Abstract
Background:
To determine if the effects of intensive lowering of systolic blood pressure (goal of less than 120 mmHg) versus standard lowering (goal of less than 140 mmHg) upon cardiovascular, renal, and safety outcomes differed by gender.
Methods:
Nine thousand three hundred and sixty-one men and women aged 50 years or older with systolic blood pressure of 130 mmHg or greater, taking 0–4 antihypertensive medications, and with increased risk of cardiovascular disease, but free of diabetes, were randomly assigned to either a systolic blood pressure target of less than 120 mmHg (intensive treatment) or a target of less than 140 mmHg (standard treatment). The primary composite outcome encompassed incident myocardial infarction, heart failure, other acute coronary syndromes, stroke, or cardiovascular-related death. All-cause mortality, renal outcomes, and serious adverse events were also assessed.
Results:
Compared with the standard treatment group, the primary composite outcome in the intensive treatment group was reduced by 16% [hazard ratio 0.84 (0.61–1.13)] in women, and by 27% in men [hazard ratio 0.73 (0.59–0.89), P value for interaction between treatment and gender is 0.45]. Similarly, the effect of the intensive treatment on individual components of the primary composite outcome, renal outcomes, and overall serious adverse events was not significantly different according to gender.
Conclusion:
In adults with hypertension but not with diabetes, treatment to a systolic blood pressure goal of less than 120 mmHg, compared with a goal of less than 140 mmHg, resulted in no heterogeneity of effect between men and women on cardiovascular or renal outcomes, or on rates of serious adverse events.
ClinicalTrials.gov number, NCT01206062.
Keywords: blood pressure, cardiovascular disease, clinical trial outcomes, gender, hypertension
INTRODUCTION
Hypertension is a highly prevalent condition that affects approximately 77.9 million, or one-third of United States adults [1], 67% of US adults aged 65 years or older [2], and 75% of adults aged 75 years or older [3]. Hypertension is associated with significantly increased risk of cardiovascular disease (CVD), including stroke, heart failure, and myocardial infarction [1]. Differences in hypertension prevalence are acknowledged with respect to gender, with the overall prevalence of hypertension being higher among US women (45 million) compared with US men (41 million) [4]. In addition, women exhibit similar hypertension prevalence as men in the 45–64 age group, but demonstrate higher prevalence than men in the greater than 65 age group [5–7]. Although current recommendations for hypertension management are similar for both genders [7], some uncertainty exists regarding blood pressure control according to gender. Although some studies report that in older age groups, women have poorer blood pressure control compared with men [8], others have reported better control in women [2]. As the prevalence of hypertension has increased in the United States [9], and the worldwide prevalence of hypertension among women is expected to exceed that of men by 2025 [10], elucidation of effective strategies to control blood pressure in both women and men is of urgent importance.
There has been vigorous discussion regarding the optimal goal for blood pressure treatment [11]. Recently, the multi-center, randomized controlled Systolic Blood Pressure Intervention Trial (SPRINT) [12] demonstrated that intensive lowering of systolic blood pressure (SBP) to a goal of less than 120 mmHg resulted in reduced nonfatal and fatal CVD events and all-cause mortality compared with standard treatment to a SBP goal of less than 140 mmHg in 9361 men and women aged 50 years or older with hypertension and increased cardiovascular risk [13]. In unadjusted analyses, the effect of the intensive treatment upon the composite primary CVD outcome was not significantly moderated by gender, which is a finding similar to that of other studies that have compared the effects of blood pressure treatment according to gender [14]. Additional examination, however, of the effects of the two treatments on individual components of the primary CVD outcome, all-cause mortality, renal outcomes and adverse events will be of interest and benefit to researchers, clinicians and patients.
In consideration of these issues, the purpose of this analysis was to determine if the blood pressure control, SAEs, and composite and individual cardiovascular and renal outcomes in the SPRINT sample differed between men and women. The large SPRINT sample was comprised of 35.6% women, and 59.6% of the sample was aged 65 years or older at baseline, which provided an excellent opportunity to examine these associations.
METHODS
Trial design and oversight, and study population
The design, eligibility criteria, procedures [12] and primary outcome results [13] for SPRINT have been described in detail previously. Briefly, SPRINT was a large, two-armed, multicenter randomized clinical trial designed to test whether intensive treatment of SBP to a goal of less than 120 mmHg would reduce CVD and unfavorable renal and cognitive outcomes compared with standard SBP treatment to a goal of less than 140 mmHg in a multiethnic sample of 9361 men and women aged 50 years or older with hypertension and increased cardiovascular risk. Participants with diabetes, polycystic kidney disease, a prior history of stroke or known dementia were excluded. Enrollment in SPRINT was conducted from November 2010 to March 2013 at 102 clinical sites in the United States, including Puerto Rico. The Institutional Review Board at each clinical site approved the study, and the trial was registered with clinicaltrials.gov (NCT01206062) prior to recruitment.
After randomization, participants were seen monthly for the first 3 months and thereafter every 3 months. All major classes of antihypertensive agents were included in the SPRINT formulary [13] and were provided at no cost to the participants, and were assessed at each study visit. Antihypertensive medications for participants in the intensive-treatment group were adjusted on a monthly basis to target the SBP goal of less than 120 mmHg. For participants in the standard-treatment group, medications were adjusted to target a SBP of 135–139 mmHg. Lifestyle modification was encouraged as part of the management strategy in both interventions.
On August 20, 2015, after analyses of the primary outcome exceeded the monitoring boundary at two consecutive time points, the Director of the National Heart, Lung and Blood Institute (NHLBI) accepted a recommendation from the independent Data Safety and Monitoring Board to inform investigators and participants of the beneficial cardiovascular outcome results, which prompted the process of ending the treatments early. Thus, this analysis reflects 3.26 years of the originally planned 5 years of follow-up.
Outcome measures
The primary outcome for SPRINT consisted of a composite outcome [12] defined as the first occurrence of any of the following: nonfatal myocardial infarction; acute coronary syndrome not resulting in myocardial infarction; nonfatal stroke; nonfatal acute decompensated heart failure; or death from CVD. Detailed descriptions of the definitions of, and adjudications for these outcomes are described elsewhere [15]. Secondary outcomes included the aforementioned individual components of the primary outcome, all-cause mortality, or an additional composite, which consisted of the primary outcome or all-cause mortality.
SPRINT also investigated renal outcomes. In this investigation, chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 using the four-variable Modification of Diet in Renal Disease (MDRD) equation [16]. Incident albuminuria was defined as a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from less than 10 at baseline to greater than 10 during follow-up. In participants with CKD at baseline, the composite primary renal outcome consisted of incident reduction in the eGFR of at least 50%; long-term dialysis, or kidney transplantation.
Serious adverse events were defined as events that were fatal or life-threatening, that resulted in clinically significant or persistent disability, which required or prolonged hospitalization, or that were judged by the investigator to represent a clinically significant hazard or harm to the participant that might require medication or surgical intervention [15]. A short list of monitored conditions (hypotension, syncope, injurious falls, electrolyte abnormalities, and bradycardia) was used to report as adverse events if the conditions were evaluated in an emergency department. We also monitored occurrences of acute kidney injury or acute renal failure if they were noted on admission or occurred during a hospitalization and were reported in the hospital discharge summary as a primary or main secondary diagnosis. The Medical Dictionary for Regulatory Activities was used to classify the safety events. Coding was performed at the coordinating center, and up to three codes were assigned to each safety event. The relationship of SAEs to the intervention was assessed by the trial safety officer and reviewed monthly by the safety committee.
Other measures
Age, gender, race/ethnicity (African American, Hispanic, white, other), highest educational attainment [less than high school, high school diploma or graduate equivalent degree (GED), post high school, college graduate], living arrangement (alone versus with other adults), health insurance (yes), alcohol consumption (typical drinks per week), smoking status (never, former, current), pack-years of smoking, and vigorous physical activity (rarely or never, one to three times per month, one time per week, two to four times per week), were assessed using self-report. In addition, depressive symptoms were assessed via self-report using the Patient Health Questionnaire (PHQ-9) total score (range 0–27), with higher scores suggesting more depressive symptoms [17]. Cognitive function was assessed via standardized interview using the Montreal Cognitive Assessment (MoCA) total score (range 0–30), with higher scores suggesting better cognitive function [18].
