Abstract
Background
Because Helicobacter pylori (H. pylori) infection and Environmental Enteric Dysfunction (EED) follow a similar mode of transmission, there can be a complex interplay between H. pylori infection and EED, both of which can influence childhood growth. We sought to investigate the factors associated with H. pylori infection and identify its relationship with the fecal biomarkers of EED including Myeloperoxidase (MPO), Neopterin (NEO), Calprotectin, Reg1B and Alpha-1 antitrypsin (AAT), and nutritional status of the children.
Methodology
Data from an on-going community-based nutrition intervention study was used for this analysis. Total 319 children aged between 12–18 months were evaluated at enrolment and at the end of a 90-day nutrition intervention. Multivariable linear regression with generalized estimating equations was done to examine the association of H. pylori infection with stool biomarker of EED and nutritional status of the children.
Principal findings
One-fifth of the participants had H. pylori infection at both the time points, with 13.8% overall persistence. Children living in crowded households had higher odds of being infected by H. pylori (AOR = 2.02; 95% CI = 1.02, 4.10; p-value = 0.045). At enrolment, 60%, 99%, 69% and 85% of the stool samples were elevated compared to the reference values set for MPO, NEO, AAT and Calprotectin in the non-tropical western countries. The proportions reduced to 52%, 99%, 67%, and 77% for the same biomarkers after the nutrition intervention. Infection with H. pylori had significant positive association with fecal AAT concentrations (Coefficient = 0.26; 95% CI = 0.02, 0.49; p-value = 0.03) and inverse relationship with Reg1B concentrations measured in the stool samples (Coefficient = -0.32; 95% CI = -0.59, -0.05; p-value = 0.02). However, H. pylori infection was not associated with the indicators of childhood growth.
Conclusions
The study findings affirmed that the acquisition and persistence of H. pylori infection in the early years of life may exert an adverse impact on intestinal health, induce gut inflammation and result in increased intestinal permeability.
Author summary
Infection with H. pylori, a substantial public health burden in the tropical countries, follows the similar mode of transmission analogous to Environmental Enteric Dysfunction (EED). There can be a complex interplay between H. pylori infection and EED–both of which can influence childhood growth–but the definite role of H. pylori infection contributing to EED and subsequent growth failure is poorly understood. In this study, the authors present data from an ongoing community-based nutrition intervention study and investigated the factors associated with H. pylori infection and identify its relationship with fecal biomarkers of EED and indicators of the nutritional status of the children hailing from a resource-poor urban settlement. They demonstrated the acquisition and persistence of H. pylori infection during early childhood. The study results also corroborate that infection with H. pylori had significant positive association with fecal Alpha-1 antitrypsin concentrations and an inverse relationship with Reg1B concentrations measured in stool samples of the children. The findings revealed in this study may contribute to a better understanding of the role of H. pylori infection in contributing to EED as well as alteration of gut function in the early years of life.
Introduction
Infection with Helicobacter pylori (H. pylori) has emerged as a substantial public health burden over the past couple of decades [1]. The infection is highly prevalent in low-income countries and affects more than half of the global population [2]. The organism is obtained mostly by oral ingestion and induces chronic inflammation of the underlying gastric mucosa [3, 4]. The pathology is associated with diarrheal diseases, malnutrition and subsequent growth failure in children [5–8]. Evidence suggests that H. pylori infection is primarily acquired at the early years of life and can persist for a long period of time [9–11]. Infection acquired in the early age induces malabsorption and implicates in growth retardation [8]. The prevalence of the infection varies from around 10% to over 80% in children living in different regions of the world [12]. A birth cohort study, conducted in Bangladesh, showed that 50–60% of Bangladeshi children had the H. pylori infection by 2 years of their age [13]. Epidemiologic studies demonstrated that first two years of life is critical for growth [14] as well as for the acquisition of H. pylori infection [15]. Consistent with those reports, many recent studies have exhibited the association of growth impairment with H. pylori infection, especially among those living in resource poor settings [8, 16–18]. Conversely, H. pylori has also been found to be linked with improved nutritional status in children, although the mechanism has not yet been elucidated [16, 19]. There is also evidence of having no relationship between infection with H. pylori and nutritional status of younger children [20]. The definite role of H. pylori on nutritional status of children is paradoxical and the findings are “mixed-bag”. However, H. pylori infection can induce inflammatory responses as well as production of pro-inflammatory cytokines, and leads to malabsorption of essential nutrients [8]. Infection with H. pylori may also predisposes to multiple enteric pathogens resulting is altered intestinal health and function [8, 21].
Environmental Enteric Dysfunction (EED), an asymptomatic small intestinal pathology, has been implicated in linear growth failure of children less than two years of age [14, 22]. EED is characterized by persistent immune activation, gut inflammation and altered intestinal permeability resulting from chronic exposure to intestinal pathogens and frequent enteric infections [23–25]. The overall negative impact of EED on child growth and development, especially in their early years of life, is now well established [22, 26]. The condition has been described in the scientific literature since 1960s, but still there is no definite criteria to diagnose the ailment [22]. EED can be diagnosed through small intestinal biopsy which is considered to be the gold standard but difficult to perform in children owing to the invasiveness of the procedure [27]. However, several biomarkers have been tested as markers of EED and found to be associated with features of EED in previous studies [28–30]. Stool biomarkers including Myeloperoxidase (MPO), Neopterin (NEO), Calprotectin, Reg1B, and Alpha-1 antitrypsin (AAT) are the non-invasive alternatives proposed for the assessment of EED [27, 31]. MPO, Calprotectin, and NEO indicate intestinal inflammation, whereas AAT is a useful marker of enteric protein loss as well as intestinal permeability [32]. Reg1B is a newly proposed marker which points to epithelial tissue injury and subsequent repair in the small intestine [33]. EED is attributable to microbial contamination of food and water associated with poor sanitation and hygiene [34, 35]. Since H. pylori infection also follows the similar mode of transmission, there can be a complex interplay between the acquisition of H. pylori infection, EED and impaired growth in the first two years of life. Prior studies showed that H. pylori infection induces gastritis and results in protein losing enteropathy with evidence of resolution of the enteropathy by eradication of the infection [36, 37]. To that end, we hypothesized that H. pylori infections may contribute to and exacerbate EED and subsequent growth failure in children. But till date, no attempt was made to investigate the definite role of H. pylori infection contributing to EED and subsequent growth failure in children less than two years of age. Given the high prevalence of both H. pylori infection as well as EED in this patient population, investigation into how each condition influences the other and patient outcomes is of high importance. Therefore, we sought to investigate the factors associated with H. pylori infection and identify its relationship with fecal biomarkers of EED and indicators of the nutritional status of the children hailing from a resource-poor urban settlement in Dhaka, Bangladesh.
Methods
Ethics statement
The research protocol of this study (protocol no.: PR-16007) was approved by the Institutional Review Board of the International Center for Diarrheal Disease Research, Bangladesh (icddr,b), and written informed consent was obtained from the parents or legal guardians.
Study design, site and population
Data from the Bangladesh Environmental Enteric Dysfunction (BEED) study was used to conduct this analysis. In brief, the BEED study is an ongoing community-based nutrition intervention study that is being conducted in the Mirpur area, a suburb located in the capital city of Bangladesh. In this study, children aged between 12 to 18 months either stunted [length-for-age z score (LAZ) <2] or at risk of stunting [LAZ = −1 to −2] are being enrolled for an intervention for 90 feeding days. The enrolled children receive an egg, 150 ml of whole milk, micronutrient sprinkles and nutritional counseling daily for 6 days in a week. A total of 319 children living in the slums of Mirpur area were included in this analysis. We included only those children who completed the nutrition intervention, and had data in both the time points–at enrollment and at the end of nutrition intervention. Exclusion criteria for enrollment in BEED study are: severe acute malnutrition, severe anemia, tuberculosis, presence of any congenital anomaly or deformity, suffering from diarrhoea or history of persistent diarrhoea in the preceding month, another family member already enrolled in the BEED study, and presence of any severe or chronic disease. The methodology of BEED study has been published previously [38].
