Abstract
Background
Bone-seeking radiopharmaceuticals can deposit radiation selectively to some osteosarcoma tumours because of the bone-forming nature of this cancer.
Objectives
This is the first report of using 223-radium, an alpha-emitting calcium analogue with a high therapeutic index, in combination therapy with other agents in 15 patients with metastatic osteoblastic osteosarcoma.
Methods
Candidates for alpha-radiotherapy if 99mTc-MDP bone scan had avid bone-forming lesions and no therapy of higher priority (eg, definitive surgery). Monthly 223-radium infusions (1.49 μCi/kg or 55.13 kBq/kg) were given.
Results
The median infusion number was three and the average time to progression was 4.3 months for this cohort receiving 223-radium+other agents. Agents provided during 223-radium included (1) drugs to reduce skeletal complications: monthly denosumab (n=13) or zolendronate (n=1); (2) agents with antivascular endothelial growth factor activity, pazopanib (n=8) or sorafenib (n=1), (3) alkylating agents: oral cyclophosphamide (n=1) or ifosfamide, given as a 14-day continuous infusion (n=1, two cycles), (4) high-dose methotrexate (n=1), pegylated liposomal doxorubicin (n=1); and (5) two other combinations: nivolumab and everolimus (n=1) and rapamycin and auranofin (n=1). Radiation therapy, including stereotactic body radiotherapy (SBRT), was also given to 11 patients concurrently with 223-radium (n=2), after 223-radium completion (n=3), or both concurrently and then sequentially for other sites (n=6). After 223-radium infusions, patients without RT had a median overall survival of 4.3 months compared with those with SBRT and/or RT, who had a median overall survival of 13.5 months.
Conclusion Although only 1/15 of patients with osteoblastic osteosarcoma still remain alive after 223-radium, overall survival
Keywords: alpha emitter; bone-seeking radiopharmaceutical; osteoblastic metastases; 99mTc-MDP bone scan with SPECT CT; stereotactic body radiotherapy (SBRT),
Significance of this study.
What is already known about this subject?
Bone-seeking radiopharmaceuticals can provide targeted radiation to osteoblastic metastases.
Alpha emitters have some radiobiological advantages, including more effective tumour cell killing and less marrow toxicity than beta emitters.
It has been shown that 223-radium can be safely given as a single agent to patients with osteoblastic metastases of osteosarcoma and that imaging shows specific deposition using either 99mTc-MDP or Na18F scans.
What does this study add?
This study is the first series of patients who have been treated with 223-radium in combination with other agents, including denosumab and chemotherapy.
How might this impact on clinical practice?
Since this study shows feasibility of the approach, patients with osteoblastic bone metastases of osteosarcoma now have additional options to treat both symptomatic and asymptomatic metastases using combination therapy using an alpha-emitting bone-seeking radiopharmaceutical, 223-radium, and other agents such as pazopanib and denosumab.
Introduction
223-Radium is an alpha-emitting bone-seeking radiopharmaceutical that is effective against osteosarcoma and other bone-forming tumours.1–4 This agent was developed to treat osteoblastic metastases and has the advantage of a decay cascade that produces four high linear energy transfer (LET) alpha particles per 223-radium decay where the 223-radium is deposited in bone or a bone-forming tumour (t1/2 11.4 days). 223-Ra is also safe because rapid radon daughter decay compared with other radium isotopes reduces potential off-target effects of this gas. Preclinical experience, clinical development and current 223-radium use in prostate cancer have shown a very high therapeutic index.5–15
Osteosarcoma is a cancer occurring in young people with an event-free survival of about 60%.16 17 The pathological diagnosis requires new bone formation by tumour cells; this characteristic also facilitates bone-seeking radiopharmaceutical deposition in tumours on bone scans. 99mTc-MDP uptake on bone scan or 18FNa uptake on bone positron emission tomography (PET) is an excellent means to identify osteosarcoma tumour that avidly sequesters the bone-seeking 223-radium radiopharmaceutical,1–4 as it is an analogue of calcium. Prior limited pilot experience1 and a phase I study in osteosarcoma2–4 have demonstrated excellent tolerance of 223-radium in patients with metastatic osteosarcoma. Twice the standard 223-radium dose has been tolerated by patients with metastatic osteosarcoma.3
Major problems affecting osteosarcoma survival and quality of life (QOL) after initial treatment are the development of lung and bone metastases.16–19 We have used additional agents with 223-radium as well as palliative and/or stereotactic body radiotherapy (SBRT) as clinically indicated against metastatic osteosarcoma because patients with osteosarcoma with bone metastases have worse survival and are at risk of skeletal complications,20–22 can escape from radiopharmaceutical action by metastases that do not avidly make bone and may develop pain from metastases that incompletely respond to alpha radiotherapy. We report that combination therapy is feasible and can result in clinical benefit.
Patients and methods
Patients
Patients with osteoblastic metastases of osteosarcoma suitable for alpha radiotherapy were identified using 99mTc MDP bone scan. At our institution, we also use single-photon emission computed tomography (SPECT) to provide axial, coronal and sagittal colour fusion images to facilitate discussion of indications, risks, alternatives of treatments for bone-forming metastases (eg, 223-radium, surgery, cryoablation and/or radiotherapy including SBRT). All patients offered 223-radium had a poor prognosis and did not have other treatment options of higher priority (eg, lung surgery to completely remove metastases to obtain a clinical remission). Informed consent was obtained in the clinic after discussion of indications, risks and alternatives to facilitate prior authorisation and also in nuclear medicine at time of 223-radium administration and re-review of radiation safety precautions. A retrospective chart review and analysis medical and radiation treatment of consecutive patients with osteosarcoma treated with 223-radium in 2016–2019 was approved by Cleveland Clinic IRB (study CCF IRB 19–709).
