Highlights
-
•
A correct patient risk stratification is of paramount importance for the proper management of economic and human resources.
-
•
Clinical trials are crucial to assessing immunosuppressant prophylaxis and treatment to avoid overuse and treatment shortage.
-
•
Controlled studies may highlight a potential preventive role of immunosuppressant in the development of Covid-19 severe forms
-
•
Despite the risk of infection in rheumatic and gastroenterological diseases a conclusive link with COV-19 remains questionable
Keywords: COVID-19, Inflammatory bowel disease, Rheumatic disease, Infection, Treatment, DMARDs, Small molecules, bDMARDs, Prevention, Flow-chart
1. Introduction
Since December 2019, the new coronavirus infections COVID-19 has spread like a rising tide overwhelming the world. This unabated spread led the World Health Organisation (WHO) to classify it as a pandemic on March 11,2020 a global public health emergency that will also have serious social and economic repercussions [1]. The last time the WHO declared a pandemic was for H1N1 swine flu in 2009. At the time of writing, COVID-19 has killed more than 30,100 people and, unfortunately, this number will continue to rise. While awaiting the development and distribution of a vaccine, scientists around the world are racing against time in the search for possible therapeutic strategies based on those used in previous health emergencies [2].
Everyone, from governments to clinicians and entire populations, feels disoriented before this unknown enemy, and so scientific societies have been drawing up constantly updated guidelines in a bid to aid physicians caring for patients with autoimmune rheumatic and inflammatory bowel disease [[3], [4], [5], [6], [7], [8], [9]]. The highly contagious and phenotypically diverse SARS-CoV-2 virus has a direct impact on everybody's daily life, particularly in the case of already vulnerable patients [10].
Preliminary recommendations regarding changes in the treatment of the patients with autoimmune diseases attending our rheumatology and gastroenterology units.
2. General considerations
Patients with rheumatic and inflammatory bowel diseases (IBDs) may be at higher risk of infection [6,7,11,12]. Disease activity, co-morbidities, immunosuppressive drugs including glucocorticoids (GCs), disease-modifying antirheumatic drugs (DMARDs), conventional synthetic (csDMARDs), biological (bDMARDs), targeted synthetic DMARDs (tsDMARDs), and the biological agents currently available for treating patients with IBD are all considered risk factors for infective complications. In addition, there are the risks inherent to the individual diseases and their treatments.
The overall risk is increased if more than one of these drugs are taken, therefore preventing infections is a crucial part of the management of rheumatic and gastroenterological patients [[13], [14], [15], [16], [17]]. For example, before starting tumour necrosis factor (TNF)-α blocking agents or biological agents in general, QuantiFERON-tb is required for the diagnosis of latent or active tuberculosis, and it is necessary to screen for hepatitis B and C, and HIV infection [18]. Vaccinations are crucial for these patients, and the international guidelines highlight the need to assess their vaccination status in their initial diagnostic work-up [14,15]. Moreover, although rheumatology and IBD patients may cover the whole age spectrum, there are now a significant number of patients receiving immunosuppressants who are more than 80 years old, have a variety of co-morbidities, and are at even higher infectious risk.
3. Patients at higher risk of covid-19 and its related clinical manifestations
Rheumatological diseases and IBD are immune disorders that are associated with an increased risk of developing opportunistic and community-acquired infections such as respiratory virus infections. They are therefore also at risk of high co-morbidity and mortality rates, particularly those receiving immunosuppressive therapy [11,12,16]. This has raised concerns about the potential risk of COVID-19 infection in IBD patients (particularly those who are taking immunosuppressants or biological drugs) because of the high morbidity and mortality rates observed in the old and frail with co-morbidities. However, to the best of our knowledge, the only data concerning the risk of SARS-CoV-2 infection in IBD patients come from just one study [19], and there are no data concerning the risk in rheumatic patients.
The observational study of IBD patients was carried out in Wuhan, China, during the peak of the outbreak (between January 12,020 and February 82,020) involved 318 patients with a mean age of 39 years who answered a questionnaire covering concerns about their clinical condition and disease relapses. As a result of the early warning and strict preventive measures, none of the patients developed any significant clinical manifestation of COVID-19 infection, not even those being treated with corticosteroids (12.6%), immunosuppressants (11%) and biological agents (6%), or those with co-morbidities. At the end of the study period, none of them reported any significant respiratory symptoms or other clinical manifestations attributable to viral infection, and none had a confirmed diagnosis of COVID-19.
An international pediatric and adult registry designed to monitor and report the outcomes of COVID-19 in IBD patients (Surveillance Epidemiology of Coronavirus) Under Research Exclusion (SECURE-IBD: www.covidibd.org) recently developed by the Crohn's & Colitis Foundation, the International Organisation for the Study of IBD (IOIBD), the European Crohn's and Colitis Organisation (ECCO), and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) reported a total of 41 COVID-19 cases as of March 23, 2020: 22 with Crohn's disease (CD) and 19 with ulcerative colitis (UC) from 13 different countries) [9]. Ten were hospitalised and two died: an 82- year-old European male with mildly active UC, Alzheimer's disease and cardiovascular disease who was receiving mesalamine 4000 mg/day. and a 25-year-old male with moderately active UC being treated with infliximab 300 mg every eight weeks and methotrexate 15 mg/week.
