Abstract
Background
We aimed to evaluate whether a high carbohydrate or a high fat diet differs in alteration of the inflammatory and metabolic risk factors in cardio-renal metabolic syndrome in rats.
Methods
Twelve male Wister rats were randomly divided into two groups: one received diet 1 standard pellet rat diet (D1) containing 10% fat, 50% carbohydrate, 25% protein and another group received diet 2 (D2) containing 59% fat, 30% carbohydrate and 11% protein for 16 weeks. Weight was recorded weekly. FSG and insulin levels were measured using an enzymatic spectrophotometric and a standard ELISA kit respectively. Inflammatory parameters including TGF-β, MCP-1, TNF-α, IL-1β, IL-6 in the renal and cardiac tissues of rats were evaluated by ELISA technique.
Result
Food intake in D1 and D2 groups increased in the study period, however food intake in D2 group was significantly higher compared with D1 group. FSG, HOMA and TG concentrations in D2 group were significantly higher compared to D1 group. Moreover, TGF-β and MCP-1 concentrations in the renal tissues of D2 group and TNF-α in the cardiac tissues of D1 group were significantly higher compared with D1 group (P<0.05). Positive associations between IL-1β and TG and between HOMA, FSG with TGF-β and MCP-1 in the renal tissue of animals were also identified.
Keywords: metabolic syndrome, diet, HOMA, insulin resistance
INTRODUCTION
A cluster of interactive maladaptive cardiovascular and kidney disease risk factors including insulin resistance, obesity, dyslipidemia, hyperglycemia, hypertension, cardiac and renal failure due to over-nutrition and development of obesity contribute to what constitutes the Cardio-Renal Metabolic Syndrome (CRS) (1). According to numerous evidences, there are potent interactions between the heart and kidney disorders; renal dysfunction is an independent risk factor for the development of cardiovascular diseases (CVD) and is associated with worsened outcome in patients with different kinds of CVDs (2). Moreover, cardiovascular diseases are the leading cause of mortality, consisting of 43.6% of all deaths in patients with end-stage renal disease (3). On the other hand, cardiac dysfunction, for instance post-myocardial infarction (MI), leads to a gradual decrease in renal function as reflected by an increase in creatinine levels. This interaction between the heart and kidney, where dysfunction of either of them leads to disorder of the other, accompanied by ingredients of metabolic syndrome is usually referred to as the CRS (4). The CRS is associated with early cardiac disease because of diastolic dysfunction, vascular, and renal disease and may lead to type 2 diabetes mellitus (T2DM) especially in overweight and obese individuals (5).
Obesity as a known risk factor for numerous health problems including metabolic syndrome, diabetes, hypertension and dyslipidemia (6) is associated with morbidity and mortality and reduces life expectancy in all age groups (7, 8). Obese individuals are at higher risk of chronic kidney disease (CKD) (9, 10) and obesity has been known as a major independent risk factor of focal glomerulosclerosis (11), podocyte lesions (12), end stage renal disease (13) and even renal cell and kidney cancers (14, 15). Moreover, obesity is a known risk factor for CVD worldwide and all of obesity-associated co-morbidities like hypertension, dyslipidemia, diabetes, or insulin resistance, and elevated levels of fibrinogen and C-reactive protein are associated with increased risk of CVD events (16, 17).