Data collection
Field staff collected the socio-economic and household information of the participants from the parents or caregivers at enrollment. Anthropometry was measured by the trained field staff following standard operating procedures (SOPs) based on the manuals of WHO and CDC [39, 40]. In order to ensure the consistency of an anthropometric measurement from one rater to another, we provided refresher’s training to the field staff and estimated intra-class correlation coefficient (ICC) periodically every three months. Such training results in significant improvement of raters pertaining to anthropometric measurements at field site with a coefficient more than 0.9 for each of the scales. Indicators of nutritional status such as length-for-age z (LAZ), weight-for-age z (WAZ), and weight-for-height z (WHZ) scores were calculated using WHO anthropometry software. Blood and non-diarrheal stool samples were collected at baseline and after completion of 90-day nutrition intervention. Stool samples were obtained without using any fixative and frozen at −70°C until analysis.
Laboratory analysis
All the laboratory assays were carried out at icddr,b in Dhaka, Bangladesh. Blood samples were collected and centrifuged for 10 minutes at 4000 rotation per minute to separate the plasma. Aliquots were immediately stored at -80°C till analysis. The inflammatory markers including high sensitivity CRP (Immundiagnostik, Bensheim, Germany) and AGP (Alpco, Salem, NH, USA) were analyzed from the plasma samples. Fecal biomarkers including AAT (Biovendor, Chandler, North Carolina), NEO (GenWay Biotech, San Diego, California), Reg1B (TechLab, Blacksburg, Virginia), Calprotectin (BÜHLMANN fCAL, Schönenbuch, Switzerland), and MPO (Alpco, Salem, New Hampshire) were measured in the stool samples using kits available for enzyme-linked immunosorbent assay (ELISA) following the instructions given by the manufacturers. Calibration curves were used to quantify the levels of each biomarker. In this study, fecal antigen test for H. pylori was employed to detect the H. pylori in the stool samples. This is a well-recognized non-invasive technique for the detection of H. pylori infection in the children [41]. Stool was analyzed for H. pylori antigen through ELISA using Amplified IDEIA™ Hp StAR™ (OXOID Limited, Hampshire, United Kingdom). Dual wavelength of 450/630nm was used following the instruction of the manufacturer.
Variables used in this analysis
We used H. pylori infection as the exposure variable. It was a binary categorical variable categorized based on the absorbance values derived from the stool ELISA results. Stool specimens with absorbance values ≥0.15 were considered positive and specimens with absorbance values <0.15 were considered negative for infection with H. pylori. Fecal biomarkers (e.g. MPO, NEO, Calprotectin, REG1B and AAT) and the indicators of nutritional status (e.g. LAZ, WAZ, and WHZ) were the outcome variables in our analyses. The covariates such as treatment of drinking water, source of drinking water, source of cooking water, hand washing practice after toilet, hand washing practice after helping the child to defecate, hand washing practice before cooking, separate space for kitchen, animal exposure at households, educational status of mothers and heads of households, and crowded living conditions were categorical variables. Crowded living condition was defined if more than 4 household members sleep in a single room[42]. Markers of systemic inflammation (e.g. CRP and AGP) were also included as covariates in this analysis (see the list of variables in S1 Table). We also divided the children enrolled in this study into four groups based on their infection with H. pylori and created a categorical variable–H. pylori infection status. The categories of the variable are: a) children who had infection at enrollment but got cleared by the end of study, b) who acquired new infection during the study, c) children who remained infected at enrollment and at the end of nutrition intervention, and d) who remained non-infected in both the time points.
Statistical analyses
Demographic and socio-economic characteristics were described by frequency with proportions for categorical variables, mean with standard deviation for symmetric continuous variables and median with inter-quartile ranges (IQR) for asymmetric continuous data. T-test, Wilcoxon rank-sum test and Pearson’s chi-square test were applied to compare the baseline characteristics between the stunted and at risk of stunting children. The univariate Pearson’s chi-square test was used to measure the differences in the prevalence of H. pylori infection both in stunted and at risk of stunting children at both the time points. We have identified the factors associated with H. pylori infection in non-diarrheal stool samples during enrollment using logistic regression model. Variables were assessed individually and were included in the multivariable logistic regression model if the p-value was found <0.2 in bivariate analysis. Education of household head was included in the model because of its previously reported association with the H. pylori infection in children [16, 42]. Additionally, the model was adjusted for age, sex, and nutritional status of the enrolled participants at enrolment.
Stool concentrations of all the fecal biomarkers (AAT, Reg1B, MPO, Calprotectin and NEO) were log-transformed. We then examined the association between H. pylori infection and stool biomarker concentrations and subsequently the association between H. pylori infection and indicators of nutritional status (LAZ, WAZ, and WLZ) of the children using multivariable linear regression with generalized estimating equations (GEE). In both the analyses, the family was Gaussian, identity was the link function and the correlation matrix was unstructured. The correlation matrix was selected based on the lowest quasi-likelihood under independence model criterion (QIC) value. Multicollinearity among the independent variables was checked for all the models using variance inflation factor (VIF) values. At first, bivariate analysis was done to explore the unadjusted effect of the variables on the outcomes using individual GEE model. Variables were included in the multivariable models if the p-value was found <0.2 in the bivariate analyses. In addition, all the estimates were adjusted for age and sex of the enrolled participants. We also performed multivariable linear regression analysis to test the association between H. pylori infection status and biomarker values at the end of nutrition intervention. The biomarker values were log-transformed prior to analysis and the models were adjusted for age, sex, and nutritional status of the children at enrollment. Herein, we considered the children who remained non-infected as the reference group. A complete case analysis was applied for all the analyses and statistical significance was defined as a two-sided p-value<0.05. The statistical analyses were conducted using R version 3.5.1 (https://www.r-project.org, Foundation for Statistical Computing, Vienna, Austria) software.
Results
A total of 319 children were included in this analysis. Among them 154 were stunted and 165 were at risk of being stunted children. The mean (±SD) age of the children was 14.5 (±2.1) months and 47.3% of the enrolled children were male. Almost 80% of the mothers received formal education. Water treatment rate was higher in the families of the children who are at risk of being stunted compared to the families of stunted children and it was found statistically significant (p = 0.047). The living condition of stunted children was more crowded than that of their peers. Compared to their counterparts, stunted children were more exposed to animals at the household level. The monthly family income of the stunted children was lower than that of at risk of stunting children. Table 1 describes the baseline characteristics of the enrolled children.
Table 1. Descriptive characteristics of the stunted and at risk of being stunted children at enrollment.