Although not confirmed in a large series of patients or in other countries, such data do not seem to support the assumption that IBD patients who adopt all of the appropriate preventive measures are at greater risk of developing or dying of COVID-19 than of developing and dying of endemic influenza.
Although their findings still need to be confirmed, a number of clinical trials conducted first in China and now in Italy have revealed many potentially attractive therapeutic options for COVID-19. These are based on the use of already existing drugs, such as antivirals (e.g., favipiravir, ribavirin, remdesivir, galidesivir), protease inhibitors (disulfiram, lopinavir, ritonavir), drugs that stimulate host immunity (pegylated interferon, chloroquine, hydroxychloroquine), immunosuppressive drugs (e.g., intravenous immunoglobulin, tocilizumab, baricitinib) and glucocorticoids [2].
It is of course fundamental to diagnose the disease as early as possible and adjust the treatment individually on the basis of the course of the disease and the type of patient.
4. Clinical classification of COVID-19 patients
COVID-19 patients can be classified into four groups on the basis of their clinical manifestations, radiological findings, and disease severity.
-
•
Mild cases, with mild clinical symptoms and no imaging findings of pneumonia;
-
•
Moderate cases, characterized by fever, respiratory tract symptoms, and imaging findings of pneumonia;
-
•
Severe cases, with a respiratory rate of ≥30 breaths/min; ≤93% oxygen saturation at rest; arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) of ≤300 mmHg or lung imaging findings of progression in 50% of lesions within 24-to 48 h;
-
•
Critical cases requiring mechanical ventilation and intensive care because of the presence of organ failure (ICU patients).
It should be noted that severe and critical cases are associated with hyperferritinemia, spike C-reactive protein (CRP) levels, hepatic dysfunction, prolonged clotting times and microthromboses reminiscent of diffuse intravascular coagulation, thus recalling the features of septic shock, cytokine-release syndrome (CRS) after chimeric antigen receptor (CAR)-T cell therapy, and macrophage activation syndrome (MAS) [20].
5. COVID-19 GI-related symptoms in IBD patients
A study conducted in Wuhan analysed gastrointestinal (GI) symptoms in patients with confirmed COVID-19, and found that 11.4% presented with at least one GI symptom (nausea, vomiting or diarrhoea): 39.2% of these patients had fever (>38.5 °C), 31.1% fatigue, 21.6% headache, and 10.8% dysponea [19]. In addition, 22.97% had severe/critical COVID-19, and 31.08% showed familial clustering, significantly higher rates than those without GI symptoms (8.14% and 20.45%). Fang et al. found that diarrhoea occurred in 49.5% of patients, in 55.2% of cases after admission and anti-viral therapy, and estimated that 22.2% of the patients presented diarrhoea before diagnosis [21,22].
The clinical case analyses of the digestive manifestations and pathological findings of patients with COVID-19 published in China have been reviewed by Tian et al. [23]. GI symptoms are frequent, and their prevalence increased during the later stages of the Chinese epidemic. Diarrhoea was the most frequent symptom in both children and adults, had a mean duration of 4.1 ± 2.5 days, and was observed both before and after diagnosis. Vomiting was more frequent in children. Both adults and children have digestive symptoms in the absence of respiratory symptoms. Pan et al. found that 48.5% of their patients had digestive symptoms, and 20% a specific symptom (diarrhoea, vomiting or abdominal pain) excluding anorexia, which is consistent with the results of the systematic analysis [24].
Unfortunately, there are no data concerning the risk of relapse of IBD and related symptoms in patients with SARS-CoV-2 infection but, given that worsening symptoms or relapsing IBD are usually characterized by diarrhoea, nausea, vomiting and fever, these may mimic and hide a COVID-19 manifestation. As this could be misinterpreted as a pure relapse, it would be appropriate to investigate other more specific symptoms of COVID-19, such as headache, dyspnoea, or changes in smell and taste before planning an examination or treating such patients.
6. Expert recommendations for the management of rheumatic and IBD patients
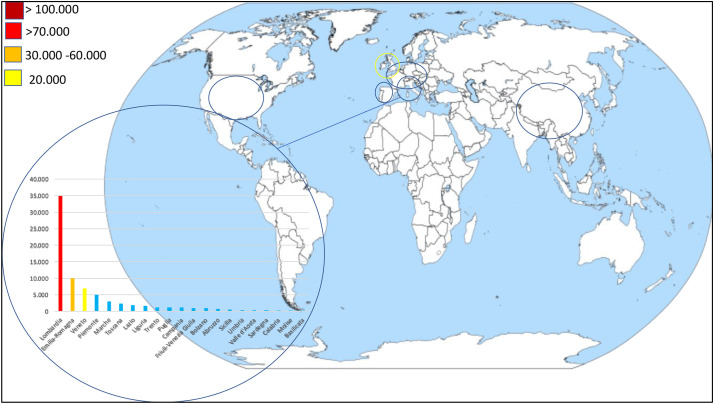
At the time of writing, Italy has the third highest number of COVID-19 cases after USA and Spain (Fig. 1 ); consequently, its experience may be useful for other countries that have not yet had to face the full force of the disease. Lombardy is the Italian region with the highest number of recorded cases (34.507 as 1 of April1, or 47.3% of the total); the corresponding figures in the neighbouring regions of Emilia-Romagna and Veneto are respectively 10,008 (13.6%) and 6935 (9.4%). To the best of our knowledge, there are still no studies that have considered how to handle IBD and rheumatic patients with COVID-19.