Characteristics of the patients with metastatic osteosarcoma who received 223-radium monthly are summarised in table 1. Once a patient was identified as being suitable for 223-radium, the interval from submission of insurance prior authorisation request and/or appeal letter to radiopharmaceutical administration was 2 days to almost 2 months; most patients were able to get 223-radium within 1 month of initial consultation. Of note, 223-radium is an option in the National Comprehensive Cancer Network (NCCN) guidelines for treatment of relapsed osteosarcoma.
Table 1.
Metastatic osteosarcoma patient characteristics and 223-radium alpha radiotherapy cycles
| Patient | Age (years) | Relapses | Time to 223-radium | 223-Radium infusions (#) | Alkaline phosphatase | ||
| Pre | Post | % Change | |||||
| A | 36 | 2 | 20 | 3 | 97 | 65 | −33% |
| B | 15 | 2 | 39 | 6 | 112 | 80 | −29% |
| C | 26 | 1 | 25 | 2 | 1714 | 715 | −62% |
| D | 24 | 2 | 21 | 3 | 85 | 59 | −31% |
| E | 23 | 4 | 16 | 6 | 70 | 48 | −325% |
| F | 15 | 4 | 33 | 1 | 310 | nd | --- |
| G | 11 | 2 | 13 | 5 | 1370 | 67 | −95% |
| H | 16 | 3 | 18 | 2 | 380 | 232 | −39% |
| I | 20 | 3 | 15 | 2 | 133 | nd | -- |
| J | 24 | 2 | 52 | 2 | 131 | 125 | −5% |
| K | 18 | 1 | 18 | 6 | 94 | 50 | −47% |
| L | 14 | 1 (TNTC-LM) | 16 | 4 | 198 | 70 | −655% |
| M | 11 | 0 (TNTC-B) | 3 | 5 | 269 | 168 | −38% |
| N | 25 | 1 (TNTC-B) | 18 | 1 | 653 | 220 | −66% |
| O | 19 | 2 | 20 | 2 | 403 | 1041 | 1.58 |
| Median | 19 | 2 | 20 | 3 | 198 | 80 | −60% |
| Mean | 20 | 2 | 21.9 | 3 | 401 | 226 | −44% |
Time to radium: months from diagnosis to 223-Ra infusion 1.
TNTC-B, too numerous to count—bone metastases; TNTC-LM, too numerous to count—lung metastases.
223-Radium administration
223-Radium (1.49 μCi/kg or 55.13 kBq/kg; Xofigo, Bayer) was administered intravenously in the Cleveland Clinic Nuclear Medicine Department by a short infusion of 1–2 min into a central line (eg, subcutaneous port) followed by normal saline flushes (10 cc)×2. Because of rapid bone and osteoblastic metastasis uptake and short range of alpha emitters bound to bone, air travel was permitted on the same day of infusion. Patients were provided with a letter concerning radioactive drug treatment in case of radioisotope detection while travelling. Since there is some elimination of unbound 223-radium alpha emitting radiopharmaceutical into GI tract for several days, patients were advised to flush toilet twice after bowel movements for 1 week.
Concomitant anticancer agents
Agents to prevent skeletal complications were given on different days than 223-radium at standard dosages. Denosumab (120 mg) was given subcutaneously monthly or zolendronate 4 mg intravenously over 60 min monthly. Calcium carbonate+vitamin D supplementation (500 mg calcium+500 U vitamin D) orally two times per day was recommended for all patients receiving denosumab or zolendronate.
Pazopanib was used at a minimum of 400 mg and a maximum of 800 mg orally daily, depending on body size and GI tolerance. Sorafenib was used at 400 mg two times per day. Ifosfamide+mesna was used as a slow continuous infusion regimen (1 g/m2/day×14 days) to reduce potential for thrombocytopenia, nephrotoxicity and encephalopathy.23–26 Oral cyclophosphamide was given as 25–50 mg q AM and dose adjusted to keep ANC>1000. High dose methotrexate was given in a standard manner.27 28 Pegylated liposomal doxorubicin was used at a dose of 30 mg/m2 per month. Nivolumab and everlimus were given in standard doses in one patient and another received sirolimus 4 mg per day and auranofin 3 mg two times per day), both standard doses of these agent.29
External beam radiation therapy (RT)
SBRT or other RT simulation was performed in treatment position with 1.5 mm CT axial slices. Various modes of immobilisation were used based on the location of the tumour to be treated. A gross tumour volume was defined based on all available imaging. Typically, a 1 cm anatomically constrained clinical target volume was used, with consideration of inclusion of the regional bone. If the patient had received previous radiotherapy or was planned for multiple courses of SBRT in close proximity, a dose accumulation was created to evaluate all radiation plans and the contributory dose to the organs at risk. A treatment plan was developed for each site within 7–10 days of simulation.
Live-time daily cone-beam CT was used for image guidance for all treatments in the presence of a physician and physicist at the treatment console prior to treatment each day. SBRT was delivered in five fractions or less using a fraction size of 5–10 Gy per fraction delivered over sequential days. A maximum of four sites were treated with SBRT during a treatment session. Two patients with lung hilum and mediastinal treatment fields had RT given as 3 Gy×5 fractions for a total of 45 Gy.
Data capture was done using REDCap and analysis of clinical benefit (improvement in pain and/or non-progression at sites of RT and/or SBRT), and survival was done using retrospective chart review with institutional review board (IRB) approval. Because Response Criteria in Solid Tumors(RECIST) has not been useful in assessing response of osteosarcoma, a bone-forming tumour, this was not used in this study.4 30 Only one patient had before and after Na18F -PET-CT scans for analysis of Na18F PET Response Criteria in Solid Tumors (NAFCIST).4 Overall survival was calculated using the method of Kaplan and Meier.
Results
A total of 15 patients with osteoblastic metastases osteosarcoma were treated in a 2.5-year period: 6 in 2016, 7 in 2017, 1 in 2018 and 1 in 2019. Patient characteristics including age, number of relapses before 223-radium therapy, months from initial diagnosis to 223-radium and total number of monthly doses of 223-radium are detailed in table 1. The median age was 19 years old. For this cohort, an average interval of 1.5 years had elapsed from initial diagnosis and alpha radiotherapy of osteoblastic metastases. Because initial osteosarcoma chemotherapy takes 8–10 months, all of these patients had early relapse or persistent active disease and/or progressive osteosarcoma at the end of initial chemotherapy.