The complex heart-kidney bidirectional relationship in CRS involves numerous pro-inflammatory and metabolic mediators which, via bloodstream in the midst of the prevailing metabolic condition, reach target tissues and deliver the adverse effects of the disease (18). These pro-inflammatory mediators had a potent role in maladaptive remodelling of cardiac and renal tissues and are involved in tissue injuries; From the pathological point of view, several of these key inflammatory mediators play an important role in the pathogenesis of obesity related CRS and inhibition of their expression is a target of therapeutic approaches to mimic the adverse effects of the CRS; among them, transforming growth factor (TGF)-β is expressed at high concentrations in both the heart and kidneys and is a potent activator of fibroblasts, known to induce myo-fibroblastic activation and increased collagen deposition and wound contraction (19). TGF-β is a key mediator of fibrosis in myocardial injury (20) and has a key role in glomerular and tubule-interstitial pathobiology in chronic kidney diseases, mediating apoptosis and epithelial-to-mesenchymal trans-differentiation (EMT) in chronic progressive renal disease (21). Therapeutic strategies to inhibit TGF-β expression are increasingly investigated with the aim of developing novel renal and cardiac events. It has been shown that abrogation of TGF-β signalling using neutralizing antibodies or oral pharmacological inhibitors has promising results in animal models of cardiac remodelling and renal failure (22, 23). Moreover, TGF-β and monocyte chemoattractant protein (MCP)-1 act in a close relationship with each other in the pathogenesis of renal and cardiac events; MCP-1 is a potent inducer of TGF-β production by infarcted heart and damaged kidneys and enhances the fibrogenic potential of mature macrophages by inducing TGF-β and stimulating collagen synthesis, while TGF-β exerts potent pro-fibrotic actions by inducing myo-fibroblast activation and stimulating the synthesis of various extracellular matrix proteins (24). MCP-1 plays a key role in the pathophysiological processes of cardiovascular and renal biology. As a key attractant for mononuclear cells it is involved in the development and progression of cardiovascular diseases like atherosclerosis, restenosis and thrombosis and its production is up-regulated in patients with congestive heart failure and dilated cardiomyopathy (25). Moreover, MCP-1 has a major role in the pathology of numerous renal diseases and the role of MCP-1 has been revealed in whole cascade of the monocyte-associated inflammation from release of monocytes from the bone marrow, building a chemokine gradient for adhesion and infiltration, stimulation, and differentiation of monocytes and macrophages in the kidney (26). Alongside with the pro-inflammatory role of TGF-β and MCP-1 in the cardio-renal injury, it closely acts in parallel with several other pro-inflammatory cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β in the cardio-renal inflammation linking these injuries with metabolic syndrome. Their pro-inflammatory roles linking them to obesity and cardiovascular or renal disorders have been implicated in previous studies (27-29).
Diet and dietary ingredients are one of the main inducers of metabolic syndrome and its adverse effects. By induction of obesity and feeding high fat or high carbohydrate diets or a combination of these two diets are the most common types of diets for inducing metabolic syndrome in animals such as Wistar rats as the most common rodent strains used (30). Many researchers have employed different types of high-fat diets that vary between 20% and 60% of total energy (31). High fat diets have been extensively used to induce metabolic syndrome in experimental animals (32, 33). Studies have also indicated that high-fat diet is effective in inducing hyperglycemia, insulin resistance and dyslipidemia (34). Studies evaluating the role of high carbohydrate diet on inducing metabolic syndrome are scarce while most of the studies used the combination of these two diets for induction of metabolic syndrome and the animals experienced abdominal obesity, hypertension, dyslipidemia and impaired glucose tolerance (35, 36).
To our review of literature, there was no study evaluating the role of numerous potent inflammatory factors in relation with CRS in rats or to evaluate the differential effects of fat-enriched or carbohydrate-enriched diets on the metabolic or pro-inflammatory ingredients of the cardio-renal metabolic syndrome in animals. Therefore, the current experimental project aimed to evaluate these two hypotheses.
ANIMALS AND METHODS
Animals and diets
Male Wistar rats (250-300 g, n=12) were purchased from the Pasteur Institute, Animal Care Center (Karaj, Iran). Each cage included six rats with 12 hours dark/light under standard situation as temperature-controlled environment (25±2¯C) with ad libitum availability to food and water. The study protocol was approved by the veterinary Ethics Committee of Tabriz University of Medical Sciences (TBZMED.REC.1394.747). After one week of acclimatization and feeding a standard laboratory chow diet, rats were randomly divided into two groups, one group received diet 1 or standard pellet rat diet (D1) containing 10% fat, 50% carbohydrate, 25% protein and another group received diet 2 (D2) containing 59% fat, 30% carbohydrate and 11% protein (Table 1). Each group was fed for 16 weeks. Moreover, all rats were weighed weekly by digital scale (PAND industries, px3000, 5kg ±1g). The study procedure and experimental protocol have been published before. Here, the procedure is explained briefly (37-39).