Fig. 1.
COVID-19 dashboard: most affected areas and detailed Italian situation as of 1 April 2020.
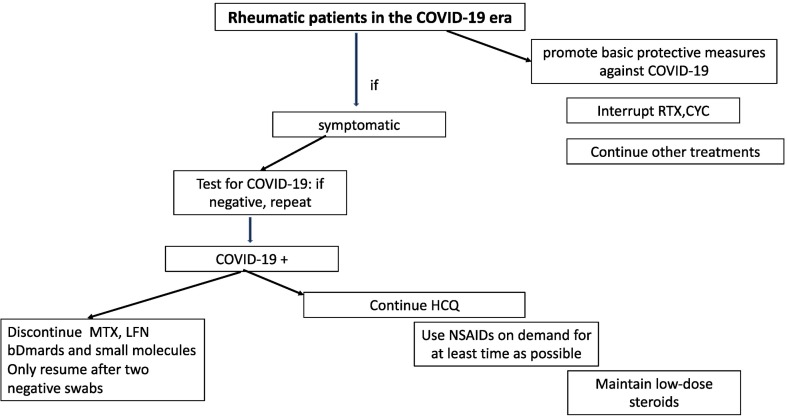
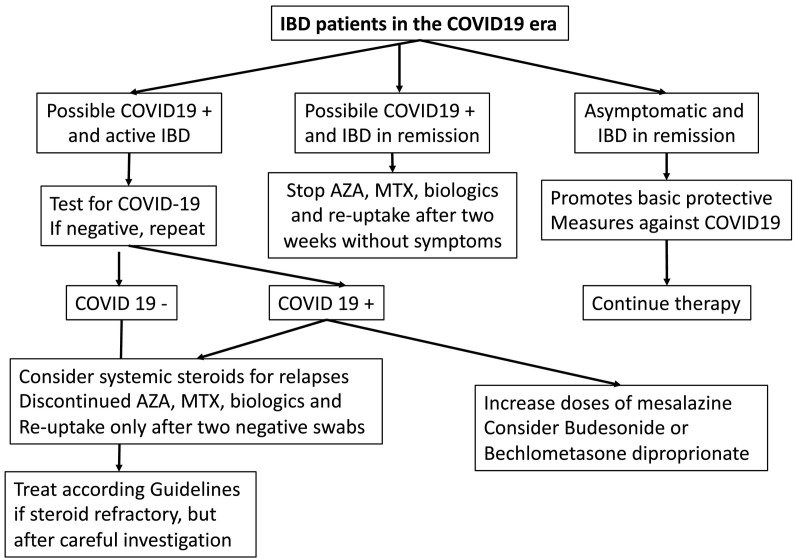
Given the experience of Milan's Luigi Sacco University Hospital (a reference centre for infectious emergencies in Lombardy and Italy), we believe that it is worth sharing the strategic therapeutic plan for autoimmune patients treated with conventional treatments and immunosuppressive/modulatory drugs developed by our gastroenterology, rheumatology and infectious disease units (Fig. 2 , Fig. 3 , Table 1 ).
Fig. 2.
Flow chart to manage rheumatic patients. MTX Methotrexate HCQ hydroxychloroquine RTX rituximab LFN leflunomide bDMARDS Biologic disease- modifying anti-rheumatic drugs NSAIDs Non-steroidal anti-inflammatory drug CYC cyclophosphamide.
Fig. 3.
Flow chart to manage IBD patients. IBD inflammatory bowel disease AZA Azathioprine MTX Methotrexate.
Table 1.
What to do with rheumatic/gastroenterological immunosuppressive treatment in COVID-19- and COVID-19+ patients and infection-related risk.
| Drugs | COVID-19 negative | COVID-19 positive | Infectious risk |
|---|---|---|---|
| Methotrexate | Continue | Discontinue | H |
| Leflunomide | Continue | Discontinue | H |
| Hydroxychloroquine | Continue | Continue | L |
| Rituximab | Discontinue | Discontinue | VH |
| Tocilizumab | Continue | Discontinue | H |
| Sarilumab | Continue | Discontinue | H |
| Anti-TNF-α agents | Continue | Discontinue | H |
| Abatacept | Continue | Discontinue | H |
| JAK inhibitors | Continue | Discontinue | H |
| Anakinra | Continue | Discontinue | H |
| Cyclophosphamide | Discontinue | Discontinue | VH |
| Steroids* | Continue | Maintain low dosage | H |
| Ustekinumab | Continue | Discontinue | H |
| Baricitinb/Tofacitinib | Continue | Discontinue | H |
| NSAIDs | Continue | On demand | NA |
| Mesalazine | Continue | Continue | None |
| Vedolizumab | Continue | Discontinue | H |
| Azathioprine 6-mercaptopurine |
Continued | Discontinued | H |
| Budesonide, Beclomethasone dipropionate | Continued | Continued | None |
| Cyclosporine A | Continued | Discontinued | H |
*In the case of IBD relapse, consider systemic administration.
H high L low VH very high NA not assessed.
General measures.