As detailed in table 1, only 3/15 patients received a planned six doses of 223-radium; the median and an average 223-radium doses were three and three per patient. The most common reason for <6 monthly doses of 223-radium was development of new or progressive non-osteoblastic metastases and a clinical decision to stop 223-radium. For example, if a new lung metastasis did not make bone (not avid on bone scan with SPECT), 223-radium would be unlikely to be deposited into the nodule and stopping monthly 223-radium was recommended.
All patients were able to get an agent useful for the prevention of complications of skeletal metastases (monthly denosumab n=13 or zolendronate n=1); one patient s/p prior denosumab refused monthly zolendronate. There were no adverse events related to hypocalcaemia, hypophosphataemia, fevers, or jaw necrosis attributable to denosumab or zolendronate during or after 223-radium therapy in this cohort. Table 2 lists concurrent agents during 223-radium, SBRT timing and survival status at the time of this report. Patients who had no SBRT and/or only one agent had worse survival (n=4 median 4.3 months) than patients treated with two or more agents and/or SBRT (n=11, medial survival of 13.5 months).
Table 2.
Combination therapy with 223-radium: other agents and SBRT
| Patient | Combinations of agents | RT timing† | RT sites† | Survival from 223-Ra (months) |
| A | Den+pazopanib | A | Face+skull base | 29 |
| B | Den+pazopanib | C+A | Bone+lung | 33 |
| C | Den+pazopanib+rapamycin/auranofin | C+A | Bone+lung | 11 |
| D | Den+Ifos* then pazopanib | A | Lung | >30 |
| E | Den+sorafenib | C+A | Lung | 16 |
| F | Den | C | Lung | 6 |
| G | Den+pazopanib | C+A | Bone | 13 |
| H | ‡HDMTX | N | None | 4 |
| I | Zolendronate | N | None | 4 |
| J | Den+nivolumab+everolimus | N | None | 10 |
| K | Den+pazopanib | C | Bone | 14 |
| L | Den+pazopanib+oral CPM | C+A | Bone, lung, liver | 11 |
| M | Den+pazopanib, then sorafenib | C+A | Bone | 6 |
| N | Den | C+A | Bone, lung | 5 |
| O | Den+liposomal doxorubicin | A | Bone | 4 |
*Ifos+mesna 1 g/m2/day×14 days via continuous infusion.
†n=34 SBRTs, n=16 other RTs. RT timing: A=after 223-radium, C=concurrent with 223-radium, N=no radiotherapy.
‡Patient H had no Den or zolendronate because of prior Den treatment and refused zolendronate.
CPM, cyclophosphamide; Den, denosumab; HTMTX, high-dose methotrexate; Ifos, ifosfamide; RT, radiotherapy; SBRT, stereotactic body radiation therapy.
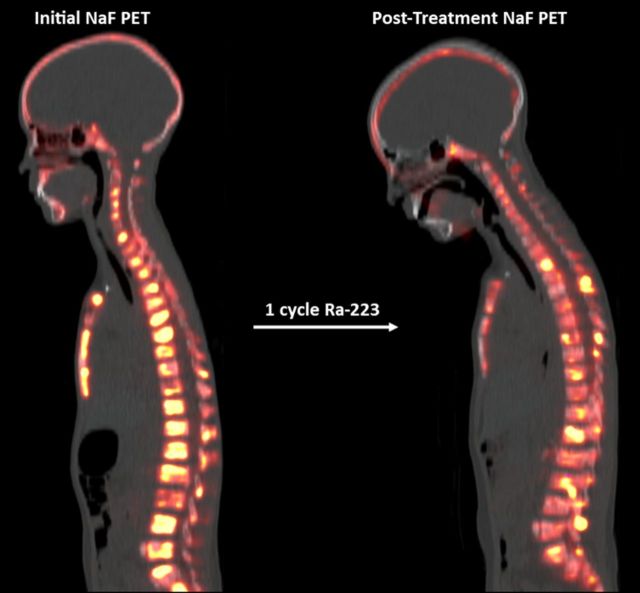
In one patient with TNTC osteoblastic bone metastases (figure 1), Na18F bone PET-CT provided data showing approximately 50% decrease in Na18F bone-PET in osteoblastic lesions (table 3). This would be considered a partial response by NAFCIST4 and was associated with dramatic improvement in pain and regaining ability to walk. Figure 2 shows another patient with TNTC lung metastases, as well as one left lower lobe lung metastasis and a bone metastases in the left femur and both proximal humerus with 99mTc-MDP avidity on 99mTc-MDP SPECT. Hence, although lung surgery was not possible, 223-radium+SBRT could provide meaningful treatment of areas of active osteosarcoma.
Figure 1.
TNTC bone metastases demonstrate a partial response by NAFCIST after 223-radium. Ten-year-old previously treated with on doxorubicin, cisplatin and high-dose methotrexate developed massive progression of bone metastases (TNTC) evidenced on Na18F PET-CT. This patient (patient M in tables 1–3) was then started on 223-radium, and after one cycle, a PR by Na18F PET Response Criteria in Solid Tumors (NAFCIST) was observed (table 3). Radiographical response was associated with excellent improvement in pain and regaining ability to walk. PET, positron emission tomography; TNTC, too numerous to count.
Table 3.
