Abstract
Context
Patients with Cushing's syndrome (CS) of any etiology experience a number of physical and psychological symptoms which impact negatively on health-related quality of life (HRQoL).
Subjects and methods
HRQoL was measured using CushingQoL questionnaire.
Results
The first part of our study was a cross-sectional analysis of 141 patients with CS over a 10-year period. CushingQoL score was lower in pituitary CS compared to adrenal CS. Remission and older age were associated with better outcome on item 7 (physical appearance anxiety). In a multivariate regression analysis after adjustment for etiology, remission status, age, UFC, duration of hypercortisolism and presence of hypercortisolism-associated comorbidities the female gender was the only negative predictor associated with poorer outcome on each of the three scores. The presence of hypercortisolism-associated comorbidities independently predicted poorer outcome on the psychological and the global subscales.
The second part of our research was a prospective study of 27 patients with adrenal adenoma. Achievement of remission independently predicted improvement of the total score of any patient.
Conclusion
Studying in details and understanding the mechanisms of the impaired HRQoL in patients with CS is the only way to become aware of the problem and create methods that could help these patients.
Keywords: adrenalectomy, adrenal adenoma, Quality of life, Cushing's syndrome
INTRODUCTION
In the recent years it has become evident that suffering from Cushing's syndrome (CS) negatively impacts patients’ welfare, duration and health-related quality of life (HRQoL), even after achieving endocrine “cure”. Prolonged hypercortisolism causes a wide variety of physical (e.g., pain, easy bruising, and trouble sleeping) and psychological symptoms (e.g., cognitive impairments, irritability, depressive mood), resulting altogether in decreased self-perception of well-being (1-4). Achievement of disease control supposes restoring life expectancy to levels similar to those seen among the general population. However, it is still debatable whether the achievement of biochemical remission is related to better HRQoL. Several studies suggest that it is not always accompanied by self-perceived improvement and “cure” (5-11). Chronic hypercortisolism has some irreversible effects on neurological function with psychological changes, cardiovascular system and metabolic parameters, thus explaining the longstanding impairment of health and HRQoL of patients with CS.
It has been proven with several questionnaires which have been used to evaluate the quality of life in CS (short form-36 (SF-36), visual analogue scale; Hospital Anxiety and Depression Scale (HADS), Nottingham Health Profile (NHP), Multidimensional Fatigue Index (MFI-20), the World Health Organization Quality of Life Scale (WHOQOL-BREF), General Health Questionnaire 28 (GHQ-28), the Functional Assessment of Cancer Therapy (FACT) and the Social Adjustment Scale (SAS1 and SAS2). All of these questionnaires, however, were designed to evaluate either general health or specific topics (fatigue, depression, etc.) and are not disease-specific. The CushingQoL disease-generated questionnaire has been validated by Webb et al. taking into account the specific problems of patients with CS (12). This questionnaire is proven to be feasible, reliable, valid and more informative than all the generic questionnaires since it approaches problems specifically related to CS. And yet interpretation of the questionnaire is dependent on cultural factors associated with nationality of the patients (13).
Based on the literature data, in a significant proportion of patients with CS achieving of stable clinical and biochemical remission is not sufficient to restore the quality of life comparable to that of the general population. We hypothesized that additional contributing factors such as CS comorbidities and concomitant diseases, gender and age, duration of follow-up could also significantly influence the process of restoration of HRQoL in these subjects.
The primary objective of the present study was to assess the HRQol using the disease specific questionnaire (CushingQoL) in a large Bulgarian cohort of patients with different forms of CS, treated and followed-up in a single tertiary center. The secondary objective was to identify reliable predictors of the impaired HRQoL even after achieving biochemical remission in CS patients.
MATERIALS AND METHODS
The study was performed in two stages. The first part was carried out in a cross-sectional design - we investigated 160 patients with different forms and in different stages of the disease (active disease or remission) of CS who have been hospitalized at the Department of Endocrinology of the Medical University – Sofia, Bulgaria over a 10-year period of time (March 2007–March 2017). The study was approved by the local Ethics Committee. Exclusion criteria were exogenous hypercortisolism, oncological and psychiatric diseases as well as other severe or disabling conditions. The patients with malignant forms of CS (n=19) were also excluded from the common analysis. All participants were older than 18 years and signed written informed consent at study entry. All of them fulfilled the questionnaire during their hospitalization for a routine work-up.
The second stage was prospective follow-up of 27 patients with adrenal adenoma (AA) who had active disease at baseline. Of all the patients with this diagnosis (n=40) 8 were with already performed adrenalectomy at the time of fulfilling the questionnaire for the first time, another 5 were lost to follow-up after surgery.
