Abstract
Alternative Lengthening of Telomeres (ALT) is a mechanism of telomere maintenance observed in many of the most recalcitrant cancer subtypes. Telomeres in ALT cancer cells exhibit a distinctive nucleoprotein architecture shaped by the mismanagement of chromatin that fosters cycles of DNA damage and replicative stress that activates homology directed repair (HDR). Mutations in specific chromatin remodeling factors appear to be key determinants of the emergence and survival of ALT cancer cells. Yet, these may represent vulnerabilities for the targeted elimination of ALT cancer cells that infiltrate tissues and organs to become devastating tumors. In this review, we examine recent findings that provide new insights of those factors and mechanisms that mediate telomere length maintenance and survival of ALT cancer cells.
Keywords: Telomere, Homology Directed Repair, DNA Damage, Replicative Stress, Cancer
INTRODUCTION
THE PHYSIOLOGICAL BASIS OF ALT
Telomeres are specialized ribonucleoprotein structures that demarcate the ends of chromosomes and protect from recognition by factors that sense and repair DNA double strand breaks [1]. They are comprised of tandem arrays of a hexameric 5’-TTAGGG-3’ DNA sequence to which constituents of the Shelterin complex bind [1]. The 3’end of each telomere contains a protruding tract of single stranded DNA that folds back and invades the duplex DNA forming a lasso-like T-Loop, the base of which hides the sequestered chromosome end [2-4]. These properties form the bedrock of telomere structure and regulate chromosome end-protection that is an essential element of genome preservation. In normal somatic human cells, the tract of TTAGGG sequence extends over ranges of 3-15 kilobases (kb) in length. Due to the End-Replication Problem, telomeres are progressively eroded with each cell division, until reaching a point when critically short telomeres pose serious threats to genomic stability. Diminished loading of Shelterin proteins, particularly Telomeric repeat binding factor 2 (TRF2), and reduced capacity to form the T-loop exposes chromosome ends that can then be detected by the primary DNA damage sensor, ataxia-telangiectasia mutated (ATM) kinase [4,5], This promotes aberrant fusion of chromosomes via exposed telomere ends by the Non-Homologous End Joining (NHEJ) pathway of DNA repair. To prevent this, cells activate a cell-cycle exit strategy known as Replicative Senescence that halts proliferation to prevent entry into mitosis and fusion of telomeres [6,7] (Figure 1). Yet, these cells are not eliminated and undergo a process of metabolic and physiological reprogramming that enables their survival [8].
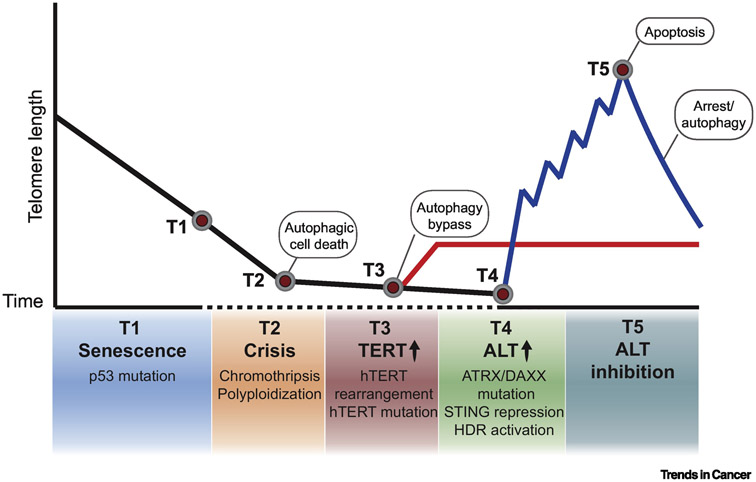
FIGURE 1. TELOMERE MAINTENANCE DURING CANCER EVOLUTION.
In normal somatic cells, telomeres shorten overtime after each cell division due to incomplete replication of terminal DNA sequences. Progressive and irreversible loss of telomeres eventually reaches a critical point when cellular senescence halts proliferation to prevent genomic instability. (T1) To bypass this barrier, cells exhibit deregulation of p53 and retinoblastoma (Rb) proteins to gain proliferative activity. (T2) However, due to telomere dysfunction-induced chromothripsis and polyploidization, cells undergo crisis and are eliminated by autophagic death. Thus, cancer cells adopt telomere maintenance mechanisms to achieve replicative immortality. (T3) The majority of cancers reactivate the specialized reverse transcriptase telomerase to synthesize de novo telomeric DNA. Telomerase re-expression arises from hTERT (catalytic subunit of telomerase) promoter mutations and upstream genomic rearrangements. (T4) 10-15% of cancers utilize the recombination-mediated pathway termed Alternative Lengthening of Telomeres (ALT). ALT telomeres display a permissive chromatin state, stochastic DNA damage and replication stress, as well as inactivation of type I interferon response – all of which contribute to a favorable environment for homology-directed repair (HDR) pathways that mediate telomere maintenance. (T5) The inhibition of ALT based telomere maintenance has been shown and often elicits strong cytotoxic effects without telomere shortening. Yet, there have been instances where telomere shortening correlates with proliferative arrest and even the activation of cytoprotective autophagy.
However, cells can bypass senescence associated anti-proliferative checkpoints by deregulating the p53 and Retinoblastoma tumor suppression pathways (Figure 1). This fosters a burst of proliferative activity that further destabilizes the genome due to continued shortening and eventual fusion of telomeres in mitosis, culminating in proliferative crisis [9,10]. At this time, cells are eliminated en masse. This prevents the propagation of unstable genomes shaped during aberrant mitoses by telomere dysfunction induced chromothripsis and polyploidization that can accelerate tumorigenesis [10-12]. Recently, several surprising aspects of the pathway that eliminates cells in crisis have emerged [13]. It was shown that shards of broken telomeric DNA formed during mitosis escape into the cytosol, where they activate the DNA sensing cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway. Through a mechanism that is currently unknown, cGAS-STING communicates with the macroautophagy machinery to mediate the systemic eradication of cells by autophagic cell death (Figure 1). As with the proliferative blockade imposed at senescence, cells can bypass autophagy possibly by inactivating components of the pathway, though this remains to be determined [13]. Should this occur, the activation of a telomere maintenance mechanism ultimately ensues and is thought to be a defining event in cancer cell evolution (Figure 1).
In most cases, a specialized reverse transcriptase complex known as telomerase is reactivated [14]. Two constituents of telomerase, the non-coding telomerase template RNA (hTR) and dyskerin are constitutively expressed. The number of molecules of active telomerase holoenzyme is limited due to silencing of the TERT promoter or low basal transcription rates [15,16]. This is alleviated by the acquisition of TERT promoter mutations [17,18] or genomic rearrangements [19] upstream of the TERT promoter that elevate TERT mRNA expression and telomerase activity. Once active, telomerase maintains the shortest telomeres and confers a proliferative advantage, enabling immortalization [18]. TERT expression appears to be permanently repressed in some tumors that are largely derived from mesenchymal-adrenergic lineage. Presumably due to selective pressure for survival, cells engage cellular DNA repair pathways particularly mediators of homologous recombination and homology directed repair to maintain telomere length by the Alternative Lengthening of Telomeres pathway, commonly referred to as ALT [20]. In addition to osteosarcoma, pancreatic neuroendocrine (PanNETs) and soft tissue tumors like leiomyosarcoma, ALT is prevalent in low grade astrocytoma and secondary glioma, as well as several pediatric cancers such as neuroblastoma, diffuse intrinsic pontine glioma (DIPG) and glioblastoma [19,21-24]. Clinical surveys currently indicate that ALT is present in ~5-15% of all cancers, though this is likely an underestimation due to deficiencies in direct clinical diagnostics for ALT detection.