Table 1.
Composition of high carbohydrate diet (D1) and high fat diet (D2)
| D1 diet | D2 diet | ||
|---|---|---|---|
| Ingredient | Percentage | Ingredient | Percentage |
| Protein | 25 % | Butter | 28 % |
| Fat | 10% | Chow diet (D1) | 28% |
| Carbohydrate | 50 % | Sugar | 14 % |
| Ash | 10 % | Yolk egg | 19 % |
| - | White egg | 11 % | |
| Energy | 100 | Total | 100 |
Table 2.
Weight, glycemic factors and serum lipids in treatment groups
| Parameters | D1 group | D2 group | P-value † |
|---|---|---|---|
| Baseline weight (kg) | 0.220±0.004 | 0.217±0.006 | 0.718 |
| Terminal weight (kg) | 0.308±0.014 | 0.428±0.007 | 0.001 |
| FSG (mmol/L) | 7.33±1.32 | 10.23±0.71 | 0.001 |
| Insulin (pmol/L) | 87.92±24.79 | 64.93±2.22 | 0.470 |
| HOMA-IR | 0.64 ± 0.13 | 1.21±0.36 | 0.005 |
| TG (mg/dL) | 41.60 ± 5.01 | 54.02±10.51 | 0.03 |
| TC (mg/dL) | 74.02 ± 3.15 | 73.38 ±7.68 | 0.94 |
| LDL-C (mg/dL) | 27.82 ± 5.88 | 23.96± 5.81 | 0.32 |
| HDL-C (mg/dL) | 30.08 ±7.54 | 42.50 ± 7.73 | 0.17 |
| AIP | 2.49 ± 1.12 | 0.77 ± 0.23 | 0.17 |
D1, high carbohydrate diet, D2, High fat diet, FSG, Fasting serum glucose, HOMA-IR, Homeostatic Model Assessment for Insulin Resistance. Data are presented as means ± SD. †p value indicated intra-group differences compared by independent samples t-test. P < 0.05 was considered as statistically significant.
Preparation of blood, heart and kidney tissue samples
After an overnight fasting, the rats were anesthetized with Ketamin (6.6 mg/kg) and Xylazine (0.3mg/kg) intraperitoneally. Blood samples were obtained from cardiac puncture and centrifuged at 10000 g at 4°C or 20 min; sera were separated and stored in an ultra-low temperature freezer (Jal-Tajhiz Production, Iran) at -80 °C until assaying. Finally, after rats were sacrificed by decapitation, the left nephrectomy specimens and heart samples were homogenized in phosphate buffered saline (PBS) and centrifuged at 10000 g at 4°C for 20 min, and clear supernatants were collected for biochemical assays.
ELISA
Total protein concentration in the tissue supernatants was measured by protein assay kit (Pars Azmun, Tehran, Karaj); accordingly, insulin, TNF-α, MCP-1, IL-1β, IL-6 and TGF-β concentrations in the supernatants were also determined using routine ELISA kits (Hangzhou Eastbiopharm, Zhejiang, China). Serum lipids were measured using the Autoanalyser (Alcyon 300 USA). Atherogenic index was measured with formula (40): Atherogenic index = ((Total Cholesterol-HDL))/HDL. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the formula: fasting insulin (microU/L) x fasting glucose (nmol/L)/22.5.
Statistical analysis
All statistical analyses were performed using SPSS software, version 16. Kolmogorov–Smirnov test was performed for normality of the distribution of variables. Data are expressed as the mean ± SD. The data were analyzed using independent sample t-test for comparisons between two groups. P < 0.05 was considered as statistically significant.