Decrease in Na18F bone PET uptake of many osteoblastic metastases after 223-radium (patient M)
| Bone lesion location | SUV pre | SUV post | Difference | |
| 223-Radium | 223-Radium×2 | SUV | Per cent less | |
| Skull base (clivus) | 9.3 | 5.1 | −4.2 | −46% |
| C-spine (C3) | 21.2 | 8.2 | −13 | −61% |
| T-spine (T2) | 26.9 | 7.8 | −19.1 | −71% |
| T-spine (T12) | 30.1 | 25.3 | −4.8 | −16% |
| L-spine (L4) | 24.9 | 10.6 | −14.3 | −57% |
| Sacrum | 26.8 | 18.8 | -8 | −30% |
| Pelvis (femoral head) | 24.7 | 6.9 | −17.8 | −72% |
| Ribs (postleft sixth) | 18.5 | 6 | −12.5 | −68% |
| Humerus (proximal right) | 35.8 | 19.5 | −16.3 | −46% |
| Ankle (left distal tibia) | 32.6 | 16 | −16.6 | −51% |
| Median | 25.8 | 9.4 | −15.3 | −54% |
| Mean | 25.1 | 12.4 | −12.7 | −51.80% |
Alkaline phosphatase was 269 before 223-radium and 168 after dose two at the time of the aforementioned sodium-fluoride PET study.
PET, positron emission tomography.
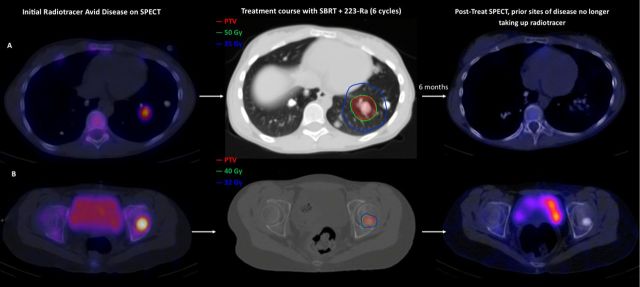
Figure 2.
Active sites of disease treated with SBRT and 223-Ra with reduced radiotracer uptake on post-treatment SPECT. (A) Sixteen-year-old with TNTC lung metastases (patient L in tables 1 and 2) exhibited two active sites of bone-forming disease on 99mTc-MDP bone scan with SPECT: left lower lobe lung and left femur. The lung lesion (A) and the femoral lesion (B) were treated simultaneously with SBRT and six cycles of 223-radium. No radiotracer uptake was observed on the post-treatment SPECT in either lesion. This patient achieved disease stability and excellent quality of life (attended school) while on therapy with 223-radium with denosumab, oral cyclophosphamide and SBRT. PTV, planning target volume; SBRT, stereotactic body radiation therapy; SPECT, single-photon emission computed tomography.
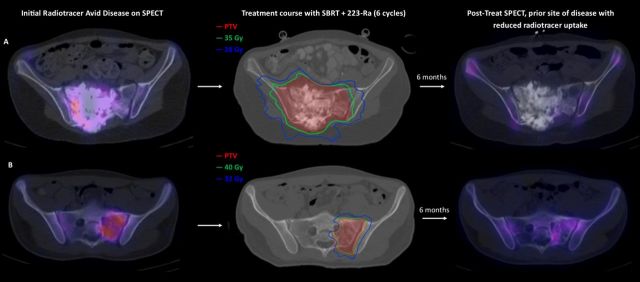
223-Radium+SBRT seemed particularly useful in controlling disease in sites for which surgery was not possible or indicated because of location. Figure 3 illustrates SBRT plans in two patients with extensive sacrum bone metastases, a location in which surgery is difficult and would affect QOL (eg, loss of bowel and bladder function). figure 3 (top) shows SBRT plan for right sacrum using 7 Gy×five fractions (35 Gy); figure 3 (bottom) shows left the sacrum SBRT plan using 8 Gy×five fractions (40 Gy). Both patients had concurrent 223-radium with SBRT of the sacrum bone metastasis; neither of these patients had relapse in the sacrum (follow-up 40 and 14 months).
Figure 3.
Sacrum metastases not amenable to surgical resection are treated with SBRT and 223-Ra with improvement in the patient’s quality of life. (A) A 15-year-old patient (patient B in tables 1–3) with osteosarcoma recurrence in the right sacrum evidenced on SPECT. This patient was treated with SBRT and six cycles of 223-Ra. The patient was able to continue to attend school while undergoing SBRT. The post-treatment SPECT revealed reduced radiotracer uptake in the sacral disease. (B) A 17-year-old patient (patient K in tables 1–3) with osteosarcoma recurrent in the left sacrum associated with severe pain. This patient was treated with SBRT and six cycles of 223-Ra. Again, the post-treatment SPECT showed reduced radiotracer uptake in the sacral disease. Complete and durable resolution of pain without local relapse was observed in both patients. PTV, planning target volume; SBRT, stereotactic body radiation therapy; SPECT, single-photon emission computed tomography.
There were no hospitalisations related to 223-radium administration. QOL during the 223-radium and combination therapy was excellent in 14/15 patients. The one exception was a patient with extensive prior radiation and no concurrent or later SBRT who had continued thrombocytopenia, pain and poor performance; this patient received only one dose of 223-radium. The other 14/15 patients had acceptable neutrophil and platelet count recovery (ANC>1000 and platelets>50K) within 1 month of 223-radium administration and remained ambulatory outpatients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0.
Two patients had pain flare reactions with increased bone pain after the first dose of 223-radium. One was related to chest wall pain from an osteoblastic lesion; this patient elected to stop therapy rather than try dexamethasone with 223-radium dose 2. The other patient who had a flare reaction was able to wean off opiates within 2 weeks and received six doses of 223-radium. This patient eventually had disease in other areas and lived for 29 months after an initial 223-radium infusion. There were no flare reactions during 223-radium cycles 2–6 in any patient.