| Variables | Stunted (n = 154) |
At risk of stunting (n = 165) | Total (N = 319) |
p-value |
|---|---|---|---|---|
| Socio-demographic variables | ||||
| Age in month, mean (SD) | 14.6 (2.1) | 14.4 (2.0) | 14.5 (2.1) | 0.39 |
| Gender (Male), n (%) | 88 (57.1%) | 63 (38.2%) | 151 (47.3%) | 0.001 |
| LAZ, mean (SD) | -2.9 (0.7) | -1.6 (0.3) | -2.2 (0.8) | <0.001 |
| WAZ, mean (SD) | -2.3 (0.8) | -1.4 (0.7) | -1.8 (0.9) | <0.001 |
| WHZ, mean (SD) | -1.1 (0.8) | -0.87 (0.9) | -0.98 (0.9) | 0.01 |
| Mothers received education, n (%) | 120 (77.9%) | 131 (79.4%) | 251 (78.7%) | 0.75 |
| Household head received education, n (%) | 98 (68.1%) | 117 (74.5%) | 215 (71.4%) | 0.22 |
| Water treatment, n (%) | 84 (54.5%) | 108 (65.5%) | 192 (60.2%) | 0.047 |
| Separate space for kitchen, n (%) | 124 (80.5%) | 145 (87.9%) | 269 (84.3%) | 0.07 |
| Always wash hand before cooking, n (%) | 14 (9.1%) | 24 (14.6%) | 38 (11.9%) | 0.13 |
| Always wash hand after toilet, n (%) | 103 (66.9%) | 126 (76.4%) | 229 (71.8%) | 0.06 |
| Always wash hand after child defecation, n (%) | 85 (55.2%) | 99 (60%) | 184 (57.7%) | 0.39 |
| Improved toilet, n (%) | 99 (64.3%) | 108 (65.5%) | 207 (64.9%) | 0.83 |
| Crowded living conditions, n (%) | 43 (27.9%) | 31 (18.8%) | 74 (23.2%) | 0.05 |
| Animal exposure in household, n (%) | 15 (9.9%) | 8 (4.9%) | 23 (7.3%) | 0.09 |
| Monthly family income (USD)*, mean (SD) | 167.3 (84.1) | 190.6 (117.3) | 179.4 (103.1) | 0.04 |
| Markers of systemic inflammation | ||||
| CRP (mg/l), median (IQR) | 1.2 (0.4, 3.2) | 1.0 (0.6, 4.1) | 1.1 (0.5, 3.6) | 0.69 |
| AGP (mg/dl), median (IQR) | 96.3 (70.8, 127.6) | 85.1 (64.1, 122.5) | 92.6 (66.5, 125.6) | 0.11 |
| Fecal biomarkers of EED | ||||
| MPO (ng/mL), median (IQR) | 2740.5 (1457.2, 5505.2) | 2266.0 (1427.0, 4758.0) | 2438.0 (1434.0, 5398.0) | 0.27 |
| NEO (nmol/L), median (IQR) | 2902.0 (1907.0, 4269.0) | 3226.0 (1732.0, 5149.0) | 3068.0 (1850.0, 4548.0) | 0.26 |
| AAT (mg/g), median (IQR) | 0.46 (0.23, 0.68) | 0.47 (0.25, 0.64) | 0.46 (0.24, 0.67) | 0.97 |
| Calprotectin (μg/g), median (IQR) | 524.2 (266.2, 1025.5) | 672.4 (327.9, 1074.8) | 598.3 (300.1, 1041.5) | 0.09 |
| Reg1B (μg/mL), median (IQR) | 62.2 (33.4, 91.7) | 51.9 (30.8, 85.1) | 57.7 (31.4, 89.3) | 0.31 |
*1 USD = 84.21 BDT was used as conversion rate
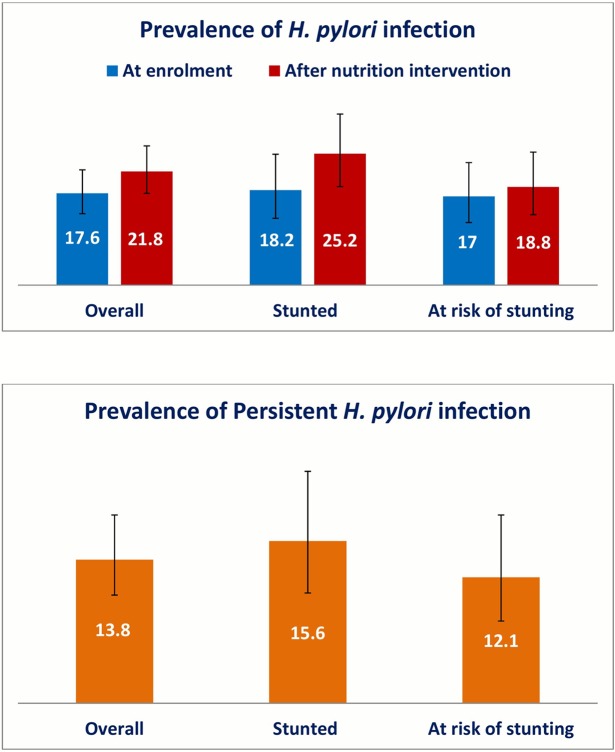
Prevalence of H. pylori infection
The prevalence of infection with H. pylori at enrollment and at the end of nutrition intervention for both the stunted and at risk of being stunted children is presented in Fig 1. Although the proportion of H. pylori positivity was higher in the stool samples of the stunted children compared to at risk of stunting children at both the time points, the difference was not statistically significant (p-value>0.05). The prevalence was lower at enrollment compared to that of at the end of nutrition intervention, but it was not statistically different (p-value>0.05). The prevalence of persistent H. pylori infection as defined by the positivity of H. pylori infection at both the time points was 13.8% in this cohort of children (Fig 1). Persistence of H. pylori infection was more frequent in stunted children (15.6%) compared to at risk of being stunted children (12.1%). Here again, the difference was not found statistically significant (p-value>0.05).
Fig 1. Prevalence of Helicobacter pylori infection in children living in Bangladesh.
Distribution of fecal biomarkers in the stool samples
Overall, the fecal biomarker levels were much higher in the study participants compared to that of the standard in the non-tropical countries where the reference values for MPO, NEO, AAT and Calprotectin are <2,000 ng/mL, <70 nmol/L, <0.27 mg/g, <200 μg/g, respectively [43]. At enrolment, 60%, 99%, 69% and 85% of the stool samples were elevated compared to the reference values set for MPO, NEO, AAT and Calprotectin in the non-tropical western countries. The proportions reduced to 52%, 99%, 67%, and 77% for the same biomarkers after the nutrition intervention. In a recent study, the median values of fecal Reg1B concentration was found 30.8 and 16.5 μg/mL in the children of Bangladesh and Peru, respectively [44]. We have observed much higher concentrations of Reg1B compared to those findings in the stool samples of the children enrolled in this study. The median (IQR) concentration of Reg1B was 57.7 (31.1, 89.3) μg/mL at enrollment and it decreased to 48.3 (17.4, 82.6) μg/mL at the end of nutrition intervention. However, all the fecal biomarker values were reduced significantly after the 90-day nutrition intervention (p-value <0.05).
At both the time points, the concentrations of MPO, AAT and Calprotectin were higher in the stool samples of the H. pylori infected children. But only the difference in fecal AAT concentrations between infected and non-infected children at enrolment was found statistically significant (p-value = 0.04). Fecal NEO and Reg1B concentrations were lower in H. pylori infected children, which was found statistically insignificant at enrollment but significant only for Reg1B at the end of nutrition intervention (p-value = 0.01). There was no statistically significant difference in the fecal biomarker concentrations of children with persistent infection compared to those without persistent infection (p-value>0.05).
Factors associated with H. pylori infection
Multivariable logistic regression model demonstrated that children living in crowded households had higher odds of being infected by H. pylori (AOR = 2.02; 95% CI = 1.02, 4.10; p-value = 0.045) in this cohort after controlling for the age, sex, nutritional status at enrollment, mother’s education, education received by household head, and water treatment. No other socio-demographic factor demonstrated any statistically significant association with the H. Pylori infection (Table 2).