At each visit, trained clinical staff measured blood pressures with an automated blood pressure device (Omron-HEM-907 XL; Omron Healthcare, Lake Forest, Ilinois, USA) using standardized procedures [13,15]. Blood pressure measurement requirements included measuring blood pressure early during the visit and not following stressful exam components such as blood draws, proper positioning of the participant in a chair with back support, and proper cuff size determination. The Manual of Procedures (MOP) stated that participants should be resting, not completing questionnaires, and not speaking with study staff during the 5-min rest period or while BP measurements were being taken. The MOP also stated that staff should leave the room during the 5-min rest period, and provide a script that staff could use to explain that they would be absent during the 5-min rest period and would then enter the room and obtain the measurements without speaking to the participant.
Additional baseline clinical variables were assessed using standardized procedures [15], including fasting serum glucose (mg/dl), total cholesterol (mg/dl), low-density lipoproteins (LDL; mg/dl) and high-density lipoproteins (HDL; mg/dl), and statin and aspirin use. Baseline height and weight were measured using standardized protocols, and baseline body mass index (BMI) was calculated as weight in kilograms/height in square meters.
Statistical analysis
Descriptive summary statistics were generated to compare the baseline characteristics of the sample according to treatment and gender. Means and standard deviations, or medians and interquartile ranges were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Analysis of variance and independent two-sample t-tests, Pearson’s chi-square tests, or Wilcoxon rank-sum tests, were used, whenever appropriate, to examine differences between genders.
The association between gender, treatment and time until the first occurrence of the primary CVD outcome was examined using Cox proportional hazards regression, with stratification by clinical site. Follow-up time was censored on the date of the last event ascertainment. Main effects in the model included treatment group and gender, and we also tested for a gender by treatment interaction using a likelihood ratio test for interaction. These analyses were repeated for each component of the primary outcome, as well as the secondary outcomes and renal outcomes. We also compared SAEs according to treatment and gender assessed over the course of the study, using Cox Proportional Hazard models.
No adjustments were made for multiple testing. Nominal P values are reported throughout the results as simple guides to possible associations. All analyses were conducted at the SPRINT Coordinating Center with the use of SAS software version 9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
The mean (SD) age of the sample (n = 9361) was 67.9 (9.4) years. Table 1 displays the baseline descriptive characteristics for the total sample, partitioned by gender and treatment group. Collectively, referring to Table 1 column ‘I’, compared with men, women were older, more likely to be African American and Hispanic, reported lower education, were less likely to live with other adults, were more likely to have never smoked, reported fewer pack-years of smoking, fewer alcoholic drinks each week, and reported lower vigorous and less-vigorous physical activity. Women also exhibited significantly higher SBP, lower DBP, lower prevalence of CVD, higher prevalence of CKD, lower (more favorable) Framingham Risk Score, higher body mass index, higher total cholesterol, LDL and HDL, and lower glucose compared with men. Also, compared with men, women had higher (less favorable) PHQ-9 scores, higher total number of antihypertensive medications, lower (less favorable) MoCA scores, and were less likely to have health insurance. Finally, women were less likely than men to use statins or aspirin.
TABLE 1.
Baseline clinical characteristics by gender and treatment
| Women | Men | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | A: overall total sample (N = 9361) | B: all women (N = 3332) | C: standard women (N = 1648) | D: intensive women (N = 1684) | E: all men (N = 6029) | F: standard men (N = 3035) | G: intensive men (N = 2994) | H: P value for gender treatment subgroup (C, D, F, G) | I: P value for all women versus all men (B, E) | J: P value for standard versus intensive women (C, D) | K: P value for standard versus intensive men (F, G) |
| Age in yearsa | 67.9 (9.4) | 67.9 (9.5) | 68.7 (9.6) | 68.4 (9.5) | 67.6 (9.4) | 67.5 (9.4) | 67.7 (9.3) | <0.0001 | <0.001 | 0.28 | 0.41 |
| Self-reported race or ethnic groupb | <0.0001 | <0.001 | 0.29 | 0.47 | |||||||
| African American | 2802 (29.9) | 1271 (38.1) | 641 (38.9) | 630 (37.4) | 1531 (25.4) | 782 (25.8) | 749 (25.0) | ||||
| Hispanic | 984 (10.5) | 454 (13.6) | 229 (13.9) | 225 (13.4) | 530 (8.8) | 252 (8.3) | 278 (9.3) | ||||
| Other | 176 (1.9) | 55(1.7) | 21 (1.3) | 34 (2.0) | 121 (2.0) | 57 (1.9) | 64 (2.1) | ||||
| White | 5399 (57.7) | 1552 (46.6) | 757 (45.9) | 795 (47.2) | 1552 (46.6) | 1944 (64.1) | 1903 (63.6) | ||||
| Educationb | <0.0001 | <0.001 | 0.50 | 0.93 | |||||||
| Less than high school | 876 (9.4) | 387 (11.7) | 197 (12.0) | 190 (11.3) | 489 (8.1) | 248 (8.2) | 241 (8.1) | ||||
| High school diploma or G.E.D | 1520 (16.3) | 598 (18.0) | 282 (17.2) | 316 (18.8) | 922 (15.3) | 464 (15.3) | 458 (15.3) | ||||
| Post-high school | 3313 (35.5) | 1248 (37.6) | 613 (37.3) | 635 (37.9) | 2065 (34.3) | 1027 (33.9) | 1038 (34.7) | ||||
| College graduate | 3635 (38.9) | 1088 (32.8) | 552 (33.6) | 536 (32.0) | 2548 (42.3) | 1292 (42.6) | 1255 (41.9) | ||||
| Lives with other adultsb | 6627 (70.9) | 2005 (60.4) | 997 (60.7) | 1008 (60.1) | 4623 (76.8) | 2315 (76.4) | 2307 (77.1) | <0.0001 | <0.001 | 0.73 | 0.50 |
| Insuredb | 8360 (89.6) | 2920 (88.1) | 1452 (88.5) | 1468 (87.6) | 5440 (90.4) | 2729 (90.2) | 2711 (90.7) | 0.0033 | <0.001 | ||
| Smoking statusb | <0.0001 | <0.001 | 0.63 | 0.59 | |||||||
| Never | 4122 (44.2) | 1825 (55.0) | 901 (54.8) | 924 (55.1) | 2297 (38.2) | 1171 (38.7) | 1126 (37.7%) | ||||
