Abstract
The novel capsid-binding antiviral pleconaril inhibits in vitro replication of most rhinoviruses and enteroviruses. Oral pleconaril treatment was studied in 2 parallel randomized, double-blind, placebo-controlled trials. Among 1363 picornavirus-infected participants (65%) in the studies combined, the median time to alleviation of illness was 1 day shorter for pleconaril recipients than for placebo recipients (P > .001). Cold symptom scores and frequency of picornavirus cultured from nasal mucus specimens were lower among pleconaril recipients by day 2 of treatment. No treatment effects were seen in those without picornavirus infection. Pleconaril was associated with a higher incidence of nausea (6% vs. 4%) and diarrhea (9% vs. 7%) and with small increases in mean serum cholesterol levels and platelet counts, compared with baseline measurements. A subsequent 6-week prophylaxis study found that pleconaril induces cytochrome P-450 3A enzymes, which metabolize a variety of drugs, including ethinyl estradiol. Early pleconaril treatment was well tolerated and significantly reduced the duration and severity of colds due to picornaviruses in adults.
The majority of common colds are due to picornaviruses, principally rhinoviruses (>100 serotypes) and, less often, enteroviruses (≤67 serotypes), which together cause ∼50% of colds annually [1]. The incidence of colds due to picornaviruses increases to 60%–80% during peak months in the fall and spring in the Northern Hemisphere [1, 2]. No antiviral therapy of proven value for colds due to picornaviruses is currently available, and prior studies of investigational antivirals did not show therapeutic benefit for established colds [3, 4]. The pathogenesis of cold symptoms is not fully understood [5], and the importance of ongoing viral replication to symptom causation remains uncertain.
Pleconaril is a novel, orally absorbed viral capsid—function inhibitor that specifically inhibits the replication of ∼90% of rhinoviruses and >99% of enteroviruses [6–8]. In experimentally induced human coxsackievirus A21 infections, oral pleconaril significantly reduced viral shedding and illness measures [9]. More recently, retrospective analysis of 2 phase II randomized, double-blind, placebo-controlled studies found that pleconaril treatment provided clinical benefit for colds due to picornaviruses in previously healthy adults [10]. Consequently, 2 large, randomized, double-blind, placebo-controlled, multicenter trials were conducted to evaluate the efficacy and safety of oral pleconaril for treatment of naturally occurring colds presumptively due to picornaviruses in adults.
Methods
Participants. Participants in both studies were otherwise healthy adults (age, ≥18 years) with self-diagnosed colds who were enrolled within 24 h after symptom onset. Participants had moderate to severe rhinorrhea and ≤1 other respiratory symptom (nasal congestion, cough, or sore throat) that was rated moderate or greater in severity.
Subjects were excluded if they had fever (oral temperature, >37.8°C), if they had allergic rhinitis that had been treated within the previous 2 weeks, if they had received asthma treatment within the previous 2 months, or if they had chronic cough, any known immunodeficiency, or an underlying medical condition that would confound the study results. Pregnant or nursing women were excluded, and urine pregnancy tests were done at entry. Smokers were allowed.
The institutional review board of each participating site approved the protocol, and written informed consent was obtained from each participant at the time of enrollment into the study. Participants were compensated for participation.
Study design and drug administration. Two prospective, multicenter, randomized, double-blind trials of identical design were conducted from August through November 2000; each enrolled participants from geographically diverse areas of the United States (150 sites) and Canada (47 sites). Participants were randomized in a 1 : 1 ratio to receive either pleconaril at 400 mg (two 200-mg tablets; Picovir; ViroPharma) or matching placebo tablets 3 times per day for 5 days. To enhance oral absorption, participants were instructed to take the study medication within 15 min after a meal or snack. Randomization was stratified by the subject's preenrollment smoking status and preenrollment use of cold symptom–relief medication to ensure that these subjects were balanced between the treatment arms. Acetaminophen and dextromethorphan were provided for disabling symptoms, because these agents were unlikely to affect the prominent nasopharyngeal symptoms of colds. The concomitant use of prescription and other over-the-counter cold symptom–relief medications was not permitted.
Clinical monitoring. Participants were evaluated at enrollment and again on study days 3, 6 (end of treatment), and 18 (end of the study) for clinical assessment and obtainment of samples for laboratory testing. Study personnel contacted participants every other day by telephone until their cold had resolved or through day 18.
Participants recorded the severity of 6 individual cold symptoms (rhinorrhea, nasal congestion, sore throat, cough, malaise, and myalgia) in study diaries twice daily, grading each as “not present,” “mild” (noticeable but not bothersome), “moderate” (bothersome), or “severe” (limiting usual activities), which were scored as 0, 1, 2, and 3, respectively, for data analysis. Once per day, subjects also recorded data on the number of facial tissues used, sleep disturbance, impairment of daily activity as a result of cold symptoms, and use of cold symptom–relief medications or other medications for any reason. Safety laboratory studies (hematological study, clinical chemistry, and urinalysis) and physical assessments were done at enrollment and on study day 6.
Virology assessments. Nasal mucus samples were obtained for virological studies at baseline and on study days 3 and 6. Subjects were asked to blow nasal mucus directly onto plastic wrap; mucus was induced, if necessary [11]. The sample was transferred into a tube containing viral transport medium (Starswab Multitrans Collection and Transport System; Starplex Scientific) and shipped for storage at -80°C until assayed.