Table 2. Factors associated with Helicobacter pylori infection in children during enrollment.
| Variables | OR (95% CI) | p-value | AOR (95% CI) | p-value |
|---|---|---|---|---|
| Age | 1.11 (0.96, 1.27) | 0.16 | 1.08 (0.93, 1.26) | 0.29 |
| Sex (female) | 0.68 (0.38, 1.21) | 0.19 | 0.62 (0.33, 1.17) | 0.14 |
| Nutritional status (At risk of stunting) | 0.92 (0.52, 1.64) | 0.78 | 1.24 (0.66, 2.33) | 0.51 |
| Crowding (> 4 people sleep per room) | 1.94 (1.04, 3.64) | 0.04 | 2.02 (1.02, 4.10) | 0.045 |
| Mother received education | 0.55 (0.29, 1.05) | 0.07 | 0.62 (0.28, 1.37) | 0.24 |
| Household head received education | 0.71 (0.38, 1.34) | 0.29 | 0.99 (0.47, 2.13) | 0.99 |
| Treatment of water | 0.66 (0.37, 1.18) | 0.16 | 0.88 (0.46, 1.67) | 0.69 |
Association of H. pylori infection with the fecal biomarkers of EED
Table 3 showed the association of H. pylori infection with the fecal biomarkers of EED. No significant association was observed between the infection and fecal levels of MPO, NEO, and Calprotectin, both in bivariate and multivariable analysis using GEE. However, H. pylori infection was significantly associated with the fecal concentrations of AAT (Coefficient = 0.26; 95% CI = 0.02, 0.49; p-value = 0.03) after adjusting for age, sex, nutritional status at enrollment, mother’s education, crowded living conditions, water treatment, hand washing practice of mother after toilet, CRP, and AGP. A statistically significant negative association was observed between H. pylori infection and fecal concentrations of REG1B (Coefficient = -0.32; 95% CI = -0.59, -0.05; p-value = 0.02) after adjustment for age, sex, nutritional status at enrollment, mother’s education, and hand washing practice of mother after defecating the child.
Table 3. Association of Helicobacter pylori infection with the fecal biomarkers of EED using GEE¶.
| Variables | AAT, mg/g | MPO, ng/mL | NEO, nmol/L | Calprotectin, μg/g | Reg1B, μg/mL |
|---|---|---|---|---|---|
| Age in days | -0.004(-0.05, 0.04) | -0.02 (-0.07, 0.03) | -0.05 (-0.10, -0.001)* | -0.02 (-0.07, 0.04) | -0.08 (-0.13, -0.02)* |
| Sex (female) | -0.14 (-0.34, 0.06) | 0.12 (-0.09, 0.33) | 0.03 (-0.18, 0.24) | -0.02 (-0.20, 0.25) | 0.12 (-0.10, 0.35) |
| Nutritional status (At risk of stunting) |
-0.07 (-0.27, 0.13) | -0.02 (-0.24, 0.19) | 0.06 (-0.15, 0.28) | -0.10 (-0.33, 0.12) | -0.03 (-0.25, 0.20) |
| Mothers received education | 0.19 (-0.06, 0.45) | 0.20 (-0.07, 0.47) | 0.29 (0.01, 0.56)* | ||
| Water treatment | 0.01 (-0.22, 0.23) | ||||
| Separate space for kitchen | -0.24 (-0.56, 0.08) | ||||
| Animal exposure in household | -0.42 (-0.84, -0.002) | ||||
| Always wash hand after child defecation | -0.14 (-0.39, 0.11) | -0.18 (-0.45, 0.09) | |||
| Always wash hand after toilet | 0.21 (-0.03, 0.45) | 0.26 (0.02, 0.49)* | -0.01 (-0.27, 0.29) | -0.02 (-0.31, 0.28) | |
| Crowding | -0.09 (-0.33, 0.15) | 0.15 (-0.10, 0.40) | |||
| CRP | 0.01 (-0.01, 0.03) | 0.02 (-0.01, 0.04) | -0.03 (-0.05, -0.01)* | 0.01 (-0.01, 0.04) | |
| AGP | 0.001 (-0.001, 0.003) | 0.001 (-0.001, 0.003) | 0.003 (0.0003, 0.005)* | ||
| Helicobacter pylori infection | 0.26 (0.02, 0.49)* | 0.14 (-0.11, 0.39) | -0.02 (-0.26, 0.23) | -0.07 (-0.34, 0.20) | -0.32 (-0.59, -0.05)* |
¶Each column represents an individual model. The adjusted coefficient with 95% confidence interval (CI) has been reported.
The asterisk (*) denotes the statistical significance with a p-value < 0.05.
Abbreviations used: AAT, alpha-1 antitrypsin; MPO, myeloperoxidase; NEO, neopterin; CRP, C-reactive protein; AGP, Alpha-1-acid glycoprotein.
We observed statistically significant association between H. pylori infection status and biomarkers of EED at the end of nutrition intervention (Table 4). The multivariable linear regression analyses showed that children who were infected at enrolment had significantly higher fecal concentrations of AAT (p-value = 0.03), MPO (p-value = 0.01), and calprotectin (p-value = 0.02) at the end of nutrition intervention compared to the children who remained non-infected. Children who acquired infection during study (p-value = 0.03) and who remained infected in the both the time points (p-value = 0.006) had significantly lower concentrations of fecal Reg1B compared to the reference group. Fecal NEO concentration was significantly lower (p-value < 0.001) in children who acquired infection during the study in comparison to the children who had no infection at all.
Table 4. Association of Helicobacter pylori infection status with the fecal biomarkers of EED using multivariable linear regression analysis¶.
| Variables | AAT, mg/g | MPO, ng/mL | NEO, nmol/L | Calprotectin, μg/g | Reg1B, μg/mL |
|---|---|---|---|---|---|
| Age in days | -0.03 (-0.09, 0.03) | -0.04 (-0.08, 0.01) | -0.08 (-0.14, -0.02) * | -0.05 (-0.11, 0.01) | -0.11 (-0.19, -0.03) * |
| Sex (female) | -0.11(-0.31, 0.09) | -0.003 (-0.20, 0.19) | 0.16 (-0.04, 0.36) | -0.12 (-0.36, 0.12) | 0.08 (-0.23, 0.39) |
| Nutritional status (At risk of stunting) |
-0.19(-0.39, 0.01) | 0.05(-0.15, 0.25) | -0.08 (-0.28, 0.12) | 0.03 (-0.21, 0.27) | -0.06 (-0.39, 0.27) |
|
Helicobacter pylori infection status (Ref: non-infected) |
|||||
| Infected at enrolment | 0.58(0.05, 1.11) * | 0.67 (0.16, 1.18) * | 0.19 (-0.32, 0.70) | 0.73 (0.10, 1.36) * | 0.11 (-0.73, 0.95) |
| Infected acquired during study | 0.33(-0.04, 0.70) | 0.30(-0.07, 0.67) | -0.72 (-1.09, -0.35) * | 0.29 (-0.14, 0.72) | -0.68 (-1.27, -0.10) * |
| Remained infected | -0.09(-0.38, 0.20) | 0.14(-0.15, 0.43) | -0.09 (-0.38, 0.20) | 0.27 (-0.08, 0.62) | -0.67 (-1.14, -0.20) * |
¶Multivariable linear regression was applied considering the biomarker values at the end of nutrition intervention as the outcome variables. Each column represents an individual model. Biomarker values were log-transformed prior to analysis. Adjusted coefficient values with 95% CI have been reported in the table.
The asterisk (*) sign indicates the statistical significance.
Association of H. pylori infection with nutritional status of the children
In multivariable analysis, infection with H. pylori was not associated with LAZ score of the children after adjusting for age, sex, maternal height, mother’s education, education received by of household head, crowded living conditions, separate space for kitchen, water treatment, hand washing practice of mother after toilet, and monthly family income. H. pylori infection did not have any significant association with WAZ score of the children after controlling for the variables named age, sex, maternal height, mother’s education, education received by of household head, crowded living conditions, separate space for kitchen, water treatment, improved toilet, hand washing practice of mother after toilet, hand washing practice of mother after defecating child, Animal exposure, AGP, and monthly family income. No statistically significant association was observed between the H. pylori infection and WLZ score of the children after adjustment for the above-mentioned confounding variables (Table 5).