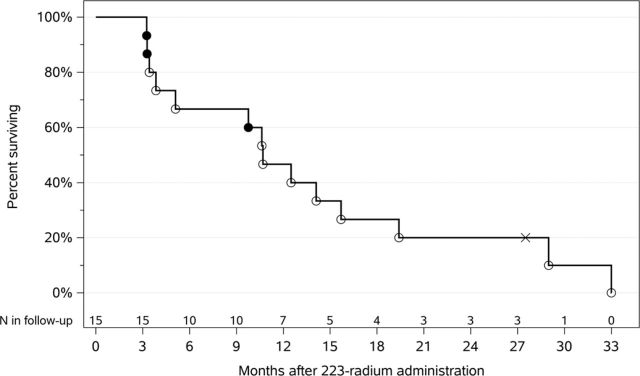
No patients were admitted for fever and neutropenia during 223-radium cycles including the patient that received two cycles of continuous infusion ifosfamide/mesna. This patient survived >2.5 years and remains alive and has been treated with 10 cycles of ifosfamide/mesna via continuous infusion (total dose 140 g/m2) with good renal function. Overall survival was 40% at 1 year and 20% at 2 years (figure 4). Although few patients had no additional palliative radiation (n=3), this was associated with short survival (3, 3 and 9 months; figure 4). After 223-radium infusions, patients without RT had a median overall survival of 4 months compared with those with SBRT and/or RT who had a medial overall survival of 13 months. Overall survival was 47% at 12 months and 20% at 24 months (figure 4). In general, 223-radium infusions were easily done in the outpatient setting, and combination therapy, including bone-seeking agents, chemotherapy and external beam radiation during and after 223-radium, was well tolerated.
Figure 4.
Overall survival of patients with osteoblastic osteosarcoma metastases after 223-radium in combination with other agents. Kaplan-Meier survival curve shows those that had additional radiation (o), no additional radiation (closed circles) and still surviving (x). Approximately 47% and 20% were surviving at 1 and 2 years, respectively; one patient remains alive for >2.5 years.
Discussion
We report the first clinical evaluation of 223-radium in bone-forming osteosarcoma with other anticancer therapies, including radiotherapy and SBRT. Using a standard dose of 1.49 μCi/kg (or 55.13 kBq/kg) 223-radium, it is feasible and safe to combine 223-radium, other agents and radiotherapy including SBRT. In the oligometastatic setting, it is generally accepted that local control surgery, if possible with acceptable morbidity, is the best means to treat second and subsequent recurrences of osteosarcoma.31 Systemic therapy with local control is needed not only in the upfront setting as described by Jaffe32 but also has been shown by recent analysis of Children’s Oncology Group data of seven phase II trials showing an event-free survival of 12% at 4 months.18 Since bone metastases in osteosarcoma are not only associated with a worse prognosis16 17 20 21 but also associated with the morbidity of osseous involvement and difficult local control strategies, we sought to use 223-radium in the setting of osteoblastic osteosarcoma metastases.
Although alpha emitters have some advantages compared with beta emitters for treatment of cancer, alpha emitters have shorter range than beta emitters. Alpha emitters have high LET,15 less haematological toxicity and a superior safety profile which facilitates not only administration but many fewer radioactivity precautions.1 5–8 13 33 Furthermore, 223-radium has been associated with clinical benefits, including improved lactate dehydrogenase (LDH), prostate specific antigen (PSA) and alkaline phosphatase and a survival advantage in those with osteoblastic bone metastases of prostate cancer.9–11 14 Because of these qualities, it would seem that 223-radium is a ‘designer drug’ for osteoblastic osteosarcoma metastases.1–4 33 However, if osteosarcoma metastases are not osteoblastic (ie, metastases do not make bone), other agents would be needed for cancer control. When used as a single agent in metastatic osteosarcoma, 2× the standard dose of 223-radium could be given safely to patients with osteosarcoma,3 all of whom previously at diagnosis had aggressive upfront multiagent chemotherapy, which for most patients includes cisplatin, doxorubicin, high-dose methotrexate and/or ifosfamide.17 19 34
Our osteosarcoma cohort was particularly challenging with early onset of new metastases, recurrence or persistent metastatic disease (median time from diagnosis, 18 months; median relapses=2). Furthermore, while only 3/15 had too numerous to count (TNTC) metastases, the others often presented with a wide variety of unresectable osseous and pulmonary sites of osteosarcoma. In this setting, we sought to maintain QOL using not only 223-radium to specifically treat the osteoblastic parts of numerous metastases but also other agents to decrease skeletal complications, as well as improve effectiveness of alpha radiotherapy and radiotherapy including SBRT.
Of note, there is a small series demonstrating utility of SBRT for metastatic Ewing and osteosarcoma with a local control of 85% after a 2-year follow-up for survivors.35 This series reported a median SBRT dose of 40 Gy delivered over five fractions; however, a range of dose and fractionation schemes were used. Although our experience is retrospective with treatment biases, the significant survival advantage of 9 months seen with the use of SBRT, along with 223-radium certainly supports consideration of the combined 223-radium+radiotherapy approach in patients with unresectable, metastatic osteosarcoma. Because of heterogeneity in the uptake of the bone seeking radiopharmaceutical and size of lesions treated with external beam radiation, it would seem that an advantage of 223-radium would be to target smaller less heterogeneous osteoblastic metastases and micrometastases, as well as to try to maintain QOL in the setting of TNTC bone metastases.
Despite numerous bone metastases, none of our patients had a pathological fracture and all were ambulatory during 223-radium cycles. We chose denosumab instead of zolendronate because of osteosarcoma biology considerations,36 37 as well as observations of osteosarcoma metastases becoming more 99mTc-MDP avid after monthly treatment with the antirank ligand antibody+calcium and vitamin D supplements two times per day. On the other hand, zolendronate may also be a similarly useful adjunct to not only prevent skeletal complications and is now inexpensive (generic) and could also delay progression.38
Agents which inhibit vascular endothelial growth factor (VEGF) are associated with minimal haematological toxicity and would seem suitable for combination with 223-radium. VEGF inhibition is associated with growth delay after radiotherapy39 and metastatic potential of osteosarcoma cells.40 Furthermore, VEGF pathway genes are sometimes amplified in osteosarcoma41 42 and are correlated with a poor outcome. However, bevacizumab can be associated with wound issues.43 Thus, use of tyrosine kinase inhibitors (TKIs) with anti-VEGF activity would seem to be an attractive option for inhibiting metastatic osteosarcoma after 223-radium. There are reports of activity of pazopanib against osteosarcoma44–46 and, similarly, sorafenib with activity in osteosarcoma47–50; these were successfully used in combination with 223-radium in our report. Since regorafenib has properties that improve response to radiotherapy in paediatric malignancy models51 and a recent study showed a high response rate of regorafenib against osteosarcoma,52 regorafenib would seem to be an attractive combination agent with 223-radium in the future. Usefulness of regorafenib may be limited by rash and GI toxicity, but this is variable in individual patients with some tolerating the agent well, while others seem to tolerate pazopanib or sorafenib better. Children’s Oncology Group has an expansion cohort currently receiving cabozanitinib (COG ADVL1622), and future activity could be expected using this TKI too.