The presence of picornavirus RNA in nasal mucus samples was identified using a real-time, quantitative RT-PCR assay (TaqMan; Applied Biosystems). The PCR primers and probe used in the TaqMan assay were derived from conserved sequences within the 5′ nontranslated region of sequenced human rhinovirus (HRV) genomes. The forward primer sequence was 5′-GTGAAGAGCC(G/C)C(A/G)TGTGCT-3′, corresponding to nucleotides 414–432 of HRV89. The reverse primer sequence was 5′-GCT(G/C)CAGGGTTAAGGTTAGCC-3′, corresponding to the reverse complement of nucleotides 461–481 of HRV89. The double-labeled fluorescent probe sequence was 5′-(FAM)-TGAGTCCTCCGGCCCCTGAATG-(TAMRA)-3′, corresponding to nucleotides 438–459 of HRV89. In this assay, the lower limit of detection for the virus (HRV1B) used to generate the standard curve was 10 pfu/mL, or 10,550 genome equivalents/mL (211 genome equivalents per reaction).
If all 3 samples obtained from a patient had negative or indeterminate results for this assay, the baseline sample was retested by a modification of an enzyme-linked oligosorbent RT-PCR assay, which detects all prototype rhinoviruses and culturable enteroviruses [12–15]. A patient was considered to be positive for picornavirus infection if nasal mucus specimens tested positive with either RT-PCR assay on any sampling day.
For subjects who had positive RT-PCR results, an aliquot of the baseline mucus sample (200 μL) was submitted for viral culture on monolayers of HeLa-I cells by a previously described technique [16]. If culture of the baseline sample yielded positive results, aliquots of samples obtained on days 3 and 6 were also cultured.
TaqMan assays were performed at ViroMed Biosafety Laboratories (St. Paul, Minnesota), enzyme-linked oligosorbent RT-PCR assays were performed at ViroPharma Incorporated (Exton, Pennsylvania), and viral cultures were performed at the University of Virginia (Charlottesville) or the University of Rochester Medical Center (Rochester, New York). Study personnel at each laboratory were blinded to treatment and sample collection day.
Efficacy end points. The primary efficacy population included any randomized participant with ≤1 nasal mucus sample that tested positive for picornavirus RNA on any sampling day by either quantitative or qualitative RT-PCR methods. The secondary efficacy population included all randomized participants. These participants are referred to as the intent-to-treat infected (ITT-I) and intent-to-treat (ITT) populations, respectively.
The primary end point was the time from initiation of therapy to alleviation of illness, defined as the number of days until complete resolution of rhinorrhea and the other 5 cold symptoms self-assessed as absent or mild for ≤48 h without use of cold symptom–relief medication. Prospectively defined secondary end points were the time to subject-assessed “no cold,” times to complete resolution of individual symptoms, total cold symptom severity scores, tissue counts, proportion of nights with disturbed sleep, duration of cold symptom–relief medication use, and frequency of viral shedding in nasal mucus. Other end points were the time to ≤50% reduction in symptom score and changes in viral RNA levels over time.
Data analysis. The distribution of time to resolution of symptom scores was estimated by the Kaplan-Meier method [17], and the Wilcoxon-Gehan statistic [18] was used to test the difference in median resolution times between treatment groups. These analyses included stratification for smoking status and preenrollment use of cold symptom–relief medication. Combined analyses of both studies also included stratification by study. In these time-to-event analyses, subjects who discontinued the study were included up to the point of the last recorded observation. The distribution of time to ≤50% reduction from baseline in total cold symptom severity score was analyzed in the same manner.
The treatment effect for change from baseline in daily total symptom severity score and total symptom severity score over the 18-day study was analyzed by analysis of covariance, with effects for treatment, study, smoking status, preenrollment use of cold symptom–relief medication, and baseline total symptom severity score. The last observation carried forward was used to impute missing individual symptom severity scores.
Treatment effect for presence of picornavirus by culture and percentage of subjects using cold medications was evaluated using Fisher's exact test. Analysis of variance was used to compare the treatment groups for reduction in virus levels (measured by PCR) from baseline to days 3 and 6, proportion of nights with sleep disturbance, and tissue use.
All study participants who received ≤1 dose of study medication were included in the safety analysis. Adverse events that began or worsened at any time after receipt of the first dose of study drug through 5 days after the last dose were summarized. All analyses were done using SAS statistical software, version 6.12 (SAS Institute) [19], and a 2-sided test at the 5% level was used for all comparisons.
Sample size. Calculations indicated that enrollment of 1000 subjects in each study was required to detect a 25% relative difference between treatment groups in the proportion of picornavirus-infected subjects reaching the primary end point (or an estimated 2-day difference in median time) with 90% power (2-sided test at the 5% level of significance [20]).
Results
Study population. The 2 studies randomized 2096 participants (1046 in the pleconaril group and 1050 in the placebo group); >90% of subjects completed treatment (table 1). The most common reason that subjects did not complete treatment was an adverse event (3% of subjects each in the pleconaril and placebo groups). Overall, 65% of participants were infected with picornavirus, with a narrow range (62%–68%) across treatment groups in each study. The pleconaril and placebo groups were similar at baseline with regard to relevant demographic and illness characteristics (table 2). The mean age of the ITT-I population was 36 years, 69% were female, and 28% were smokers. The median time from symptom onset to receipt of the first dose of study drug was 20 h.
Table 1.
Disposition of subjects in 2 studies of efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses.
