Abstract
The host cellular environment is a key determinant of pathogen infectivity. Viral gene expression and viral particle production of glucose-6-phosphate dehydrogenase (G6PD)–deficient and G6PD-knockdown cells were much higher than their counterparts when human coronavirus (HCoV) 229E was applied at 0.1 multiplicity of infection. These phenomena were correlated with increased oxidant production. Accordingly, ectopic expression of G6PD in G6PD-deficient cells or addition of antioxidant (such as α-lipoic acid) to G6PD-knockdown cells attenuated the increased susceptibility to HCoV 229E infection. All experimental data indicated that oxidative stress in host cells is an important factor in HCoV 229E infectivity
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency worldwide [1]. Accumulating evidence indicates that G6PD deficiency affects cells other than those of erythrocytes. For example, G6PD deficiency is now known to affect oocyte development and/or survival as well as growth and senescence of human foreskin fibroblasts (HFFs). Moreover, human G6PD-deficient neutrophils exhibit impaired production of nitric oxide, superoxide, and hydrogen peroxide, which may explain the impaired bactericidal effect of these cells [2]. Taken together, these findings indicate that G6PD deficiency causes abnormal cellular redox, thus affecting cells other than red cells. Oxidative stress is known to affect viral proliferation and virulence [3]; however, the effect of G6PD deficiency on viral infectivity has not been thoroughly studied
In addition to being major sources of intracellular oxidants, pulmonary cells are exposed to 8000 L of oxygen-rich air daily as well as toxic particles [4]. Recent studies indicate that diesel exhaust exacerbates influenza virus infections in respiratory epithelial cells [5]. Human coronavirus (HCoV) 229E, a common pathogen for respiratory tract infection, is a large, enveloped RNA virus and has a high affinity with airway cells. Recent identification of a novel coronavirus as a causative agent of severe acute respiratory syndrome (SARS) has received substantial clinical attention. Because pulmonary cells are under high oxidative stress and because HCoV 229E is an interesting viral pathogen affecting pulmonary cells, it would be of interest to investigate how oxidative stress affects HCoV 229E infection of airway cells
MethodsDulbecco’s modified Eagle medium (DMEM), trypsin, penicillin, streptomycin, Lipofectamine 2000 transfection reagents, and dichlorofluorescin (DCF) diacetate were purchased from Invitrogen. The G6PD antibody was acquired from Genesis Biotech (Taiwan). Anti-actin and anti-CD13 antibodies were purchased from Santa Cruz Biotechnologies, and antibiotic G418 sulfate and α-lipoic acid were purchased from Promega
G6PD-deficient (HFF1), normal (HFF3), and G6PD-overexpressing fibroblasts (LGIN and LKGIN) were prepared as described elsewhere [6]. Lung carcinoma cells (A549) and human lung fibroblasts (MRC-5) were obtained from the American Type Culture Collection. All cells were cultured in DMEM supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100U/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 with or without 300 μg/mL G418, depending on whether or not they were transfected