It is essential to promote the basic protective measures against COVID-19 recommended by the WHO for everybody [25]:
-
•
Stay at home and follow the directions of the local health authority;
-
•
Maintain social distancing of at least two meters from anyone;
-
•
Wash hands frequently and thoroughly with soap and water or clean them using an alcohol-based hand rub;
-
•
Avoid touching the eyes, nose and mouth
-
•
Practice respiratory hygiene by covering the mouth and nose with a bent elbow or tissue when coughing or sneezing (and immediately dispose of the used tissue);
-
•
Seek medical care early if symptomatic.
In addition, people should be educated to wear a mask in order to avoid infecting other people, avoid public toilets, and use gloves for shopping and all other outside activities.
Given the speed at which the pandemic is evolving, all of our patients have been asked to keep themselves informed and been told that our units would provide them with behavioural indications that will enable them to comply fully with all of the provisions issued by the Italian government and regional institutions.
7. Pharmacological treatment of COVID-19-negative rheumatology patients
All patients should be warned not to discontinue or reduce their treatment without consulting their rheumatologist unless they have specific symptoms. The first challenge for rheumatologists is to decide whether to interrupt or continue a treatment: although it is true that the treatment is designed to control disease activity, it is also undeniable that the same treatment may expose patients, such other infection, to an increased risk for COVID-19.
It is known that infections are a major concern in patients with overt inflammatory arthritis as they can contribute to disease flares [26]. One prospective cohort study of patients with inflammatory polyarthritis showed that the risk of hospitalisation because of infection is 2–4 times higher than in the healthy population [27]. Similarly, an analysis by the US CORRONA registry of rheumatoid arthritis (RA) patients has shown that each 0.6 unit increase in the 28-joint Disease Activity Score (DAS28) corresponds to a 25% increase in the rate of infections requiring hospitalisation and a 4% increase in the rate of outpatient infections [28].
7.1. Rituximab
Although there are no published data supporting it, we prefer to discontinue the use of rituximab (RTX) infusions because of the related risk of infections. RTX is a monoclonal antibody that acts by binding the CD20+ surface antigen, thus leading to the depletion of CD20-positive B cells. It was originally approved for the treatment of a broad variety of hematological disease such as B-cell lymphoma, but it has been increasingly used in various autoimmune diseases in which B cells are thought to play a key role, and is now also approved for the treatment of severe active RA and granulomatosis with severe active polyangitis and microscopic polyangitis. In addition to infections. Which remain a major concern for patients receiving RTX, another rare but potentially fatal complication associated with RTX infusion is cytokine release syndrome (CRS), a life-threatening condition generated by uncontrolled immune activation that leads to multi-organ failure and eventually death [29]. COVID-19 can induce such a “cytokine storm” [2,[30], [31], [32]] of over-active effector T cells and the bulk production of pro-inflammatory cytokines, thus leading to acute lung injury (ALI) and ARDS [33].
7.2. Cyclophosphamide
Cyclophosphamide is a highly potent immunosuppressant that has demonstrated efficacy in autoimmune diseases. Given its increased risk of opportunistic infections and potential hematological alterations such as leukopenia and thrombocytopenia we prefer to discontinue it although, at now, there aren't data supporting it [34,35].
7.3. Corticosteroids
Another important and much debated issue is how to deal with the use of corticosteroids, which do not seem to add any benefit in terms of the clinical outcome of COVID-19, and may actually slow virus clearance. We therefore consider it appropriate to reduce their administration to the minimum effective dose, but not below 5–10 mg of prednisone per day.
7.4. NSAIDs
A great tumult arose about the use of NSAIDs after the French Minister of Health»s statement advising against the use of ibuprofen in patients with COVID-19. The European Medicines Agency (EMA) has reiterated that up to now there is no scientific evidence of a link between ibuprofen and the worsening of COVID-19, although the situation will have to be monitored over time [36].
We believe that it is best to be prudent and that it is up to each individual rheumatologist to choose which NSAID or analgesic to use for each specific case. We therefore recommend their use on demand, albeit for as short a time as possible.
7.5. Chloroquine/hydroxychloroquine
A recent study found that chloroquine, a long used antimalarial drug, had a potent in vitro antiviral effect in a COVID-19 assay by increasing the endosomal pH required for virus/cell fusion and interfering with the glycosylation of cell receptors, thus adding to its well-known immune-modulating action [37]. Chloroquine is being considered for inclusion in the next versions of the guidelines for the prevention, diagnosis and treatment of COVID-19 pneumonia to be issued by the National Health Commission of the People's Republic of China [38] and the Italian guidelines for the treatment of COVID-19 [3].
8. Pharmacological treatment in COVID-19-negative gastroentero-logy patients
8.1. Immunosuppressive and biological treatments
Although the available data do not suggest that there is a specific risk of SARS-CoV-2 infection and morbidity in IBD patients receiving immunosuppressive treatment, it is known that opportunistic infections have deleterious effects on such patients, which suggests that the risks and benefits of the treatment should be balanced before continuing its administration. There is evidence showing that IBD patients have impaired innate mucosal immunity, but they should not be considered as having altered immunocompetence per se. The current guidelines recommend screening patients who have to start immunosuppressive or biological therapy for some, but not all viruses: for example, screening for HBV, HCV and HIV is recommended, but screening for herpes simplex virus (HSV), Epstein Barr virus (EBV), and varicella zoster virus (VZV) is not, although it is recommended that patients should be vaccinated against VZV using inactivated vaccine before starting any immunomodulatory therapy because of the greater risk of developing severe viral infection [12]. Given that there is no vaccine or specific treatment for COVID-19, it seems that the only preventive measure that could be taken is to discontinued immunosuppressive treatment while bearing the associated risks in mind.