CS was confirmed by elevated urinary free cortisol (UFC), abnormal late-night serum (LNSeC) and/or salivary cortisol (LNSC) and lack of suppression of serum cortisol after overnight 1 mg Dexamethasone (DXM) test. Cushing's disease (CD) was diagnosed by: elevated or normal ACTH; abnormal 2 day/8 mg Dexamethasone suppression test, DDAVP or CRH test; positive MRI and/or postsurgical corticotropic insufficiency. Ectopic ACTH secretion (EACTHS) was proven by either histological examination after surgery for the patients being already operated on or dynamic tests suggestive of ectopic ACTH production in subjects whose source of ACTH secretion was not found by the routine work up. ACTH-independent forms of CS were confirmed by suppressed ACTH and positive adrenal CT/MRI imaging.
Remission of CS was defined by normalized overnight 1 mg DXM suppression test (serum cortisol levels <50 mmol/L), normal 24 h UFC rates and absence of clinical signs and symptoms of active hypercortisolism. Hypocortisolism was defined as low serum morning cortisol or the use of glucocorticoid replacement therapy. Hypopituitarism was defined as deficiency of at least one of the hormones secreted by the anterior pituitary.
Since CS is a rare condition with a very low incidence, a long period of time was necessary for a single tertiary center to achieve a representative sample of patients. Data from the medical records and CushingQoL questionnaire were collected. Demographic (age, gender), clinical (activity of hypercortisolism, presence of comorbidities and related conditions, duration since establishment of the diagnosis) and biochemical data were correlated both to the score of the questionnaire (physical, psychological and global) and to the individual score of each separate question.
Questionnaire
A special disease generated questionnaire was used to assess the health-related quality of life of patients with CS (CushingQoL), designed and validated by Webb et al. (12) after cultural and linguistic adaptation in Bulgarian language and approval by the original authors. It comprises 12 questions divided into two scales—physical (PhS) (3 items) and psychological (PsS) (9 items). Each item has 5 possible answers, rated on a scale of 1 to 5. The score is the sum of all the item responses and can range from 12 (worst HRQoL) to 60 points (best HRQoL) for the global score (Gs); from 3 to 15 for the physical score (Phs); from 9 to 45 for the psychological score (Pss). The score can be interpreted if the number of unanswered items does not exceed 3 (25% of the questions). To simplify interpretation of scores, standardization on a scale from 0 (worst HRQoL) to 100 (best HRQoL) can be done with the following formulas: Y= (x) - min/(max-min)x100 where Y is the recalculated score. X is the sum of all the item responses within the study score; ‘min’ is the minimum (min=12 for the Gs; min=3 for the Phs and min=9 for the Pss), and ‘max’ is the maximum possible score (max=60 for the Gs, max=15 for the Phs, max=45 for the Pss). The questions belonging to the physical and to the psychological subscales are shown in Table 1.
Table 1.
Questions belonging to the physical and to the psychological subscales
| Psychosocial issues subscale | Physical problems subscale |
|---|---|
| 2. I have pain that keeps me from leading a normal life | 1. I have trouble sleeping |
| 5. I am more irritable, I have sudden mood swings and angry outbursts | 3. My wounds take a long time to heal |
| 6. I have less self-confidence, I feel more insecure | 4. I bruise easily |
| 7. I am worried about the changes in my physical appearance due to my illness | |
| 8. I feel less like going out or seeing relatives or friends | |
| 9. I had to give up my social or leisure activities due to my illness | |
| 10. My illness affects my everyday activities such as working or studying | |
| 11. It is difficult for me to remember things | |
| 12. I am worried about my health in the future |
Hormonal assays
The 24-h urinary excretion of free cortisol (UFC, nmol/24 h), as well as the serum cortisol were measured by highly sensitive and specific RIA (Immunotech, Beckman Coulter Co., France). Intra-assay and inter-assay coefficients of variations were for serum ≤ 5.8% and 9.2%, respectively; for urine samples ≤ 8.9% and 13.3%, respectively; for urine extracts ≤ 9.4% and 12.6%, respectively. Analytical sensitivity was 5 nM. Extremely low cross reactivities were obtained against other naturally occurring steroids (aldosterone, corticosterone, cortisone, 11-DOC, progesterone, etc.) or therapeutic drugs (prednisolone, prednisone, spironolactone, etc.) after dichloromethane extraction of the urine samples before testing. Salivary cortisol was determined by fully automated highly sensitive competitive electrochemiluminescence immunoassay (ECLIA) using Elecsys Cortisol reagent kit (Roche).
Plasma morning ACTH was determined by highly-sensitive and specific IRMA method (ACTH Thermo Scientific BRAHMS, Germany) with analytical sensitivity 0.26 pmol/L and functional sensitivity, measured by 20%th intertest variation coefficient – 0.52 pmol/L. These characteristics guaranteed accurate measurement of the low ACTH levels (< 0.5 pmol/L).
Statistical methods
All statistical analyses were performed by SPSS version 25. Descriptive methods were used to summarize and present data - for non-metric data: absolute and relative (%) frequencies; for metric data: mean and standard deviation. Hypothesis testing was done by the following methods: for nonmetric data chi-square test with Fisher's exact probability was applied, for metric paired groups’ data - Student's t-test, for metric independent groups - two-independent samples t-test. ANCOVA model was used to justify the influence of heterogeneous factor of studied patients (demographic, clinical and biochemical characteristics). The significance level of 0.05 was used. The influence of different factors on CushingQol scores was assessed by linear regression in the cross-sectional group with a significance level set up at p < 0.1.