Other agents with activity against osteosarcoma with a higher therapeutic index used with acceptable toxicity in our report also included high-dose methotrexate,53 ifosfamide/mesna as a continuous infusion at 1 g/m2/day×14 days, which is associated with minimal thrombocytopenia,23–26 53 oral cyclophosphamide,27 28 54 pegylated liposomal doxorubicin and auranofin with sirolimus.29 Since mammalian target of rapamycin (mTOR) inhibitors may be useful against sarcoma cancer stem cells55 56 as well as having some radiosensitisation qualities,57 these agents could also be used in combination with either 223-radium or SBRT.
We used an approach in patients with metastatic osteoblastic osteosarcoma to not only reduce disease burden but also maintain QOL. Combination therapy as used in this cohort could maintain QOL and, in some cases, disease control for limited amounts of time, but long-term efficacy of the bone seeking alpha-emitting radiopharmaceutical seemed limited by populations of cells that escape by no longer having the ‘osteoblastic phenotype’. What is needed are other means to efficiently eliminate disease burden and to maintain QOL. Future directions could include immune agents such as mifamurtide,58 59 anti-CD47,60 61 perturbation of metastatic phenotypes62 63 and a novel radiopharmaceutical with tumour seeking characteristics, CLR-131 (NCT03478346).
Conclusion
For patients with osteosarcoma with an osteoblastic phenotype, 223-radium would seem to be a ‘designer drug’. Although logistics of getting the bone-seeking radiopharmaceutical for a rare cancer can be complicated, it is an option in the NCCN guidelines and provides a minimally toxic means to ameliorate metastatic burden. We were able to provide combination therapy with 223-radium and other agents including SBRT to prevent skeletal complications and to maintain QOL. Nevertheless, because risk of relapse remains high and survival is poor, additional novel means to inhibit metastatic dissemination and to reduce burden of disease with a high therapeutic index are needed for metastatic osteosarcoma.
Acknowledgments
The authors acknowledge radiation oncology physicists, nuclear medicine personnel (especially Rick Full) and paediatric infusion nurses and nurse coordinators at the Cleveland Clinic (especially Allison Ochocki and Shauna Sartoski), who make cancer care look easy and friendly to our patients and their families.
Footnotes
Twitter: @PAndersonMDPhD, @Viveksubbiah
Contributors: All authors actively participated in the multidisciplinary care, treatment and/or analysis of these patients who received 223-radium and also external beam radiotherapy treatments and/or consultations as well as medical therapy. PMA and SW captured and analyzed data using REDCap. VS was instrumental in providing experience and expertise in the use of 223-radium and the selection of innovative combinations of agents and scans to show efficacy as well as revisions of the manuscript.
Funding: This work was funded by the Anderson Sarcoma Research Fund (T54628).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. de-identified data can be obtained from Peter Anderson MD, PhD (andersp@ccf.org)
References
- 1.Anderson PM, Subbiah V, Rohren E. Bone-Seeking radiopharmaceuticals as targeted agents of osteosarcoma: samarium-153-EDTMP and radium-223. Adv Exp Med Biol 2014;804:291–304. 10.1007/978-3-319-04843-7_16 [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V, Anderson P, Rohren E. Alpha emitter radium 223 in high-risk osteosarcoma: first clinical evidence of response and blood-brain barrier penetration. JAMA Oncol 2015;1:253–5. 10.1001/jamaoncol.2014.289 [DOI] [PubMed] [Google Scholar]
- 3.Subbiah V, Anderson PM, Kairemo K, et al. . Alpha particle Radium 223 dichloride in high-risk osteosarcoma: a phase I dose escalation trial. Clin Cancer Res 2019;25:3802–10. 10.1158/1078-0432.CCR-18-3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kairemo K, Rohren EM, Anderson PM, et al. . Development of sodium fluoride PET response criteria for solid tumours (NAFCIST) in a clinical trial of radium-223 in osteosarcoma: from RECIST to PERCIST to NAFCIST. ESMO Open 2019;4:e000439 10.1136/esmoopen-2018-000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson S, Larsen RH, Fosså SD, et al. . First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451–9. 10.1158/1078-0432.CCR-04-2244 [DOI] [PubMed] [Google Scholar]
- 6.Nilsson S, Franzén L, Parker C, et al. . Bone-Targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587–94. 10.1016/S1470-2045(07)70147-X [DOI] [PubMed] [Google Scholar]
- 7.Nilsson S, Strang P, Aksnes AK, et al. . A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012;48:678–86. 10.1016/j.ejca.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 8.Nilsson S, Franzén L, Parker C, et al. . Two-Year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer 2013;11:20–6. 10.1016/j.clgc.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, et al. . Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 10.Sartor O, Coleman R, Nilsson S, et al. . Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014;15:738–46. 10.1016/S1470-2045(14)70183-4 [DOI] [PubMed] [Google Scholar]
- 11.Humm JL, Sartor O, Parker C, et al. . Radium-223 in the treatment of osteoblastic metastases: a critical clinical review. Int J Radiat Oncol Biol Phys 2015;91:898–906. 10.1016/j.ijrobp.2014.12.061 [DOI] [PubMed] [Google Scholar]