One of the signature markers associated with ALT is the loss of expression of alpha-thalassemia/mental retardation syndrome, X-linked (ATRX) and death domain associated protein (DAXX) [21]. Missense mutations and exon deletions that respectively produce a non-functional or truncated ATRX protein have been identified in whole genome sequencing (WGS) and whole exome sequencing (WES) of a wide range of tumor types that invariably become classified as ALT positive, as well as in 70%-80% of ALT cell lines [25]. Similar inactivating mutations in DAXX have also been detected, particularly in vivo, with some ALT cancer cell lines harboring a chimeric fusion protein formed between DAXX and kinesin motor protein KIFC3 [26]. Interestingly, these inactivating mutations, particularly in DAXX, have been observed late in tumor development and correlate with metastatic disease. ATRX and DAXX form a multifunctional chromatinremodeling histone chaperone complex responsible for the replication independent deposition of histone H3.3 at telomeres and pericentromeric chromatin [27]. In the absence of functional ATRX-DAXX, failure to assemble chromatin with histone H3.3 can have pleiotropic effects on transcription, sister chromatid cohesion and chromatin compaction [27-29]. ATRX deficiency has also been implicated in alterations of sub- telomeric DNA methylation on the basis that it interacts with DNA methyltransferase 1 (DNMT1) [30]. Remarkably, heterozygous mutations in histones H3.1 and H3.3 that result in amino acid changes at lysine 27 (K27M) or vicinal to lysine 36 (G34R) have been identified in a low percentage of high grade pediatric glioblastoma and diffuse intrinsic pontine glioma (DIPG) [23,24]. These changes disrupt epigenome-wide chromatin organization by altering the targeting and activity of Enhancer of Zeste 2 (EZH2) and SET domain containing 2 (SETD2) lysine methyltransferases complexes, respectively [31]. Recurrent mutations in isocitrate dehydrogenase (IDH1), a metabolic factor that regulates the production of α-ketoglutarate that is required for the enzymatic demethylation of histones and DNA, correlate with ALT in low-grade astrocytoma and GBMs [32,33]. Conversion of arginine 132 to histidine (R132H) disrupts IDH1 metabolic activity in these tumors and has serious ramifications for all chromatin-templated processes including DNA repair [34,35]. While these genetic aberrations in core constituents and/or regulators of chromatin are not necessarily mutually exclusive[24], it is intriguing that they functionally converge through their capacity to reconfigure chromatin structure at telomeres in these cancers.
These findings indicate that at multiple points during ALT cancer cell evolution, these mutations promote considerable re-modification and expansion of telomeric chromatin, which loses its DNA repair refractory state and becomes permissive for homology directed repair (HDR) mechanisms. Evidence of chromatin adaptation in ALT cells was shown by genome-wide alterations in nucleosome density, histone modification and the enhanced association of variant histone MacroH2A1.2 [36-38]. However, there is clearly much more to be learned regarding how the ALT phenotype emerges. One critical outcome of the reconfiguration of chromatin is that telomere integrity is challenged and subject to recurrent replicative stress and stochastic DNA double strand breaks [25,39]. The inability to adequately compensate for the loss of ATRX’s myriad of functional activities may represent the basis of this since experimental restoration of ATRX appears to alleviate the strain of replicative stress on ALT telomeres [40]. These sources of endogenous damage come with benefits and costs for the cell as they diminish the integrity of telomeres, posing a challenge for genomic stability. However, it is likely that recurrent cycles of DNA damage provide the trigger and DNA substrates necessary to potentiate ALT associated HDR in successive generations. However, prolonged DNA damage signaling and genomic stability may lead to anti-proliferative signaling that leads to apoptosis or cell cycle arrest. To overcome this, it appears that ALT cells also inactivate the type I interferon response by repressing the expression of STING that can mediate the eradication of damaged cells by autophagic cell death or interferon stimulated death mechanisms [41].
ADVANCING TOWARDS AN INTEGRATED MODEL OF ALT
The first evidence of ALT in human cancer came from the observation of telomere length stabilization in cells lacking detectable telomerase [20]. Previous to this, it was established that telomerase deficient budding yeast (Saccharomyces cerevisiae) could bypass senescence by engaging the homologous recombination (HR) DNA repair machinery [42-44]. Taking cues from yeast, it was subsequently demonstrated that human cancer cell lines which lack telomerase transfer telomeric DNA sequences between sister chromatids or separate chromosomes by HR related mechanisms [45]. Through imaging of individual proteins and unbiased proteomics profiling of telomere composition [46,47], an undoubted constitutive and selective association of HR factors with ALT telomeres has been determined. They include the central recombinase RAD51, single-stranded DNA binding Replication Protein A (RPA)[48], the DNA resection MRE11-RAD50-NBS1 (MRN) complex [49], Bloom helicase (BLM) [50] and several other HR accessory factors such as FANCM, BRCA1, BRCA2, RAD51AP1 and RAD52 [51-55]. These proteins are frequently sequestered together with clusters of telomeres and Shelterin subunits within specialized Promyelocytic Leukemia (PML) foci, referred to as ALT associated PML bodies (APBs) [56]. APBs have become indelible markers of recombination-mediated repair and extension of telomeres in ALT cancer cells (Box 1, Figure 2). They are thought to be sites of recombination, telomeric synthesis, and generation of extra-chromosomal telomeric sequences (ECTS), such as C-circles [53,57].
Box 1. ALT-associated PML Bodies: where telomeres cluster for recombination?
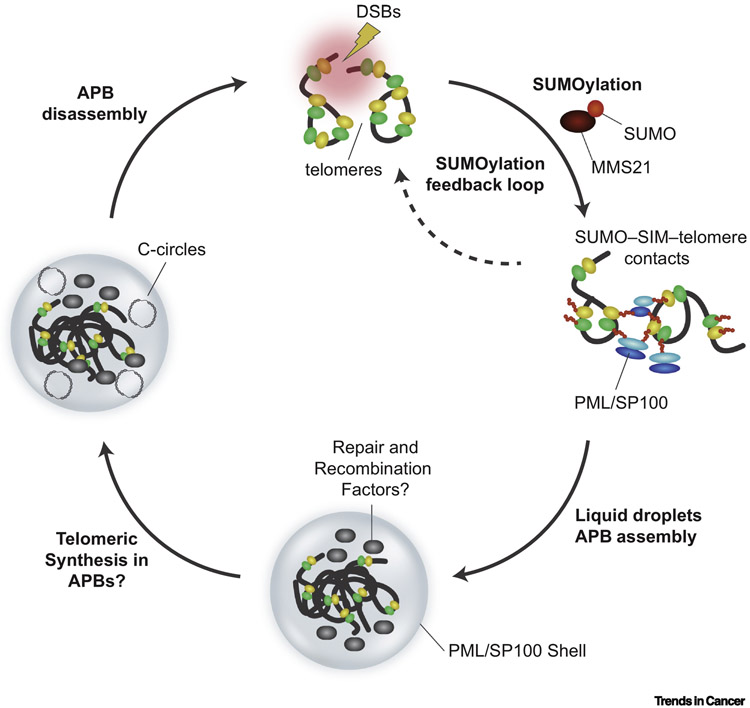
One of the defining characteristics of cancer cells that activate ALT is the clustering and encapsulation of telomeres within nuclear PML bodies forming specialized structures termed ALT associated PML Bodies – often simply referred to as APBs [56]. These are observed within approximately 5-10% of cells within ALT cancer derived in vitro cell cultures and at a similar frequency within ALT tumors. In fact, fluorescence in situ hybridization (FISH) based detection of APBs in paraffin-embedded tumor sections remains the best determinant of ALT status in cancer [96]. Structurally, APBs are comprised of PML/SP100 oligomers that forms a ~ 1 μm diameter shell surrounding 2-5 individual telomeres [97]. In addition to linear chromosomal telomeres, numerous telomeric and HDR proteins including TRF1, TRF2, the MRN complex, RPA2, BRCA1, RAD51, RAD52 and the BTR and SMC5/6 complexes localize within APBs [48-50,68,72,98]. Evidence suggests that the formation of APBs is a dynamic process that is coordinated within the cell-cycle where their numbers peak during G2-phase and is stimulated by telomeric damage from DSBs or acute replicative stress [59,99,100]. An emerging model suggests a crucial role for SUMOylation as a driver of APB formation. SUMO-modification of PML and telomere associated proteins is mediated by the MMS21 SUMO E3 ligase. This fosters stabilizing electrostatic interactions between SUMO modified protein and SUMO-interaction motifs (SIMs) within HDR proteins that stimulates liquid phase separation to form membrane-less nuclear compartment, aka APBs [73,101]. These SUMO-SIM interactions are also linked with the coordinated sequestration of specific protein groups, such as HR factors[102]. The assumption is that the compartmentalization of telomeres within APBs provides the optimal environment for telomere recombination and DNA synthesis mediated extension. This is supported by observations where significant amounts of nascent single stranded DNA and extra-chromosomal telomeric DNA sequences, such as C-circles, accumulate and are detected within APBs [53]. Similarly, alterations in APB levels often correlate with rates of telomere-sister chromatid (t-SCE) exchange events – another although less robust indicator of ALT associated telomere recombination [58,72]. Recently, artificial formation of APB like structures through SUMO-SIM was shown to promote ALT-like phenotypes via a BTR dependent mechanism [73]. PML proteins fulfill additional functions within cells including the sequestration and degradation of damaged proteins or DNA [103]. Therefore, APBs might act as sensors of telomere damage or repositories of damaged proteins and aberrant DNA byproducts. In addition, the similarity in core composition with senescence associated heterochromatic foci (SAHF) raises the possibility that APBs detect anomalies in chromatin structure [104,105]. Thus, despite the strong correlations with ALT and telomere recombination, the diversity of PML protein dynamics and regulation suggest APBs may potentially have additional roles in ALT cancer cells beyond facilitating DNA transactions at telomeres.