RESULTS
I. Cross-sectional group analysis
In total 160 patients with CD fulfilled the questionnaire for a period of ten years (between March 2007 and March 2017). The distribution of the different etiological forms is shown in Figure 1. The mean scores of the patients with EACTHS were: Gs- 46.18 ± 8.9, Phs- 41.6 ± 15.2, Pss- 47.7 ± 8.99, for the patients with ACC: Gs- 48.26 ± 22.5, Phs- 47.2 ± 23.5, Pss- 51.3 ± 24.6. However, as the questionnaire is not specific for patients with malignant diseases, those with ACC and EACTHS were excluded from the data analysis. All patients with benign primary adrenal forms of CS were analyzed as a common group. The characteristics of the studied patients (n = 141) are shown in Table 2. Within the group of Cushing's disease (n=90) 9 patients underwent radiotherapy, 18 had recurrence of the disease and 7 were diagnosed with hypopituitarism.
Figure 1.
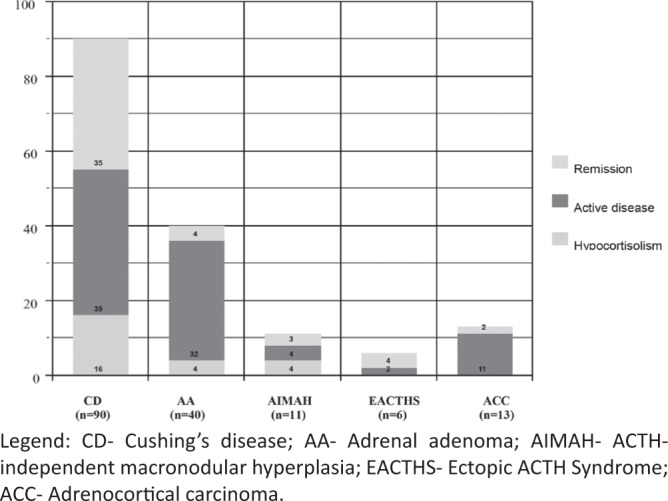
Distribution of the different etiological forms.
Table 2.
Demographic, clinical and biochemical characteristics of studied patients (n = 141) with CS divided into three groups depending on the disease activity
| Active disease (n=69) | Remission (n=72) | ||
|---|---|---|---|
| Eucortisolism (n=48) | Hypocortisolism (n=24) | ||
| Etiology: Pituitary/Adrenal | 9/30 | 35/13 | 16/8 |
| Gender (females) | 63 (91.2%) | 39 (79.6%) | 22 (91%) |
| Age (years – mean ± SD) | 47.4 ± 13 | 45.6 ± 11.59 | 49 ± 11 |
| Time since diagnosis (years) | 3.18 ± 4.52 | 9.69 ± 8.05 | 11.33 ± 12 |
| UFC (nmol/24h) | 417.7 (76 – 2000) | 132 (25 – 210) | 45 (19 – 207) |
| Hypertension | 57 (83%) | 30 (63%) | 4 (17%) |
| Diabetes | 26 (38%) | 20 (42%) | 3 (13%) |
| Osteoporosis | 18 (26%) * | 6 (12.5%) ** | 11 (45%) *** |
| Dyslipidemia | 47 (68%) | 24 (50%) | 10 (42%) |
| Global score | 44.9± 17.9 | 47.5±22.9 | |
| Physical score | 43.6 ± 23.1 | 44.8±25.3 | |
| Psychological score | 45.3± 19.1 | 48.3±23.8 | |
Legend: Missing data for:
27 pts;
13 pts;
4 pts.
Comparison between the different etiological groups
CushingQoL score was lower in pituitary CS compared to adrenal CS for the global score as well as for the two subscales, however, it did not reach statistical significance. The score did not significantly differ between these groups on any item of the questionnaire (Global Score Pituitary CS vs. Adrenal CS, p = 0.5). (Table 3).
Table 3.