RESULTS
Changes in weight, serum lipids, glycemic status and food intake
Baseline weight in two groups was identical whereas after dietary treatment, rats in D2 group achieved significantly more weight compared with rats in D1 group (P < 0.001). D2 group also led to the significantly higher concentrations of FSG, insulin resistance and TG concentrations compared with D1 group (P < 0.05). Rats in D1 and D2 groups experienced higher food intake during the study period while the food intake in D2 group was significantly higher compared with rats in D1 group (Figure 1, P < 0.05).
Figure 1.
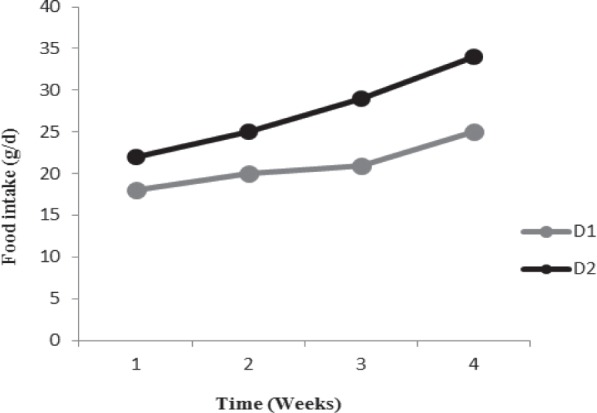
Increase of food intake across the groups during the study period. D1: high carbohydrate diet; D2: high fat diet.
Changes in TNF-α, TGF-β, MCP-1, IL-6 and IL-1β concentrations in the cardiac and renal tissues of treatment groups
The comparison of inflammatory factors including TNF-α, TGF-β, MCP-1, IL-6 and IL-1β in the renal and cardiac tissues of D1 and D2 groups is presented in Table 3. In the cardiac tissue, only the TNF-α concentrations in D1 group were significantly higher compared with D2 group whereas, in the renal tissue, TGF-β and MCP-1 concentrations in D2 group were significantly higher compared with D1 group (P <0.05). No significant difference between other inflammatory parameters of cardiac or renal tissues between groups was identified. Also, we assessed the association between pro-inflammatory parameters and markers of metabolic syndrome in renal tissues (Table 4). Renal IL-6 concentrations were in positive relationship with serum TG (r=0.57, P= 0.009), while, renal TNF-α and MCP-1 were in negative association with HOMA-IR and serum FSG concentrations.
Table 3.
Pro-inflammatory factors in cardiac and renal tissues of treatment groups
| Parameters | Cardiac Tissues | Renal Tissues | ||||
|---|---|---|---|---|---|---|
| D1 group | D2 group | P-value † | D1 group | D2 group | P-value † | |
| TNF-α (ng/mg Protein) | 8.28 ±1.04 | 5.96±0.66 | 0.003 | 0.08±0.02 | 0.11±0.06 | 0.25 |
| TGF-β (ng/mg Protein) | 1.12± 0.39 | 1.11±0.3 | 0.39 | 0.08±0.04 | 0.14±0.05 | 0.027 |
| MCP-1 (ng/mg Protein) | 0.62±0.15 | 0.68±0.07 | 0.12 | 0.05±0.22 | 0.09±0.02 | 0.05 |
| IL-6 (ng/mg Protein) | 0.53± 0.12 | 0.42±0.34 | 0.98 | 0.06±0.02 | 0.07±0.03 | 0.35 |
| IL-1β (ng/mg Protein) | 0.43 ±0.11 | 0.55±0.15 | 0.43 | 0.59±0.21 | 0.55±0.17 | 0.69 |
D1, high carbohydrate diet, D2, High fat diet, Data are presented as means ± SD. †p value indicated intra-group differences compared by independent samples t-test. P < 0.05 was considered as statistically significant.
Table 4.
