiment 2b indicated that this inhibition was specific to preparatory processes related to movement, given that similar effects were not observed when the participants prepared to perform a visual imagery task.
Discussion
The aim of the current study was to compare neurophysiological signatures of motor preparation during actual and imagined movements. We found similar patterns of CS dynamics, but also some notable differences between the actual and imagined conditions. Specifically, excitability decreased during the preparation phase for both actual and imagined trials, but only actual actions revealed a difference between right and left finger responses (Duque and Ivry 2009). Preparatory inhibition was also observed when the targeted muscle was not involved in the task. Interestingly, this signature of inhibition was not present when participants were engaged in a non-motor imagery task. These results suggest that CS inhibition is a signature of neural processes associated with the preparation of movements, whether they are imagined or actually executed.
Similarities Between Actual and Imagined Movement Preparation
We first replicated previous results showing that CS excitability during the preparation of actual movements is inhibited prior to the expected time of an imperative signal (Duque et al. 2010). Consistent with this earlier work, the attenuation of MEP amplitude was greater in the late stage of preparation in comparison to the early stage. Also, this inhibition was greater when the targeted right index finger was the agonist of the forthcoming movement, compared to when this finger was not involved in the forthcoming action (Labruna et al. 2014; Greenhouse, Saks, et al. 2015a). We also observed preparatory inhibition in a muscle that was not involved in the task (Greenhouse, Sias, et al. 2015b), with suppressed MEPs elicited from a hand muscle when the participants prepared a foot movement. This latter result is suggestive of a relatively broad form of motor inhibition that is evident when preparing any movement, even when the target limb is not a potential effector (Lebon et al. 2016).
The current experiments provide the first evidence that CS excitability is also inhibited during the preparation of imagined movements, mirroring the decrease across a delay period that is observed for actual movements. Interestingly, we found a positive correlation between the magnitude of inhibition during the preparation of actual and imagined trials: The greater the MEP decreased prior to actual movements, the greater the MEP decreased during the preparation of imagined movements. This correlation was observed in both right and left finger movement conditions. This pattern strongly points toward neural mechanisms that are shared between actual and imagined movement (Jeannerod 2001), and that these mechanisms are operative as part of the preparation for movement execution or motor imagery.
The difference between the early and late probe may reflect the recruitment of inhibitory mechanisms as planning processes unfold, with significant decreases in the MEPs only observed near the onset time of the imperative signal. This could arise from the activity of neurons in premotor and sensorimotor areas that exhibit sustained increases or decreases in discharge rates during delayed tasks (Wise et al. 1983; Crammond and Kalaska 2000; Meftah el et al. 2009; Kaufman et al. 2014). Alternatively, the dynamics here may reflect changes in motor cortex that show modest changes in activity during the early stages of preparation in delay response tasks (Cohen et al. 2010). We envision that this recruitment process is a continuous process and modulated by task constraints: For example, the time course of preparatory inhibition is sensitive to the duration of the delay period, evident at probes as early as 100 ms if the delay period is short (Lebon et al. 2016).
The results of the second experiment also underscore similarities between actual and imagined movements. Participants had to prepare to move, or prepare to imagine a movement of the right or the left foot, while MEPs were recorded in the right index finger. Here, too, a decrease of CS excitability was observed during the preparation of actual and imagined movements. Interestingly, no inhibition was found when the participants prepared to imagine a neutral picture (not involving a movement). Thus, the inhibition of MEPs did not reflect a general process associated with task preparation or motor attention, but a specific neural process inherent to movement preparation. However, one could question the relationship between preparatory inhibition and the urge to perform an action. While we did not observe any correlation between RTs and MEP decrease during actual movement preparation, we could not measure response initiation in the visualization task (experiment 2b). Future research can explore this relationship further.
Specificity of Imagined Movement Preparation
In contrast to actual movement preparation, the decrease of CS excitability during preparation of imagined trials was equivalent when on trials in which left or right finger movements were prepared. This dissociation suggests that inhibitory signals recruited during the preparation of an imaginary movement may lack the specificity observed when preparing actual movements.
Prior work has shown that preparatory inhibition in actual movement trials is evident at the spinal level (Touge et al. 1998; Duque and Ivry 2009). It may be that the inhibition observed in advance of imagined movements does not extend to more peripheral parts of the nervous system. Previous studies have shown that alpha-motoneuron excitability is not altered when people produce imagined movements (Hashimoto and Rothwell 1999), or that the manifestation of imagined movements at the spinal level is very weak (Grospretre et al. 2016). Similarly, signals associated with the preparation of an imagined movement may not be sufficient to modulate spinal excitability, and thus, fail to exhibit a difference between conditions in which the targeted muscle is selected or not selected for the forthcoming movement.
This finding supports the hypothesis suggesting that only additional inhibition is required when an actual movement is expected after the imperative signal. Therefore, the generic inhibition present during the preparation of imagined movements would help to select the appropriate motor representation.