Comparison of the scores of the different subscales according to the etiology and the activity of the disease
| Etiology | p value | Disease activity | p value | |||
|---|---|---|---|---|---|---|
|
Pituitary
CS |
Adrenal
CS |
Active
disease |
Remission | |||
| Global score | 45.2± 18 | 47.8 ± 23 | p = 0.5 | 44.9 | 47.4 | p = 0.2 |
| Physical score | 43.2 ± 23 | 46 ± 25 | p = 0.5 | 43.5 | 44.8 | p = 0.3 |
| Psychological score | 45.9 ± 19 | 48.4 ± 24 | p = 0.2 | 45.3 | 48.3 | p = 0.2 |
| Sleep | 2.71 | 2.6 | p = 0.6 | 2.7 | 2.7 | p = 0.8 |
| Pain | 2.91 | 3.14 | p = 0.3 | 3.1 | 2.9 | p = 0.9 |
| Wound healing | 2.96 | 3.46 | p = 0.06 | 3.1 | 3.1 | p = 0.7 |
| Easy bruising | 2.5 | 2.6 | p = 0.05 | 2.4 | 2.7 | p = 0.2 |
| Irritability | 2.89 | 2.86 | p = 0.7 | 2.8 | 2.9 | p = 0.8 |
| Self confidence | 3 | 3.12 | p = 0.9 | 2.9 | 3.2 | p = 0.2 |
| Physical appearance | 2.46 | 2.28 | p = 0.6 | 2.2 | 2.5 | p = 0.04 |
| Leisure time | 2.9 | 2.94 | p = 0.4 | 2.7 | 3.1 | p = 0.2 |
| Social activities | 3.38 | 3.48 | p = 0.9 | 3.38 | 3.45 | p = 0.2 |
| Everyday activities | 3.04 | 2.94 | p = 0.7 | 3.0 | 2.99 | p = 0.4 |
| Memory | 3.12 | 3.38 | p = 0.7 | 3.15 | 3.27 | p = 0.5 |
| Worries on future health | 2.07 | 2.3 | p = 0.2 | 2.0 | 2.3 | p = 0.1 |
Comparison between patients with active hypercortisolism and those in remission
Patients with active disease scored significantly worse than those in remission only for item 7 (physical appearance, p=0.04), (Global Score Pituitary CS vs. Adrenal CS, p = 0.2) (Table 3).
Analysing only active patients, HRQoL did not differ between patients with different etiology on any of the items of the CushingQoL. The subgroup analysis of the patients in remission demonstrated significantly better outcome on item 12 (worries about future health; p=0.025) for patients with adrenal CS compared to those with CD.
Comparison between the genders
According to the gender women had significantly lower scores of all subscales compared to men, corresponding to lower QoL (Fig. 2). Correlations between all the items separately are presented in Figure 3.
Figure 2.
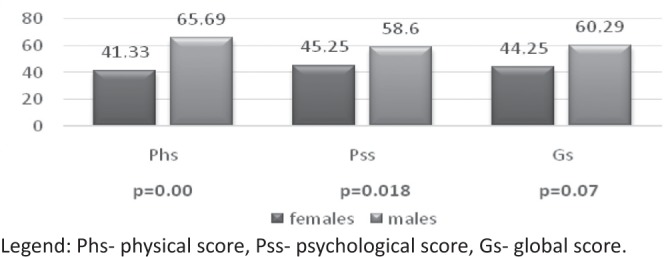
Mean global, physical and psychological subscales scores in women and men.
Figure 3.
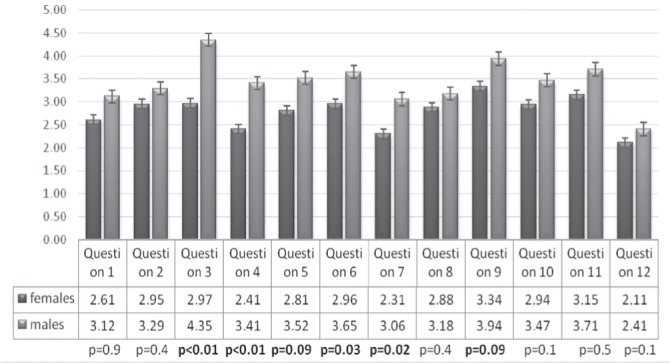
Correlations between all the items separately.
Comparison according to the age
Older age was associated with better outcome on item 7 (physical appearance anxiety, p<0.01).
Comparison according to the presence of hypercortisolism-associated comorbidities
Some co-morbidities were associated with poorer outcome on certain items: arterial hypertension – on items 5 (irritability, p=0.028) and 8 (leisure time, p<0.01); diabetes mellitus - on item 4 (easy bruising, p=0.028); osteoporosis - on item 10 (everyday activities, p=0.031).
No significant dependence of the CushingQoL scores was observed either on the UFC levels (Global scale: p=0.9; Phs: p=0.9; Pcs: p=0.9) or on the duration of active disease (Global scale: p=0.7; Phs: p=0.8; Pcs: p=0.4).
In order to find factors with independent influence on the Cushing QoL scores, we performed a multivariate regression analysis. After adjustment for etiology, remission status, age, UFC, duration of hypercortisolism and presence of hypercortisolism-associated comorbidities no association was observed between etiology of the disease, remission status, age, hormonal findings and duration of the disease. Female gender was associated with poorer outcome on each of the three scores. Presence of hypercortisolism-associated comorbidities independently predicted poorer outcome on the psychological and the global subscales (Table 4).
Table 4.