| Former | 3973 (42.6) | 1055 (31.8) | 532 (32.4) | 523 (31.2) | 2918 (48.5) | 1464 (48.4) | 1454 (48.6) | ||||
| Current | 1240 (13.3) | 440 (13.3) | 210 (12.8) | 230 (13.7) | 800 (13.3) | 391 (12.9) | 409 (13.7) | ||||
| Pack-years of smokinga | 12.3 (20.4) | 7.8 (15.5) | 7.5 (14.8) | 8.0 (16.2) | 14.9 (22.2) | 14.6 (22.0) | 15.1 (22.4) | <0.0001 | <0.001 | 0.43 | 0.39 |
| Alcohol: drinks/typical weeka | 2.0 (1.7) | 1.6 (1.6) | 1.6 (1.4) | 1.6 (1.8) | 2.1 (1.7) | 2.1 (1.8) | 2.1 (1.6) | <0.0001 | <0.001 | 0.40 | 0.36 |
| Vigorous physical activityb | <0.0001 | <0.001 | 0.86 | 0.27 | |||||||
| Rarely or never | 2520 (27.1) | 1171 (35.3) | 583 (35.5) | 588 (35.2) | 1349 (22.5) | 659 (21.8) | 690 (23.1) | ||||
| One to three times per month | 1512 (16.2) | 526 (15.9) | 268 (16.3) | 258 (15.4) | 986 (16.4) | 515 (17.1) | 471 (15.8) | ||||
| One time per week | 1005 (10.8) | 351 (10.6) | 166 (10.1) | 185 (11.1) | 654 (10.9) | 326 (10.8) | 328(11.0) | ||||
| Two to four times per week | 3024 (32.5) | 959 (28.9) | 471 (28.7) | 488 (29.2) | 2065 (34.4) | 1062 (35.2) | 1003 (33.6) | ||||
| More than five times per week | 1253 (13.5) | 306 (9.2) | 154 (9.4) | 152 (9.1) | 947 (15.8) | 458 (15.2) | 489 (16.4) | ||||
| SBP (mmHg)a | 139.7 (15.6) | 141.2 (16.8) | 141.2 (16.9) | 141.3 (16.8) | 138.8 (14.8) | 138.8 (14.4) | 138.8 (15.1) | <0.001 | <0.001 | 0.99 | 0.99 |
| DBP (mmHg)a | 78.1 (11.9) | 77.6 (12.2) | 77.3 (12.3) | 78.0 (12.1) | 78.4 (11.8) | 78.4 (11.8) | 78.4(11.8) | 0.003 | <0.001 | 0.14 | 0.84 |
| Cardiovascular diseaseb | 1877 (20.1) | 510 (15.3) | 266 (16.1) | 244 (14.5) | 1367 (22.7) | 671 (22.1) | 696 (23.2) | <0.001 | <0.001 | 0.19 | 0.29 |
| Chronic kidney diseaseb | 2646 (28.4) | 1058 (32.0) | 521 (31.9) | 537 (32.2) | 1588 (26.4) | 795 (26.3) | 793 (26.5) | <0.001 | <0.001 | 0.85 | 0.88 |
| Framingham 10-year CVD risk scorea | 24.8 (12.6) | 16.7 (7.9) | 16.8 (7.9) | 16.6 (7.7) | 29.3 (12.4) | 29.2 (12.4) | 29.4 (12.5) | <0.001 | <0.001 | 0.51 | 0.41 |
| BMI (kg/m2)a | 29.9 (5.8) | 30.1 (6.6) | 30.0 (6.5) | 30.2 (6.7) | 29.7 (5.3) | 29.7 (5.3) | 29.7 (5.3) | 0.02 | <0.001 | 0.46 | 0.67 |
| Total cholesterol (mg/dl)a | 190.1 (41.2) | 205.8 (41.1) | 205.7 (40.5) | 205.9 (41.8) | 181.5 (38.6) | 181.6 (38.6) | 181.4 (38.5) | <0.001 | <0.001 | 0.90 | 0.90 |
| LDL (mg/dl)a | 112.4(35.1) | 122.4 (36.3) | 122.2 (35.8) | 122.7 (36.8) | 106.8 (33.1) | 106.7 (33.0) | 106.9 (33.3) | <0.001 | <0.001 | 0.71 | 0.80 |
| HDL (mg/dl)a | 52.9 (14.5) | 59.4 (15.6) | 59.6 (15.8) | 59.3 (15.4) | 49.3 (12.4) | 49.2 (12.4) | 49.4 (12.4) | <0.001 | <0.001 | 0.67 | 0.48 |
| Fasting glucose (mg/dl)a | 98.8 (13.5) | 97.1 (13.5) | 97.2 (13.5) | 97.0 (13.4) | 99.8 (13.5) | 99.7 (13.2) | 99.9 (13.8) | <0.001 | 0.002 | 0.65 | 0.52 |
| eGFR MDRD (ml/min per 1.73 m2)a | 71.8 (20.6) | 70.3 (21.4) | 70.2 (21.6) | 70.4 (21.2) | 72.5 (20.1) | 72.5 (19.9) | 72.5 (20.3) | <0.001 | <0.001 | 0.81 | 0.99 |
| PHQ-9 total score (range 0 to 27)a,c | 3.1 (4.2) | 3.5 (4.3) | 3.5 (4.4) | 3.6 (4.2) | 2.8 (4.1) | 2.8 (4.1) | 2.8 (4.0) | <0.001 | <0.001 | 0.68 | 0.88 |
| MoCA total score (range 0–30)a,d | 22.9 ± 4.1 | 22.7 ((4.5) | 22.6 (4.6) | 22.7 (4.4) | 23.0 (3.9) | 23.0 (3.8) | 23.0 (3.9) | 0.0002 | <0.001 | 0.74 | 0.79 |
| Use of ACE inhibitorsb | 3456 (36.9) | 1046 (31.4) | 498 (30.2) | 548 (32.5) | 2410 (40.0) | 1195 (39.4) | 1215 (40.6) | <0.001 | <0.001 | 0.15 | 0.34 |
| Use of aldosterone receptor blockersb | 179 (1.9) | 93 (2.8) | 51 (3.1) | 42 (2.5) | 86 (1.4) | 43 (1.4) | 43 (1.4) | <0.001 | <0.001 | 0.30 | 0.95 |
| Use of alpha-1 blockersb | 422 (4.5) | 45 (1.4) | 20 (1.2) | 25 (1.5) | 377 (6.3) | 187 (6.2) | 190 (6.3) | <0.001 | <0.001 | 0.50 | 0.77 |
| Use of angiotensin II antagonistsb | 1985 (21.2) | 875 (26.3) | 438 (26.6) | 437 (26.0) | 1110 (18.4) | 554 (18.3) | 556 (18.6) | <0.001 | <0.001 | 0.68 | 0.75 |
| Use of beta blockers with intrinsic sympathomimetic activityb | 5 (0.1) | 1 (0.0) | 0 (0.0) | 1 (0.1) | 4 (0.1) | 3 (0.1) | 1 (0.0) | 0.51 | 0.47 | 0.32 | 0.32 |
| Use of beta blockers without intrinsic sympathomimetic activityb | 2881 (30.8) | 1101 (33.0) | 535 (32.5) | 566 (33.6) | 1780 (29.5) | 864 (28.5) | 916 (30.6) | 0.001 | 0.0004 | 0.48 | 0.07 |
| Use of CCBs-Dihydropyridines | 2782 (29.7) | 970 (29.1) | 478 (29.0) | 492 (29.2) | 1812 (30.1) | 927 (30.5) | 885 (29.6) | 0.65 | 0.34 | 0.89 | 0.40 |
| Use of CCBs-non-Dihydropyridinesb | 499 (5.3) | 206 (6.2) | 111 (6.7) | 95 (5.6) | 293 (4.9) | 148 (4.9) | 145 (4.8) | 0.02 | 0.006 | 0.19 | 0.95 |
| Use of central alpha-2 agonists and other centrally acting drugsb | 199 (2.1) | 100 (3.0) | 48 (2.9) | 52 (3.1) | 99 (1.6) | 42 (1.4) | 57 (1.9) | <0.001 | <0.001 | 0.77 | 0.11 |
| Use of combined alpha blockers and beta blockersb | 416 (4.4) | 151 (4.5) | 75 (4.6) | 76 (4.5) | 265 (4.4) | 122 (4.0) | 143 (4.8) | 0.55 | 0.76 | 0.96 | 0.15 |
| Use of direct vasodilatorsb | 142 (1.5) | 53 (1.6) | 25 (1.5) | 28 (1.7) | 89 (1.5) | 42 (1.4) | 47 (1.6) | 0.88 | 0.66 | 0.74 | 0.55 |
| Use of loop diureticsb | 425 (4.5) | 190 (5.7) | 95 (5.8) | 95 (5.6) | 235 (3.9) | 118 (3.9) | 117 (3.9) | 0.001 | <0.001 | 0.88 | 0.97 |
| Use of potassium-sparing diureticsb | 404 (4.3) | 207 (6.2) | 117 (7.1) | 90 (5.3) | 197 (3.3) | 105 (3.5) | 92 (3.1) | <0.001 | <0.001 | 0.04 | 0.40 |
| Use of renin inhibitorsb | 30 (0.3) | 16 (0.5) | 30 (0.3) | 8 (0.5) | 14 (0.2) | 6 (0.2) | 8 (0.3) | 0.23 | 0.04 | 0.96 | 0.58 |
| Use of Thiazide diureticsb | 3659 (39.1) | 1472 (44.2) | 3659 (39.1) | 754 (45.8) | 2187 (36.3) | 1119 (36.9) | 1068 (35.7) | <0.001 | <0.001 | 0.07 | 0.33 |
| Number of antihypertensive medications prescribedb | 1.8 (1.0) | 1.9 (1.0) | 1.8 (1.0) | 1.9 (1.0) | 1.8 (1.0) | 1.8 (1.1) | 1.8 (1.0) | <0.001 | <0.001 | 0.64 | 0.30 |
| Statin useb | 4054 (43.7) | 1236 (35.8) | 639 (39.2) | 597 (47.8) | 2818 (46.7) | 1437 (47.8) | 1381 (46.4) | <0.001 | <0.001 | 0.04 | 0.31 |
| Aspirin useb | 4756 (51.0) | 1464 (43.9) | 718 (43.7) | 746 (44.5) | 3292 (54.6) | 1632 (54.0) | 1660 (55.6) | <0.001 | <0.001 | 0.64 | 0.21 |
Data are presented as mean (standard deviation).