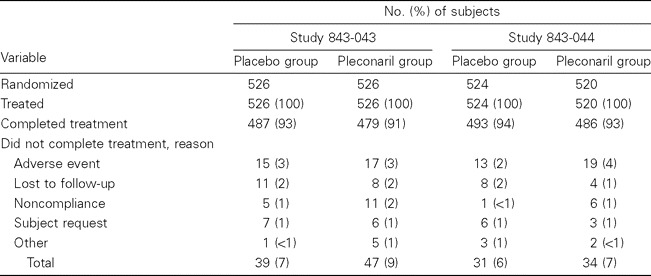
Table 2.
Demographic and clinical characteristics of picornavirus-infected subjects (intent-to-treat infected population) at baseline in 2 studies.

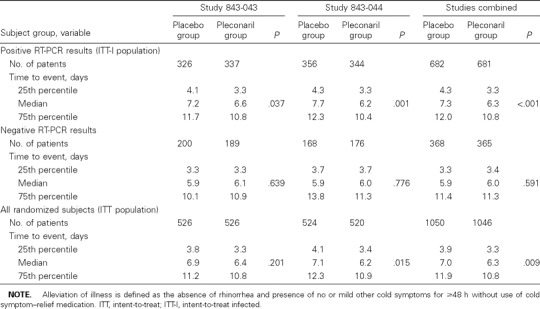
Illness resolution. Among the picornavirus-infected (ITT-I) population, the time to reach the primary end point of illness alleviation was significantly shorter among pleconaril-treated subjects than among placebo recipients in each study (figure 1). Overall, pleconaril-treated patients experienced a 1-day reduction in the median duration of illness, compared with placebo-treated subjects (P > .001; table 3). The treatment effect was similar for subjects who had positive results of viral cultures at baseline (combined median time to illness alleviation, 7.9 days for the placebo group and 6.8 days for the pleconaril group; P = .002) and for those with negative results of viral culture at baseline (7.0 days for the placebo group and 6.0 days for the pleconaril group; P = .048). No treatment effect was observed among participants without detectable picornavirus infection (table 3). In the ITT population, the magnitude of the treatment benefit was smaller than that observed among ITT-I subjects but favored pleconaril in both studies.
Figure 1.
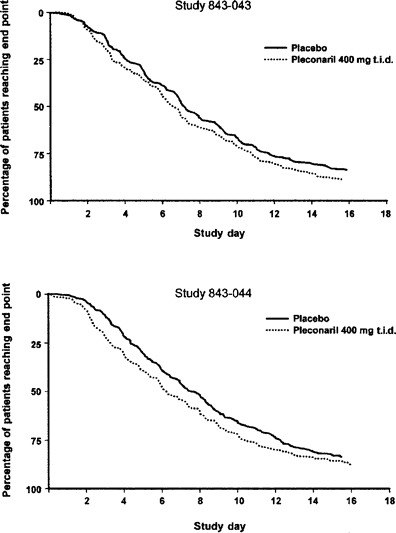
Kaplan-Meier analyses of time to alleviation of illness (primary efficacy end point) among picornavirus-infected subjects. Marks on the x axis represent end of study day.
Table 3.
Data on the primary efficacy end point (number of days to alleviation of illness) in 2 studies of oral pleconaril for treatment of colds due to picornaviruses.
Among ITT-I subjects, the self-assessed times to “no cold” and to resolution of each individual cold symptom were reduced in pleconaril recipients (table 4). The total cold symptom severity score was reduced by 19% over the duration of the study for pleconaril recipients (table 4), who also experienced significant reductions from baseline in symptom severity scores by day 2 of treatment, compared with placebo recipients (figure 2). A reduction in total symptom severity score of ≤50% occurred earlier among pleconaril recipients than among placebo recipients (combined medians of 2.9 and 3.9 days, respectively; P > .001). Pleconaril treatment was associated with fewer tissues used for nose blowing (24% reduction), fewer nights of sleep disturbance (1 night reduction), and fewer days of cold symptom–relief medication use (table 4).
Table 4.
Data for secondary end points used as efficacy measures in 2 studies of oral pleconaril for treatment of colds due to picornaviruses among picornavirus-infected subjects (intent-to-treat infected subjects).
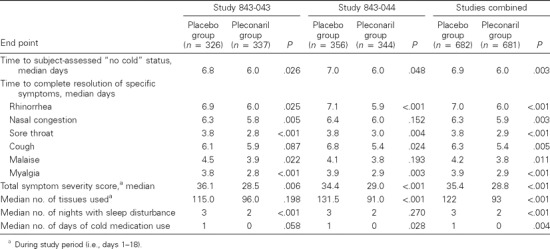
Figure 2.
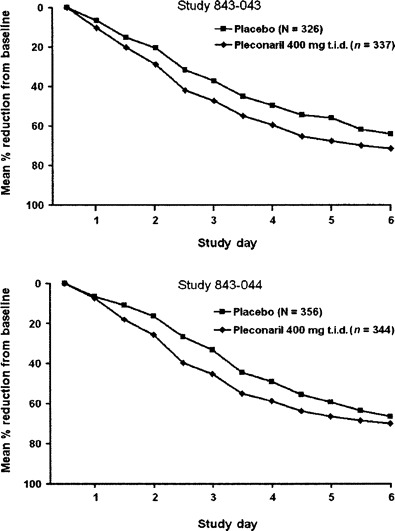
Change from baseline value in total cold symptom severity scores among picornavirus-infected subjects, days 1–6. Pleconaril recipients experienced significant (P > .05) reductions from baseline by day 2 of treatment in each study.