Some recommendations have been suggested in the case of IBD patients in COVID-19 epidemic areas [39]. As IBD patients taking immunosuppressants should be considered as being at potentially high risk of SARS-CoV-2 infection (particularly if they are in an active stage of the disease, malnourished, elderly, or have co-morbidities such as hypertension or diabetes), specific care should be taken to implement preventive strategies, including the avoidance of jobs at higher risk of transmission the restriction of social activities, and limitations on hospital medical examinations.
Patients can continue their treatment if they are in a stable condition. Expert opinion suggests continuing the use of immunosuppressants, particularly thiopurines and biological agents, because any interruption may be associated with an increased risk of recurrence. This may involve therapeutic measures that also prevent hospitalisation and, in some cases, even the possibility of surgery because these are conditions that increase the risk of infectious complications, including COVID-19. It is also worth remembering that, even if they are discontinued, these drugs continue to have effects for some weeks.
Biological drugs (infliximab, adalimumab, golimumab, vedolizumab, ustekinumab) should not be discontinued for the same reasons as those indicated above, although it may be necessary to favour domiciliary treatment with adalimumab injections if infliximab infusions are not accessible [39]. It is generally not recommended to change treatments or increase the doses of immunosuppressants in epidemic areas unless it is really necessary, but temporary discontinuation can be considered provided that the risk of relapse is taken into account [39]. If it is suspected that a patient receiving immunosuppressive or biological treated is undergoing a relapse with fever and needs to be seen as outpatient, treatment should be discontinued if COVID-19 cannot be ruled out. Patients with biological agents who are at low risk of relapse, such as those in long-term and stable clinical remission, can postpone the infusion, although those at high risk should continue. It is also necessary to remember that, although the risk of a relapse of CD and UC during the first year after the withdrawal of a biological drug or immunosuppressant is 20–50%, it is less for shorter periods of discontinuation (albeit unpredictably so), which means that the risk needs to be assessed before postponing an infusion. In the absence of precise and objective diagnostic instruments that can help decision making (e.g. an endoscopic evaluation of mucosal healing), it is possible to rely on calprotectin or CRP levels. Finally, the factors favouring a reduced risk of relapse are immunomodulatory co-treatment, the absence of complex perianal or severe rectal disease, no history of intestinal or colonic stricture, intra-abdominal abscesses or fistulae, and a limited extent of the disease in the past [40]. In the absence of these, it is wiser not to stop biological treatment.
8.2. Mesalazine
There are no data that contraindicate the use of oral or rectal mesalazine, which can and should be continued if it is already being used.
8.3. Corticosteroids
Although the Chinese experience suggests that the short-term use of low-dose steroids (≤0.5–1 mg/kg for seven days) may be beneficial in controlling overwhelming inflammation and cytokine-related lung injury as much as possible, it is better to avoid systemic steroids, especially if they are administered in combination with immunosuppressants [41,42]. However, in the case of severe IBD exacerbations, their use may be necessary but their therapeutic effects must be balanced against the possible risk of COVID-19 infection.
9. Pharmacological treatment in COVID-19-positive rheumatic and IBD patients
Any patient who develops symptoms of any infection such as fever, cough and/or shortness of breath should be tested (pharyngeal and/or nasal swab, sputum, bronchoalveolar lavage fluid) and given medical care. As SARS-CoV-2 preferentially proliferates in type II alveolar cells, a higher positive rate of nucleic acids is found in the lower respiratory, and so a specimen taken from this area is to be preferred. However, an initial negative test should be repeated on subsequent days because peak viral shedding occurs 3–5 days after disease onset.
In the case of a positive test, immunosuppressive treatment with traditional DMARDs other than chloroquine or hydroxychloroquine, biological DMARDs (bDMARDs), small molecules, and biological agents for IBD should be discontinued throughout the course of the infection. We believe that a temporary suspension of even one month does not worsen disease activity, whereas, at now, given the well-known infectious risk relating to these therapies, continuing immunosuppressive therapy would lead to the possibility of a rapid evolution of the infection. We resume biological agents after patients have undergone two negative tests and their full blood counts, creatinine, bilirubin, albumin, LDH, AST/ALT, CK, CRP, IL-6, troponin T and ferritin levels, pro-thrombin (INR) and lipid profiles have normalized.
Our prudent attitude, guided by current scientific evidence on immunosuppressant infectious risk-related, does not preclude the possibility that future data will instead highlight the protective role of these therapies in the development of severe forms of COVID-19 [43].