For G6PD-RNAi plasmids, the complementary oligonucleotides G6PD-143S (5′-ACACACATATTCATCATCGAAGCTT-GGATGATGAATATGTGTGT-3′) and G6PD–143AS (5′-GGA-TACACACATATTCATCATCCAAGCTTCGATGATGAATA-TGTGTGT-3′) were annealed and ligated into pTOPO-U6 to generate pTOPO G6PD-143. The plasmid was tested and then removed to insert into the pCI-neo mammalian expression vector, as described elsewhere [7]
For the virus and plaque assay, HCoV 229E (provided by M. M. Lai, Academia Sinica) was propagated in MRC-5 cells as described elsewhere [8]. The viral titer was calculated by plaque formation on A549 cells
G6PD activity and Western blot analysis was done as described elsewhere [6]. Cell viability (MTT assay) was determined as described elsewhere [9], using the following formula: (A490 − A650) treated cells × 100/(A490 − A650) control cells, where A indicates absorbance. To measure cell-specific receptors for HCoV 229E, cell-surface membrane receptors for CD13 were quantitated by flow cytometry
Total RNA from HCoV-infected cells was isolated by use of the RNeasy Mini Kit (Qiagen), and reverse-transcription polymerase chain reaction (RT-PCR) was performed using SuperScript III reverse transcriptase (Invitrogen), in accordance with the manufacturer’s instructions. To quantify the DNA fragment, 2 HCoV 229E–specific oligonucleotide primers of nucleocapsid were used: 5′-AGGCGCAAGAATTCAGAACCAGAG-3′ and 5′-AGCAGGACTCTGATTACGAGAAAG-3′. Quantitative PCR was performed as described elsewhere [10]
Cellular NADPH was quantified by high-performance liquid chromatography, as described elsewhere [11]. Cells (4×105) were acidified with 1% (wt/vol) metaphosphoric acid and then filtered, and intracellular glutathione (GSH) was chromatographed, both as described elsewhere [12]. Reactive oxygen species (ROS) were detected by monitoring DCF fluorescence, as described elsewhere [13]
Statistical differences were analyzed by Student’s t test. Differences were considered statistically significant at P<.05
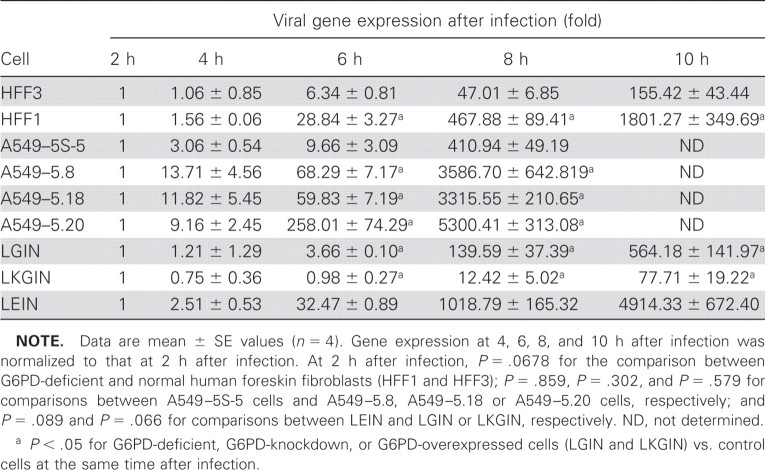
ResultsTo investigate whether G6PD deficiency affects viral infection, G6PD-deficient fibroblasts (HFF1) and normal fibroblasts (HFF3) were subjected to HCoV 229E infection at a low viral inoculum (MOI of 0.1); viral gene expression was determined by quantitative PCR. Because the actual nucleocapsid gene expression did not differ significantly between HFF1 and HFF3 at 2 h after infection, gene expression at 2 h after infection was normalized to 1. In HFF1, viral gene expression at 4, 6, 8, and 10 h after infection was 2-, 29-, 468-, and 1801-fold higher than at 2 h after infection, respectively. However, in HFF3, viral gene expression at 4, 6, 8, and 10 h after infection was 1-, 6-, 47-, and 155-fold higher than at 2 h after infection, respectively (table 1). To determine whether the enhanced viral gene expression of G6PD-deficient fibroblasts after HCoV 229E infection was cell specific, G6PD-knockdown cells derived from human A549 lung epithelial cells were used. Three stable G6PD-knockdown cell lines—A549–5.8, A549–5.18, and A549–5.20—were selected, as was a control cell line transfected with pCI-neo vector only, A549–5S-5 (figure 1A). Likewise, viral gene expression was similar in G6PD-knockdown A549 cells and revealed a much higher increase than in control cells (table 1). These data indicate that host cellular G6PD activity modulating viral gene expression and the enhanced susceptibility to HCoV 229E infection in G6PD-deficient fibroblasts is not cell-type specific
Table 1.