Correlation matrix between metabolic syndrome markers and inflammatory factors in the renal tissue
| Parameter | r (†P) | LDL | HDL | TG | TC | FSG | HOMA-IR |
|---|---|---|---|---|---|---|---|
| IL-6 | r | 0.005 | -0.12 | 0.57 | 0.28 | -0.18 | 0.09 |
| P | 0.98 | 0.6 | 0.009 | 0.22 | 0.42 | 0.81 | |
| IL-1β | r | -0.17 | -0.22 | 0.28 | -0.02 | -0.4 | -0.1 |
| P | 0.46 | 0.34 | 0.22 | 0.93 | 0.07 | 0.67 | |
| TNF-α | r | -0.12 | -0.24 | 0.04 | 0.04 | 0.58 | 0.506 |
| P | 0.59 | 0.32 | 0.85 | 0.83 | 0.008 | 0.023 | |
| TGF-β | r | -0.23 | -0.31 | 0.03 | 0.01 | 0.39 | 0.27 |
| P | 0.32 | 0.18 | 0.87 | 0.94 | 0.08 | 0.25 | |
| MCP-1 | r | -0.19 | -0.03 | 0.01 | -0.06 | 0.58 | 0.45 |
| P | 0.41 | 0.91 | 0.96 | 0.78 | 0.007 | 0.04 |
IL, interleukin; TNF- α, tumor necrosis factor α; TGF-β, transforming growth factor β; MCP-1 monocyte chemoattractant protein 1. FSG, Fasting serum glucose, HOMA-IR, Homeostatic Model Assessment for Insulin Resistance.† P values obtained by Pearson correlation analysis. P < 0.05 was considered as statistically significant.
DISCUSSION
In the current study, we evaluated the differential effects of dietary carbohydrate or fat enriched diet on pro-inflammatory factors in the cardiac and renal tissues of male Wistar rats with cardio-renal metabolic syndrome. According to our findings, high fat diet induced more pronounced features of cardio-renal metabolic syndrome in rats. Moreover, there was an association between inflammatory parameters of the renal tissue with markers of glycemic status and lipids while no association between these parameters in the cardiac tissue was found.
The most common feature of the cardio-renal metabolic syndrome is insulin resistance, metabolic dyslipidemia alongside with cardiac and renal complications (41). In the current study, we used dietary approaches to induce the cardio-renal metabolic syndrome and a high fat diet revealed the biggest effect on renal and cardiac tissue inflammation and insulin resistance. Numerous previous studies revealed the major potential of a high fat diet in inducing metabolic syndrome and its related metabolic disorders while very scarce experimental studies used high carbohydrate diet and most of them used the combination of high fat and high carbohydrate dietary regimens (30). In the study by Castro et al. the high fat diet for 13 weeks led to a marked increase in the blood pressure, heart rate and major elevations in visceral lipid store in Wistar rats and the researchers suggested that a high fat diet could be the best nutritional intervention procedure in inducing metabolic syndrome (42). Other studies also revealed that high-fat diet is effective in promoting hyperglycemia, insulin resistance and dyslipidemia either independently or concurrently (43).
The potential effects of a high fat diet in increasing the pro-inflammatory parameters including TGF-β and MCP-1 in a renal tissue of rats in the current study was also similar to the findings of previous studies highlighting the pathogenic role of MCP-1 in high-fat diet-induced renal damage; in the study by Decleves et al. (44) a high-fat diet led to renal damage and hypertrophy and increased MCP-1 concentrations in the renal tissue and urine of C57BL/6J mice; thereby, the authors suggested that the enzyme adenosine monophosphate-activated protein kinase (AMPK) was the most important mediator of this high fat diet induced damages. They also revealed that the early increase in glomerular MCP-1 contributes to the subsequent recruitment of macrophages that would then contribute to an enhanced release of pro-inflammatory TNF-α, and the most renal profibrotic factor, TGF-β. TGF-β is a potent activator of renal damage and injury and it has been suggested that hyperlipidemia induced by high fat diet is the most important activator of TGF-beta1/Smad signalling pathway and eventually renal damage (45). TGF-β and MCP-1 are potentially linked with obesity, metabolic syndrome and diabetes mellitus; it has been suggested that TGF-β has a common target molecule for the development of CVD, renal insufficiency and metabolic syndrome (46).