Data are presented as number (percentage).
Higher scores suggest worse function.
Higher scores suggest better function.
Achieved blood pressure
Figure 1 illustrates the differences in mean SBP between the intensive and standard treatments for women (Panel A) and men (Panel B) at each clinic visit. Not surprisingly, the smaller sample sizes with wider confidence intervals for the year-4 visit and onward reflect the early stopping of the treatments. In both genders, a marked difference in SBP between the treatments was achieved by the 6-month assessment, and was sustained throughout the course of the study. Also, the mean number of antihypertensive medications was stable from the 6-month assessment until the end of the study, for both study treatment groups and for women and men. Similar blood pressures were achieved in both genders. At the 3-year assessment; the mean (SD) SBP for women in the intensive treatment group was 120.1 mmHg (12.8), and for men in the intensive treatment group the mean (SD) SBP was 120.6 mmHg (14.7). At the 3-year assessment, the mean (SD) SBP for women in the standard treatment group was 136.5 (14.8), and mean (SD) SBP for men was 135.5 (12.7).
FIGURE 1.
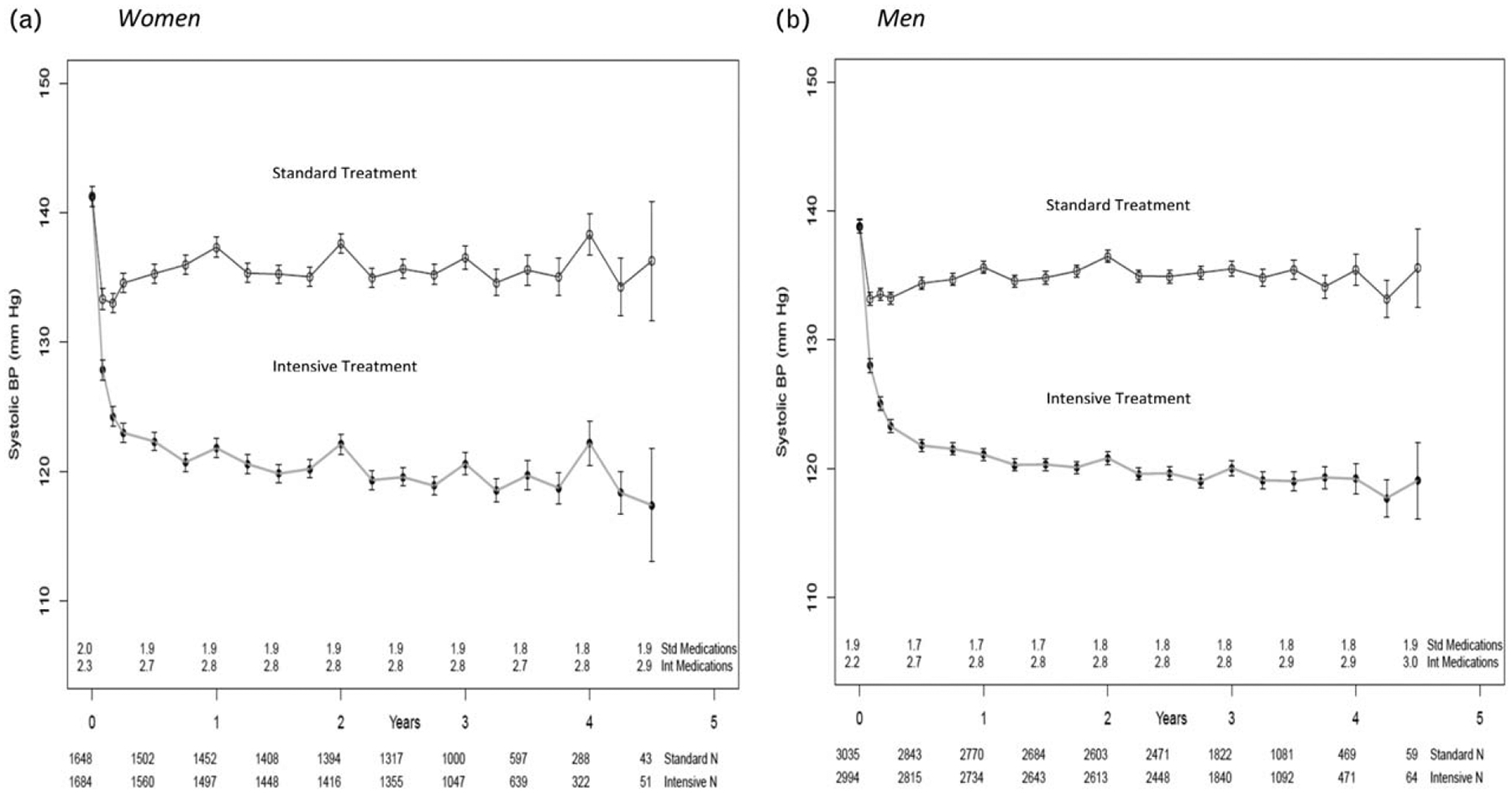
Mean systolic blood pressure in (a) women and (b) men in the two treatment arms over the course of the trial. In both (a) and (b) the mean number of medications is the number of blood pressure medications administered at the exit of each visit and the bars represent 95% confidence intervals.
Primary composite cardiovascular disease outcome, and individual cardiovascular disease outcomes
Figure 2 portrays the results of the Cox Proportional Hazards models that examined the effects of treatment and gender upon the primary CVD outcome, as well as the secondary CVD outcomes. The beneficial effects of the intensive treatment on the primary outcome [women: hazard ratio 0.84 (0.61–1.13); men: hazard ratio 0.73 (0.59–0.89), P value for interaction = 0.45], was consistent across both genders. In addition, for several individual CVD outcomes, including all-cause death, myocardial infarction, heart failure, cardiovascular death, nonfatal MI, nonfatal heart failure, and a composite of the primary CVD outcome and all-cause death, the hazard ratios for the effect of the intensive treatment versus the standard treatment were in a similar favorable direction suggestive of lower risk in both women and men, with no heterogeneity of effect according to gender (all interaction P values greater than 0.05). For other individual CVD outcomes, such as nonmyocardial infarction acute coronary syndrome (women: hazard ratio 0.75; men: hazard ratio 1.09), all stroke (women: hazard ratio 1.21; men: hazard ratio 0.75), and all nonfatal stroke (women: hazard ratio 1.28; men: hazard ratio 0.71), the hazard ratios for the genders suggested a difference, although the treatment group by gender interactions did not reach significance (all interaction P values greater than 0.05) suggesting no heterogeneity of effect.
FIGURE 2.