10. Reorganisation of rheumatology and gastroenterology clinics
In accordance with the provisions of the Italian government and regional institutions, all deferred medical services were suspended except for those required for urgent clinical conditions (within three days) and those that could only be delayed for ten days. Consequently, all of the scheduled clinical and instrumental evaluations of stable patients in regular follow-up were re-scheduled, although is still possible for patients to interact with their dedicated rheumatology and gastroenterology team via phone interviews and social networks, thus making it possible to monitor their clinical condition [44]. They can also send in the results of routine laboratory tests and report any problems with their clinical condition or current therapies. Electronic intensive care unit (e-ICU) monitoring programs, which allow nurses and physicians to monitor the status of sicker patients are ideal, but patients who report symptoms suggesting a recurrence are preferably assessed by means of a telephone interview in order to clarify the severity of the episode and make therapeutic decisions [45].
Patients complaining symptoms suggesting a severe relapse are invited to visit the hospital (while strictly complying with all of the necessary procedures) with the aim of assessing the need for hospitalisation, and the consequent diagnostic and therapeutic procedures.
Patients on the surgical list were contacted by our reference surgeon and re-scheduling was considered, while ensuring a non-COVID path for urgent interventions.
The following procedures were defined for patients enrolled in controlled clinical trials:
- they can only be admitted to hospital if they are being treated with intravenously administered drugs;
- if they are being treated with subcutaneous drugs and unpostponable visit is planned, the Sponsor is asked to have the drug delivered to their home;
- the possibility of carrying out laboratory tests at trusted laboratories close to each patient's home is verified.
The de novo enrolment of patients with rheumatic disease and/or IBD is continued only if the study drug is the only therapeutic alternative.
For the more than 1000 patients administered biological drugs at our rheumatology and gastroenterology units, the following general measures have been taken:
- they are received by a nurse who provides them with an alcohol solution for hand cleansing, a mask and gloves;
- they then undergo the planned outpatient examination by a doctor from the rheumatology or gastroenterology team;
- infusions are administered in special rooms with armchairs separated by more than one meter (as mortality is higher among elderly patients [46], they are allocated single rooms in order to avoid any other possible contact);
- if necessary, the time interval between infusions is extended.
These procedures are coordinated by three dedicated nurses.
Many of our patients come from other parts of Lombardy or other Italian regions (>20%), and if they cannot reach our centers because of the current restrictions, they are directed to the other centers of the Italian network close to their homes. If this is not possible, the possibility of postponing the infusion in the weeks following the scheduled date is evaluated provided that this is compatible with the patient's clinical condition and the pharmacokinetic characteristics of the drug involved.
In the case of subcutaneous biological therapies, Lombardy's regional government has activated alternative means of delivering the medicines for home use in order to limit the need for patients (particularly the most vulnerable and those living in the more remote location) to attend regional healthcare facilities. The Pharmaceutical Service of Milan (ATS Milan) is available to support the planning of deliveries. In the case of patients resident (and domiciled) outside the region and unable to attend a hospital in Lombardy for objective reasons, the ATS assists patient-centered distribution logistics by activating the patients' local hospital network; in the case of patients domiciled in Lombardy but unable to attend a regional hospital for objective reasons, the ATS schedules the collection of drugs from the hospital pharmacy and their delivery to each patient's home by a courier; in the case of patients resident, domiciled or simply present in the territory of the ATS who are being treated at a hospital outside Lombardy and for whom movement is not recommended, the ATS coordinates with hospital facilities in Lombardy in order to guarantee the continuation of the therapeutic program.
11. Do anti-rheumatic drugs play a prophylactic or therapeutic role in patients with COVID-19 infection?
Some recent studies have reported that chloroquine has an antiviral effect on COVID-19 assays, and chloroquine and its analogue hydroxychloroquine are well known to rheumatologists. The repositioning of old drugs to antiviral treatment is a remarkable strategy because the drugs have well-known safety profiles, side effects, schedules and drug interactions [37,47]. Recent findings have shown that hydroxychloroquine is three times more potent than chloroquine [48]. This in vitro antiviral effect is related to alterations in the endosomal pH required for virus/cell fusion and the glycosylation of SARS-CoV-2 receptors, and the regulation of the immune system [49], and indicates the use of chloroquine/hydroxychloroquine in mild forms of the disease. Depending on studies a different therapeutic regimen is suggested [50].
The Italian Society of Infectious and Tropical disease (Lombardy section) recommend the use of 500 mg of chloroquine twice a day for ten days, or 200 mg of hydroxychloroquine 200 mg per day [3][. However, we would like to point out that, although studies of antimalarials and COVID-19 are promising and have a mechanistic justification, data and controlled studies to support the use of anti-malarial for COVID-19 patients, are still lacking [51,52].
Unjustified tweets come from politicians like President Trump that have emphasized the use of these drugs as: “a real chance to be one of the biggest game changers in the history of medicine”, have led to a current shortage of HCQ for our rheumatic patients. Another raising question if as whether these drugs can be used to prevent COVID-19. Given the lack of scientific evidence, the Italian Society of Infectious and Tropical Diseases (SIMIT) has pronounced against this possibility [3] and we believe that, even though they have a good safety profile, it is necessary to evaluate them further in clinical trials.