Analyses of the combined study results were conducted with a Cox regression model to assess 2-way interaction effects between treatment and the variables age, sex, race, smoking status, and preenrollment cold medication use. The only interaction with significant impact on treatment effect was smoking status (P = .013). The time to reach the primary end point was shorter for pleconaril recipients than for placebo recipients among nonsmokers (6.0 vs. 7.3 days; P > .001), but it was not different among smokers (8.3 vs. 7.4 days; P = .692). However, additional analysis regarding effects of pleconaril for smokers is limited by the fact that smokers constituted only 28% of the study population.
Virologic analysis. Among subjects with detectable picornavirus RNA in baseline nasal mucus samples, 827 (65%) of 1263 subjects had positive results of viral cultures. Acid stability testing of 69 randomly selected isolates determined that 68 (99%) were acid-labile and presumed rhinoviruses, whereas 1 was acid-stable and a presumed enterovirus.
Among those who had positive results of culture at baseline, fewer pleconaril recipients had positive culture results on day 3 (range, day 2–4) than did placebo recipients (53% vs. 72%; P > .001; figure 3). Additional analysis of the subset of subjects who had samples obtained for culture on day 2 revealed that significantly fewer pleconaril recipients than placebo recipients had positive viral culture results (27 [60%] of 45 subjects vs. 49 [84%] of 58 subjects; P = .007).
Figure 3.
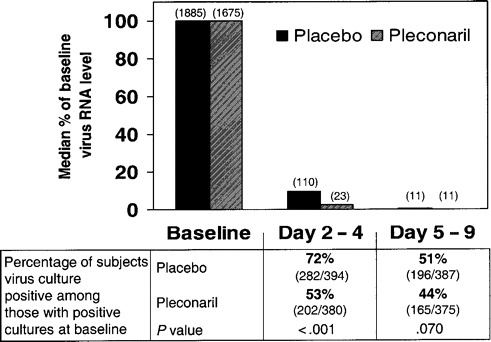
Antiviral activity in studies 843-043 and 843-044 combined: change in viral RNA levels from baseline values, as determined using the RT-PCR TaqMan assay (Applied Biosystems; numbers in parentheses are median virus levels at each time point in relative plaque-forming units per milliliter derived from the HRV1B standard curve) and results of viral cultures for subjects with positive culture results at baseline.
Nasal mucus viral RNA levels decreased rapidly in both pleconaril and placebo treatment groups. Subjects in the pleconaril group showed a larger median percentage reduction from baseline in virus levels on study day 3, compared with subjects in the placebo group (97.7% vs. 90.3%; P > .001). By day 6 (range, day 5–9), the median percentage reduction in virus levels was >99% in both treatment groups.
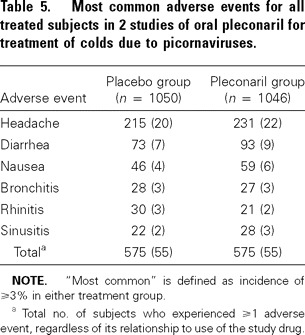
Safety. Pleconaril was generally well tolerated. The most commonly reported adverse events were headache, diarrhea, and nausea (table 5); ≤95% of adverse events were mild or moderate in severity. Four subjects receiving pleconaril and 2 receiving placebo reported a serious adverse event, none of which was considered by the investigators to be related to study drug except for 1 case of inadvertent overdose of pleconaril (with no adverse sequelae).
Table 5.
Most common adverse events for all treated subjects in 2 studies of oral pleconaril for treatment of colds due to picornaviruses.
No differences were noted in vital signs or physical examination findings in either treatment group, and there were no clinically significant laboratory abnormalities (data not shown). The only laboratory findings associated with pleconaril use were small median increases from baseline values in the pleconaril group for nonfasting serum cholesterol levels (an increase of 5 mg/dL [or 3%], compared with a decrease of 4 mg/dL [or 2%] in placebo recipients; P > .001) and for platelet counts (an increase of 15 × 103 platelets/mm3 [or 6%], compared with an increase of 7 × 103 platelets/mm3 [or 3%] in placebo recipients; P > .001).
Discussion
These prospective, double-blind, placebo-controlled studies found that early pleconaril treatment significantly reduces the duration and severity of colds due to picornaviruses in adults; these findings establish pleconaril as the first antiviral to have proven therapeutic value for such illnesses. These results confirm and extend those of an earlier retrospective analysis of adults with colds due to picornaviruses, which found a 1.5-day reduction in the time to alleviation of illness in pleconaril recipients, compared with placebo recipients [10]. Pleconaril use was associated with significant reductions in symptom severity scores and the frequency of recovery of picornaviruses within 1 day after initiation of therapy. In addition, pleconaril therapy resulted in a significant reduction in the duration of each individual cold symptom monitored in the study, a finding consistent with the hypothesis that ongoing viral replication is an important contributor to the pathogenesis of cold symptoms.
The rapid decrease in viral RNA levels in both pleconaril and placebo groups illustrates the importance of early initiation of antiviral therapy. We also observed that substantial proportions of both placebo recipients and, less often, pleconaril recipients continued to have positive culture results on study day 6 or later, although at very low levels of viral RNA. This prolonged recovery of virus is consistent with earlier data from natural and experimentally induced rhinovirus infections [21, 22], but it raises the issue of emergence of drug-resistant variants. In the current studies, viruses with reduced susceptibility to pleconaril (≤10-fold change from baseline value) were recovered during or after therapy from ∼10% of patients who received pleconaril. However, subgroup analyses indicate that clinical benefit for these participants was as good as or better than that for pleconaril recipients with no reduction in virus susceptibility to the drug (unpublished data). Further phenotypic and genetic characterization of viruses from these and other pleconaril trials is ongoing, to determine relationships between in vitro susceptibility and clinical outcomes.