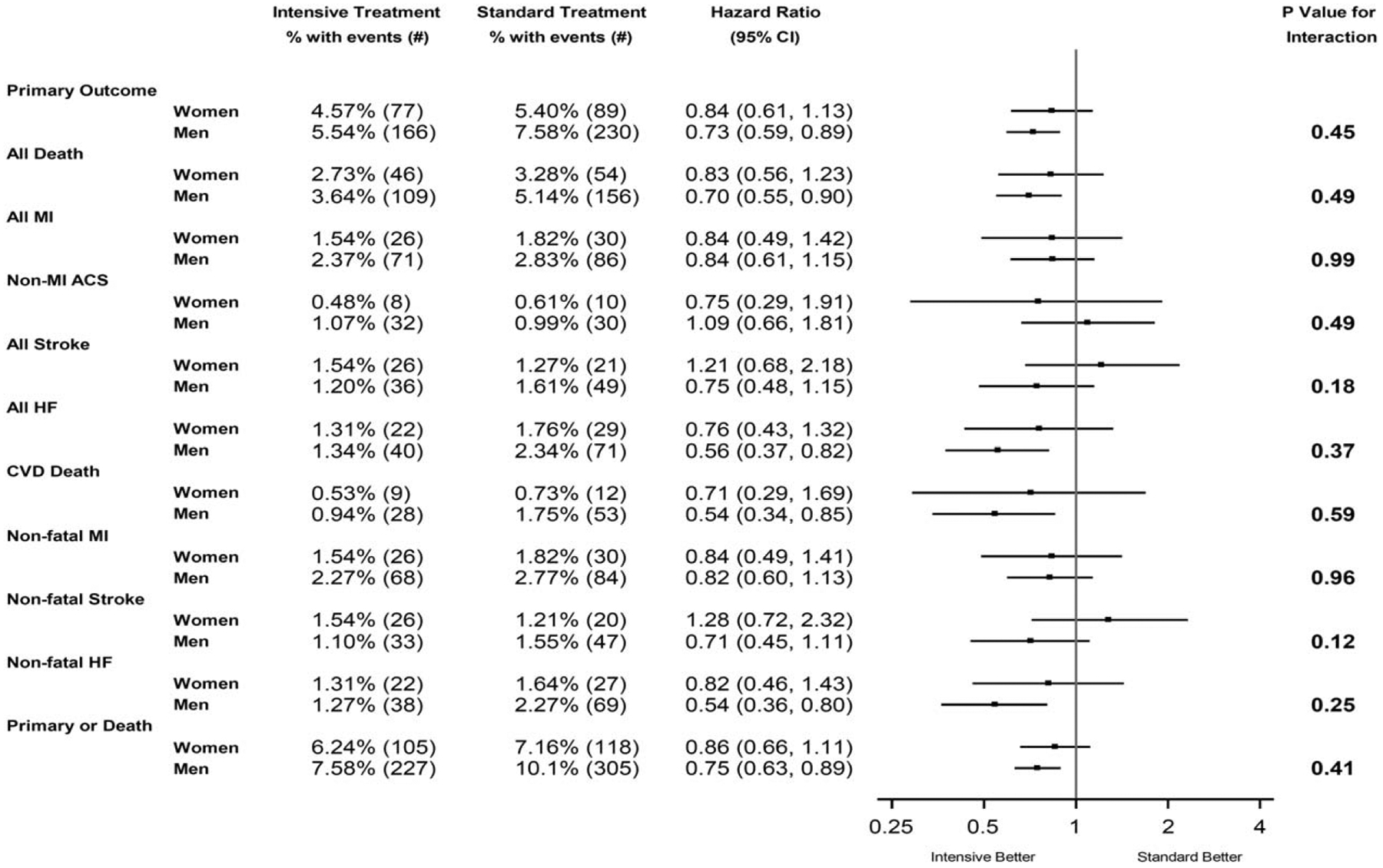
Forest plot of primary and secondary cardiovascular disease outcomes according to treatment group and gender. The primary outcome was the first occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes. ACS, acute coronary syndrome; HF, heart failure; MI, myocardial infarction.
Renal outcomes
Figure 3 depicts the results of the Cox Proportional Hazards models that investigated the effects of treatment group and gender upon the renal outcomes. Collectively, in both women and men, the rates of renal outcomes were low. As reported previously, compared with the standard treatment, in participants without CKD at baseline, intensive treatment resulted in significantly higher rates of the CKD composite outcome (hazard ratio3.49; P < 0.001) [12]. In participants with CKD at baseline, the effect of the intensive treatment versus the standard treatment upon the composite CKD outcome suggested a difference, but the treatment group by gender interaction was not significant [women: hazard ratio 1.43 (0.47, 4.80); men: hazard ratio 0.61 (0.21, 1.67), P value for interaction, 0.27). In participants without CKD at baseline, the effect of the intensive versus the standard treatment upon the composite CKD outcome was similar according to gender [women: hazard ratio 3.15 (1.86, 5.62), men: hazard ratio 3.76 (2.34, 6.33), P value for interaction 0.64]. In participants with CKD at baseline, the effect of the intensive versus standard treatment upon incident albuminuria was similar in women and men [women: hazard ratio 0.94 (0.55, 1.61); men: hazard ratio 0.73 (0.45, 1.18), P value for interaction = 0.50]. In non-CKD participants, the effects of the two treatments upon incident albuminuria were similar in both genders [women: hazard ratio 0.77 (0.51, 1.14); men: hazard ratio 0.80 (0.60, 1.06), P value for interaction=0.87].
FIGURE 3.
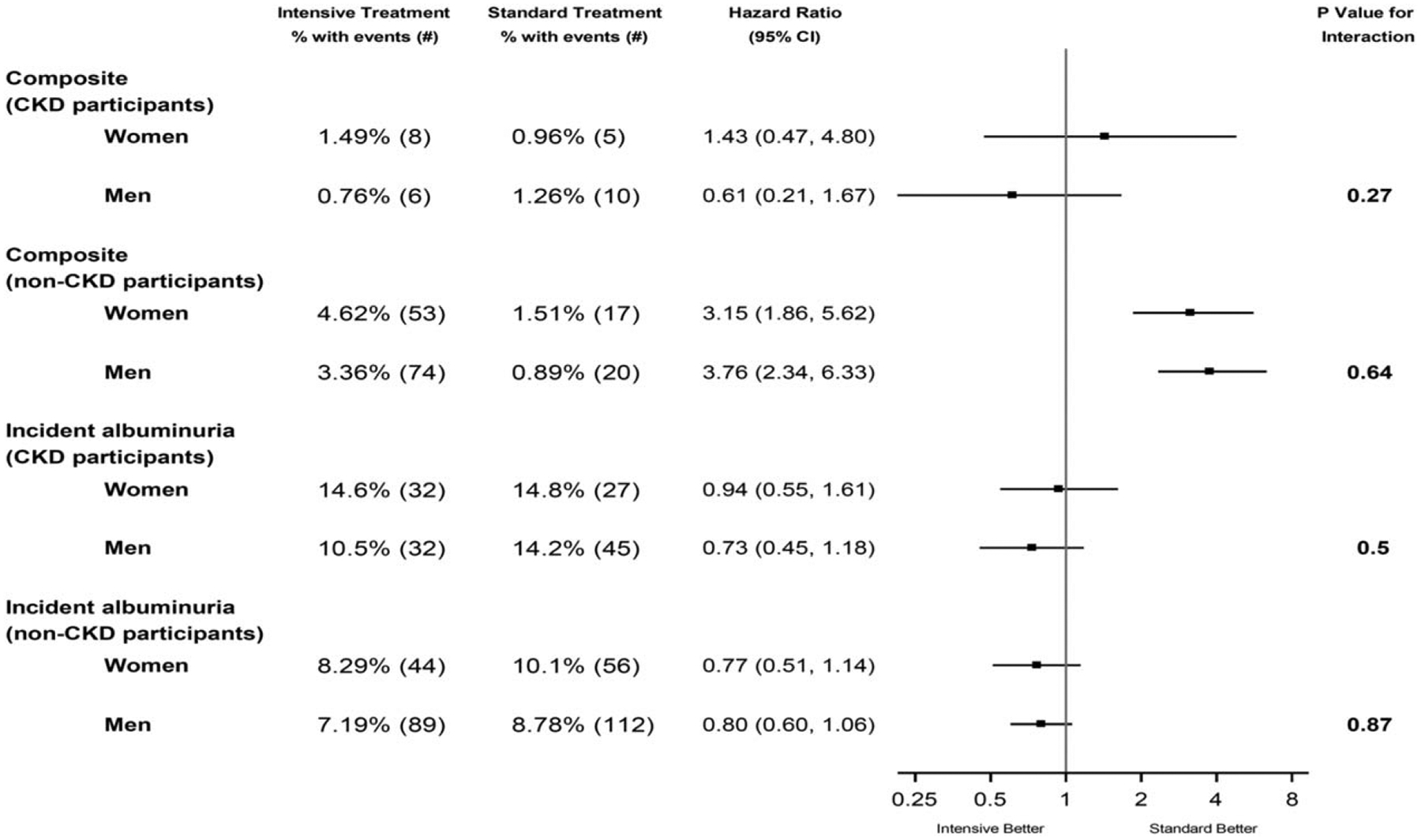
Forest plot of renal outcomes according to treatment group and gender. The composite renal outcome for participants with CKD at baseline was the first occurrence of a reduction in the estimated GFR of 50% or more, long-term dialysis, or kidney transplantation. CKD, chronic kidney disease, defined as estimated glomerular filtration rate of less than 60 ml/min/1.73 m2 using the Modification of Diet in Renal Disease equation. Incident albuminuria is defined as a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from less than 10 at baseline to greater than 10 during follow-up. The denominators for number of patients represent those without albuminuria at baseline.