Hypercortisolism-associated comorbidities as independent predictors of the outcome (multivariate regression analysis)
| Physical score | Psychological score | Global score | ||||
|---|---|---|---|---|---|---|
| B | p | B | p | B | p | |
| Constant | 100.73 | 0.002 | 45.237 | 0.093 | 59.109 | 0.026 |
| Etiology | -0.115 | 0.986 | -0.286 | 0.958 | -0.243 | 0.964 |
| Remission status | 0.708 | 0.919 | -4.100 | 0.489 | -2.898 | 0.617 |
| Gender | -24.00 | 0.016 | -14.121 | 0.092 | -16.591 | 0.044 |
| Age | 0.015 | 0.950 | 0.167 | 0.425 | 0.129 | 0.528 |
| UFC | -0.002 | 0.656 | -0.001 | 0.823 | -0.001 | 0.759 |
| Duration | -0.209 | 0.594 | -0.078 | 0.814 | -0.111 | 0.733 |
| Hypertension | 6.266 | 0.358 | 8.468 | 0.145 | 7.917 | 0.163 |
| Diabetes | -8.514 | 0.204 | 0.809 | 0.886 | -1.522 | 0.783 |
| Osteoporosis | -2.442 | 0.482 | -0.496 | 0.866 | -0.982 | 0.733 |
| Dyslipidemia | 0.376 | 0.951 | 11.087 | 0.036 | 8.409 | 0.102 |
In the subgroup with pituitary CS we performed a multivariate regression analysis with the following variables: presence of hypopituitarism, recidives, radiotherapy and ACTH levels. Higher levels of ACTH were a significant predictor of lower psychological and global score of the questionnaire (Table 5).
Table 5.
Multivariate regression analysis in patients with CD after adjustment for presence of hypopituitarism, recidives and radiotherapy
| Global score | Physical score | Psychological score | ||||
|---|---|---|---|---|---|---|
| B | p | B | p | B | p | |
| Constant | 43.475 | 0.029 | 24.011 | 0.320 | 49.963 | 0.016 |
| Radiotherapy | 3.573 | 0.771 | 2.269 | 0.880 | 4.007 | 0.752 |
| Hypopituitarism | 2.697 | 0.853 | 13.582 | 0.447 | -0.931 | 0.951 |
| Recidives | -3.611 | 0.586 | -5.302 | 0.516 | -3.047 | 0.657 |
| ACTH | -0.132 | 0.067 | -0.036 | 0.680 | -0.163 | 0.029 |
II. Prospective group analysis
Twenty-seven patients with ACTH-independent CS (23 women/4 men) were studied prospectively. All of them underwent unilateral adrenalectomy and achieved remission. The median follow-up from surgery was 3 years (0.5 - 10). Eleven patients were receiving GC replacement therapy at the time of follow-up. They had baseline evaluation (at the state of active disease) and re-evaluation (at the state of stable at least 6 months remission after surgery). The change of the scores of the three scales is shown in Table 6. All the patients had significantly ameliorated scores of the three scales (p<0.01). The mean individual change of scores (the difference between the scores at re-evaluation and baseline) was higher in patients in remission not receiving GC therapy versus patients with hypocortisolism for all the scales. The greatest improvement was observed in the physical score. The average change in the scores for each individual item is shown in Figure 4.
Figure 4.
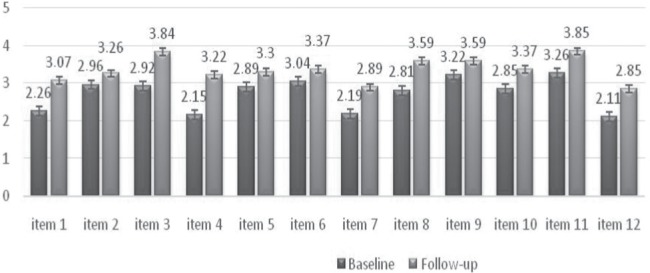
Average change in the scores for each item.
Table 6.
Comparison between the scores in active disease and in remission
| Global score | Physical score | Psychological score | ||
|---|---|---|---|---|
|
The whole group
(n=27) |
Baseline | 42.59 ± 24 | 34.23 ± 25 | 45.37 ± 26 |
| Follow-up | 58.18 ± 26 | 57.10 ± 27 | 58.54 ± 27 | |
| Change | 15.6 (p<0.01) | 22.8 (p<0.01) | 13.2 (p<0.01) | |
|
Patients in remission
(n=16) |
Baseline | 48.18 ± 30 | 40.63 ± 31 | 50.69 ± 31 |
| Follow-up | 64.84 ± 30 | 65.10 ± 29 | 64.76 ± 31 | |
| Change | 15.6 (p<0.01) | 24.9 (p<0.01) | 14.1 (p<0.01) | |
|
Pts with hypocortisolism
(n=11) |
Baseline | 34.47 ± 15 | 25.10 ± 11 | 37.63 ± 18 |
| Follow-up | 48.48 ± 20 | 45.45 ± 23 | 49.49 ± 21 | |
| Change | 14.1 (p<0.01) | 20.5 (p<0.01) | 11.8 (p<0.01) |
DISCUSSION
The present study demonstrates the longterm deleterious effects of endogenous hypercortisolism on the health related quality of life. To the best of our knowledge our study includes the largest number of patients (n=160) analyzed in a single tertiary center for a 10-year period.