Pleconaril was shown to be safe and generally well tolerated. Compared with placebo, there were only small (2%) excess frequencies of headaches, nausea, and diarrhea in patients receiving pleconaril. The small increases from baseline in cholesterol levels and platelet counts are not clinically significant, an observation that was confirmed in a subsequent 6-week prophylaxis study (unpublished observations). In that study, an excess of mild or moderate menstrual disorders (most commonly breakthrough bleeding or spotting) was reported from women taking oral contraceptives and pleconaril. Subsequent investigations revealed that pleconaril induces hepatic cytochrome P-450 3A enzymes. Pleconaril reduced the area under the curve of plasma levels of ethinyl estradiol by 34% following single-dose administration (G. Rhodes, personal communication). Retrospective review of all randomized, placebo-controlled trials in which pleconaril was administered for 5–7 days revealed that menstrual irregularities were reported by 3.5% of 310 pleconaril-treated women who were using oral contraceptives and by none of 291 placebo-treated women. None of the menstrual irregularities led to discontinuation of treatment. Additional studies are ongoing to better characterize the magnitude and duration of cytochrome P-450 3A induction and to determine the clinical significance for coadministration of pleconaril with other drugs metabolized by cytochrome P-450 3A.
One limitation of the current studies is that most participants were generally healthy young adults. Other studies have established that rhinoviruses can cause both upper and lower respiratory tract complications, including asthma exacerbations in both adults and children [23–25], acute exacerbations of chronic obstructive pulmonary disease [26], acute otitis media in children [27], and sinusitis in adults [13]. Others at risk for lower respiratory complications due to rhinovirus infection include patients with cystic fibrosis [28], elderly individuals [29], and immunocompromised persons [30]. The positive findings in the current trials indicate that studies of pleconaril should be extended to children, smokers, and those with underlying airway disease.
In summary, early pleconaril treatment of colds due to picornaviruses reduces the duration and severity of illness in adults. Pleconaril at this dosage was well tolerated, although additional data are needed to better characterize its potential for drug interactions.
Pleconaril Respiratory Infection Study Group
The following members of the Pleconaril Respiratory Infection Study Group enrolled subjects and participated in the studies described herein: Regina Medical Center, Regina, Saskatchewan, Canada: G. Achyuthan; Arizona Clinical Research Center, Tucson: A. Adamczyk; Brandywine Clinical Research, Downingtown, PA: L. Alwine; Credit Valley Hospital, Mississauga, Ontario, Canada: E. Amer; Bluegrass Clinical Research, Louisville, KY: K. Anderson; New Orleans Institute of Clinical Investigation, New Orleans, LA: J. Angelo; Health Sciences Center, Winnipeg, Manitoba, Canada: F. Aoki; Private practice, Philadelphia, PA: P. Arcuri; MedSource, Richmond, VA: M. Armstrong; The Johns Hopkins University, Lutherville, MD: P. Auwaeter; Scripps Clinic for Clinical Trials, La Jolla, CA: G. Babikian; Novabyss, Sherbrooke, Quebec, Canada: J. Bachand; Pinnacle Research Center of Texas, Ft. Worth: S. Barclay; Humbr River Hospital, Toronto, Ontario, Canada: J. Barkin; Private practice, Saskatoon, Saskatchewan, Canada: K. Bayly; Medical Associates Research Group, San Diego: M. Bennett; Clinical Research, Cedar Rapids, IA: J. Benz; Clinical Research Institute, Minneapolis, MN: G. Berman; Vanderbilt University, Nashville, TN: I. Biaggioni; Primary Physicians Research, Pittsburgh, PA: M. Blatter; Hoag Memorial Hospital, Newport Beach, CA: B. Bock; Harleysville Medical Associates, Harleysville, PA: B. Bock; First Care Family Doctors South, Fayetteville, AR: M. Bonner; Private practice, Bellevue, WA: S. Bonvallet; Antigonish Clinical Trials Research Group, Antigonish, Nova Scotia, Canada: W. Booth; California Research Foundation, San Diego, CA: M. Brandon; BBM Clinical Research, Courtice, Ontario, Canada: E. R. Brankston; Alpine Clinical Research Center, Boulder, CO: P. Brownstone; Cowan Avenue Medical Clinic, St. John's, Newfoundland, Canada: W. Button; Internal Medicine Associates, West Grove, PA: D. Callahan; Private practice, Regina, Saskatchewan, Canada: M. Cameron; Georgia Research Associates, Atlanta: J. Capo; The Asthma and Allergy Institute, Mobile, AL: L. Caputo; Southern Clinical Research, Augusta, GA: A. Carr; Northgate Medical Centre, North Bay, Ontario, Canada: J. Carter; Delta Medical, Dewitt, MI: T. Chiambretti; University of Texas Medical Branch, Galveston: T. Chonmaitree; Renstar Medical Research, Ocala, FL: S. Clevinger; Central Austin Internists, Austin, TX: T. Coats; Memorial Health University Medical Center, Savannah, GA: K. Cobb; Clinical Research Consultants, Trumbull, CT: S. Cohen; MSHJ Research Associates, Halifax, Nova Scotia, Canada: H. Conter; Community Research Management Associates, Cincinnati, OH: B. Corser; Acadia Medical Centre, Saskatoon, Saskatchewan, Canada: D. Dattani; Ferrell Duncan Clinic, Springfield, MO: S. Daugherty; Lakeside Medical Clinic, Saskatoon, Saskatchewan, Canada: H. Daverne; Heartland Medical, New Tazewell, TN: G. Day; Herridge Community Health Clinic, Ottawa, Ontario, Canada: F. Diaz-Mitoma; Brooke Army Medical Center, Ft. Sam Houston, TX: D. Dooley; Scripps Clinic Encintas, Encintas, CA: M. Drehobl; Scripps Clinic Rancho Bernardo, San Diego, CA: M. Drehobl; Wisconsins Veterans Home, King, WI: P. Drinka; St. Luke's Family Health Care, Boise, ID: J. Eck; Family Medical Center North, Bismarck, ND: R. Emery; The Center for Pharmaceutical Research, Kansas City, MO: J. Ervin; Internal Medicine Group, Cheyenne, WY: K. Evans; Meridien Research, St. Petersburg, FL: M. Farmer; VAPA Clinical Research, Richmond, VA: B. Feinstein; Stony Creek, Ontario, Canada: D. Fraser; Meridian Clinical Research, Omaha, NE: T. Free; North Texas Clinical Research, Irving: W. Gaman; Allergy and Asthma Practice, Laguna Niguel, CA: R. Gehling; Family & Internal Medicine, Lebanon, KY: D. George; Geny Research Corp., Natick, MA: N. Gershman; University Clinical Research, Pembroke Pines, FL: L. Gilderman; Christiana Care Health Service, Wilmington, DE: J. Gill; West Tropicana Medical Center, Las Vegas, NV: I. Goldsmith; Toronto, Ontario, Canada: B. Green; Cooper Hospital/University Medical Center, Camden, NJ: R. Greenman; Oklahoma State University, Tulsa, OK: S. Grogg; Institute of Healthcare Assessment, San Diego, CA: H. Guy; Southeastern Clinical Research, Chattanooga, TN: F. Hamilton; Tampa Bay Medical Research, Clearwater, FL: J. Hampsey; Miami Valley Clinical Trial Resources, Franklin, OH: C. Hanshaw; Whitehills Medical Clinic, St. John's, Newfoundland, Canada: R. Hart; St. Joseph's Medical Center, Tacoma, WA: R. Harvey; Comprehensive Clinical Research, Berlin, NJ: D. Hassman; University of Virginia Health System, Charlottesville: F. Hayden; N.C. Children's & Adult's Clinical Research Foundation, Chapel Hill, NC: F. Henderson; New Hanover Medical Research, Wilmington, NC: C. Herring; West Texas Medical Associates, San Angelo: D. Herrington; NSDEA, New Hyde Park, NY: K. Hershon; Fleetwood Medical Associates, Fleetwood, PA: R. Hippert; Heart of America Research Institute, Mission, KS: J. Holmes; Radiant Research, Portland, OR: M. Hosko; Saskatoon, Saskatchewan, Canada: E. Howlett; Olive Branch, MS: R. Huling; C.A.R.E. Clinical Research, St. Louis, MO: T. Hyers; Summerhill Medical Clinic, Manuels, Newfoundland, Canada: F. Jardine; Montana Health Research, Billings, MT: F. Kahn; Palm Beach Research Center, Sunrise, FL: M. Kaufman; SouthEast Research Associates, Marietta, GA: R. Kaufmann; Connor Research Group, Camp Hill, PA: J. Kearney; Belvedere Medicentre, Edmonton, Alberta, Canada: A. Kelly; Urgicare Medical Centre, Richmond Hill, Ontario, Canada: M.G. Kennedy; West Coast Clinical Trials, Long Beach, CA: K. Kim; Advanced Clinical Research, Salt Lake City, UT: J. Kirstein; Heartland Research Associates, Wichita, KS: T. Klein; Heartland Healthcare, Granite City, IL: K. Konzen; Northeast Clinical Research, Allentown, PA: N. Kopyt; Montreal, Quebec, T. Korin; Rocky Mountain Center, Wheat Ridge, CO: R. Lapidus; Manna Research, Weston, Ontario, Canada: B. Lasko; Highland Clinic, APMC, Shreveport, LA: J. Laviolette; Mellennium Clinical Research, Arlington, VA: S. Lee-Rugh; Invascor Clinical Research, Longueuil, Quebec, Canada: J. Lenis; Welstar Health System, Marietta, GA: A. Lentnek; New Mexico Clinical Research, Albuquerque: M. Lewiecki; Odyssey Research Services, Fargo, ND: M. Lillestol; FPA Clinical Research, Kissimmee, FL: M. Link; Encompass Clinical Research, Spring Valley, CA: R. Lipetz; Wichita Clinic, Wichita, KS: R. Loeffler; Medical Group at Marple Commons, Broomall, PA: M. Logan; Hampton Roads Center, Virginia Beach, VA: B. Lubin; New England Center for Clinical Research, Cranston, RI: F. Maggiacomo; Toronto, Ontario, Canada: M. Maleki-Yazdi; Centre Medical Halles de Ste-Foy, Ste-Foy, Quebec, Canada: A. Martel; Odyssey Research Services, Minot, ND: S. Mattson; New Orleans Clinical Trial Management, Kenner, LA: C. Mayorga; Coastal Medical Research