Serious adverse events
Table 2 presents SAEs for the study, partitioned by gender and treatment. There were no differences in overall SAEs (Table 2, Section ‘A’) by randomized group either in women or in men and there was not a gender by treatment group interaction (P value for interaction = 0.24). For conditions of interest, SAEs only (Section ‘B’), women in the intensive treatment group were at more risk than women in the standard treatment group of developing an acute kidney injury or acute renal failure (hazard ratio = 2.01; 95% CI 1.26, 3.21). For conditions of interest, emergency room visits or SAEs (Section ‘C’), women in the intensive treatment group were at higher risk to experience syncope or an electrolyte abnormality than women in the standard treatment group. In addition, women in the intensive treatment were more likely to experience a serum sodium less than 130 mmol/l, or a serum potassium less than 3.0 mmol/l than women in the standard treatment. Women in the intensive treatment group were less likely than women in the standard treatment group to experience orthostatic hypotension (Section ‘D’).
TABLE 2.
Serious adverse events,a conditions of interest, and monitored clinical measures, according to treatment group and gender
| Standard treatment | Intensive treatment | Hazard ratio (95% Cl) for intensive to standard treatment | |||||
|---|---|---|---|---|---|---|---|
| Description | Women, N (%) with events | Men, N (%) with events | Women, N (%) with events | Men, N (%) with events | Women, hazard ratio (95% Cl) | Men, hazard ratio (95% Cl) | P value for interaction between treatment arm and gender |
| A: total serious adverse eventsa | 576 (35) | 1166 (38.4) | 637 (37.8) | 1162 (38.8) | 1.11 (0.99, 1.25) | 1.01 (0.93, 1.10) | 0.24 |
| Conditions of interest | |||||||
| B: serious adverse event only | |||||||
| Hypotension | 18 (1.1) | 40 (1.3) | 30 (1.8) | 69 (2.3) | 1.64 (0.91, 2.94) | 1.74 (1.18, 2.57) | 0.87 |
| Syncope | 27 (1.6) | 46 (1.5) | 40 (2.4) | 57 (1.9) | 1.48 (0.90, 2.43) | 1.25 (0.85, 1.84) | 0.62 |
| Bradycardia | 23 (1.4) | 45 (1.5) | 22 (1.3) | 56 (1.9) | 0.98 (0.54, 1.77) | 1.25 (0.85, 1.86) | 0.42 |
| Electrolyte abnormality | 56 (3.4) | 48 (1.6) | 76 (4.5) | 62 (2.1) | 1.29 (0.91, 1.84) | 1.30 (0.90, 1.90) | 0.91 |
| Injurious fallb | 46 (2.8) | 55 (1.8) | 49 (2.9) | 53 (1.8) | 1.05 (0.69, 1.58) | 0.97 (0.66, 1.41) | 0.78 |
| Acute kidney injury (AKI) or acute renal failure (ARF)c | 28 (1.7) | 87 (2.9) | 55 (3.3) | 138 (4.6) | 2.01 (1.26, 3.21) | 1.61 (1.23, 2.11) | 0.49 |
| C: emergency room visit or SAE | |||||||
| Hypotension | 25 (1.5) | 54 (1.8) | 41 (2.4) | 103 (3.4) | 1.60 (0.97, 2.63) | 1.93 (1.39, 2.68) | 0.56 |
| Syncope | 36 (2.2) | 64 (2.1) | 58 (3.4) | 90 (3) | 1.61 (1.06, 2.45) | 1.42 (1.03, 1.95) | 0.66 |
| Bradycardia | 27 (1.6) | 49 (1.6) | 28 (1.7) | 66 (2.2) | 1.05 (0.62, 1.80) | 1.36 (0.94, 1.97) | 0.38 |
| Electrolyte abnormality | 61 (3.7) | 66 (2.2) | 94 (5.6) | 76 (2.5) | 1.49 (1.07, 2.06) | 1.16 (0.84, 1.62) | 0.24 |
| Injurious fallb | 135 (8.2) | 182 (6) | 165 (9.8) | 170 (5.7) | 1.21 (0.96, 1.53) | 0.94 (0.76, 1.15) | 0.10 |
| AKI or ARFc | 31 (1.9) | 89 (2.9) | 56 (3.3) | 145 (4.8) | 1.83 (1.17, 2.88) | 1.66 (1.27, 2.16) | 0.78 |
| D: monitored clinical events | |||||||
| Laboratory measured | |||||||
| Serum sodium less than 130 mmol/l | 50 (3) | 53 (1.7) | 95 (5.6) | 94 (3.1) | 1.83 (1.29, 2.58) | 1.80 (1.29, 2.52) | 0.84 |
| Serum sodium more than 150 mmol/l | 0 (0) | 0 (0) | 1 (0.1) | 5 (0.2) | — | — | — |
| Serum potassium less than 3 mmol/l | 37 (2.2) | 38 (1.3) | 65 (3.9) | 52 (1.7) | 1.70 (1.14, 2.56) | 1.38 (0.91, 2.10) | 0.43 |
| Serum potassium greater than 5.5 mmol/l | 67 (4.1) | 106 (3.5) | 71 (4.2) | 113 (3.8) | 1.01 (0.72, 1.42) | 1.08 (0.83, 1.40) | 0.89 |
| Orthostatic hypotensione | |||||||
| Alone | 347 (21.1) | 510 (16.8) | 301 (17.9) | 476 (15.9) | 0.84 (0.72, 0.98) | 0.93 (0.82, 1.06) | 0.30 |
| With dizziness | 30 (1.8) | 41 (1.4) | 35 (2.1) | 27 (0.9) | 1.18 (0.72, 1.93) | 0.66 (0.41, 1.08) | 0.12 |
A serious adverse event was defined as an event that was fatal or life-threatening, which resulted in clinically significant or persistent disability, which required or prolonged a hospitalization, or that was judged by the investigator to represent a clinically significant hazard or harm to the participant that might require medical or surgical intervention to prevent one of the other events listed above.
An injurious fall was defined as a fall that resulted in evaluation in an emergency department or that resulted in hospitalization.
Acute kidney injury or acute renal failure were coded if the diagnosis was listed in the hospital discharge summary and was believed by the safety officer to be one of the top three reasons for admission or continued hospitalization. A few cases of acute kidney injury were noted in an emergency department if the participant presented for one of the other conditions of interest.
Adverse laboratory measures were detected on routine or unscheduled tests; routine laboratory tests were performed at 1 month, then quarterly during the first year, then every 6 months.
Orthostatic hypertension was defined as a drop in SBP of at least 20mmHg or in DBP of at least 10mmHg at 1 min after the participant stood up, as compared with the value obtained whenever the participant was seated. Standing blood pressures were measured at screening, baseline, 1 month, 6 months, 12 months, and yearly thereafter. Participants were asked if they felt dizzy at the time the orthostatic measure was taken.
For conditions of interest, SAEs only, men in the intensive treatment group were at higher risk than men in the standard treatment group to experience hypotension (hazard ratio 1.74; 95% CI 1.18, 2.57) or acute kidney injury or acute renal failure. For emergency room visits or SAEs for conditions of interest, men in the intensive treatment group were at increased risk to experience hypotension, syncope, or an acute kidney injury or acute renal failure compared with men in the standard treatment. Men in the intensive treatment group were also at increased risk to exhibit a serum sodium less than 130 mmol/l than men in the standard treatment. There did not appear to be a gender by treatment interaction for any SAE, emergency department visit, or monitored clinical event.