Table 5. Association of Helicobacter pylori infection with the indicators of nutritional status in children¶.
| Variables | LAZ | WAZ | WLZ |
|---|---|---|---|
| Age | -0.02 (-0.06, 0.2) | -0.02 (-0.07, 0.03) | -0.03 (-0.08, 0.02) |
| Sex (female) | 0.34 (0.17, 0.51) * | 0.33 (0.14, 0.53) * | 0.24 (0.04, 0.43) * |
| Mothers received education | 0.18 (-0.06, 0.41) | 0.26 (-0.02, 0.53) | 0.18 (-0.10, 0.46) |
| Household head received education | 0.19 (-0.03, 0.40) | 0.18 (-0.07, 0.43) | 0.16 (-0.09, 0.41) |
| Separate space for kitchen | 0.02 (-0.23, 0.28) | 0.14 (-0.18, 0.44) | 0.08 (-0.24, 0.40) |
| Water treatment | 0.001 (-0.20, 0.19) | 0.22 (0.001, 0.45) | 0.26 (0.04, 0.49) * |
| Improved toilet | 0.08 (-0.13, 0.29) | 0.19 (-0.03, 0.40) | |
| Always wash hand after toilet | 0.14 (-0.06, 0.34) | 0.02 (-0.25, 0.29) | |
| Always wash hand after child defecation | -0.11 (-0.34, 0.13) | -0.14 (-0.35, 0.07) | |
| Monthly family income | 5.2e-06 (-4.8e-06, 0.00001) | 6.2e-06 (-4.5e-06, 0.00002) | 5.7e-06 (-5.2e-06, 0.00002) |
| Crowded living condition | -0.07 (-0.28, 0.14) | -0.10 (-0.33, 0.14) | -0.07 (-0.30, 0.17) |
| Animal exposure in the households | -0.40 (-0.76, -0.03) * | -0.32 (-0.70, 0.05) | |
| Maternal height | 0.02 (0.01, 0.04) * | 0.02 (0.01, 0.04) * | 0.02 (-0.002, 0.04) |
| AGP | -0.002 (-0.002, -0.001) * | -0.002 (-0.003, -0.001) * | |
| Helicobacter pylori infection | 0.05 (-0.05, 0.15) | 0.08 (-0.04, 0.20) | 0.06 (-0.09, 0.21) |
¶Each row represents an individual model. Adjusted coefficient values with 95% confidence interval have been reported in the table.
The asterisk (*) sign indicates the statistical significance.
Discussion
Our study results revealed that children living in crowded households had higher odds of being infected by H. pylori. We observed nearly one-fifth of the participants had H. pylori infection at both the time points, with 13.8% overall persistence. Infection with H. pylori was positively associated with fecal AAT concentrations. An inverse association was observed between the infection and fecal Reg1B concentrations of these children. In addition, a positive association was reported between H. pylori infection and fecal concentrations of AAT, MPO, and calprotectin at the end of nutrition intervention in children who were infected at enrollment compared to the children who remained non-infected during the study period. On the other hand, fecal Reg1B concentration measured at the end of the study was lower in children who acquired infection during the study and who remained infected in both the time points. Past evidence has been limited to the association between H. pylori infection and childhood growth only, and no research was done to explore the role of H. pylori infection on the changes in EED biomarkers. To our knowledge this is the first attempt to investigate the relationship between H. pylori infection, fecal biomarkers of EED, and subsequent child growth in Bangladeshi children living in an urban community. Our findings, which were based on a well-designed community-based nutrition intervention study, provided an accurate estimate of the burden of H. pylori infection as well as its persistence in the children of an urban area in Bangladesh. Moreover, the results of the study reinforce the hypothesis that H. pylori infection may contribute to the exacerbation of altered gut health as well as EED in young children living in poor environment.
It is known that H. pylori can persist at a high rate in the gastrointestinal tract of people living in resource limited settings [11, 45, 46]. The infection is inversely associated with the living conditions as well as the practice of hygiene and sanitation [47]. Previous reports indicated that children from the households with greater number of inhabitants are more prone to have H. pylori infection [2, 48, 49]. Moreover, it is hypothesized that the infection with H. pylori transmits through oral-oral route and within families [50–52]. Our finding also supports the transmission within families and goes in line with the previous evidence of having association between H. pylori infection and excessive household members.
Approximately one-fifth of our study participants, irrespective of their nutritional status, were infected at enrollment. The prevalence increased at the end of nutrition intervention, although it was statistically insignificant. However, the prevalence rate that we have observed was lower compared to the prior researches conducted in this country [11, 52]. A birth cohort study conducted in rural Bangladesh reported the seroprevalence as 47.6% in children at the end of two years of age [53]. Another study that followed the children of an urban slum up to 2 years of age stated 50% of H. pylori positivity using the fecal antigen test in Bangladesh [54]. Earlier studies conducted in India, Argentina, Ethiopia, and Brazil have reported the prevalence of H. pylori infection as 22%, 25%, 48%, and 55%, respectively in children during their early years [9, 16, 54]. Perhaps, improvements in the living standard including access to safe water, improved sanitation, and better hygiene practice played a potential role in reducing the prevalence of the infection in this community [55]. Moreover, the study participants enrolled in this study are from 12 to 18 months of age. The younger age of the participants can be another possible explanation for such lower rate of positivity compared to earlier studies, because the frequency of H. pylori acquisition increases with the increase of age up to 10 years of age [53, 56]. However, since we observed the prevalence of H. pylori infection in children less than two years of age, it substantiates the evidence of the acquisition of H. pylori infection in early childhood, even before two years of age. Our results also confirm the persistence of H. pylori infection in the under-2 children enrolled in this study.
In this study, the fecal biomarker values were much elevated in this cohort of children compared to the reference values for those living in western countries. This finding is consistent with the previous documents published earlier using data from both urban and rural areas of Bangladesh [57, 58]. However, such elevation indicates the widespread perturbation of gut health and integrity in children living in resource limited urban settlement in Bangladesh.
We observed that infection with H. pylori was associated with the increased levels of AAT in the stool samples of the children. Additionally, fecal AAT measured at the end of nutrition intervention was higher in children who were infected at enrollment but recovered by the end of study. AAT is a protein that is released by neutrophils during infection or inflammatory conditions [59, 60]. Since AAT is not produced in the intestine, its presence in the gut lumen can be considered as a measure of gut inflammation [59]. AAT also reflects protein loss due to disruption of mucosal barrier and has been established as a marker of increased intestinal permeability [58, 59]. Because AAT can be used for assessing both intestinal inflammation and increased gut permeability, two important domains of EED, several studies measured fecal AAT as a biomarker of EED [14, 22, 27, 61]. Prior report showed that fecal AAT as well as “EED composite score” comprising AAT were associated with impaired linear growth in children [22, 62]. To that end, AAT measured in the stool samples can be a better readout for diagnosing EED. However, there remains paucity of research investigating the relationship between H. pylori infection and fecal concentrations of AAT. An old case report of a 37 year old man with H. pylori infection documented an elevated level of AAT in his stool samples [63]. Another report showed that H. pylori infection in children was associated with acute gastritis and protein losing enteropathy [36, 64]. They observed resolution of enteropathy as well as improvement of the gastritis after clearance of the organism, and therefore, hypothesized the protein losing enteropathy as a consequence of gastritis caused by H. pylori [36]. Recent evidence has shown that H. pylori bacteria shed outer membrane vesicles (OMV) [65]. OMV is known to be responsible for inducing intestinal barrier dysfunction and tight junction disruption [66]. In accordance with above mentioned old reports as well as the recent evidence pertaining to OMV, our result also corroborates that acquisition of H. pylori infection may contribute to increased intestinal permeability and gut inflammation in infected children. This finding is in favor of our hypothesis and would contribute to advance further understanding on the complex interplay between infection with H. pylori, impaired intestinal health and EED.
Similar to AAT, children with H. pylori infection had higher concentrations of MPO and calprotectin at the end of nutrition intervention. Both MPO and calprotectin are released from neutrophils and indicates intestinal inflammation [32, 67, 68]. MPO has been tested as a biomarker of EED in several studies [14, 22, 27, 28]. Previously, MPO was found to be associated with markers of intestinal permeability including AAT [32, 57, 59]. A positive association of calprotectin with small intestinal bacterial overgrowth has also been determined in another study [59]. Recent reports suggest that H. pylori may colonize in the human gut [69]. Subsequently, the organism may elicit immune responses in the intestine and contributes to intestinal damage as well as pathogenesis of inflammatory bowel disease [69]. Therefore, the elevation of AAT, MPO, and calprotectin in children infected at baseline indicates that infection with H. pylori may exerts an adverse impact on intestinal health. Moreover, it also highlights that the effect may persist for a considerable period of time, even after the resolution of infection. We do not know how long the effect may continue and what can be the long-term consequences, particularly on childhood growth. More longitudinal studies are needed to investigate the exact effect of infection with H. pylori on the domains of EED and growth of the children.