Our cross-sectional group analysis showed that the impairment of the HRQoL was similar for both etiologies (CD and adrenal CS) when analyzing separately only patients with active hypercortisolism (n=69). The patients with CD scored worse than those with adrenal CS, however, without reaching statistical significance. A lot of studies have estimated the association of QoL with the etiological form of CS and could not show any statistically significant correlation. Recent data from ERCUSYN failed to find a significant difference in HRQoL between patients with adrenal and pituitary form when comparing active patients with both etiologies (14). Wagenmakers et al. estimated the HRQoL in CS patients and healthy controls using 7 different questionnaires and no item of any questionnaire differed significantly between these two groups (15). Webb et al. did not demonstrate any differences in the total CushingQoL score between CD (n=60) and adrenal forms of CS (n=18) two years after treatment (12). All these data come to show the undeniable fact that the hypercortisolism itself is the main factor that contributes to the impaired quality of life in patients with CS.
Divergent results in regard to the correlation between “remission state” and HRQoL have been reported. In the original validation study (12) significant differences were observed in CushingQoL scores between those with active and controlled disease (based on UFC levels, p = 0.009). Similar results were shown by Santos et al. (16). However, other research groups failed to find statistically significant differences in CushingQoL scores between patients with fully controlled and those with uncontrolled hypercortisolism (Colao et al.) (17). In our cross-sectional group the patients in remission scored better than those with active hypercortisolism for all the scores however without reaching statistical significance. The only item that differed significantly between the two groups was item 8 (leisure time). As an underlying cause we can suspect depressed mood and anxiety - common but often underestimated symptoms which, if remain undiagnosed, may progress to depression and anxiety disorders.
In a separate analysis including only patients in remission (n=72) with both etiologies remitted patients with adrenal form scored significantly better than those with CD only for item 12 (worries about future health), however it did not affect either the physiological, or the global score. This result is not surprising having in mind the patients’ awareness about the recurring nature of CD (18). Another hypothesis can be that the worse HRQoL in CD may be due to complications of pituitary surgery, for example hypopituitarism, although we did not find any correlations between these parameters. Other study, however, found that patients treated for CD without hypopituitarism had better scores on several questionnaires, including the CushingQoL, than those with hormone deficiencies (Wagenmakers et al.).
In our study the female gender was found to be the only negative independent predictor of HRQoL affecting the three integral scores (Gs, Pss and Phs) of the questionnaire, having in mind the clear female preponderance (88% of the patients). Literature data concerning the association of QoL with the gender in subjects with CS are controversial (12, 16). In general, HRQoL is usually more impaired in women than in men and this tendency has been proven in many studies using different questionnaires as tools for assessing the HRQoL (19-21). The presence of gender-specific symptoms such as menstrual disturbances and women's concerns about their reproductive health can partly explain this observation.
CS, regardless of the etiology, is associated with persistent physical morbidity (22). Metabolic disturbances (diabetes, hypertension, dyslipidemia) and osteoporosis comprise the most common hypercortisolism-associated comorbidities. They lead to increased cardiovascular risk, fatigability, myopathy and bone fragility which exert negative impact on well-being and HRQoL. We assessed the individual influence of all these conditions and none of them was correlated with a significant worsening of the scores of the three main subscales. And yet they did affect some of the items of the questionnaire. Diabetes mellitus was associated with worse outcome on item 4 (wound healing) which is not surprising having in mind the aberrations in the proliferative, remodelling, and maturation phases of wound angiogenesis in patients with diabetes (23, 24). The simultaneous presence of hypercortisolism and hyperglycemia may have additive effect. Regarding the hypertension many questionnaires assessing its influence on the HRQoL have been created. The majority of them show that this condition is an independent risk factor for a worse Qol although considered to be almost always a clinically silent disease (25, 26). In agreement with this statement in our study the hypertension affected only item 5 (irritability). We assessed the influence of the hypercortisolism-associated comorbidities in multivariate regression analysis after adjustment for etiology, remission status, age, hormonal results and duration of hypercortisolism. The presence of hypercortisolism-associated comorbidities independently predicted a poorer outcome on the psychological and the global subscales.
Another factor related to HRQoL is the age of the patients. Our results showed a negative correlation between the advancing age and the worries about the physical appearance (item 7). However, it did not affect either the psychological or the global scale score of the questionnaire which is in accordance with other studies (Santos et al.). This result is not surprising as older adults are less concerned about their physical appearance, compared to young individuals.
The CushingQoL questionnaire is proven to be sensitive enough to reflect the subtle changes in the quality of life of patients with CS (16). It is well-known that improvement after successful therapy of CS is often incomplete due to persistent physical and psychological co-morbidities. In order to assess the effect of the remission on HRQoL we performed a prospective study including the patients with an adrenal adenoma treated with unilateral adrenalectomy. They fulfilled the questionnaire twice – at diagnosis of hypercortisolism (baseline visit) and after achieving of stable remission (follow-up visit). The improvement of the scores at re-evaluation was significant for all the three subscales analyzed (physical, psychological and global score) compared to baseline, however neither of them reached normal values typical for the healthy individuals. Different studies using different questionnaires published in the literature confirm that HRQoL in cured individuals remains worse compared to the normal population (5-11). The persistent changes in the physical and mental state of patients with CS after cure of the disease might explain not fully recovery of the QoL (27, 28).