Increased viral gene (nucleocapsid) expression in glucose-6-phosphate dehydrogenase (G6PD)–deficient fibroblasts (HFF1 and LEIN) and in G6PD-knockdown cells (A549–5.8, A549–5.18, and A549–5.20), compared with normal fibroblasts (HFF3), vector-only controls (A549–5S-5), and G6PD-overexpressed cells (LGIN and LKGIN)
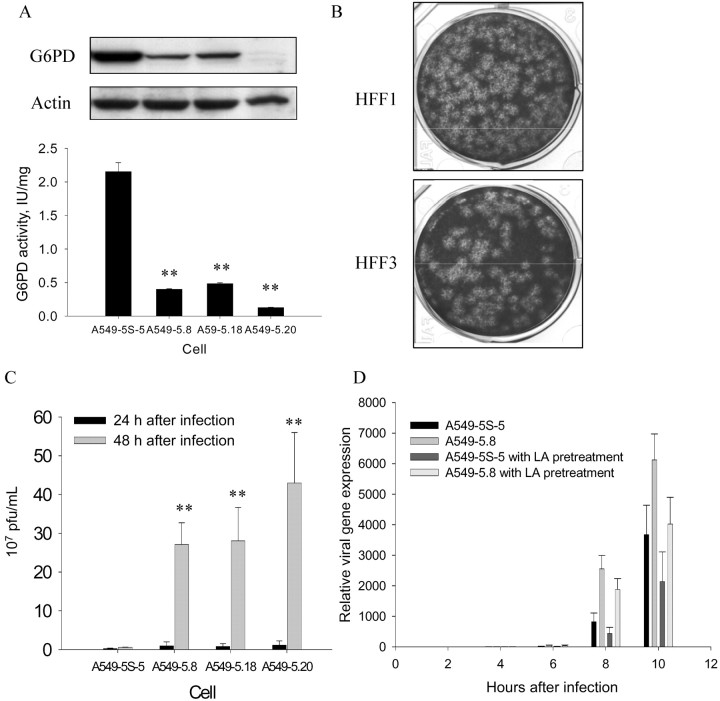
Figure 1.
Elevated viral particle production in glucose-6-phosphate dehydrogenase (G6PD)–deficient cells and protective effect of antioxidants against virus infection at 48 h after infection with human coronavirus (HCoV) 229E. A G6PD activity and Western blot analysis. The indicated A549 vector and G6PD-knockdown cells were harvested for G6PD activity assay and Western blotting of G6PD protein. The activity of G6PD is given in international units per milligram of protein in cell lysate; for infection and plaque assay, an MOI of 0.1 was used to measure virus titer. B and C Viral particle production. Viral particle production was visualized by plaque formation (B) and was higher in G6PD-deficient human foreskin fibroblasts (HFF1) at 24 h after infection than in normal fibroblasts (HFF3). Similar data (C) indicated that viral particle production was higher in G6PD-knockdown A549 cells (A549–5.8, A549–5.18, and A549–5.20) than in control cells (A549–5S-5).Pub _bookmark Command="[Quick Mark]"> D Viral gene (nucleocapsid) expression. Viral gene (nucleocapsid) expression in cells pretreated with an antioxidant, lipoic acid (LA), and in control cells was determined by quantitative polymerase chain reaction at 2, 4, 6, 8, and 10 h after infection with HCoV 229E. Data are mean ± SE values (n=4). **P<.01 for HFF1 vs. HFF3 and for vector-only A549–5S-5 vs. G6PD-knockdown A549–5.8, A549–5.18, or A549–5.20 epithelial cells infected with HCoV 229E) at an MOI of 0.1
Next, the effect of G6PD deficiency on production of viral particles was determined. In this experiment, viral particles were quantified by plaque assay at 24 and 48 h after infection in G6PD-deficient cells and control cells. The number of viral particles from HFF1 was found to be 3-fold higher than that in HFF3 at an MOI of 0.1 at 24 h after infection (figure 1B) and even at 48 h after infection. Similar findings were also observed in G6PD-knockdown A549 cells (figure 1C). These data indicate that host cellular G6PD activity modulates viral particle production
An MTT assay to investigate how enhanced viral production affects cellular viability revealed that cell viability was significantly lower (8%) in HFF1 than in HFF3 at an MOI of 0.1 at 48 h after infection (P<.05), and the difference increased to 18% at 72 h after infection (data not shown). Similarly, G6PD-knockdown epithelial cells exhibited a significantly higher rate of cell death than control cells at 48 and 72 h after infection (data not shown). These data demonstrate that both G6PD-deficient fibroblasts and G6PD-knockdown A549 cells are more susceptible to HCoV 229E–induced cell death
HCoV 229E requires a receptor (CD13) to infect cells, and this study further investigated whether enhanced viral infection in G6PD-deficient cells is attributable to elevated CD13 expression on these cells. Flow cytometry and Western blot analysis revealed that G6PD-deficient fibroblasts and G6PD-knockdown epithelial cells did not differ significantly in CD13 expression compared with their controls (data not shown). These data indicate that enhanced susceptibility to HCoV 229E infection in G6PD-deficient and G6PD-knockdown cells is not attributable to increased expression of viral receptor CD13
Because G6PD-deficient cells were favored for viral replication, the redox status of G6PD-deficient cell was determined by testing the cellular ROS level and by quantifying cellular NADPH and intracellular GSH levels. In the basal condition, G6PD-deficient fibroblasts (HFF1) had lower levels of NADPH (314.7 nmol/g of protein) and intracellular GSH (28.0 μmol/g of protein) than control HFF3 cells (403.4 nmol/g of protein and 35.7 μmol/g of protein, respectively). This phenomenon was also observed in G6PD-knockdown A549–5.8 cells (511.2 nmol/g of protein for NADPH and 72.7 μmol/g of protein for GSH) compared with control A549–5S-5 cells (686.1 nmol/g of protein and 85.7 μmol/g of protein, respectively). All of these data confirmed that G6PD-deficient cells had less reducing power than control cells. Moreover, DCF staining revealed significantly higher ROS production and significantly lower GSH levels in virus-infected G6PD-knockdown epithelial cells than in control cells (GSH, 49.5 vs. 66.3 μmol/g of protein). Taken together, the diminished reducing power and enhanced ROS production indicated that viral infection caused more oxidative stress in G6PD-knockdown epithelial cells than in control cells