We observed a negative relationship between H. pylori infection and fecal Reg1B concentrations. Reg1B is expressed in human paneth cells and known to have antibacterial effect [70]. Reg1B is also involved in the cellular growth and regeneration [44], and hence, it is supposed to be high in children as they are in the growing stage. A study conducted in Bangladesh also showed that Reg1B concentrations was much higher in children compared to adults [71]. Perhaps, such high amount of Reg1B concentrations with its antimicrobial effect can play a potential role in contributing to the inverse relationship between H. pylori and fecal Reg1B concentrations in young children. However, further insight is required to elucidate the interaction between H. pylori infection and fecal Reg1B concentrations in children during their early years of life.
Observational studies, conducted in different parts of the world, revealed the adverse impact of H. pylori infection on childhood growth and development [72, 73]. Linear growth deficits were much more influenced compared to ponderal growth in H. pylori positive children [72]. In contrast, some studies indicated an absence of association between H. pylori infection and nutritional status of the infected children [74–76]. This is what we found as well in our study. There was an insignificant relationship between H. pylori infection and the indicators of nutritional status in the children. The children enrolled in this study received nutrition intervention for a period of 90-feeding days. All the fecal biomarker values reduced significantly at the end of intervention indicating improvement of gut health among the children. Probably, the effect of nutrition intervention limited the role of H. pylori infection in the children of this cohort. Moreover, we have observed the children only for a shorter period of time, which do not allow postulating the effect of H. pylori infection on the growth of the children. The lower prevalence of H. pylori infection in the study population can be another cause for such relationship. Timing of growth failure resulting from infection with H. pylori should also be taken into consideration. The exact time to potentiate intestinal damage and subsequent growth failure by H. pylori is unknown. Additionally, child growth is a complex phenomenon that depends on multiple factors. It is always challenging to control all the confounders to assess growth of young children in community based epidemiological studies. Inability to control all the confounding variables can be another explanation of having insignificant relationship between the infection and growth of children in this study. Therefore, a longitudinal study starting from birth including collection of all the potential confounders’ information would be better to elucidate the effect of H. pylori infection on childhood growth.
Our study has several shortcomings. First, the participants were malnourished and hailed from a low-resource setting. A comparison group comprising healthy children from improved socio-economic condition would be helpful to understand the impact of H. pylori on EED and nutritional status of children. Second, we could not include some of the variables (e.g. birth weight, breast feeding status, pathogen burden, etc.) that were found to be the significant contributors of childhood growth in earlier studies. The strength of the study includes the comprehensive approach to understand the interactions between H. pylori infection and consequent gut function during early childhood.
In conclusion, the study results affirmed the acquisition and persistence of H. pylori infection during early childhood in the children living in an urban settlement in Dhaka, Bangladesh. Children living in crowded households were more likely to be affected by the infection with H. pylori. The indicators of childhood growth were not associated with the infection caused by H. pylori in this cohort of children. However, the children with H. pylori infection had higher concentrations of AAT, and lower concentrations of Reg1B in their stool samples. This result suggests that H. pylori infection may exert an adverse impact on the intestinal health and function, induce loss of gut integrity and result in intestinal inflammation as well as increased gut permeability. This finding would help better understand the etiology and pathophysiology of EED in young children living in resource poor settings. The study results also indicate the importance of H. pylori infection in contributing to altered gut function and implicate the importance of further research regarding the role of H. pylori infection on EED as well as nutritional status of children during early years of life.
Supporting information
(DOC)
(DOCX)
Acknowledgments
The authors express thanks to the staff and participants of the BEED study as well as the field and laboratory staffs at icddr,b for their valuable contributions. icddr,b is also grateful to the Government of Bangladesh, Canada, Sweden and the UK for providing unrestricted support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This protocol is supported by the Bill and Melinda Gates Foundation under its Global Health Program. Project investment ID is OPP1136751. (https://www.gatesfoundation.org/How-We-Work/Quick-Links/GrantsDatabase/Grants/2015/11/OPP1136751). The funders had no role in the study design; collection, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit and publication of the manuscript.
References
- 1.Begue RE, Gonzales JL, Correa-Gracian H, Tang S. Dietary risk factors associated with the transmission of Helicobacter pylori in Lima, Peru. The American journal of tropical medicine and hygiene. 1998;59(4):637–40. 10.4269/ajtmh.1998.59.637 [DOI] [PubMed] [Google Scholar]
- 2.Aitila P, Mutyaba M, Okeny S, Ndawula Kasule M, Kasule R, Ssedyabane F, et al. Prevalence and Risk Factors of Helicobacter pylori Infection among Children Aged 1 to 15 Years at Holy Innocents Children’s Hospital, Mbarara, South Western Uganda. Journal of tropical medicine. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suerbaum S, Michetti P. Helicobacter pylori infection. New England Journal of Medicine. 2002;347(15):1175–86. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 4.Megraud F. Epidemiology of Helicobacter pylori infection. Gastroenterology Clinics of North America. 1993;22(1):73–88. [PubMed] [Google Scholar]
- 5.Isenbarger DW, Bodhidatta L, Hoge CW, Nirdnoy W, Pitarangsi C, Umpawasiri U, et al. Prospective study of the incidence of diarrheal disease and Helicobacter pylori infection among children in an orphanage in Thailand. The American journal of tropical medicine and hygiene. 1998;59(5):796–800. 10.4269/ajtmh.1998.59.796 [DOI] [PubMed] [Google Scholar]
- 6.Quiñonez JM, Chew F, Torres O, Bégué RE. Nutritional status of Helicobacter pylori-infected children in Guatemala as compared with uninfected peers. The American journal of tropical medicine and hygiene. 1999;61(3):395–8. 10.4269/ajtmh.1999.61.395 [DOI] [PubMed] [Google Scholar]
- 7.Vilchis J, Duque X, Mera R, Morán S, Torres J, González-Cossío T, et al. Association of Helicobacter pylori infection and height of Mexican children of low socioeconomic level attending boarding schools. The American journal of tropical medicine and hygiene. 2009;81(6):1091–6. 10.4269/ajtmh.2009.09-0107 [DOI] [PubMed] [Google Scholar]
- 8.Franceschi F, Annalisa T, Di Rienzo Teresa D, Ianiro G, Franco S, Viviana G, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World Journal of Gastroenterology: WJG. 2014;20(36):12809 10.3748/wjg.v20.i36.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parente JML, Da Silva BB, Palha-Dias MP, Zaterka S, Nishimura NF, Zeitune JM. Helicobacter pylori infection in children of low and high socioeconomic status in northeastern Brazil. The American journal of tropical medicine and hygiene. 2006;75(3):509–12. [PubMed] [Google Scholar]