Group, San Luis Obispo, CA: F. Mazzone; Allergy and Asthma Research Center of El Paso, El Paso, TX: R. Menendez; Santa Barbara Clinical Research, Santa Barbara, CA: M. Merrin; Atlanta Research Professionals, Dunwoody, GA: A. Miller; SMBD-Jewish General Hospital, Montreal, Quebec, Canada: M. Miller; Topsail Road Medical Clinic, St. John's, Newfoundland, Canada: K. Misik; Palm Beach Research Center, West Palm Beach, FL: B. Miskin; Riverview Medical Clinic, Riverview, New Brunswick, Canada: K. Mitton; Research Solutions, Fayetteville, AR: R. Montgomery; Glasgow Family Practice, Newark, DE: J. Navarro; Victoria Square Medical Clinic, Regina, Saskatchewan, Canada: G. O'Byrne; Commonwealth Medical Clinic, Mt. Pearl, Newfoundland, Canada: D. O'Keefe; Rosedale Medical Group, Hamilton, Ontario, Canada: J. A. Opie; Advanced Clinical Research, Boise, ID: D. Orchard; NFLD Drive Family Practice, St. John's, Newfoundland, Canada: P. O'Shea; Radiant Research, Greer, SC: D. Owens; Essential Health Care, Bardstown, KY: L. Oxnard; Central Kentucky Research, Lexington: J. Pappas; South Florida Clinical Research, Hollywood: A. Patron; Scripps Clinic Group, La Jolla, CA: M. Perlman; Longmont Medical Research, Longmont, CO: L. Pfeifer; Treasure Coast Infectious Disease Consultants, Vero Beach, FL: G. Pierone; Clinical Research, Seaforth Medical, Montreal, Quebec, Canada: B. Pynn; Encompass Clinical Research–North Coast, Encintas, CA: J. Quigley; Metrolina Medical Research Associates, Charlotte, NC: G. Raad; TPS Neshaminy Bensalem Medical, Bensalem, PA: M. Radbill; University Clinical Research, Deland, FL: B. Rankin; TQM Research Center, Cincinnati, OH: R. Rechtin; R/D Clinical Research, Lake Jackson, TX: H. Resnick; Medical Parameters, Martinez, GA: R. Rhoades; Research Solutions, Little Rock, AR: K. Roberts; University of Missouri Columbia, Columbia, MO: P. Robinson; Multi-Specialty Research Associates, Raleigh, NC: J. Rubino; Westside Family Medical Center, Kalamazoo, MI: G. Ruoff; Southern Drug Research, Tallassee, AL: M. Russell; New Orleans Clinical Trial Management, Covington, LA: R. Saguiguit; Los Angeles Clinical Research, Encino, CA: G. Saliba; International Clinical Research, San Diego, CA: R. Sanzone; University of Iowa, Iowa City: M. Schilling; Radiant Research, Lakewood, WA: J. Schmidt; Hill Top Research, Columbus, OH: D. Schumacher; Coastal Carolina Research Center, Mt. Pleasant, SC: V. Scott; Benchmark Research, Fort Worth, TX: W. Seger; Asthma, Nasal Disease & Allergy Research Center of New England, Providence, RI: G. Settipane; Central California Medical Research, Fresno, CA: G. Sevel; University of Alberta Hospital, Edmonton, Alberta, Canada: S. Shafran; Millennium Clinical Research, Washington, D.C.: M. Shepard; Gain Medical Centre, Coquitlam, British Columbia, Canada: D. Shu; Quest Clinical Trials, Markham, Ontario, Canada: I. Siegel; Montana Medical Research, Missoula, MT: W. Sinclair; Wake Forrest University School of Medicine, Winston-Salem, NC: J. Spangler; Hackensack University Medical Center, Hackensack, NJ: S. Sperber; Brookdale Clinic, Peterborough, Ontario, Canada: D. Spink; Grants Pass, OR: R. Steinbrenner; GFI Research Center, Evansville, IN: R. Stoltz; Primary Physicians Research, Johnstown, PA: C. Stotler; Novabyss, Sherbrooke, Quebec, Canada: C. St-Pierre; Central New York Clinical Research, Manlius: C. Stringer; North Penn Family Medicine, Landsdale, PA: M. Sussman; Zoom International, St. Jerome, Quebec, Canada: G. Tellier; Act Medical Research-Malvern, Scarborough, Ontario, Canada: A. Teplinsky; Maricopa Medical Center, Phoenix, AZ: B. Tiffany; Polyclinic Professional Center, Charlottetown, Prince Edward Island, Canada: D. Tweel; J & S Studies, Bryan, TX: B. Tyler; Health Research Associates, Cleveland, OH: A. Varner; P. W. Clinical Research, Winston-Salem, NC: P. Vrooman; Advanced Clinical Research, Bountiful, UT: R. Wade; Coastal Clinical Research, Mobile, AL: J. Walker; Clinical Trials North Houston, Houston, TX: S. Weakley; Diablo Clinical Research, Walnut Creek, CA: R. Weinstein; Warminster Medical Associates, Warminster, PA: G. Weisman; Paradigm Clinical Trials, Oshawa, Ontario, Canada: P. Whitsitt; QualSite Clinical Research, Wheat Ridge, CO: T. Wirecki; P. W. Clinical Research, Winston-Salem, NC: W. Wray; TPS Founders Medical Group NE, PA: G. Yeoman; Northern California Research, Fair Oaks: D. Young; The Male Health Centres, Barrie, Ontario, Canada: J. Zadra; Act Medical Research-Malton, Mississauga, Ontario, Canada: B. Zidel; Act Medical Research, Cadillac, MI: M. Ziter; Group North, Windsor, Windsor, Ontario, Canada: P. Ziter; University of Virginia Elson Student Health Center, Charlottesville, VA.