- 12.Parker C, Finkelstein SE, Michalski JM, et al. . Efficacy and safety of radium-223 dichloride in symptomatic castration-resistant prostate cancer patients with or without baseline opioid use from the phase 3 ALSYMPCA trial. Eur Urol 2016;70:875–83. 10.1016/j.eururo.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 13.Parker CC, Coleman RE, Sartor O, et al. . Three-Year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized Alpharadin in symptomatic prostate cancer trial. Eur Urol 2018;73:427–35. 10.1016/j.eururo.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Sartor O, Coleman RE, Nilsson S, et al. . An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol 2017;28:1090–7. 10.1093/annonc/mdx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruland Øyvind S, Nilsson S, Fisher DR, et al. . High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006;12:6250s–7. 10.1158/1078-0432.CCR-06-0841 [DOI] [PubMed] [Google Scholar]
- 16.Smeland S, Bielack SS, Whelan J, et al. . Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American osteosarcoma study) cohort. Eur J Cancer 2019;109:36–50. 10.1016/j.ejca.2018.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janeway KA, Barkauskas DA, Krailo MD, et al. . Outcome for adolescent and young adult patients with osteosarcoma: a report from the children's Oncology Group. Cancer 2012;118:4597–605. 10.1002/cncr.27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagmay JP, Krailo MD, Dang H, et al. . Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through children's cancer group, pediatric Oncology group, and children's Oncology group: learning from the past to move forward. J Clin Oncol 2016;34:3031–8. 10.1200/JCO.2015.65.5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marina NM, Smeland S, Bielack SS, et al. . Comparison of MAPIE versus map in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016;17:1396–408. 10.1016/S1470-2045(16)30214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacci G, Longhi A, Bertoni F, et al. . Bone metastases in osteosarcoma patients treated with neoadjuvant or adjuvant chemotherapy: the Rizzoli experience in 52 patients. Acta Orthop 2006;77:938–43. 10.1080/17453670610013268 [DOI] [PubMed] [Google Scholar]
- 21.Spraker-Perlman HL, Barkauskas DA, Krailo MD, et al. . Factors influencing survival after recurrence in osteosarcoma: a report from the children's Oncology Group. Pediatr Blood Cancer 2019;66:e27444. 10.1002/pbc.27444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen EL, Yoo CH, Gutkin PM, et al. . Outcomes for pediatric patients with osteosarcoma treated with palliative radiotherapy. Pediatr Blood Cancer 2020;67:e27967. 10.1002/pbc.27967 [DOI] [PubMed] [Google Scholar]
- 23.Meazza C, Casanova M, Luksch R, et al. . Prolonged 14-day continuous infusion of high-dose ifosfamide with an external portable pump: feasibility and efficacy in refractory pediatric sarcoma. Pediatr Blood Cancer 2010;55:617–20. 10.1002/pbc.22596 [DOI] [PubMed] [Google Scholar]
- 24.Anderson P. Continuously improving ifosfamide/mesna: a winning combination. Pediatr Blood Cancer 2010;55:599–600. 10.1002/pbc.22652 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Kawedia JD, Myers AL, et al. . Physical and chemical stability of high-dose ifosfamide and mesna for prolonged 14-day continuous infusion. J Oncol Pharm Pract 2014;20:51–7. 10.1177/1078155213478284 [DOI] [PubMed] [Google Scholar]
- 26.Martin-Liberal J, Alam S, Constantinidou A, et al. . Clinical activity and tolerability of a 14-day infusional ifosfamide schedule in soft-tissue sarcoma. Sarcoma 2013;2013:1–6. 10.1155/2013/868973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanova M, Ferrari A, Bisogno G, et al. . Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European rhabdomyosarcoma protocol. Cancer 2004;101:1664–71. 10.1002/cncr.20544 [DOI] [PubMed] [Google Scholar]
- 28.Ferrari A, Grosso F, Stacchiotti S, et al. . Response to vinorelbine and low-dose cyclophosphamide chemotherapy in two patients with desmoplastic small round cell tumor. Pediatr Blood Cancer 2007;49:864–6. 10.1002/pbc.20682 [DOI] [PubMed] [Google Scholar]
- 29.Parrales A, McDonald P, Ottomeyer M, et al. . Comparative oncology approach to drug repurposing in osteosarcoma. PLoS One 2018;13:e0194224. 10.1371/journal.pone.0194224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guenther LM, Rowe RG, Acharya PT, et al. . Response evaluation criteria in solid tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood Cancer 2018;65:1–2. 10.1002/pbc.26896 [DOI] [PubMed] [Google Scholar]
- 31.Bielack SS, Kempf-Bielack B, Branscheid D, et al. . Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma Study Group patients. J Clin Oncol 2009;27:557–65. 10.1200/JCO.2008.16.2305 [DOI] [PubMed] [Google Scholar]
- 32.Jaffe N, Carrasco H, Raymond K, et al. . Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer 2002;95:2202–10. 10.1002/cncr.10944 [DOI] [PubMed] [Google Scholar]
- 33.Targeted Alpha Therapy Working Group, Parker C, Lewington V, et al. . Targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncol 2018;4:1765–72. 10.1001/jamaoncol.2018.4044 [DOI] [PubMed] [Google Scholar]
- 34.Bielack SS, Smeland S, Whelan JS, et al. . Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative map: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 2015;33:2279–87. 10.1200/JCO.2014.60.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown LC, Lester RA, Grams MP, et al. . Stereotactic body radiotherapy for metastatic and recurrent Ewing sarcoma and osteosarcoma. Sarcoma 2014;2014:1–9. 10.1155/2014/418270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L, McManus MM, Hughes DPM. Understanding the biology of bone sarcoma from early initiating events through late events in metastasis and disease progression. Front Oncol 2013;3:230. 10.3389/fonc.2013.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera-Valentin RK, Zhu L, Hughes DPM. Bone sarcomas in pediatrics: progress in our understanding of tumor biology and implications for therapy. Paediatr Drugs 2015;17:257–71. 10.1007/s40272-015-0134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conry RM, Rodriguez MG, Pressey JG. Zoledronic acid in metastatic osteosarcoma: encouraging progression free survival in four consecutive patients. Clin Sarcoma Res 2016;6:6. 