To further confirm that enhanced susceptibility to HCoV 229E infection is modulated by cellular G6PD activity, G6PD-overexpressing fibroblasts (LGIN and LKGIN) were used to test the effects of G6PD replenishment on HCoV 229E–induced cell death and viral gene expression. The expression of CD13 in LGIN and LKGIN did not differ significantly from that in the control LEIN (data not shown). However, LGIN and LKGIN showed less susceptibility to HCoV 229E–induced cell death (12% vs. 18%) than LEIN at 72 h after infection (data not shown). Subsequent determination of viral gene expression revealed significantly less expression in LGIN and LKGIN than in LEIN control cells (table 1). Taken together, these data provide compelling evidence that cellular G6PD activity modulates cellular susceptibility to viral infection
To evaluate whether ectopic application of antioxidant protects G6PD-knockdown cells from viral infection, α-lipoic acid, a potent antioxidant, was applied in culture medium for 5 h before virus infection. The viral gene expression in G6PD-knockdown cells pretreated with antioxidant was lower than that in untreated cells (figure 1D). Additionally, A549 cells pretreated with antioxidant were 23% less susceptible to virus-induced cell death than control cells 48 h after infection at an MOI of 0.1 (data not shown). Taken together, these data further demonstrate the association between susceptibility to HCoV 229E infection and cellular redox status
DiscussionAlthough G6PD status reportedly affects the clinical course of viral diseases such as AIDS [14] and hepatitis [15], this study has demonstrated, for the first time, that oxidative stress increases susceptibility of G6PD-deficient cells to viral infection. Additionally, these findings demonstrate that enhanced viral infection in G6PD-deficient cells is ameliorated by antioxidant agents, such as lipoic acid. These data provide further evidence that host redox status is important in viral infectivity
Increased viral infection in G6PD-deficient cells may be partly attributable to increased viral receptors in these cells or increased production of viral particles. Because G6PD-deficient cells do not express higher CD13 on the cellular surface than control cells, it is unlikely that enhanced susceptibility of G6PD-deficient cells to HCoV 229E infection is due to an increase in human receptor CD13 expression on these cells. Conversely, enhanced production of viral particles in G6PD-deficient cells was clearly demonstrated by increased plaque formation (figure 1) and elevated viral gene expression (table 1) after virus infection. Thus, G6PD-deficiency provides a more suitable milieu for viral replication than that provided by non-G6PD-deficient cells
One condition favoring viral replication is high oxidative stress. More ROS is produced by G6PD-knockdown cells than their normal counterparts, and cellular GSH content was lower than that of control cells during viral infection. The low GSH content in G6PD-knockdown cells has been associated with low NADPH levels in these cells. Because these changes in redox status of G6PD-knockdown cells are associated with increased viral gene expression (table 1), these findings are consistent with the postulate that increased oxidative stress in G6PD-knockdown cells promotes viral gene expression
Because increased susceptibility of G6PD-knockdown cells to HCoV 229E infection correlates with ROS production, antioxidant pretreatment should attenuate the phenomena. Indeed, the enhanced susceptibility of these cells to viral infection can be attenuated by pretreating G6PD-knockdown cells with lipoic acid (figure 1D). The current finding that lipoic acid diminishes ROS production in G6PD-knockdown cells supports the postulate that oxidative stress contributes to the enhanced susceptibility of these cells to HCoV 229E infection. Moreover, this finding also suggests that antioxidant treatment may protect G6PD-deficient subjects from viral infection