DISCUSSION
Although recruitment of women has generally improved in recent CVD prevention trials, women historically have been underrepresented as participants in cardiovascular research studies [19]. Thus, the purpose of this investigation was to examine in SPRINT whether the effects of the intensive treatment upon cardiovascular and renal outcomes, and SAEs differed in women and men. Collectively, we observed similar effects of the intensive treatment upon cardiovascular and renal outcomes in men and women over 3 years of follow-up. These findings provide important evidence regarding consideration of gender in developing and implementing strategies for blood pressure control, as well as studying the effects of antihypertensive therapy upon adverse outcomes. Indeed, it has been estimated that the eligibility criteria for SPRINT may represent approximately 16.8 million US adults who may potentially benefit from intensive therapy [20].
Although current recommendations for blood pressure management are ‘gender-neutral’, as stated by Daugherty et al. [8], uncertainty persists regarding whether determinants of blood pressure control differ in men and women [21–23]. As stated by Doumas et al. [7], the degree to which observed differences in control are affected by biological differences or treatment strategies is not certain. In this analysis, at baseline, SBP was slightly higher in women than in men, although men and women were taking similar total numbers of antihypertensive medications. We also found that women were more likely than men to be taking diuretics at baseline, and that women were less likely than men to be taking angiotensin-converting enzyme (ACE) inhibitors, which is similar to the findings of other reports [24]. Similar blood pressure deltas between the intensive and standard treatment groups were achieved in both women and men during the first year of intervention and were maintained throughout the study. Indeed, as noted earlier, in the intensive treatment group, the mean achieved SBP for women was slightly lower than for men at the 3-year point (Fig. 1). Additionally, for both genders, similar total numbers of antihypertensive medications were required to achieve the goal blood pressures. The current results suggest that whenever the same treatment strategy is employed for both genders, similar blood pressure goals may be targeted and sustained successfully with similar numbers of antihypertensive medications.
The incidence of cardiovascular and renal events did not differ significantly according to gender in the two treatments. Also, as reported previously from SPRINT, although incident CKD was higher in the intensive treatment group compared with the standard treatment group in the non-CKD subgroup, there was no significant difference in incident CKD according to gender in either treatment group [25]. These results are similar to those found in a review of 31 randomized clinical trials of CVD prevention that focused on blood pressure treatment [14,26]. Of these 31 trials, five trials compared ‘more intensive’ versus ‘less intensive’ blood pressure treatment regimens. In all the trials reviewed, whereas mean baseline blood pressures were higher in women compared with men, both genders experienced similar reductions in blood pressure, and derived similar reductions in cardiovascular events [14]. In the SPRINT sample, women also displayed higher baseline SBP compared with men. It must be noted, however, that of the five trials that compared more intensive versus less intensive treatment, four trials focused on reduction of DBP. The current study is responsive to recommendations for gender-specific analyses of cardiovascular trials [27], and the current findings may suggest that intensive treatment for blood pressure is of similar benefit to women and men, and may confirm current identical recommendations for blood pressure management for both genders [5].
In middle-aged and older adults, it is plausible that intensive pharmacological treatment for hypertension may result in increased unfavorable side effects, and that women may experience more adverse events than men [28,29]. This possible disparity in adverse events may partly be because of gender-specific conditions such as meno-pause and its treatment [30], which may be associated with increased salt-sensitivity, endothelial dysfunction, visceral adiposity [31] and renin–angiotensin–aldosterone system activation [32,33]. Furthermore, clinicians and patients often express reluctance to initiate or maintain tight blood pressure control because of concerns regarding orthostatic hypotension, falls and fractures [34,35]. In this analysis, the risk of SAEs was similar in both genders with no heterogeneity of effect in women and men. Women and men in the intensive treatment group experienced more selected SAEs compared with their counterparts in the standard treatment group, and overall there were no SAE gender by treatment group interactions. The results of this investigation may assist patients and providers in assessing the benefits versus risks of intensive blood pressure control, and may inform providers regarding possible adverse effects associated with more intensive treatment of blood pressure.
This study had several strengths, including its randomized design, a large, geographically diverse, multiethnic sample, rigorous monitoring of blood pressure and antihypertensive medications, as well as rigorous oversight and monitoring of SAEs. Several limitations of this investigation, however, must be noted, one of the most prominent of which is the sample size of women of 35.6% (n = 3332), which was lower than the planned 50% enrollment [13,30,36,37]. The percentage of women in SPRINT is consistent with that found by Melloni et al. [38], who, examining the representation of women in 156 trials of CVD prevention from 1970 to 2006, found that overall, women constituted 30% of participants. The lower than planned percentage of women in SPRINT may have been partly reflective of the inclusion of participants from the Veterans Affairs system, which has a small percentage of women. If the Veterans Affairs Clinical Center Network is excluded, the percentage of women in SPRINT is 42.7%, which is similar to the findings of Melloni et al. [38], which showed that women constituted 44% of participants over the past decade. Nonetheless, it must be noted that in ACCORD [35], which was similar to SPRINT in design and included Veterans Affairs participants, the percentage of women was higher (47%). Thus, whereas the results of this investigation provide important information, our analyses may have been underpowered to detect differences according to gender. In interpretation of these results, it is important to note that SPRINT did not stratify participants according to gender, as with most randomized controlled trials examining major cardiovascular outcomes, and was not powered to formally test treatment differences in several subgroups of interest, including gender. Furthermore, the SPRINT trial was stopped early for benefit [12,13]. Thus, it is not surprising that the 95% confidence interval for the primary outcome in the women subgroup contained 1.00 (hazard ratio 0.84, 95% CI 0.61–1.13). This was also true for other outcomes such as all death, cardiovascular death, and nonfatal heart failure (Fig. 2). The point estimate of the hazard ratio for the primary outcome, however, was in the same direction as observed in the men subgroup (hazard ratio 0.73) and the formal interaction test of gender by treatment assignment revealed no heterogeneity of effect.
Wenger et al. [30] have postulated that the low cardiovascular event rate in women may have resulted from the possibility that the women enrollees may have had lower cardiovascular risk at baseline compared with men at baseline, thus constituting selection bias [30]. It is also plausible that the cardiovascular outcomes in women participants may have been more favorable if women had younger baseline age. However, the SPRINT data reveal complex baseline differences in cardiovascular risk factors according to gender. While women displayed more favorable baseline Framingham 10-year risk scores and lower pack-years of smoking compared with men, women also displayed older age, higher SBP [14,26], higher body mass index, and were more likely to live alone. In addition, women had higher percentages of CKD compared with men, and had higher percentages of African-Americans and Hispanic Americans compared with men. Interestingly, at least one commentary has suggested that the Framingham risk score may underestimate cardiovascular risk in women [39]. Women also had significantly lower prevalences than men of modifiable factors which may be addressed in clinical practice, such as of use of statin [40] and aspirin [41], both of which have been associated with lower rates of CVD in both genders [42,43]. However, the benefit of aspirin for primary prevention of CVD remains uncertain [44]. Thus, interpretation of the cardiovascular risk of women versus men at the onset of the SPRINT trial must be performed with care.
Additional limitations of SPRINT include that treatment assignment was not blinded, and that self-report of adverse events at any visit was included in the design. Because the intervention group had more pro re nata (PRN) visits for blood pressure management they had greater opportunity to report SAE events. Thus, ascertainment bias may have affected the SPRINT SAE results and these should be interpreted with caution. Primary outcome data in SPRINT, however, were collected with the same frequency in both treatment groups and these analyses are not at risk for this type of bias. In addition, although the eligibility criterion of at least 50 years of age likely resulted in a high percentage of women who were postmenopausal and the mean age of women participants was 67.9 years, we did not assess menopausal status directly. Thus, this factor cannot be controlled in the SPRINT analyses. SPRINT also excluded individuals with diabetes [45], a prior history of stroke, or persons who were institutionalized. Thus, these results are not generalizable to these populations [46,47], and partly may account for the low rates observed for events such as nonmyocardial acute coronary syndrome and nonfatal stroke. Also, as noted earlier, although the smaller sample size of women participants suggests that further research is needed to further clarify the role of gender in CVD prevention, it is unlikely that a separate trial of intensive blood pressure treatment like SPRINT done only in women will ever be conducted because of ethical concerns of denying the standard group from potentially beneficial treatment. Thus, clinical practice decision makers will have to rely on the best evidence available to make decisions with women patients regarding intensive blood treatment.