When analyzing the items of the questionnaire separately all the three items belonging to the physical subscale, but not all of those belonging to the psychological one, improved significantly. The items that did not undergo significant changes in cured patients were those related to the pain and irritability, mood swings and angry outbursts. It is well-known that chronic cortisol excess leads to structural brain abnormalities (mainly loss of brain volume) that result in a large spectrum of psychological disturbances. Although it is proven that the brain volume loss is at least partially reversible following correction of hypercortisolism (29), these structural changes might explain the persistence of low quality of life in cured patients. Many studies showed a significant association between anxiety and depression scores and HRQoL parameters in CD patients (5,30).
Obviously the results of different studies exploring HRQoL in CS seem to be various and often contradictory. That makes it really difficult to find predictors for ameliorating QoL of patients with CS unlike other rare diseases, for example acromegaly (31). The reasons seem to be of different origin, however, one of the most serious remains the irreversible hypercortisolism-associated psychological disorders and their impact on the mood of the patients and subsequently on the way of analyzing the subjective questions. What is well-known so far is that the impaired HRQoL persists despite endocrine remission in CS of any etiology.
In conclusion, tools like disease-specific questionnaires represent the most appropriate means by which specific programs can be created in order to help not only through the most difficult periods in the course of the disease, but also during the treatment, in the post-treatment period and when remission is achieved. However, in such a rare disease more data are necessary to help optimizing management and long-term outcome.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing's syndrome: a Consensus Statement. J Clin Endocrinol Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 2.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing's syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–919. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 3.Sonino N, Fava GA. Psychosomatic aspects of Cushing's disease. Psychotherapy and Psychosomatics. 1998;67:140–146. doi: 10.1159/000012274. [DOI] [PubMed] [Google Scholar]
- 4.Santos A, Resmini E, Pascual JC, Crespo I, Webb SM. Psychiatric Symptoms in Patients with Cushing's Syndrome: Prevalence, Diagnosis and Management. Drugs. 2017;77(8):829–842. doi: 10.1007/s40265-017-0735-z. [DOI] [PubMed] [Google Scholar]
- 5.van Aken MO, Pereira AM, Biermasz NR, van Thiel SW, Hoftijzer HC, Smit JW, Roelfsema F, Lamberts SW, Romijn JA. Quality of life in patients after long-term biochemical cure of Cushing's disease. J Clin Endocrinol Metab. 2005;90:3279–3286. doi: 10.1210/jc.2004-1375. [DOI] [PubMed] [Google Scholar]
- 6.Hawn MT, Cook D, Deveney C, Sheppard BC. Quality of life after laparoscopic bilateral adrenalectomy for Cushing's disease. Surgery. 2002;132:1064–1068. doi: 10.1067/msy.2002.128482. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay JR, Nansel T, Baid S, Gumowski J, Nieman LK. Long-term impaired quality of life in Cushing's syndrome despite initial improvement after surgical remission. J Clin Endocrinol Metab. 2006;91:447–453. doi: 10.1210/jc.2005-1058. [DOI] [PubMed] [Google Scholar]
- 8.Heald AH, Ghosh S, Bray S, Gibson C, Anderson SG, Buckler H, Fowler HL. Long-term negative impact on quality of life in patients with successfully treated Cushing's disease. Clinical Endocrinology. 2004;61:458–465. doi: 10.1111/j.1365-2265.2004.02118.x. [DOI] [PubMed] [Google Scholar]
- 9.Forget H, Lacroix A, Cohen H. Persistent cognitive impairment following surgical treatment of Cushing's syndrome. Psychoneuroendocrinology. 2002;27:367–383. doi: 10.1016/s0306-4530(01)00059-2. [DOI] [PubMed] [Google Scholar]
- 10.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing's syndrome after correction of hypercortisolism. Journal of Clinical Endocrinology and Metabolism. 1997;82:912–919. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 11.Sonino N, Navarrini C, Ruini C, Ottolini F, Paoletta A, Fallo F, Boscaro M, Fava GA. Persistent psychological distress in patients treated for endocrine disease. Psychotherapy and Psychosomatics. 2004;73:78–83. doi: 10.1159/000075538. [DOI] [PubMed] [Google Scholar]
- 12.Webb SM, Badia X, Barahona MJ, Colao A, Strasburger CJ, Tabarin A, van Aken MO, Pivonello R, Stalla G, Lamberts SW, Glusman JE. Evaluation of health-related quality of life in patients with Cushing's syndrome with a new questionnaire. Eur J Endocrinol. 2008;158(5):623–630. doi: 10.1530/EJE-07-0762. [DOI] [PubMed] [Google Scholar]