- 10.Muhsen K, Goren S, Cohen D. H elicobacter pylori Infection in Early Childhood and Growth at School Age. Helicobacter. 2015;20(6):410–7. 10.1111/hel.12227 [DOI] [PubMed] [Google Scholar]
- 11.Sarker S, Mahalanabis D, Hildebrand P, Rahaman M, Bardhan P, Fuchs G, et al. Helicobacter pylori: prevalence, transmission, and serum pepsinogen II concentrations in children of a poor periurban community in Bangladesh. Clinical infectious diseases. 1997;25(5):990–5. 10.1086/516070 [DOI] [PubMed] [Google Scholar]
- 12.Soltani J, Amirzadeh J, Nahedi S, Shahsavari S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iranian journal of pediatrics. 2013;23(1):13 [PMC free article] [PubMed] [Google Scholar]
- 13.Bhuiyan TR, Islam MMT, Uddin T, Chowdhury MI, Janzon A, Adamsson J, et al. Th1 and Th17 responses to Helicobacter pylori in Bangladeshi infants, children and adults. PLoS One. 2014;9(4):e93943 10.1371/journal.pone.0093943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arndt MB, Richardson BA, Ahmed T, Mahfuz M, Haque R, John-Stewart GC, et al. Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. The American journal of tropical medicine and hygiene. 2016;95(3):694–701. 10.4269/ajtmh.16-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenbacher D, Inceoglu J, Bode G, Brenner H. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. The Journal of pediatrics. 2000;136(6):744–8. [PubMed] [Google Scholar]
- 16.Janjetic MA, Mantero P, Rua EC, Balcarce N, de Palma GZ, Catalano M, et al. Dietary and anthropometric indicators of nutritional status in relation to Helicobacter pylori infection in a paediatric population. British Journal of Nutrition. 2015;113(7):1113–9. 10.1017/S0007114515000483 [DOI] [PubMed] [Google Scholar]
- 17.Thomas J, Dale A, Bunn J, Harding M, Coward W, Cole T, et al. Early Helicobacter pylori colonisation: the association with growth faltering in The Gambia. Archives of disease in childhood. 2004;89(12):1149–54. 10.1136/adc.2002.015313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mera RM, Correa P, Fontham EE, Reina JC, Pradilla A, Alzate A, et al. Effects of a new Helicobacter pylori infection on height and weight in Colombian children. Annals of epidemiology. 2006;16(5):347–51. 10.1016/j.annepidem.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 19.Marini E, Maldonado-Contreras AL, Cabras S, Hidalgo G, Buffa R, Marin A, et al. Helicobacter pylori and intestinal parasites are not detrimental to the nutritional status of Amerindians. The American journal of tropical medicine and hygiene. 2007;76(3):534–40. [PubMed] [Google Scholar]
- 20.Mahalanabis D, Rahman MM, Sarker SA, Bardhan PK, Hildebrand P, Beglinger C, et al. Helicobacter pylori infection in the young in Bangladesh: prevalence, socioeconomic and nutritional aspects. International journal of epidemiology. 1996;25(4):894–8. 10.1093/ije/25.4.894 [DOI] [PubMed] [Google Scholar]
- 21.Salama RI, Emara MH, Mostafa HM, Abd-Elsalam S, Alnabawy SM, Elshweikh SA, et al. Helicobacter pylori infection and risk of salmonella infection. Medicine. 2019;98(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. The American journal of tropical medicine and hygiene. 2013;88(2):390–6. 10.4269/ajtmh.2012.12-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosek MN, Ahmed T, Bhutta Z, Caulfield L, Guerrant R, Houpt E, et al. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine. 2017;18:109–17. 10.1016/j.ebiom.2017.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahim SM, Das S, Gazi MA, Mahfuz M, Ahmed T. Association of intestinal pathogens with faecal markers of environmental enteric dysfunction among slum‐dwelling children in the first 2 years of life in Bangladesh. Tropical Medicine & International Health. 2018;23(11):1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George CM, Burrowes V, Perin J, Oldja L, Biswas S, Sack D, et al. Enteric infections in young children are associated with environmental enteropathy and impaired growth. Tropical Medicine & International Health. 2018;23(1):26–33. [DOI] [PubMed] [Google Scholar]
- 26.de Morais MB, da Silva GAP. Environmental enteric dysfunction and growth. Jornal de pediatria. 2019. [DOI] [PubMed] [Google Scholar]
- 27.George CM, Oldja L, Biswas SK, Perin J, Lee GO, Ahmed S, et al. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. The American journal of tropical medicine and hygiene. 2015;93(2):269–75. 10.4269/ajtmh.14-0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddin MI, Islam S, Nishat NS, Hossain M, Rafique TA, Rashu R, et al. Biomarkers of environmental enteropathy are positively associated with immune responses to an oral cholera vaccine in Bangladeshi children. PLoS neglected tropical diseases. 2016;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick BJ, Lee GO, Seidman JC, Haque R, Mondal D, Quetz J, et al. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. The American journal of tropical medicine and hygiene. 2017;96(2):465–72. 10.4269/ajtmh.16-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George CM, Oldja L, Biswas S, Perin J, Lee GO, Kosek M, et al. Geophagy is associated with environmental enteropathy and stunting in children in rural Bangladesh. The American journal of tropical medicine and hygiene. 2015;92(6):1117–24. 10.4269/ajtmh.14-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal NT, Sadiq K, Syed S, Akhund T, Umrani F, Ahmed S, et al. Promising Biomarkers of Environmental Enteric Dysfunction: A Prospective Cohort study in Pakistani Children. Scientific reports. 2018;8(1):2966 10.1038/s41598-018-21319-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahim SM, Das S, Sanin KI, Gazi MA, Mahfuz M, Islam MM, et al. Association of Fecal Markers of Environmental Enteric Dysfunction with Zinc and Iron Status among Children at First Two Years of Life in Bangladesh. The American journal of tropical medicine and hygiene. 2018;99(2):489–94. 10.4269/ajtmh.17-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syed S, Ali A, Duggan C. Environmental enteric dysfunction in children: a review. Journal of pediatric gastroenterology and nutrition. 2016;63(1):6 10.1097/MPG.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. 2016;138(6):e20160641 10.1542/peds.2016-0641 [DOI] [PubMed] [Google Scholar]
- 35.Etheredge AJ, Manji K, Kellogg M, Tran H, Liu E, McDonald CM, et al. Markers of Environmental Enteric Dysfunction Are Associated With Neurodevelopmental Outcomes in Tanzanian Children. Journal of pediatric gastroenterology and nutrition. 2018;66(6):953–9. 10.1097/MPG.0000000000001978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill ID, Sinclair-Smith C, Lastovica A, Bowie M, Emms M. Transient protein losing enteropathy associated with acute gastritis and campylobacter pylori. Archives of disease in childhood. 1987;62(12):1215–9. 10.1136/adc.62.12.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landzberg BR, Pochapin MB. Protein-losing enteropathy and gastropathy. Current treatment options in gastroenterology. 2001;4(1):39–49. 10.1007/s11938-001-0045-z [DOI] [PubMed] [Google Scholar]
- 38.Mahfuz M, Das S, Mazumder RN, Rahman MM, Haque R, Bhuiyan MMR, et al. Bangladesh environmental enteric dysfunction (BEED) study: protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ open. 2017;7(8):e017768 10.1136/bmjopen-2017-017768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Organization WH. Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee. 1995. [PubMed]
- 40.Control CfD, Prevention. National Health and Nutrition Examination Survey (NHANES) anthropometry procedures manual; 2009. USA: CDC; 2012. [Google Scholar]
- 41.Oderda G, Rapa A, Ronchi B, Lerro P, Pastore M, Staiano A, et al. Detection of Helicobacter pylori in stool specimens by non-invasive antigen enzyme immunoassay in children: multicentre Italian study. Bmj. 2000;320(7231):347–8. 10.1136/bmj.320.7231.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami S. Helicobacter pylori infection in children: population‐based age‐specific prevalence and risk factors in a developing country. Acta Paediatrica. 2010;99(2):279–82. 10.1111/j.1651-2227.2009.01542.x [DOI] [PubMed] [Google Scholar]