Also, the SPRINT participants were highly educated, with 38.9% of participants having graduated from college. This characteristic may have aided both adherence to the treatments and retention in the trial. In addition, although lifestyle modification [48] strategies such as smoking cessation, alcohol consumption, dietary [49] and physical activity [50] recommendations were provided to SPRINT participants as ‘background’ therapy, these approaches were not emphasized, and dietary patterns were not assessed.
In conclusion, this investigation found that the primary results of SPRINT were consistent in women and men, and it provides additional evidence supporting intensive blood pressure management and control in women. In order to translate these findings into practice, clinicians will have to weigh evidence of the efficacy of intensive lowering of blood pressure with its potential adverse events, as well as incorporate collaborative patient-centered approaches to maximize the benefits of blood pressure management in women.
Supplementary Material
ACKNOWLEDGEMENTS
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN-268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the co-authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list, http://links.lww.com/HJH/A879: ClinicalTrials.gov Identifier: NCT01206062. We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439; OSU: UL1RR025755; U Penn: UL1RR024134 and UL1TR000003; Boston: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752; UL1TR000073 and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; UT Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337 COBRE Award NIGMS.
Sources of funding support: The sources of support information for this analysis are listed above.
Abbreviations:
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- BMI
body mass index
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- GED
graduate equivalent degree
- HDL
high-density lipoproteins
- LDL
low-density lipoproteins
- MDRD
Modification of Diet in Renal Disease
- MoCA
Montreal Cognitive Assessment
- MOP
Manual of Procedures
- PHQ-9
Patient Health Questionaire-9-item
- SAEs
serious adverse events
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation 2013; 127:143–152. [DOI] [PubMed] [Google Scholar]
- 2.Ostchega Y, Dillon CF, Hughes JP, Carroll M, Yoon S. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc 2007; 55:1056–1065. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics: 2016 Update: a report from the American Heart Association. Circulation 2016; 133:447–454. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics: 2017 update: a report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 6.McDonald M, Hertz RP, Unger AN, Lustik MB. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States Adults aged 65 and older. J Gerontol A Biol Sci Med Sci 2009; 64A:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep 2013; 15:321–330. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty SL, Masoudi FA, Ellis JL, Ho PM, Schmittdiel JA, Tavel HM, et al. Age-dependent gender differences in hypertension management. J Hypertens 2011; 29:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 2008; 52:818–827. [DOI] [PubMed] [Google Scholar]
- 10.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 11.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2005; 387:957–967. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull F, Woodward M, Neal B, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J 2008; 29:2669–2680. [DOI] [PubMed] [Google Scholar]
- 15.Systolic Blood Pressure Intervention Trial (SPRINT). SPRINT Protocol. Systolic Blood Pressure Intervention Trial (SPRINT), 2012. Available: https://www.sprinttrial.org/public/Protocol_Current.pdf. [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699. [DOI] [PubMed] [Google Scholar]
- 19.Westerman S, Wenger N. Women and heart disease, the underrecognized burden: sex differences, biases, and unmet clinical and research challenges. Clin Sci (Lond) 2016; 130:551–563. [DOI] [PubMed] [Google Scholar]
- 20.Bress AP, Tanner RM, Hess R, ColantonioF LD. Shimbo D, Muntner P. Generalizability of SPRINT results to the U.S. adult population. J Am Coll Cardiol 2016; 67:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong KL, Tso AWK, Lam KSL, Cheung BMY. Gender difference in blood pressure control and cardiovascular risk factors in americans with diagnosed hypertension. Hypertension 2008; 51: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 22.Pimenta E Hypertension in women. Hypertens Res 2012; 35:148–152. [DOI] [PubMed] [Google Scholar]
- 23.August P, Oparil S. Hypertension in women. J Clin Endocrinol Metab 1999; 84:1862–1866. [DOI] [PubMed] [Google Scholar]
- 24.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension 2008; 51:1149–1155. [DOI] [PubMed] [Google Scholar]
- 25.Beddhu S, Rocco MV, Toto R. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oparil S SY 11–3 hypertension in women: more dangerous than in men? J Hypertens 2016; 34 (Suppl 1):e366. [Google Scholar]
- 27.Blauwet LA, Redberg RF. The role of sex-specific results reporting in cardiovascular disease. Cardiol Rev 2007; 15:275–278. [DOI] [PubMed] [Google Scholar]
- 28.Hage FG, Mansur SJ, Xing D, Oparil S. Hypertension in women. Kidney Int Suppl 2013; 3:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neaton JD, Grimm RH Jr, Prineas RJ. Treatment of mild hypertension study: final results. JAMA 1993; 270:713–724. [PubMed] [Google Scholar]
- 30.Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ. Women, hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med 2016; 129:1030–1036. [DOI] [PubMed] [Google Scholar]
- 31.Foy CG, Hsu FC, Haffner SM, Norris JM, Rotter JI, Henkin LF, et al. Visceral fat and prevalence of hypertension among african americans and hispanic americans: findings From the IRAS Family Study. Am J Hypertens 2008; 21:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension. Hypertension 2008; 51:952–959. [DOI] [PubMed] [Google Scholar]
- 33.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 2004; 286:R233–R249. [DOI] [PubMed] [Google Scholar]
- 34.Stokes GS. Management of hypertension in the elderly patient. Clin Interv Aging 2009; 4:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis KL, Palermo L, Vittinghoff E, Evans GW, Atkinson HH, Hamilton BP, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD Trial. J Gen Intern Med 2014; 29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buse JB. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. Am J Cardiol 2007; 99 (12 Suppl):S21–S33. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey TM, Snyder JK, Lovato LC, Roumie CL, Glasser SP, Cosgrove NM, et al. Recruitment strategies and challenges in a large intervention trials: Systolic Blood Pressure Intervention Trial. Clin Trials 2016; 13:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010; 3: 135–142. [DOI] [PubMed] [Google Scholar]
- 39.Sibley C, Blumenthal RS, Merz CNB, Mosca L. Limitations of current cardiovascular disease risk assessment strategies in women. J Womens Health (Larchmt) 2006; 1:54–56. [DOI] [PubMed] [Google Scholar]
- 40.Karalis DG, Wild RA, Maki KC, Gaskins R, Jacobson TA, Sponseller CA, et al. Gender differences in side effects and attitudes regarding statin use in the Understanding Statin Use in America and Gaps in Patient Education (USAGE) study. J Clin Lipidol 2016; 10:833–841. [DOI] [PubMed] [Google Scholar]
- 41.Rivera CM, Song J, Copeland L, Buirge C, Ory M, McNeal CJ. Underuse of aspirin for primary and secondary prevention of cardiovascular disease events in women. J Womens Health (Larchmt) 2012; 21:379–387. [DOI] [PubMed] [Google Scholar]
- 42.Adelman EE, Lisabeth L, Brown DL. Gender differences in the primary prevention of stroke with aspirin. Womens Health (Lond) 2011; 7: 341–352. [DOI] [PubMed] [Google Scholar]
- 43.Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol 2012; 59: 572–582. [DOI] [PubMed] [Google Scholar]
- 44.McKibben RA, Al Rifai M, Mathews LM, Michos ED. Primary prevention of atherosclerotic cardiovascular disease in women. Curr Cardiovasc Risk Rep 2015; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayan K, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the united states. JAMA 2003; 290:1884–1890. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt H, Ghazi L, Calhoun D, Oparil S. BP targets in hypertension: what should we do now that SPRINT is out? Curr Cardiol Rep 2016; 18:98. [DOI] [PubMed] [Google Scholar]
- 47.Cushman WC, Whelton PK, Fine LJ, Wright JT Jr, Reboussin DM, Johnson KC, et al. SPRINT Trial results: latest news in hypertension management. Hypertension 2016; 67:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 49.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of dietary approaches to stop hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis 2014; 24:1253–1261. [DOI] [PubMed] [Google Scholar]
- 50.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelly GA, Ray CA. Exercise and hypertension. Med Sci Sports Exerc 2004; 36:533–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