- 13.Winter SD, Depaoli S, Tiemensma J. Assessing differences in how the CushingQoL is interpreted across countries: comparing patients from the U.S. and the Netherlands. Front Endocrinol (Lausanne) 2018;9:368. doi: 10.3389/fendo.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valassi E, Feelders R, Maiter D, Chanson P, Yaneva M, Reincke M, Krsek M, Tóth M, Webb SM, Santos A, Paiva I, Komerdus I, Droste M, Tabarin A, Strasburger CJ, Franz H, Trainer PJ, Newell-Price J, Wass JA, Papakokkinou E, Ragnarsson O, ERCUSYN Study Group Worse Health-Related Quality of Life at long-term follow-up in patients with Cushing's disease than patients with cortisol producing adenoma. Data from the ERCUSYN. Clin Endocrinol (Oxf) 2018;88(6):787–798. doi: 10.1111/cen.13600. Epub 2018. Erratum in: Clin Endocrinol (Oxf). 2018;89(5):678. [DOI] [PubMed] [Google Scholar]
- 15.Wagenmakers MA, Netea-Maier RT, Prins JB, Dekkers T, den Heijer M, Hermus AR. Impaired quality of life in patients in long-term remission of Cushing's syndrome of both adrenal and pituitary origin: a remaining effect of long-standing hypercortisolism? Eur J Endocrinol. 2012;167(5):687–695. doi: 10.1530/EJE-12-0308. [DOI] [PubMed] [Google Scholar]
- 16.Santos A, Resmini E, Martínez-Momblán MA, Crespo I, Valassi E, Roset M, Badia X, Webb SM. Psychometric performance of the CushingQoL questionnaire in conditions of real clinical practice. Eur J Endocrinol. 2012;167(3):337–342. doi: 10.1530/EJE-12-0325. [DOI] [PubMed] [Google Scholar]
- 17.Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM, Pasireotide B2305 Study Group A 12-month phase 3 study of pasireotide in Cushing's disease. New Engl J Med. 2012;366:914–924. doi: 10.1056/NEJMoa1105743. [DOI] [PubMed] [Google Scholar]
- 18.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A. Endocrine Society. Treatment of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20:562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 20.Norris CM, Ghali WA, Galbraith PD, Graham MM, Jensen LA, Knudtson ML, APPROACH Investigators Women with coronary artery disease report worse health-related quality of life outcomes compared to men. Health Qual Life Out. 2004;2:21. doi: 10.1186/1477-7525-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badia X, Schiaffino A, Alonso J, Herdman M. Using the EuroQoI 5-D in the Catalan general population: feasibility and construct validity. Qual Life Res. 1998;7:311–322. doi: 10.1023/a:1024933913698. [DOI] [PubMed] [Google Scholar]
- 22.Ferraù F, Korbonits M. Metabolic comorbidities in Cushing's syndrome. Eur J Endocrinol. 2015;173(4):M133–157. doi: 10.1530/EJE-15-0354. [DOI] [PubMed] [Google Scholar]
- 23.Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazziotti G, Formenti AM, Frara S, Maffezzoni F, Doga M, Giustina A. Diabetes in Cushing Disease. Curr Diab Rep. 2017;17(5):32. doi: 10.1007/s11892-017-0860-9. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho MV, Siqueira LB, Sousa AL, Jardim PC. The influence of hypertension on quality of life. Arq Bras Cardiol. 2013;100(2):164–174. doi: 10.5935/abc.20130030. [DOI] [PubMed] [Google Scholar]
- 26.Schmieder RE, Jumar A, Fronk EM, Alexandre AF, Bramlage P. Quality of life and emotional impact of a fixed-dose combination of antihypertensive drugs in patients with uncontrolled hypertension. J Clin Hypertens (Greenwich) 2017;19(2):126–134. doi: 10.1111/jch.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing's syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–919. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 28.Sonino N, Navarrini C, Ruini C, Ottolini F, Paoletta A, Fallo F, Boscaro M, Fava GA. Persistent Psychological Distress in Patients Treated for Endocrine Disease. Psychother Psychosom. 2004;73:78–83. doi: 10.1159/000075538. [DOI] [PubMed] [Google Scholar]
- 29.Bourdeau I, Bard C, Noël B, Leclerc I, Cordeau MP, Bélair M, Lesage J, Lafontaine L, Lacroix A. Loss of brain volume in endogenous Cushing's syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002;87(5):1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- 30.Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM, ERCUSYN Study Group The European Registry on Cushing's syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165(3):383–392. doi: 10.1530/EJE-11-0272. [DOI] [PubMed] [Google Scholar]
- 31.Vandeva S, Yaneva M, Natchev E, Elenkova A, Kalinov K, Zacharieva S. Disease control and treatment modalities have impact on quality of life in acromegaly evaluated by Acromegaly Quality of Life (AcroQoL) Questionnaire. Endocrine. 2015;49(3):774–782. doi: 10.1007/s12020-014-0521-6. [DOI] [PubMed] [Google Scholar]