Patients with severe COVID-19 infection requiring ICU hospitalisation, experience a pattern of pneumonia that can rapidly progress into respiratory failure, and have a picture of cytokine disruption with high levels of IL-6, IL-2, IL-7, IL-10 and TNF-α [2,33]. On the basis of this, a number of trials are evaluating the possibility of using tocilizumab, an IL-6 receptor blocker licensed for RA and cytokine release syndrome, to treat COVID-19 at an expected dose of 8 mg/kg to be repeated after 12 h. A multicenter RCT approved in China currently involves patients with COVID-19 pneumonia and high IL- 6 levels (ChiCTR2000029765), and a phase II and a phase III studies have been approved by the Italian Drug Regulatory Agency (AIFA) for patients with pneumonia and early respiratory failure that has a one-month reduction in mortality as its primary outcome (TOCIVID-19).
Other therapies used in rheumatology, such as anti-IL-1 or JAK-1 inhibitors and interferon (IFN) may also be used. The antiviral properties of the small molecules seem to be attributable to their ability to interfere with lipid-dependent virus attachment to host cells [53,54,55]. IFN is also one of the most potent innate immune responses preventing viral replication during the early phases of infection [56]. As a selective inhibitor of JAK-1 and JAK-2, baricitinib can dampen the host inflammatory response to the cytokine storm (including IL-6 and IFN-gamma) responsible for the more severe forms of interstitial pneumonia during COVID-19 [57].
In our opinion, strong pre-clinical and clinical studies are needed to determine the safety and effectiveness of these drugs in the treatment of COVID-19.
12. Conclusions
As specialists, we can be of help in the management of COVID-19 for various reasons. Our patients are at high risk of infection because of their underlying disease, co-morbidities, and the immunosuppressive strategies used to treat them [2,13,54,55]. We are responsible for managing our patients by evaluating the most appropriate treatment case by case, which can lead to savings for the healthcare system, reduce the stress on ICUs, and allow the appropriate allocation of resources [58,59]. We also have extensive knowledge of the efficacy, mode of action, and safety of the drugs currently being tested in clinical trials involving COVID-19 patients [58].
The still open questions that only time and trials can clarify are:
Can these drugs play a protective role against Sars -COV- 2?
Can drugs such as tocilizumab also be used chronically or only in the acute stages of infection?
After how long can we resume suspended therapies in our patients?
Will screening for COVID-19 enter clinical routine in the same way as screening for TB and hepatitis B/C before starting immunosuppressive therapies?
Are there any markers of disease progression that can be used in COVID-19 patients to identify those who may develop the disease or its more severe forms?
Given that SARS-CoV-2 is a new virus, there are no data concerning the antibody response profile of COVID-19 patients; studies investigating the dynamics of total antibodies, IgM and IgG antibodies against SARS-CoV-2 are therefore required. Is it possible that antibody testing may play a key role in allowing rapid screening, a timely diagnosis, and an accurate clinical prognosis?
References
- 1.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 3.SIMIT Vademecum. http://www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf Available from.
- 4.NHS Clinical guide for the management of rheumatology patients during the coronavirus pandemic. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/clinical-guide-rheumatology-patients-v1-19-march-2020.pdf Available from.
- 5.EULAR: EULAR guidance for patients COVID-19 outbreak. https://www.eular.org/eular_guidance_for_patients_covis19_outbreak.cfn Available from.
- 6.ACR update Covid 19 https://www.rheumatology.org/Announcements Available from.
- 7.ECCO Taskforce. https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/1st_interview_COVID-19%20ECCOTaskforce_published.pdf Available from.
- 8.ECCO Taskforce. https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/2nd_Interview_COVID-19_ECCO_Taskforce_published.pdf Available from.
- 9.ECCO Taskforce. https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/3rd_Interview_COVID-19_ECCO_Taskforce_published.pdf Available from.
- 10.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueroa-Parra G., Aguirre-Garcia G.M., Gamboa-Alonso C.M., Camacho-Ortiz A., Galarza-Delgado D.A. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217322. [DOI] [PubMed] [Google Scholar]
- 12.Rahier J.F., Magro F., Abreue C. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. Journal Crohn Colitis. 2014;6:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Winthrop K.L. Infection and malignancy in rheumatic diseases. Rheum Dis Clin North Am. 2017;43:xiii–xiv. doi: 10.1016/j.rdc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Furer V., Rondaan C., Heijstek W. Update of EULAR recommendations for Vaccination in Adult Patients with Autoimmune Inflammatory Rheumatics diseases. Ann Rheum Dis. 2019;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 15.Van Assen S., Agmon-Levin N., Elkayam O. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–422. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 16.Kirchgesner J., Lemaitre M., Carrat F., Zureik M., Carbonnel F., Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Meroni P.L., Zavaglia D. Girmenia C Vaccinations in adults with rheumatoid arthritis in an era of new disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2018;36:317–328. [PubMed] [Google Scholar]
- 18.Bartalesi F., Scirè C., Requena-Méndez A., Abad M.A., Buonfrate D., Caporali R. Recommendations for infectious disease screening in migrants to Western Europe with inflammatory arthropathies before starting biologic agents. Results from a multidisciplinary task force of four European societies (SIR, SER, SIMET, SEMTSI) facing the largest impact of the flow of migrants today. Clin Exp Rheumatol. 2017;35:752–765. [PubMed] [Google Scholar]
- 19.An Ping, Ji Mengyao, Ren Haixia, Su Juan, Kang Jian, Yin Anning, Zhou Qian, Shen Linyong, Zhao Liang, Jiang Xiaoda, Xiao Yong, Tan Wei, Lv Xiaoguang, Li Jiao, Liu Shuzhong, Zhou Jing, Chen Hongbin, Xu Yaqing, Liu Jun, Chen Mingkai, Cao Jiwang, Zhou Zhongyin, Shen Lei, Tan Shiyun, yu Honggang, Dong Weiguo, Ding Yijuan. Protection of 318 Inflammatory Bowel Disease Patients from the Outbreak and Rapid Spread of COVID-19 Infection in Wuhan, China (2/20/2020) https://ssrn.com/abstract=3543590 Available at SSRN:
- 20.Dholaria B.R., Bachmeier C.A., Locke F. Mechanisms and management of chimeric antigen receptor T-cell therapy related toxicities. BioDrugs. 2019;33:45–60. doi: 10.1007/s40259-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;24 doi: 10.1136/gutjnl-2020-320926. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang D., Ma J., Guan J. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Chin J Dig. 2020 [Google Scholar]
- 23.Tian Y., Rong L., Nian W., He Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan L., Mi M., Gang Ren H. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. https://journals.lww.com/ajg/Documents/COVID_Digestive_Symptoms_AJG_Preproof.pdf Available from. [DOI] [PMC free article] [PubMed]
- 25.WHO - Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public Available from.