FIGURE 2. THE BIOGENESIS AND FUNCTION OF APBs.
SUMOylation of Shelterin components, TRF1 and TRF2, recruits PML/SP100 via SUMO-SIM interactions. SUMOylation-induced APB condensates lead to clustering of telomeres and DNA repair factors within the PML/SP-100 shell. A positive SUMOylation feedback loop enhances APB formation. APBs act as a platform to concentrate repair and recombination factors to perhaps facilitate telomeric DNA synthesis, particularly of extra-chromosomal C-circles. APBs are subsequently disassembled as a result of deSUMOylation events.
In addition, ALT cells exhibit high occurrence of telomeric sister chromatid exchanges (T-SCEs) as an outcome of elevated crossover events [58]. There are at least two independent and potentially competing HDR mechanisms that underpin ALT, both of which utilize exposed DNA ends at stochastic telomeres or stalled replication forks as primers. RAD51 dependent homologous recombination facilitates error-free repair of telomeres [45,59]. Later in G2/M phase, a RAD52-Proliferating Cellular Nuclear Antigen (PCNA)-Replication Factor C 1-5 complex (RFC)-DNA polymerase δ (Polδ) dependent pathway mediates conservative DNA synthesis that extends telomere length [54,60,61] (Figure 3 and Box 2). Recent studies have shed light on the complex regulation of these HDR mechanisms.
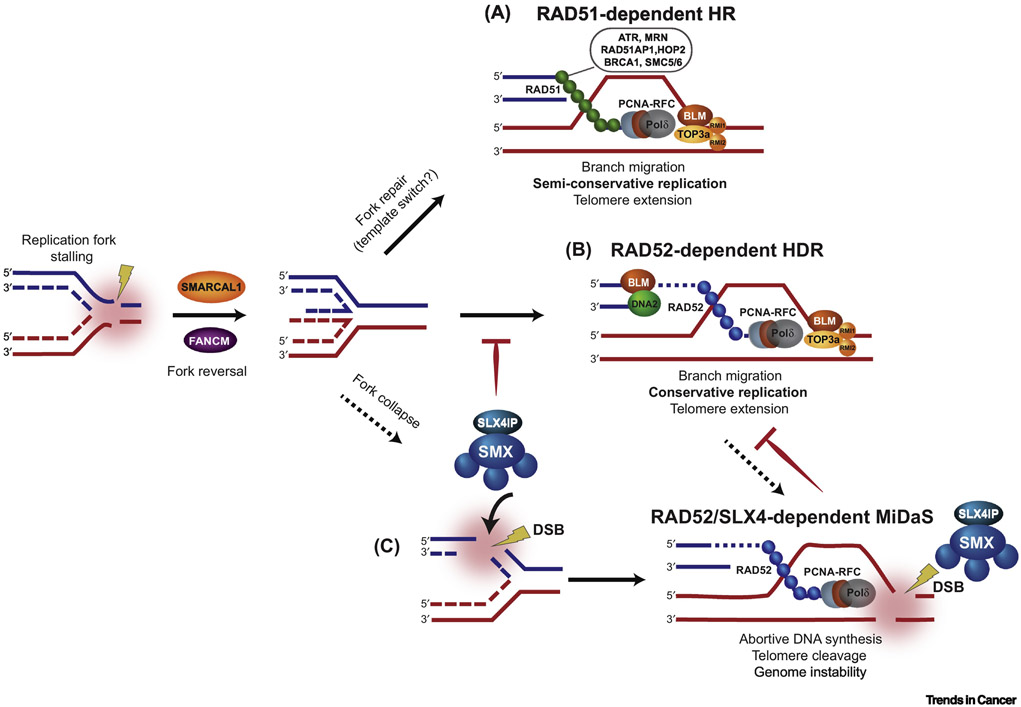
FIGURE 3. HOMOLOGY DIRECTED REPAIR MECHANISM OF ALT.
Telomeres pose as a challenge for the replication machinery, leading to replication fork stalling. The stalled replication fork can undergo fork reversal and restart by SMARCAL1 and FANCM. Here, replication stress is relieved at telomeres and ALT activity is restrained. However, unresolved replication stress leads to fork collapse, which provides direct DSB substrates for ALT-mediated recombination and telomere synthesis by RAD51-dependent (A) and RAD52-dependent (B and C) mechanisms. (A) Rad51-dependent HR: HR components, such as ATR, MRN, RAD51AP1, HOP2, BRCA1, and SMC5/6, facilitate the loading of RAD51 onto a telomeric end for subsequent long-range homology search. This results in semi-conservative replication, in which both recipient and donor telomeric strands contain the original template and newly synthesized telomeric DNA. (B) RAD51-dependent HDR: BLM-DNA2 resect the telomeric end to initiate PCNA-RFC-Polδ-mediated DNA synthesis. The outcome is conservative replication, which leads to newly synthesized DNA on both strands of the recipient telomere. In (A) and (B), the BTR complex (BLM-TOP3A-RMI1/2) initiates telomere dissolution for long-tract ALT-mediated telomere synthesis. (C) RAD52/SLX4-dependent Mitotic DNA synthesis (MiDaS): SLX4IP can antagonize the BTR complex to favor SMX (SLX1-SLX4, MUS81-EME1)-dependent resolution. The SMX complex promotes resolution of telomeric recombination intermediates in the absence of telomere extension.
Box 2. Learning the rules of ALT DNA Synthesis from yeast.
Break induced replication (BIR) has long been implicated in ALT. Yet, the molecular details of this non-canonical DNA synthesis pathway have derived from studies of natural and engineered DSBs in yeast. Cells lacking functional telomerase, through deletion or mutation of the est1 gene, outgrew senescence by engaging either a rad51-dependent recombination pathway (type I survivors), or a rad51-independent break-induced replication process (type II survivors) [42-44]. Both pathways are dependent on rad52 and thepol32 subunit of DNA Polδ [42,106].
In human ALT cancer cells, it was demonstrated that the targeted introduction of DNA nicks and DSBs at telomeres through FokI endonuclease fused to TRF1 activated HDR responses that mirrored those in yeast [59,60]. This was a major breakthrough that provided the first molecular details of BIR-related DNA synthesis in ALT cancer cells. Like BIR in yeast, BIR-related DNA synthesis in ALT cancer cells was determined to be RAD51 independent and largely restricted to G2 and M-phases [60]. This and subsequent independent studies established that RAD52-PCNA-RFC mediated loading of Polδ is essential for conservative synthesis of both leading and lagging DNA strands [54,60,61]. BIR-like DNA synthesis during G2 proceeds unidirectionally to the end of the telomere, resulting in net telomere extension.