- 43.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759–66. 10.1016/j.ebiom.2015.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson KM, Buss J, Easley R, Yang Z, Korpe PS, Niu F, et al. REG1B as a predictor of childhood stunting in Bangladesh and Peru–. The American of Clinical Nutrition. 2013;97(5):1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology. 2016;150(1):64–78. 10.1053/j.gastro.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cover TL. Helicobacter pylori diversity and gastric cancer risk. MBio. 2016;7(1):e01869–15. 10.1128/mBio.01869-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y, Wan JH, Li XY, Zhu Y, Graham D, Lu NH. Systematic review with meta‐analysis: the global recurrence rate of Helicobacter pylori. Alimentary pharmacology & therapeutics. 2017;46(9):773–9. [DOI] [PubMed] [Google Scholar]
- 48.Wangda S, Richter JM, Kuenzang P, Wangchuk K, Choden T, Tenzin K, et al. Epidemiology of Helicobacter pylori infection in asymptomatic schoolchildren in Bhutan. Helicobacter. 2017;22(6):e12439. [DOI] [PubMed] [Google Scholar]
- 49.O'ryan ML, Lucero Y, Rabello M, Mamani N, Salinas AM, Peña A, et al. Persistent and transient Helicobacter pylori infections in early childhood. Clinical Infectious Diseases. 2015;61(2):211–8. 10.1093/cid/civ256 [DOI] [PubMed] [Google Scholar]
- 50.Kayali S, Manfredi M, Gaiani F, Bianchi L, Bizzarri B, Leandro G, et al. Helicobacter pylori, transmission routes and recurrence of infection: state of the art. Acta Bio Medica Atenei Parmensis. 2018;89(8-S):72–6. 10.23750/abm.v89i8-S.7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mamishi S, Eshaghi H, Mahmoudi S, Bahador A, Hosseinpour Sadeghi R, Najafi M, et al. Intrafamilial transmission of Helicobacter pylori: genotyping of faecal samples. British journal of biomedical science. 2016;73(1):38–43. 10.1080/09674845.2016.1150666 [DOI] [PubMed] [Google Scholar]
- 52.Sarker SA, Rahman MM, Mahalanabis D, Bardhan PK, Hildebrand P, Beglinger C, et al. Prevalence ofHelicobacter pylori infection in infants and family contacts in a poor Bangladesh community. Digestive diseases and sciences. 1995;40(12):2669–72. 10.1007/bf02220458 [DOI] [PubMed] [Google Scholar]
- 53.Kienesberger S, Perez-Perez GI, Olivares AZ, Bardhan P, Sarker SA, Hasan KZ, et al. When is Helicobacter pylori acquired in populations in developing countries? A birth-cohort study in Bangladeshi children. Gut microbes. 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhuiyan TR, Qadri F, Saha A, Svennerholm A-M. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. The Pediatric infectious disease journal. 2009;28(2):79–85. 10.1097/INF.0b013e31818a5d9d [DOI] [PubMed] [Google Scholar]
- 55.Salih BA. Helicobacter pylori infection in developing countries: the burden for how long? Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. 2009;15(3):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding Z, Zhao S, Gong S, Li Z, Mao M, Xu X, et al. Prevalence and risk factors of Helicobacter pylori infection in asymptomatic Chinese children: a prospective, cross‐sectional, population‐based study. Alimentary pharmacology & therapeutics. 2015;42(8):1019–26. [DOI] [PubMed] [Google Scholar]
- 57.Campbell RK, Schulze KJ, Shaikh S, Mehra S, Ali H, Wu L, et al. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. Journal of pediatric gastroenterology and nutrition. 2017;65(1):40–6. 10.1097/MPG.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fahim SM, Das S, Sanin KI, Gazi MA, Mahfuz M, Islam MM, et al. Association of Fecal Markers of Environmental Enteric Dysfunction with Zinc and Iron Status among Children at First Two Years of Life in Bangladesh. The American journal of tropical medicine and hygiene. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS neglected tropical diseases. 2018;12(1):e0006205 10.1371/journal.pntd.0006205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Serres F, Blanco I. Role of alpha‐1 antitrypsin in human health and disease. Journal of internal medicine. 2014;276(4):311–35. 10.1111/joim.12239 [DOI] [PubMed] [Google Scholar]
- 61.Campbell RK, Schulze K, Shaikh S, Mehra S, Ali H, Wu L, et al. Biomarkers of environmental enteric dysfunction among children in rural Bangladesh. Journal of pediatric gastroenterology and nutrition. 2017;65(1):40 10.1097/MPG.0000000000001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lima AA, Leite AM, Moura AF, Lima NL, Oria RB, Soares AM, et al. , editors. Risk factors, gut function biomarkers and growth deficit associated with environmental enteropathy and malnutrition: The case-control mal-ed study in Fortaleza, Ceara, Brazil. American Journal of Tropical Medicine and Hygiene; 2015: AMER SOC TROP MED & HYGIENE 8000 WESTPARK DR, STE 130, MCLEAN, VA 22101 USA. [Google Scholar]
- 63.Badov D, Lambert J, Finlay M, Balazs N. Helicobacter pylori as a pathogenic factor in Menetrier's disease. The American journal of gastroenterology. 1998;93(10):1976 10.1111/j.1572-0241.1998.00347.x [DOI] [PubMed] [Google Scholar]
- 64.Sullivan P, Thomas J, Eastham E, Lunn P, Neale G. Helicobacter pylori and protein losing enteropathy. Archives of disease in childhood. 1990;65(3):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lekmeechai S, Su Y-C, Brant M, Alvarado-Kristensson M, Vallström A, Obi I, et al. Helicobacter pylori outer membrane vesicles protect the pathogen from reactive oxygen species of the respiratory burst. Frontiers in microbiology. 2018;9 10.3389/fmicb.2018.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chmiela M, Walczak N, Rudnicka K. Helicobacter pylori outer membrane vesicles involvement in the infection development and Helicobacter pylori-related diseases. Journal of biomedical science. 2018;25(1):78 10.1186/s12929-018-0480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biological and Pharmaceutical Bulletin. 2003;26(6):753–60. 10.1248/bpb.26.753 [DOI] [PubMed] [Google Scholar]
- 68.Vaos G, Kostakis ID, Zavras N, Chatzemichael A. The role of calprotectin in pediatric disease. BioMed research international. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World journal of gastroenterology: WJG. 2014;20(21):6374 10.3748/wjg.v20.i21.6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Beelen Granlund A, Østvik AE, Brenna Ø, Torp SH, Gustafsson BI, Sandvik AK. REG gene expression in inflamed and healthy colon mucosa explored by in situ hybridisation. Cell and tissue research. 2013;352(3):639–46. 10.1007/s00441-013-1592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Easley R, Peterson K, Haque R, Petri W, Lyerly D, Buss J. Reg1B and malnutrition in Bangladeshi children. Age (in years).1:5. [Google Scholar]
- 72.Dror G, Muhsen K. Helicobacter pylori infection and children's growth: an overview. Journal of pediatric gastroenterology and nutrition. 2016;62(6):e48–e59. 10.1097/MPG.0000000000001045 [DOI] [PubMed] [Google Scholar]
- 73.Kalach N, Bontems P, Raymond J. Helicobacter pylori infection in children. Helicobacter. 2017;22:e12414. [DOI] [PubMed] [Google Scholar]
- 74.Pacifico L, Osborn JF, Tromba V, Romaggioli S, Bascetta S, Chiesa C. Helicobacter pylori infection and extragastric disorders in children: a critical update. World Journal of Gastroenterology: WJG. 2014;20(6):1379 10.3748/wjg.v20.i6.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Özçay F, Demir H, Özen H, Gürakan F, Saltik IN, Yüce A, et al. Normal growth in young children with Helicobacter pylori infection. Journal of pediatric gastroenterology and nutrition. 2002;35(1):102 10.1097/00005176-200207000-00024 [DOI] [PubMed] [Google Scholar]
- 76.Chiu N-C, Lin C-Y, Chi H, Yeung C-Y, Ting W-H, Chan W-T, et al. Helicobacter pylori infection is not associated with failure to thrive: a case control study. Therapeutics and clinical risk management. 2017;13:273 10.2147/TCRM.S123148 [DOI] [PMC free article] [PubMed] [Google Scholar]