Neural Dynamics Between Preparation and Implementation of Imagined Movements
During the implementation phase, and in accord with previous studies (for review, see Stinear 2010; Ruffino et al. 2017), MEPs increased when participants imagined a movement of the cued effector, i.e., the right index finger in the current study. We did not observe any increase in right FDI when imagining left hand movement or foot movement, nor when picturing neutral images (non-motor task).
Interestingly, we noticed a specific pattern of (in)activation during motor imagery: Although mean MEP amplitude significantly increased during the implementation phase in comparison to baseline, there were some participants who failed to show this pattern. It is possible that these participants were unable imagine movements or failed to follow directions. However, the correlation between imagined and actual movement in the late stage of preparation argues against this latter hypothesis. Indeed, the low level of inhibition during the preparation of actual movements did not reflect a lack of attention or preparation given that the amount of inhibition was not correlated with RT. Alternatively, the level of inhibition during preparation may reflect individual differences in how movements are executed or imagined.
A negative correlation was also observed in the motor imagery condition between the magnitude of inhibition during the preparation phase and increase in excitability during the implementation phase. Participants who strongly inhibited the motor system during the preparation of imagined movement showed a greater increase in CS excitability when imagining the movement. Overall, this neural pattern suggests that the preparation and implementation phases were tightly interrelated during motor imagery.
Our results did show an interesting dissociation between the preparation and the implementation of a mental simulation. The amplitude of inhibition during preparation was similar whether the effector was involved or not involved in the task, whereas during mental simulation (i.e., post-imperative phase), an increase in CS excitability was only observed when the muscle was the “agonist” for the imagined movement. This finding suggests that even if preparation and motor imagery are both internal processes, they are 2 different stages in motor simulation.
Neural Mechanisms Underlying Inhibition During Motor Preparation
Taken together, the generic inhibition observed when people prepare imagined or actual movements may facilitate the selection of the appropriate motor representation. Several neural regions have been associated with preparatory inhibition. The lateral prefrontal cortex has been hypothesized to indirectly inhibit the motor cortex to prevent execution, perhaps as part of a monitoring process to ensure that planned actions are appropriate given the current context or goal (Aron 2007; Kranczioch et al. 2009). Using a similar task as employed here, disruption of this area with repetitive TMS has been shown to reduce preparatory inhibition in effectors involved or not in the forthcoming action (Duque et al. 2012). Another candidate would be premotor regions. The supplementary motor area is activated in both preparation and implementation phases of imagined and actual movements (Cunnington et al. 1996; Hetu et al. 2013), although, to date, a specific contribution to inhibitory effects has not been described. Dorsal premotor cortex (PMd) has also been implicated in preparatory inhibition, either through its effects on cortical targets (Koch et al. 2006) or through direct projections to spinal regions (Fetz et al. 1999, 2002). Disruption of PMd with repetitive TMS also reduces preparatory inhibition, although this effect was only observed in the effector involved on the forthcoming action (Duque et al. 2012). As such, it may be that the contribution of PMd to preparatory inhibition is minimal during imagery, given the absence of a difference between conditions in which right FDI was involved or not. Subcortically, motor inhibition figures prominently in models of the basal ganglia (Aron 2007). Future work will be required to assess the contribution of different neural regions to preparatory inhibition, and ask how these might differ between actual and imagined movements.
Conclusion
Motor imagery has provided a useful method to examine the neural mechanisms underlying movement processes. The results presented here provide evidence that motor preparation engages a generic inhibition when the forthcoming action is about to be actually executed or imagined, whereas neural mechanisms that induce additional inhibition in advance of an actual movement are not relevant for motor imagery. Taken together, motor imagery in delayed tasks requires the neural system to be prepared to select the appropriate motor representation.
Notes
Conflict of Interest: None declared.
Funding
This work was supported by the France-Berkeley Fund FBF #2015-34 and the National Institute of Health (NS074917).