Despite these clear similarities with BIR in yeast, there are several aspects of ALT DNA synthesis that remain to be determined. First, the unwinding of DNA strands ahead of the migrating replisome involves helicase activity that in yeast models was shown to rely on pif1 gene [107]. The identity of the BIR helicase in human ALT cells remains elusive. This is a key unanswered question since the specific capacity of this factor to sustain telomeres exclusively in ALT cancer cells by BIR could represent a bona fide ALT-specific therapy target. Second, though strand invasion or annealing with telomeres on sister chromatids is likely to prime DNA synthesis, extra-chromosomal telomeric DNA species (ECTS) have also been suggested as potential substrates to initiate synthesis.
There is evidence of diverse subtypes of DNA synthesis at ALT telomeres [55]. These alternative substrates could potentially be utilized in those distinct contexts, as was proposed [108]. Finally, it is very well established that BIR is an inherently mutagenic HDR mechanism due to template switching and/or slippage of Polδ [109,110]. ALT telomeres harbor a significant portion of variant TTAGGG repeat content [111]. One might speculate that these variant sequences could be produced by low-fidelity polymerase activity and/or the absence of proofreading mechanisms during BIR-like telomere extension. These variant sequences displace Shelterin and recruit orphan nuclear receptors that provide scaffolds of intrachromosomal contacts and lead to what are referred to as Targeted Telomere Insertions (TTIs) [112-114]. Thus, this low-fidelity DNA synthesis mechanism could promote trans-generational telomere instability.
Turning bad into good: using replicative stress for telomere maintenance
The intrinsic difficulty of replicating long tracts of GC rich telomeric sequences has been implicated as the basis for constitutive HDR activity at ALT telomeres. Stalling of replication forks poses a threat to genome stability [62]. This can arise due to polymerase collision with DNA nicks, DNA gaps or polymerase barriers like G-quadruplexes that can readily form within ALT telomeres. These replisome collisions are detected by the DNA damage sensor kinase, Ataxia-Telangiectasia and Rad3 related (ATR) [60]. This initiates the processing of the stalled forks and downstream signaling to attenuate telomere replication such as recruitment of RPA to single stranded telomeric DNA. Subsequent, repair and restart of these stalled forks involves dedicated remodeling enzymes like SMARCAL1, an ATP-dependent strand annealing helicase [63], and/or FANCM, a DNA-dependent ATPase/Translocase subunit of the Fanconi Anemia (FA) core complex (Figure 3) [51,64,65]. FANCM also works with RNaseH1 to dissolve aberrant R-Loops that form between the TERRA lncRNA and telomeric DNA sequences, which can accumulate and destabilize replication forks and HR intermediates within ALT telomeres [66]. The activity of these proteins is essential for mediating replication fork reversal and repair. Prevention of fork remodeling, either by depletion of SMARCAL1 or disruption of FANCM, unleashes staggering increases of APB frequency and size [63]. These cells exhibit elevated RAD51-dependent clustering of telomeres, chromosomal abnormalities, and DNA damage signaling that is consistent with rampant, uncontrolled recombination. ALT cell lines deficient of these proteins, particularly FANCM, display acute cell death [64]. Thus, this initial response by SMARCAL1 and FANCM represents a critical mechanism to salvage stalled forks at ALT telomeres. It also acts as a front-line barrier to excessive telomere recombination in order to preserve ALT cancer cell survival and proliferation.
Once stabilized and repaired, an active fork can be restored by RAD51 dependent homologous recombination. RAD51 presynaptic filaments explore nuclear space, probing for identical telomeric sequences that provide the template for error-free repair [59] (Figure 3). At genomic DSBs, RAD51 dependent homology search involves the consumption of ATP and polymerization of nuclear F-actin filaments that propel homologous DNA sequences into proximity [67]. Though yet to be determined, the same processes are likely to stimulate telomere clustering in ALT. As with HR at genomic DSBs, homology search during ALT is co-regulated and stimulated by HR accessory factors like BRCA1, BRCA2, RAD51AP1 and the SMC5/6 heterodimer [52,59,68]. Both RAD51AP1 and SMC5/6 appear to be regulated through MMS21 mediated SUMOylation– with the former being regulated through SUMO-dependent post-translational stabilization in ALT cells. This implicates a role for post-translational modifications in the fine-tuning of HDR in ALT [52,68]. ALT is also distinct through the involvement of the HOP2-MND1 heterodimer that is normally associated with meiotic HR [59]. The involvement of HOP2-MND1 at telomeres could assist in overcoming reduced homology search and capture kinetics at G-C rich regions, such as those at telomeres. It was also proposed that HOP2-MND1’s role in gametogenesis is remnant of a primordial meiotic origin of ALT that is reactivated in telomerase deficient cancer cells [69]. Though undoubtedly intriguing, the basis of meiotic factors like HOP2 involvement in ALT remains unclear. Once homologous telomeric sequences have been captured, the presynaptic filament invades, aligns and combines with the double stranded partner DNA, forming the synaptic complex. This action displaces a strand of DNA, forming the Displacement Loop (D-Loop), at whose terminal 3’hydroxyl group PCNA is loaded by RFC to prime and initiate semi-conservative DNA synthesis. Given the high G-C rich content at telomeres, the translesion DNA polymerase, Polη, might be employed to initiate DNA synthesis before Polδ takes over [47]. During DNA synthesis that emanates from stalled forks, the translocase activity of FANCM works in unison with the DNA unwinding and decatenation activities of the BLM-TOP3A-RMI1/2 (BTR) complex to promote branch migration and eventual dissolution of D-loops and Holliday Junctions (HJs) [70-72]. The dissolution activity of the BTR complex is crucial as it facilitates Polδ dependent telomere extension and suppresses telomere sister chromatid exchange (t-SCE) events, thereby preserving the original orientation of telomeric DNA strands.
In addition to HJ resolution, BLM has also been shown to alleviate persistent telomere replicative stress at forks that escape reversal. Here, BLM’s helicase activity can facilitate EXO1-DNA2 dependent resection that potentially sets the stage for repair by a RAD51 independent HDR pathway [73]. Here, RAD52 could be recruited to initially stabilize these HR intermediates perhaps to limit resection, as suggested from in vitro studies [74,75]. Then, utilizing its single-strand annealing (SSA) and/or D-loop formation activities, RAD52 subsequently facilitates intra-chromosomal pairing of telomeric DNA sequences located on proximal sister chromatids and D-loop formation [53,55]. These events prime break induced replication (BIR)-related DNA synthesis at ALT telomeres. In fact, several BIR-like pathways are essential aspects of ALT telomere maintenance [55]. These conservative DNA synthesis pathways take place during G2 and M-phases, share independence from RAD51 dependent HDR and universally adopt the specialized PCNA-RFC-Polδ replisome (Box 2). The predominant pathway relies on RAD52 mediated restoration of stalled replication since ALT cells lacking any of RAD52 or the POLD3 subunit of Polδ—the homolog of yeast pol32 [76]—display attenuated DNA synthesis and extensive telomere shortening [53,55,60]. RAD52 is also necessary for a spontaneous form of BIR-related DNA synthesis during the early stages of mitosis, termed Mitotic DNA synthesis (MiDaS), which can occur on chromosome arms as well as at telomeres [77-79]. The predominance of the RAD52-dependent pathway over its RAD51 mediated counterpart has been attributed to distinct binding kinetics of either protein [55,60]. Also, the individual substrate requirements of RAD51 and RAD52 and the mechanisms of HR intermediate processing will likely dictate the commitment to either pathway.