Acknowledgments
We thank Marissa Seligman and Sandra Norris for editorial assistance in manuscript preparation.
Footnotes
Presented in part: 39th Annual Meeting of the Infectious Diseases Society of America, San Francisco, 25–28 October 2001 (abstract 414); and 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, 16–19 December 2001 (H-659).
Financial support: ViroPharma Incorporated, Exton, Pennsylvania. F.G.H. is a paid consultant for ViroPharma Incorporated and member of its Scientific Advisory Board; E.C.C.. S.A.V., S.L., S.H., D.C.P., M.C. and M.M. are employees of ViroPharma Incorporated; the other authors have no commercial or other association that would pose a conflict of interest in the writing of this manuscript.
a Members of the study group are listed at the end of the text.
References
- 1.Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–42. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arruda E, Pitkaranta A, Witek TJ, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–8. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden FG, Kaiser DL, Albrecht JK. Intranasal recombinant alfa-2b interferon treatment of naturally occurring common colds. Antimicrob Agents Chemother. 1988;32:224–30. doi: 10.1128/aac.32.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden FG, Hipskind GJ, Woerner DH, et al. Intranasal pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob Agents Chemother. 1995;39:290–4. doi: 10.1128/aac.39.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gwaltney JM, Heinz BA. Rhinovirus. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. 2nd ed. Washington, DC: American Society for Microbiology; 2002. pp. 995–1018. [Google Scholar]
- 6.Hadfield T, Diana GD, Rossmann MG. Analysis of three structurally related antiviral compounds in complex with human rhinovirus 16. Proc Natl Acad Sci USA. 1999;96:14730–5. doi: 10.1073/pnas.96.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKinlay MA, Pevear DC, Rossmann MG. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Annu Rev Microbiol. 1992;46:635–54. doi: 10.1146/annurev.mi.46.100192.003223. [DOI] [PubMed] [Google Scholar]
- 8.Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999;43:2109–15. doi: 10.1128/aac.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiff GM, Sherwood JR. Clinical activity of pleconaril in an experimentally induced coxsackievirus A21 respiratory infection. J Infect Dis. 2000;181:20–6. doi: 10.1086/315176. [DOI] [PubMed] [Google Scholar]
- 10.Hayden FG, Coats T, Kim K, et al. Oral pleconaril treatment of picornavirus-associated viral respiratory illness in adults: efficacy and tolerability in phase II clinical trials. Antivir Ther. 2002;7:53–65. [PubMed] [Google Scholar]
- 11.Powell KR, Shorr R, Cherry JD, Hendley JO. Improved method for collection of nasal mucus. J Infect Dis. 1977;136:109–11. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]
- 12.Arruda E, Hayden FG. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–9. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 13.Pitkaranta A, Arruda E, Malmberg H, Hayden FG. Detection of rhinovirus in sinus brushings of subjects with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–3. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blomqvist S, Skyttä A, Poivainen M, Hovi T. Rapid detection of human rhinoviruses in nasopharyngeal aspirates by a microwell reverse transcription-PCR-hybridization assay. J Clin Microbiol. 1999;37:2813–6. doi: 10.1128/jcm.37.9.2813-2816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesanen M, Piiparinen H, Kallio A, Vaneri A. Detection of herpes simplex virus DNA in cerebrospinal fluid samples using the polymerase chain reaction and microplate hybridization. J Virol Methods. 1996;59:1–11. doi: 10.1016/0166-0934(95)01991-x. [DOI] [PubMed] [Google Scholar]
- 16.Arruda E, Crump C, Rollins BS, Ohlin A, Hayden FG. Comparative susceptibility of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J Clin Microbiol. 1996;34:1277–9. doi: 10.1128/jcm.34.5.1277-1279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Assoc. 1958;53:457–81. [Google Scholar]
- 18.Gehan EA. Statistical methods for survival time studies. In: Staquet MJ, editor. Cancer therapy: prognostic factors and criteria. New York: Raven Press; 1975. pp. 7–35. [Google Scholar]
- 19.SAS Institute . SAS/STAT user's guide. Version 6. 4th ed. Vol. 1. Cary, NC: SAS Institute; 1989. [Google Scholar]
- 20.Freedman LS. Tables of the number of patients required in clinical trials using the log rank test. Stat Med. 1982;1:121–9. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 21.Fox JP, Cooney MK, Hall CE. The Seattle virus watch. V. Epidemiologic observations of rhinovirus infections, 1965–1969, in families with young children. Am J Epidemiol. 1975;101:122–43. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- 22.Winther B, Gwaltney JM, Mygind N, Turner RB, Hendley JO. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–7. [PubMed] [Google Scholar]
- 23.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 25.Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev. 1999;12:9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–73. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 27.Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–5. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong D, Grimwood K, Carlin JB, et al. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol. 1998;26:371–9. doi: 10.1002/(sici)1099-0496(199812)26:6<371::aid-ppul1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson KG, Kent J, Hammersley V, Esperanza C. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–23. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102(1):10–8. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