References
- Angelini M, Calbi M, Ferrari A, Sbriscia-Fioretti B, Franca M, Gallese V, Umilta MA. 2015. Motor inhibition during overt and covert actions: an electrical neuroimaging study. PLoS One. 10:e0126800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. 2007. The neural basis of inhibition in cognitive control. Neuroscientist. 13:214–228. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Gueugneau N, Bisio A, Ruggeri P, Papaxanthis C, Bove M. 2015. Motor cortical plasticity induced by motor learning through mental practice. Front Behav Neurosci. 9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmutter S, Prut Y. 2010. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol. 20:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. 2000. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 84:986–1005. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Bradshaw JL, Phillips JG. 1996. Movement-related potentials associated with movement preparation and motor imagery. Exp Brain Res. 111:429–436. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. 2007. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 25:3766–3774. [DOI] [PubMed] [Google Scholar]
- Duque J, Greenhouse I, Labruna L, Ivry RB. 2017. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 40:219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB. 2009. Role of corticospinal suppression during motor preparation. Cereb Cortex. 19:2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, Ivry RB. 2012. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 32:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB. 2010. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 30:3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Maier MA. 1999. Primate spinal interneurons: muscle fields and response properties during voluntary movement. Prog Brain Res. 123:323–330. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K. 2002. Functional properties of primate spinal interneurones during voluntary hand movements. Adv Exp Med Biol. 508:265–271. [DOI] [PubMed] [Google Scholar]
- Gentili R, Han CE, Schweighofer N, Papaxanthis C. 2010. Motor learning without doing: trial-by-trial improvement in motor performance during mental training. J Neurophysiol. 104:774–783. [DOI] [PubMed] [Google Scholar]
- Gentili RJ, Papaxanthis C. 2015. Laterality effects in motor learning by mental practice in right-handers. Neuroscience. 297:231–242. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Saks D, Hoang T, Ivry RB. 2015. a. Inhibition during response preparation is sensitive to response complexity. J Neurophysiol. 113:2792–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Sias A, Labruna L, Ivry RB. 2015. b. Nonspecific Inhibition of the motor system during response preparation. J Neurosci. 35:10675–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grospretre S, Lebon F, Papaxanthis C, Martin A. 2016. New evidence of corticospinal network modulation induced by motor imagery. J Neurophysiol. 115:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grush R. 2004. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 27:377–396. discussion 396–442. [DOI] [PubMed] [Google Scholar]
- Gueugneau N, Schweighofer N, Papaxanthis C. 2015. Daily update of motor predictions by physical activity. Sci Rep. 5:17933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Rothwell JC. 1999. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 125:75–81. [DOI] [PubMed] [Google Scholar]
- Hetu S, Gregoire M, Saimpont A, Coll MP, Eugene F, Michon PE, Jackson PL. 2013. The neural network of motor imagery: an ALE meta-analysis. Neurosci Biobehav Rev. 37:930–949. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. 1994. The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci. 17:187–245. [Google Scholar]
- Jeannerod M. 2001. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 14:S103–S109. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. 2014. Cortical activity in the null space: permitting preparation without movement. Nat Neurosci. 17:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Del Olmo MF, Cheeran B, Milton R, Alvarez Sauco M, Rothwell JC. 2006. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J Neurosci. 26:7452–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranczioch C, Mathews S, Dean PJ, Sterr A. 2009. On the equivalence of executed and imagined movements: evidence from lateralized motor and nonmotor potentials. Hum Brain Mapp. 30:3275–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Lebon F, Duque J, Klein PA, Cazares C, Ivry RB. 2014. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci. 26:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon F, Greenhouse I, Labruna L, Vanderschelden B, Papaxanthis C, Ivry RB. 2016. Influence of delay period duration on inhibitory processes for response preparation. Cereb Cortex. 26:2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meftah el M, Bourgeon S, Chapman CE. 2009. Instructed delay discharge in primary and secondary somatosensory cortex within the context of a selective attention task. J Neurophysiol. 101:2649–2667. [DOI] [PubMed] [Google Scholar]
- Michel C, Gaveau J, Pozzo T, Papaxanthis C. 2013. Prism adaptation by mental practice. Cortex. 49:2249–2259. [DOI] [PubMed] [Google Scholar]
- Munzert J, Lorey B, Zentgraf K. 2009. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 60:306–326. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE. 1996. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 16:7688–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbaugh JW, Syndulko K, Lindsley DB. 1976. Brain wave components of the contingent negative variation in humans. Science. 191:1055–1057. [DOI] [PubMed] [Google Scholar]
- Ruffino C, Papaxanthis C, Lebon F. 2017. Neural plasticity during motor learning with motor imagery practice: review and perspectives. Neuroscience. 341:61–78. [DOI] [PubMed] [Google Scholar]
- Sabate M, Gonzalez B, Rodriguez M. 2004. Brain lateralization of motor imagery: motor planning asymmetry as a cause of movement lateralization. Neuropsychologia. 42:1041–1049. [DOI] [PubMed] [Google Scholar]
- Stinear CM. 2010. Corticospinal facilitation during motor imagery In: Guillot A, Collet C, editors. The neurophysiological foundations of mental and motor imagery. Oxford: Oxford University Press. [Google Scholar]
- Stinear JW, Byblow WD. 2004. An interhemispheric asymmetry in motor cortex disinhibition during bimanual movement. Brain Res. 1022:81–87. [DOI] [PubMed] [Google Scholar]
- Tandonnet C, Garry MI, Summers JJ. 2011. Selective suppression of the incorrect response implementation in choice behavior assessed by transcranial magnetic stimulation. Psychophysiology. 48:462–469. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. 1998. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 109:489–495. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. 2010. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci. 22:225–239. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. 1964. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 203:380–384. [DOI] [PubMed] [Google Scholar]
- Wise SP, Weinrich M, Mauritz KH. 1983. Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain Res. 260:301–305. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. 2001. Motor prediction. Curr Biol. 11:R729–R732. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Shibasaki H, Ikeda A, Terada K, Nagamine T, Honda M. 1997. Cortical mechanism underlying externally cued gait initiation studied by contingent negative variation. Electroencephalogr Clin Neurophysiol. 105:390–399. [DOI] [PubMed] [Google Scholar]