Salvaging the wreckage of telomere entanglements
Stalled forks that escape or are incompatible with repair through the mechanisms outlined above must be salvaged before mitosis. These can be subjected to endonucleolytic resolution by a complex comprised of SLX1-SLX4, MUS81-EME1, XPF-ERCC1 (SMX) that cleaves, or resolves, each junction of the intermediate structure [80]. The SMX complex associates with telomeres irrespective of whether telomerase or ALT is active [81,82]. It acts as an all-purpose responder to detrimental telomere damage where it dismantles complex HR entanglements. This generates large deletions of telomeric DNA at these intermediates. Though new DSBs formed may be amenable to direct PCNA-RFC-Polδ assembly, RAD52 also stimulates MiDaS at intermediates that are resolved by SMX [55]. However, unlike RAD52 dependent DNA synthesis during G2, MiDaS that is stimulated by SLX4 appears to be abortive or non-productive [55]. Notably, in contrast with chromosomal MiDaS that is induced by acute replicative stress, several studies have found that the MUS81-EME1 endonuclease subunits of SMX are dispensable to support telomeric MiDaS [55,79]. However, the issue is not solved since one study identified a distinct class of G2 phase DNA synthesis that was dependent on FANCD2 mediated recruitment of MUS81 via nuclear orphan receptors that associate with variants of the TCAGGG sequences that are embedded within ALT telomeres [83] (see Box 2). Curiously, the latter occurred without the need for mono-ubiquitination of FANCD2, which is a central enabler of chromosomal MiDaS [84] and was previously linked with ALT telomere maintenance [85,86]. Clearly, ALT cancer cells have evolved several distinct mechanisms of non-canonical DNA synthesis to ensure safe transition through mitosis. Untangling the web of regulation and functional hierarchy of these pathways is likely to present future challenges.
However, based on current evidence, it is reasonable to assert that the SMX complex appears to fulfill a greater role in ALT cells to antagonize the HJ dissolution of the BTR complex, which seems to be essential for telomere length homeostasis [72]. In the absence of functional BTR, the promiscuous resolvase activity of SMX becomes uncontrolled and promotes unwarranted recombination, yielding excessive t-SCEs and diminished DNA synthesis. In contrast, perturbation of SMX mediated resolution disruption promotes unrestrained BLM mediated dissolution and DNA synthesis at telomeres [72]. Interestingly, an auxiliary constituent of the SMX complex, SLX4IP, was shown to directly bind and potentially antagonize BLM’s dissolution activity [87]. ALT cells lacking both SLX4 and SLX4IP exhibit staggering increases in recombination, mitotic anomalies and a severe synthetic lethal phenotype that was fully rescued by depletion of BLM [87]. Thus, the BTR dissolution and SMX resolvase activities are subject to exquisite molecular and temporal regulation that is vital in regulating telomere length homeostasis and preserving ALT cancer cell viability.
EXPOLITING ALT’S VULNERABILITIES FOR CANCER THERAPY
The recent advances made in understanding the ALT mechanism have revealed specific vulnerabilities within ALT cancer cells that might be capitalized upon for therapy development. There have been several recent instances, where inhibition or depletion of HDR factors disrupts the ALT mechanism and provokes acute death of ALT cancer cells (Figure 4). Indeed, several ALT cell lines were reported to exhibit acute sensitivity to inhibition of ATR kinase, though whether this is a general phenomenon remains unclear [88,89]. Similarly, it was shown that depletion of FANCM protein induces a potent acute apoptotic phenotype in ALT cancer cell lines [64]. Inhibition of BLM, given its multiple roles in ALT, could also be exploited to elicit a cytotoxic response [90]. Thus, targeting the FANCM-BTR complex represents a potentially viable option for therapy development. An alternative approach involving prolonged chemical stabilization of G4 quadruplexes may be sufficient to suppress HR intermediate processing and elevate telomere replicative stress, driving them to death [91]. Along similar lines, a derivative cis-platin molecule, Tetra-Pt(bpy) could inhibit single-strand annealing reactions at recombining telomeres and promote cytotoxic accumulation of G-quadruplexes [92]. The key certainly appears to lie in determining combinations that induce ALT specific synthetic lethality through either restricting BLM mediated dissolution and inducing catastrophic recombination, as was recently demonstrated by the systemic death of cells lacking both SLX4IP and SLX4 [87].
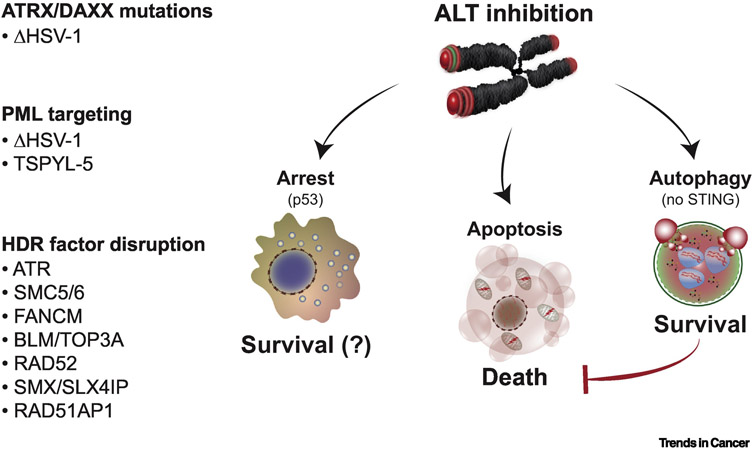
FIGURE 4. THE FATE OF ALT-INHIBITED CANCER CELLS.
ALT cancer cells must maintain a balance between pro- and anti-recombinogenic signals. A shift from this equilibrium poses a therapeutic vulnerability that can be exploited for selective killing of ALT cancers. Synthetic lethality with ATRX/DAXX is a promising target since ATRX/DAXX loss of function mutations are prevalent in ALT cancers. Similarly, ALT-associated PML bodies (APBs) is an attractive target for therapy since APBs are unique to ALT cancers and are implicated in telomere metabolism of ALT cells. Disruption of APBs with infection of Herpes Simplex Virus-1 (HSV-1) or depletion of TSPYL5 have been shown to elicit cytotoxicity. In addition, several lines of evidence report that inhibition of HDR factors perturbs ALT activity and provokes to cell death. Intriguingly, the absence of STING in ALT cancers enables activation of the pro-survival autophagic pathway. This reliance on autophagy may present a unique opportunity in ALT cancers to tip the balance in favor of apoptotic cell death.
It is not unreasonable to assume, based on the striking correlation with ALT activation, that the absence of functional ATRX-DAXX complex affords the opportunity to determine synthetic lethal interactions that eliminate ALT cancer cells. Identifying and targeting factors that functionally compensate for ATRX loss in remediation of replicative stress might yield some benefits. CRISPR based or small compound library screens to identify synthetic lethal partners of ATRX deficiency are yet to be reported. Mitigating factors might be the heterogeneity of available standard ALT cell lines in terms of tissue of origin, ploidy and somatic mutation burden. However, one interesting example of synthetic lethality with ATRX inactivation was reported in which the specific killing of ALT cancer cells was induced following infection with mutant Herpes Simplex Virus-1 (HSV-1) [93]. This was directly attributed to repression and proteasomal degradation of PML protein isoforms upon loss of ATRX and demonstrated the potential utility of viral-based therapy to kill ATRX-deficient ALT cancer cells.
Like ATRX inactivation, PML proteins hold special and unique importance in the biology of ALT cells. Shuttling of telomeres to PML bodies by SUMOylation driven liquid phase separation is a signature event in ALT (Figure 2, Box 1) [68,73]. Conventionally, this is linked with productive DNA synthesis at telomeres [53]. However, it was recently shown that the sequestration of telomeres within APBs could be a poison pill. POT1, a single stranded DNA binding constituent of Shelterin that is essential for chromosome end protection and telomere replication, is targeted for USP7 dependent proteolysis within APBs [94]. Yet, this detrimental event that could unleash ATR dependent DNA damage signaling is prevented by a newly identified ALT specific factor, Testis-Specific Y-encoded Like Protein (TSPYL5) that counteracts the destabilizing activity of Ubiquitin Specific Protease-7 (USP7) [94]. In doing so, TSPYL5 fulfills a key role in sustaining ALT cell viability since its depletion elicits rampant apoptosis due to compromised telomere function. This remarkable finding shows that disrupting APBs, or more specifically, APB-resident factors that confer protection against PML-USP7 dependent proteolysis could be a powerful approach for ALT based cancers.