10.1186/s13569-016-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solberg TD, Nearman J, Mullins J, et al. . Correlation between tumor growth delay and expression of cancer and host VEGF, VEGFR2, and osteopontin in response to radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:918–26. 10.1016/j.ijrobp.2008.06.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia S-F, Guan H, Duan X, et al. . VEGF165 is necessary to the metastatic potential of Fas(-) osteosarcoma cells but will not rescue the Fas(+) cells. J Exp Ther Oncol 2008;7:89–97. [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Yang D, Sun Y, et al. . Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer 2011;117:4925–38. 10.1002/cncr.26116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ługowska I, Woźniak W, Klepacka T, et al. . A prognostic evaluation of vascular endothelial growth factor in children and young adults with osteosarcoma. Pediatr Blood Cancer 2011;57:63–8. 10.1002/pbc.23021 [DOI] [PubMed] [Google Scholar]
- 43.Navid F, Santana VM, Neel M, et al. . A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer 2017;141:1469–77. 10.1002/ijc.30841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safwat A, Boysen A, Lücke A, et al. . Pazopanib in metastatic osteosarcoma: significant clinical response in three consecutive patients. Acta Oncol 2014;53:1451–4. 10.3109/0284186X.2014.948062 [DOI] [PubMed] [Google Scholar]
- 45.Elete KR, Albritton KH, Akers LJ, et al. . Response to pazopanib in patients with relapsed osteosarcoma. J Pediatr Hematol Oncol 2018:1. 10.1097/MPH.0000000000001375 [DOI] [PubMed] [Google Scholar]
- 46.Longhi A, Paioli A, Palmerini E, et al. . Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol 2019;58:124–8. 10.1080/0284186X.2018.1503714 [DOI] [PubMed] [Google Scholar]
- 47.Grignani G, Palmerini E, Dileo P, et al. . A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian sarcoma group study. Ann Oncol 2012;23:508–16. 10.1093/annonc/mdr151 [DOI] [PubMed] [Google Scholar]
- 48.Grignani G, Palmerini E, Ferraresi V, et al. . Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol 2015;16:98–107. 10.1016/S1470-2045(14)71136-2 [DOI] [PubMed] [Google Scholar]
- 49.Mei J, Zhu X, Wang Z, et al. . Vegfr, RET, and Raf/MEK/ERK pathway take part in the inhibition of osteosarcoma MG63 cells with sorafenib treatment. Cell Biochem Biophys 2014;69:151–6. 10.1007/s12013-013-9781-7 [DOI] [PubMed] [Google Scholar]
- 50.Armstrong AE, Walterhouse DO, Leavey PJ, et al. . Prolonged response to sorafenib in a patient with refractory metastatic osteosarcoma and a somatic PDGFRA D846V mutation. Pediatr Blood Cancer 2019;66:e27493. 10.1002/pbc.27493 [DOI] [PubMed] [Google Scholar]
- 51.Daudigeos-Dubus E, Le Dret L, Lanvers-Kaminsky C, et al. . Regorafenib: antitumor activity upon mono and combination therapy in preclinical pediatric malignancy models. PLoS One 2015;10:e0142612. 10.1371/journal.pone.0142612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffaud F, Mir O, Boudou-Rouquette P, et al. . Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2019;20:120–33. 10.1016/S1470-2045(18)30742-3 [DOI] [PubMed] [Google Scholar]
- 53.Anderson P. Chemotherapy for osteosarcoma with high-dose methotrexate is effective and outpatient therapy is now possible. Nat Clin Pract Oncol 2007;4:624–5. 10.1038/ncponc0953 [DOI] [PubMed] [Google Scholar]
- 54.Nicolini A, Mancini P, Ferrari P, et al. . Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC). Biomed Pharmacother 2004;58:447–50. 10.1016/j.biopha.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 55.Thornton KA, Chen AR, Trucco MM, et al. . A dose-finding study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcoma. Int J Cancer 2013;133:997–1005. 10.1002/ijc.28083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trucco MM, Meyer CF, Thornton KA, et al. . A phase II study of temsirolimus and liposomal doxorubicin for patients with recurrent and refractory bone and soft tissue sarcomas. Clin Sarcoma Res 2018;8:21. 10.1186/s13569-018-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy JD, Spalding AC, Somnay YR, et al. . Inhibition of mTOR radiosensitizes soft tissue sarcoma and tumor vasculature. Clin Cancer Res 2009;15:589–96. 10.1158/1078-0432.CCR-08-1019 [DOI] [PubMed] [Google Scholar]
- 58.Anderson PM, Meyers P, Kleinerman E, et al. . Mifamurtide in metastatic and recurrent osteosarcoma: a patient access study with pharmacokinetic, pharmacodynamic, and safety assessments. Pediatr Blood Cancer 2014;61:238–44. 10.1002/pbc.24686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson PM, Tomaras M, McConnell K. Mifamurtide in osteosarcoma--a practical review. Drugs Today 2010;46:327–37. 10.1358/dot.2010.46.5.1500076 [DOI] [PubMed] [Google Scholar]
- 60.Advani R, Flinn I, Popplewell L, et al. . Cd47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 2018;379:1711–21. 10.1056/NEJMoa1807315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sikic BI, Lakhani N, Patnaik A, et al. . First-In-Human, first-in-class phase I trial of the Anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 2019;37:946–53. 10.1200/JCO.18.02018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrow JJ, Bayles I, Funnell APW, et al. . Positively selected enhancer elements endow osteosarcoma cells with metastatic competence. Nat Med 2018;24:176–85. 10.1038/nm.4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhawan A, Nichol D, Kinose F, et al. . Collateral sensitivity networks reveal evolutionary instability and novel treatment strategies in ALK mutated non-small cell lung cancer. Sci Rep 2017;7:1232. 10.1038/s41598-017-00791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]