In the current study, high carbohydrate diet increased TNF-α concentrations in the cardiac tissue of rats. TNF-α is a cytokine produced mainly by macrophages, has also been found in vascular smooth muscle cells and elevation of its concentrations had been reported in various cardiac diseases, especially in congestive heart failure (CHF); in the study by Doyama K et al. (47) the elevated TNF-α concentrations have been reported in the cardiac tissue of patients with heart failure. In a similar study, the effect of long term high-carbohydrate or high-fat diet on adiposity, glucose tolerance, and secretion of TNF-α and MCP-1 by adipose tissue and liver in Swiss mice has been evaluated. According to their findings, a high carbohydrate diet but no high fat diet was able to increase the tissue TNF-α concentrations (48).
In another similar work by Ferreira et al., 30-weeks supplementation with carbohydrate - enriched diet in male Wistar rats significantly increased TNF-α and MCP-1 concentrations in the heart tissue of rats (49). The underlying mechanism linking carbohydrate intake and inflammation is related to stimulated NAPDH oxidase in poly-morphonuclear leukocytes and mononuclear cells and increased the generation of reactive oxygen species (ROS) due to reduced antioxidant capacity (50). Moreover, intravenous glucose administration increases concentrations of the inflammatory markers IL-6, IL-18, and tumor necrosis factor α which can be prevented by simultaneous infusion of the anti-oxidant glutathione (51, 52).
In conclusion, in the current work, we showed potential effects of high fat diet on inducing metabolic abnormalities related to cardio-renal syndrome including hyperglycemia, high insulin resistance, and higher inflammatory parameters including TGF-β and MCP-1 in renal tissue of rats. Although, high carbohydrate diet was also capable of inducing higher TNF-α in the cardiac tissue, but because of a more extent metabolic and inflammatory response after a high fat diet, it can be suggested as a dietary intervention tool for inducing the cardio-renal metabolic syndrome in animal models.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research has been performed by a grant from the Research Undersecretary of Tabriz University of Medical Sciences (Project number: 1395.532).
Acknowledgement
The current research was financially supported by a grant from the Tabriz University of Medical Sciences.
References
- 1.Connell AW, Sowers JR. The CardioRenal metabolic syndrome. J Am Soc Hypertens. 2014;8(8):604–606. doi: 10.1016/j.jash.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szymanski MK, Boer RA, Navis GJ, Gilst WH, Hillege HL. Animal models of cardiorenal syndrome: a review. Heart Fail Rev. 2012;17:411–420. doi: 10.1007/s10741-011-9279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahapatra HS, Lalmalsawma R, Singh NP, Kumar M, Tiwari SC. Cardiorenal syndrome. Iran J Kidney Dis. 2009;3(2):61–70. [PubMed] [Google Scholar]
- 4.Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17:2886–2891. doi: 10.1681/ASN.2006010063. [DOI] [PubMed] [Google Scholar]
- 5.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–13. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gai Z, Hiller C, Chin SH, Hofstetter L, Stieger B, Konrad D, Kullak-Ublick GA. Uninephrectomy augments the effects of high fat diet induced obesity on gene expression in mouse kidney. BBA Mol Basis Dis. 2014;1842(9):1870–1878. doi: 10.1016/j.bbadis.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Khaki Khatibi F, Yaghoubi A, Zarghami N, Rahbani M, Babaie H. Evaluation of hs-CRP, antioxidant markers and MDA in patients of coronary artery disease (CAD) containing non-smokers and non-diabetics. J Cardiovasc Thorac Res. 2011;2(4):13–18. [Google Scholar]
- 8.Hellmich N. Obesity threatens life expectancy. USA Today. 2005:2006. in. Retrieved November. [Google Scholar]