Interfering with the processing of DNA intermediates of telomere recombination has typically provoked acute cytotoxicity and apoptotic cell death (Figure 4). In those contexts, telomere shortening is often not observed. Rather, it seems that a complete systems failure ensues. This contrasts with the chronic, progressive telomere shortening and DNA damage accumulation due to disruption of key mediators of HDR like MMS21 or RAD52 that culminates in a senescence-like growth arrest [53,68]. It should be considered that this outcome could be due to the wildtype p53 status of the cell lines used in those studies and is not typical of most ALT cancers which tend to harbor mutated p53 [95]. However, in one surprising case, the perturbation of telomere maintenance in ALT cells lacking RAD51AP1, a co-regulator of RAD51, provoked an autophagic phenotype [52]. As with autophagy during crisis, telomere dysfunction and cGAS dependent sensing of telomeric DNA fragments appears to mediate autophagy in ALT cells. However, in contrast to autophagy during cellular crisis [13], this autophagic pathway conferred cell survival [52]. Only when autophagy was inactivated did the ALT cells succumb by apoptosis. The absence of STING seems to be a prime culprit for this distinction since it is required for telomere dysfunction induced autophagy during crisis [13] and is repressed in ALT cells [41]. Other factors are likely to dictate outcomes such as the particular burden of telomere damage. For instance, autophagy in crisis responds to overwhelming and catastrophic telomere de-protection during mitosis. In contrast, the RAD51AP1 deficient ALT cells displayed a relatively modest chronic telomere damage phenotype. The extent of the metabolic shock or activation of distinct signaling pathways could also prove determinative. However, should ALT cancer cells rely on autophagy for survival, this could represent a new vulnerability in ALT cells that presents an opportunity for therapeutic evaluation (Figure 4).
CONCLUDING REMARKS
The recent years have proven tremendously exciting, having delivered immense progress in our understanding of the ALT mechanism. The discovery of the DNA synthesis pathways and PCNA-RFC-Polδ replisome that mediates ALT telomere extension has been pivotal in this respect. With this, the field is well positioned to decipher with precision how the related HDR pathways cooperate to replicate, extend and maintain functional telomeres in cancer cells that rely on ALT. Of the many outstanding questions, one of the next challenges lies in determining how the differential regulation of the RAD51 and RAD52 mediated pathways might eventually intersect. In addition, identifying other key regulators of ALT DNA synthesis including the mammalian counterpart of the BIR helicase are high priorities. Furthermore, distinctions between HDR at ALT telomeres and conventional HDR at DSBs are also beginning to emerge such as the role of meiotic factors and the moonlighting of additional DNA repair pathways in regulating ALT. Though the endpoints of telomere maintenance by the ALT mechanism are only beginning to emerge, they are consistent with the view that ALT cells may be vulnerable to therapeutic strategies that target their reliance on replicative stress due to ATRX-DAXX loss and perhaps compromised interferon signaling capacity due to STING repression. Though greater insight into how these aspects of ALT cancer cells might be connected are evidently needed – these recent findings are beginning to allow for the tracing of molecular events that shape ALT cancer cells, making them distinct from those that activate telomerase. The resolution of these questions will be highly important and exciting. They will provide novel insight into this unique form of telomere extension and possibly guide future investigation towards strategies that eliminate ALT-reliant cancers.
OUTSTANDING QUESTIONS.
What is the stimulus that activates ALT?
How do ALT cancer cells adapt to loss of ATRX and DAXX?
Does STING deficiency impact ALT emergence, progression and treatment?
What is the precise function of APBs in ALT cells? (see Box 1)
What is the identity of the BIR helicase? (see Box 2)
What determines choice of HDR via RAD51 or RAD52?
Is BIR-related DNA synthesis of ALT telomeres mutagenic?
Can synthetic lethal interactions of ALT associated mutations be identified to kill ALT cancer cells?
HIGHLIGHTS.
Growing consensus that ALT arises from deviations in the management of chromatin structure.
Maintaining the optimal equilibrium between HR intermediate dissolution and resolution is a key determinant of telomere length maintenance and ALT cell survival.
Inhibition and/or disruption of HDR factors elicits diverse cell fate outcomes including cellular arrest, apoptosis or even autophagy
ACKNOWLEDGEMENTS
We apologize to all those whose work we were unable to refer to in this review due to space constraints. We thank members of the O’Sullivan lab for critical reading and discussions that helped develop this review article. This work was supported with funding from American Cancer Society (RSG-18-038-01-DMC), the National Cancer Institute (NCI) (5R01CA207209-02) and UPMC Hillman Cancer Center.
GLOSSARY
- Chromothripsis:
Derived from Greek word describing chromosome shattering. Involves complex chromosomal rearrangements that result in mosaic genome organization.
- cGAS-STING pathway:
Cyclic GMP-AMP (cGAMP) synthase (cGAS), a cytosolic DNA sensor, triggers the innate immune response through activation of downstream signaling effector stimulator of interferon genes (STING). This results in the transcription of genes encoding type I interferons and other cytokines.
- CRISPR-Cas9:
Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR) is a genome editing tool derived from the natural defense mechanism of bacteria and archaea. A single guide RNA binds to the target DNA sequence, to which CRISPR associated protein 9 (Cas9) cuts the DNA at that specific site. Erroneous repair by NHEJ can mutate sequences and disrupt gene function.
- End-Replication Problem:
Sequence loss of DNA ends after each round of cell division due to incomplete lagging strand synthesis.
- Genomic Rearrangements:
Gross chromosomal alterations that arise from deletions, duplications, insertions, inversions, or translocations. Genomic rearrangements in regions upstream of the hTERT gene locus (5p15.33) can introduce enhancers that increase hTERT expression.
- Homology Directed Repair (HDR) mechanisms:
HDR utilizes long stretches of sequence homology (~200 bp) to repair double-stranded DNA lesions. This can be through homologous recombination (HR), single-strand annealing (SSA), or break-induced replication (BIR).
- Homologous Recombination (HR):
The exchange of genetic information between two strands of DNA that contain stretches of similar or identical base sequences. HR serves to generate genetic diversity during meiosis and to repair damaged DNA lesions.
- Macroautophagy:
A process in which cells form double-membrane vesicles, known as autophagosomes, that are then fused with lysosomes to degrade cellular component.
- Mesenchymal-Adrenergic lineage:
Mesenchymal lineage comprises of mesenchymal cells (MSCs), which are multipotent cells found in the bone marrow that can differentiate into a variety of cell types. The adrenergic lineage refers to a collection of neurons in the central nervous system that stain for phenylethanolamine-N-methyl-transferase (PNMT), enabling norepinephrine to epinephrine conversion. Interestingly, neuroblastoma tumors consist of heterogenous populations of mesenchymal-adrenergic fate switching.
- Non-Homologous End Joining (NHEJ):
An error prone double strand break (DSB) repair pathway that ligates broken DNA ends without sequence homology.
- Polyploidization:
Cells undergo multiplication of whole chromosomes, which leads to more than two homologous sets of chromosomes.
- R-Loops:
Nucleic acid structures consisting of a DNA-RNA hybrid with a displaced ssDNA filament that arise during transcription. R-loops can be a potential regulator of gene expression, but also a major source of genome instability.
- Shelterin:
A six-subunit protein complex (TRF1, TRF2, POT1, RAP1, TIN2, and TPP1) that protects telomeric ends from being recognized by the DNA damage machinery as double strand breaks.
- Telomerase:
A ribonucleoprotein that extends telomere length by synthesizing telomeric DNA repeats. Human telomerase consists of the subunits: telomerase reverse transcriptase (TERT), telomerase RNA (TR) and Dyskerin (DKC1).