Acknowledgments
The RNAi core laboratory of Chang Gung University is appreciated for technical support in constructing the RNAi plasmid. Ted Knoy is appreciated for his editorial assistance
Footnotes
Potential conflicts of interest: none reported
Presented in part: Experimental Biology 2007, Washington, DC, 28 April–2 May 2007 (abstract LB323)
Financial support: Chang Gung University (grant CMRPD140041); National Science Council of Taiwan (grant NSC94–2320-B182–041); Ministry of Education of Taiwan (grant EMRPD150241)
References
- 1.Ho HY, Cheng ML, Chiu DT. Glucose-6-phosphate dehydrogenase—from oxidative stress to cellular functions and degenerative disease. Redox Rep. 2007;12:109–18. doi: 10.1179/135100007X200209. [DOI] [PubMed] [Google Scholar]
- 2.Tsai KJ, Hung IJ, Chow CK, Stern A, Chao SS, Chiu DT. Impaired production of nitric oxide, superoxide, and hydrogen peroxide in glucose 6-phosphate-dehydrogenase-deficient granulocyte. FEBS Lett. 1998;436:411–4. doi: 10.1016/s0014-5793(98)01174-0. [DOI] [PubMed] [Google Scholar]
- 3.Friel H, Lederman H. A nutritional supplement formula for influenza A (H5N1) infection in human. Med Hypotheses. 2006;67:578–87. doi: 10.1016/j.mehy.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis: conclusions from the CF Antioxidant Workshop, Bethesda, Maryland, November 11–12, 200. Free Radic Biol Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito T, Okumura H, Tsukue N, Kobayashi T, Honda K, Sekizawa K. Effect of diesel exhaust particles on mRNA expression of viral and bacterial receptors in rat lung epithelial L2 cell. Toxicol Lett. 2006;165:66–70. doi: 10.1016/j.toxlet.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Cheng ML, Ho HY, Wu YH, Chiu DT. Glucose-6-phosphate dehydrogenase-deficient cells show an increased propensity for oxidant-induced senescenc. Free Radic Biol Med. 2004;36:580–91. doi: 10.1016/j.freeradbiomed.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Huang CL, Cheng JC, Liao CH, et al. Disabled-2 is a negative regulator of integrin αIIbβ3-mediated fibrinogen adhesion and cell signalin. J Biol Chem. 2004;279:42279–89. doi: 10.1074/jbc.M402540200. [DOI] [PubMed] [Google Scholar]
- 8.Wentworth DE, Tresnan DB, Turner BC, et al. Cells of human aminopeptidase N (CD13) transgenic mice are infected by human coronavirus-229E in vitro, but not in viv. Virology. 2005;335:185–97. doi: 10.1016/j.virol.2005.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggieri A, Di Trani L, Gatto I, et al. Canine coronavirus induces apoptosis in cultured cell. Vet Microbiol. 2007;121:64–72. doi: 10.1016/j.vetmic.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerimele F, Battle T, Lynch R, et al. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr virus (EBV)-positive versus EBV-negative Burkitt’s lymphom. Proc Natl Acad Sci USA. 2005;102:175–9. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litt MR, Potter JJ, Mezey E, Mitchell MC. Analysis of pyridine dinucleotides in cultured rat hepatocytes by high-performance liquid chromatograph. Anal Biochem. 1989;179:34–6. doi: 10.1016/0003-2697(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 12.Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue sample. Anal Biochem. 1997;247:63–8. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- 13.Ho HY, Cheng ML, Lu FJ, et al. Enhanced oxidative stress and accelerated cellular senescence in glucose-6-phosphate dehydrogenase (G6PD)-deficient human fibroblast. Free Radic Biol Med. 2000;29:156–69. doi: 10.1016/s0891-5849(00)00331-2. [DOI] [PubMed] [Google Scholar]
- 14.Reinke CM, Thomas JK, Graves AH. Apparent hemolysis in an AIDS patient receiving trimethoprim/sulfamethoxazole: case report and literature review. J Pharm Technol. 1996. pp. 256–62. [DOI] [PubMed]
- 15.Gotsman I, Muszkat M. Glucose-6-phosphate dehydrogenase deficiency is associated with increased initial clinical severity of acute viral hepatitis. J Gastroenterol Hepatol. 2001;16:1239–43. doi: 10.1046/j.1440-1746.2001.02611.x. [DOI] [PubMed] [Google Scholar]