- 9.Sharifi MH, Eftekhari MH, Ostovan MA, Rezaianazadeh A. Effects of a therapeutic lifestyle change diet and supplementation with Q10 plus L-carnitine on quality of life in patients with myocardial infarction: A randomized clinical trial. J Cardiovasc Thorac Res. 2017;9(1):21–28. doi: 10.15171/jcvtr.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen HM, Liu ZH, Zeng C, Li SJ, Wang QW, Li LS. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48(5):772–779. doi: 10.1053/j.ajkd.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bergström A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A. Obesity and renal cell cancer–a quantitative review. Brit J Cancer. 2001;85(7):984–990. doi: 10.1054/bjoc.2001.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson KM, Cho E. Obes Cancer. Springer; 2016. Obesity and kidney cancer; pp. 81–93. [DOI] [PubMed] [Google Scholar]
- 16.Sokhanvar S, Mazaki RRS, Mousavinasab N, Golmohammadi Z. The association between serum lipoprotein (a) and other cardiac risk factors with the severity of coronary artery disease. J Cardiovasc Thorac Res. 2011;3(1):35–39. [Google Scholar]
- 17.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cadiovasc Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Otero-Losada M, Llambí HG, Ottaviano G, Cao G, Müller A, Azzato F, Ambrosio G, Milei J. Cardiorenal involvement in metabolic syndrome induced by cola drinking in rats: Proinflammatory cytokines and impaired antioxidative protection. Mediators of Inflammation. 2016;2016:1–11. doi: 10.1155/2016/5613056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 20.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta. Mol Genet Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- 21.Böttinger EP, Bitzer M. TGF-beta signalling in renal disease. J Am Soc Nephrol. 2002;13(10):2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 22.Meredith A, Boroomand S, Carthy J, Luo Z, McManus B. 1,25 Dihydroxyvitamin D3 inhibits TGFβ1- mediated primary human cardiac myofibroblast activation. PLoS ONE. 2015;10(6):e0128655. doi: 10.1371/journal.pone.0128655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagita M. Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol Dial Transplant. 2012;27(10):3686–3691. doi: 10.1093/ndt/gfs381. [DOI] [PubMed] [Google Scholar]
- 24.Dobaczewski M, Frangogiannis N. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2010;1:391–405. doi: 10.2741/s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H. Elevated circulating levels of C–C chemokines in patients with congestive heart failure. Circulation. 1998;97:1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 26.Haller HA, Bertram F, Nadrowitz J. Menne. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens. 2016;25(1):42–49. doi: 10.1097/MNH.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 27.Meldrum DR, Donnahoo KK. Role of TNF in mediating renal insufficiency following cardiac surgery: evidence of a postbypass cardiorenal syndrome. J Surg Res. 1999;85(2):185–199. doi: 10.1006/jsre.1999.5660. [DOI] [PubMed] [Google Scholar]
- 28.Cruz DN, Schmidt-Ott KM, Vescovo G, House AA, Kellum JA, Ronco C, McCullough PA. Karger Publishers: 2013.. Pathophysiology of cardiorenal syndrome type 2 in stable chronic heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI), in ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes; pp. 117–136. [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, House AA, Haapio M. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intens Care Med. 2008;34(5):957–960. doi: 10.1007/s00134-008-1017-8. [DOI] [PubMed] [Google Scholar]
- 30.Wong SK, Chin KY, Suhaimi FHJ, Fairus A, Ima-Nirwana S. Animal models of metabolic syndrome: a review. Nutr Metab. 2016;13(1):65–70. doi: 10.1186/s12986-016-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 32.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pezeshkian M, Darbin A, Rashidi MR, Vatankhah A, Golmohammadi Z, Afrasiabi A, Nouri M. The effect of atherogenic diet with or without enzyme inhibitors on the incidence and progress of atherosclerosis in rabbits. J Cardiovasc Thorac Res. 2011;3(1):7–10. [Google Scholar]