- TERT promoter mutations (TPM):
Hotspot mutations in the TERT promoter, which encodes the catalytic subunit of telomerase, leads to telomerase activation. In familial and sporadic melanomas, two common non-coding mutations are located at −124 and −146 nucleotides upstream from ATG within the hTERT promoter region. The prevalence of hTERT promotor mutations varies according to cancer type and histology.
- Type I interferon response:
Production of Type I interferons (IFNs), a family of monomeric cytokines, induces transcription of pleiotropic genes that play a role in the anti-microbial, anti-tumorigenesis, and inflammatory response.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.de Lange T (2018) Shelterin-Mediated Telomere Protection. Anmi. Rev. Genet 52, 223–247 [DOI] [PubMed] [Google Scholar]
- 2.Griffith JD et al. (1999) Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 [DOI] [PubMed] [Google Scholar]
- 3.Doksani Y et al. (2013) Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell 155, 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Ly D et al. (2018) Telomere Loop Dynamics in Chromosome End Protection. Mol. Cell 71, 510–525.e6 [DOI] [PubMed] [Google Scholar]
- 5.Denchi EL and de Lange T (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 [DOI] [PubMed] [Google Scholar]
- 6.d'Adda di Fagagna F et al. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 [DOI] [PubMed] [Google Scholar]
- 7.Fumagalli M et al. (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol 14, 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodier F et al. (2009) Persistent DNA damage signalling triggers senescence- associated inflammatory cytokine secretion. Nat. Cell Biol 11, 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi MT et al. (2012) A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat. Struct. Mol. Biol 19, 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi MT et al. (2015) Cell death during crisis is mediated by mitotic telomere deprotection. Nature 522, 492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davoli T et al. (2010) Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 141, 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maciejowski J et al. (2015) Chromothripsis and Kataegis Induced by Telomere 12 Crisis. Cell 163, 1641–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassour J et al. (2019) Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim NW et al. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 15.Cristofari G and Lingner J (2006) Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 25, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen SB et al. (2007) Protein composition of catalytically active human telomerase from immortal cells. Science 315, 1850–1853 [DOI] [PubMed] [Google Scholar]
- 17.Akıncılar SC et al. (2016) Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov 6, 1276–1291 [DOI] [PubMed] [Google Scholar]
- 18.Chiba K et al. (2017) Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 357, 1416–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peifer M et al. (2015) Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526, 700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan TM et al. (1997) Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med 3, 1271–1274 [DOI] [PubMed] [Google Scholar]
- 21.Heaphy CM et al. (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333, 425–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaphy CM et al. (2011) Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol 179, 1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartzentruber J et al. (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 [DOI] [PubMed] [Google Scholar]
- 24.Mackay A et al. (2017) Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 32, 520–537.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovejoy CA et al. (2012) Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 8, e1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason-Osann E et al. (2018) Identification of a novel gene fusion in ALT positive osteosarcoma. Oncotarget 9, 32868–32880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law MJ et al. (2010) ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell 143, 367–378 [DOI] [PubMed] [Google Scholar]
- 28.Goldberg AD et al. (2010) Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramamoorthy M and Smith S (2015) Loss of ATRX Suppresses Resolution of Telomere Cohesion to Control Recombination in ALT Cancer Cells. Cancer Cell 28, 357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwase S et al. (2011) ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat. Struct. Mol. Biol 18, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis PW et al. (2013) Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturm D et al. (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22, 425–437 [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee J et al. (2018) Mutant IDH1 Cooperates with ATRX Loss to Drive the Alternative Lengthening of Telomere Phenotype in Glioma. Cancer Res. 78, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang L et al. (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Núñez FJ et al. (2019) IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci Transl Med 11, eaaq1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang FTM et al. (2015) CHK1-driven histone H3.3 serine 31 phosphorylation is important for chromatin maintenance and cell survival in human ALT cancer cells. Nucleic Acids Res. 43, 2603–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Episkopou H et al. (2014) Alternative Lengthening of Telomeres is characterized by reduced compaction of telomeric chromatin. Nucleic Acids Res. 42, 4391–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J et al. (2019) The macroH2A1.2 histone variant links ATRX loss to alternative telomere lengthening. Nat. Struct. Mol. Biol 26, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cesare AJ et al. (2009) Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat. Struct. Mol. Biol 16, 1244–1251 [DOI] [PubMed] [Google Scholar]
- 40.Clynes D et al. (2015) Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun 6, 7538–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y-A et al. (2017) Extrachromosomal telomere repeat DNA is linked to ALT development via cGAS-STING DNA sensing pathway. Nat. Struct. Mol. Biol 24, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 42.Lundblad V and Szostak JW (1989) A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 [DOI] [PubMed] [Google Scholar]
- 43.Teng SC and Zakian VA (1999) Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol. Cell. Biol 19, 8083–8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundblad V and Blackburn EH (1993) An alternative pathway for yeast telomere maintenance rescues estl- senescence. Cell 73, 347–360 [DOI] [PubMed] [Google Scholar]
- 45.Dunham MA et al. (2000) Telomere maintenance by recombination in human cells. Nat. Genet 26, 447–450 [DOI] [PubMed] [Google Scholar]
- 46.Déjardin J and Kingston RE (2009) Purification of proteins associated with specific genomic Loci. Cell 136, 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García-Expósito L et al. (2016) Proteomic Profiling Reveals a Specific Role for Translesion DNA Polymerase η in the Alternative Lengthening of Telomeres. Cell Rep 17, 1858–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grudic A et al. (2007) Replication protein A prevents accumulation of single-stranded telomeric DNA in cells that use alternative lengthening of telomeres. Nucleic Acids Res. 35, 7267–7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu G et al. (2003) Assembly of functional ALT-associated promyelocytic leukemia bodies requires Nijmegen Breakage Syndrome 1. Cancer Res. 63, 2589–2595 [PubMed] [Google Scholar]
- 50.Acharya S et al. (2014) Association of BLM and BRCA1 during Telomere Maintenance in ALT Cells. PLoS ONE 9, e 103819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan X et al. (2017) FANCM, BRCA1, and BLM cooperatively resolve the replication stress at the ALT telomeres. Proc. Natl. Acad. Sci. U.S.A 114, E5940–E5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barroso-González J et al. (2019) RAD51AP1 Is an Essential Mediator of Alternative Lengthening of Telomeres. Mol. Cell 76, 11–26.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J-M et al. (2019) Alternative Lengthening of Telomeres through Two Distinct Break-Induced Replication Pathways. Cell Rep 26, 955–968.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min J et al. (2017) Alternative Lengthening of Telomeres Mediated by Mitotic DNA Synthesis Engages Break-Induced Replication Processes. Mol. Cell. Biol 37, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma P et al. (2019) RAD52 and SLX4 act nonepistatically to ensure telomere stability during alternative telomere lengthening. Gems Dev. 33, 221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeager TR et al. (1999) Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 59, 4175–4179 [PubMed] [Google Scholar]
- 57.Henson JD et al. (2009) DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat. Biotechnol 27, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 58.Londoño-Vallejo JA et al. (2004) Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 64, 2324–2327 [DOI] [PubMed] [Google Scholar]