- 26.Galloway J.B., Hyrich K.L., Mercer L.K., Dixon W.G., Fu B., Ustianowski A.P. Anti -TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology biologics register with special emphasis on risks in the elderly. Rheumatology Oxf Engl. 2010;50:124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin J., Lunt M., Bunn D., DPM Symmons, Silman A.J. Risk and predictors of infection leading to hospitalization in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis. 2007;66:308–312. doi: 10.1136/ard.2006.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Au K., Reed G., Curtis J.R., Kremer J.M., Greenberg J.D., Strand V. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 29.Williams M., Khalid T., Hughes S., Bonney D., Wynn R. Rituximab-induced Cytokine Storm in the Absence of Overt Lymphoproliferative Disease. J Pediatr Hematol Oncol. 2016;38:29–31. doi: 10.1097/MPH.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 30.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo A.W.I., Tang N.L.S., To KF How the SARS coronavirus causes disease: Host or organ- ism? J Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteleone G., Sarzi Puttini P., Ardizzone S. Preventing COVID-19-induced pneumonia with anti-cytokine therapy. The Lancet Rheumatology. 2020 doi: 10.1016/S2665-9913(20)30093-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R., Fehr A.R., Vijay R. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV- infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D., Yang Y., Kuang F., Qing S., Hu B., Yu X. Risk of infection with different immunosuppressive drugs combined with glucocorticoids for the treatment of idiopathic membranous nephropathy: A pairwise and network meta-analysis. Int Immunopharmacol. 2019;70:354–361. doi: 10.1016/j.intimp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013 Oct;22(12):1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 36.Day M. Covid-19: European drugs agency to review safety of ibuprofen. BMJ. 2020;368:m1168. doi: 10.1136/bmj.m1168. [DOI] [PubMed] [Google Scholar]
- 37.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 39.Mao R., Liang J., Shen J., Ghosh S., Zhu L.R., Yang H. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(5):426–428. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis E. Tailoring Biologic or Immunomodulator Treatment Withdrawal in Inflammatory Bowel Disease. Front Med (Lausanne) 2020;6:302. doi: 10.3389/fmed.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou W., Liu Y., Tian D. Potential benefits of precise corticosteroids therapy for severe 2019- nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monti S., Balduzzi S., Delvino P. Clinical Course of COVID-19 in a Series of Patients with Chronic Arthritis Treated with Immunosuppressive Targeted Therapies. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollander J.E., Carr B.G. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 45.Smith A.C., Thomas E., Snoswell C.L., Haydon H., Mehrotra A., Clemensen J. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19) J Telemed Telecare. 2020 doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 47.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open- label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keyaerts E., Vijgen L., Maes P., Neyts J., Ranst M.V. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Bioph Res Co. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortegiani Andrea, Ingoglia Giulia, Ippolito Mariachiara. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. pii: S0883-9441(20)30390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scuccimarri R., Sutton E., Fitzcharles M.A. Hydroxychloroquine: a potential ethical dilemma for rheumatologists during the COVID-19 pandemic. J Rheumatol. 2020 doi: 10.3899/jrheum.200369. http://www.jrheum.org/content/early/2020/04/01/jrheum.200369 pii: jrheum.200369. [DOI] [PubMed] [Google Scholar]
- 52.Molina J.M., Delaugerre C., Goff J.L., Mela-Lima B., Ponscarme D. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 Infection. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baglivo M., Baronio M., Natalini G., Beccari T. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020;91:161–164. doi: 10.23750/abm.v91i1.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caso F., Costa L., Ruscitti P., Navarinic L., Del Puente A., Giacomelli R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borden E.C., Sen G.C., Uze G., Silverman R.H., Ransohoff R.M., Foster G.R. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GAM Sanchez, Reinhardt A., Ramsey S., Wittkowski H., Hashkes P.J., Berkun Y. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. 2018;128:3041–3052. doi: 10.1172/JCI98814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marotto D., Sarzi-Puttini P. What is the role of rheumatologists in the era of COVID-19? Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020 doi: 10.1016/j. [DOI] [PMC free article] [PubMed] [Google Scholar]