- 34.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 35.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and beta-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322–1332. doi: 10.1017/S0007114510002308. [DOI] [PubMed] [Google Scholar]
- 36.Panchal SK, Poudyal H, Iyer A, Nazer R, Alam A, Diwan V. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodelling in rats. J Cardiovasc Pharmacol. 2011;57:51–64. doi: 10.1097/FJC.0b013e3181feb90a. [DOI] [PubMed] [Google Scholar]
- 37.Hajiluian G, Nameni G, Shahabi P, Mesgari-Abbasi M, Sadigh-Eteghad S, Farhangi MA. Vitamin D administration, cognitive function, BBB permeability and neuroinflammatory factors in high-fat diet-induced obese rats. Int J Obes (Lond) 2017;41(4):639–644. doi: 10.1038/ijo.2017.10. [DOI] [PubMed] [Google Scholar]
- 38.Nameni G, Farhangi MA, Hajilouian G, Shahabi P, Mesgari-Abbasi M. Insulin deficiency: A possible link between obesity and cognitive function. Int J Dev Neurosci. 2017;59:15–20. doi: 10.1016/j.ijdevneu.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Nameni G, Hajiluian G, Shahabi P, Farhangi MA, Mesgari-Abbasi M, Hemmati MR. The impact of vitamin D supplementation on neurodegeneration, TNF-α concentration in hypothalamus, and CSF-to-plasma ratio of insulin in high-fat-diet-induced obese rats. J Mol Neurosci. 2016;61(2):247–255. doi: 10.1007/s12031-016-0864-y. [DOI] [PubMed] [Google Scholar]
- 40.Yokozawa T, Cho EJ, Sasaki S, Satoh A, Okamoto T, Sei Y. The Protective Role of Chinese Prescription Kangen-karyu Extract on Diet-Induced Hypercholesterolemia in Rats. Biological & Pharmaceutical Bulletin. 2006;29(4):760–765. doi: 10.1248/bpb.29.760. [DOI] [PubMed] [Google Scholar]
- 41.Connell AW, Sowers JR. The CardioRenal Metabolic Syndrome. J Am Soc Hypertens. 2014;8(8):604–606. doi: 10.1016/j.jash.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro UGM, Santos RAS, Silva ME, Lima WG, Campagnole-Santos MJ, Alzamora AC. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 2013;12:136–147. doi: 10.1186/1476-511X-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 44.Declèves AE, Mathew AV, Cunard R, Sharma K. AMPK Mediates the Initiation of Kidney Disease Induced by a High-Fat Diet. J Am Soc Nephrol. 2011;22(10):1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen ZX, Xie XY, Yu RC, Zhang J, Lei MX. Hyperlipidemia induced by high fat diet ingestion activates TGF-beta/Smad signalling pathway in the kidney of diabetic rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(10):906–912. [PubMed] [Google Scholar]
- 46.Aihara K, Ikeda Y, Yagi S, Akaike M, Matsumoto T. Transforming Growth Factor-β1 as a common target molecule for development of cardiovascular diseases, renal insufficiency and metabolic syndrome. Cardiol Res Pract. 2011;2011:1–9. doi: 10.4061/2011/175381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyama K, Fujiwara H, Fukumoto M, Tanaka M, Fujiwara Y, Oda T, Inada T, Ohtani S, Hasegawa K, Fujiwara T, Sasayama S. Tumour necrosis factor is expressed in cardiac tissues of patients with heart failure. Int J Cardiol. 1996;3(3):217–225. doi: 10.1016/0167-5273(96)02607-1. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira AV, Mario EG, Porto LC, Andrade SP, Botion LM. High-carbohydrate diet selectively induces tumor necrosis factor-α production in mice liver. Inflammation. 2011;34(2):139–145. doi: 10.1007/s10753-010-9217-0. [DOI] [PubMed] [Google Scholar]
- 49.Ferreira ALA, Ferron AJT, Moreto F, Francisqueti FV, Kitawara KAH, Lo ATC, Ferraz APCR, Garcia JL, Ghiraldeli L, Correa CR. Heart inflammation is associated to carbohydrate-enriched diet in an experimental model of obesity. FASEB J. 2017;31(1):11–17. [Google Scholar]
- 50.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 51.Esposito K, Nappo F, Marfella R. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 52.Gode S, Aksu T, Demirel A, Sunbul M, Gul M, Bakır I, Yeniterzi M. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res. 2016;8(4):140–146. doi: 10.15171/jcvtr.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