- 59.Cho NW et al. (2014) Interchromosomal homology searches drive directional ALT telomere movement and synapsis. Cell 159, 108–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dilley RL et al. (2016) Break-induced telomere synthesis underlies alternative telomere maintenance. Nature 539, 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roumelioti F-M et al. (2016) Alternative lengthening of human telomeres is a conservative DNA replication process with features of break-induced replication. EMBO Rep. 17, 1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeman MK and Cimprich KA (2014) Causes and consequences of replication stress. Nat Cell Biol. 16, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox KE et al. (2016) SMARCAL1 Resolves Replication Stress at ALT Telomeres. Cell Rep 14, 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva B et al. (2019) FANCM limits ALT activity by restricting telomeric replication stress induced by deregulated BLM and R-loops. Nat Commim 10, 2253–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu R et al. (2019) The FANCM-BLM-TOP3A-RMI complex suppresses alternative lengthening of telomeres (ALT). Nat Commim 10, 2252–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arora R et al. (2014) RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commim 5, 5220–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrank BR et al. (2018) Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature 559, 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potts PR and Yu H (2007) The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat. Struct. Mol. Biol 14, 581–590 [DOI] [PubMed] [Google Scholar]
- 69.Dilley RL and Greenberg RA (2015) ALTernative Telomere Maintenance and Cancer. Trends Cancer 1, 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bizard AH and Hickson ID (2014) The dissolution of double Holliday junctions. Cold Spring Harb Perspect Biol 6, a016477–a016477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhattacharyya S et al. (2009) Telomerase-associated protein 1, HSP90, and topoisomerase IIalpha associate directly with the BLM helicase in immortalized cells using ALT and modulate its helicase activity using telomeric DNA substrates. J. Biol. Chem 284, 14966–14977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sobinoff AP et al. (2017) BLM and SLX4 play opposing roles in recombination- dependent replication at human telomeres. EMBO J. 36, 2907–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Min J et al. (2019) Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Gems Dev. 33, 814–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan Z et al. (2019) Rad52 Restrains Resection at DNA Double-Strand Break Ends in Yeast. Mol. Cell DOI: 10.1016/j.molcel.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malacaria E et al. (2019) Rad52 prevents excessive replication fork reversal and protects from nascent strand degradation. Nat Commun 10, 1412–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lydeard JR et al. (2010) Break-induced replication requires all essential DNA replication factors except those specific for pre-RC assembly. Gems Dev. 24, 1133–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhowmick R et al. (2016) RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Mol. Cell 64, 1117–1126 [DOI] [PubMed] [Google Scholar]
- 78.Sotiriou SK et al. (2016) Mammalian RAD52 Functions in Break-Induced Replication Repair of Collapsed DNA Replication Forks. Mol. Cell 64, 1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Özer Ö et al. (2018) Human cancer cells utilize mitotic DNA synthesis to resist replication stress at telomeres regardless of their telomere maintenance mechanism. Oncotarget 9, 15836–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West SC et al. (2015) Resolution of Recombination Intermediates: Mechanisms and Regulation. Cold Spring Harb. Symp. Onant. Biol 80, 103–109 [DOI] [PubMed] [Google Scholar]
- 81.Sarkar J et al. (2015) SLX4 contributes to telomere preservation and regulated processing of telomeric joint molecule intermediates. Nucleic Acids Res. 43, 5912–5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson JSJ et al. (2013) Localization-dependent and -independent roles of SLX4 in regulating telomeres. Cell Rep 4, 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu M et al. (2019) Nuclear receptors regulate alternative lengthening of telomeres through a novel noncanonical FANCD2 pathway. Sci Adv 5, eaax6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minocherhomji S et al. (2015) Replication stress activates DNA repair synthesis in mitosis. Nature 528, 286–290 [DOI] [PubMed] [Google Scholar]
- 85.Root H et al. (2016) FANCD2 limits BLM-dependent telomere instability in the alternative lengthening of telomeres pathway. Hum. Mol. Genet 25, 3255–3268 [DOI] [PubMed] [Google Scholar]
- 86.Fan Q et al. (2009) A role for monoubiquitinated FANCD2 at telomeres in ALT cells. Nucleic Acids Res. 37, 1740–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panier S et al. (2019) SLX4IP Antagonizes Promiscuous BLM Activity during ALT Maintenance. Mol. Cell 76, 27–43.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flynn RL et al. (2015) Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 347, 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deeg KI et al. (2016) Cancer Cells with Alternative Lengthening of Telomeres Do Not Display a General Hypersensitivity to ATR Inhibition. Front Oncol 6, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen GH et al. (2013) A small molecule inhibitor of the BLM helicase modulates chromosome stability in human cells. Chem. Biol 20, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan Z et al. (2015) Telomere G-Quadruplex as a Potential Target to Accelerate Telomere Shortening by Expanding the Incomplete End-Replication of Telomere DNA. Curr Top Med Chem 15, 1940–1946 [DOI] [PubMed] [Google Scholar]
- 92.Zheng X-H et al. (2017) A Cisplatin Derivative Tetra-Pt(bpy) as an Oncotherapeutic Agent for Targeting ALT Cancer. J. Natl. Cancer Inst 109, 331. [DOI] [PubMed] [Google Scholar]
- 93.Han M et al. (2019) Synthetic lethality of cytolytic HSV-1 in cancer cells with ATRX and PML deficiency. J. Cell Sci 132, jcs222349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Episkopou H et al. (2019) TSPYL5 Depletion Induces Specific Death of ALT Cells through USP7-Dependent Proteasomal Degradation of POT1. Mol Cell 75, 469–482.e6 [DOI] [PubMed] [Google Scholar]
- 95.Henson JD et al. (2002) Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 [DOI] [PubMed] [Google Scholar]
- 96.Venturini L et al. (2008) ALT-associated promyelocytic leukaemia body (APB) detection as a reproducible tool to assess alternative lengthening of telomere stability in liposarcomas. J. Pathol 214, 410–414 [DOI] [PubMed] [Google Scholar]
- 97.Draskovic I et al. (2009) Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc. Natl Acad. Sci. U.S.A 106, 15726–15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luca P et al. (2019) BRCA2 Deletion Induces Alternative Lengthening of Telomeres in Telomerase Positive Colon Cancer Cells. Genes (Basel) 10, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grobelny JV et al. (2000) ALT-associated PML bodies are present in viable cells and are enriched in cells in the G(2)/M phase of the cell cycle. J. Cell Sci 113, 4577–4585 [DOI] [PubMed] [Google Scholar]
- 100.Fasching CL et al. (2007) DNA damage induces alternative lengthening of telomeres (ALT) associated promyelocytic leukemia bodies that preferentially associate with linear telomeric DNA. Cancer Res. 67, 7072–7077 [DOI] [PubMed] [Google Scholar]
- 101.Chung I et al. (2011) De novo assembly of a PML nuclear subcompartment occurs through multiple pathways and induces telomere elongation. J. Cell. Sci 124, 3603–3618 [DOI] [PubMed] [Google Scholar]
- 102.Psakhye I and Jentsch S (2012) Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151, 807–820 [DOI] [PubMed] [Google Scholar]
- 103.Dellaire G and Bazett-Jones DP (2004) PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26, 963–977 [DOI] [PubMed] [Google Scholar]
- 104.Chandra T and Narita M (2013) High-order chromatin structure and the epigenome in SAHFs. Nucleus 4, 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osterwald S et al. (2015) PML induces compaction, TRF2 depletion and DNA damage signaling at telomeres and promotes their alternative lengthening. J. Cell. Sci 128, 1887–1900 [DOI] [PubMed] [Google Scholar]
- 106.Lydeard JR et al. (2007) Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 448, 820–823 [DOI] [PubMed] [Google Scholar]
- 107.Saini N et al. (2013) Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 502, 389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Natarajan S and McEachern MJ (2002) Recombinational telomere elongation promoted by DNA circles. Mol. Cell. Biol 22, 4512–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deem A et al. (2011) Break-induced replication is highly inaccurate. PLoS Biol. 9,el000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sakofsky CJ et al. (2014) Break-induced replication is a source of mutation clusters underlying kataegis. Cell Rep 7, 1640–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee M et al. (2014) Telomere extension by telomerase and ALT generates variant repeats by mechanistically distinct processes. Nucleic Acids Res. 42, 1733–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conomos D et al. (2012) Variant repeats are interspersed throughout the telomeres and recruit nuclear receptors in ALT cells. J. Cell Biol 199, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conomos D et al. (2014) NuRD-ZNF827 recruitment to telomeres creates a molecular scaffold for homologous recombination. Nat. Struct. Mol. Biol 21, 760–770 [DOI] [PubMed] [Google Scholar]
- 114.Marzec P et al. (2015) Nuclear-receptor-mediated telomere insertion leads to genome instability in ALT cancers. Cell 160, 913–927 [DOI] [PubMed] [Google Scholar]